Surgical Resection and Immediate Reconstruction with a Bilayer Wound Collagen Matrix of a Rare Oral Angiosarcoma: A Case Report
Abstract
1. Introduction
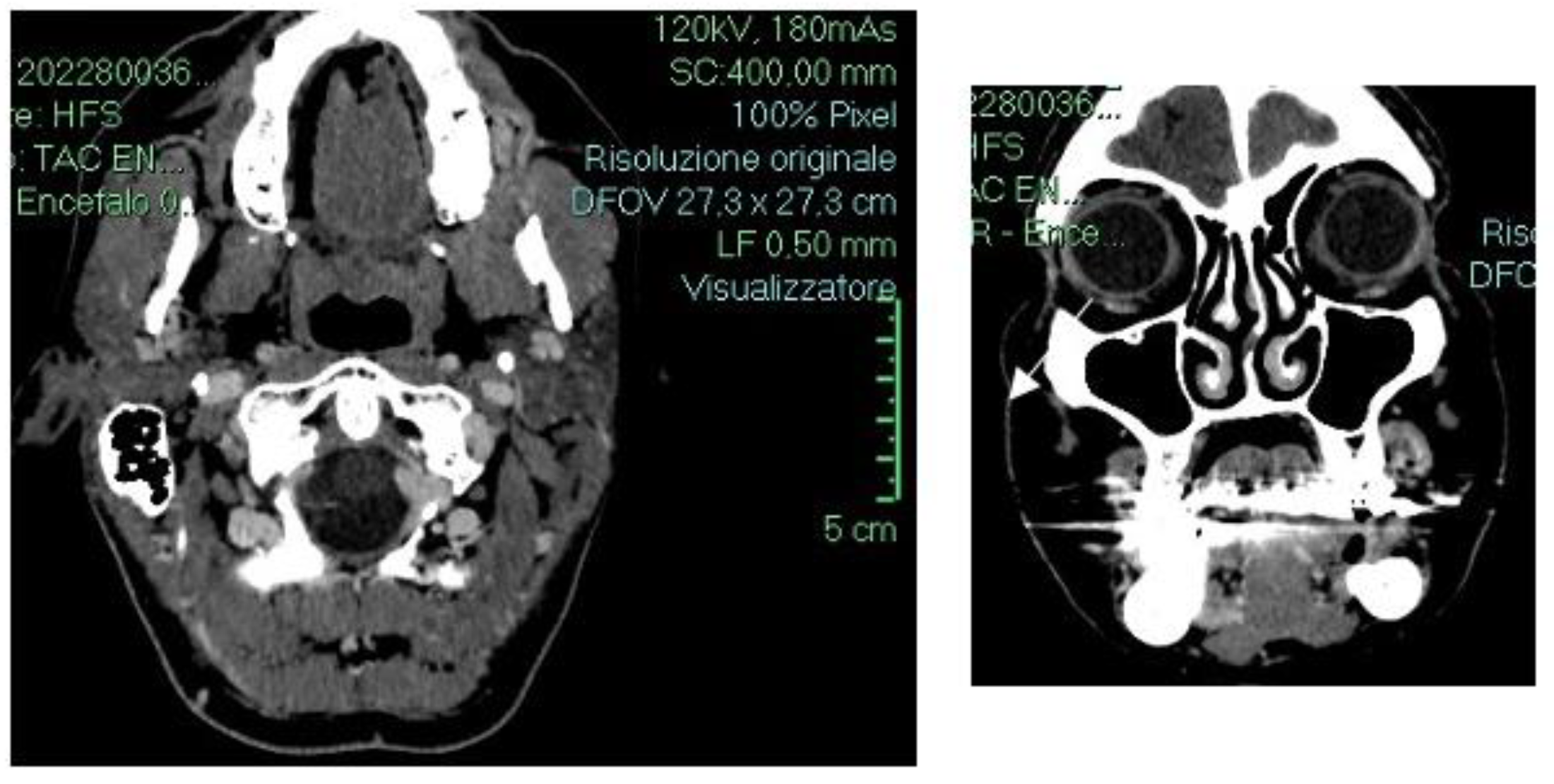
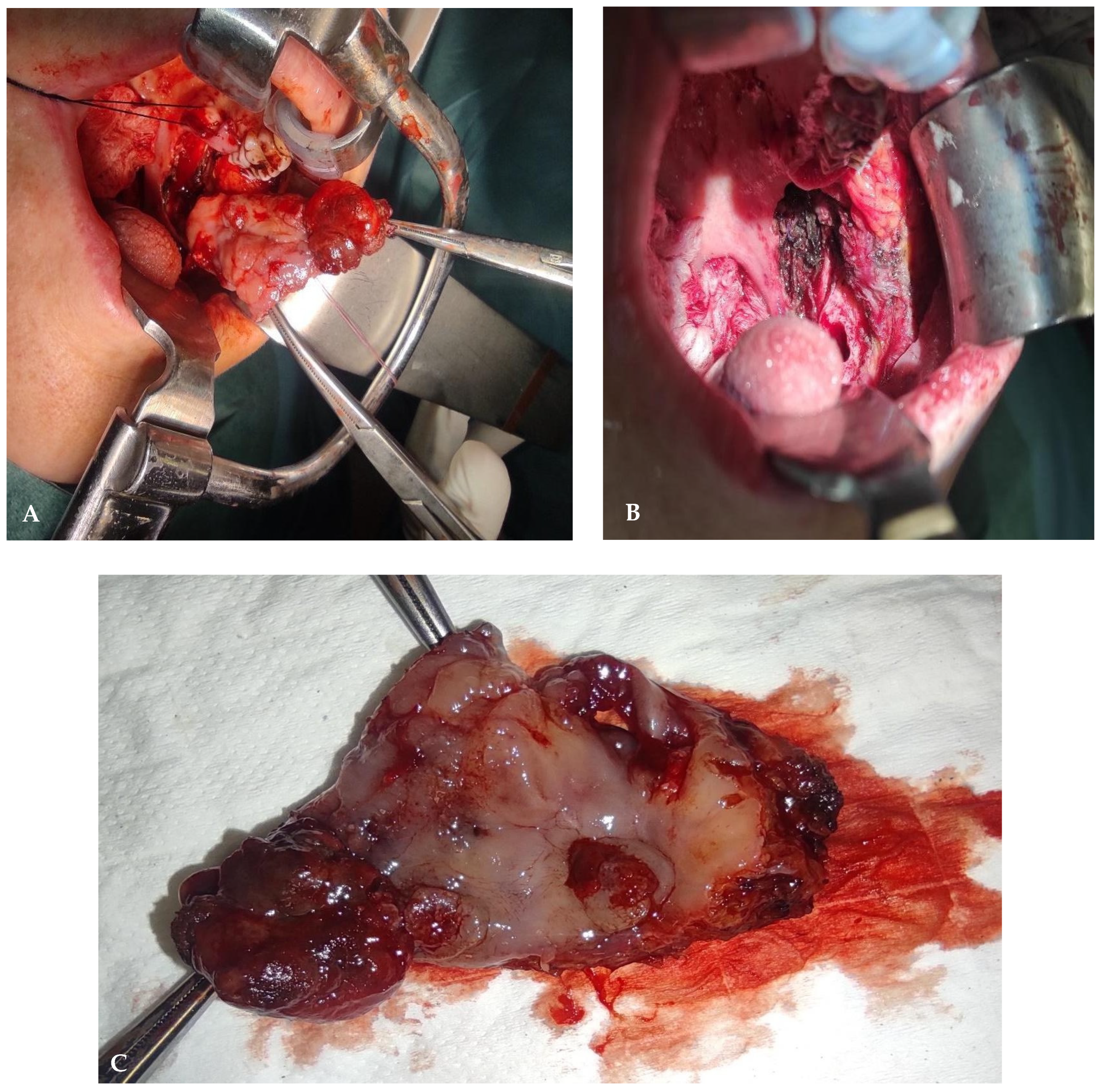
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, S.W.; Lasota, J.; Miettinen, M.M. Angiosarcoma of soft tissue. In WHO Classification Tumours of Soft Tissue and Bone; Fletcher, C.D.M., Unni, K.K., Mertens, F., Eds.; IARC Press: Lyon, France, 2002; pp. 175–177. [Google Scholar]
- Meis-Kindblom, J.M.; Kindblom, L.G. Angiosarcoma of soft tissue: A study of 80 cases. Am. J. Surg. Pathol. 1998, 22, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zheng, J.; Zhou, Q.; Ye, W.; Wang, Y.; Zhu, H.; Wang, L.; Zhang, Z. Angiosarcomas of the head and neck: A clinico-immunohistochemical study of 8 consecutive patients. Int. J. Oral Maxillofac. Surg. 2010, 39, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Coindre, J.M.; Terrier, P.; Guillou, L.; Le Doussal, V.; Collin, F.; Ranchère, D.; Sastre, X.; Vilain, M.O.; Bonichon, F.; N’Guyen Bui, B. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: A study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001, 91, 1914–1926. [Google Scholar] [CrossRef] [PubMed]
- Mark, R.J.; Poen, J.C.; Tran, L.M.; Fu, Y.S.; Juillard, G.F. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer 1996, 77, 2400–2406. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.J.; Radden, B.G.; Gibbons, S.D.; Busmanis, I.; Cook, R.M. Primary angiosarcoma of the oral cavity. Br. J. Oral Maxillofac. Surg. 1991, 29, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, F.; Maurina, T.; Degano-Valmary, S.; Spicarolen, T.; Chaigneau, L. Angiosarcoma: A case report of gingival disease with both palatine tonsils localization. Rare Tumors 2016, 8, 5907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iacomino, E.; D’Amario, M.; Tucci, C.; di Marco, G.P.; Sollima, L.; Capogreco, M. An unexpected synovial sarcoma of the parotid gland: A rare localization. J. Biol. Regul. Homeost. Agents 2022, 36, 71–79. [Google Scholar] [CrossRef]
- Rouhani, P.; Fletcher, C.D.; Devesa, S.S.; Toro, J.R. Cutaneous soft tissue sarcoma incidence patterns in the U.S.: An analysis of 12,114 cases. Cancer 2008, 113, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Illuminati, G.; Pasqua, R.; Nardi, P.; Fratini, C.; Calio, F.C.; Ricco, J.B. Intravascular Ultrasound-Assisted Endovascular Exclusion of Penetrating Aortic Ulcers. Ann. Vasc. Surg. 2021, 70, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.A.; Hornicek, F.J.; Kaufman, A.M.; Harmon, D.C.; Springfield, D.S.; Raskin, K.A.; Mankin, H.J.; Kirsch, D.G.; Rosenberg, A.E.; Nielsen, G.P.; et al. Treatment and outcome of 82 patients with angiosarcoma. Ann. Surg. Oncol. 2007, 14, 1953–1956. [Google Scholar] [CrossRef] [PubMed]
- Fury, M.G.; Antonescu, C.R.; Van Zee, K.J.; Brennan, M.F.; Maki, R.G. A 14-year retrospective review of angiosarcoma: Clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005, 11, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.; Barry, M.; Kell, M.R. Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV breast cancer impacts on survival. Ann. Surg. Oncol. 2013, 20, 2828–2834. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, F.S.-L.; Rodriguez, J.C.; Asa’Ad, F.; Wang, H.-L. Comparison of two soft tissue substitutes for the treatment of gingival recession defects: An animal histological study. J. Appl. Oral Sci. 2019, 27, e20180584. [Google Scholar] [CrossRef] [PubMed]
- Terada, T. Angiosarcoma of the oral cavity. Head Neck Pathol. 2011, 5, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Cigna, E.; Rizzo, M.I.; Greco, A.; De Virgilio, A.; Palmieri, A.; Maruccia, M.; Ribuffo, D.; Scuderi, N.; De Vincentiis, M. Retromolar Trigone Reconstructive Surgery: Prospective Comparative Analysis Between Free Flaps. Ann. Surg. Oncol. 2014, 22, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, Z.; Liu, F.; Huang, S.; Dai, W.; Sun, C. Vascularized free forearm flap versus free anterolateral thigh perforator flaps for reconstruction in patients with head and neck cancer: Assessment of quality of life. Head Neck 2013, 35, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Girod, D.A.; Sykes, K.; Jorgensen, J.; Tawfik, O.; Tsue, T. Acellular dermis compared to skin grafts in oral cavity reconstruction. Laryngoscope 2009, 119, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.; Khoo, T.; Shah, J.Y. Biologic and synthetic skin substitutes: An overview. Indian J. Plast. Surg. 2010, 43, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.K.; Louis, M.R.; Gimenez, A.; Reece, E.M. The basics of integra dermal regeneration template and its expanding clinical applications. Semin. Plast. Surg. 2019, 33, 185–189. [Google Scholar] [CrossRef]
- Srivastava, A.; Maniakas, A.; Myers, J.; Chambers, M.S.; Cardoso, R. Reconstruction of intraoral oncologic surgical defects with Integra® bilayer wound matrix. Clin. Case Rep. 2021, 9, 213–219. [Google Scholar] [CrossRef]
- Movaniya, P.N.; Makwana, T.R.; Desai, N.N.; Makwana, K.G.; Patel, H.B. Efficacy of Collagen Membrane Graft in Intraoral Surgery—An Evaluative Study. Ann. Maxillofac. Surg. 2021, 11, 42–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horta, R.; Nascimento, R.; Silva, A.; Amarante, J. The Retromolar Trigone. J. Craniofacial Surg. 2016, 27, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Lindenmayer, A.E.; Chaubal, A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens-evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod. Pathol. 1994, 7, 82–90. [Google Scholar] [PubMed]
- De Young, B.R.; Swanson, P.E.; Argenyi, Z.B.; Ritter, J.H.; Fitsgibbon, J.F.; Stahl, D.J.; Hoover, W.; Wick, M.R. CD31 immunoreactivity in mesenchymal neoplasms of the skin and subcutis: Report of 145 cases and review of putative immunohistologic markers of endothelial differentiation. J. Cutan. Pathol. 1995, 22, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Varvares, M.A.; Poti, S.; Kenyon, B.; Christopher, K.; Walker, R.J. Surgical margins and primary site resection in achieving local control in oral cancer resections. Laryngoscope 2015, 125, 2298–2307. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ali, S.N.; Farid, M.; Pancholi, M.; Rayatt, S.; Yap, L.H. Use of dermal regeneration template (Integra) for reconstruction of full-thickness complex oncologic scalp defects. J. Craniofac Surg. 2010, 21, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Stevenson, J.; Parry, M.; Tsuda, Y.; Kaneuchi, Y.; Jeys, L. The adequacy of resection margin for non-infiltrative soft-tissue sarcomas. Eur. J. Surg. Oncol. 2021, 47, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Seike, S.; Tomita, K.; Ikeda, J.-I.; Morii, E.; Isomura, E.T.; Kubo, T. Possible malignant transformation of arteriovenous malformation to angiosarcoma: Case report and literature review. J. Surg. Case Rep. 2019, 2019, rjz375. [Google Scholar] [CrossRef]
- Welkoborsky, H.-J.; Deichmüller, C.; Bauer, L.; Hinni, M.L. Reconstruction of large pharyngeal defects with microvascular free flaps and myocutaneous pedicled flaps. Curr. Opin. Otolaryngol. Head Neck Surg. 2013, 21, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.W.; Koljenović, S.; Hardillo, J.A.; Ten Hove, I.; Meeuwis, C.A.; Sewnaik, A.; Dronkers, E.A.; Bakker Schut, T.C.; Langeveld, T.P.; Molenaar, J.; et al. Resection margins in oral cancer surgery: Room for improvement. Head Neck. 2016, 38 (Suppl. S1), E2197–E2203. [Google Scholar] [CrossRef]
- Iacomino, E.; Junquera, L.M.; Vendettuoli, M.; González, A.M.; Olay, S.; Corbacelli, A. Distraction of oral scars contractures following caustic ingestion. A form of conservative treatment. Med. Oral 2003, 8, 61–65. [Google Scholar] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacomino, E.; Fratini, C.; Sollima, L.; Eibenstein, A.; Barbato, C.; de Vincentiis, M.; Minni, A.; Zoccali, F. Surgical Resection and Immediate Reconstruction with a Bilayer Wound Collagen Matrix of a Rare Oral Angiosarcoma: A Case Report. Diseases 2024, 12, 117. https://doi.org/10.3390/diseases12060117
Iacomino E, Fratini C, Sollima L, Eibenstein A, Barbato C, de Vincentiis M, Minni A, Zoccali F. Surgical Resection and Immediate Reconstruction with a Bilayer Wound Collagen Matrix of a Rare Oral Angiosarcoma: A Case Report. Diseases. 2024; 12(6):117. https://doi.org/10.3390/diseases12060117
Chicago/Turabian StyleIacomino, Enzo, Chiara Fratini, Laura Sollima, Alberto Eibenstein, Christian Barbato, Marco de Vincentiis, Antonio Minni, and Federica Zoccali. 2024. "Surgical Resection and Immediate Reconstruction with a Bilayer Wound Collagen Matrix of a Rare Oral Angiosarcoma: A Case Report" Diseases 12, no. 6: 117. https://doi.org/10.3390/diseases12060117
APA StyleIacomino, E., Fratini, C., Sollima, L., Eibenstein, A., Barbato, C., de Vincentiis, M., Minni, A., & Zoccali, F. (2024). Surgical Resection and Immediate Reconstruction with a Bilayer Wound Collagen Matrix of a Rare Oral Angiosarcoma: A Case Report. Diseases, 12(6), 117. https://doi.org/10.3390/diseases12060117






