Anatomy and Pathologies of the Spinous Process
Abstract
1. Introduction
2. Anatomy
3. Pathologies Affecting the Spinous Process
3.1. Congenital
3.1.1. Hyperplasia and Hypoplasia
3.1.2. Fusion Abnormalities
3.1.3. Apophysis
3.1.4. Other Normal Variants
3.2. Degenerative/Inflammatory
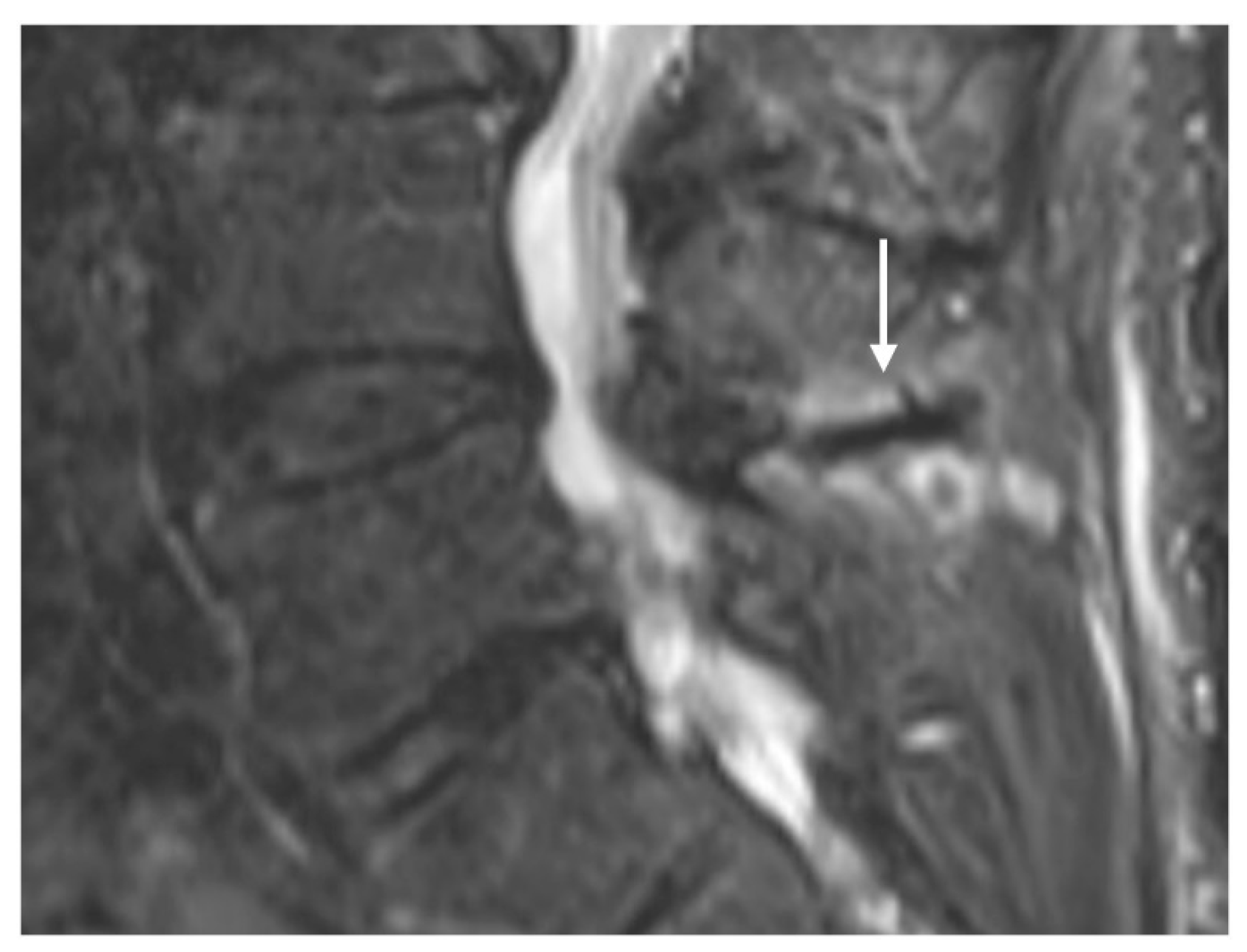
Baastrup Disease
3.3. Neoplastic
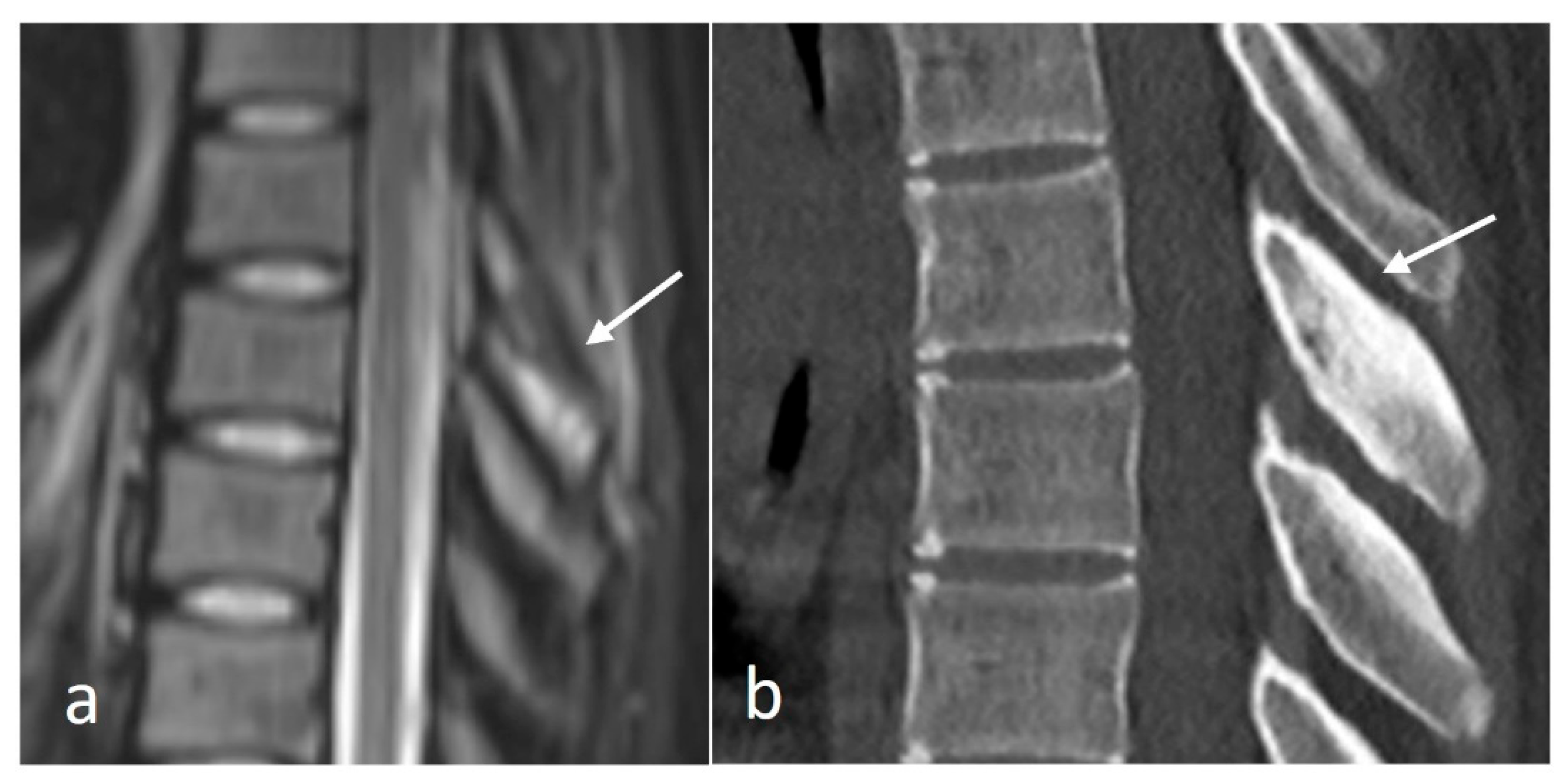
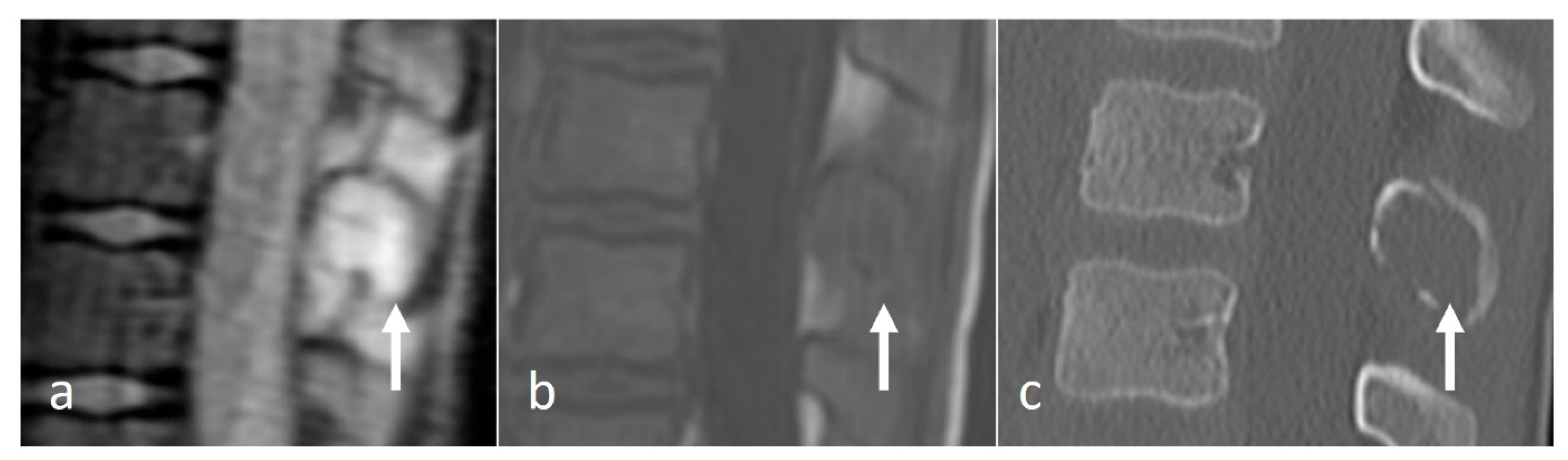
3.3.1. Osteoid Osteoma
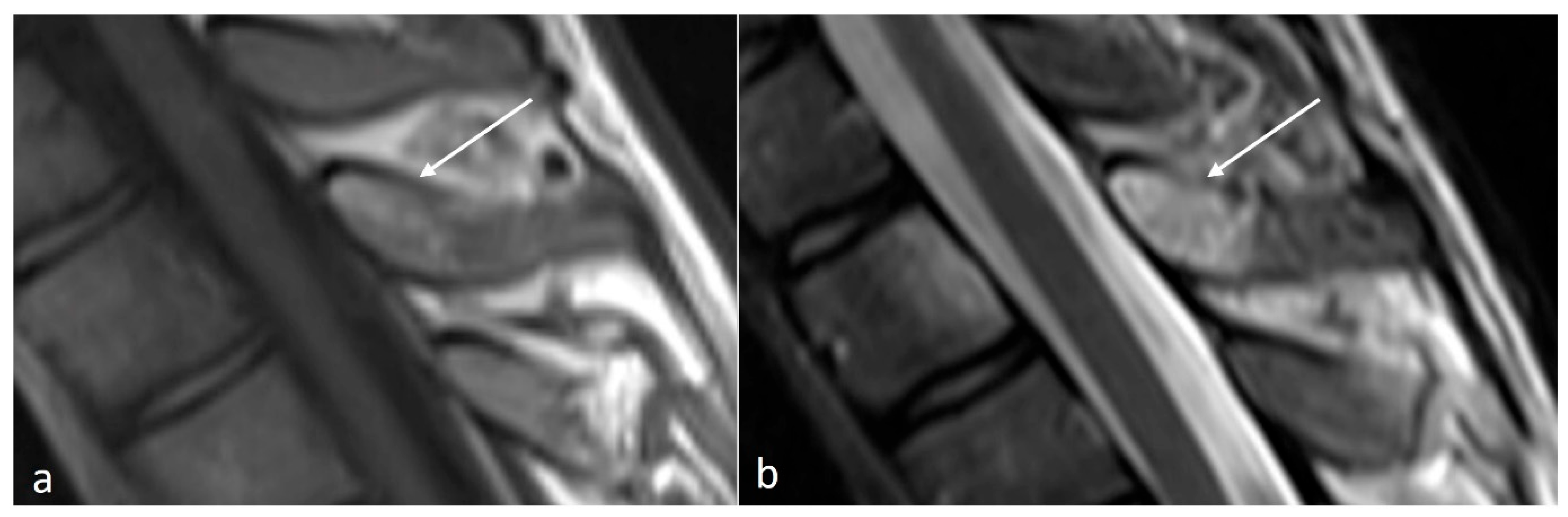
3.3.2. Osteochondroma
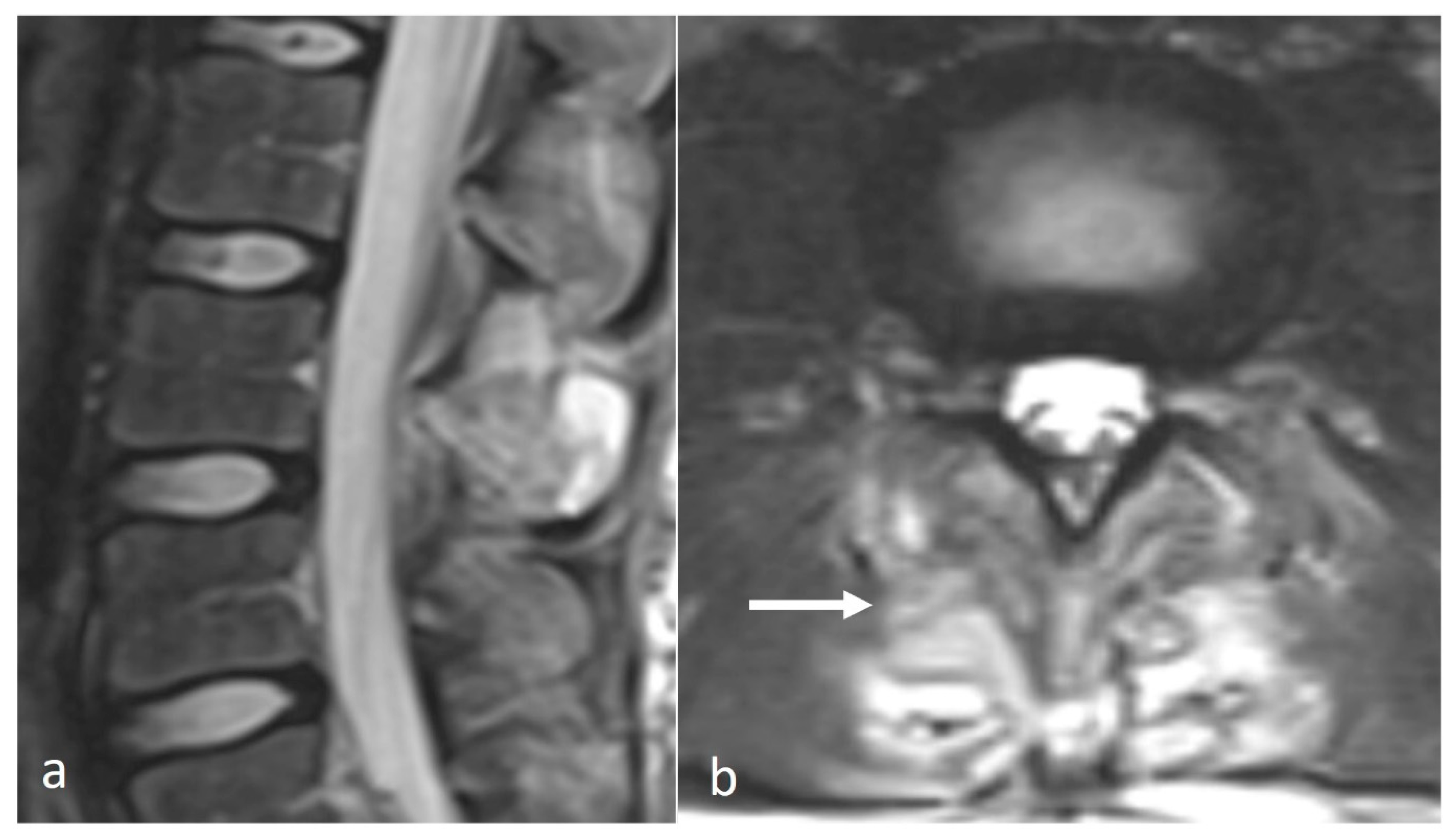
3.3.3. Giant Cell Tumour (GCT)
3.3.4. Aneurysmal Bone Cyst (ABC)
3.3.5. Haemangioma
3.3.6. Fibrous Dysplasia (FD)
3.3.7. Chondroblastoma
3.3.8. Metastases
3.3.9. Multiple Myeloma and Plasmacytoma
3.3.10. Eosinophilic Granuloma (EG)
3.4. Trauma
Fracture, Apophyseal Injury and Non-Union
3.5. Metabolic
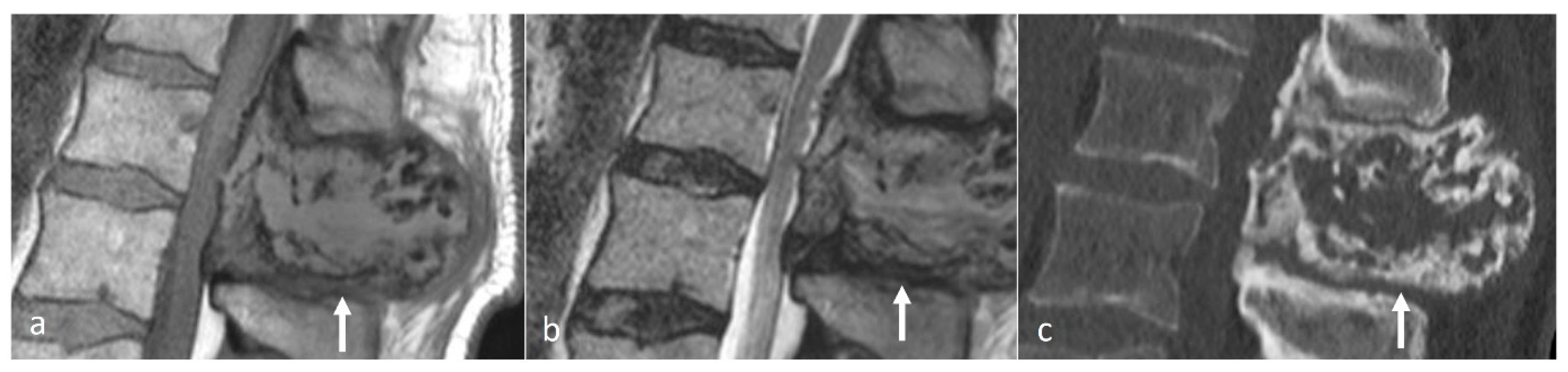
Paget’s Disease
3.6. Osteomyelitis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, H.-J.; Jeon, Y.-H.; Rho, M.-H.; Lee, E.-J.; Park, N.-H.; Park, S.-I.; Jo, J.-H. Incidental findings of the lumbar spine at MRI during herniated intervertebral disk disease evaluation. AJR Am. J. Roentgenol. 2011, 196, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Ariyaratne, S.; Jenko, N.; Iyengar, K.P.; James, S.; Mehta, J.; Botchu, R. Primary Osseous Malignancies of the Spine. Diagnostics 2023, 13, 1801. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Jyotsnarani, Y.; Uppin, S.G.; Susarla, R. Imaging features of primary tumors of the spine: A pictorial essay. Indian J. Radiol. Imaging 2016, 26, 279–289. [Google Scholar] [CrossRef] [PubMed]
- McMinn, R.M.H. (Ed.) Last’s Anatomy: Regional and Applied; Elsevier: Chatswood, Australia, 2003. [Google Scholar]
- Butler, P. Applied Radiological Anatomy; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Bogduk, N. Clinical Anatomy of the Lumbar Spine and Sacrum; Elsevier Health Sciences: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Farooqi, R.R.; Mehmood, M.; Kotwal, H.A. Hyperplasia of Lamina and Spinous Process of C5 Vertebrae and Associated Hemivertebra at C4 Level. J. Orthop. Case Rep. 2017, 7, 79–81. [Google Scholar] [CrossRef]
- Keats, T.E.; Anderson, M.W. Atlas of Normal Roentgen Variants That May Simulate Disease, 9th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Mellado, J.M.; Larrosa, R.; Martín, J.; Yanguas, N.; Solanas, S.; Cozcolluela, M.R. MDCT of variations and anomalies of the neural arch and its processes: Part 1—Pedicles, pars interarticularis, laminae, and spinous process. AJR Am. J. Roentgenol. 2011, 197, W104–W113. [Google Scholar] [CrossRef]
- Pfeiffer. Isolated Hypoplasia of Spinous Process Cause of Back Pain. JAMA 1963, 184, 193. [Google Scholar] [CrossRef]
- Chen, J.J.; Branstetter, B.F.; Welch, W.C. Multiple posterior vertebral fusion abnormalities: A case report and review of the literature. AJR Am. J. Roentgenol. 2006, 186, 1256–1259. [Google Scholar] [CrossRef]
- Koehler, S.M.; Rosario-Quinones, F.; Mayer, J.; McAnany, S.; Schiller, A.L.; Qureshi, S.; Hecht, A.C. Understanding acute apophyseal spinous process avulsion injuries. Orthopedics 2014, 37, e317–e321. [Google Scholar] [CrossRef]
- Ravikanth, R.; Pottangadi, R. Nonunited secondary ossification centers of the spinous processes of vertebrae at multiple levels presenting as aberrant articulations in an adult. J. Craniovertebral Junction Spine 2018, 9, 216–217. [Google Scholar] [CrossRef]
- Kwong, Y.; Rao, N.; Latief, K. MDCT Findings in Baastrup Disease: Disease or Normal Feature of the Aging Spine? Am. J. Roentgenol. 2011, 196, 1156–1159. [Google Scholar] [CrossRef]
- Ariyaratne, S.; Jenko, N.; Iyengar, K.P.; James, S.; Mehta, J.; Botchu, R. Primary Benign Neoplasms of the Spine. Diagnostics 2023, 13, 2006. [Google Scholar] [CrossRef] [PubMed]
- Mallepally, A.R.; Mahajan, R.; Pacha, S.; Rustagi, T.; Marathe, N.; Chhabra, H.S. Spinal osteoid osteoma: Surgical resection and review of literature. Surg. Neurol. Int. 2020, 11, 308. [Google Scholar] [CrossRef] [PubMed]
- Rajakulasingam, R.; Murphy, J.; Botchu, R.; James, S.L. Osteochondromas of the cervical spine-case series and review. J. Clin. Orthop. Trauma 2020, 11, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Saifuddin, A.; Tyler, P.; Hargunani, R. Musculoskeletal MRI, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 752–755, 773–776. [Google Scholar]
- Bhojraj, S.Y.; Nene, A.; Mohite, S.; Varma, R. Giant cell tumor of the spine: A review of 9 surgical interventions in 6 cases. Indian J. Orthop. 2007, 41, 146–150. [Google Scholar] [CrossRef]
- Tafti, D.; Cecava, N.D. Fibrous Dysplasia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ilaslan, H.; Sundaram, M.; Unni, K.K. Vertebral chondroblastoma. Skeletal Radiol. 2003, 32, 66–71. [Google Scholar] [CrossRef]
- Nishida, J.; Kato, S.; Murakami, H.; Ehara, S.; Satoh, T.; Okada, K.; Shimamura, T. Tetraparesis caused by chondroblastoma of the cervical spine: A case report. Spine 2003, 28, E173–E178. [Google Scholar] [CrossRef]
- Ziu, E.; Viswanathan, V.K.; Mesfin, F.B. Spinal Metastasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Matamalas, A.; Valverde, C.; Benavente, S.; Casas-Gomila, L.; Romagosa, C.; González-Tartière, P.; Pellisé, F. Team Approach: Metastatic Disease of the Spine. JBJS Rev. 2018, 6, e6. [Google Scholar] [CrossRef]
- Barzilai, O.; Fisher, C.G.; Bilsky, M.H. State of the Art Treatment of Spinal Metastatic Disease. Neurosurgery 2018, 82, 757–769. [Google Scholar] [CrossRef]
- Guillevin, R.; Vallee, J.-N.; Lafitte, F.; Menuel, C.; Duverneuil, N.-M.; Chiras, J. Spine metastasis imaging: Review of the literature. J. Neuroradiol. 2007, 34, 311–321. [Google Scholar] [CrossRef]
- Lasocki, A.; Gaillard, F.; Harrison, S.J. Multiple myeloma of the spine. Neuroradiol. J. 2017, 30, 259–268. [Google Scholar] [CrossRef]
- Posthuma de Boer, J.; van Wulfften Palthe, A.F.Y.; Stadhouder, A.; Bloemers, F.W. The Clay Shoveler’s Fracture: A Case Report and Review of the Literature. J. Emerg. Med. 2016, 51, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Solaroğlu, İ.; Kaptanoğlu, E.; Okutan, Ö.; Beşkonaklı, E. Multiple isolated spinous process fracture (Clay-shoveler’s fracture) of cervical spine: A case report. Turk. J. Trauma Emerg. Surg. 2007, 13, 162–164. [Google Scholar]
- Weston, W.J. Clay shoveller’s disease in adolescents (Schmitt’s disease); a report of two cases. Br. J. Radiol. 1957, 30, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.M.; Teixeira, A.; Frada, R.; Sousa, R.; Veigas, T.; Miranda, A. Multiple contiguous spinous process fractures, a case report and literature review. Trauma Case Rep. 2022, 42, 100683. [Google Scholar] [CrossRef]
- Schroeder, J.E.; Kaplan, L.; Hasharoni, A.; Hiller, N.; Barzilay, Y. A Hard Fall: An Isolated Fracture of Lumbarized S1 Spinous Process: A Case Report and Review of the Literature. Spine 2009, 34, E864. [Google Scholar] [CrossRef]
- Nakamae, T.; Kamei, N.; Fujimoto, Y.; Yamada, K.; Ujigo, S.; Adachi, N. Spinous Process Fractures in Osteoporotic Vertebral Fractures: A Cross-Sectional Study. Spine Surg. Relat. Res. 2021, 6, 139–144. [Google Scholar] [CrossRef]
- Longo, U.G.; Ciuffreda, M.; Locher, J.; Maffulli, N.; Denaro, V. Apophyseal injuries in children’s and youth sports. Br. Med. Bull. 2016, 120, 139–159. [Google Scholar] [CrossRef]
- Rosenbaum, H.D.; Hanson, D.J. Geographic Variation in the Prevalence of Paget’s Disease of Bone. Radiology 1969, 92, 959–963. [Google Scholar] [CrossRef]
- Pestka, J.M.; Seitz, S.; Zustin, J.; Püschel, K.; Amling, M.; Barvencik, F. Paget disease of the spine: An evaluation of 101 patients with a histomorphometric analysis of 29 cases. Eur. Spine J. 2012, 21, 999–1006. [Google Scholar] [CrossRef]
- Collins, D.H. Paget’s disease of bone; incidence and subclinical forms. Lancet 1956, 271, 51–57. [Google Scholar] [CrossRef]
- Mirra, J.M.; Brien, E.W.; Tehranzadeh, J. Paget’s disease of bone: Review with emphasis on radiologic features, Part I. Skeletal Radiol. 1995, 24, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Dell’Atti, C.; Cassar-Pullicino, V.N.; Lalam, R.K.; Tins, B.J.; Tyrrell, P.N.M. The spine in Paget’s disease. Skeletal Radiol. 2007, 36, 609–626. [Google Scholar] [CrossRef] [PubMed]
- Zlatkin, M.B.; Lander, P.H.; Hadjipavlou, A.G.; Levine, J.S. Paget disease of the spine: CT with clinical correlation. Radiology 1986, 160, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Vande Berg, B.C.; Malghem, J.; Lecouvet, F.E.; Maldague, B. Magnetic resonance appearance of uncomplicated Paget’s disease of bone. Semin. Musculoskelet. Radiol. 2001, 5, 69–77. [Google Scholar] [CrossRef]
- Graeber, A.; Cecava, N.D. Vertebral Osteomyelitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariyaratne, S.; Jenko, N.; Iyengar, K.P.; Davies, M.; Azzopardi, C.; Hughes, S.; Botchu, R. Anatomy and Pathologies of the Spinous Process. Diseases 2024, 12, 302. https://doi.org/10.3390/diseases12120302
Ariyaratne S, Jenko N, Iyengar KP, Davies M, Azzopardi C, Hughes S, Botchu R. Anatomy and Pathologies of the Spinous Process. Diseases. 2024; 12(12):302. https://doi.org/10.3390/diseases12120302
Chicago/Turabian StyleAriyaratne, Sisith, Nathan Jenko, Karthikeyan P. Iyengar, Mark Davies, Christine Azzopardi, Simon Hughes, and Rajesh Botchu. 2024. "Anatomy and Pathologies of the Spinous Process" Diseases 12, no. 12: 302. https://doi.org/10.3390/diseases12120302
APA StyleAriyaratne, S., Jenko, N., Iyengar, K. P., Davies, M., Azzopardi, C., Hughes, S., & Botchu, R. (2024). Anatomy and Pathologies of the Spinous Process. Diseases, 12(12), 302. https://doi.org/10.3390/diseases12120302






