The Impact of the Dialysis Event Prevention Bundle on the Reduction in Dialysis Event Rate in Patients with Catheters: A Retrospective and Prospective Cohort Study
Abstract
:1. Background
2. Subjects and Methods
2.1. Study Setting and Design
2.2. Ethics Approval and Informed Consent
2.3. Participant Selection
2.4. Study Interventions and Implementation
2.5. Outcomes
2.6. Statistical Analyses
3. Results
3.1. Patients’ Demographic and Clinical Data
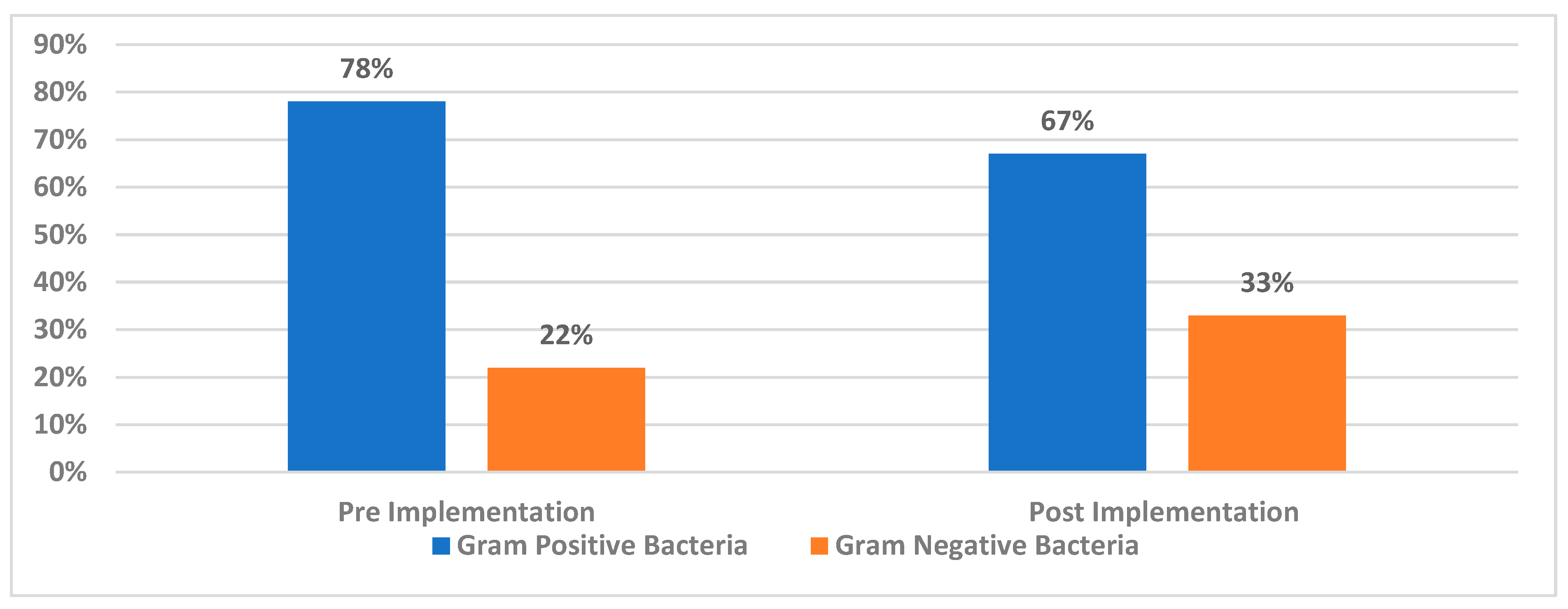
3.2. Prevalence of Gram-Positive and Gram-Negative Bacteria in Blood-Positive Cultures
3.3. The Differences Between Pre-Implementation and Post-Implementation Bacterial Prevalence
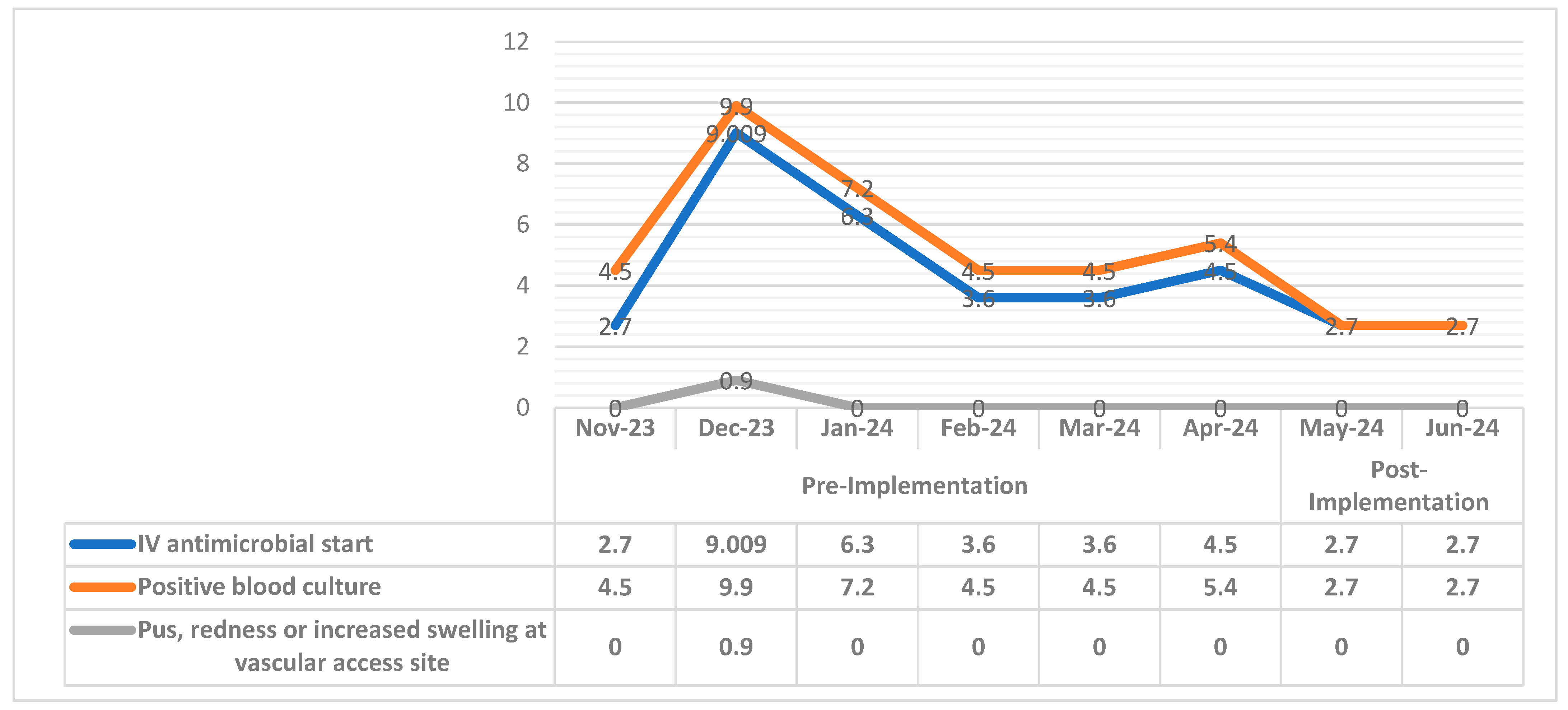
3.4. Dialysis Events Rate for Pre- and Post-Implementation Period
3.5. Relation Between Dialysis-Related Events and (Patients, Age Category, Permanent Catheter Site)
3.6. Dialysis Event Prevention Bundle Compliance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mousa, D.; Alharbi, A.; Helal, I.; Al-Homrany, M.; Alhujaili, F.; Alhweish, A.; Marie, M.A.; Al Sayyari, A. Prevalence and Associated Factors of Chronic Kidney Disease among Relatives of Hemodialysis Patients in Saudi Arabia. Kidney Int. Rep. 2021, 6, 817–820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, D.B.; Arduino, M.J.; Patel, P.R. Hemodialysis-Associated Infections. Chronic Kidney Dis. Dial. Transplant. 2019, 389–410.e8. [Google Scholar] [CrossRef] [PubMed Central]
- Suri, R.S.; Larive, B.; Sherer, S.; Eggers, P.; Gassman, J.; James, S.H.; Lindsay, R.M.; Lockridge, R.S.; Ornt, D.B.; Rocco, M.V.; et al. Risk of vascular access complications with frequent hemodialysis. J. Am. Soc. Nephrol. 2013, 24, 498–505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Center for Disease Control and Prevention. NHSN Dialysis Event Surveillance Protocol. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/8pscdialysiseventcurrent.pdf (accessed on 1 January 2024).
- Center for Disease Control and Prevention. Audit Tools and Checklists for Dialysis Safety. Available online: https://www.cdc.gov/dialysis-safety/hcp/tools/index.html (accessed on 1 January 2023).
- General Directorate of Infection Prevention and Control. MOH Healthcare-associated infection (HAI). In Surveillance Manual, 2nd ed.; Ministry of Health: Riyadh, Saudi Arabia, 2023; Available online: https://jed-s3.bluvalt.com/psj1-ifn-s3-ifn01/files/07/Guidelines/MOH_Surveillance_Manual_2nd_Edition_2023%5B1%5D.pdf (accessed on 1 November 2023).
- Gahlot, R.; Nigam, C.; Kumar, V.; Yadav, G.; Anupurba, S. Catheter-related bloodstream infections. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 162–167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weikert, B.; Kramer, T.S.; Schwab, F.; Graf-Allgeier, C.; Wolke, S.I.; Gastmeier, P.; Geffers, C. Effect of a multimodal prevention strategy on dialysis-associated infection events in outpatients receiving haemodialysis: The DIPS stepped wedge, cluster-randomized trial. Clin. Microbiol. Infect. 2024, 30, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- General Directorate of Infection Prevention and Control of Healthcare Facilities (GDIPC), Version 5. 2024. Available online: https://www.moh.gov.sa/Ministry/Rules/Documents/National-Guide-Auditors-Infection-Control-Version-5.pdf (accessed on 7 August 2024).
- Sax, H.; Allegranzi, B.; Uçkay, I.; Larson, E.; Boyce, J.; Pittet, D. ‘My five moments for hand hygiene’: A user-centred design approach to understand, train, monitor and report hand hygiene. J. Hosp. Infect. 2007, 67, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Storr, J.; Twyman, A.; Zingg, W.; Damani, N.; Kilpatrick, C.; Reilly, J.; Price, L.; Egger, M.; Grayson, M.L.; Kelley, E.; et al. Core components for effective infection prevention and control programmes: New WHO evidence-based recommendations. Antimicrob. Resist. Infect. Control 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borgert, M.J.; Goossens, A.; Dongelmans, D.A. What are effective strategies for the implementation of care bundles on ICUs: A systematic review. Implement. Sci. 2015, 10, 119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, M.; Satoh, N.; Nakamura, M.; Horita, S.; Seki, G.; Moriya, K. Bacteremia in hemodialysis patients. World J. Nephrol. 2016, 5, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, B.; Carlton, D.; Saddekni, S.; Hamrick, K.; Oser, R.; Westfall, A.O.; Allon, M. Bacteremia associated with tunneled dialysis catheters: Comparison of two treatment strategies. Kidney Int. 2000, 57, 2151–2155. [Google Scholar] [CrossRef] [PubMed]
- Krishnasami, Z.; Carlton, D.; Bimbo, L.; Taylor, M.E.; Balkovetz, D.F.; Barker, J.; Allon, M. Management of hemodialysis catheter-related bacteremia with an adjunctive antibiotic lock solution. Kidney Int. 2002, 61, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Almenara-Tejederas, M.; Rodríguez-Pérez, M.A.; Moyano-Franco, M.J.; de Cueto-López, M.; Rodríguez-Baño, J.; Salgueira-Lazo, M. Tunneled catheter-related bacteremia in hemodialysis patients: Incidence, risk factors and outcomes. A 14-year observational study. J. Nephrol. 2023, 36, 203–212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gork, I.; Gross, I.; Cohen, M.J.; Schwartz, C.; Moses, A.E.; Elhalel, M.D.; Benenson, S. Access-related infections in two haemodialysis units: Results of a nine-year intervention and surveillance program. Antimicrob. Resist. Infect. Control 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lavallée, J.F.; Gray, T.A.; Dumville, J.; Russell, W.; Cullum, N. The effects of care bundles on patient outcomes: A systematic review and meta-analysis. Implement. Sci. 2017, 12, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kotwal, S.; Cass, A.; Coggan, S.; Gray, N.A.; Jan, S.; McDonald, S.; Polkinghorne, K.R.; Rogers, K.; Talaulikar, G.; Di Tanna, G.L.; et al. Multifaceted intervention to reduce haemodialysis catheter related bloodstream infections: REDUCCTION stepped wedge, cluster randomised trial. BMJ. 2022, 377, e069634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, D.W.; van Eps, C.; Mudge, D.W.; Wiggins, K.J.; Armstrong, K.; Hawley, C.M.; Campbell, S.B.; Isbel, N.M.; Nimmo, G.R.; Gibbs, H. Randomized, controlled trial of topical exit-site application of honey (Medihoney) versus mupirocin for the prevention of catheter-associated infections in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Power, A.; Duncan, N.; Singh, S.K.; Brown, W.; Dalby, E.; Edwards, C.; Lynch, K.; Prout, V.; Cairns, T.; Griffith, M.; et al. Sodium citrate versus heparin catheter locks for cuffed central venous catheters: A single-center randomized controlled trial. Am. J. Kidney Dis. 2009, 53, 1034–1041. [Google Scholar] [CrossRef] [PubMed]


| Category | Number (N.) | Percent (%) |
|---|---|---|
| Gender | ||
| Males | 57 | 51% |
| Female | 54 | 49% |
| Age Category: | ||
| 18–20 years | 2 | 1.8% |
| 20–40 years | 22 | 19.8% |
| 41–60 years | 43 | 38.6% |
| 61–90 years | 44 | 39.6% |
| Comorbidities | ||
| Patients With Comorbidities (DM, HTN, or both) | 88 | 79.3% |
| Patients Without Comorbidities | 23 | 21.6% |
| Permanent Catheter Site: | ||
| Jugular Permanent Catheter (JPC) | 106 | 95.5% |
| Femoral Permanent Catheter (FPC) | 4 | 3.6% |
| Trans-hepatic Permanent Catheter (THPC) | 1 | 0.9% |
| Bacteria | Pre-Implementation | Post-Implementation | Total | Percentage | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| November-23 | December-23 | January-24 | February-24 | March-24 | April-24 | May-24 | June-24 | |||
| Gram-Positive Bacteria | ||||||||||
| Staphylococcus epidermidis | 2 | 6 | 4 | 1 | 3 | 6 | 0 | 2 | 24 | 52.20% |
| Staphylococcus hominis | 2 | 3 | 0 | 1 | 1 | 0 | 1 | 0 | 8 | 17.40% |
| Staphylococcus haemolyticus | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 4.30% |
| Staphylococcus lugdunensis | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2.17% |
| Staphylococcus capitis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2.17% |
| Gram Negative Bacteria | ||||||||||
| Klebsiella oxytoca | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.17% |
| Citrobacter koseri | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.17% |
| Pseudomonas aeruginosa | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 3 | 6.52% |
| Eschrichia coli | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2.17% |
| Pantoea species | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2.17% |
| Stenotrophomonas maltophilia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2.17% |
| Enterobacter cloacae | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2.17% |
| Comamonas acidovorans | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2.17% |
| Dialysis Event Rate | Group | N | Mean | Std. Deviation | p-Value | T |
|---|---|---|---|---|---|---|
| IV antimicrobial start | Pre-Implementation | 6 | 4.95150 | 2.332722 | 0.003 | 1.295 |
| Post-Implementation | 2 | 2.70000 | 0.000000 | |||
| Positive blood culture | Pre-Implementation | 6 | 6.00000 | 2.179908 | 0.039 | 2.031 |
| Post-Implementation | 2 | 2.70000 | 0.000000 | |||
| Pus, redness or increased swelling at vascular access site | Pre-Implementation | 6 | 0.15000 | 0.367423 | 0.004 | 1.048 |
| Post-Implementation | 2 | 0.00000 | 0.000000 |
| (1) Relation Between IV Antimicrobial Start and (Comorbidities, Age, Permanent Catheter Site) | No. | % | p-Value | Chi-Square |
| Comorbidities | ||||
| Patients With Comorbidities (DM, HTN, or both) | 88 | 79.3% | 0.736 | 1.982 |
| Patients Without Comorbidities | 23 | 21.6% | ||
| Age Category: | ||||
| 18–20 years | 2 | 1.8% | 0.005 | 4.173 |
| 20–40 years | 22 | 19.8% | ||
| 41–60 years | 43 | 38.7% | ||
| 61–90 years | 44 | 39.6% | ||
| Permanent Catheter Site: | ||||
| Jugular Permanent Catheter (JPC) | 106 | 95.5% | 0.002 | 4.904 |
| Femoral Permanent Catheter (FPC) | 4 | 3.6% | ||
| Trans-hepatic Permanent Catheter (THPC) | 1 | 0.9% | ||
| (2) Relation between Positive blood culture and (Comorbidities, age, permanent catheter site) | No. | % | p-value | Chi-Square |
| Comorbidities | ||||
| Patients With Comorbidities (DM, HTN, or both) | 88 | 79.3% | 0.000 | 5.012 |
| Patients Without Comorbidities | 23 | 21.6% | ||
| Age Category: | ||||
| 18–20 years | 2 | 1.8% | 0.320 | 1.723 |
| 20–40 years | 22 | 19.8% | ||
| 41–60 years | 43 | 38.7% | ||
| 61–90 years | 44 | 39.6% | ||
| Permanent Catheter Site: | ||||
| Jugular Permanent Catheter (JPC) | 106 | 95.5% | 0.078 | 1.884 |
| Femoral Permanent Catheter (FPC) | 4 | 3.6% | ||
| Trans-hepatic Permanent Catheter (THPC) | 1 | 0.9% | ||
| (3) Relation between pus, redness, or increased swelling at vascular access site and (Comorbidities, age, permanent catheter site) | No. | % | p-value | Chi-Square |
| Comorbidities | ||||
| Patients With Comorbidities (DM, HTN, or both) | 88 | 79.3% | 0.034 | 4.50 |
| Patients Without Comorbidities | 23 | 21.6% | ||
| Age Category: | ||||
| 18–20 years | 2 | 1.8% | 0.021 | 4.97 |
| 20–40 years | 22 | 19.8% | ||
| 41–60 years | 43 | 38.7% | ||
| 61–90 years | 44 | 39.6% | ||
| Permanent Catheter Site: | ||||
| Jugular Permanent Catheter (JPC) | 106 | 95.5% | 0.002 | 3.98 |
| Femoral Permanent Catheter (FPC) | 4 | 3.6% | ||
| Trans-hepatic Permanent Catheter (THPC) | 1 | 0.9% | ||
| Dialysis Event Prevention Bundle’s Main Components | Total Opportunities (May 2024) | Staff Compliance (May 2024) | Total Opportunities (June 2024) | Staff Compliance (June 2024) |
|---|---|---|---|---|
| Hemodialysis catheter connection | 1332 | 1185 | 1332 | 1150 |
| Hemodialysis catheter disconnection | 1332 | 1200 | 1332 | 1215 |
| Hemodialysis catheter exit site care | 1332 | 1200 | 1332 | 1250 |
| Dialysis station routine disinfection | 1332 | 1260 | 1332 | 1280 |
| Hemodialysis injectable medication preparation | 1332 | 1230 | 1332 | 1200 |
| Hemodialysis injectable medication administration | 1332 | 1265 | 1332 | 1240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlHulays, R.H.; Ghazy, A.A.; Taha, A.E. The Impact of the Dialysis Event Prevention Bundle on the Reduction in Dialysis Event Rate in Patients with Catheters: A Retrospective and Prospective Cohort Study. Diseases 2024, 12, 301. https://doi.org/10.3390/diseases12120301
AlHulays RH, Ghazy AA, Taha AE. The Impact of the Dialysis Event Prevention Bundle on the Reduction in Dialysis Event Rate in Patients with Catheters: A Retrospective and Prospective Cohort Study. Diseases. 2024; 12(12):301. https://doi.org/10.3390/diseases12120301
Chicago/Turabian StyleAlHulays, Reem Hamed, Amany A. Ghazy, and Ahmed E. Taha. 2024. "The Impact of the Dialysis Event Prevention Bundle on the Reduction in Dialysis Event Rate in Patients with Catheters: A Retrospective and Prospective Cohort Study" Diseases 12, no. 12: 301. https://doi.org/10.3390/diseases12120301
APA StyleAlHulays, R. H., Ghazy, A. A., & Taha, A. E. (2024). The Impact of the Dialysis Event Prevention Bundle on the Reduction in Dialysis Event Rate in Patients with Catheters: A Retrospective and Prospective Cohort Study. Diseases, 12(12), 301. https://doi.org/10.3390/diseases12120301






