Electrical Storm Induced by Cardiac Resynchronization: Efficacy of the Multipoint Pacing Stimulation
Abstract
1. Introduction
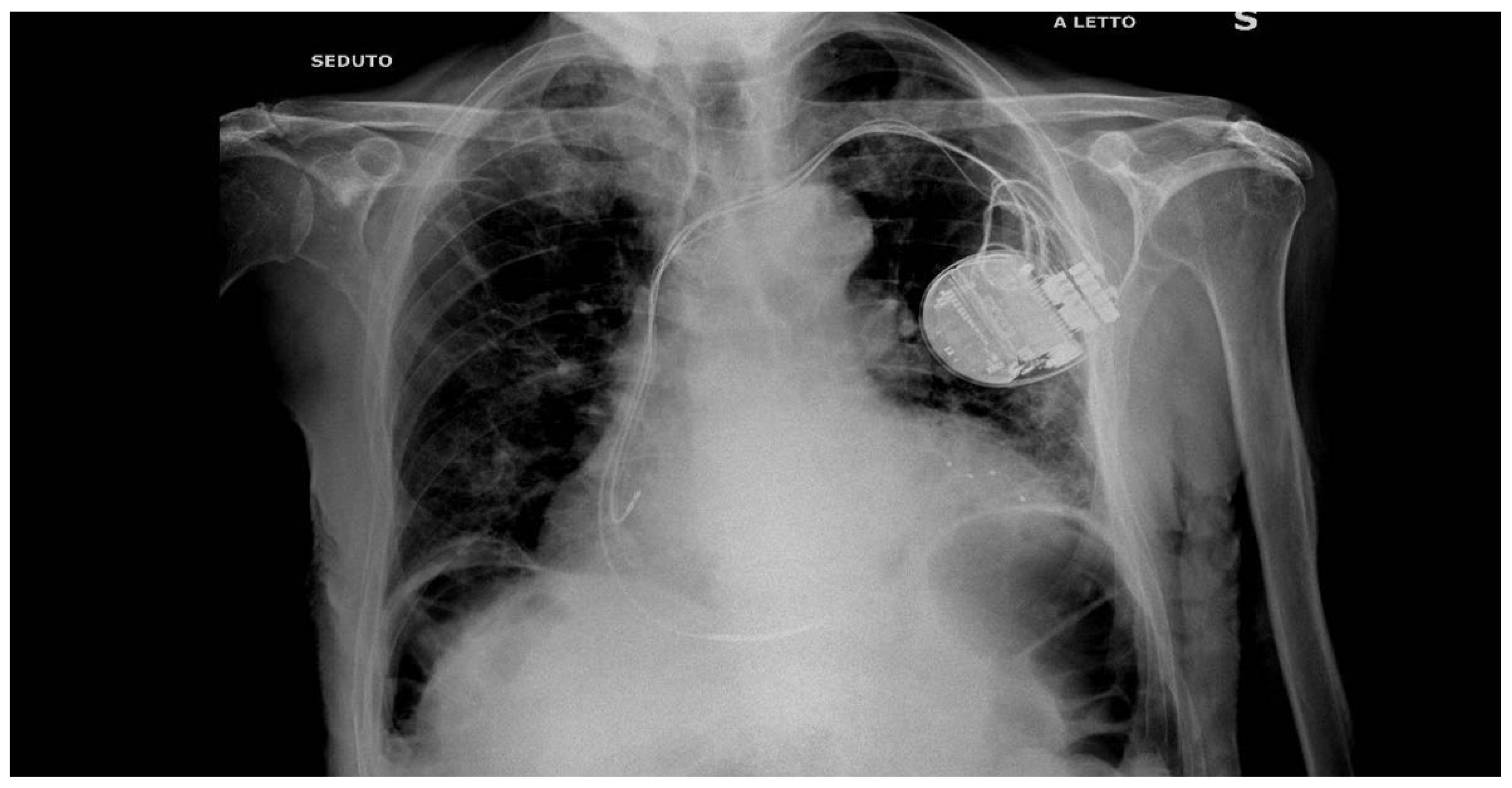
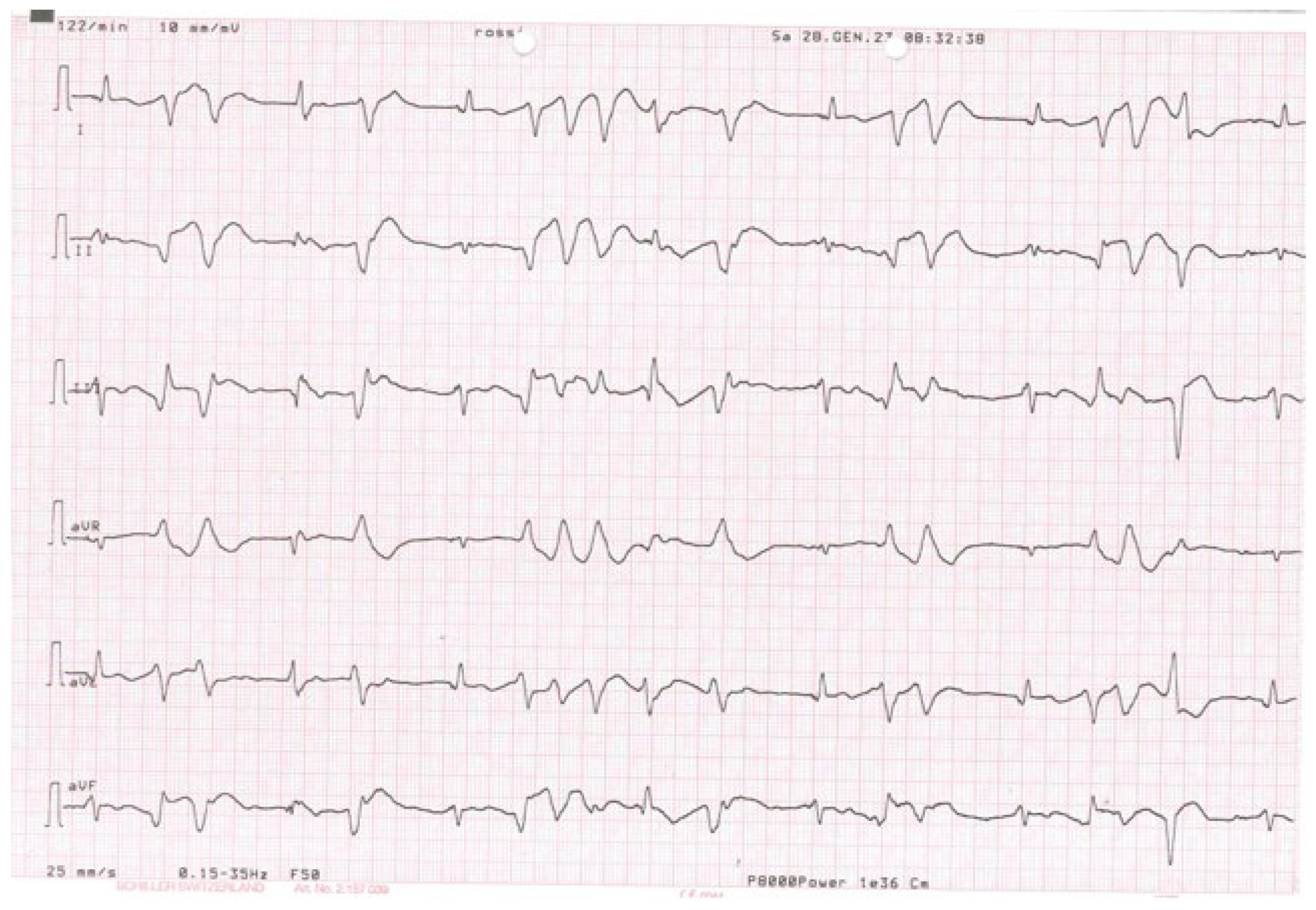
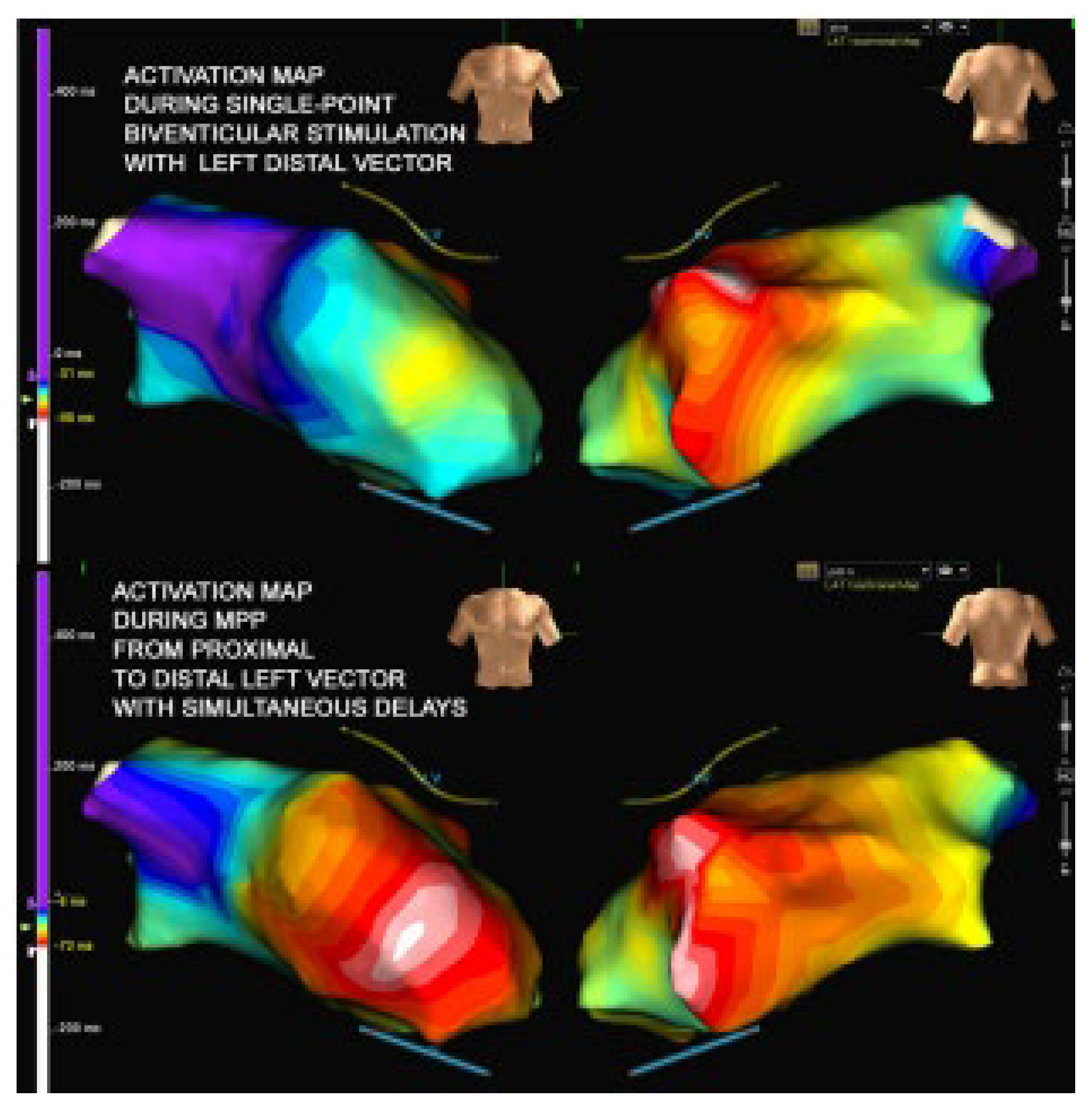
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cleland, J.G.; Daubert, J.C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. Cardiac resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Comparison of medical therapy, Pacing and Defibrillation inHeart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Daubert, J.C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. Long-term effects of cardiac resynchronization therapy on mortality in heart failure the CARE-HF trial extension phase. Eur. Heart J. 2006, 27, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Mykytsey, A.; Maheshwari, I.P.; Dhar, G.; Razminia, M.; Zheutlin, T.; Wang, T.; Kehoe, R. Ventricular tachycardia induced by ventricular pacing in severe ischemic cardiomyopathy. J. Cardiovasc. Electrophysiol. 2005, 16, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Nayak, H.M.; Verdino, R.J.; Russo, A.M.; Gesterfeld, E.P.; Hsia, H.H.; Lin, D.; Dixit, S.; Cooper, J.M.; Callans, D.J.; Marchlinski, F.E. Ventricular tachycardia storm after initiation of biventricular pacing: Incidence, clinical characteristics management and outcome. J. Cardiovasc. Electrophysiol. 2008, 19, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.M.; Brugada, J.; Antezelevitch, C. Potential proarrhythmic effects of biventricular pacing. J. Am. Coll. Cardiol. 2005, 46, 2340–2347. [Google Scholar] [CrossRef] [PubMed]
- Kantharia, B.K.; Patel, J.A.; Nagra, B.S.; Ledley, G.S. Electrical storm of monomorphic ventricular tachycardia after a cardiac-resynchronization-therapy-defibrillator upgrade. Europace 2006, 8, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Roque, C.; Trevisi, N.; Silberbauer, J.; Oloriz, T.; Mizuno, H.; Baratto, S.; Bisceglia, C.; Sora, N.; Marzi, A.; Radinovic, A.; et al. Electrical storm induced by cardiac resynchronization therapy is determined by pacing on epicardial scar and can be successfully managed by catheter ablation. Circ. Arrhytm. Electrophysiol. 2014, 7, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Asvestas, D.; Balasubramanian, R.; Sopher, M.; Paisey, J.; Babu, G.G. Extended bipolar left ventricular pacing as a possible therapy for late electrical storm induced by cardiac resynchronization therapy. J. Electrocardiol. 2017, 50, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Gras, D.; Clementy, N.; Ploux, S.; Guyomar, Y.; Legallois, D.; Segreti, L.; Blangy, H.; Laurent, G.; Bizeau, O.; Fauquenbergue, S. Lazaraus a for the BioCONTINUE Study Investigators. CRT-D replacement strategy: Results of the BioCONTINUE study. J. Interv. Card. Electrophysiol. 2023, 66, 1201–1209. [Google Scholar] [CrossRef]
- Vieweg, W.V.R.; Wood, M.A.; Fernandez, A.; Beatty-Brooks, M.; Masnain, M.; Padwag, A.K. Proarrhythmic risk until antipsychotic and antidepressant drugs: Implications in the elderly. Drugs Aging 2009, 26, 997–1012. [Google Scholar] [CrossRef]
- Massetti, C.M.; Cheng, C.M.; Shape, B.A.; Meier, C.R.; Guglielmo, B.S. The FDA extended warning for intravenous haloperidol and torsades de pointes: How should Institutions respond? J. Hosp. Med. 2010, 5, E8–E16. [Google Scholar] [CrossRef] [PubMed]
- Tanti, A.; Micallef, B.; Saijj, J.V.; Serracino-Inglett, A.I.; Borg, J.J. QT shortening: A proarrhythmic safety surrogate measure or an inappropriate surrogate indicator of it? Curr. Med. Res. Opin. 2022, 38, 1473–1483. [Google Scholar] [CrossRef]
- Metha, V.S.; Elliott, M.K.; Sidhu, B.S.; Gould, J.; Porter, B.; Niederer, S.; Rinaldi, C.A. Multipoint pacing for cardiac resynchronization therapy in patients with heart failure: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2021, 32, 2577–2589. [Google Scholar]
- Menardi, E.; Ballari, G.P.; Goletto, C.; Rossetti, G.; Vado, A. Characterization of ventricular activation pattern and acute hemodynamics during multipoint left ventricular pacing. Heart Rhythm 2015, 12, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Senes, J.; Mascia, G.; Bottoni, N.; Oddone, D.; Donateo, P.; Grimaldi, T.; Minneci, C.; Bertolozzi, I.; Brignole, M.; Puggioni, E.; et al. Is His-optimized superiot to conventional cardiaca resynchronization therapy in improving heart failure. Results from a propensity-method study. Pace 2021, 44, 1532–1539. [Google Scholar] [CrossRef]
- Gonzales-Mates, C.E.; Rodriguez-Queralto, O.; Zakeret, F.; Jimenez, J.; Casteigt, B.; Valles, E. Conduction system stimulation to avoid left ventricular dysfunction. Circ. Arrhyt. Electrophysiol. 2024, 17, e012473. [Google Scholar]
- Kutyifa, V.; Zareba, W.; McNitt, S.; Singh, J.; Hall, W.J.; Polonsky, S.; Goldenberg, I.; Huang, D.T.; Merkely, B.; Wang, P.J.; et al. Left ventricular lead location and the risk of ventricular arrhythmias in the MADIT-CRT trial. Eur. Heart J. 2013, 34, 184–190. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonella, A.; Casile, C.; Menardi, E.; Feola, M. Electrical Storm Induced by Cardiac Resynchronization: Efficacy of the Multipoint Pacing Stimulation. Diseases 2024, 12, 105. https://doi.org/10.3390/diseases12050105
Gonella A, Casile C, Menardi E, Feola M. Electrical Storm Induced by Cardiac Resynchronization: Efficacy of the Multipoint Pacing Stimulation. Diseases. 2024; 12(5):105. https://doi.org/10.3390/diseases12050105
Chicago/Turabian StyleGonella, Anna, Carmelo Casile, Endrj Menardi, and Mauro Feola. 2024. "Electrical Storm Induced by Cardiac Resynchronization: Efficacy of the Multipoint Pacing Stimulation" Diseases 12, no. 5: 105. https://doi.org/10.3390/diseases12050105
APA StyleGonella, A., Casile, C., Menardi, E., & Feola, M. (2024). Electrical Storm Induced by Cardiac Resynchronization: Efficacy of the Multipoint Pacing Stimulation. Diseases, 12(5), 105. https://doi.org/10.3390/diseases12050105






