Abstract
Cystic Echinococcosis (CE) is a zoonotic disease caused by the larval stage of the tapeworm Echinococcus granulosus sensu lato (s.l.). This study aims to investigate the use of two monoclonal antibodies (mAbEmG3 and mAbEm2G11) by immunohistochemistry (IHC) to confirm the diagnosis of CE in human patients, in particular in those cases in which other techniques fail to provide a correct or conclusive diagnosis. For this purpose, a survey on 13 patients was performed. These subjects were referred to Sardinian hospitals (Italy) from 2017 to 2022 and were suspected to be affected by CE. Our findings from these 13 patients showed the detection of E. granulosus sensu stricto by IHC in 12 of 13 echinococcal cysts, as one sample was of a non-parasitological origin. The results confirmed that IHC, by means of the mAbEmG3 and mAbEm2G11, is a reliable diagnostic tool that showed a very high performances when tested on strain of E. granulosus s.l. from Sardinia.
1. Introduction
Echinococcosis is a zoonotic disease caused by the larval stage (metacestodes) of tapeworms of the genus Echinococcus belonging to Cestoda class and Taeniidae family [1,2]. In humans, the species with the most medical importance are E. granulosus sensu lato (s.l.), the causative agent of cystic echinococcosis (CE), and E. multilocularis, the etiological agent of alveolar echinococcosis (AE) [3]. These two helminths represent a serious health problem worldwide give different clinical symptoms and have, host specificity, pathogenicity, and geographic distributions [4,5,6,7].
E. granulosus s.l. has a cosmopolitan distribution, except for Antarctica. It has been eliminated in Iceland and New Zealand through comprehensive control programs, and is highly endemic in Mediterranean areas [8,9]. Italy presents an overall incidence rate of human CE equal to 1.6/105 inhabitants per year [5,10]. Differences have been recorded among the Italian administrative divisions, the regions, with the highest incidence in the islands of Sardinia and Sicily corresponding to 6.8/105 and 4.0/105, respectively [11]. Overall, around 900 new cases of CE are expected in Italy every year [12].
In contrast, E. multilocularis has mainly been detected in Asia, Japan, China, North America, Central, and Eastern Europe [5,13]. There has been no evidence of its presence in the Mediterranean areas until recently, when the first human case of AE in Italy was described in a report [14].
The life cycles of both parasites involve two mammalian host species [15,16]. The definitive host is represented by carnivores, mainly dogs for E. granulosus s.l. and foxes or other wild canids and dogs for E. multilocularis, which harbor the adult stages in the intestine and release eggs through feces into the environment. The intermediate host is typically represented by ungulates for E. granulosus s.l., and by rodents for E. multilocularis. Humans are dead-end hosts as they play no role in maintaining the life cycles of the parasites [17,18].
If embryonated eggs are ingested with contaminated food, they hatch in the small intestine and release a larval stage, known as the oncosphere. After penetrating the mucosa, the oncosphere travels via the bloodstream or the lymphatic system; in most cases it reaches the liver; followed by the lungs; and, less frequently, other sites like the spleen, kidneys, heart, bones, and central nervous system [19]. In the organs, the oncosphere further develops into the metacestode stage, which slowly grows, forming a cyst-like parasitic structure for E. granulosus s.l. and a tumor-like tissue mass for E. multilocularis [15,20].
The taxonomic subdivision of E. granulosus s.l. comprises five species: E. granulosus sensu stricto (s.s.; G1 and G3 genotype), E. ortleppi (G4), E. equinus (G5), E. canadensis (G6–G8 and G10), and E. felidis [21,22]. On the other hand, several studies have reported little variance among E. multilocularis genotypes [23].
CE diagnosis [24] in humans is mainly performed using imaging techniques (ultrasound, conventional radiography, magnetic resonance, and computed tomography), which are very useful tools [25,26]. However, they frequently need to be supported by other examinations, in particular in the case of differential diagnosis [27], neoplasia [7], or abscess [28]. Serological tests (enzyme linked immunosorbent assay (ELISA) and immunochromatographic test (ICT), along with immunoblotting (IB)) [29,30] are able to detect IgG antibodies directed against E. granulosus s.l. and E. multilocularis, and represent a valid tool to support doubtful radiological exams. However, these serological tests are often limited by cross-reactivity with other helminthic diseases, especially in the case of CE assays [31]. Moreover, in early and/or late stage cysts, a low performance has been reported [32]. However, in vivo investigations may be supported by further analyses performed directly on the cyst after surgical enucleation. Molecular analysis along with histopathology are the most reliable techniques to confirm the disease [26]. While molecular analysis is useful to differentiate species and genotypes following DNA analysis, histopathology presents a high specificity for recognizing the typical feature of the parasitic tissue, for example using hematoxylin–eosin (H/E) staining or immunohistochemistry (IHC), to directly detect the zoonotic agent using monoclonal antibodies (mAbs). The molecular analysis has to be performed on fresh or promptly frozen biological material. Indeed, protocols of DNA extraction on paraffin fixed formalin embed (PFFE) tissues often fail due to degraded genomic material in the presence of formalin [33]. Consequently, molecular analysis has several limits, such as having to perform retrospective surveys on stored PFFE samples [34].
Conversely, IHC presents several advantages for the direct detection of the parasite for both diagnostic and research purposes. Moreover, IHC is able to distinguish between E. granulosus s.l. and E. multilocularis using two different mAbs [35]. In detail, mAbEm2G11 is directed against the mucin-type Em2-glycoprotein specific for E. multilocularis [36], while mAbEmG3 is directed against an Echinococcus spp. specific antigen that has not been characterized yet [35,37]. Compared with molecular analysis, a dual staining approach using these two mAbs has another advantage. In the case of degenerated lesions and degraded DNA, where PCR fails, IHC represents a more sensitive alternative [35].
The main objective of this research was to test and investigate the reliability of a diagnostic tool in a small number of samples, which had previously been set up with a big sample size [35]. For this purpose, a survey was performed on samples of E. granulosus s.l. that originated from Sardinia. In detail, in this study, we wanted to test an IHC protocol with two monoclonal antibodies (mAbEmG3 and mAbEm2G11) able to confirm the clinical diagnosis of CE in Sardinia. In particular, in those cases in which radiological techniques and immunological analyses usually failto provide a correct or conclusive diagnostic answer. An early and prompt identification of CE allows for an adequate medical treatment and the correct follow-up of patients. In addition, the data obtained from this survey aimed to provide important information from an epidemiological point of view and may contribute to filling the gap that still exists regarding Echinococcosis for a specific geographical area, such as Sardinia.
2. Materials and Methods
2.1. Patients
A total of 13 patients were included in this retrospective study. These subjects referred to different Sardinian Hospitals (Italy) from 2017 to 2022 with symptoms compatible with CE. Several investigations on these patients and their samples have already been performed and described [38]. The previously published information on the 13 studied samples can be summarized as follows: clinical and laboratory analyses were carried out using imaging techniques, serological analysis, parasitological examination, and molecular characterization. A cystic lesion was evidenced in all subjects, but 12 specimens presented the pathognomonic signs of an echinococcal cyst: an oval or round shape lesion, laminated and germinal layers, and the presence of daughter cysts and fluid. Moreover, microscopic investigation evidenced the presence of protoscoleces in 11 cystic liquid samples, confirming their fertile status. Finally, the Sanger sequencing of mtgenes cox1 and nad5 of the successfully amplified fragments of these 11 samples led to clearly distinguishing between G1 (n = 9) and G3 (n = 2) genotypes of E. granulosus s.l. (Table 1).

Table 1.
Findings on clinical and laboratory investigations previously published (*) [38] compared with the immunohistochemistry results of 13 patients suspected of CE and their cystic samples.
These previous data [38] were very useful as a comparison with those obtained in this study and were used to confirm the new findings (displayed in Table 1).
2.2. Histopathological Analysis
An aliquot of each parasitic sample, collected during previous examination, was promptly fixed in 10% formalin and embedded in paraffin, following routine appropriate laboratory methods. Briefly, sections of 3–4 µm were serially cut from paraffin blocks, and the slices were collected on glass slides in a thermostatic bath and then subjected to two different protocols using an automatic stainer. To highlight the nucleus and cytoplasm of the cells, H/E staining was performed. Subsequently, to determine the parasitic source of the cyst in question, an IHC procedure [39] was used by two different mAbs, mAbEmG3 and mAbEm2G11, which were able to identify Echinococcus spp. and E. multilocularis [35], respectively. The monoclonal antibodies were produced in vitro from murine cell lines, as already described [36], and are available upon request (peter.deplazes@uzh.ch). Moreover, the IHC method required that the tissue slice was placed on a positively charged glass slide.
3. Results
3.1. Patients
The 13 patients involved in the study comprised 8 males and 5 females—their age ranged between 18 and 78 years with a mean of 51.5 (standard deviation of ±18.8). Three patients, although residents of Italy, came from other countries, such as Romania, Morocco, and Ghana.
3.2. Histopathological Analysis
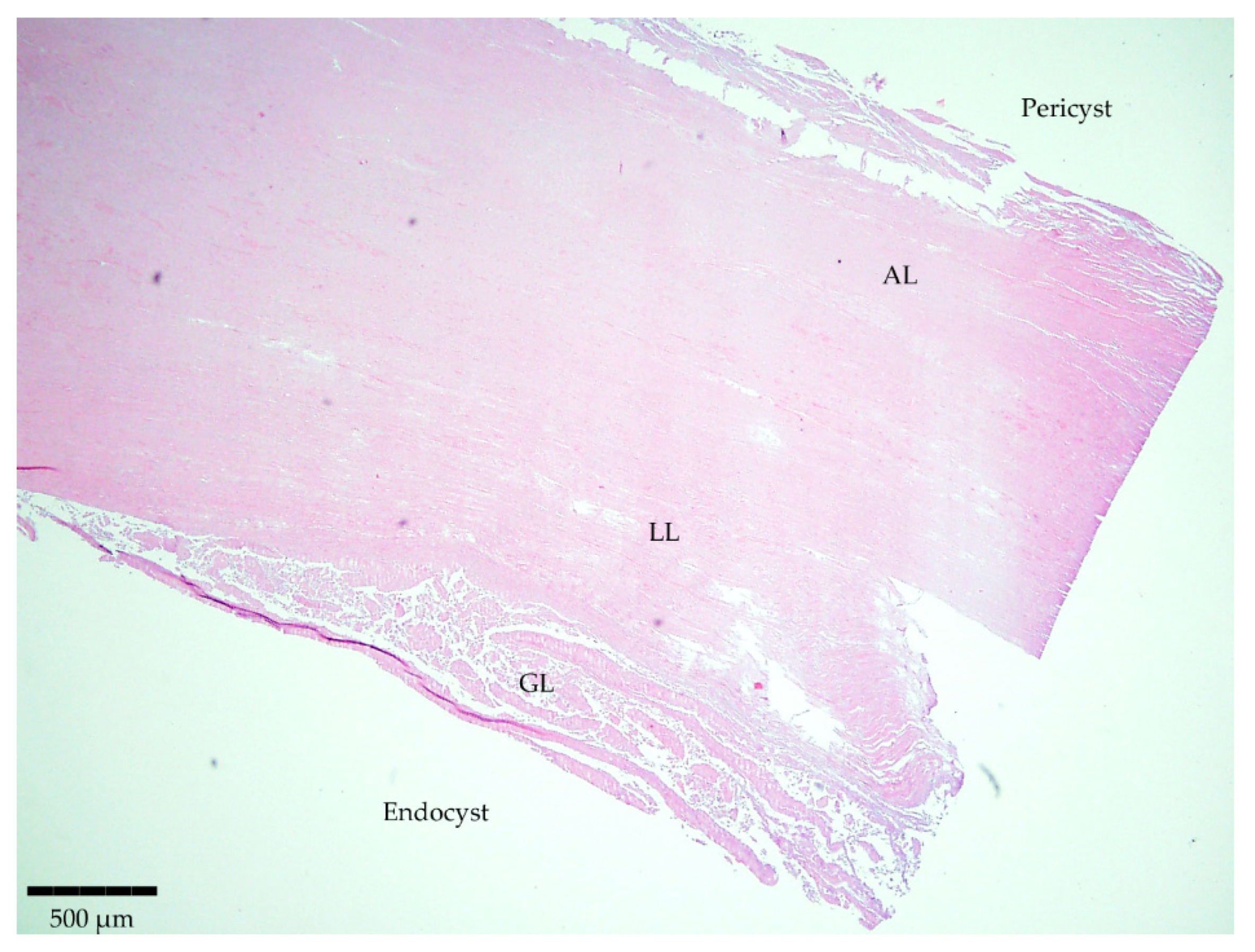
Staining of the sliced samples with H/E evidenced the typical parasite features for 12 samples out of the 13 involved in the study (Table 1). The structure was characterized by separate layers (Figure 1). Firstly, the host-produced granulomatous reaction surrounded all parasitic cystic structures with an adventitial layer (AL), besides one thick acellular and laminated layer (LL) and a cellular germinal layer (GL). Finally, brood capsules with protoscoleces could be observed in fertile cysts. Only one histological section was considered negative as it showed non-parasitic characteristics.
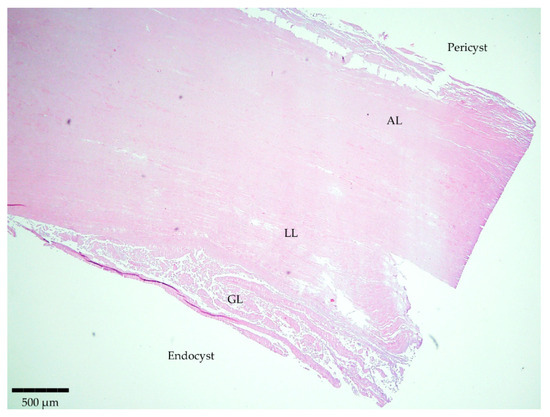
Figure 1.
Microscopic observation of a histopathological section stained with H/E evidencing the typical parasite features. Magnitude 4×; scale bar: 500 µm. Legend: adventitial layer (AL), laminated layer (LL), and germinal layer (GL).
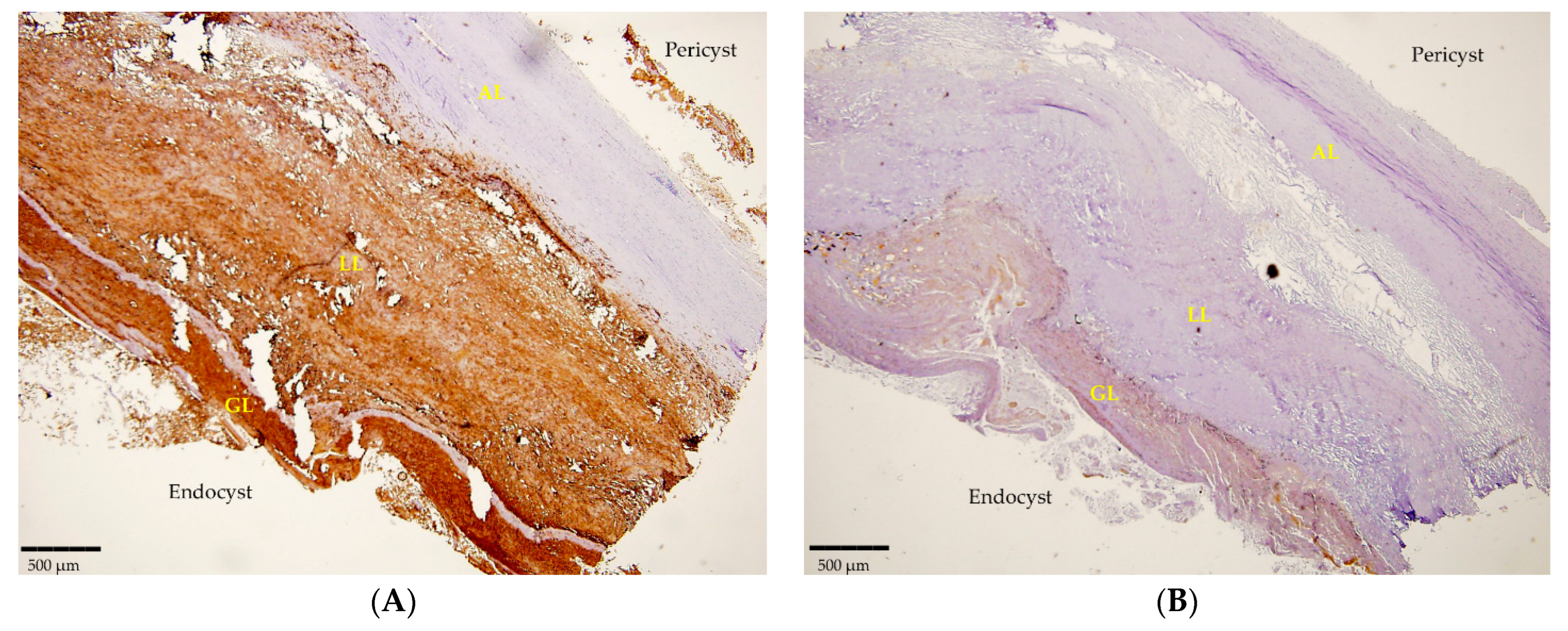
Likewise, 12 histological sections that were examined clearly revealed the positivity detected by mAbs in IHC. The mAbEmG3 displayed and confirmed the specificity for E. granulosus s.s. samples, evidenced by the typical brownish color of the reactive layers of the GL, comprising protoscoleces, and LL, visible using optical microscopy (Figure 2A). Furthermore, small antigenic particles of E. granulosus (SPEGS) were often detected outside of the laminated layer in the host tissue surrounding the parasite lesions. One negative slide was also evidenced. In addition, after incubation with mAbEm2G11, all preparations were negative (Figure 2B).
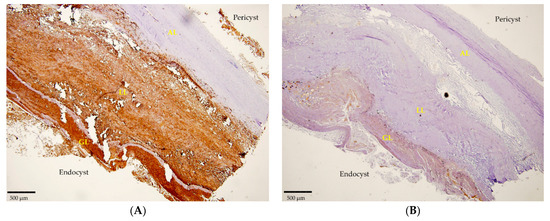
Figure 2.
(A) Section of a cyst positive for E. granulosus s.s. detected by IHC using mAbEmG3. (B). Negative IHC section of E. granulosus s.s. cyst incubated with mAbEm2G11. Magnitude 4×; scale bar: 500 µm. Legend: adventitial layer (AL), laminated layer (LL), germinal layer (GL).
4. Discussion
The diagnosis of CE is considered challenging as cases can be asymptomatic for years and there are no pathognomonic signs of the disease until the parasitic lesion reaches a considerable size; Sometimes, cyst formation can be confused with another disorder; thus, it becomes necessary to perform a differential diagnosis.
This survey aimed to test the reliability of a diagnostic tool, previously set up [35], on samples of E. granulosus s.l. originated from Sardinia, as, to our knowledge, the IHC protocol with two monoclonal antibodies (mAbEmG3 and mAbEm2G11) has never been used on a Sardinian strain. However, the three patients who were not born in Sardinia but were residents at the time of diagnosis lacked further data to confirm the origin of the infection.
IHC is useful to support the analytical process for providing a clear diagnostic picture of CE. In detail, in this study, we wanted a test able to confirm the clinical diagnosis of CE in Sardinia; in particular in those cases in which radiological techniques and immunological analyses failed to provide a correct or conclusive diagnostic answer. Early and prompt identification of CE allows for adequate medical treatment and the correct follow-up of patients. In addition, data obtained from this survey aimed to provide important information from an epidemiological point of view, and may contribute to filling the gap in knowledge regarding Echinococcosis in a specific geographical area, such as Sardinia. For this reason, 13 patients suspected to harbor an echinococcal cyst after being abdominally examined using imaging techniques were involved in this study.
Despite radiological techniques being considered the most reliable exams for CE detection [26,32], sometimes, they fail to perform a correct diagnosis of CE. Indeed, other pathologies such as abscess or neoplasia could be wrongly detected and confused with an hydatid cyst; in particular, if the inner biologic material is liquid or even lacking [7]. It has been reported that hepatic CE and pulmonary cysts can radiologically look like malignant or infectious diseases such as neoplasia or tuberculosis [40].
Immunological analyses support radiological tools by providing a correct and conclusive diagnostic response; however, they occasionally lack sensitivity and specificity [29,31,41] in particular for cases of early (CE1) or late stage (CE4/5) CE and cross-reaction for other parasitosis [32].
Noticeably, improved detection of the CE etiological agent has been found through investigations performed directly on parasitological material using PCR and sequencing. All of the detected isolates were previously characterized as E. granulosus s.s. and resulted in G1 (n = 9) and G3 (n = 2) genotypes, confirming the presence of a higher concentration of G1 in Sardinia [38].
The histopathological analysis was able to shed light on this complex diagnostic picture (Table 1). In this study, a total of 12 samples were positivite for CE; in contrast, 1 sample was diagnosed as being of a non-parasitological origin, as it resulted in a neoplasia. The distinctive characteristic structure of an Echinococcus cyst was shown by H/E, while a positive reaction by IHC was evidenced by mAbEmG3 (Figure 2A). As reported in a previous study [35], a high sensitivity and specificity were reported for this newly established mAb as it was able to detect all CE samples demonstrating 100% positivity and was of capable of identifying PCR negative samples that were stage CE5 and sterile. Moreover, the sample of a non-parasitological source was confirmed negative. Furthermore, mAbEmG3 was able to identify E. granulosus s.l. in only one step, avoiding amplification and sequencing protocols. Further, as established by other studies [35,36,42], after incubation with mAbEm2G11, all preparations were negative (Figure 2B), confirming the affinity and specificity of this antibody to E. multilocularis.
Hence, to have a consistent and conclusive diagnosis, a multidisciplinary approach is required, and the contribution of different techniques is the only way to guarantee the correct identification of this zoonosis. A conclusive and early diagnosis of CE in human patients is often essential so that correct clinical and pharmacological management and follow up of the patient can be performed [26]. Moreover, several accurate laboratory and clinical investigations in the direction of a differential diagnosis need to be performed during these examinations [27]. The need for a reliable diagnostic tool becomes necessary, in particular for unclear and difficult cases.
Moreover, we believe the present findings improve the knowledge on histopathology in the scientific and diagnostic field of echinococcosis. In this study, we also wanted to test the newly established mAbs on E. granulosus s.s. strains with a Sardinian origin. Even if Sardinia, similar to other Mediterranean areas, has only been characterized by the presence of E. granulosus s.l. [38], the need to develop techniques able to discriminate between CE and AE [43] is of vast importance in several geographic zones worldwide [44]; in particular, because of the recent findings of an autochthonous case of human AE in Italy [14].
5. Conclusions
Our findings confirm that IHC, by means of mAbEmG3 and mAbEm2G11, is a reliable diagnostic tool to confirm the diagnosis of CE in human patients. As a very high performance was presented upon detection of the E. granulosus s.l. Sardinian strain, IHC is particularly useful in those cases in which other techniques fail to provide a correct or conclusive diagnosis.
Author Contributions
Conceptualization, C.S. and G.M.; methodology, A.P., P.A.K., P.D., A.M.F. and A.C.; software, C.S.; validation, C.S., A.P., A.M.F., A.C., P.A.K. and P.D.; formal analysis, C.S. and A.P.; investigation, C.S., A.M.F. and A.C.; resources, C.S., P.A.K. and P.D.; data curation, C.S. and A.P.; writing—original draft preparation, C.S.; writing—review and editing, C.S., P.A.K. and P.D.; visualization, C.S., G.M., P.A.K. and P.D.; supervision, C.S., P.A.K. and P.D.; project administration, C.S.; funding acquisition, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All of the actions linked to human samples and the related information handled at IZS of Sardinia were performed in conformity with the standards of policy and the ethics committee of the Local Health Authority of Sassari (Italy) (Protocol n◦ 1136), as required by the National Health Service since 26 March 2013. Moreover, all specimens from the patients involved in the study were accompanied by a medical request and their privacy was always respected, according to the current legislation. Finally, their management was approved by the Declaration of Helsinki of 1975, revised in 2013.
Informed Consent Statement
Informed consent was obtained from all of the subjects involved in the study.
Data Availability Statement
The authors are available to share data related to the manuscript; no further data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Thompson, R.C.A. Chapter Two—Biology and Systematics of Echinococcus. In Advances in Parasitology; Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Echinococcosis, Part A.; Academic Press: Cambridge, MA, USA, 2017; Volume 95, pp. 65–109. [Google Scholar]
- Nakao, M.; Lavikainen, A.; Yanagida, T.; Ito, A. Phylogenetic Systematics of the Genus Echinococcus (Cestoda: Taeniidae). Int. J. Parasitol. 2013, 43, 1017–1029. [Google Scholar] [CrossRef]
- Woolsey, I.D.; Miller, A.L. Echinococcus Granulosus Sensu Lato and Echinococcus Multilocularis: A Review. Res. Vet. Sci. 2021, 135, 517–522. [Google Scholar] [CrossRef]
- Nakao, M.; Yanagida, T.; Konyaev, S.; Lavikainen, A.; Odnokurtsev, V.A.; Zaikov, V.A.; Ito, A. Mitochondrial Phylogeny of the Genus Echinococcus (Cestoda: Taeniidae) with Emphasis on Relationships among Echinococcus Canadensis Genotypes. Parasitology 2013, 140, 1625–1636. [Google Scholar] [CrossRef]
- Deplazes, P.; Rinaldi, L.; Alvarez Rojas, C.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.M.; Lahmar, S.; Cringoli, G.; et al. Chapter Six—Global Distribution of Alveolar and Cystic Echinococcosis. In Advances in Parasitology; Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Echinococcosis, Part A.; Academic Press: Cambridge, MA, USA, 2017; Volume 95, pp. 315–493. [Google Scholar]
- Knapp, J.; Gottstein, B.; Saarma, U.; Millon, L. Taxonomy, Phylogeny and Molecular Epidemiology of Echinococcus Multilocularis: From Fundamental Knowledge to Health Ecology. Vet. Parasitol. 2015, 213, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Gemmell, M.A.; Meslin, F.-X.; Pawlowski, Z.S.; World Health Organization. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; World Organization for Animal Health: Paris, France, 2001; ISBN 978-92-9044-522-7. [Google Scholar]
- Craig, P.S.; McManus, D.P.; Lightowlers, M.W.; Chabalgoity, J.A.; Garcia, H.H.; Gavidia, C.M.; Gilman, R.H.; Gonzalez, A.E.; Lorca, M.; Naquira, C.; et al. Prevention and Control of Cystic Echinococcosis. Lancet Infect. Dis. 2007, 7, 385–394. [Google Scholar] [CrossRef]
- Gessese, A.T. Review on Epidemiology and Public Health Significance of Hydatidosis. Vet. Med. Int. 2020, 2020, 8859116. [Google Scholar] [CrossRef] [PubMed]
- Casulli, A. Recognising the Substantial Burden of Neglected Pandemics Cystic and Alveolar Echinococcosis. Lancet Glob. Health 2020, 8, e470–e471. [Google Scholar] [CrossRef]
- Brundu, D.; Piseddu, T.; Stegel, G.; Masu, G.; Ledda, S.; Masala, G. Retrospective Study of Human Cystic Echinococcosis in Italy Based on the Analysis of Hospital Discharge Records between 2001 and 2012. Acta Trop. 2014, 140, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Piseddu, T.; Brundu, D.; Stegel, G.; Loi, F.; Rolesu, S.; Masu, G.; Ledda, S.; Masala, G. The Disease Burden of Human Cystic Echinococcosis Based on HDRs from 2001 to 2014 in Italy. PLoS Neglected Trop. Dis. 2017, 11, e0005771. [Google Scholar] [CrossRef]
- Eckert, J.; Deplazes, P.; Kern, P. Alveolar Echinococcosis (Echinococcus multilocularis): And Neotropical Forms of Echinococcosis (Echinococcus vogeli and Echinococcus oligarthrus). In Oxford Textbook of Zoonoses: Biology, Clinical Practice, and Public Health Control; Palmer, S.R., Soulsby, L., Torgerson, P., Brown, D.W.G., Eds.; Oxford University Press: Oxford, UK, 2011; ISBN 978-0-19-857002-8. [Google Scholar]
- Tamarozzi, F.; Ronzoni, N.; Degani, M.; Oliboni, E.; Tappe, D.; Gruener, B.; Gobbi, F. Confirmed Autochthonous Case of Human Alveolar Echinococcosis, Italy, 2023. Emerg Infect. Dis. 2024, 30, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Moro, P.; Schantz, P. Echinococcus Species (Agents of Cystic, Alveolar, and Polycystic Echinococcosis). In Principles and Practice of Pediatric Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1356–1362.e2. ISBN 978-1-4377-2702-9. [Google Scholar]
- Moro, P.; Schantz, P.M. Echinococcosis: A Review. Int. J. Infect. Dis. 2009, 13, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Deplazes, P. Biological, Epidemiological, and Clinical Aspects of Echinococcosis, a Zoonosis of Increasing Concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef]
- Kołodziej-Sobocińska, M. Factors Affecting the Spread of Parasites in Populations of Wild European Terrestrial Mammals. Mamm. Res. 2019, 64, 301–318. [Google Scholar] [CrossRef]
- Bhutani, N.; Kajal, P. Hepatic Echinococcosis: A Review. Ann. Med. Surg. 2018, 36, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.-S.; Kim, J.-G.; Han, X.; Kang, I.; Kong, Y. Comparison of Echinococcus Multilocularis and Echinococcus Granulosus Hydatid Fluid Proteome Provides Molecular Strategies for Specialized Host-Parasite Interactions. Oncotarget 2017, 8, 97009–97024. [Google Scholar] [CrossRef]
- Kinkar, L.; Laurimäe, T.; Sharbatkhori, M.; Mirhendi, H.; Kia, E.B.; Ponce-Gordo, F.; Andresiuk, V.; Simsek, S.; Lavikainen, A.; Irshadullah, M.; et al. New Mitogenome and Nuclear Evidence on the Phylogeny and Taxonomy of the Highly Zoonotic Tapeworm Echinococcus Granulosus Sensu Stricto. Infect. Genet. Evol. 2017, 52, 52–58. [Google Scholar] [CrossRef]
- Romig, T.; Ebi, D.; Wassermann, M. Taxonomy and Molecular Epidemiology of Echinococcus Granulosus Sensu Lato. Vet. Parasitol. 2015, 213, 76–84. [Google Scholar] [CrossRef]
- Nakao, M.; Xiao, N.; Okamoto, M.; Yanagida, T.; Sako, Y.; Ito, A. Geographic Pattern of Genetic Variation in the Fox Tapeworm Echinococcus Multilocularis. Parasitol. Int. 2009, 58, 384–389. [Google Scholar] [CrossRef]
- Mihmanli, M.; Idiz, U.O.; Kaya, C.; Demir, U.; Bostanci, O.; Omeroglu, S.; Bozkurt, E. Current Status of Diagnosis and Treatment of Hepatic Echinococcosis. World J. Hepatol. 2016, 8, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.A.; Ali, R.M.A.; Khan, S.; Saqib, M.; Qamar, W.; Li, L.; Fu, B.-Q.; Yan, H.-B.; Jia, W.-Z. Past and Present of Diagnosis of Echinococcosis: A Review (1999–2021). Acta Trop. 2023, 243, 106925. [Google Scholar] [CrossRef]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert Consensus for the Diagnosis and Treatment of Cystic and Alveolar Echinococcosis in Humans. Acta Tropica 2010, 114, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, C.; Steverding, D.; Wang, X.; Shi, F.; Yang, Y. Differential Diagnosis of Cystic and Alveolar Echinococcosis Using an Immunochromatographic Test Based on the Detection of Specific Antibodies. Parasitol. Res. 2013, 112, 3627–3633. [Google Scholar] [CrossRef]
- Krige, J.E.J.; Beckingham, I.J. Liver Abscesses and Hydatid Disease. BMJ 2001, 322, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Peruzzu, A.; Mastrandrea, S.; Fancellu, A.; Bonelli, P.; Muehlethaler, K.; Masala, G.; Santucciu, C. Comparison and Evaluation of Analytic and Diagnostic Performances of Four Commercial Kits for the Detection of Antibodies against Echinococcus Granulosus and Multilocularis in Human Sera. Comp. Immunol. Microbiol. Infect. Dis. 2022, 86, 101816. [Google Scholar] [CrossRef]
- Maleki, F.; Akhlaghi, L.; Tabatabaie, F. Evaluation of Hydatid Cyst Antigen for Serological Diagnosis. Med. J. Islam. Repub. Iran. 2023, 37, 87. [Google Scholar] [CrossRef]
- Kronenberg, P.A.; Deibel, A.; Gottstein, B.; Grimm, F.; Müllhaupt, B.; Meyer Zu Schwabedissen, C.; Aitbaev, S.; Omorov, R.A.; Abdykerimov, K.K.; Minbaeva, G.; et al. Serological Assays for Alveolar and Cystic Echinococcosis-A Comparative Multi-Test Study in Switzerland and Kyrgyzstan. Pathogens 2022, 11, 518. [Google Scholar] [CrossRef]
- Brunetti, E.; Tamarozzi, F.; Macpherson, C.; Filice, C.; Piontek, M.S.; Kabaalioglu, A.; Dong, Y.; Atkinson, N.; Richter, J.; Schreiber-Dietrich, D.; et al. Ultrasound and Cystic Echinococcosis. Ultrasound Int. Open 2018, 4, E70–E78. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, E.; Moutet, M.-L.; Baulard, C.; Bacq-Daian, D.; Sandron, F.; Mesrob, L.; Fin, B.; Delépine, M.; Palomares, M.-A.; Jubin, C.; et al. Performance Comparison of Three DNA Extraction Kits on Human Whole-Exome Data from Formalin-Fixed Paraffin-Embedded Normal and Tumor Samples. PLoS ONE 2018, 13, e0195471. [Google Scholar] [CrossRef]
- Knapp, J.; Lallemand, S.; Monnien, F.; Felix, S.; Valmary-Degano, S.; Courquet, S.; Demonmerot, F.; Heyd, B.; Turco, C.; Doussot, A.; et al. Molecular Diagnosis of Alveolar Echinococcosis in Patients Based on Frozen and Formalin-Fixed Paraffin-Embedded Tissue Samples. Parasite 2022, 29, 4. [Google Scholar] [CrossRef]
- Reinehr, M.; Micheloud, C.; Grimm, F.; Kronenberg, P.A.; Grimm, J.; Beck, A.; Nell, J.; Meyer zu Schwabedissen, C.; Furrer, E.; Müllhaupt, B.; et al. Pathology of Echinococcosis: A Morphologic and Immunohistochemical Study on 138 Specimens With Focus on the Differential Diagnosis Between Cystic and Alveolar Echinococcosis. Am. J. Surg. Pathol. 2020, 44, 43. [Google Scholar] [CrossRef]
- Deplazes, P.; Gottstein, B. A Monoclonal Antibody against Echinococcus Multilocularis Em2 Antigen. Parasitology 1991, 103, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, P.A.; Reinehr, M.; Eichenberger, R.M.; Hasler, S.; Laurimäe, T.; Weber, A.; Deibel, A.; Müllhaupt, B.; Gottstein, B.; Müller, N.; et al. Monoclonal Antibody-Based Localization of Major Diagnostic Antigens in Metacestode Tissue, Excretory/Secretory Products, and Extracellular Vesicles of Echinococcus Species. Front. Cell. Infect. Microbiol. 2023, 13, 1162530. [Google Scholar] [CrossRef] [PubMed]
- Santucciu, C.; Bonelli, P.; Peruzzu, A.; Fancellu, A.; Farà, A.; Mastrandrea, S.; Drocchi, G.; Cossu, A.; Profili, S.; Porcu, A.; et al. Genetic Characterization of Echinococcus Granulosus Sensu Stricto Isolated from Human Cysts from Sardinia, Italy. Diseases 2023, 11, 91. [Google Scholar] [CrossRef]
- Magaki, S.; Hojat, S.A.; Wei, B.; So, A.; Yong, W.H. An Introduction to the Performance of Immunohistochemistry. Methods Mol. Biol. 2019, 1897, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Çobanoğlu, U.; Aşker, S.; Mergan, D.; Sayır, F.; Bilici, S.; Melek, M. Diagnostic Dilemma in Hydatid Cysts: Tumor-Mimicking Hydatid Cysts. Turk. Thorac. J. 2015, 16, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Tamarozzi, F.; Longoni, S.S.; Vola, A.; Degani, M.; Tais, S.; Rizzi, E.; Prato, M.; Scarso, S.; Silva, R.; Brunetti, E.; et al. Evaluation of Nine Commercial Serological Tests for the Diagnosis of Human Hepatic Cyst Echinococcosis and the Differential Diagnosis with Other Focal Liver Lesions: A Diagnostic Accuracy Study. Diagnostics 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Barth, T.F.E.; Herrmann, T.S.; Tappe, D.; Stark, L.; Grüner, B.; Buttenschoen, K.; Hillenbrand, A.; Juchems, M.; Henne-Bruns, D.; Kern, P.; et al. Sensitive and Specific Immunohistochemical Diagnosis of Human Alveolar Echinococcosis with the Monoclonal Antibody Em2G11. PLoS Negl. Trop. Dis. 2012, 6, e1877. [Google Scholar] [CrossRef] [PubMed]
- Barth, T.F.E.; Casulli, A. Morphological Characteristics of Alveolar and Cystic Echinococcosis Lesions in Human Liver and Bone. Pathogens 2021, 10, 1326. [Google Scholar] [CrossRef]
- Romig, T. Epidemiology of Echinococcosis. Langenbecks Arch. Surg. 2003, 388, 209–217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).