Predictors of Occult Metastasis and Prognostic Factors in Patients with cN0 Oral Cancer Who Underwent Elective Neck Dissection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.2. Study Variables
2.3. Data Analyses
3. Results
3.1. Patient Characteristics
3.2. Variables Predicting the OM for cN0 ND
3.2.1. Variables for OM
3.2.2. Logistic Multivariate Analysis of the Parameters
3.3. The Factors Affecting Prognosis after SOHND
3.3.1. Association between Clinical Factors and OS and DFS (Table 3)
| Variables | No. of Patients (%) | OS (%) | p † | DFS (%) | p † | |
|---|---|---|---|---|---|---|
| Age | ≥65 | 44 (51.2) | 80.1 | 0.402 | 82.0 | 0.900 |
| Median (years) | <65 | 42 (48.8) | 86.7 | 82.4 | ||
| Sex | Male | 55 (64.0) | 82.0 | 0.837 | 79.7 | 0.582 |
| Female | 31 (36.0) | 85.8 | 86.8 | |||
| Tobacco consumption | Present | 9 (10.5) | 75.0 | 0.891 | 83.3 | 0.683 |
| None | 77 (89.5) | 83.7 | 82.0 | |||
| Alcohol consumption | Present | 36 (41.9) | 87.2 | 0.516 | 78.8 | 0.559 |
| None | 50 (58.1) | 80.6 | 84.8 | |||
| Primary site | Tongue | 40 (46.5) | 81.6 | 0.716 | 80.8 | 0.071 |
| Lower gingiva | 37 (43.0) | 85.1 | 85.9 | |||
| Buccal mucosa | 5 (5.8) | 100 | 100 | |||
| Other | 4 (1.2) | 66.7 | 37.5 | |||
| T classification | T1 | 6 (7.0) | 100 | 0.045 * | 83.3 | 0.257 |
| T2 | 33 (38.4) | 89.9 | 87.1 | |||
| T3 | 12 (14.0) | 54.9 | 58.3 | |||
| T4a | 31 (36.0) | 84.5 | 85.9 | |||
| T4b | 4 (4.7) | 75.0 | 75.0 | |||
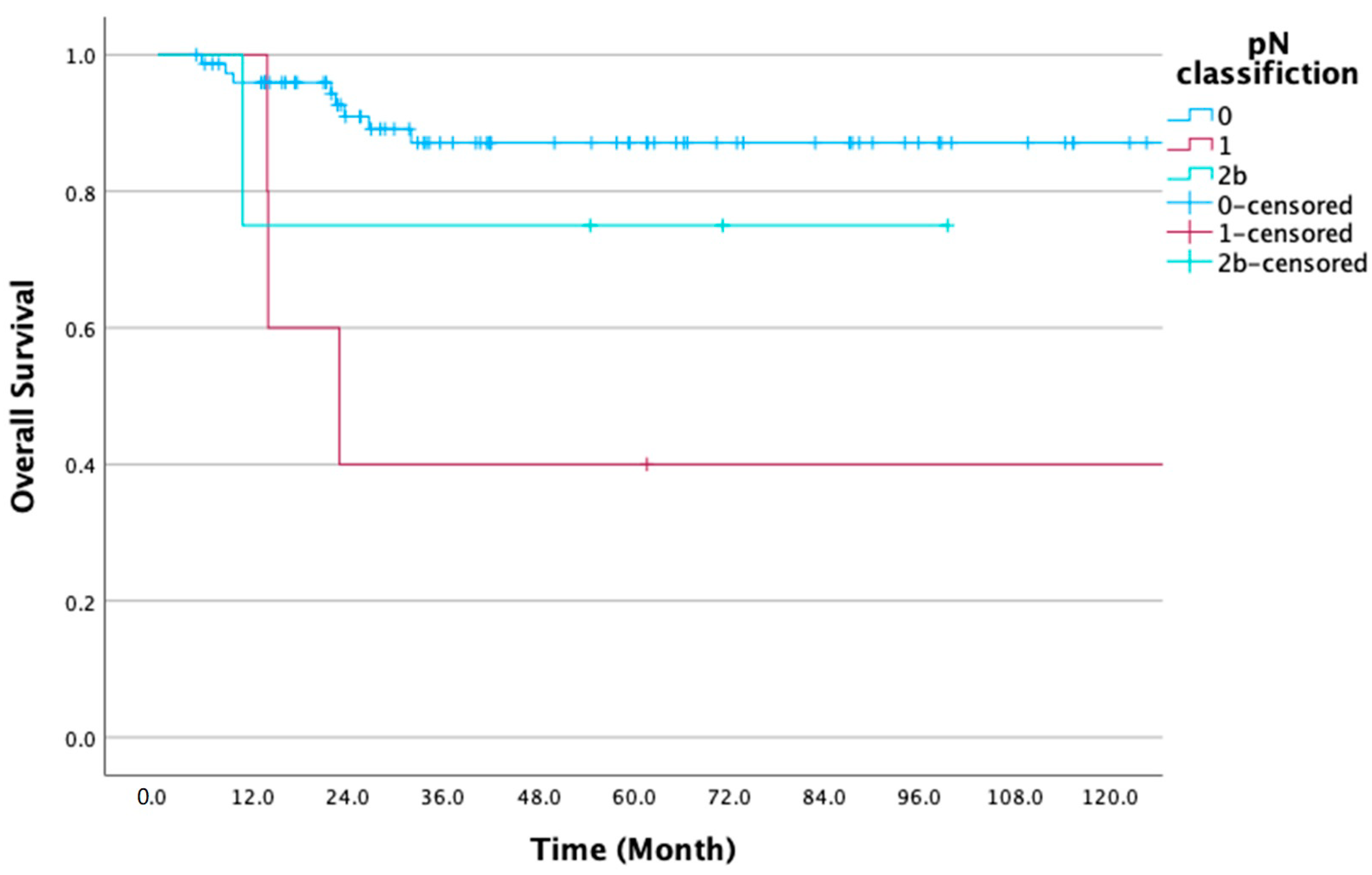
| pN classification | N0 | 77 (89.5) | 87.1 | 0.006 ** | 85.5 | 0.009 ** |
| N1 | 5 (5.8) | 40.0 | 40.0 | |||
| N2b | 4 (4.7) | 75.0 | 75.0 | |||
| pN | Present | 9 (10.5) | 55.6 | 0.007 ** | 55.6 | 0.011 * |
| None | 77 (89.5) | 87.1 | 85.5 | |||
| Surgical margin | <5 | 36 (41.9) | 73.7 | 0.043 * | 69.3 | 0.013 * |
| (mm) | ≥5 | 50 (58.1) | 90.2 | 91.1 | ||
| Histological grade | G1 | 52 (60.5) | 84.2 | 0.686 | 83.6 | 0.621 |
| G2 | 30 (34.9) | 80.0 | 77.8 | |||
| G3 | 4 (4.7) | 100 | 100 | |||
| BMI (kg/m2) | <18.5 | 9 (10.5) | 75.0 | 0.250 | 76.2 | 0.732 |
| ≥18.5–<25 | 57 (66.3) | 81.2 | 82.8 | |||
| ≥25 | 20 (23.3) | 93.8 | 83.6 | |||
| NLR | ≥1.74 | 65 (75.6) | 84.0 | 0.547 | 82.3 | 0.723 |
| <1.74 | 21 (24.4) | 80.4 | 81.0 | |||
| Laterality of neck dissection | Unilateral | 79 (91.9) | 86.2 | 0.023 * | 83.1 | 0.339 |
| Bilateral | 7 (8.1) | 57.1 | 71.4 | |||
| Reconstructive surgery | Present | 56 (65.1) | 77.1 | 0.050 | 76.8 | 0.097 |
| None | 30 (34.9) | 96.2 | 92.8 | |||
| Lymphatic invasion | Present Absent unknown | 8 (9.3) 76 (88.4) 2 | 62.5 87.4 | 0.057 | 62.5 85.3 | 0.080 |
| Vascular invasion | Present Absent Unknown | 10 (11.6) 73 (84.9) 3 | 68.6 88.2 | 0.049 * | 58.3 87.9 | 0.006 ** |
| Perineural invasion | Present Absent | 21 (24.4) 65 (75.6) | 78.6 85.6 | 0.648 | 74.1 85.4 | 0.420 |
| YK classification | 1 2 3 4C 4D Unknown | 6 (7.0) 11 (12.8) 49 (57.0) 14 (16.3) 1 (1.2) 5 | 83.3 100.0 86.0 85.7 0 | 0.181 | 83.3 100.0 82.2 85.7 0 | 0.209 |
3.3.2. Cox Multivariate Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brennan, P.A.; Subramaniam, S.; Tsioryannis, C.; Green, B. An Update on the Latest Evidence for Managing the Clinically Negative Neck (CN0) in Oral Squamous Cell Carcinoma. Oral Dis. 2017, 23, 287–291. [Google Scholar] [CrossRef]
- Majoufre, C.; Faucher, A.; Laroche, C.; De Bonfils, C.; Siberchicot, F.; Renaud-Salis, J.-L.; Pinsolle, J. Supraomohyoid Neck Dissection in Cancer of the Oral Cavity. Am. J. Surg. 1999, 178, 73–77. [Google Scholar] [CrossRef]
- Chegini, S.; Schilling, C.; Walgama, E.S.; Yu, K.M.; Thankappan, K.; Iyer, S.; Cariati, P.; Balasubramanian, D.; Kanatas, A.; Lai, S.Y.; et al. Neck Failure Following Pathologically Node-Negative Neck Dissection (PN0) in Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Br. J. Oral Maxillofac. Surg. 2021, 59, 1157–1165. [Google Scholar] [CrossRef]
- Koyama, L.K.S.; Matos, L.L.; Kulcsar, M.A.V.; de Araújo Filho, V.J.F.; Cernea, C.R. Oral Cancer Treatment: Still an Indication for Elective Neck Dissection? ORL J. Otorhinolaryngol. Relat. Spec. 2018, 80, 96–102. [Google Scholar] [CrossRef]
- Kaneko, S.; Yoshimura, T.; Ikemura, K.; Shirasuna, K.; Kusukawa, J.; Ohishi, M.; Shiba, R.; Sunakawa, H.; Tominaga, K.; Sugihara, K.; et al. Primary Neck Management among Patients with Cancer of the Oral Cavity without Clinical Nodal Metastases: A Decision and Sensitivity Analysis. Head Neck 2002, 24, 582–590. [Google Scholar] [CrossRef]
- Kerrebijn, J.D.F.; Freeman, J.L.; Irish, J.C.; Witterick, I.J.; Brown, D.H.; Rotstein, L.E.; Gullane, P.J. Supraomohyoid Neck Dissection. Is It Diagnostic or Therapeutic? Head Neck 1999, 21, 39–42. [Google Scholar] [CrossRef]
- Haidari, S.; Obermeier, K.T.; Kraus, M.; Otto, S.; Probst, F.A.; Liokatis, P. Nodal Disease and Survival in Oral Cancer: Is Occult Metastasis a Burden Factor Compared to Preoperatively Nodal Positive Neck? Cancers 2022, 14, 4241. [Google Scholar] [CrossRef]
- Shimamoto, H.; Oikawa, Y.; Osako, T.; Hirai, H.; Mochizuki, Y.; Tanaka, K.; Tomioka, H.; Harada, H. Neck Failure after Elective Neck Dissection in Patients with Oral Squamous Cell Carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 32–36. [Google Scholar] [CrossRef]
- Ding, Z.; Xiao, T.; Huang, J.; Yuan, Y.; Ye, Q.; Xuan, M.; Xie, H.; Wang, X. Elective Neck Dissection Versus Observation in Squamous Cell Carcinoma of Oral Cavity With Clinically N0 Neck: A Systematic Review and Meta-Analysis of Prospective Studies. J. Oral Maxillofac. Surg. 2019, 77, 184–194. [Google Scholar] [CrossRef]
- Mizrachi, A.; Migliacci, J.C.; Montero, P.H.; McBride, S.; Shah, J.P.; Patel, S.G.; Ganly, I. Neck Recurrence in Clinically Node-Negative Oral Cancer: 27-Year Experience at a Single Institution. Oral Oncol. 2018, 78, 94–101. [Google Scholar] [CrossRef]
- Bera, R.N.; Singh, A.K.; Tripathi, R.; Sharma, N.K. Influence of Site, Size, Depth of Invasion and Histologic Grading on the Occurrence of Cervical Level IIb Metastasis and Extranodal Extension in Clinically N0 Neck of Patients with OSCC: A Single Center Retrospective Analysis. J. Maxillofac. Oral Surg. 2022, 21, 1078–1087. [Google Scholar] [CrossRef]
- Struckmeier, A.-K.; Buchbender, M.; Moest, T.; Lutz, R.; Agaimy, A.; Kesting, M. Occult Metastasis Is No Burden Factor in Oral Squamous Cell Carcinoma Patients When Adhering to a Standardized Approach in Neck Dissection. Clin. Oral Investig. 2024, 28, 113. [Google Scholar] [CrossRef]
- Yamamoto, E.; Kohama, G.; Sunakawa, H.; Iwai, M.; Hiratsuka, H. Mode of invasion, bleomycin sensitivity, and clinical course in squamous cell carcinoma of the oral cavity. Cancer 1983, 51, 2175–2180. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefèbvre, J.-L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative Irradiation with or without Concomitant Chemotherapy for Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative Concurrent Radiotherapy and Chemotherapy for High-Risk Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef]
- Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: Executive summary. Am. J. Clin. Nutr. 1998, 68, 899–917. [Google Scholar] [CrossRef]
- Grimm, M.; Rieth, J.; Hoefert, S.; Krimmel, M.; Rieth, S.; Teriete, P.; Kluba, S.; Biegner, T.; Munz, A.; Reinert, S. Standardized Pretreatment Inflammatory Laboratory Markers and Calculated Ratios in Patients with Oral Squamous Cell Carcinoma. Eur. Arch. Otorhinolaryngol. 2016, 273, 3371–3384. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, M.; Li, J.; Feng, Z.; Qin, L.; Han, Z. Diagnostic Value of Magnetic Resonance Imaging in Cervical Lymph Node Metastasis of Oral Squamous Cell Carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 582–592. [Google Scholar] [CrossRef]
- Xu, F.; Peng, L.; Feng, J.; Zhu, X.; Pan, Y.; Hu, Y.; Gao, X.; Ma, Y.; He, Y. A Prediction Model of Nodal Metastasis in cN0 Oral Squamous Cell Carcinoma Using Metabolic and Pathological Variables. Cancer Imaging 2023, 23, 34. [Google Scholar] [CrossRef]
- Laimer, J.; Lauinger, A.; Steinmassl, O.; Offermanns, V.; Grams, A.E.; Zelger, B.; Bruckmoser, E. Cervical Lymph Node Metastases in Oral Squamous Cell Carcinoma-How Much Imaging Do We Need? Diagnostics 2020, 10, 199. [Google Scholar] [CrossRef]
- Ishibashi, N.; Yamagata, K.; Sasaki, H.; Seto, K.; Shinya, Y.; Ito, H.; Shinozuka, K.; Yanagawa, T.; Onizawa, K.; Bukawa, H. Real-Time Tissue Elastography for the Diagnosis of Lymph Node Metastasis in Oral Squamous Cell Carcinoma. Ultrasound Med. Biol. 2012, 38, 389–395. [Google Scholar] [CrossRef]
- Wu, C.-N.; Chuang, H.-C.; Lin, Y.-T.; Fang, F.-M.; Li, S.-H.; Chien, C.-Y. Prognosis of Neutrophil-to-Lymphocyte Ratio in Clinical Early-Stage Tongue (CT1/T2N0) Cancer. OncoTargets Ther. 2017, 10, 3917–3924. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, J.; Zhong, Z. Prediction of Lymph Node Metastasis in Oral Tongue Squamous Cell Carcinoma Using the Neutrophil-to-Lymphocyte Ratio and Platelet-to-Neutrophil Ratio. J. Clin. Lab. Anal. 2021, 35, e23684. [Google Scholar] [CrossRef] [PubMed]
- Müller-Richter, U.; Betz, C.; Hartmann, S.; Brands, R.C. Nutrition Management for Head and Neck Cancer Patients Improves Clinical Outcome and Survival. Nutr. Res. 2017, 48, 1–8. [Google Scholar] [CrossRef]
- Saroul, N.; Puechmaille, M.; Lambert, C.; Hassan, A.S.; Biau, J.; Lapeyre, M.; Mom, T.; Bernadach, M.; Gilain, L. Prognosis in Head and Neck Cancer: Importance of Nutritional and Biological Inflammatory Status. Otolaryngol. Head Neck Surg. 2022, 166, 118–127. [Google Scholar] [CrossRef]
- Yamagata, K.; Fukuzawa, S.; Uchida, F.; Terada, K.; Ishibashi-Kanno, N.; Bukawa, H. Does the Geriatric Nutrition Risk Index Predict the Prognosis of Patients with Oral Squamous Cell Carcinoma? Br. J. Oral Maxillofac. Surg. 2022, 60, 475–481. [Google Scholar] [CrossRef]
- Liaw, G.-A.; Yen, C.-Y.; Chiang, W.-F.; Lee, C.-H.; Yang, C.; Chiou, C.-T.; Liu, S.-Y. Outcome of Treatment with Total Main Tumor Resection and Supraomohyoid Neck Dissection in Oral Squamous Cell Carcinoma. J. Formos. Med. Assoc. 2006, 105, 971–977. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferreli, F.; Festa, B.M.; Costantino, A.; Malvezzi, L.; Colombo, G.; Spriano, G.; Mercante, G.; De Virgilio, A. Prevalence of Occult Level 2b Nodal Metastases in CN0 Squamous Cell Carcinoma of the Oral Cavity: A Systematic Review and Meta-Analysis. Oral Oncol. 2021, 122, 105540. [Google Scholar] [CrossRef]
- Elsheikh, M.N.; Rinaldo, A.; Ferlito, A.; Fagan, J.J.; Suárez, C.; Lowry, J.; Paleri, V.; Khafif, A.; Olofsson, J. Elective Supraomohyoid Neck Dissection for Oral Cavity Squamous Cell Carcinoma: Is Dissection of Sublevel IIB Necessary? Oral Oncol. 2008, 44, 216–219. [Google Scholar] [CrossRef]
- McDonald, C.; Kent, S.; Schache, A.; Rogers, S.; Shaw, R. Health-Related Quality of Life, Functional Outcomes, and Complications after Sentinel Lymph Node Biopsy and Elective Neck Dissection in Early Oral Cancer: A Systematic Review. Head Neck 2023, 45, 2754–2779. [Google Scholar] [CrossRef]
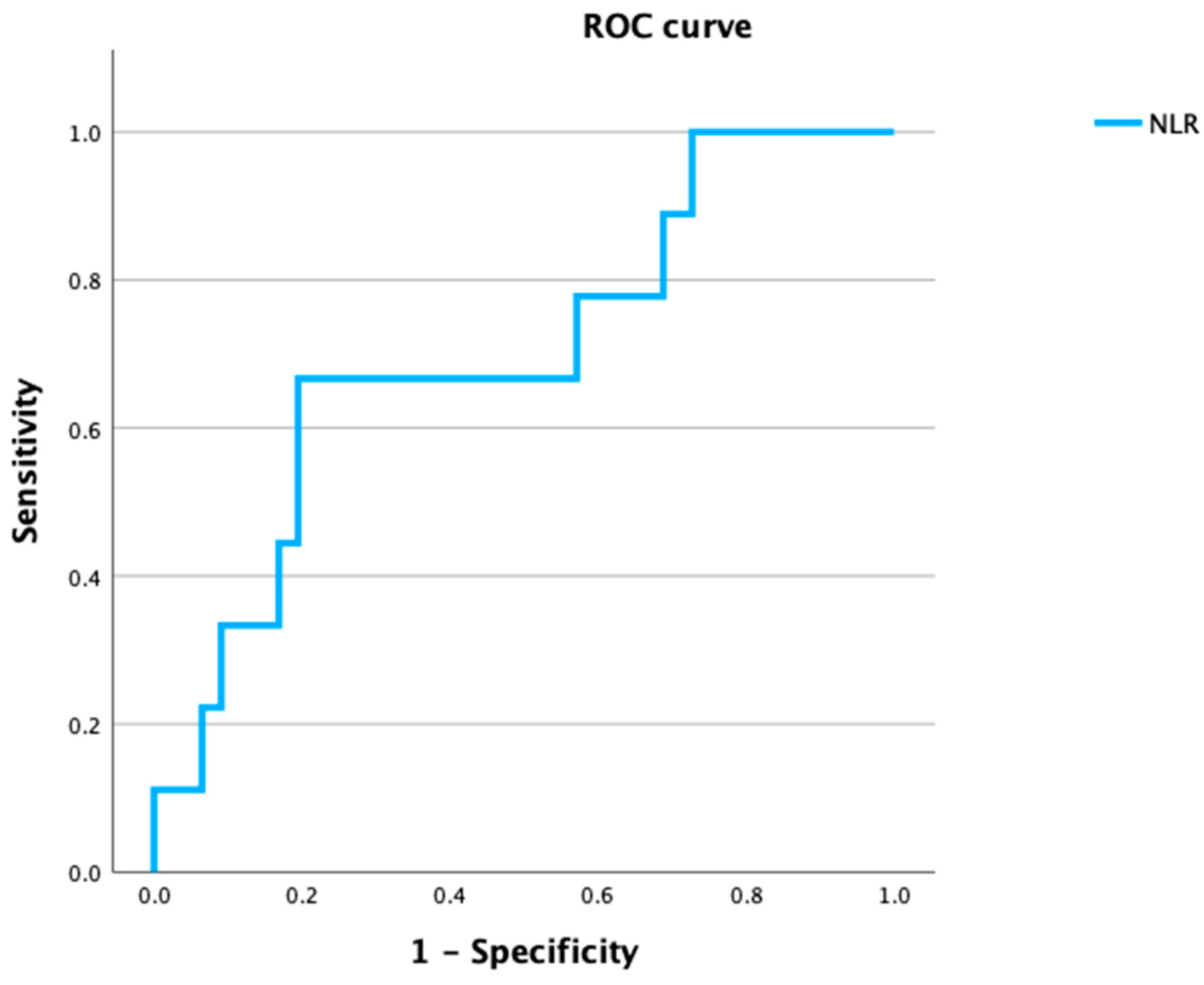
| Variable | Total No. of Patients | No Metastasis No. of Patients (%) n = 77 | Occult Metastasis No. of Patients (%) n = 9 | p-Value | |
|---|---|---|---|---|---|
| Sex | Male | 55 | 49 (63.6) | 6 (66.7) | 1.000 |
| Female | 31 | 28 (36.4) | 3 (33.3) | ||
| Age (years) | <65 | 42 | 38 (49.4) | 4 (44.4) | 1.000 |
| ≥65 | 44 | 39 (50.6) | 5 (55.6) | ||
| BMI (kg/m2) | <18.5 | 9 | 8 (10.4) | 1 (11.1) | 0.656 |
| ≥18.5–<25 | 57 | 50 (64.9) | 7 (77.8) | ||
| ≥25 | 20 | 19 (24.7) | 1 (11.1) | ||
| Lymphocyte count (/mm3) | <1518 | 39 | 38 (49.4) | 1 (11.1) | 0.036 * |
| ≥1518 | 47 | 39 (50.6) | 8 (88.9) | ||
| NLR | <1.74 | 21 | 15 (19.4) | 6 (66.7) | 0.006 ** |
| ≥1.74 | 65 | 62 (80.5) | 3 (33.3) | ||
| Tabaco consumption | Present | 27 | 26 (33.8) | 1 (11.1) | 0.158 |
| Never | 59 | 51 (66.2) | 8 (88.9) | ||
| Alcohol consumption | Present | 38 | 33 (42.9) | 5 (55.6) | 0.353 |
| Never | 48 | 44 (57.1) | 4 (44.4) | ||
| Primary site | Tongue | 40 | 36 (46.8) | 4 (44.4) | 0.656 |
| Lower gingiva | 37 | 34 (44.2) | 3 (33.3) | ||
| Buccal mucosa | 5 | 4 (5.2) | 1 (11.1) | ||
| Other | 4 | 3 (3.9) | 1 (11.1) | ||
| T classification | T1 | 6 | 6 (7.8) | 0 (0) | 0.286 |
| T2 | 33 | 31 (40.3) | 2 (22.2) | ||
| T3 | 12 | 9 (11.7) | 3 (33.3) | ||
| T4a | 31 | 28 (36.4) | 3 (33.3) | ||
| T4b | 4 | 3 (3.9) | 1 (11.1) | ||
| Histological grade | G1 | 52 | 47 (61.0) | 5 (55.6) | 0.680 |
| G2 | 30 | 26 (33.8) | 4 (44.4) | ||
| G3 | 4 | 4 (5.2) | 0 (0) | ||
| YK classification | 1 | 6 | 5 (6.5) | 1 (11.1) | 0.755 |
| 2 | 11 | 11 (14.3) | 0 (0) | ||
| 3 | 49 | 43 (55.8) | 6 (66.7) | ||
| 4C | 14 | 12 (15.6) | 2 (22.2) | ||
| 4D | 1 | 1 (1.3) | 0 (0) | ||
| Unknown | 5 | ||||
| Lymphatic invasion | Present | 8 | 6 (7.8) | 2 (22.2) | 0.203 |
| Absent | 76 | 69 (89.6) | 7 (77.8) | ||
| Unknown | 2 | ||||
| Vascular invasion | Present | 10 | 6 (7.8) | 4 (44.4) | 0.006 ** |
| Absent | 73 | 69 (89.6) | 4 (44.4) | ||
| Unknown | 2 | ||||
| Perineural invasion | Present | 21 | 18 (23.4) | 3 (33.3) | 0.682 |
| Absent | 65 | 59 (76.6) | 6 (66.7) |
| B | Wald | OR | 95% CI | p | |
|---|---|---|---|---|---|
| NLR (≤1.74 vs. >1.74) | 2.269 | 6.199 | 9.674 | 1.621–57.741 | 0.013 * |
| Vascular invasion (Present vs. Absent) | 2.146 | 5.482 | 8.548 | 1.419–51.511 | 0.019 * |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p Values † | HR (95% CI) | p Values † |
| pN | ||||
| Present vs. None | 4.465 (1.343–14.847) | 0.015 * | 4.709 (1.401–15.822) | 0.012 * |
| Laterality of neck dissection | ||||
| Bilateral vs. Unilateral | 4.104 (1.101–15.308) | 0.036 * | 4.392 (1.163–16.589) | 0.029 * |
| Surgical margin (mm) | ||||
| <5 vs. ≥5 | 3.236 (0.973–10.767) | 0.056 | ||
| T classification | ||||
| T1,2 vs. T3,4 | 0.362 (0.098–1.339) | 0.128 | ||
| Vascular invasion | ||||
| Present vs. None | 3.570 (0.922–13.822) | 0.065 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p Values † | HR (95% CI) | p Values † |
| pN | ||||
| Present vs. None | 3.994 (1.251–12.751) | 0.019 * | 1.244 (0.192–8.067) | 0.819 |
| Surgical margin (mm) | ||||
| <5 vs. ≥5 | 3.933 (1.232–12.554) | 0.021 * | 3.991 (1.031–15.453) | 0.045 * |
| Vascular invasion | ||||
| Present vs. None | 4.608 (1.386–15.322) | 0.013 * | 2.889 (0.526–15.882) | 0.222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamagata, K.; Fukuzawa, S.; Noguchi, A.; Takaoka, S.; Uchida, F.; Ishibashi-Kanno, N.; Bukawa, H. Predictors of Occult Metastasis and Prognostic Factors in Patients with cN0 Oral Cancer Who Underwent Elective Neck Dissection. Diseases 2024, 12, 39. https://doi.org/10.3390/diseases12020039
Yamagata K, Fukuzawa S, Noguchi A, Takaoka S, Uchida F, Ishibashi-Kanno N, Bukawa H. Predictors of Occult Metastasis and Prognostic Factors in Patients with cN0 Oral Cancer Who Underwent Elective Neck Dissection. Diseases. 2024; 12(2):39. https://doi.org/10.3390/diseases12020039
Chicago/Turabian StyleYamagata, Kenji, Satoshi Fukuzawa, Atsuro Noguchi, Shohei Takaoka, Fumihiko Uchida, Naomi Ishibashi-Kanno, and Hiroki Bukawa. 2024. "Predictors of Occult Metastasis and Prognostic Factors in Patients with cN0 Oral Cancer Who Underwent Elective Neck Dissection" Diseases 12, no. 2: 39. https://doi.org/10.3390/diseases12020039
APA StyleYamagata, K., Fukuzawa, S., Noguchi, A., Takaoka, S., Uchida, F., Ishibashi-Kanno, N., & Bukawa, H. (2024). Predictors of Occult Metastasis and Prognostic Factors in Patients with cN0 Oral Cancer Who Underwent Elective Neck Dissection. Diseases, 12(2), 39. https://doi.org/10.3390/diseases12020039






