Elevated Lipoprotein(a) Level Influences Familial Hypercholesterolemia Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
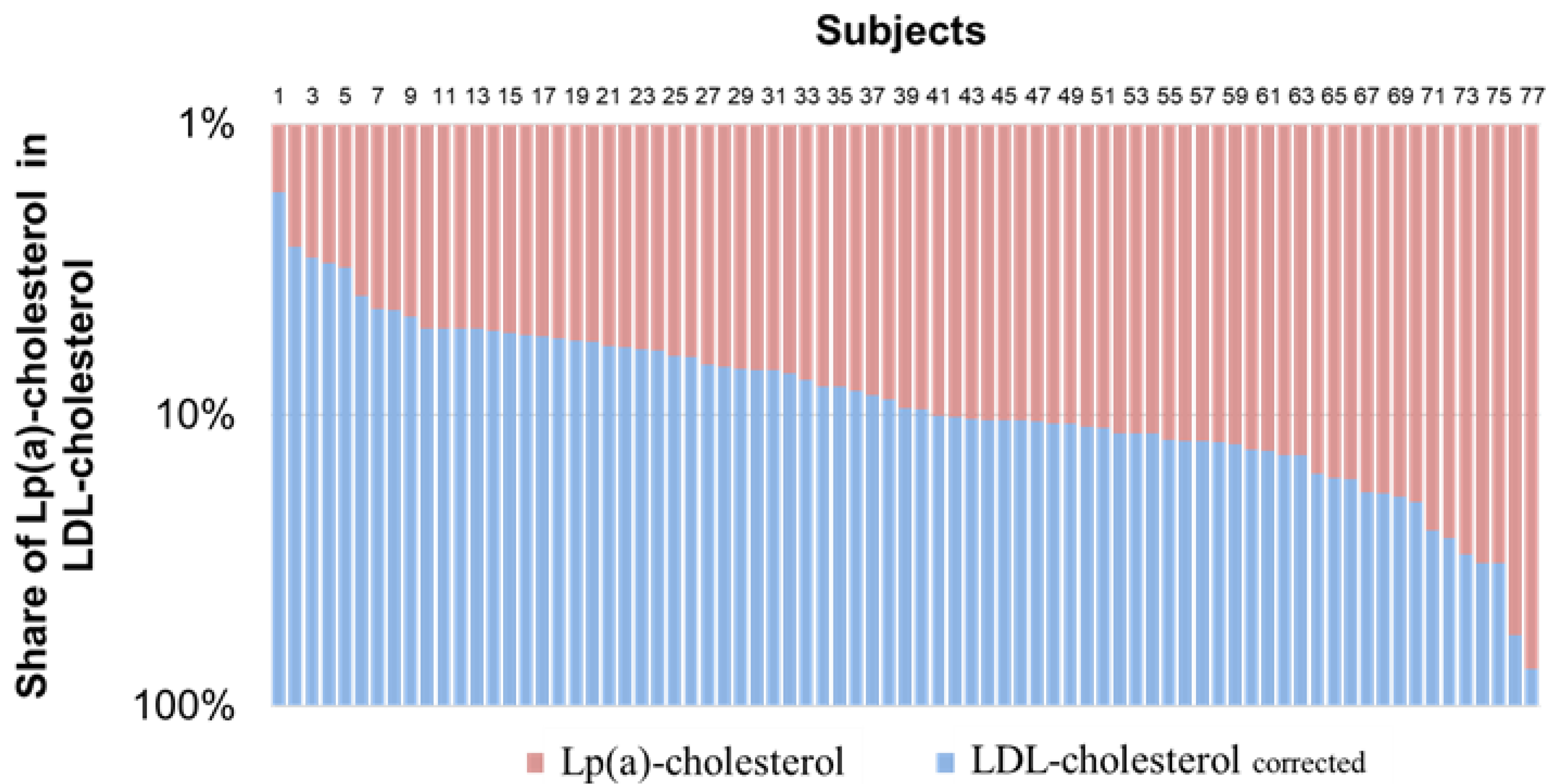
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsimikas, S.; Fazio, S.; Ferdinand, K.C.; Ginsberg, H.N.; Koschinsky, M.; Marcovina, S.M.; Moriarty, P.M.; Rader, D.J.; Remaley, A.T.; Reyes-Soffer, G.; et al. NHLBI Working Group Recommendations to Reduce Lipoprotein(a)-Mediated Risk of Cardiovascular Disease and Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Akioyamen, L.E.; Genest, J.; Shan, S.D.; Reel, R.L.; Albaum, J.M.; Chu, A.; Tu, J.V. Estimating the prevalence of heterozygous familial hypercholesterolaemia: A systematic review and meta-analysis. BMJ Open 2017, 7, e016461. [Google Scholar] [CrossRef] [PubMed]
- Vuorio, A.; Watts, G.F.; Schneider, W.J.; Tsimikas, S.; Kovanen, P.T. Familial hypercholesterolemia and elevated lipoprotein(a): Double heritable risk and new therapeutic opportunities. J. Intern. Med. 2019, 287, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Familial Hypercholesterolaemia (FH): Report of a Second WHO Consultation, Geneva. 4 September 1998. Available online: https://apps.who.int/iris/handle/10665/66346 (accessed on 17 March 2021).
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Yeang, C.; Witztum, J.L.; Tsimikas, S. ‘LDL-C’ = LDL-C + Lp(a)-C: Implications of achieved ultra-low LDL-C levels in the proprotein convertase subtilisin/kexin type 9 era of potent LDL-C lowering. Curr. Opin. Lipidol. 2015, 26, 169–178. [Google Scholar] [CrossRef]
- Langsted, A.; Kamstrup, P.R.; Benn, M.; Tybjærg-Hansen, A.; Nordestgaard, B.G. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: A prospective cohort study. Lancet Diabetes Endocrinol. 2016, 4, 577–587. [Google Scholar] [CrossRef]
- Dahlen, G.H. Incidence of Lp(a) among Populations; Academic Press: Cambridge, MA, USA, 1990; pp. 151–173. [Google Scholar]
- Afanasieva, O.I.; Ezhov, M.V.; Razova, O.; Afanasieva, M.; Utkina, E.; Pokrovsky, S. Apolipoprotein(a) phenotype determines the correlations of lipoprotein(a) and proprotein convertase subtilisin/kexin type 9 levels in patients with potential familial hypercholesterolemia. Atherosclerosis 2018, 277, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus Statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.S.; Kovanen, P.T.; Goldstein, J.L. Regulation of Plasma Cholesterol by Lipoprotein Receptors. Science 1981, 212, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Kraft, H.G.; Menzel, H.J.; Hoppichler, F.; Vogel, W.; Utermann, G. Changes of genetic apolipoprotein phenotypes caused by liver transplantation. Implications for apolipoprotein synthesis. J. Clin. Investig. 1989, 83, 137–142. [Google Scholar] [CrossRef] [PubMed]
- McConnell, J.P.; Guadagno, P.A.; Dayspring, T.D.; Hoefner, D.M.; Thiselton, D.L.; Warnick, G.R.; Harris, W.S. Lipoprotein(a) mass: A massively misunderstood metric. J. Clin. Lipidol. 2014, 8, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Fatica, E.M.; Meeusen, J.W.; Vasile, V.C.; Jaffe, A.S.; Donato, L.J. Measuring the contribution of Lp(a) cholesterol towards LDL-C interpretation. Clin. Biochem. 2020, 86, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Pang, J.; Hooper, A.J.; Bell, D.A.; Burnett, J.R.; Watts, G.F. Effect of Lipoprotein(a) on the Diagnosis of Familial Hypercholesterolemia: Does It Make a Difference in the Clinic? Clin. Chem. 2019, 65, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- van Capelleveen, J.C.; van der Valk, F.M.; Stroes, E.S. Current therapies for lowering lipoprotein (a). J. Lipid Res. 2016, 57, 1612–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wissen, S.; Smilde, T.J.; Trip, M.D.; De Boo, T.; Kastelein, J.J.P.; Stalenhoef, A.F.H. Long term statin treatment reduces lipoprotein(a) concentrations in heterozygous familial hypercholesterolaemia. Heart 2003, 89, 893–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, H.; Umemoto, T. Atorvastatin decreases lipoprotein(a): A meta-analysis of randomized trials. Int. J. Cardiol. 2012, 154, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Papademetriou, V.; Stavropoulos, K.; Papadopoulos, C.; Koutsampasopoulos, K.; Dimitriadis, K.; Tsioufis, K. Role of PCSK9 Inhibitors in High Risk Patients with Dyslipidemia: Focus on Familial Hypercholesterolemia. Curr. Pharm. Des. 2019, 24, 3647–3653. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, S.; Afanasieva, O.; Ezhov, M.V. Lipoprotein(a) apheresis. Curr. Opin. Lipidol. 2016, 27, 351–358. [Google Scholar] [CrossRef] [PubMed]



| Variable | Meaning |
|---|---|
| Relatives with LDL-C levels >95th percentile | 153 (74%) |
| Family history of coronary heart disease | 118 (57%) |
| Coronary heart disease | 70 (34%) |
| Stroke/transient ischemic attack | 11 (5%) |
| Genetic testing | 58 (28%) |
| 35 (60%) |
| 2 (3%) |
| 1 (2%) |
| 20 (35%) |
| Tendon xanthomas | 25 (15%) |
| Corneal arcus | 10 (5.5%) |
| Lipid profile | |
| Highest LDL-C level, mmol/L | 6.5 ± 2.4 |
| Lipoprotein(a), mg/dL | 17 [7;52] |
| LDL-Ccorrected, mmol/L | 6.2 ± 2.4 |
| Lipoprotein(a) ≥ 30 mg/dL | 77 (37%) |
| FH Diagnosis | Unlikely | Possible | Probable | Definite | ||
|---|---|---|---|---|---|---|
| Lp(a) < 40 mg/dL | n = 141 | Diagnosis | 0 | 49 | 56 | 36 |
| Diagnosis corrected | 5 | 52 | 49 | 35 | ||
| Reclassification of subjects | +5 | +3 | −7 | −1 | ||
| Total % of reclassified points | 15% | |||||
| Total % of reclassified diagnosis | 11% | |||||
| Lp(a) ≥ 40 mg/dL | n = 65 | Diagnosis | 0 | 21 | 20 | 24 |
| Diagnosis corrected | 8 | 25 | 10 | 22 | ||
| Reclassification of subjects | +8 | +4 | −10 | −2 | ||
| Total % of reclassified points | 51% | |||||
| Total % of reclassified diagnosis | 34% | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chubykina, U.V.; Ezhov, M.V.; Afanasieva, O.I.; Klesareva, E.A.; Pokrovsky, S.N. Elevated Lipoprotein(a) Level Influences Familial Hypercholesterolemia Diagnosis. Diseases 2022, 10, 6. https://doi.org/10.3390/diseases10010006
Chubykina UV, Ezhov MV, Afanasieva OI, Klesareva EA, Pokrovsky SN. Elevated Lipoprotein(a) Level Influences Familial Hypercholesterolemia Diagnosis. Diseases. 2022; 10(1):6. https://doi.org/10.3390/diseases10010006
Chicago/Turabian StyleChubykina, Uliana V., Marat V. Ezhov, Olga I. Afanasieva, Elena A. Klesareva, and Sergei N. Pokrovsky. 2022. "Elevated Lipoprotein(a) Level Influences Familial Hypercholesterolemia Diagnosis" Diseases 10, no. 1: 6. https://doi.org/10.3390/diseases10010006
APA StyleChubykina, U. V., Ezhov, M. V., Afanasieva, O. I., Klesareva, E. A., & Pokrovsky, S. N. (2022). Elevated Lipoprotein(a) Level Influences Familial Hypercholesterolemia Diagnosis. Diseases, 10(1), 6. https://doi.org/10.3390/diseases10010006








