Prognostic Implications of Initial Radiological Findings of Pulmonary Fibrosis in Patients with Acute SARS-CoV-2 Infection: A Prospective Multicentric Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Biochemical Analysis
2.4. COVID-19 Severity
2.5. HRCT Imaging
2.6. Definitions
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Literature Findings
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alrajhi, N.N. Post-COVID-19 pulmonary fibrosis: An ongoing concern. Ann. Thorac. Med. 2023, 18, 173–181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Z.; Peng, F.; Zhou, Y. Pulmonary fibrosis: A short- or long-term sequelae of severe COVID-19? Chin. Med. J. Pulm. Crit. Care Med. 2023, 1, 77–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duong-Quy, S.; Vo-Pham-Minh, T.; Tran-Xuan, Q.; Huynh-Anh, T.; Vo-Van, T.; Vu-Tran-Thien, Q.; Nguyen-Nhu, V. Post-COVID-19 Pulmonary Fibrosis: Facts—Challenges and Futures: A Narrative Review. Pulm. Ther. 2023, 20, 295–307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tran, S.; Ksajikian, A.; Overbey, J.; Li, P.; Li, Y. Pathophysiology of Pulmonary Fibrosis in the Context of COVID-19 and Implications for Treatment: A Narrative Review. Cells 2022, 11, 2489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tabassum, S.; Haider, S.; Shaukat, S. Spectrum of HRCT Scan Chest Findings in COVID-19 Patients as Categorized by Modified CO-RADS Classification. Pak. J. Med. Sci. 2022, 38, 838–843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Younus, S.; Maqsood, H.; Sattar, A.; Younas, A.; Shakeel, H.A. A novel chest CT severity score in COVID-19 and its correlation with severity and prognosis of the lung disease: A retrospective cohort study. Ann. Med. Surg. 2022, 82, 104692. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ravindra Naik, B.; Sakalecha, A.K.; Sunil, B.N.; Chaithanya, A.; Kale, R.M.; Uhasai, K. Computed Tomography Severity Scoring on High-Resolution Computed Tomography Thorax and Inflammatory Markers with COVID-19 Related Mortality in a Designated COVID Hospital. Cureus 2022, 14, e24190. [Google Scholar] [CrossRef] [PubMed]
- Kanne, J.P.; Little, B.P.; Schulte, J.J.; Haramati, A.; Haramati, L.B. Long-term Lung Abnormalities Associated with COVID-19 Pneumonia. Radiology 2023, 306, e221806. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, S.J.; Baldwin, M.M.; Daynes, E.; Evans, A.R.; Greening, N.J.; Jenkins, R.G.; Lone, I.N.; McAuley, H.; Mehta, P.; Newman, J.; et al. Respiratory sequelae of COVID-19: Pulmonary and extrapulmonary origins, and approaches to clinical care and rehabilitation. Lancet Respir. Med. 2023, 11, 709–725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfortmueller, C.A.; Spinetti, T.; Urman, R.D.; Luedi, M.M.; Schefold, J.C. COVID-19-associated acute respiratory distress syndrome (CARDS): Current knowledge on pathophysiology and ICU treatment—A narrative review. Best Pr. Res. Clin. Anaesthesiol. 2021, 35, 351–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cabrera-Benitez, N.E.; Laffey, J.G.; Parotto, M.; Spieth, P.M.; Villar, J.; Zhang, H.; Slutsky, A.S. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: A significant contributor to poor outcome. Anesthesiology 2014, 121, 189–198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Savin, I.A.; Zenkova, M.A.; Sen’kova, A.V. Pulmonary Fibrosis as a Result of Acute Lung Inflammation: Molecular Mechanisms, Relevant In Vivo Models, Prognostic and Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 14959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoon, H.Y.; Uh, S.T. Post-Coronavirus Disease 2019 Pulmonary Fibrosis: Wait or Needs Intervention. Tuberc. Respir. Dis. 2022, 85, 320–331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, H.; Zhang, N.; Liu, Y.; Yang, X.; He, Y.; Li, Q.; Shen, X.; Zhu, Y.; Yang, Y. The Interaction Between Pulmonary Fibrosis and COVID-19 and the Application of Related Anti-Fibrotic Drugs. Front. Pharmacol. 2022, 12, 805535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatterjee, S.; Nalla, L.V.; Sharma, M.; Sharma, N.; Singh, A.A.; Malim, F.M.; Ghatage, M.; Mukarram, M.; Pawar, A.; Parihar, N.; et al. Association of COVID-19 with Comorbidities: An Update. ACS Pharmacol. Transl. Sci. 2023, 6, 334–354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahman, T.; Nahar, N.U.; Ibrahim; Islam, I.; Bhowmik, B.; Shirin, M.; Khan, M.R.; Akkas, N.; Molla, M.A. Early Prediction and HRCT Evaluation of Post COVID-19 Related Lung Fibrosis. Microbiol. Insights 2023, 16, 11786361231190334. [Google Scholar] [CrossRef] [PubMed]
- Mulet, A.; Tarrasó, J.; Rodríguez-Borja, E.; Carbonell-Asins, J.A.; Lope-Martínez, A.; Martí-Martinez, A.; Murria, R.; Safont, B.; Fernandez-Fabrellas, E.; Ros, J.A.; et al. Biomarkers of Fibrosis in Patients with COVID-19 One Year After Hospital Discharge: A Prospective Cohort Study. Am. J. Respir. Cell Mol. Biol. 2023, 69, 321–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Safont, B.; Tarraso, J.; Rodriguez-Borja, E.; Fernández-Fabrellas, E.; Sancho-Chust, J.N.; Molina, V.; Lopez-Ramirez, C.; Lope-Martinez, A.; Cabanes, L.; Andreu, A.L.; et al. Lung Function, Radiological Findings and Biomarkers of Fibrogenesis in a Cohort of COVID-19 Patients Six Months After Hospital Discharge. Arch. Bronconeumol. 2022, 58, 142–149. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tarraso, J.; Safont, B.; Carbonell-Asins, J.A.; Fernandez-Fabrellas, E.; Sancho-Chust, J.N.; Naval, E.; Amat, B.; Herrera, S.; Ros, J.A.; Soler-Cataluña, J.J.; et al. Lung function and radiological findings 1 year after COVID-19: A prospective follow-up. Respir. Res. 2022, 23, 242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554776/ (accessed on 25 August 2024).
- Lee, H.; Choi, H.; Yang, B.; Lee, S.-K.; Park, T.S.; Park, D.W.; Moon, J.-Y.; Kim, T.-H.; Sohn, J.W.; Yoon, H.J.; et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur. Respir. J. 2021, 58, 2004125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kewalramani, N.; Heenan, K.-M.; McKeegan, D.; Chaudhuri, N. Post-COVID Interstitial Lung Disease—The Tip of the Iceberg. Immunol. Allergy Clin. N. Am. 2023, 43, 389–410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arai, T.; Kurahara, Y.; Moda, M.; Kobayashi, T.; Matsuda, Y.; Kagawa, T.; Sugawara, R.; Tsuyuguchi, K.; Inoue, Y. COVID-19 in Patients with Pre-Existing Interstitial Lung Disease: Potential Value of a Steroid-Based Treatment Strategy. J. Clin. Med. 2023, 12, 4940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, B.-G.; Lee, H.; Jeong, C.Y.; Yeom, S.W.; Park, D.W.; Park, T.S.; Moon, J.-Y.; Kim, T.-H.; Sohn, J.W.; Yoon, H.J.; et al. Risk of newly diagnosed interstitial lung disease after COVID-19 and impact of vaccination: A nationwide population-based cohort study. Front. Public Health 2024, 11, 1295457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trongtrakul, K.; Tajarernmuang, P.; Limsukon, A.; Theerakittikul, T.; Niyatiwatchanchai, N.; Surasit, K.; Glunriangsang, P.; Liwsrisakun, C.; Bumroongkit, C.; Pothirat, C.; et al. The National Early Warning Score 2 with Age and Body Mass Index (NEWS2 Plus) to Determine Patients with Severe COVID-19 Pneumonia. J. Clin. Med. 2024, 13, 298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scott, L.J.; Tavaré, A.; Hill, E.M.; Jordan, L.; Juniper, M.; Srivastava, S.; Redfern, E.; Little, H.; Pullyblank, A. Prognostic value of National Early Warning Scores (NEWS2) and component physiology in hospitalised patients with COVID-19: A multicentre study. Emerg. Med. J. 2022, 39, 589–594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monk, M.; Torres, J.; Vickery, K.; Jayaraman, G.; Sarva, S.T.; Kesavan, R.; Page, K.N. A Comparison of ICU Mortality Scoring Systems Applied to COVID-19. Cureus 2023, 15, e35423. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schoenmakers, T.; Leers, M.P.G.; Gorissen, S.H.M.; van Loo, I.H.M.; van Rosmalen, F.; Aydeniz, E.; Schellens, J.; Driessen, M.; Deneer, R.; de Venne, W.P.H.G.V.-V.; et al. The laboratory parameters-derived CoLab score as an indicator of the host response in ICU COVID-19 patients decreases over time: A prospective cohort study. Sci. Rep. 2024, 14, 8220. [Google Scholar] [CrossRef]
- Bartoszewicz, K.; Bartoszewicz, M.; Gradkowski, W.; Stróż, S.; Stasiak-Barmuta, A.; Czaban, S.L. Analysis of prognostic factors in critically ill patients with COVID-19. PLoS ONE 2024, 19, e0302248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, J.; Liu, X.; Xiao, W.; Lu, J.; Guan, L.; Fang, Z.; Chen, J.; Sun, B.; Cai, Z.; Sun, X.; et al. Phospholipid remodeling and its derivatives are associated with COVID-19 severity. J. Allergy Clin. Immunol. 2023, 151, 1259–1268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Picchi, S.G.; Lassandro, G.; Corvino, A.; Tafuri, D.; Caruso, M.; Faggian, G.; Cocco, G.; Pizzi, A.D.; Gallo, L.; Quassone, P.; et al. COVID-19: Correlation between HRCT findings and clinical prognosis and analysis of parenchymal pattern evolution. J. Clin. Imaging Sci. 2023, 13, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hui, J.Y.-H.; Hon, T.Y.-W.; Yang, M.K.-W.; Cho, D.H.-Y.; Luk, W.-H.; Chan, R.Y.-Y.; Chan, K.-S.; Loke, T.K.-L.; Chan, J.C.-S. High-resolution computed tomography is useful for early diagnosis of severe acute respiratory syndrome-associated coronavirus pneumonia in patients with normal chest radiographs. J. Comput. Assist. Tomogr. 2004, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.L.; Ooi, G.C.; Khong, P.L.; Zhou, L.J.; Tsang, K.W.T.; Nicolaou, S. High-resolution CT findings of severe acute respiratory syndrome at presentation and after admission. Am. J. Roentgenol. 2004, 182, 39–44. [Google Scholar] [CrossRef] [PubMed]
| Variables | Fibrosis (n = 60) | No Fibrosis (n = 60) | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 58.0 ± 13.2 | 55.4 ± 15.8 | 0.326 |
| Age category | 0.319 | ||
| <40 years | 5 (8.3%) | 8 (13.3%) | |
| 40–59 years | 39 (65.00%) | 31 (51.7%) | |
| ≥60 years | 16 (26.7%) | 21 (35.00%) | |
| Gender | 0.353 | ||
| Men | 33 (54.6%) | 38 (63.3%) | |
| Women | 27 (45.4%) | 22 (36.7%) | |
| Place of origin | 0.274 | ||
| Rural | 11 (18.2%) | 16 (26.7%) | |
| Urban | 49 (81.8%) | 44 (73.3%) | |
| COVID-19 vaccination | 0.458 | ||
| Yes | 27 (45.5%) | 23(38.3%) | |
| No | 33 (54.5%) | 37 (61.7%) | |
| Smoking | 0.425 | ||
| No | 44 (72.7%) | 40 (66.7%) | |
| Past smoker | 16 (27.37%) | 20 (33.3%) | |
| Days since symptom onset (median, IQR) | 2.7 (1.1–4.5) | 2.8 (1.3–4.9) | 0.518 |
| Days of hospitalization (mean ± SD) | 13.9 ± 6.6 | 9.0 ± 7.5 | <0.001 |
| Oxygen saturation (mean ± SD) | 91.6 ± 2.7 | 94.2 ± 3.4 | <0.001 |
| Developed severe disease | 27 (45.0%) | 13 (21.7%) | 0.006 |
| Variables | Normal Range | Fibrosis (n = 60) | No Fibrosis (n = 60) | p-Value |
|---|---|---|---|---|
| WBC (×103/L) | 4.0–10.0 | 16.7 ± 4.6 | 12.3 ± 5.9 | <0.001 |
| Hemoglobin (g/dL) | 12.0–16.0 | 10.8 ± 1.3 | 13.5 ± 1.6 | <0.001 |
| Neutrophils (×103/L) | 2.0–7.0 | 12.4 ± 3.1 | 9.4 ± 4.9 | 0.001 |
| Lymphocytes (×103/L) | 1.0–3.0 | 3.9 ± 1.4 | 3.9 ± 2.7 | 0.899 |
| Platelets (×103/uL) | 150–400 | 328.4 ± 68.2 | 274.3 ± 55.9 | <0.001 |
| ESR (mm/h) | <20 | 47.9 ± 9.2 | 19.6 ± 5.9 | <0.001 |
| Fibrinogen (mg/dL) | 200–400 | 602.9 ± 98.8 | 348.2 ± 72.1 | <0.001 |
| CRP (mg/L) | <5 | 120.8 ± 32.2 | 22.5 ± 9.6 | <0.001 |
| LDH | 100–250 | 420.4 ± 60.2 | 234.7 ± 45.3 | <0.001 |
| AST (U/L) | 0–40 | 84.3 ± 22.5 | 28.8 ± 10.6 | <0.001 |
| ALT (U/L) | 0–40 | 68.6 ± 21.1 | 26.8 ± 9.3 | <0.001 |
| Urea (mg/dL) | 15–45 | 59.2 ± 15.3 | 35.9 ± 10.5 | <0.001 |
| Creatinine (mg/dL) | 0.6–1.2 | 1.4 ± 0.3 | 0.9 ± 0.2 | <0.001 |
| Blood glucose (mg/dL) | 70–140 | 182.3 ± 40.6 | 118.3 ± 29.8 | <0.001 |
| D-dimers (ug/mL) | 0.0–0.5 | 3.6 ± 1.0 | 0.6 ± 0.2 | <0.001 |
| Variables | Fibrosis (n = 60) | No Fibrosis (n = 60) | p-Value |
|---|---|---|---|
| HRCT score | 12.4 ± 4.3 | 7.9 ± 3.2 | <0.001 |
| SIRI | 2.9 ± 1.1 | 1.2 ± 0.6 | <0.001 |
| SII | 950.3 ± 310.4 | 460.2 ± 289.8 | <0.001 |
| PNI | 38.5 ± 6.9 | 48.3 ± 5.4 | <0.001 |
| SOFA | 7.3 ± 2.7 | 3.9 ± 2.1 | <0.001 |
| APACHE II | 17.84± 5.3 | 10.3 ± 4.0 | <0.001 |
| NEWS 2 | 6.5 ± 2.0 | 3.1 ± 1.5 | <0.001 |
| Laboratory Parameter | Best Cutoff Value | Sensitivity | Specificity | AUC | p-Value |
|---|---|---|---|---|---|
| HRCT score | 9.7 | 0.857 | 0.798 | 0.885 | <0.001 |
| SIRI | 2.0 | 0.763 | 0.749 | 0.819 | 0.006 |
| SII | 675.3 | 0.824 | 0.766 | 0.854 | <0.001 |
| PNI | 42.6 | 0.701 | 0.684 | 0.739 | 0.033 |
| SOFA | 6.1 | 0.877 | 0.831 | 0.907 | <0.001 |
| APACHE II | 16.5 | 0.903 | 0.865 | 0.926 | <0.001 |
| NEWS 2 | 5.4 | 0.836 | 0.793 | 0.874 | 0.001 |
| Compound Score 1 | 25.5 | 0.925 | 0.889 | 0.947 | <0.001 |
| Compound Score 2 | 23.1 | 0.892 | 0.857 | 0.913 | <0.001 |
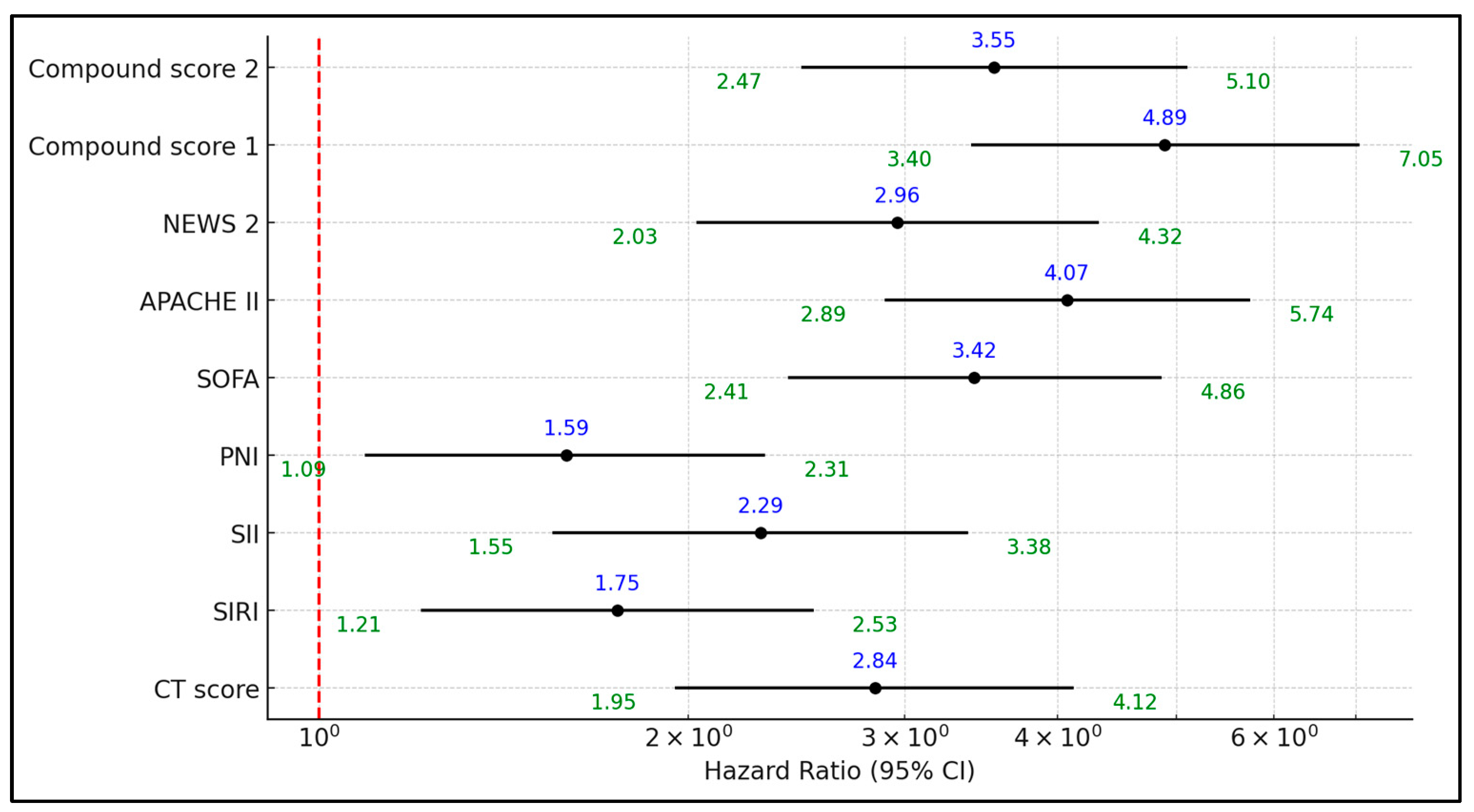
| Factors Above the Best Cutoff | Hazard Ratio | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|
| HRCT score | 2.84 | 1.95 | 4.12 | 0.001 |
| SIRI | 1.75 | 1.21 | 2.53 | 0.003 |
| SII | 2.29 | 1.55 | 3.38 | <0.001 |
| PNI | 1.59 | 1.09 | 2.31 | 0.016 |
| SOFA | 3.42 | 2.41 | 4.86 | <0.001 |
| APACHE II | 4.07 | 2.89 | 5.74 | <0.001 |
| NEWS 2 | 2.96 | 2.03 | 4.32 | <0.001 |
| Compound Score 1 | 4.89 | 3.4 | 7.05 | <0.001 |
| Compound Score 2 | 3.55 | 2.47 | 5.1 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cîrjaliu, R.-E.; Gurrala, S.V.; Nallapati, B.; Krishna, V.; Oancea, C.; Tudorache, E.; Marc, M.; Bratosin, F.; Bogdan, I.; Rosca, O.; et al. Prognostic Implications of Initial Radiological Findings of Pulmonary Fibrosis in Patients with Acute SARS-CoV-2 Infection: A Prospective Multicentric Study. Diseases 2024, 12, 285. https://doi.org/10.3390/diseases12110285
Cîrjaliu R-E, Gurrala SV, Nallapati B, Krishna V, Oancea C, Tudorache E, Marc M, Bratosin F, Bogdan I, Rosca O, et al. Prognostic Implications of Initial Radiological Findings of Pulmonary Fibrosis in Patients with Acute SARS-CoV-2 Infection: A Prospective Multicentric Study. Diseases. 2024; 12(11):285. https://doi.org/10.3390/diseases12110285
Chicago/Turabian StyleCîrjaliu, Roxana-Elena, Sri Vidhya Gurrala, Balaji Nallapati, Vamsi Krishna, Cristian Oancea, Emanuela Tudorache, Monica Marc, Felix Bratosin, Iulia Bogdan, Ovidiu Rosca, and et al. 2024. "Prognostic Implications of Initial Radiological Findings of Pulmonary Fibrosis in Patients with Acute SARS-CoV-2 Infection: A Prospective Multicentric Study" Diseases 12, no. 11: 285. https://doi.org/10.3390/diseases12110285
APA StyleCîrjaliu, R.-E., Gurrala, S. V., Nallapati, B., Krishna, V., Oancea, C., Tudorache, E., Marc, M., Bratosin, F., Bogdan, I., Rosca, O., Barata, P. I., Hangan, L. T., Chirilă, S. I., & Fildan, A.-P. (2024). Prognostic Implications of Initial Radiological Findings of Pulmonary Fibrosis in Patients with Acute SARS-CoV-2 Infection: A Prospective Multicentric Study. Diseases, 12(11), 285. https://doi.org/10.3390/diseases12110285








