Comparison of Three-Bag Method Acetylcysteine Versus Two-Bag Method Acetylcysteine for the Treatment of Acetaminophen Toxicity: An Updated Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection Criteria
2.3. Outcome Measures
2.4. Assessment of Study Quality and Publication Bias
2.5. Data Synthesis and Statistical Analysis
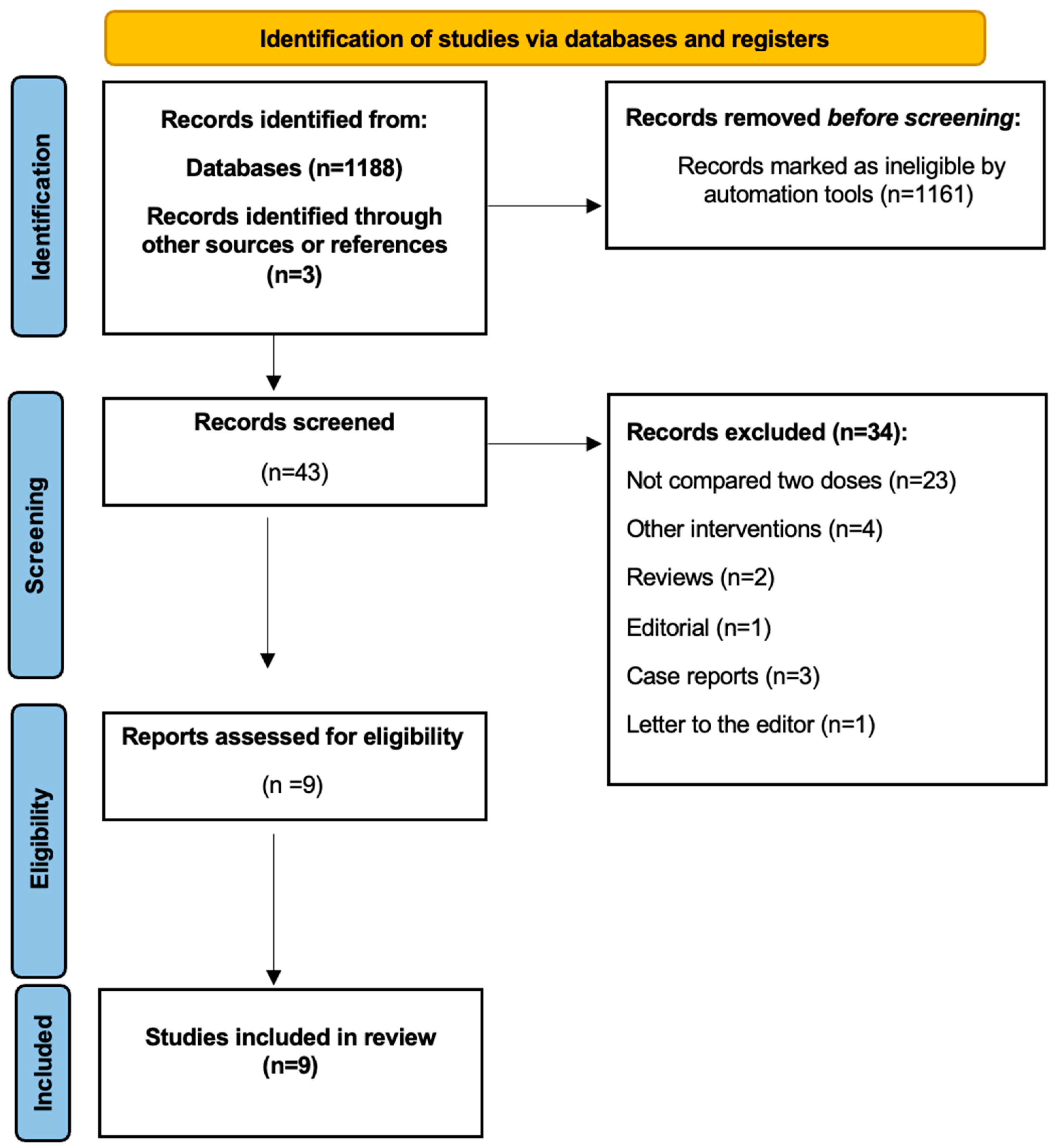
3. Results
3.1. Included Studies
3.2. Study Characteristics and Quality Assessment
3.3. Results of the Meta-Analysis
3.3.1. Anaphylactic Reactions
3.3.2. Gastrointestinal Symptoms
3.3.3. Liver Toxicity
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Model List of Essential Medicines—22nd List, 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Therapeutic Goods Administration (TGA). Recommended Paracetamol Doses; Therapeutic Goods Administration: Woden, Australia, 2023.
- Budnitz, D.S.; Lovegrove, M.C.; Crosby, A.E. Emergency Department Visits for Overdoses of Acetaminophen-Containing Products. Am. J. Prev. Med. 2011, 40, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.S.; Curry, S.C. Evaluation and Treatment of Acetaminophen Toxicity. Adv. Pharmacol. 2019, 85, 263–272. [Google Scholar] [PubMed]
- Ershad, M.; Naji, A.; Vearrier, D. N-Acetylcysteine. Adv. Pharmacol. 2019, 85, 173–182. [Google Scholar]
- Hayes, B.D.; Klein-Schwartz, W.; Doyon, S. Frequency of Medication Errors with Intravenous Acetylcysteine for Acetaminophen Overdose. Ann. Pharmacother. 2008, 42, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Institute for Safe Medication Practices Canada. Preventing Errors with Intravenous Acetylcysteine. ISMP Can. Saf. Bull. 2023, 23, 1–13. Available online: https://ismpcanada.ca/wp-content/uploads/ISMPCSB2023-i7-Acetylcysteine.pdf (accessed on 14 June 2024).
- Cole, J.B.; Thomas, M.; Zhang, W. Is Two Better Than Three? A Systematic Review of Two-Bag Intravenous N-Acetylcysteine Regimens for Acetaminophen Poisoning. West. J. Emerg. Med. 2023, 24, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- McNulty, R.; Lim, J.M.E.; Chandru, P.; Gunja, N. Fewer Adverse Effects with a Modified Two-Bag Acetylcysteine Protocol in Paracetamol Overdose. Clin. Toxicol. 2017, 56, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Alrossies, A.S. Evaluation of a Shorter 12h Acetylcysteine Regimen and Development of a Simpler Acetylcysteine (SNAP) Protocol for the Treatment of Paracetamol Poisoning. Doctoral Dissertation, Newcastle University, Callaghan, Australia, 2022. [Google Scholar]
- Johnson, M.T.; Brewer, K.L.; Young, R.S. Evaluation of a Simplified N-Acetylcysteine Dosing Regimen for the Treatment of Acetaminophen Toxicity. Ann. Pharmacother. 2011, 45, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Graudins, A. Simplification of the Standard Three-Bag Intravenous Acetylcysteine Regimen for Paracetamol Poisoning Results in a Lower Incidence of Adverse Drug Reactions. Clin. Toxicol. 2016, 54, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Aki, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 25 April 2016).
- Review Manager (RevMan). Computer Program, Version 5.2; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2012. [Google Scholar]
- Bateman, D.N.; Dear, J.W.; Thanacoody, H.K.R.; Thomas, S.H.L. Effectiveness of the Modified Two-Bag Acetylcysteine Protocol for the Treatment of Paracetamol Poisoning. BMJ 2014, 348, g1236. [Google Scholar] [CrossRef]
- Wong, A.; Homer, N.; Dear, J.W.; Choy, K.W.; Doery, J.; Graudins, A. Paracetamol Metabolite Concentrations Following Low-Risk Overdose Treated with an Abbreviated 12-h Versus 20-h Acetylcysteine Infusion. Clin. Toxicol. 2018, 56, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.E.; Rasmussen, D.N.; Petersen, T.S.; Macias-Perez, I.M.; Pavliv, L.; Kaelin, B.; Dart, R.C.; Dalhoff, K. Fewer Adverse Effects Associated with a Modified Two-Bag Intravenous Acetylcysteine Protocol Compared to Traditional Three-Bag Regimen in Paracetamol Overdose. Clin. Toxicol. 2018, 56, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Daoud, A.; Dalhoff, K.P.; Christensen, M.B.; Bøgevig, S.; Petersen, T.S. Two-Bag Intravenous N-Acetylcysteine, Antihistamine Pretreatment, and High Plasma Paracetamol Levels Are Associated with a Lower Incidence of Anaphylactoid Reactions to N-Acetylcysteine. Clin. Toxicol. 2019, 57, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.C.; James, R.; Young, R. High-Dose N-Acetylcysteine Treatment Does Not Affect the Odds of Hepatotoxicity in Acute Massive Acetaminophen Overdose. J. Clin. Pharmacol. 2021, 61, 298–304. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Graudins, A.; Wong, A. A Two-Bag Acetylcysteine Regimen Is Associated with Shorter Delays and Interruptions in the Treatment of Paracetamol Overdose. Clin. Toxicol. 2022, 60, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Sudanagunta, S.; Camarena-Michel, A.; Pennington, S.; Leonard, J.; Hoyte, C.; Wang, G.S. Comparison of Two-Bag Versus Three-Bag N-Acetylcysteine Regimens for Pediatric Acetaminophen Toxicity. Ann. Pharmacother. 2022, 57, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Cumberland Pharmaceuticals Inc. Acetadote® (Acetylcysteine) Injection [Package Insert]. NDA 21-539/S-004, 2006. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/021539s004lbl.pdf (accessed on 12 December 2024).



| # | Author, Year | Study Design | N-Acetylcysteine Dose | Number of Patients | NOS |
|---|---|---|---|---|---|
| 1 | Bateman, 2014 [16] | RCT | Two-bag regimen
| 108 patients | 5 |
Three-bag regimen
| 109 patients | ||||
| 2 | Wong, 2015 [12] | Retrospective | Two-bag regimen
| 210 patients | 5 |
Three-bag regimen
| 389 patients | ||||
| 3 | McNulty, 2017 [9] | Prospective | Two-bag regimen
| 163 patients | 5 |
Three-bag regimen
| 313 patients | ||||
| 4 | Wong, 2018 [17] | Retrospective | Two-bag regimen
| 8 patients | 4 |
Three-bag regimen
| 21 patients | ||||
| 5 | Schmidt, 2018 [18] | Retrospective | Two-bag regimen
| 493 patients | 6 |
Three-bag regimen
| 274 patients | ||||
| 6 | Daoud, 2019 [19] | Retrospective | Two-bag regimen
| 2951 patients | 5 |
Three-bag regimen
| 1364 patients | ||||
| 7 | Lewis, 2019 [20] | Retrospective | Standard-dose IV NAC:
| 135 patients | 3 |
High-dose IV NAC:
| 117 patients | ||||
| 8 | O’Callaghan, 2022 [21] | Retrospective | Two-bag regimen
| 598 patients | 4 |
Three-bag regimen
| 271 patients | ||||
| 9 | Sudanagunta 2023 [22] | Retrospective | Two-bag regimen
| 93 patients | 5 |
Three-bag regimen
| 150 patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrashed, M.; Alyousef, A.; Badreldin, H.A.; Bin Saleh, K.; Al Harbi, S.; Albekairy, A.M.; Alghamdi, A.; Al-Nahdi, A.; Alonazi, D.; Alnuhait, M.; et al. Comparison of Three-Bag Method Acetylcysteine Versus Two-Bag Method Acetylcysteine for the Treatment of Acetaminophen Toxicity: An Updated Systematic Review and Meta-Analysis. Diseases 2024, 12, 332. https://doi.org/10.3390/diseases12120332
Alrashed M, Alyousef A, Badreldin HA, Bin Saleh K, Al Harbi S, Albekairy AM, Alghamdi A, Al-Nahdi A, Alonazi D, Alnuhait M, et al. Comparison of Three-Bag Method Acetylcysteine Versus Two-Bag Method Acetylcysteine for the Treatment of Acetaminophen Toxicity: An Updated Systematic Review and Meta-Analysis. Diseases. 2024; 12(12):332. https://doi.org/10.3390/diseases12120332
Chicago/Turabian StyleAlrashed, Mohammed, Abdulrahman Alyousef, Hisham A. Badreldin, Khalid Bin Saleh, Shmeylan Al Harbi, Abdulkareem M. Albekairy, Abrar Alghamdi, Amal Al-Nahdi, Dhay Alonazi, Mohammed Alnuhait, and et al. 2024. "Comparison of Three-Bag Method Acetylcysteine Versus Two-Bag Method Acetylcysteine for the Treatment of Acetaminophen Toxicity: An Updated Systematic Review and Meta-Analysis" Diseases 12, no. 12: 332. https://doi.org/10.3390/diseases12120332
APA StyleAlrashed, M., Alyousef, A., Badreldin, H. A., Bin Saleh, K., Al Harbi, S., Albekairy, A. M., Alghamdi, A., Al-Nahdi, A., Alonazi, D., Alnuhait, M., Alshammari, A., & Alqahtani, T. (2024). Comparison of Three-Bag Method Acetylcysteine Versus Two-Bag Method Acetylcysteine for the Treatment of Acetaminophen Toxicity: An Updated Systematic Review and Meta-Analysis. Diseases, 12(12), 332. https://doi.org/10.3390/diseases12120332





