Evaluation of Natural and Vaccine-Induced Anti-SARS-CoV-2 Immunity: A Comparative Study between Different Groups of Volunteers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Antibody Dosage Anti Spike-SARS-CoV-2
2.3. Statistical Analysis
3. Results
3.1. COVID-19 Infected Patients
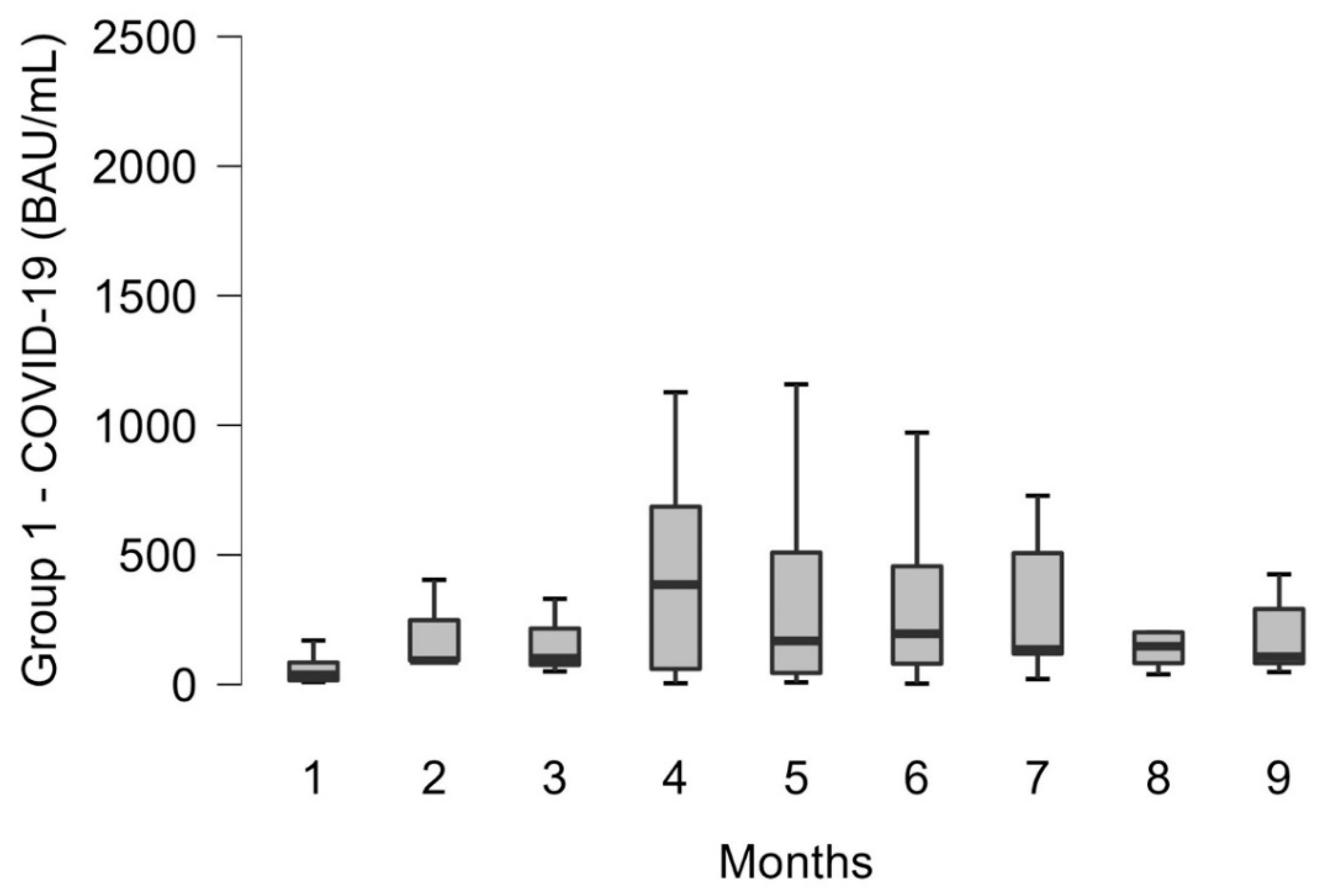
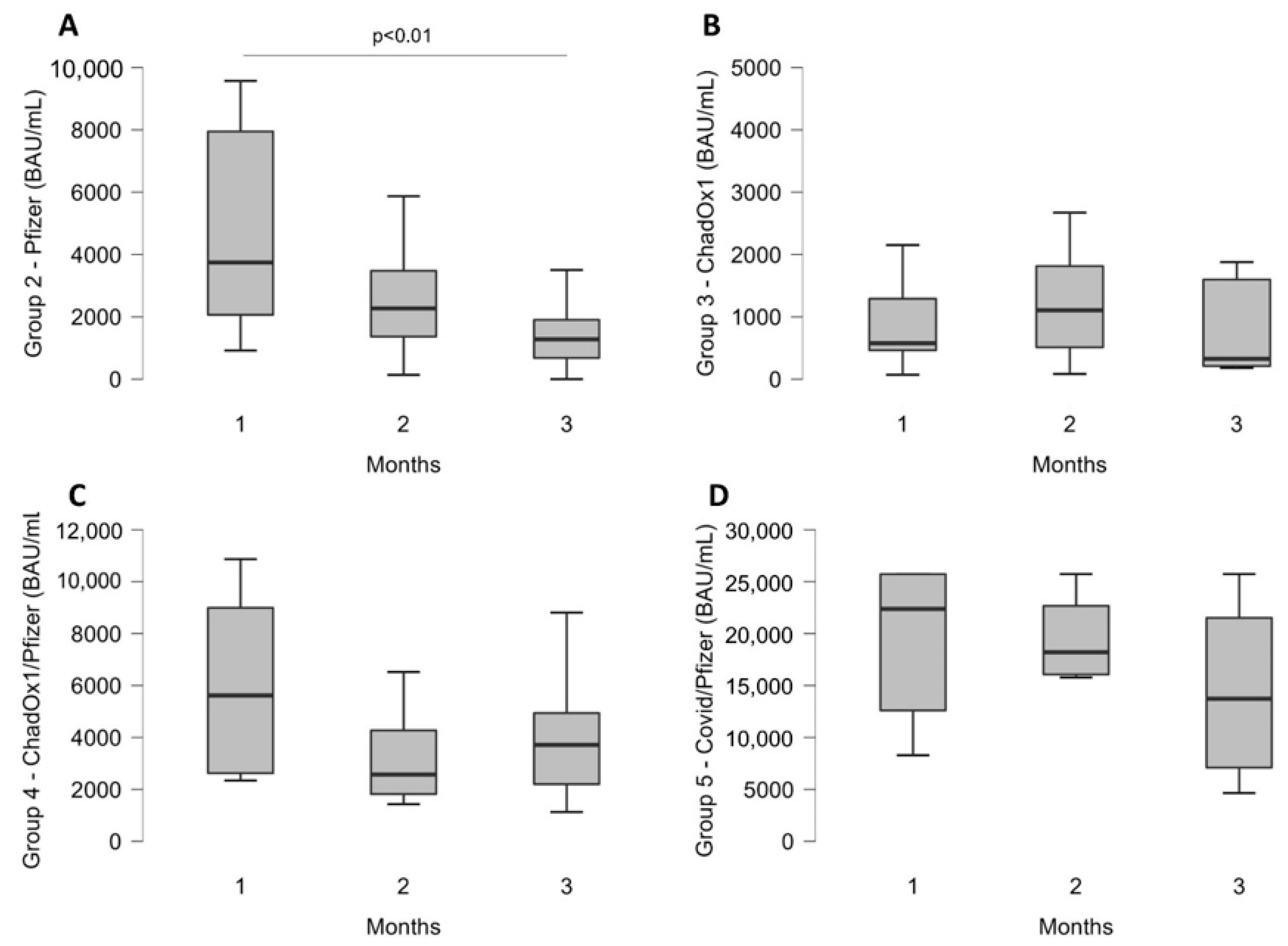
3.2. Trend over Time for Each Group
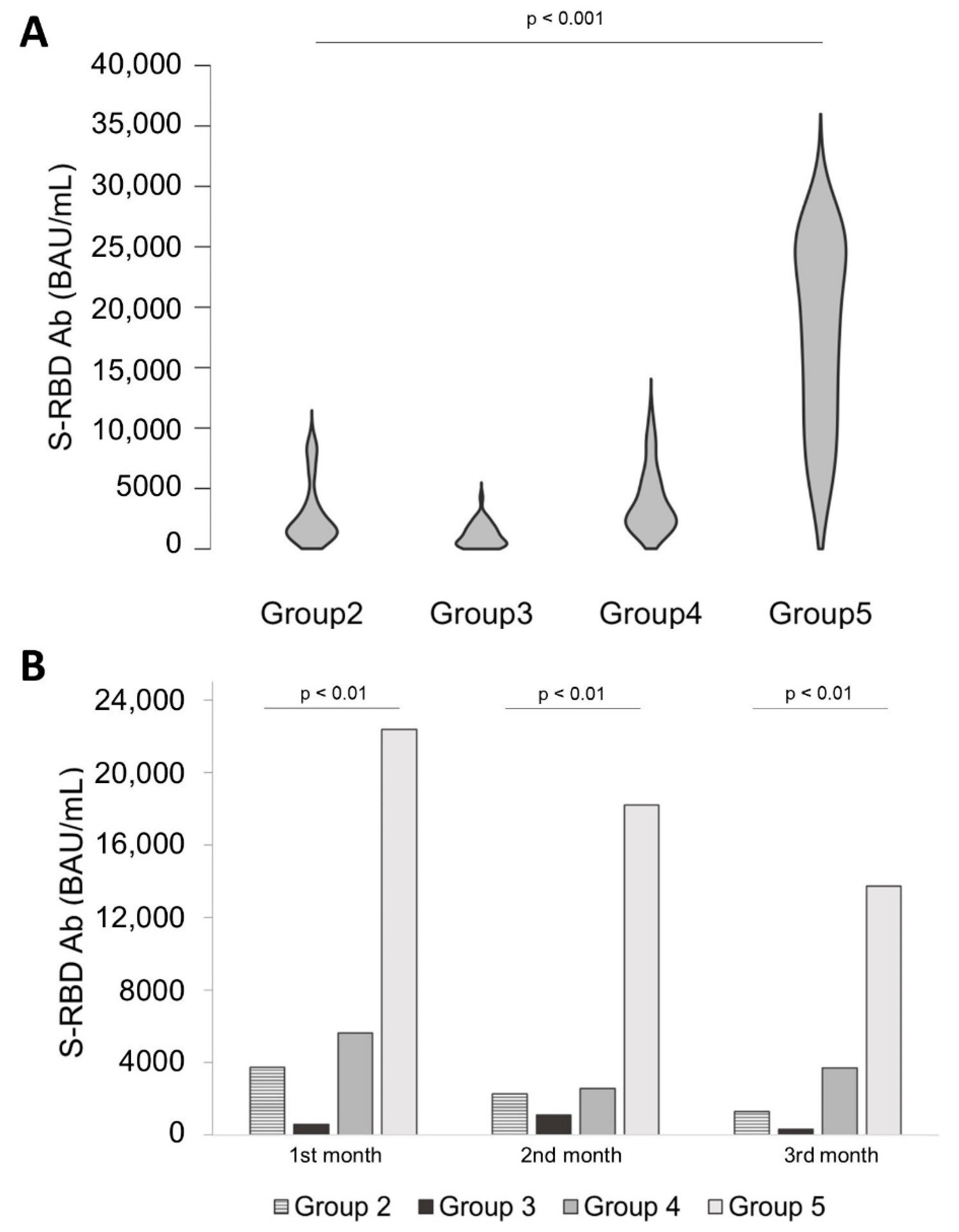
3.3. Comparison of Antibody Levels over Time between All Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Addetia, A.; Crawford, K.H.D.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.-L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020, 58, e02107-20. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Cristiano, A.; Nuccetelli, M.; Pieri, M.; Sarubbi, S.; Pelagalli, M.; Calugi, G.; Tomassetti, F.; Bernardini, S. Serological Anti-SARS-CoV-2 Neutralizing Antibodies Association to Live Virus Neutralizing Test Titers in COVID-19 Paucisymptomatic/Symptomatic Patients and Vaccinated Subjects. Int. Immunopharmacol. 2021, 101, 108215. [Google Scholar] [CrossRef] [PubMed]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for Antibody as a Protective Correlate for COVID-19 Vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Padoan, A.; Cosma, C.; Bonfante, F.; Della Rocca, F.; Barbaro, F.; Santarossa, C.; Dall’Olmo, L.; Pagliari, M.; Bortolami, A.; Cattelan, A.; et al. Neutralizing Antibody Titers Six Months after Comirnaty Vaccination: Kinetics and Comparison with SARS-CoV-2 Immunoassays. Clin. Chem. Lab. Med. 2021, 60, 456–463. [Google Scholar] [CrossRef]
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody Responses to SARS-CoV-2 in Patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Ballin, M.; Nordström, A. Effectiveness of Heterologous ChAdOx1 NCoV-19 and MRNA Prime-Boost Vaccination against Symptomatic COVID-19 Infection in Sweden: A Nationwide Cohort Study. Lancet Reg. Health Eur. 2021, 11, 100249. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, F.; Nuccetelli, M.; Sarubbi, S.; Gisone, F.; Ciotti, M.; Spinazzola, F.; Ricotta, C.; Cagnoli, M.; Borgatti, M.; Iannetta, M.; et al. Evaluation of S-RBD and High Specificity ACE-2-Binding Antibodies on SARS-CoV-2 Patients after Six Months from Infection. Int. Immunopharmacol. 2021, 99, 108013. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadeq, D.W.; Shurrab, F.M.; Ismail, A.; Amanullah, F.H.; Thomas, S.; Aldewik, N.; Yassine, H.M.; Abdul Rahim, H.F.; Abu-Raddad, L.; Nasrallah, G.K. Comparison of Antibody Immune Responses between BNT162b2 and MRNA-1273 SARS-CoV-2 Vaccines in Naïve and Previously Infected Individuals. J. Travel Med. 2021, 28, taab190. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent Antibody Responses to SARS-CoV-2 in Convalescent Individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef] [PubMed]
- L’Huillier, A.G.; Meyer, B.; Andrey, D.O.; Arm-Vernez, I.; Baggio, S.; Didierlaurent, A.; Eberhardt, C.S.; Eckerle, I.; Grasset-Salomon, C.; Huttner, A.; et al. Antibody Persistence in the First 6 Months Following SARS-CoV-2 Infection among Hospital Workers: A Prospective Longitudinal Study. Clin. Microbiol. Infect. 2021, 27, e1–e784. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; Cosma, C.; Padoan, A. SARS-CoV-2 Antibody Assay after Vaccination: One Size Does Not Fit All. Clin. Chem. Lab. Med. 2021, 59, e380–e381. [Google Scholar] [CrossRef] [PubMed]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.-B.; Massardier-Pilonchéry, A.; et al. Immunogenicity and Efficacy of Heterologous ChAdOx1-BNT162b2 Vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Nicolai, E.; Ciotti, M.; Nuccetelli, M.; Sarubbi, S.; Pelagalli, M.; Bernardini, S. Antibody Response to COVID-19 Vaccine: A Point of View That Can Help to Optimize Dose Distribution. Int. Immunopharmacol. 2022, 102, 108406. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, D.; Jang, H.; Abreu, R.B.; Hanley, H.B.; Gattiker, J.L.; Jefferson, A.M.; Ross, T.M. SARS-CoV-2 MRNA Vaccines Elicit Different Responses in Immunologically Naïve and Pre-Immune Humans. Front. Immunol. 2021, 12, 728021. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct Antibody and Memory B Cell Responses in SARS-CoV-2 Naïve and Recovered Individuals Following MRNA Vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef] [PubMed]
- Filia, A.; Rota, M.F.; D’Ancona, P.F. National COVID-19 Vaccination Plan. Available online: https://www.epicentro.iss.it/en/vaccines/covid-19-vaccination-plan#writers (accessed on 10 August 2021).
| Infection/ Vaccine | Age | Gender | No | Schedule | Period | Antibody Monitoring | |
|---|---|---|---|---|---|---|---|
| Group 1 | COVID-19 infection | 43 ± 18 yrs | m 57 (46 ± 17 yrs) f 76 (41 ± 18 yrs) | 133 | Previous SARS-CoV-2 infection | April 2020 and September 2021 | From 1st month since negative swab to 9 months after |
| Group 2 | Pfizer BioNTech | 49 ± 18 yrs | m 85 (53 ± 17 yrs) f 87 (46 ± 17 yrs) | 172 | Double dose of Pfizer-BioNTech Cominarty (BNT162b2) vaccine | 2nd dose: 21 days later | Since administration of 2nd dose |
| Group 3 | ChadOx1 nCoV19 | 59 ± 13 yrs | m 17 (53 ± 14 yrs) f 21 (63 ± 10 yrs) | 38 | Double dose of Vaxzevria (ChAdOx1-S) AstraZeneca) vaccine | 2nd dose: 4–12 weeks after | Since administration of 2nd dose |
| Group 4 | ChadOx1 nCoV19 + Pfizer BioNTech/heterologous | 43 ± 14 yrs | m 16 (43 ± 16 yrs) f 15 (42 ± 10 yrs) | 32 | First dose (priming) of the vaccine Vaxzevria (ChAdOx1-S) AstraZeneca and a second dose (booster) of the vaccine Cominarty (BNT162b2) Pfizer-BioNTech | Booster dose: 8–12 weeks from the priming (since the ministerial circular of 14 June 2021) | Since administration of booster dose |
| Group 5 | COVID-19 infection + booster dose | 44 ± 17 yrs | m 10 (52 ± 15 yrs) f 18 (40 ± 17) yrs | 28 | Previous SARS-CoV-2 infection and a single dose of anti-SARS-CoV-2/COVID19 vaccine (in this study with Cominarty (BNT162b2) Pfizer-BioNTech preparation) | Vaccine between 4–8 months since infection | Since administration of vaccine |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Over 10 years | Under 10 years |
| Previous infection of COVID-19 in the last 9 months | Under immunosuppressor therapy (in the last 5 months) |
| Full schedule vaccination (and type of vaccine) | Under immunomodulator therapy (in the last 5 months) |
| Less than 30 days from the second dose or the booster dose | Chronic disease |
| Negative SARS-COV-2 antigen screening test | Immunodeficiency |
| Ig anti-RBD > 0.823 BAU/ML | Ig anti-RBD < 0.823 BAU/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schipani, M.C.; Tomassetti, F.; Polidori, I.; Ricci, P.; Frassanito, M.L.; Seraceni, S.; Morello, M.; Nicolai, E.; Aquaro, S.; Bernardini, S.; et al. Evaluation of Natural and Vaccine-Induced Anti-SARS-CoV-2 Immunity: A Comparative Study between Different Groups of Volunteers. Diseases 2022, 10, 25. https://doi.org/10.3390/diseases10020025
Schipani MC, Tomassetti F, Polidori I, Ricci P, Frassanito ML, Seraceni S, Morello M, Nicolai E, Aquaro S, Bernardini S, et al. Evaluation of Natural and Vaccine-Induced Anti-SARS-CoV-2 Immunity: A Comparative Study between Different Groups of Volunteers. Diseases. 2022; 10(2):25. https://doi.org/10.3390/diseases10020025
Chicago/Turabian StyleSchipani, Maria Caterina, Flaminia Tomassetti, Isabella Polidori, Paola Ricci, Maria Loredana Frassanito, Silva Seraceni, Maria Morello, Eleonora Nicolai, Stefano Aquaro, Sergio Bernardini, and et al. 2022. "Evaluation of Natural and Vaccine-Induced Anti-SARS-CoV-2 Immunity: A Comparative Study between Different Groups of Volunteers" Diseases 10, no. 2: 25. https://doi.org/10.3390/diseases10020025
APA StyleSchipani, M. C., Tomassetti, F., Polidori, I., Ricci, P., Frassanito, M. L., Seraceni, S., Morello, M., Nicolai, E., Aquaro, S., Bernardini, S., Pieri, M., & Calugi, G. (2022). Evaluation of Natural and Vaccine-Induced Anti-SARS-CoV-2 Immunity: A Comparative Study between Different Groups of Volunteers. Diseases, 10(2), 25. https://doi.org/10.3390/diseases10020025










