Primary Malignant Melanoma of The Endocervix Uteri and Outpatient Hysteroscopy as a Diagnostic Tool: Case Report and Literature Overview
Abstract
1. Introduction
2. Materials and Methods
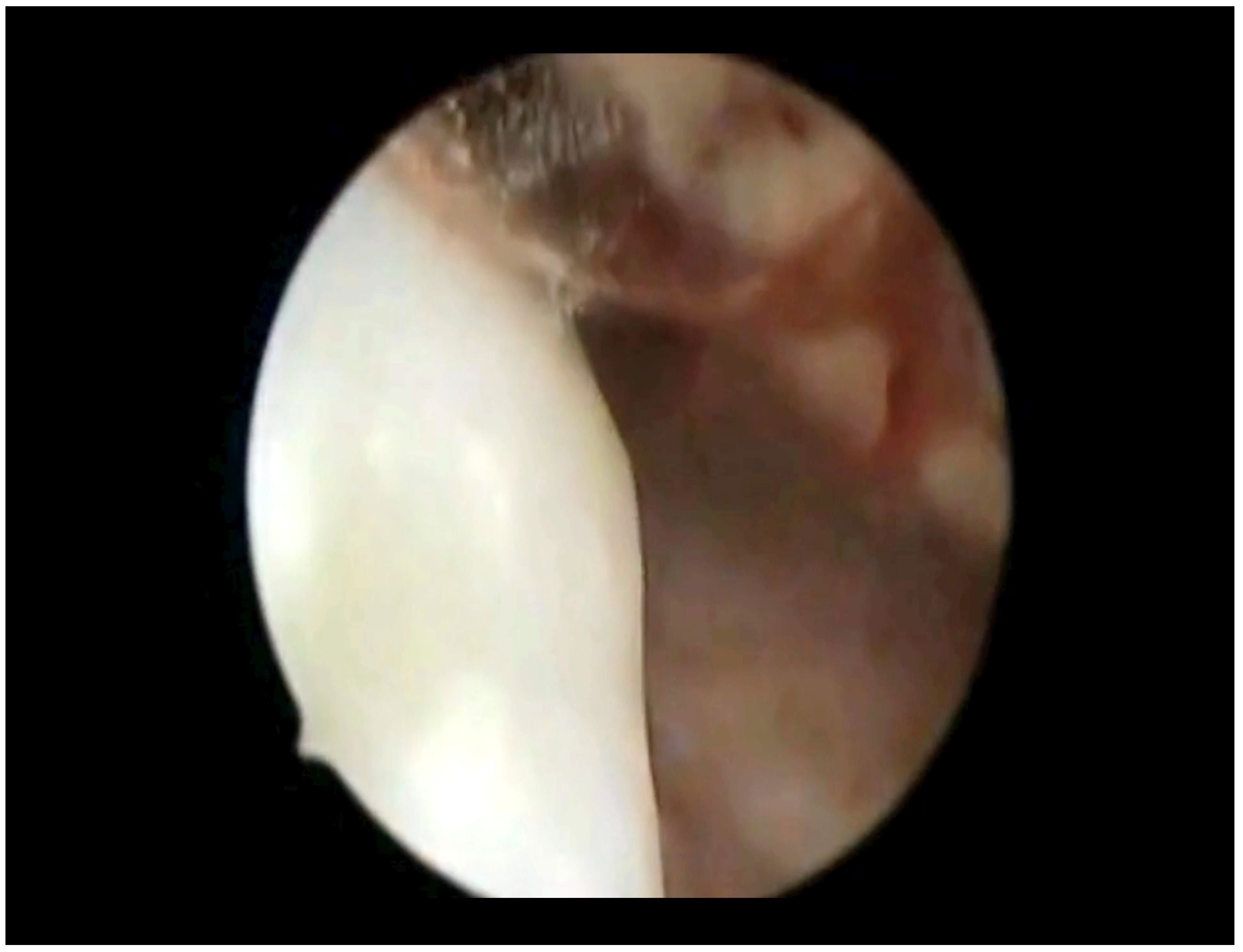
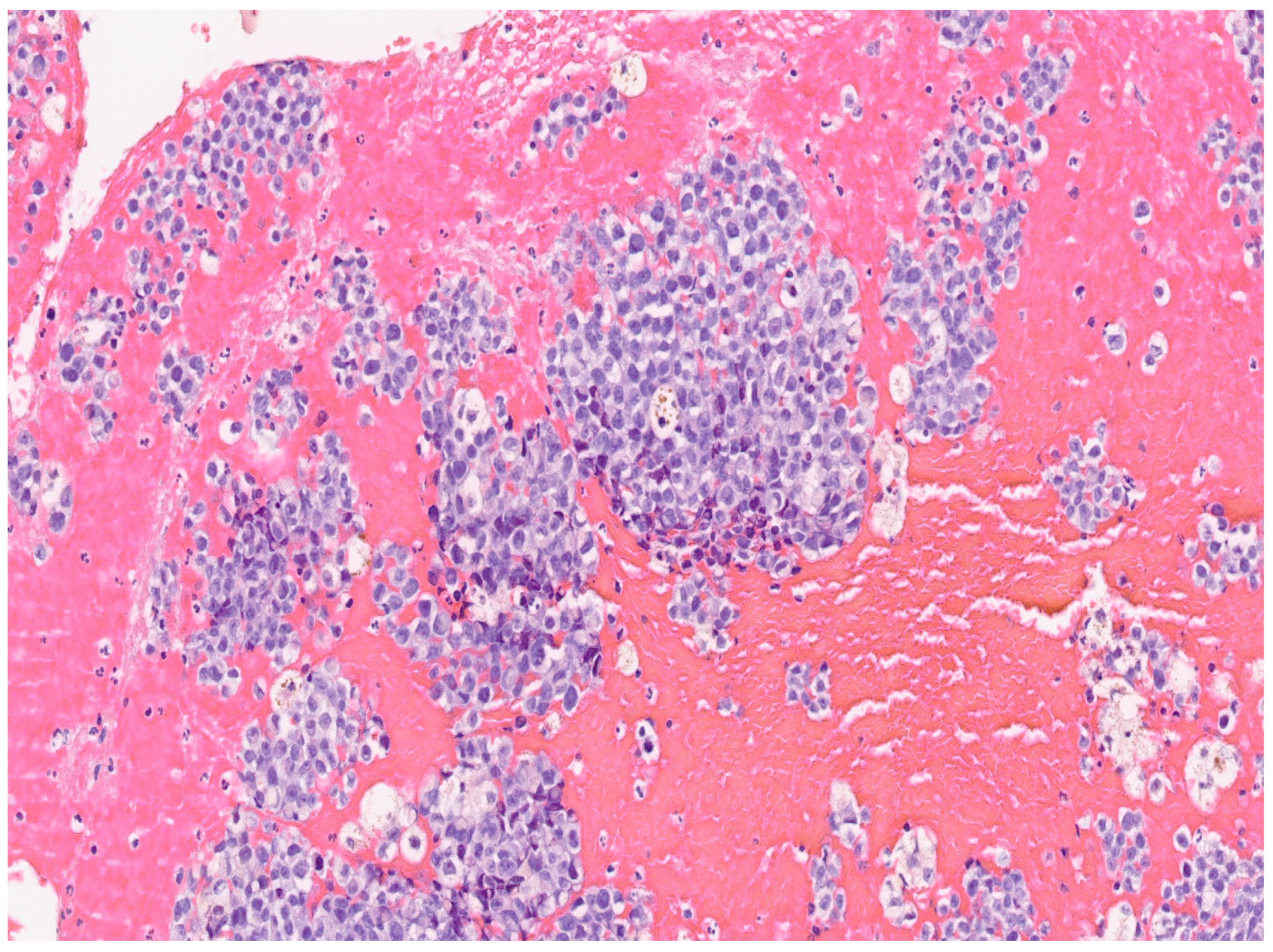
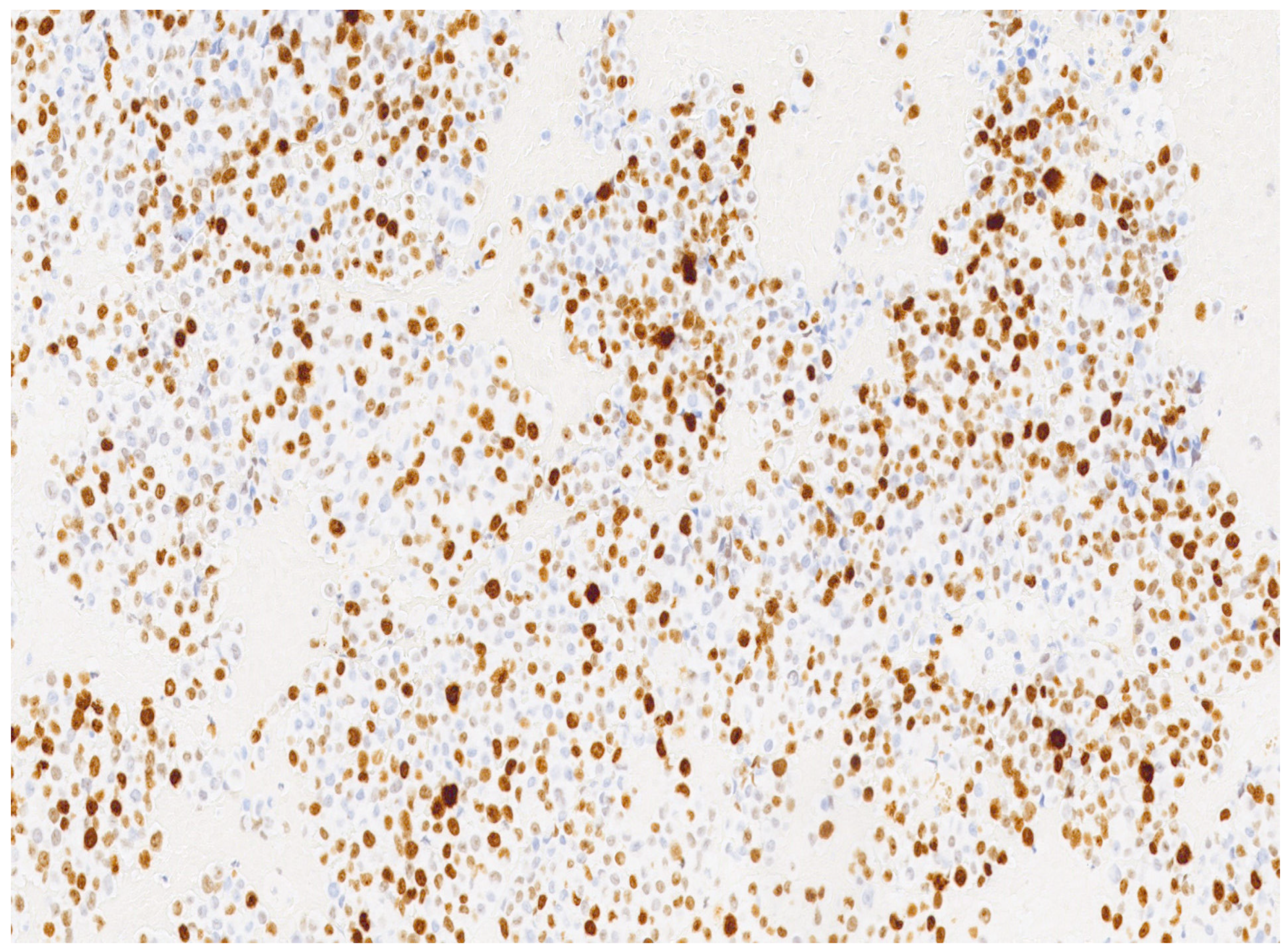
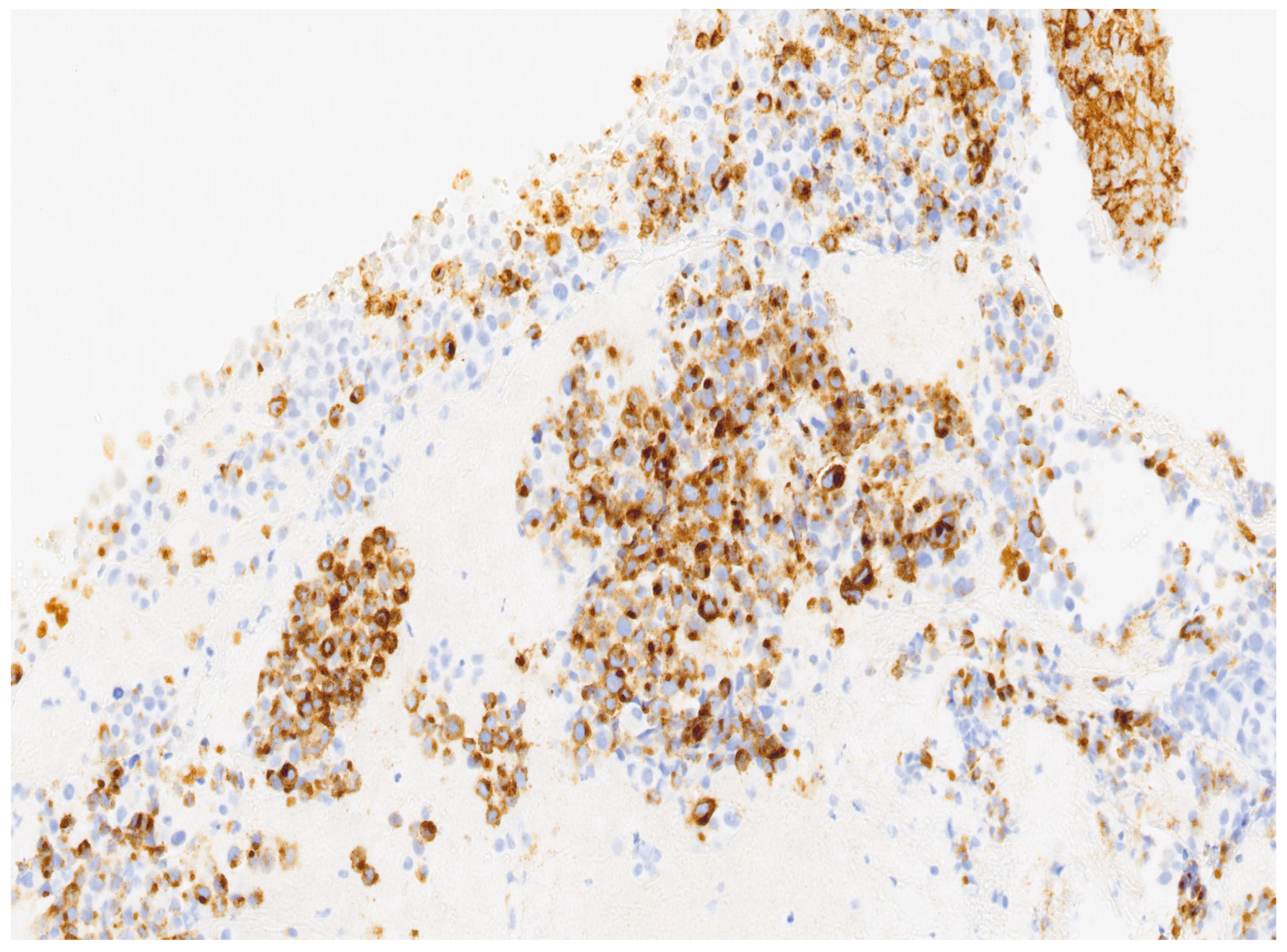
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tranoulis, A.; Laios, A.; Munot, S.; Theophilou, G.; Pathak, D.; Nugent, D. Multidisciplinary Approach in the Management of Primary Malignant Melanoma of the Uterine Cervix: Diagnostic and Management Challenges. J. Gynecol. Surg. 2018, 34, 209–213. [Google Scholar] [CrossRef]
- Simões, M.; Cunha, V.; Nabais, H.; Riscado, I.; Jorge, A.F. Primary malignant melanoma of the uterine cervix-case report and review. Eur. J. Gynaecol. Oncol. 2011, 32, 448–451. [Google Scholar] [PubMed]
- Udager, A.M.; Frisch, N.K.; Hong, L.J.; Stasenko, M.; Johnston, C.M.; Liu, J.R.; Chan, M.P.; Harms, P.W.; Fullen, D.R.; Orsini, A.; et al. Gynecologic melanomas: A clinicopathologic and molecular analysis. Gynecol. Oncol. 2017, 147, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Patrick, R.J.; Fenske, N.A.; Messina, J.L. Primary mucosal melanoma. J. Am. Acad. Dermatol. 2007, 56, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Kechagias, K.S.; Zafeiri, M.; Katsikas Triantafyllidis, K.; Kyrtsonis, G.; Geropoulos, G.; Lyons, D.; Burney Ellis, L.; Bowden, S.; Galani, A.; Paraskevaidi, M.; et al. Primary Melanoma of the Cervix Uteri: A Systematic Review and Meta-Analysis of the Reported Cases. Biology 2023, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Vlajkovic, S.; Jovanovic, P.; Stefanovic, V. Primary mucosal melanomas: A comprehensive review. Int. J. Clin. Exp. Pathol. 2012, 5, 739–753. [Google Scholar] [PubMed]
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A.; et al. Melanoma. Nat. Rev. Dis. Primers. 2015, 1, 15003. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Kumar, N.; Ahuja, A.; Jain, A.; Ray, R.; Sarkar, C.; Gupta, S.D. Primary malignant melanoma at unusual sites: An institutional experience with review of literature. Melanoma Res. 2010, 20, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Jalpota, Y. Primary malignant melanoma of the uterine cervix. Indian. J. Pathol. Microbiol. 2009, 52, 575–576. [Google Scholar] [CrossRef]
- Uzüm, N.; Köse, F.; Ataoğlu, O. Metastatic malignant melanoma of the uterine cervix: First diagnosed on liquid-based cytology. Diagn. Cytopathol. 2008, 36, 769–772. [Google Scholar] [CrossRef]
- Min, K.J.; Kim, Y.S.; Hong, J.H.; Lee, J.K.; Yang, D.S. Primary malignant melanoma of uterine cervix: A suggestion of new scheme of treatment combination. Chin. J. Cancer Res. 2014, 26, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Piura, B. Management of primary melanoma of the female urogenital tract. Lancet Oncol. 2008, 9, 973–981. [Google Scholar] [CrossRef]
- Ye, Y.; Fu, A.; Cai, J.; Ma, Z.; Chen, Y.; Zou, X.; Li, H.; Chen, Y.; Zhao, S.; Chen, C. Primary malignant melanoma of the cervix: A comprehensive analysis of case reports in the Chinese population. Cancer Med. 2023, 12, 14052–14061. [Google Scholar] [CrossRef] [PubMed]
- Indini, A.; Di Guardo, L.; Cimminiello, C.; Lorusso, D.; Raspagliesi, F.; Del Vecchio, M. Investigating the role of immunotherapy in advanced/recurrent female genital tract melanoma: A preliminary experience. J. Gynecol. Oncol. 2019, 30, e94. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Uhara, H.; Mikoshiba, Y.; Kobayashi, A.; Uchiyama, R.; Tateishi, K.; Yamamoto, H.; Okuyama, R. Nivolumab-induced organizing pneumonia in a melanoma patient. Jpn. J. Clin. Oncol. 2016, 46, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Anko, M.; Nakamura, M.; Kobayashi, Y.; Tsuji, K.; Nakada, S.; Nakamura, Y.; Funakoshi, T.; Banno, K.; Aoki, D. Primary malignant melanoma of the uterine cervix or vagina which were successfully treated with nivolumab. J. Obstet. Gynaecol. Res. 2020, 46, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, A.; Esmaeil Pour, M.A.; Mousavi, S.; Khalili-Toosi, A.R.; Amin, A. Primary Malignant Melanoma of the Genitourinary System: A Systemic Review and Report of Eight Cases. Cureus 2022, 14, e30444. [Google Scholar] [CrossRef] [PubMed]
- Stabile, G.; Ripepi, C.; Sancin, L.; Restaino, S.; Mangino, F.P.; Nappi, L.; Ricci, G. Management of Primary Uterine Cervix B-Cell Lymphoma Stage IE and Fertility Sparing Outcome: A Systematic Review of the Literature. Cancers 2023, 15, 3679. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, A.; Laibangyang, A.; Yazdani, A.; Hurwitz, J.; Chuang, L. Recurrent cervical cancer after trachelectomy diagnosed by hysteroscopy: A case report. Gynecol. Oncol. Rep. 2023, 45, 101134. [Google Scholar] [CrossRef]
- Gadducci, A.; Carinelli, S.; Guerrieri, M.E.; Aletti, G.D. Melanoma of the lower genital tract: Prognostic factors and treatment modalities. Gynecol. Oncol. 2018, 150, 180–189. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbé, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Lorigan, P.; et al. Efficacy and Safety of Nivolumab Alone or in Combination With Ipilimumab in Patients With Mucosal Melanoma: A Pooled Analysis. J. Clin. Oncol. 2017, 35, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Zhang, X.; Xu, Q. Primary malignant melanoma of the cervix: A case report. Oncol. Lett. 2014, 8, 2661–2663. [Google Scholar] [CrossRef] [PubMed]
- Osamura, R.Y.; Watanabe, K.; Oh, M. Melanin-containing cells in the uterine cervix: Histochemical and electron-microscopic studies of two cases. Am. J. Clin. Pathol. 1980, 74, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Norris, H.J.; Taylor, H.B. Melanomas of the vagina. Am. J. Clin. Pathol. 1966, 46, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, S.; Bajetta, E.; Carcangiu, M.L.; Formisano, B.; Ducceschi, M.; Buzzoni, R. A literature overview of primary cervical malignant melanoma: An exceedingly rare cancer. Crit. Rev. Oncol. Hematol. 2012, 81, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Dealberti, D.; Riboni, F.; Cosma, S.; Pisani, C.; Montella, F.; Saitta, S.; Calagna, G.; Di Spiezio Sardo, A. Feasibility and Acceptability of Office-Based Polypectomy with a 16F Mini-Resectoscope: A Multicenter Clinical Study. J. Minim. Invasive Gynecol. 2016, 23, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Luerti, M.; Vitagliano, A.; Di Spiezio Sardo, A.; Angioni, S.; Garuti, G.; De Angelis, C.; Italian School of Minimally Invasive Gynecological Surgery Hysteroscopists Group. Effectiveness of Hysteroscopic Techniques for Endometrial Polyp Removal: The Italian Multicenter Trial. J. Minim. Invasive Gynecol. 2019, 26, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Nappi, L.; Angioni, S.; De Feo, V.; Greco, P.; Stabile, G.; Greco, F.; D’Alterio, M.N.; Sorrentino, F. Diagnostic accuracy of hysteroscopy vs dilation and curettage (D&C) for atypical endometrial hyperplasia in patients performing hysterectomy or serial follow-up. Clin. Exp. Obstet. Gynecol. 2022, 49, 24. [Google Scholar] [CrossRef]
- Kechagias, K.S.; Kalliala, I.; Bowden, S.J.; Athanasiou, A.; Paraskevaidi, M.; Paraskevaidis, E.; Dillner, J.; Nieminen, P.; Strander, B.; Sasieni, P.; et al. Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: Systematic review and meta-analysis. BMJ 2022, 378, e070135. [Google Scholar] [CrossRef]
- Noguchi, T.; Ota, N.; Mabuchi, Y.; Yagi, S.; Minami, S.; Okuhira, H.; Yamamoto, Y.; Nakamura, Y.; Ino, K. A Case of Malignant Melanoma of the Uterine Cervix with Disseminated Metastases throughout the Vaginal Wall. Case Rep. Obstet. Gynecol. 2017, 2017, 5656340. [Google Scholar] [CrossRef]
- Yuan, G.; Wu, L.; Li, B.; An, J. Primary malignant melanoma of the cervix: Report of 14 cases and review of literature. Oncotarget 2017, 8, 73162–73167. [Google Scholar] [CrossRef]
- Clark, K.C.; Butz, W.R.; Hapke, M.R. Primary malignant melanoma of the uterine cervix: Case report with world literature review. Int. J. Gynecol. Pathol. 1999, 18, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Min, A.; Fu, A.; Huang, M.; Wang, H.; Chen, H. Primary Malignant Melanoma of the Cervix: An Integrated Analysis of Case Reports and Series. Front. Oncol. 2022, 12, 913964. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Yang, A.; Zhang, Y.; Tao, L.; Zou, H.; Ren, Y.; Liang, W.; Jiang, J.; Zhao, J.; Zhang, W.; et al. Primary Cervical Malignant Melanoma: 2 Cases and a Literature Review. Int. J. Gynecol. Pathol. 2019, 38, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Cantuaria, G.; Angioli, R.; Nahmias, J.; Estape, R.; Penalver, M. Primary malignant melanoma of the uterine cervix: Case report and review of the literature. Gynecol. Oncol. 1999, 75, 170–174. [Google Scholar] [CrossRef]
- Borcoman, E.; Le Tourneau, C. Pembrolizumab in cervical cancer: Latest evidence and clinical usefulness. Ther. Adv. Med. Oncol. 2017, 9, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, M.B.; Broach, V.; Shoushtari, A.N.; Carvajal, R.D.; Alektiar, K.; Kollmeier, M.A.; Abu-Rustum, N.R.; Leitao, M.M., Jr. Combined immunotherapy and radiation for treatment of mucosal melanomas of the lower genital tract. Gynecol. Oncol. Rep. 2016, 16, 42–46. [Google Scholar] [CrossRef]
- Chanal, J.; Kramkimel, N.; Guegan, S.; Moguelet, P.; Fourchotte, V.; Avril, M.F. Locally Advanced Unresectable Vaginal Melanoma: Response With Anti-Programmed Death Receptor 1. J. Low. Genit. Tract. Dis. 2016, 20, e4–e5. [Google Scholar] [CrossRef]
- Shah, V.; Panchal, V.; Shah, A.; Vyas, B.; Agrawal, S.; Bharadwaj, S. Immune checkpoint inhibitors in metastatic melanoma therapy (Review). Med. Int. 2024, 4, 13. [Google Scholar] [CrossRef]
- Trichkova, K.P.; Görtler, F.; Bjørge, L.; Schuster, C. Assessment of Variables Related to the Risk of Severe Adverse Events in Cutaneous Melanoma Patients Treated with Immune Checkpoint Inhibitors. Cancers 2024, 16, 250. [Google Scholar] [CrossRef]
- Wang, D.; Xu, T.; Zhu, H.; Dong, J.; Fu, L. Primary malignant melanomas of the female lower genital tract: Clinicopathological characteristics and management. Am. J. Cancer Res. 2020, 10, 4017–4037. [Google Scholar] [PubMed]
- Diakosavvas, M.; Fasoulakis, Z.N.; Kouroupi, M.; Theodora, M.; Inagamova, L.; Tsatsaris, G.; Nikolaou, P.; Frangia-Tsivou, K.; Giatromanolaki, A.; Kontomanolis, E.N. Primary Malignant Melanoma of the Cervix: A Case Report and a Review of the Literature. Case Rep. Oncol. Med. 2020, 2020, 7206786. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dealberti, D.; Bosoni, D.; Spissu, F.; Pisani, C.; Pizio, C.; Nappi, L.; Sorrentino, F.; Carlucci, S.; Stabile, G. Primary Malignant Melanoma of The Endocervix Uteri and Outpatient Hysteroscopy as a Diagnostic Tool: Case Report and Literature Overview. Diseases 2024, 12, 126. https://doi.org/10.3390/diseases12060126
Dealberti D, Bosoni D, Spissu F, Pisani C, Pizio C, Nappi L, Sorrentino F, Carlucci S, Stabile G. Primary Malignant Melanoma of The Endocervix Uteri and Outpatient Hysteroscopy as a Diagnostic Tool: Case Report and Literature Overview. Diseases. 2024; 12(6):126. https://doi.org/10.3390/diseases12060126
Chicago/Turabian StyleDealberti, Davide, David Bosoni, Federica Spissu, Carla Pisani, Corinna Pizio, Luigi Nappi, Felice Sorrentino, Stefania Carlucci, and Guglielmo Stabile. 2024. "Primary Malignant Melanoma of The Endocervix Uteri and Outpatient Hysteroscopy as a Diagnostic Tool: Case Report and Literature Overview" Diseases 12, no. 6: 126. https://doi.org/10.3390/diseases12060126
APA StyleDealberti, D., Bosoni, D., Spissu, F., Pisani, C., Pizio, C., Nappi, L., Sorrentino, F., Carlucci, S., & Stabile, G. (2024). Primary Malignant Melanoma of The Endocervix Uteri and Outpatient Hysteroscopy as a Diagnostic Tool: Case Report and Literature Overview. Diseases, 12(6), 126. https://doi.org/10.3390/diseases12060126









