Assessment of Kidney Function Discrepancies in Pediatric CAKUT Patients Using Bedside Schwartz Equation and Renal Scintigraphy
Abstract
1. Introduction
2. Methodology
2.1. Study Design
2.2. Patient Population
- o
- GFR Category:
- ▪
- G1 is defined as GFR ≥ 90 mL/min/1.73 m2;
- ▪
- G2 is defined as GFR 60–89 mL/min/1.73 m2;
- ▪
- G3a is defined as GFR 45–59 mL/min/1.73 m2;
- ▪
- G3b is defined as GFR 30–44 mL/min/1.73 m2;
- ▪
- G4 is defined as GFR 15–29 mL/min/1.73 m2;
- ▪
- G5 is defined as GFR < 15 mL/min/1.73 m2;
- o
- Albuminuria using albumin to creatinine ratio (ACR- approximate equivalent) category:
- ▪
- A1 is defined as < 3 mg/mmol or < 30 mg/g;
- ▪
- A2 is defined as 3–30 mg/mmol or 30–300 mg/g;
- ▪
- A3 is defined as 30 mg/mmol or >300 mg/g.
- Children presenting with urinary tract infections during the assessment;
- Children who did not undergo scintigraphy concomitant with the measurement of creatinine levels;
- Children without informed consent from the caregivers for performing scintigraphy.
2.3. Data Collection
2.4. Estimation of Glomerular Filtration Rate (eGFR)
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Types of CAKUT
3.3. Chronic Kidney Disease Staging
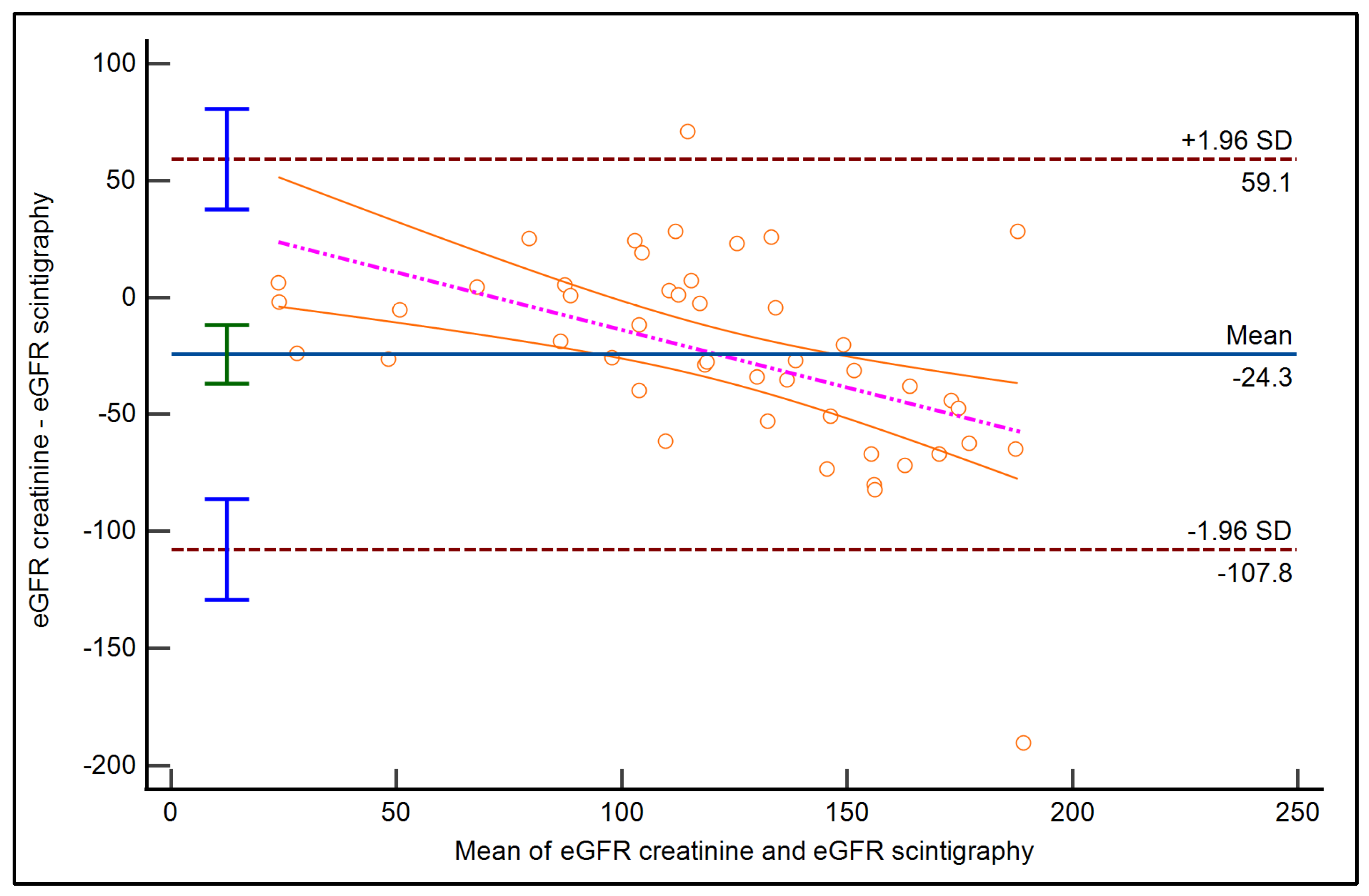
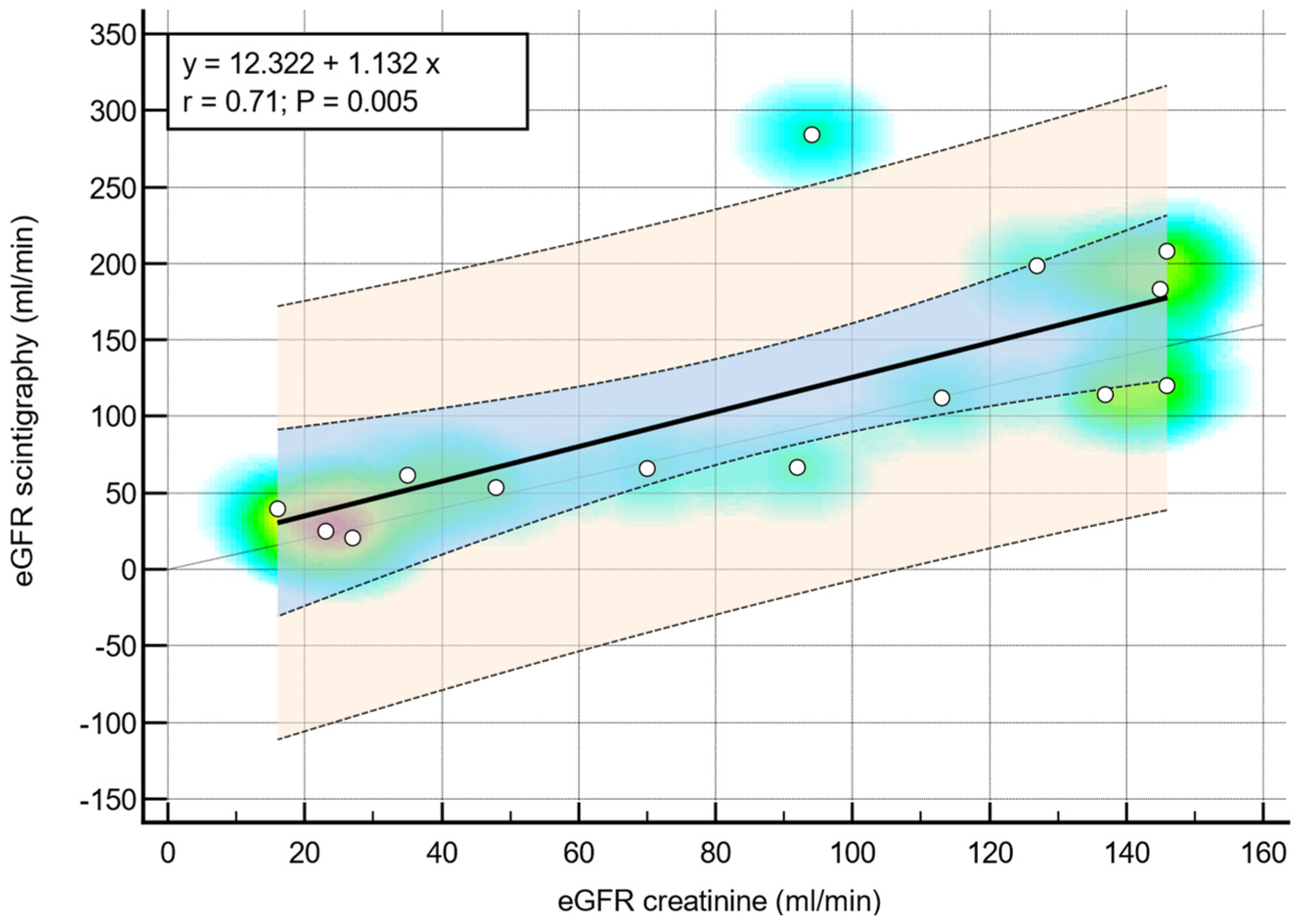
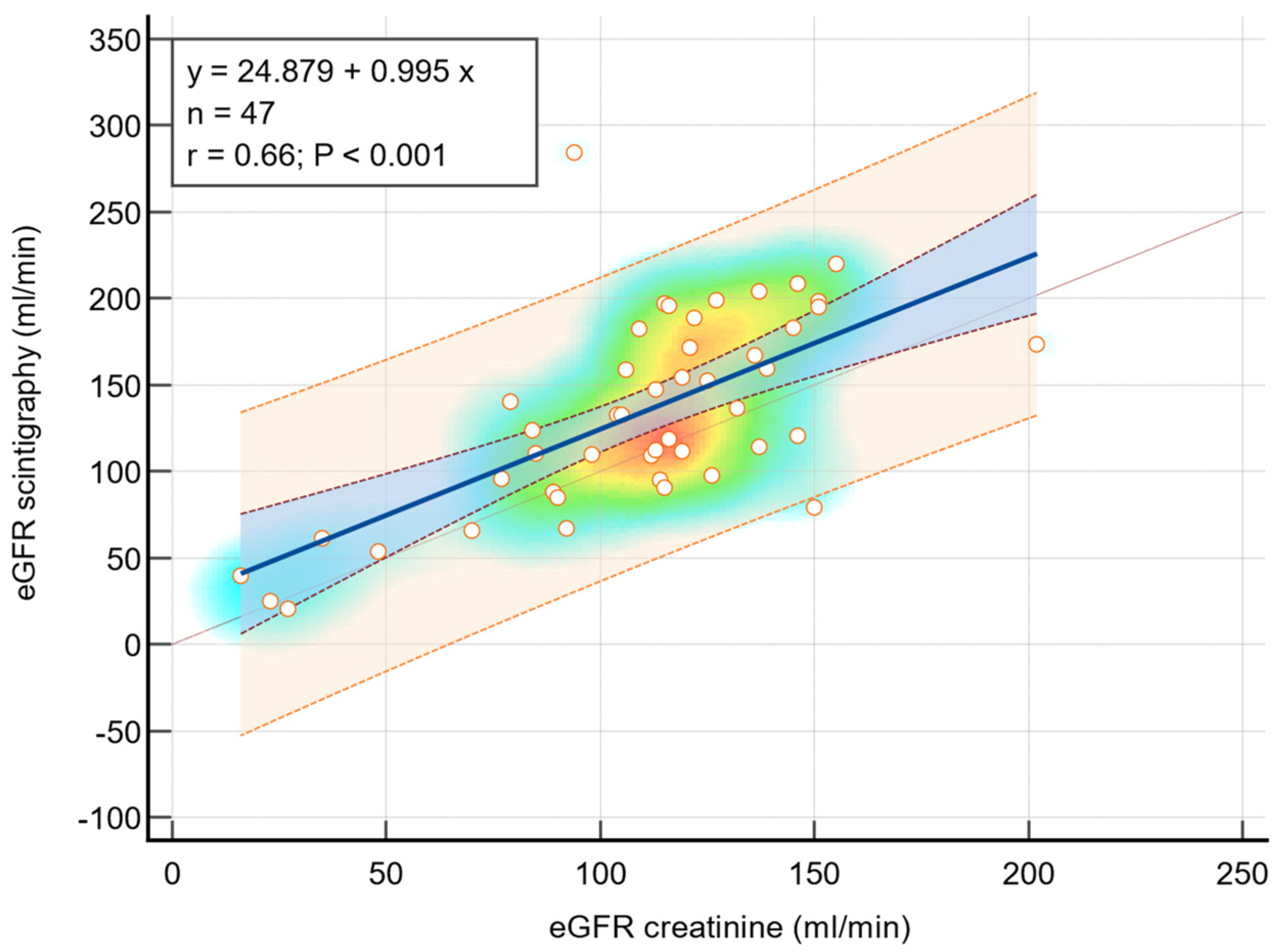
3.4. GFR Estimation Using Serum Creatinine and Scintigraphy
3.5. Correlation Between Renal Scintigraphy and eGFR
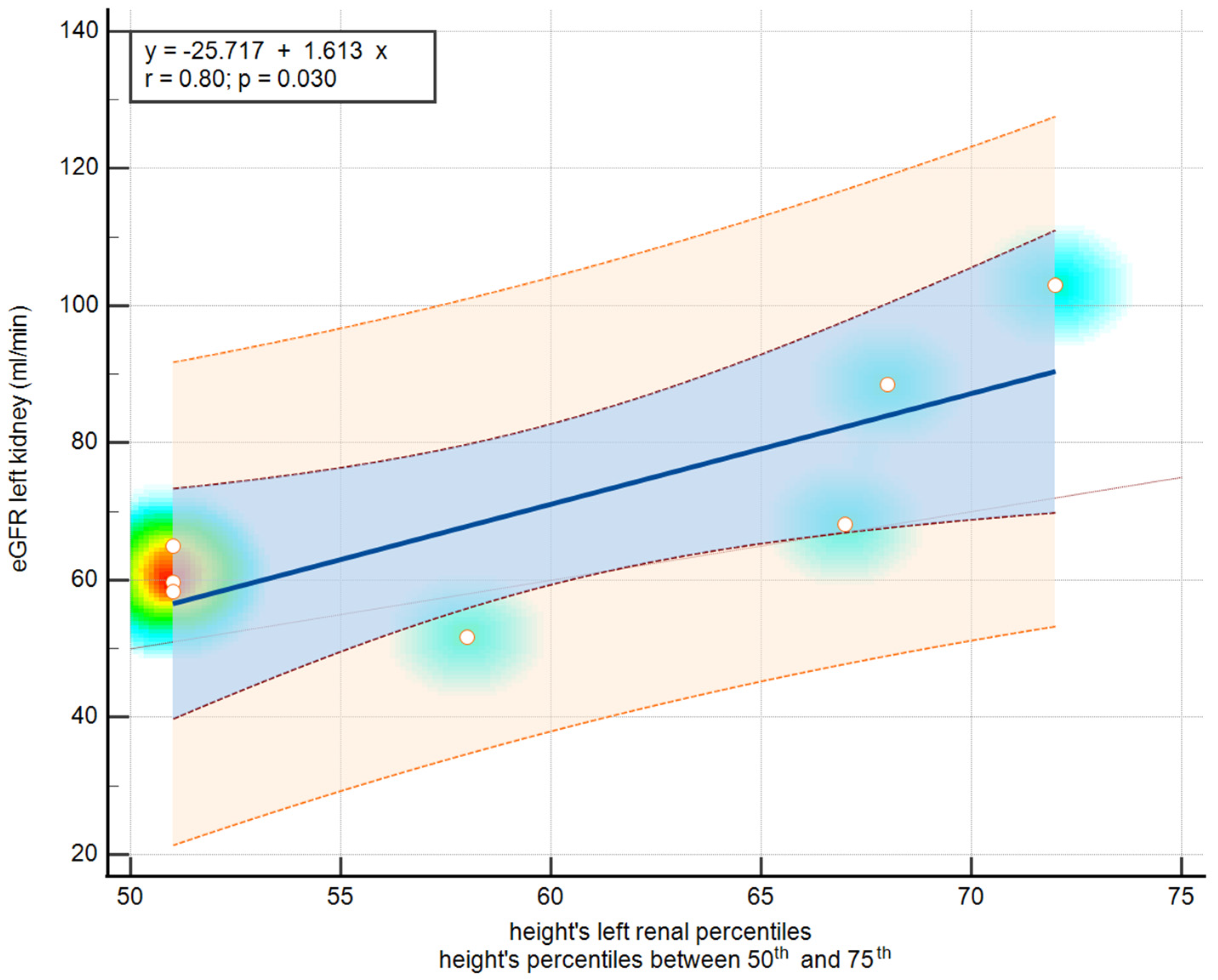
3.6. Correlation Between Renal Scintigraphy and Ultrasound Features
4. Discussion
4.1. Limitations of the Study
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebert, N.; Bevc, S.; Bökenkamp, A.; Gaillard, F.; Hornum, M.; Jager, K.J.; Mariat, C.; Eriksen, B.O.; Palsson, R.; Rule, A.D.; et al. Assessment of Kidney Function: Clinical Indications for Measured GFR. Clin. Kidney J. 2021, 14, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- McCulloch, M.; Luyckx, V.A.; Cullis, B.; Davies, S.J.; Finkelstein, F.O.; Yap, H.K.; Feehally, J.; Smoyer, W.E. Challenges of Access to Kidney Care for Children in Low-Resource Settings. Nat. Rev. Nephrol. 2021, 17, 33–45. [Google Scholar] [CrossRef]
- Dastidar, R.; Sikder, K.; Das, B. A Comparative Study on the Efficacy Between Cystatin C and Creatinine-Based Equations for Early Detection of Renal Damage in Patients of Eastern India. Ind. J. Clin. Biochem. 2023. [Google Scholar] [CrossRef]
- Viteri, B.; Calle-Toro, J.S.; Furth, S.; Darge, K.; Hartung, E.A.; Otero, H. State-of-the-Art Renal Imaging in Children. Pediatrics 2020, 145, e20190829. [Google Scholar] [CrossRef]
- Carrero, J.-J.; Fu, E.L.; Sang, Y.; Ballew, S.; Evans, M.; Elinder, C.-G.; Barany, P.; Inker, L.A.; Levey, A.S.; Coresh, J.; et al. Discordances Between Creatinine- and Cystatin C-Based Estimated GFR and Adverse Clinical Outcomes in Routine Clinical Practice. Am. J. Kidney Dis. 2023, 82, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sever, Z.; Shammas, A.; Gheisari, F.; Vali, R. Pediatric Nephro-Urology: Overview and Updates in Diuretic Renal Scans and Renal Cortical Scintigraphy. Semin. Nucl. Med. 2022, 52, 419–431. [Google Scholar] [CrossRef]
- Gottlieb, E.R.; Estiverne, C.; Tolan, N.V.; Melanson, S.E.F.; Mendu, M.L. Estimated GFR with Cystatin C and Creatinine in Clinical Practice: A Retrospective Cohort Study. Kidney Med. 2023, 5, 100600. [Google Scholar] [CrossRef]
- Conkar, S.; Mir, S.; Karaslan, F.N.; Hakverdi, G. Comparing Different Estimated Glomerular Filtration Rate Equations in Assessing Glomerular Function in Children Based on Creatinine and Cystatin C. J. Clin. Lab. Anal. 2018, 32, e22413. [Google Scholar] [CrossRef]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef]
- Blaufox, M.D.; De Palma, D.; Taylor, A.; Szabo, Z.; Prigent, A.; Samal, M.; Li, Y.; Santos, A.; Testanera, G.; Tulchinsky, M. The SNMMI and EANM Practice Guideline for Renal Scintigraphy in Adults. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2218–2228. [Google Scholar] [CrossRef] [PubMed]
- Caroli, A.; Remuzzi, A.; Lerman, L.O. Basic Principles and New Advances in Kidney Imaging. Kidney Int. 2021, 100, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Kumar, D.; Khanna, M.; Al Heidous, M.; Sheikh, A.; Virmani, V.; Palaniappan, Y. Multi-Modality Imaging Review of Congenital Abnormalities of Kidney and Upper Urinary Tract. World J. Radiol. 2016, 8, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Guarino, S.; Capalbo, D.; Martin, N.; Campana, G.; Rambaldi, P.F.; Miraglia Del Giudice, E.; La Manna, A.; Marzuillo, P. In Children with Urinary Tract Infection Reduced Kidney Length and Vesicoureteric Reflux Predict Abnormal DMSA Scan. Pediatr. Res. 2020, 87, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Haffner, D. Strategies for Optimizing Growth in Children with Chronic Kidney Disease. Front. Pediatr. 2020, 8, 399. [Google Scholar] [CrossRef]
- Fried, J.G.; Morgan, M.A. Renal Imaging: Core Curriculum 2019. Am. J. Kidney Dis. 2019, 73, 552–565. [Google Scholar] [CrossRef]
- Murugapoopathy, V.; Gupta, I.R. A Primer on Congenital Anomalies of the Kidneys and Urinary Tracts (CAKUT). Clin. J. Am. Soc. Nephrol. 2020, 15, 723–731. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Winkelmayer, W.C. Erratum: Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2017, 7, e1. [Google Scholar] [CrossRef]
- Growth Charts—Homepage. Available online: https://www.cdc.gov/growthcharts/index.htm (accessed on 16 May 2024).
- A Health Professional’s Guide to Using Growth Charts. Paediatr. Child Health 2004, 9, 174–188. [CrossRef]
- Majd, M.; Bar-Sever, Z.; Santos, A.I.; De Palma, D. The SNMMI and EANM Procedural Guidelines for Diuresis Renography in Infants and Children. J. Nucl. Med. 2018, 59, 1636–1640. [Google Scholar] [CrossRef]
- Obrycki, Ł.; Sarnecki, J.; Lichosik, M.; Sopińska, M.; Placzyńska, M.; Stańczyk, M.; Mirecka, J.; Wasilewska, A.; Michalski, M.; Lewandowska, W.; et al. Kidney Length Normative Values in Children Aged 0–19 Years—A Multicenter Study. Pediatr. Nephrol. 2022, 37, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Muñoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New Equations to Estimate GFR in Children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.; Younis, J.; Moustafa, H.; Elantably, I. Comparison of Glomerular Filtration Rate Measurement Methods between Radionuclide in Vivo Scintigraphic Gates’ and Plasma Sampling. Open Access Maced. J. Med. Sci. 2019, 7, 2734–2738. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Barai, S.; Gambhir, S.; Parasar, D.S.; Ora, M.; Gupta, A.; Sharma, R.K. Comparison of Glomerular Filtration Rate Estimated by Plasma Clearance Method with Modification of Diet in Renal Disease Prediction Equation and Gates Method. Indian J. Nephrol. 2012, 22, 103–107. [Google Scholar] [CrossRef]
- Liu, J.-L.; Wang, X.-W.; Liu, C.-H.; Gao, D.M.X.-J.; Jiang, X.-Y.; Mao, J.-H.; Zhu, G.-H.; Zhang, A.-H.; Wang, M.; Dang, X.-Q.; et al. Genetic Spectrum of CAKUT and Risk Factors for Kidney Failure: A Pediatric Multicenter Cohort Study. Nephrol. Dial. Transpl. 2022, 38, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, R.L. CAKUT: A Pediatric and Evolutionary Perspective on the Leading Cause of CKD in Childhood. Pediatr. Rep. 2023, 15, 143–153. [Google Scholar] [CrossRef]
- Societatea Română de Pediatrie. Protocoale de Diagnostic Și Tratament în Pediatrie; Amaltea: Bucharest, Romania, 2024; ISBN 978-973-162-234-7. [Google Scholar]
- Bilukha, O.; Couture, A.; McCain, K.; Leidman, E. Comparison of Anthropometric Data Quality in Children Aged 6–23 and 24–59 Months: Lessons from Population-Representative Surveys from Humanitarian Settings. BMC Nutr. 2020, 6, 60. [Google Scholar] [CrossRef]
- Ramayani, O.R.; Eyanoer, P.C.; Ritarwan, K.; Siregar, B.; Siregar, R.S. Prevalence of Recurrent Urinary Tract Infection in Children with Congenital Anomalies of the Kidney and Urinary Tract (CAKUT). IOP Conf. Ser. Earth Environ. Sci. 2018, 125, 012112. [Google Scholar] [CrossRef]
- Rodriguez, M.M. Congenital Anomalies of the Kidney and the Urinary Tract (CAKUT). Fetal Pediatr. Pathol. 2014, 33, 293–320. [Google Scholar] [CrossRef]
- Calderon-Margalit, R.; Efron, G.; Pleniceanu, O.; Tzur, D.; Stern-Zimmer, M.; Afek, A.; Erlich, T.; Derazne, E.; Kark, J.D.; Keinan-Boker, L.; et al. Congenital Anomalies of the Kidney and Urinary Tract and Adulthood Risk of Urinary Tract Cancer. Kidney Int. Rep. 2021, 6, 946–952. [Google Scholar] [CrossRef]
- Miyakita, H.; Hayashi, Y.; Mitsui, T.; Okawada, M.; Kinoshita, Y.; Kimata, T.; Koikawa, Y.; Sakai, K.; Satoh, H.; Tokunaga, M.; et al. Guidelines for the Medical Management of Pediatric Vesicoureteral Reflux. Int. J. Urol. 2020, 27, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, L.; De Chiara, L.; Innocenti, S.; Errichiello, C.; Romagnani, P.; Becherucci, F. Chronic Kidney Disease in Children: An Update. Clin. Kidney J. 2023, 16, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Xu, Y.; Zheng, J.; Wang, X.; Li, Y.; Wang, Y.; Zhu, B.; Zhao, L.; Zhao, H.; Yu, J. Prevalence of and Risk Factors for Chronic Kidney Disease in Ten Metropolitan Areas of China: A Cross-Sectional Study Using Three Kidney Damage Markers. Ren. Fail. 2023, 45, 2170243. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Kunwar, L.; Bc, B.; Gupta, A. Correlation of Ultrasonographic Parameters with Serum Creatinine and Estimated Glomerular Filtration Rate in Patients with Echogenic Kidneys. J. Nepal Health Res. Counc. 2020, 18, 495–499. [Google Scholar] [CrossRef]
- He, K.; Wan, D.; Li, S.; Yuan, G.; Gao, M.; Han, Y.; Li, Z.; Hu, D.; Meng, X.; Niu, Y. Non-Contrast-Enhanced Magnetic Resonance Urography for Measuring Split Kidney Function in Pediatric Patients with Hydronephrosis: Comparison with Renal Scintigraphy. Pediatr. Nephrol. 2024, 39, 1447–1457. [Google Scholar] [CrossRef]
- Miftari, R.; Nura, A.; Topçiu-Shufta, V.; Miftari, V.; Murseli, A.; Haxhibeqiri, V. Impact of Gate 99mTc DTPA GFR, Serum Creatinine and Urea in Diagnosis of Patients with Chronic Kidney Failure. Acta Inf. Med. 2017, 25, 99–102. [Google Scholar] [CrossRef]
- Pottel, H.; Dubourg, L.; Goffin, K.; Delanaye, P. Alternatives for the Bedside Schwartz Equation to Estimate Glomerular Filtration Rate in Children. Adv. Chronic Kidney Dis. 2018, 25, 57–66. [Google Scholar] [CrossRef]
- Marzuillo, P.; Carreras-Badosa, G.; Martínez-Calcerrada, J.-M.; Guarino, S.; Palma, P.L.; Petrone, D.; Miraglia Del Giudice, E.; Bassols, J.; López-Bermejo, A. Body Surface Area-Based Kidney Length Percentiles Misdiagnose Small Kidneys in Children with Overweight/Obesity. Pediatr. Nephrol. 2023, 38, 1523–1532. [Google Scholar] [CrossRef]
- Groen In ’t Woud, S.; Westland, R.; Feitz, W.F.J.; Roeleveld, N.; van Wijk, J.A.E.; van der Zanden, L.F.M.; Schreuder, M.F. Clinical Management of Children with a Congenital Solitary Functioning Kidney: Overview and Recommendations. Eur. Urol. Open Sci. 2021, 25, 11–20. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Luo, J.; Chen, W.; Yang, M.; Wang, L.; Zhu, N.; Yuan, W.; Gu, L. Choosing an Appropriate Glomerular Filtration Rate Estimating Equation: Role of Body Mass Index. BMC Nephrol. 2021, 22, 197. [Google Scholar] [CrossRef]
| Characteristic | Sample Size (n = 94) |
|---|---|
| Male gender (%) | 54; (57.44%) |
| Place of origin from rural areas (%) | 52; (55.31%) |
| Age at the moment of scintigraphy examination (months) | 112; (61, 147) |
| Weight at the moment of examination (kg) | 28; (19, 49.25) |
| Weight percentiles | 58; (25, 79.50) |
| Height at the moment of examination (cm) | 136; (110.87, 150) |
| Height percentiles | 65; (30.25, 92.25) |
| Body surface area (BSA) (m2) | 1.02; (0.77, 1.43) |
| Anomaly or Pathology (CAKUT) | Sample Size (n = 94) |
|---|---|
| Kidney anomalies | |
| Renal hypoplasia | 12 (12.77%) |
| Solitary kidney | 4 (4.26%) |
| Polycystic kidney disease | 6 (6.38%) |
| Renal dysplasia | 6 (6.38%) |
| ‘Horseshoe’ kidneys | 2 (2.12%) |
| Duplex Kidney (Duplicated collecting system) | 16 (17.02%) |
| Collecting system anomalies | |
| Ureteropelvic junction stenosis | 32 (34.04%) |
| Bladder anomalies | |
| Vesicoureteral reflux * | 26 (27.65%) |
| Urethral anomalies | |
| Posterior urethral valve | 8 (8.51%) |
| Stages | Sample Size (n = 94) |
|---|---|
| CKD G1 | 76 (80.85%) |
| CKD G2 | 8 (8.51%) |
| CKD G3a | 0 |
| CKD G3b | 4 (4.25%) |
| CKD G4 | 4 (4.25%) |
| CKD G5 | 2 (2.12%) |
| CKD A1 | 94 (100%) |
| GFR Measurement Method | Mean GFR (mL/min/1.73 m2) | Standard Deviation (SD) | Mean Difference vs. Iohexol (mL/min/1.73 m2) | p-Value for Difference | Correlation with Iohexol GFR (r) | p-Value for Correlation |
|---|---|---|---|---|---|---|
| Iohexol Plasma Clearance | 129.7 | 53.8 | N/A | N/A | N/A | N/A |
| eGFR from Schwartz Equation | 114.2 | 42.3 | −15.5 | 0.023 | 0.86 | <0.001 |
| GFR from Renal Scintigraphy | 133.1 | 56.5 | 3.40 | 0.57 | 0.91 | <0.001 |
| BSA’s Percentiles | Right Kidney Scintigraphy | Left Kidney Scintigraphy | p Value |
|---|---|---|---|
| <25th | 57.45 ± 37.75 | 43.90 ± 40.93 | 0.24 |
| 25th–50th | 102.02 ± 41.52 | 35.60 ± 26.05 | 0.01 |
| 50th–75th | 79.18 ± 31.14 | 74.50 ± 19.02 | 0.71 |
| >75th | 81.53 ± 40.58 | 65.89 ± 33.66 | 0.23 |
| Entire lot | 76.03 ± 40.91 | 57.46 ± 35.91 | 0.02 |
| Category | Feature | Pearson Correlation (r) | p Value |
|---|---|---|---|
| Right Renal Z Score (BSA) | <25th Percentile | 0.33 | 0.16 |
| 25th–50th Percentiles | −0.64 | 0.08 | |
| 50th–75th Percentiles | −0.40 | 0.28 | |
| >75th Percentile | 0.52 | 0.08 | |
| Right Renal Z Score (Height) | <25th Percentile | 0.15 | 0.54 |
| 25th–50th Percentiles | −0.10 | 0.84 | |
| 50th–75th Percentiles | 0.41 | 0.30 | |
| >75th Percentile | 0.16 | 0.54 | |
| Left Renal Z Score (BSA) | <25th Percentile | 0.33 | 0.19 |
| 25th–50th Percentiles | −0.95 | 0.20 | |
| 50th–75th Percentiles | 0.37 | 0.35 | |
| >75th Percentile | −0.34 | 0.15 | |
| Left Renal Z Score (Height) | <25th Percentile | 0.42 | 0.09 |
| 25th–50th Percentiles | 0.18 | 0.81 | |
| 50th–75th Percentiles | 0.80 | 0.02 | |
| >75th Percentile | −0.29 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steflea, R.M.; Jammula, G.; Kanka, A.; Streian, C.G.; Bratosin, F.; Roberta, A.C.; Susan, M.; Harich, O.O.; Boru, C.; Tanasescu, S.; et al. Assessment of Kidney Function Discrepancies in Pediatric CAKUT Patients Using Bedside Schwartz Equation and Renal Scintigraphy. Diseases 2024, 12, 265. https://doi.org/10.3390/diseases12110265
Steflea RM, Jammula G, Kanka A, Streian CG, Bratosin F, Roberta AC, Susan M, Harich OO, Boru C, Tanasescu S, et al. Assessment of Kidney Function Discrepancies in Pediatric CAKUT Patients Using Bedside Schwartz Equation and Renal Scintigraphy. Diseases. 2024; 12(11):265. https://doi.org/10.3390/diseases12110265
Chicago/Turabian StyleSteflea, Ruxandra Maria, Geethiikha Jammula, Akhila Kanka, Caius Glad Streian, Felix Bratosin, Avram Cecilia Roberta, Monica Susan, Octavia Oana Harich, Casiana Boru, Sonia Tanasescu, and et al. 2024. "Assessment of Kidney Function Discrepancies in Pediatric CAKUT Patients Using Bedside Schwartz Equation and Renal Scintigraphy" Diseases 12, no. 11: 265. https://doi.org/10.3390/diseases12110265
APA StyleSteflea, R. M., Jammula, G., Kanka, A., Streian, C. G., Bratosin, F., Roberta, A. C., Susan, M., Harich, O. O., Boru, C., Tanasescu, S., Cristescu, D.-M., Banta, A.-M., Doros, G., & Feciche, B. (2024). Assessment of Kidney Function Discrepancies in Pediatric CAKUT Patients Using Bedside Schwartz Equation and Renal Scintigraphy. Diseases, 12(11), 265. https://doi.org/10.3390/diseases12110265






