The Assessment of SF-36 Survey for Quality-of-Life Measurement after Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria and Definitions
2.3. Definitions
2.4. Data Collection Process
2.5. Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Participants’ Characteristics
3.3. Disease Characteristics
3.4. SF-36 Survey Results
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.G.K.; Noon, A.P. Epidemiology, aetiology and screening of bladder cancer. Transl. Androl. Urol. 2019, 8, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Mastroianni, R.; Iannuzzi, A.; Ragusa, A.; Tuderti, G.; Ferriero, M.; Anceschi, U.; Bove, A.M.; Brassetti, A.; Misuraca, L.; D’Annunzio, S.; et al. Health Related Quality of Life in Patients with Bladder Cancer Receiving a Radical Cystectomy. Cancers 2023, 15, 5830. [Google Scholar] [CrossRef] [PubMed]
- Waraich, T.A.; Khalid, S.Y.; Ali, A.; Kathia, U.M. Comparative Outcomes of Radical Cystectomy in Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e50646. [Google Scholar] [CrossRef]
- Nason, G.J.; Ajib, K.; Tan, G.H.; Kulkarni, G.S. Radical cystectomy-what is the optimal surgical approach? Transl. Androl. Urol. 2020, 9, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Aminoltejari, K.; Black, P.C. Radical cystectomy: A review of techniques, developments and controversies. Transl. Androl. Urol. 2020, 9, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.S.; Shan, B.L.; Shan, L.L.; Chin, P.; Murray, S.; Ahmadi, N.; Saxena, A. A systematic review and meta-analysis of quality of life outcomes after radical cystectomy for bladder cancer. Surg. Oncol. 2016, 25, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.B.; Atkinson, T.M.; Dalbagni, G.M.; Li, Y.; Vickers, A.J.; Herr, H.W.; Donat, S.M.; Sandhu, J.S.; Sjoberg, D.S.; Tin, A.L.; et al. Health-related Quality of Life for Patients Undergoing Radical Cystectomy: Results of a Large Prospective Cohort. Eur. Urol. 2022, 81, 294–304. [Google Scholar] [CrossRef]
- Westhofen, T.; Eismann, L.; Buchner, A.; Schlenker, B.; Giessen-Jung, C.; Becker, A.; Stief, C.G.; Kretschmer, A. Baseline Health-related Quality of Life Predicts Bladder Cancer-specific Survival Following Radical Cystectomy. Eur. Urol. Focus 2022, 8, 1659–1665. [Google Scholar] [CrossRef]
- Catto, J.W.F.; Downing, A.; Mason, S.; Wright, P.; Absolom, K.; Bottomley, S.; Hounsome, L.; Hussain, S.; Varughese, M.; Raw, C.; et al. Quality of Life After Bladder Cancer: A Cross-sectional Survey of Patient-reported Outcomes. Eur. Urol. 2021, 79, 621–632. [Google Scholar] [CrossRef]
- Smith, A.B.; Jaeger, B.; Pinheiro, L.C.; Edwards, L.J.; Tan, H.J.; Nielsen, M.E.; Reeve, B.B. Impact of bladder cancer on health-related quality of life. BJU Int. 2018, 121, 549–557. [Google Scholar] [CrossRef]
- Wong, F.Y.; Yang, L.; Yuen, J.W.M.; Chang, K.K.P.; Wong, F.K.Y. Assessing quality of life using WHOQOL-BREF: A cross-sectional study on the association between quality of life and neighborhood environmental satisfaction, and the mediating effect of health-related behaviors. BMC Public Health 2018, 18, 1113. [Google Scholar] [CrossRef]
- Péus, D.; Newcomb, N.; Hofer, S. Appraisal of the Karnofsky Performance Status and proposal of a simple algorithmic system for its evaluation. BMC Med. Inform. Decis. Mak. 2013, 13, 72. [Google Scholar] [CrossRef]
- Ware, J., Jr.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Kim, Y.E.; Jung, Y.S.; Ock, M.; Yoon, S.J. DALY Estimation Approaches: Understanding and Using the Incidence-based Approach and the Prevalence-based Approach. J. Prev. Med. Public Health 2022, 55, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Segura, N.; Marcos-Delgado, A.; Pinto-Carral, A.; Fernández-Villa, T.; Molina, A.J. Health-Related Quality of Life (HRQOL) Instruments and Mobility: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 16493. [Google Scholar] [CrossRef] [PubMed]
- Pazeto, C.L.; Baccaglini, W.; Tourinho-Barbosa, R.R.; Glina, S.; Cathelineau, X.; Sanchez-Salas, R. HRQOL related to urinary diversion in Radical Cystectomy: A systematic review of recent literature. Int. Braz. J. Urol. 2019, 45, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Omorphos, N.P.; Piedad, J.C.P.; Vasdev, N. Guideline of guidelines: Muscle-invasive bladder cancer. Turk. J. Urol. 2021, 47 (Suppl. S1), S71–S78. [Google Scholar] [CrossRef] [PubMed]
- Xun, Y.; Guo, Q.; Ren, M.; Liu, Y.; Sun, Y.; Wu, S.; Lan, H.; Zhang, J.; Liu, H.; Wang, J.; et al. Characteristics of the sources, evaluation, and grading of the certainty of evidence in systematic reviews in public health: A methodological study. Front. Public Health 2023, 11, 998588. [Google Scholar] [CrossRef] [PubMed]
- Hara, I.; Miyake, H.; Hara, S.; Gotoh, A.; Nakamura, I.; Okada, H.; Arakawa, S.; Kamidono, S. Health-related quality of life after radical cystectomy for bladder cancer: A comparison of ileal conduit and orthotopic bladder replacement. BJU Int. 2002, 89, 10–13. [Google Scholar] [CrossRef]
- Yang, M.; Wang, H.; Wang, J.; Ruan, M. Impact of invasive bladder cancer and orthotopic urinary diversion on general health-related quality of life: An SF-36 survey. Mol. Clin. Oncol. 2013, 1, 758–762. [Google Scholar] [CrossRef]
- Philip, J.; Manikandan, R.; Venugopal, S.; Desouza, J.; Javlé, P.M. Orthotopic neobladder versus ileal conduit urinary diversion after cystectomy--a quality-of-life based comparison. Ann. R. Coll. Surg. Engl. 2009, 91, 565–569. [Google Scholar] [CrossRef]
- Autorino, R.; Quarto, G.; Di Lorenzo, G.; De Sio, M.; Perdonà, S.; Giannarini, G.; Giugliano, F.; Damiano, R. Health related quality of life after radical cystectomy: Comparison of ileal conduit to continent orthotopic neobladder. Eur. J. Surg. Oncol. 2009, 35, 858–864. [Google Scholar] [CrossRef]
- Fujisawa, M.; Isotani, S.; Gotoh, A.; Okada, H.; Arakawa, S.; Kamidono, S. Health-related quality of life with orthotopic neobladder versus ileal conduit according to the SF-36 survey. Urology 2000, 55, 862–865. [Google Scholar] [CrossRef]
- Takenaka, A.; Hara, I.; Soga, H.; Sakai, I.; Terakawa, T.; Muramaki, M.; Miyake, H.; Tanaka, K.; Fujisawa, M. Assessment of long-term quality of life in patients with orthotopic neobladder followed for more than 5 years. Int. Urol. Nephrol. 2011, 43, 749–754. [Google Scholar] [CrossRef]
- Yoneda, T.; Adachi, H.; Urakami, S.; Kishi, H.; Shigeno, K.; Shiina, H.; Igawa, M. Health related quality of life after orthotopic neobladder construction and its comparison with normative values in the Japanese population. J. Urol. 2005, 174, 1944–1947. [Google Scholar] [CrossRef]
- Miyake, H.; Furukawa, J.; Muramaki, M.; Takenaka, A.; Fujisawa, M. Orthotopic bladder substitution following radical cystectomy in women: Comparative study between sigmoid and ileal neobladders. Urol. Oncol. 2012, 30, 38–43. [Google Scholar] [CrossRef]
- Stakhovskyi, O.E.; Semko, S.L.; Pikul, M.V.; Grechko, B.O.; Voylenko, O.A.; Kononenko, O.A.; Vitruk, I.V.; Stakhovsky, E.O. Quality of life in patients after radical cystectomy with modified ureterocutaneostomy and Bricker urinary diversion. Exp Oncol. 2020, 42, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.R.; Wright, J.L.; Holt, S.K.; Dash, A.; Gore, J.L.; Schade, G.R. Health Related Quality of Life Following Radical Cystectomy: Comparative Analysis from the Medicare Health Outcomes Survey. J Urol. 2018, 199, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Sevgi, V.; Özgür, Ç.; Talha, M.; Güven, A.; Hakan, B. The Effect of Postoperative Early Mobilization on Healing Process and Quality of Life Following Radical Cystectomy and Ileal Conduit: A Randomized Prospective Controlled Trial. J. Urol. Surg. 2021, 9, 9–19. [Google Scholar] [CrossRef]
- Francolini, G.; Ghoshal, A.; Caini, S.; Piazzini, T.; Becherini, C.; Detti, B.; Di Cataldo, V.; Valzano, M.; Visani, L.; Salvestrini, V.; et al. Quality of life after definitive treatment for bladder cancer: A systematic review and meta-analysis. Radiother. Oncol. 2024, 190, 110038. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.J.; Downing, A.; Wright, P.; Hounsome, L.; Bottomley, S.E.; Corner, J.; Richards, M.; Catto, J.W.; Glaser, A.W. Health-related quality of life after treatment for bladder cancer in England. Br. J. Cancer 2018, 118, 1518–1528. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Wu, T.Y.; Ou, C.H.; Cheng, H.L.; Tzai, T.S.; Yang, W.H.; Wang, J.D. Dynamic changes of quality of life in muscle-invasive bladder cancer survivors. BMC Urol. 2022, 22, 126. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, G.; Anwar, T.; Golzy, M.; Murray, K.S. Living with Bladder Cancer: Self-reported Changes in Patients’ Functional and Overall Health Status Following Diagnosis. Eur. Urol. Open Sci. 2020, 20, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Iwata, T.; Foerster, B.; Fossati, N.; Briganti, A.; Nasu, Y.; Egawa, S.; Abufaraj, M.; Shariat, S.F. Comparison of perioperative complications and health-related quality of life between robot-assisted and open radical cystectomy: A systematic review and meta-analysis. Int. J. Urol. 2019, 26, 760–774. [Google Scholar] [CrossRef]
- Mastroianni, R.; Tuderti, G.; Anceschi, U.; Bove, A.M.; Brassetti, A.; Ferriero, M.; Zampa, A.; Giannarelli, D.; Guaglianone, S.; Gallucci, M.; et al. Comparison of Patient-reported Health-related Quality of Life Between Open Radical Cystectomy and Robot-assisted Radical Cystectomy with Intracorporeal Urinary Diversion: Interim Analysis of a Randomised Controlled Trial. Eur. Urol. Focus 2022, 8, 465–471. [Google Scholar] [CrossRef]

| Study and Author | Country | Study Year | Study Design | Quality of Evidence |
|---|---|---|---|---|
| 1. Hara et al. [21] | Japan | 2002 | Prospective cohort | Low |
| 2. Yang et al. [22] | China | 2013 | Prospective cohort | Low |
| 3. Philip et al. [23] | United Kingdom | 2009 | Cross-sectional | Low |
| 4. Autorino et al. [24] | Italy | 2009 | Prospective cohort | High |
| 5. Fujisawa et al. [25] | Japan | 2000 | Cross-sectional | Low |
| 6. Takenaka et al. [26] | Japan | 2010 | Prospective cohort | High |
| 7. Yoneda et al. [27] | Japan | 2005 | Prospective cohort | Medium |
| 8. Miyake et al. [28] | Japan | 2012 | Cross-sectional | Medium |
| 9. Stakhovskyi et al. [29] | Ukraine | 2020 | Cross-sectional | Low |
| 10. Winters et al. [30] | United States | 2018 | Cross-sectional | Medium |
| 11. Vermişli et al. [31] | Turkey | 2021 | Randomized Trial | Medium |
| Study Number | Age (Years) | Sex | Number of Patients | Control Group | Time of Evaluation/ Follow-Up |
|---|---|---|---|---|---|
| 1. Hara et al. [21] | 58.5 (mean) | 85 (100%) men | 85 | Ileal conduit vs. Neobladder | Follow-up: 130 months (median) |
| 2. Yang et al. [22] | 66.0 (mean) | NR | 82 | Ileal conduit vs. Neobladder | At 6 months, 1 year and 2 years after surgery |
| 3. Philip et al. [23] | 65.5 vs. 73.5 | 40 (76.9%) men | 52 | Ileal conduit vs. Neobladder | 15 months (median) |
| 4. Autorino et al. [24] | 65.9 vs. 63.5 | 79 (100%) men | 79 | Ileal conduit vs. Neobladder | >12 months |
| 5. Fujisawa et al. [25] | 61.4 vs. 70.6 | 38 (67.8%) men | 56 | Ileal conduit vs. Neobladder | 31 vs. 44 months |
| 6. Takenaka et al. [26] | 62.0 (median) | 78 (90.7%) men | 86 | Continence vs. Incontinence | 89 months |
| 7. Yoneda et al. [27] | 65.6 (mean) | 47 (83.9%) men | 56 | NR | 51.6 months |
| 8. Miyake et al. [28] | 63 (median) | 32 (100%) women | 32 | Sigmoid vs. Ileal Neobladder | >12 months |
| 9. Stakhovskyi et al. [29] | 58.5 vs. 60.5 | NR | 40 | Ileal conduit vs. Neobladder | 3 months |
| 10. Winters et al. [30] | 77 (mean) | 126 (76%) | 166 | Bladder vs. Colorectal Cancer | 24 months |
| 11. Vermişli et al. [31] | 64.8 (mean) | 20 (50%) | 40 | Early vs. Late Mobilization | 3 months |
| Study Number | Ileal Conduit (Non-Orthotopic) | Neobladder (Orthotopic) | Status of Continence | Cancer Type/Staging/Grading |
|---|---|---|---|---|
| 1. Hara et al. [21] | 37 (43.5%) | Ileum 26 (30.6%) Colon 22 (25.9%) | Daytime micturition: Grade I (52%) Grade II (38%) Grade III (10%) | NR |
| 2. Yang et al. [22] | 28 (34.1%) | 54 (65.9%) | NR | 77 (93.9%) TCC 5 (6.1%) T1 45 (54.9%) T2 32 (39.0%) T3 |
| 3. Philip et al. [23] | 24 (46.1%) | 28 (53.9%) | 15% incontinence | NR |
| 4. Autorino et al. [24] | 44 (55.7%) | 35 (64.3%) | Daytime micturition: Grade I (59%) Grade II (34%) Grade III (7%) | 59 (74.6%) T2 13 (25.4%) T3 |
| 5. Fujisawa et al. [25] | 20 (35.7%) | 36 (64.3%) | Daytime micturition: Grade I (53.6%) Grade II (35.7%) Grade III (10.7%) | NR |
| 6. Takenaka et al. [26] | 0 (0%) | 86 (100%) | 27.9% incontinence | NR |
| 7. Yoneda et al. [27] | 0 (0%) | 56 (100%) | NR | NR |
| 8. Miyake et al. [28] | 0 (0%) | Ileum 14 (43.8%) Sigmoid 18 (56.2%) | Spontaneous voiding: Ileum—64.3% Sigmoid—94.4% Daytime micturition: Ileum—91.7% Sigmoid—83.3% | 21 (65.6%) T2 9 (28.1%) T3 2 (6.3%) T4 |
| 9. Stakhovskyi et al. [29] | 20 (50%) | 20 (50%) | NR | 25 (62.5%) T2 9 (22.5%) T3 6 (15.0%) T4 |
| 10. Winters et al. [30] | 156 (100%) | 0 (0%) | NR | NR |
| 11. Vermişli et al. [31] | 40 (100%) | 0 (0%) | NR | NR |
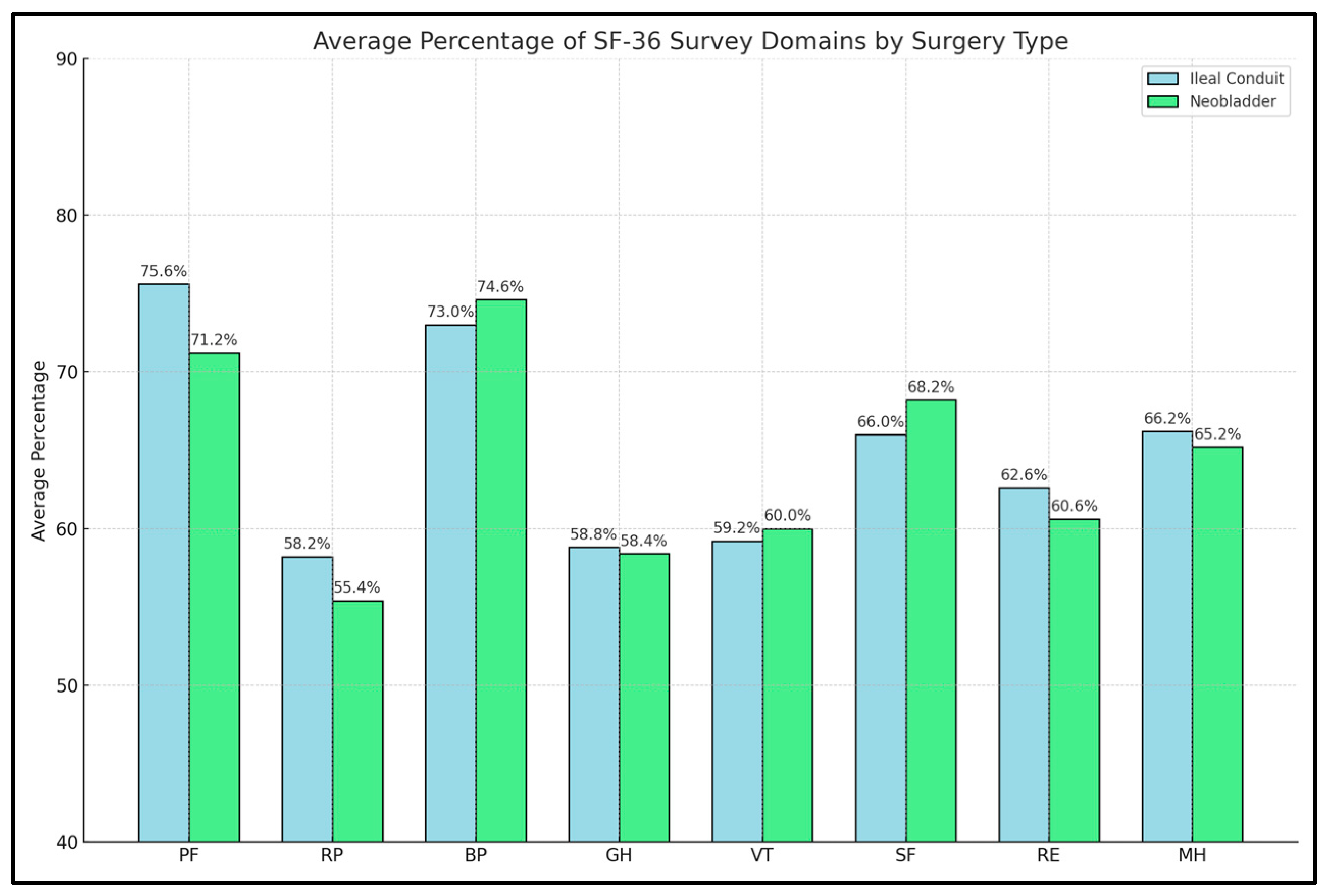
| Study Number ** | PF | RP | BP | GH | VT | SF | RE | MH | TOTAL |
|---|---|---|---|---|---|---|---|---|---|
| 1. Hara et al. [21] | 75% vs. 74% | 63% vs. 58% | 76% vs. 73% | 50% vs. 50% * | 55% vs. 55% | 52% vs. 46% | 62% vs. 66% * | 64% vs. 65% | NR |
| 2. Yang et al. [22] | 23.0 vs. 24.1 | 5.7 vs. 5.2 | 9.2 vs. 10.3 | 19.6 vs. 11.3 * | 16.3 vs. 14.2 | 7.3 vs. 4.8 * | 3.9 vs. 3.1 * | 23.1 vs. 15.1 * | 112.8 vs. 95.4 * |
| 3. Philip et al. [23] | 77% vs. 61% * | 68% vs. 59% | 78% vs. 79% | 73% vs. 68% | 61% vs. 62% | 79% vs. 79% | 84% vs. 79% | 86% vs. 79% | NR |
| 4. Autorino et al. [24] | 70% vs. 76% | 61% vs. 59% * | 71% vs. 72% | 60% vs. 60% | 54% vs. 52% | 71% vs. 60% * | 63% vs. 52% * | 69 vs. 64% | NR |
| 5. Fujisawa et al. [25] | 78% vs. 80% | 54% vs. 64% * | 71% vs. 80% | 56% vs. 64% | 62% vs. 70% | 76% vs. 81% | 55% vs. 66% * | 71% vs. 76% | NR |
| 6. Takenaka et al. [26] | 50% vs. 44% | 45% vs. 40% | 54% vs. 50% | 51% vs. 42% * | 53% vs. 50% | 50% vs. 41% * | 46% vs. 39% | 52% vs. 48% * | NR |
| 7. Yoneda et al. [27] | 75% vs. 77% | 61% vs. 79% * | 69% vs. 70% | 57% vs. 61% | 62% vs. 63% | 81% vs. 85% | 68% vs. 80% * | 70% vs. 75% | NR |
| 8. Miyake et al. [28] | 48% vs. 39% | 43% vs. 46% | 53% vs. 48% | 49% vs. 47% | 53% vs. 50% | 48% vs. 40% | 44% vs. 35% | 51% vs. 50% | NR |
| 9. Stakhovskyi et al. [29] | 78% vs. 65% | 45% vs. 37% | 69% vs. 69% | 55% vs. 50% | 64% vs. 61% | 52% vs. 75% | 49% vs. 40% | 41% vs. 42% | NR |
| 10. Winters et al. [30] | 53% vs. 51% | 42% vs. 38% | 50% vs. 56% | 52% vs. 51% | 51% vs. 48% | 70% vs. 66% | 66% vs. 62% | 73% vs. 70% | NR |
| 11. Vermişli et al. [31] | 74% vs. 66% | 70% vs. 42% * | 49% vs. 53% | 64% vs. 38% * | 66% vs. 41% * | 65% vs. 48% * | 73% vs. 45% * | 83% vs. 60% * | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbos, V.; Feciche, B.; Latcu, S.; Croitor, A.; Dema, V.; Bardan, R.; Faur, F.I.; Mateescu, T.; Novacescu, D.; Bogdan, G.; et al. The Assessment of SF-36 Survey for Quality-of-Life Measurement after Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Systematic Review. Diseases 2024, 12, 56. https://doi.org/10.3390/diseases12030056
Barbos V, Feciche B, Latcu S, Croitor A, Dema V, Bardan R, Faur FI, Mateescu T, Novacescu D, Bogdan G, et al. The Assessment of SF-36 Survey for Quality-of-Life Measurement after Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Systematic Review. Diseases. 2024; 12(3):56. https://doi.org/10.3390/diseases12030056
Chicago/Turabian StyleBarbos, Vlad, Bogdan Feciche, Silviu Latcu, Alexei Croitor, Vlad Dema, Razvan Bardan, Flaviu Ionut Faur, Tudor Mateescu, Dorin Novacescu, Gherle Bogdan, and et al. 2024. "The Assessment of SF-36 Survey for Quality-of-Life Measurement after Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Systematic Review" Diseases 12, no. 3: 56. https://doi.org/10.3390/diseases12030056
APA StyleBarbos, V., Feciche, B., Latcu, S., Croitor, A., Dema, V., Bardan, R., Faur, F. I., Mateescu, T., Novacescu, D., Bogdan, G., & Cumpanas, A. A. (2024). The Assessment of SF-36 Survey for Quality-of-Life Measurement after Radical Cystectomy for Muscle-Invasive Bladder Cancer: A Systematic Review. Diseases, 12(3), 56. https://doi.org/10.3390/diseases12030056







