Electromyography Pattern Likelihood Analysis for Flexion-Relaxation Phenomenon Evaluation
Abstract
1. Introduction
2. Measurement System, Positioning, and Acquisition Protocol
2.1. Longissimus Muscles
- Muscle: erector spinae
- Subdivision: longissimus (semispinalis back)
- Abbreviation (convention): LSXfor the left muscle and LDXfor the right muscle
- Channel (convention): Channel 1 is the LSX channel, while Channel 2 is the LDX channel
- Origin: in the lumbar region, it merges with the iliocostalis of the loins on the posterior surfaces of the transverse processes and the accessory processes of the lumbar vertebrae and on the anterior aspect of the thoraco-lumbar fascia
- Insertion: through tendons on the tips of the transverse processes of all thoracic vertebrae and on the inferior part of the ninth and tenth rib between the tubercle and the costal angle
- Function: extension of the trunk
- Reference position: pronounced with the lumbar part of the slightly flexed column
- Electrode dimensions: no more than 10 mm in the direction of the fibers
- Inter-electrode distance: 20 mm
- Electrode placement:
- −
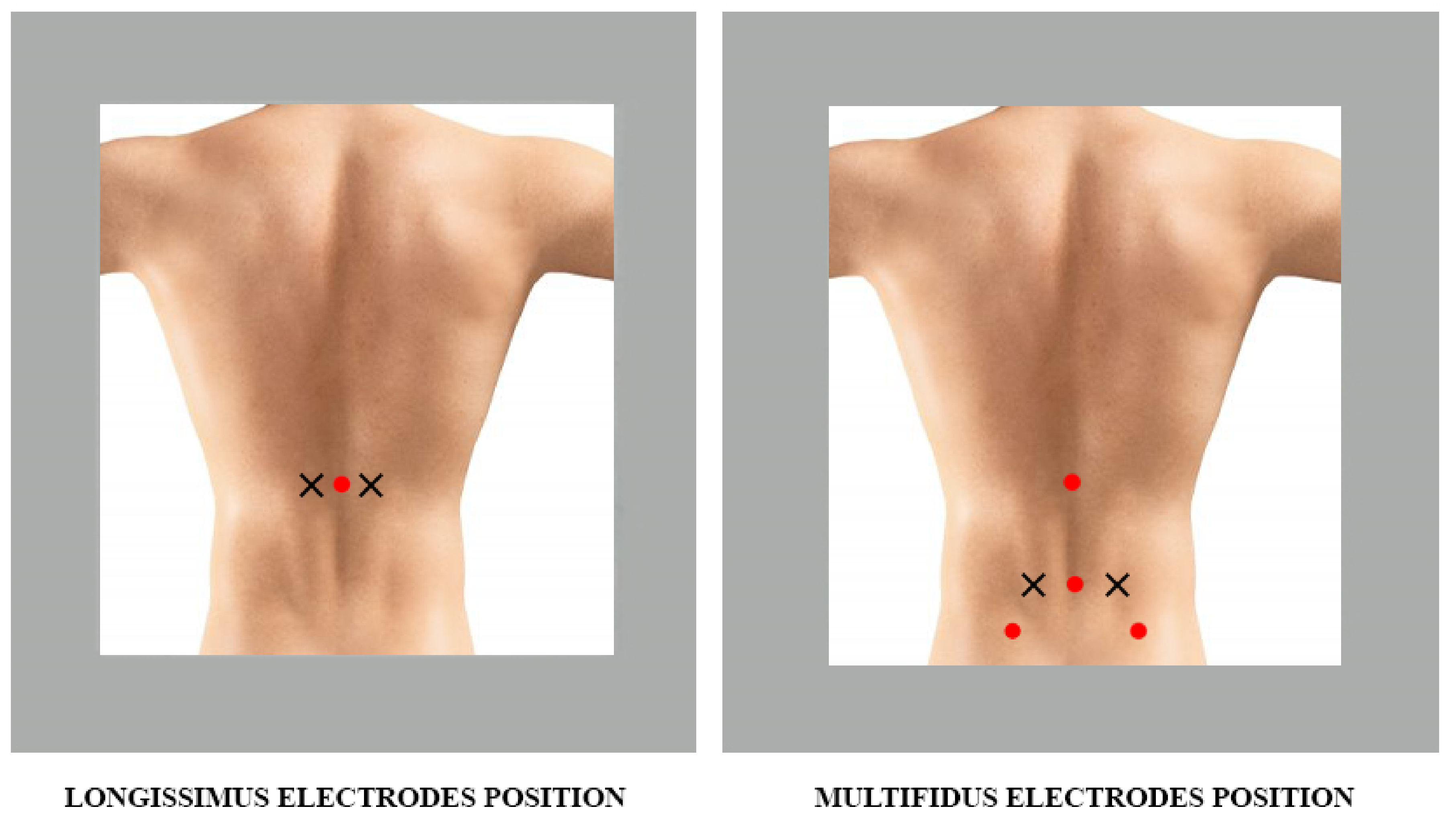
- Position: two fingers apart in a lateral direction from the spinous process L1 (Figure 2)
- −
- Fastening: double-sided tape or rings
- −
- Reference electrode: C7 spinous process
- −
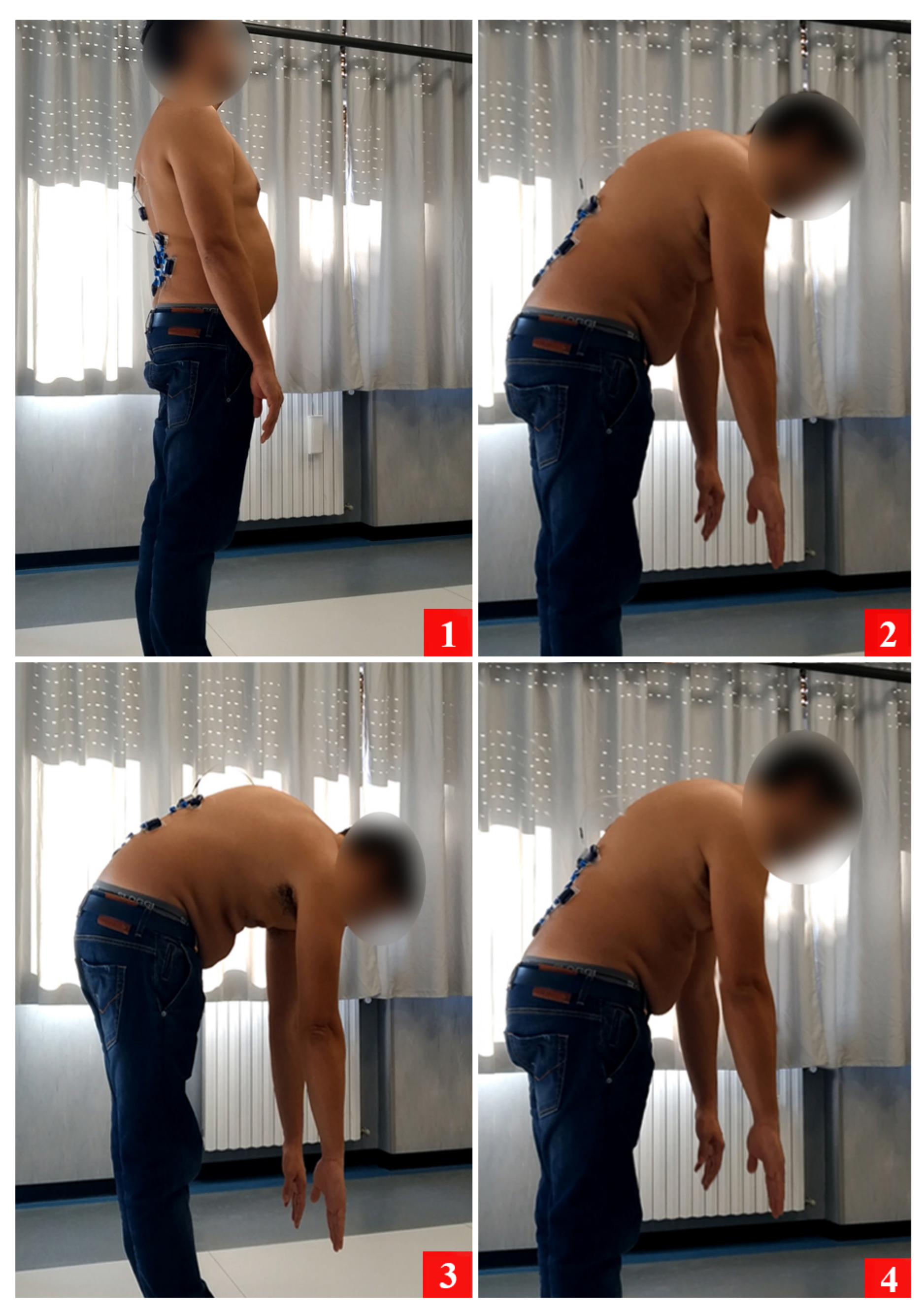
- Clinical test: lifting the trunk from the prone position
2.2. Multifidus Muscles
- Muscle: multifidus (spiny transverse)
- Abbreviation (convention): MSXfor the left muscle and MDXfor the right muscle
- Channel (convention): Channel 3 is the MSX channel, while Channel 4 is the MDX channel
- Origin: spinous processes of L1-L5
- Insertion: processes of the L4-S1 vertebrae; iliac crest and dorsal surface of the sacrum
- Function: extension
- Reference position: pronounced with the lumbar part of the slightly flexed column
- Electrode dimensions: no more than 10 mm in the direction of the fibers
- Inter-electrode distance: 20 mm
- Electrode placement:
- −
- Position: on the line connecting the caudal tip of the posterior superior iliac spine (SIPS) to the space between L1 and L2, at the level of the spinous process of L5, 2–3 cm from the medial line (Figure 2)
- −
- Fastening: double-sided tape or rings
- −
- Reference electrode: on the spinous process of C7
- −
- Clinical test: lifting the trunk from the prone position.
3. Materials and Methods
4. Experimental Design
4.1. Exclusion Criteria
- Pregnancy
- Severe structural deformities (e.g., kyphoscoliosis)
- Systemic diseases (a disease that affects multiple apparatuses or organs, often related to rheumatic diseases, or rare diseases such as genetic disorders) or neoplastic diseases (tumors)
- Significant psychiatric diseases
- Any other medical condition that could interfere with the correct execution of the protocol
4.2. Inclusion Criteria for Individuals in the Control Group
- Aged between 18 and 65 years old
- No history of musculoskeletal or abdominal pain
- Not under medical treatment
- No episodes of LBP within the last six months
- No consultation with a therapist or doctor regarding LBP problems
4.3. Inclusion Criteria for the Patient Group
- Aged between 18 and 65 years old
- Available to participate in a pain management program
- Actively suffering from LBP (LBP type should be specified, and it should also be clarified whether it is present when the test is executed)
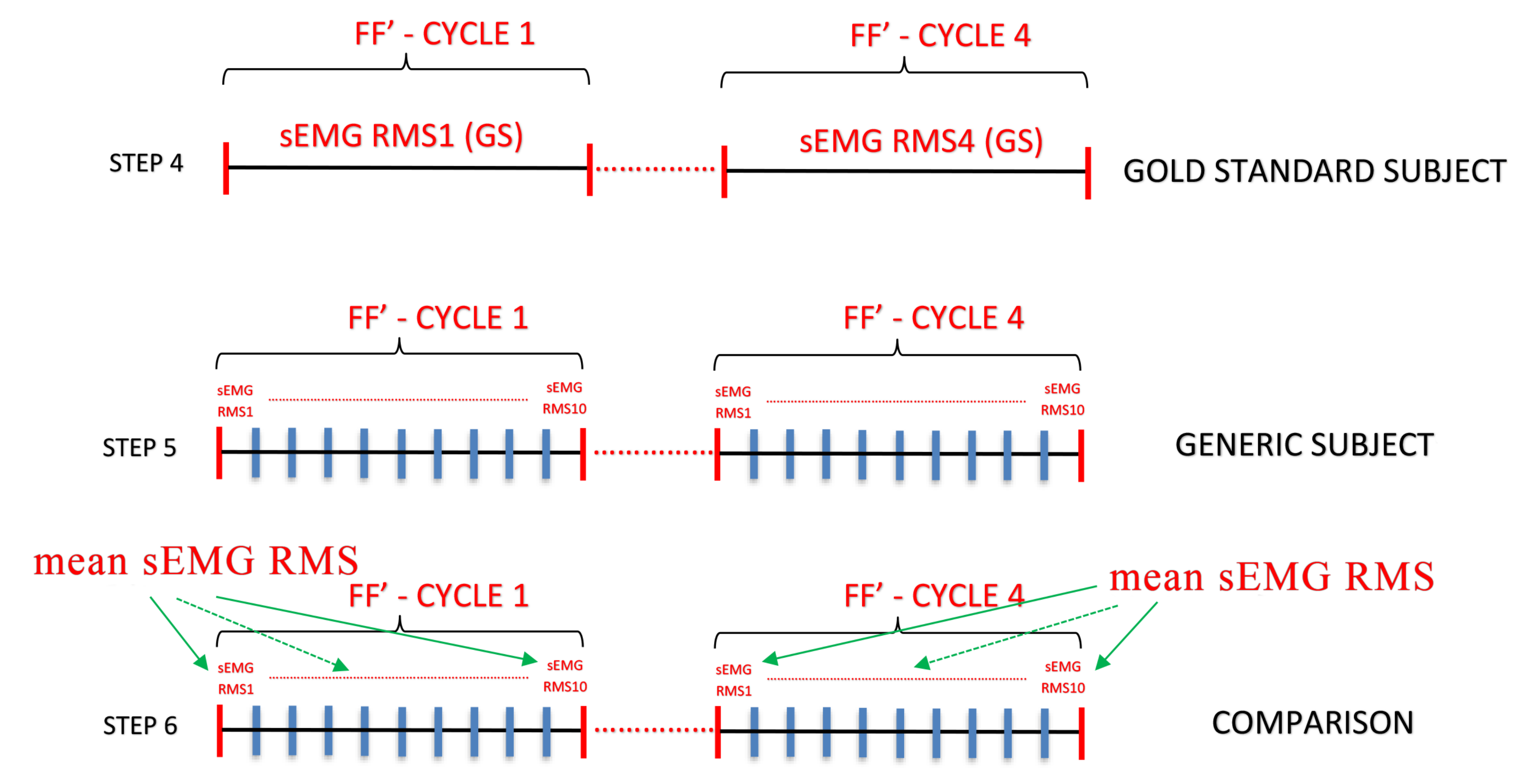
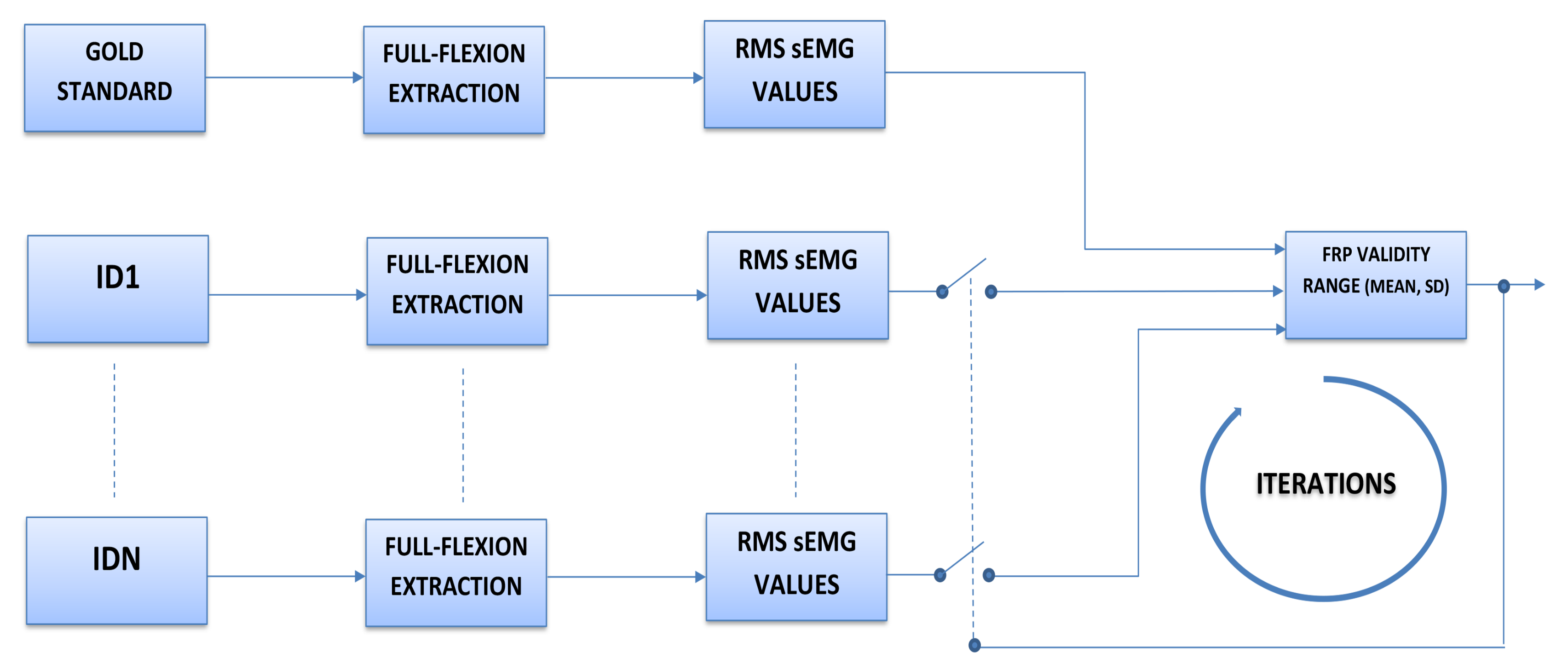
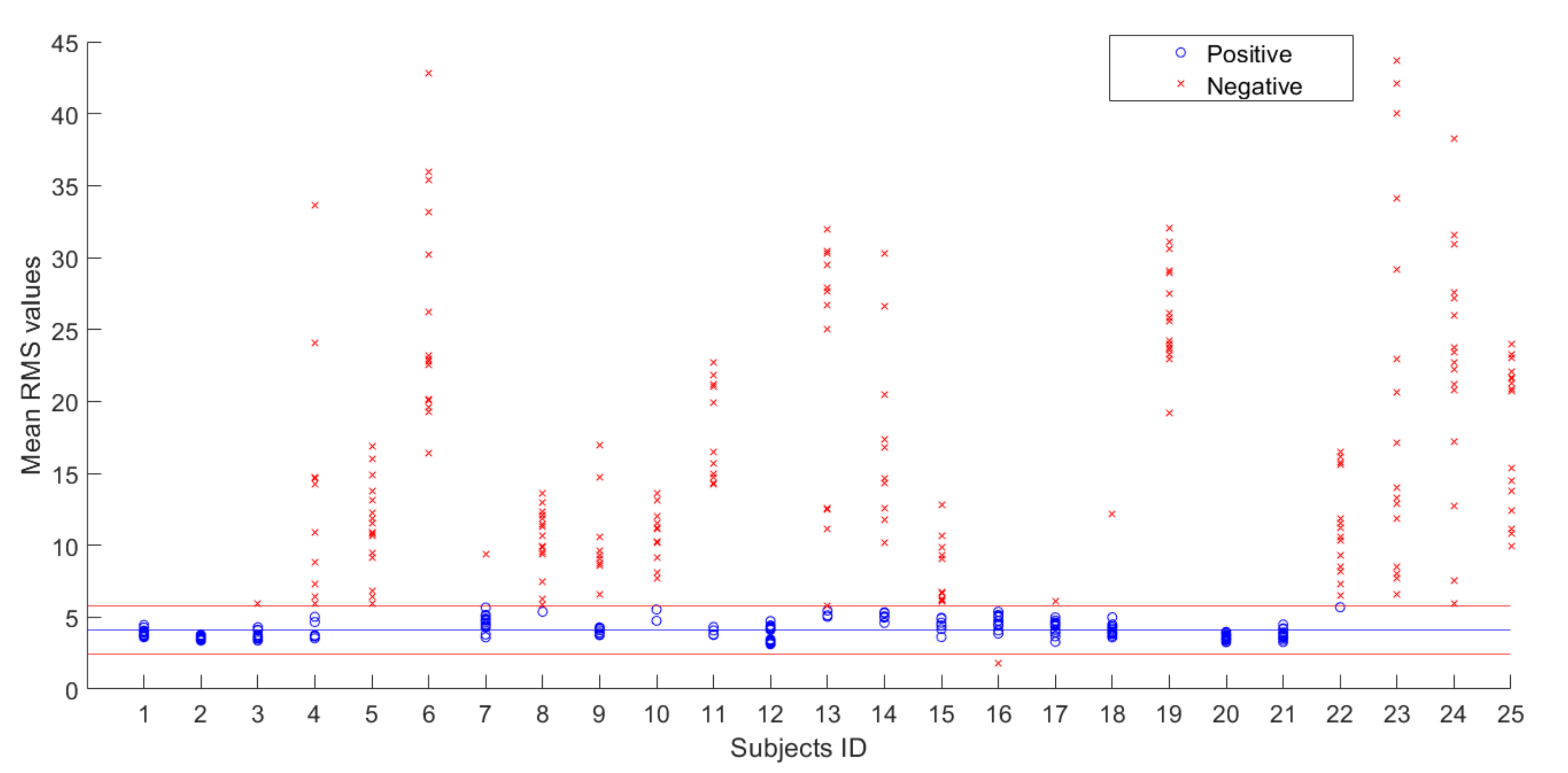
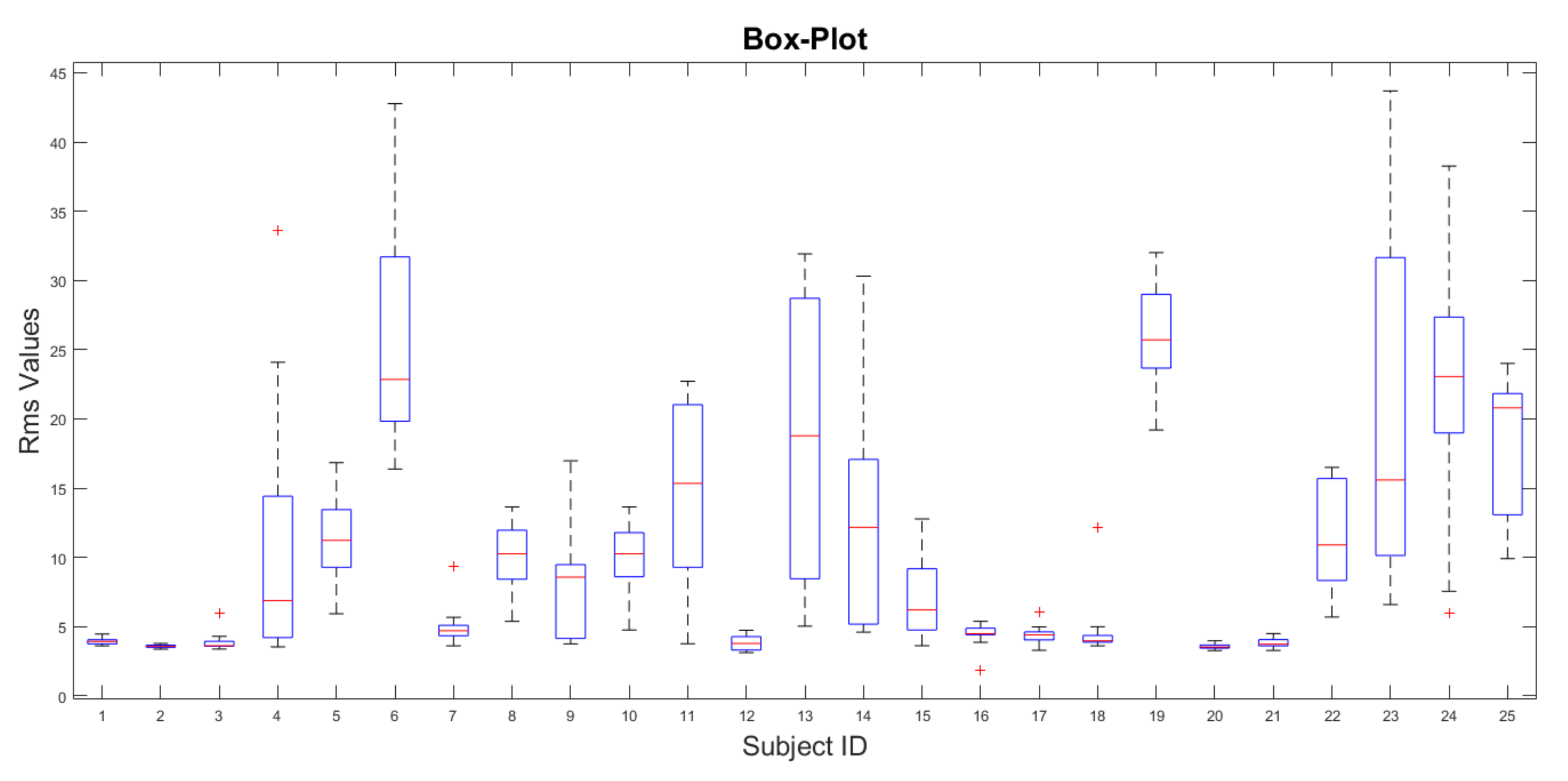
5. Results
- If the value is inside the range, it is considered as FRP positive
- If the value is outside the range, it is considered FRP negative, as reported in Table 2.
- ID6, ID8, ID22, and ID25 were not correctly identified as controls (they had no LBP, but also did not show FRP). Therefore, nine control subjects were correctly identified from a total of 13 control subjects (69.2%, against 83% found by Neblet et al. [22]).
- ID1 was not correctly identified as an LBP patient (he had LBP, but showed FRP). Therefore, eleven patients were correctly identified from a total of 12 LBP subjects (92%, against 79% found by Neblet et al. [22]).
6. Discussions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McGorry, R.W.; Lin, J.H. Flexion relaxation and its relation to pain and function over the duration of a back pain episode. PLoS ONE 2012, 7, e39207. [Google Scholar] [CrossRef]
- Oddsson, L.I.; De Luca, C.J. Activation imbalances in lumbar spine muscles in the presence of chronic low back pain. J. Appl. Physiol. 2003, 94, 1410–1420. [Google Scholar] [CrossRef]
- Kaplanis, P.; Pattichis, C.S.; Hadjileontiadis, L.; Roberts, V. Surface EMG analysis on normal subjects based on isometric voluntary contraction. J. Electromyogr. Kinesiol. 2009, 19, 157–171. [Google Scholar] [CrossRef]
- Jv, B.; De Luca, C. Muscles Alive: Their Functions Revealed by Electromyography. Williams & Wilkins, Baltimore, pp 3946Belenkii V, Gurnkel, VS, Paltsev Y (1967) Elements of control of voluntary movements. Biozika 1967, 12, 135141Carlson. [Google Scholar]
- Wolf, S.L.; Nacht, M.; Kelly, J.L. EMG feedback training during dynamic movement for low back pain patients. Behav. Ther. 1982, 13, 395–406. [Google Scholar] [CrossRef]
- Marras, W.S.; Lavender, S.A.; Leurgans, S.E.; Rajulu, S.L.; Allread, S.W.G.; Fathallah, F.A.; Ferguson, S.A. The role of dynamic three-dimensional trunk motion in occupationally-related. Spine 1993, 18, 617–628. [Google Scholar] [CrossRef]
- Lund, J.P.; Donga, R.; Widmer, C.G.; Stohler, C.S. The pain-adaptation model: A discussion of the relationship between chronic musculoskeletal pain and motor activity. Can. J. Physiol. Pharmacol. 1991, 69, 683–694. [Google Scholar] [CrossRef]
- Ahern, D.K.; Follick, M.J.; Council, J.R.; Laser-Wolston, N.; Litchman, H. Comparison of lumbar paravertebral EMG patterns in chronic low back pain patients and non-patient controls. Pain 1988, 34, 153–160. [Google Scholar] [CrossRef]
- Andersson, G.; Ortengren, R.; Herberts, P. Quantitative electromyographic studies of back muscle activity relatated to posture and loading. Orthop. Clin. N. Am. 1977, 8, 85–96. [Google Scholar]
- Dolce, J.J.; Raczynski, J.M. Neuromuscular activity and electromyography in painful backs: Psychological and biomechanical models in assessment and treatment. Psychol. Bull. 1985, 97, 502. [Google Scholar] [CrossRef]
- Floyd, W.; Silver, P. The function of the erectores spinae muscles in certain movements and postures in man. J. Physiol. 1955, 129, 184. [Google Scholar] [CrossRef]
- Larivière, C.; Gagnon, D.; Loisel, P. The comparison of trunk muscles EMG activation between subjects with and without chronic low back pain during flexion–extension and lateral bending tasks. J. Electromyogr. Kinesiol. 2000, 10, 79–91. [Google Scholar] [CrossRef]
- Sihvonen, T.; Partanen, J.; Hänninen, O.; Soimakallio, S. Electric behavior of low back muscles during lumbar pelvic rhythm in low back pain patients and healthy controls. Arch. Phys. Med. Rehabil. 1991, 72, 1080–1087. [Google Scholar]
- Triano, J.J.; Schultz, A.B. Correlation of objective measure of trunk motion and muscle function with low-back disability ratings. Spine 1987, 12, 561–565. [Google Scholar] [CrossRef]
- Nouwen, A.; Van, P.A.; Versloot, J.M. Patterns of muscular activity during movement in patients with chronic low-back pain. Spine 1987, 12, 777–782. [Google Scholar] [CrossRef]
- Roland, M. A critical review of the evidence for a pain-spasm-pain cycle in spinal disorders. Clin. Biomech. 1986, 1, 102–109. [Google Scholar] [CrossRef]
- Wolf, S.; Basmajian, J. Assessment of paraspinal electromyographic activity in normal subjects and in chronic back pain patients using a muscle biofeedback device. Int. Ser. Biomech. VIB 1978, 6B, 319–324. [Google Scholar]
- Lehman, G.J.; McGill, S.M. The importance of normalization in the interpretation of surface electromyography: A proof of principle. J. Manip. Physiol. Ther. 1999, 22, 444–446. [Google Scholar] [CrossRef]
- Halim, H.N.A.; Azaman, A.; Manaf, H.; Saidin, S.; Zulkapri, I.; Yahya, A. Gait Asymmetry Assessment using Muscle Activity Signal: A Review of Current Methods. J. Phys. Conf. Ser. 2019, 1372, 012075. [Google Scholar] [CrossRef]
- Paoletti, M.; Belli, A.; Palma, L.; Paniccia, M.; Tombolini, F.; Ruggiero, A.; Vallasciani, M.; Pierleoni, P. Data acquired by wearable sensors for the evaluation of the flexion-relaxation phenomenon. Data Brief 2020, 31, 105957. [Google Scholar] [CrossRef]
- Paoletti, M.; Belli, A.; Palma, L.; Vallasciani, M.; Pierleoni, P. A Wireless Body Sensor Network for Clinical Assessment of the Flexion-Relaxation Phenomenon. Electronics 2020, 9, 1044. [Google Scholar] [CrossRef]
- Neblett, R.; Brede, E.; Mayer, T.G.; Gatchel, R.J. What is the best surface EMG measure of lumbar flexion-relaxation for distinguishing chronic low back pain patients from pain-free controls? Clin. J. Pain 2013, 29, 334. [Google Scholar] [CrossRef]
- Neblett, R.; Mayer, T.G.; Gatchel, R.J.; Keeley, J.; Proctor, T.; Anagnostis, C. Quantifying the lumbar flexion–relaxation phenomenon: Theory, normative data, and clinical applications. Spine 2003, 28, 1435–1446. [Google Scholar] [CrossRef]
- Schinkel-Ivy, A.; Nairn, B.C.; Drake, J.D. Evaluation of methods for the quantification of the flexion-relaxation phenomenon in the lumbar erector spinae muscles. J. Manip. Physiol. Ther. 2013, 36, 349–358. [Google Scholar] [CrossRef]
- Neblett, R.; Mayer, T.G.; Brede, E.; Gatchel, R.J. Correcting abnormal flexion-relaxation in chronic lumbar pain: Responsiveness to a new biofeedback training protocol. Clin. J. Pain 2010, 26, 403. [Google Scholar] [CrossRef]
- Sella, G.E. Muscles in Motion: The SEMG of the ROM of the Human Body; GENMED Pub.: Martins Ferry, OH, USA, 2002. [Google Scholar]
- Nord, S.; Ettare, D.; Drew, D.; Hodge, S. Muscle learning therapy—Efficacy of a biofeedback based protocol in treating work-related upper extremity disorders. J. Occup. Rehabil. 2001, 11, 23–31. [Google Scholar] [CrossRef]
- Peper, E.; Gibney, K.H. Healthy Computing with Muscle Biofeedback: A Practical Manual for Preventing Repetitive Motion Injury; Biofeedback Foundation of Europe: Woerden, The Netherlands, 2000. [Google Scholar]
- Redfern, M.S.; Hughes, R.E.; Chaffin, D.B. High-pass filtering to remove electrocardiographic interference from torso EMG recordings. Clin. Biomech. 1993, 8, 44–48. [Google Scholar] [CrossRef]
- Ritvanen, T.; Zaproudina, N.; Nissen, M.; Leinonen, V.; Hänninen, O. Dynamic surface electromyographic responses in chronic low back pain treated by traditional bone setting and conventional physical therapy. J. Manip. Physiol. Ther. 2007, 30, 31–37. [Google Scholar] [CrossRef]
- Sánchez-Zuriaga, D.; López-Pascual, J.; Garrido-Jaén, D.; García-Mas, M.A. A comparison of lumbopelvic motion patterns and erector spinae behavior between asymptomatic subjects and patients with recurrent low back pain during pain-free periods. J. Manip. Physiol. Ther. 2015, 38, 130–137. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Lowery, M.; Stoykov, N. The effect of subcutaneous fat on myoelectric signal amplitude and cross-talk. Prosthet. Orthot. Int. 2003, 27, 48–54. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European recommendations for surface electromyography. Roessingh Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Nougarou, F.; Massicotte, D.; Descarreaux, M. Detection method of flexion relaxation phenomenon based on wavelets for patients with low back pain. EURASIP J. Adv. Signal Process. 2012, 2012, 151. [Google Scholar] [CrossRef]
- Paoletti, M.; Belli, A.; Palma, L.; Paniccia, M.; Tombolini, F.; Ruggiero, A.; Vallasciani, M.; Pierleoni, P. Dataset for clinical assessment of flexion-relaxation phenomenon. Mendeley Data 2020, 1. [Google Scholar] [CrossRef]
- Sihvonen, T.; Partanen, J.; Hanninen, O. Averaged (rms) surface EMG in testing back function. Electromyogr Clin. Neurophysiol. 1988, 28, 335–339. [Google Scholar]


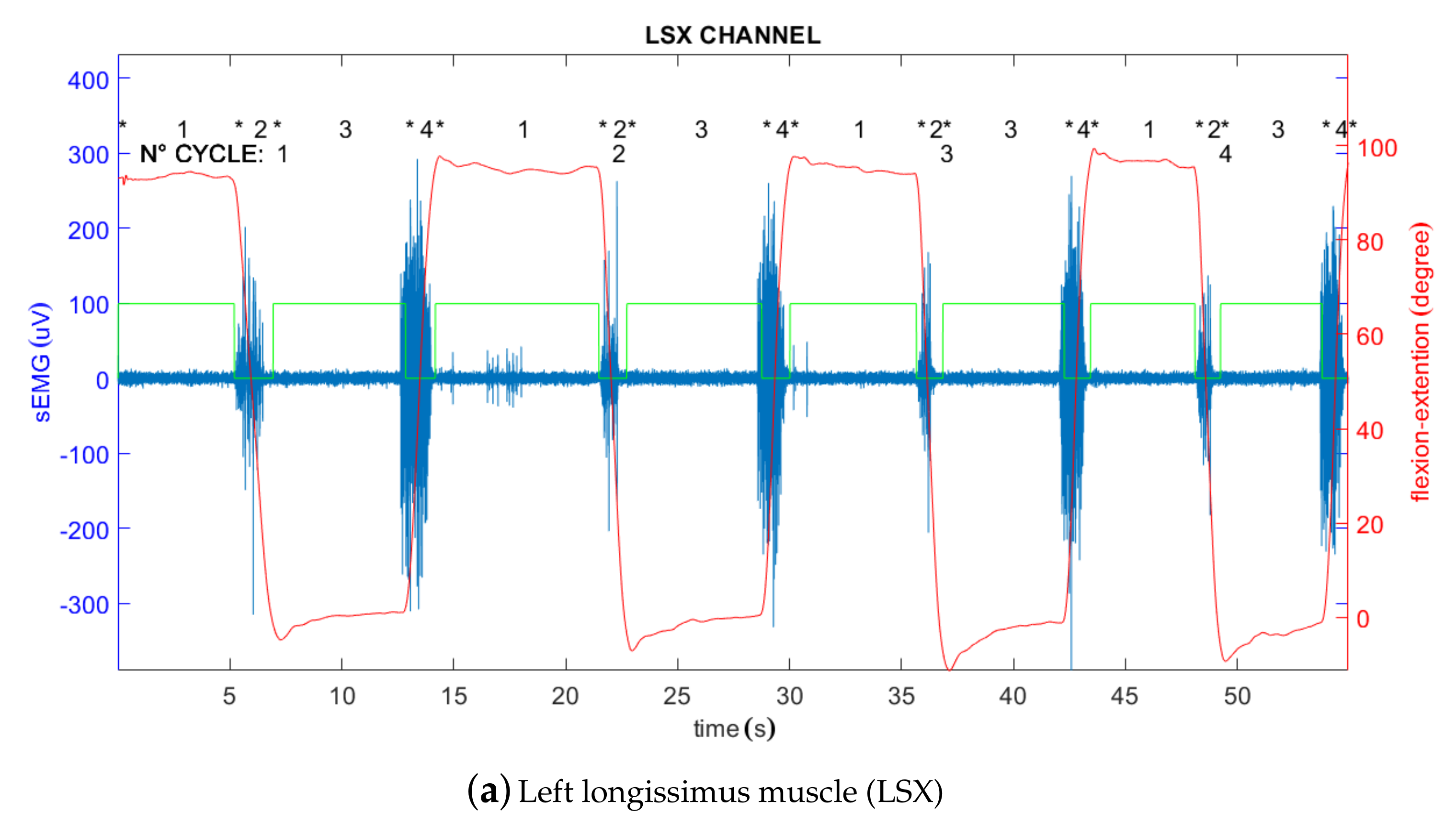
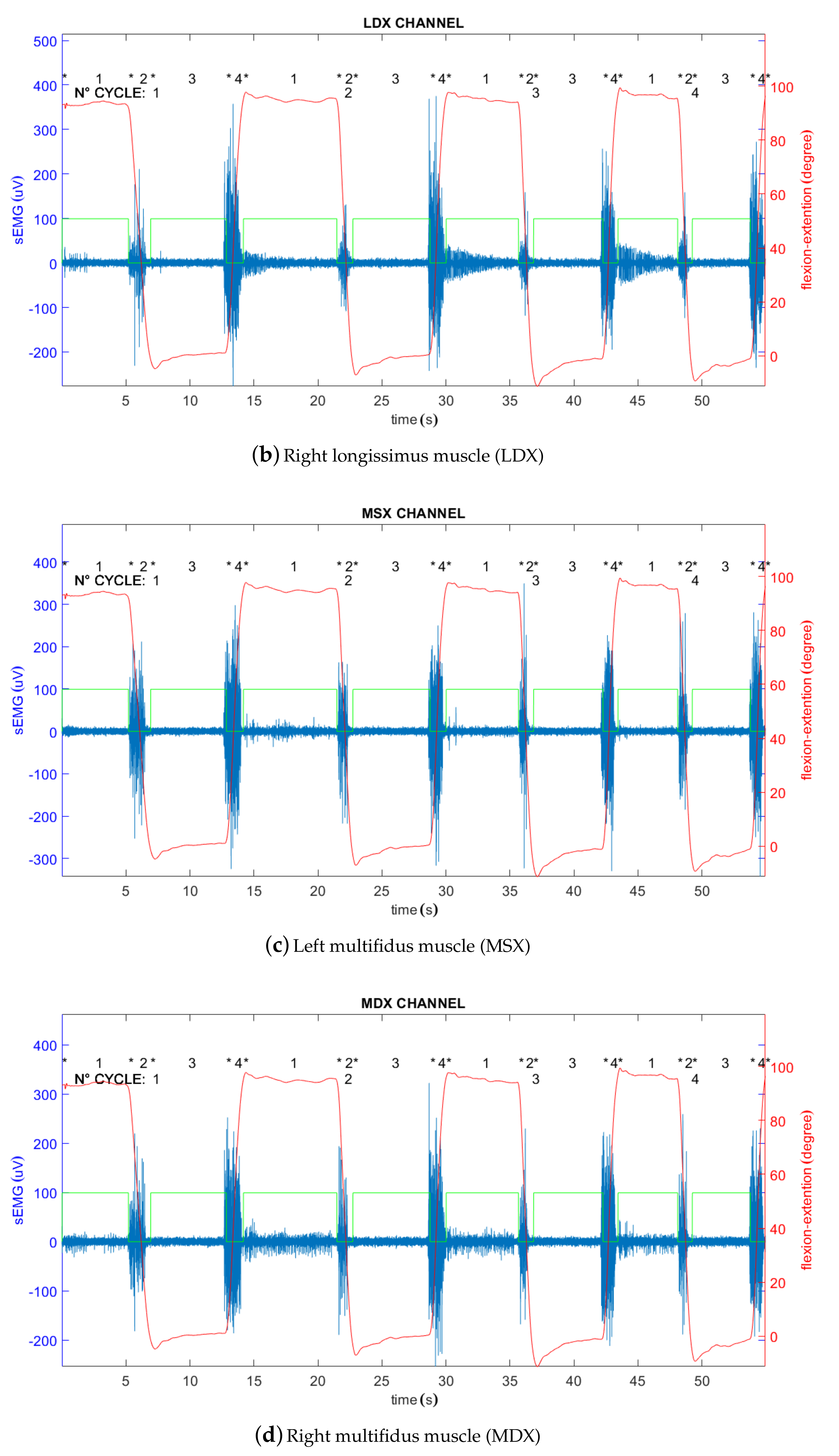

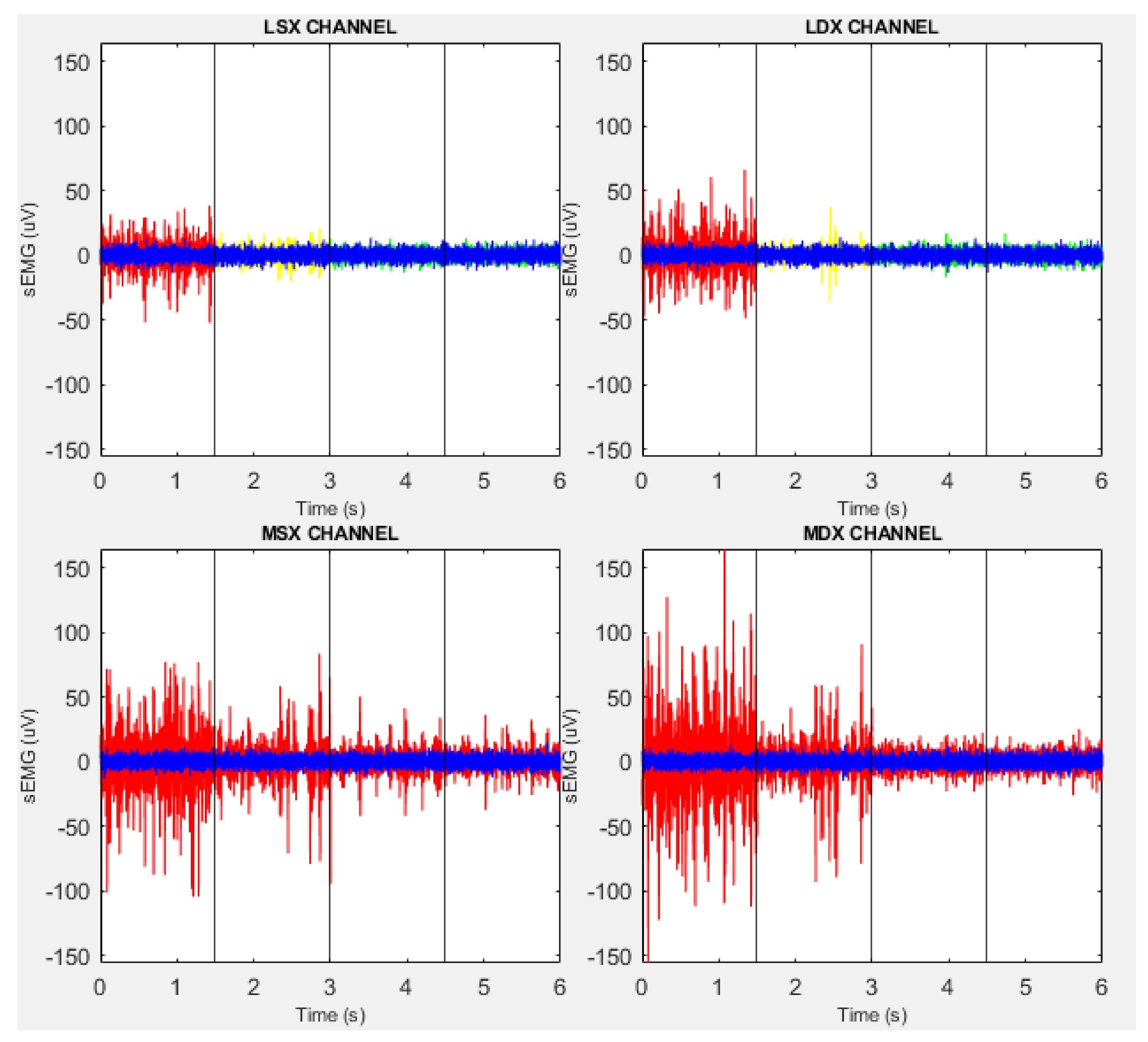
| Subject ID | RMS (LSX) | RMS (LDX) | RMS (MSX) | RMS (MDX) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 1 | 3.79 | 3.80 | 3.93 | 3.92 | 3.71 | 4.06 | 4.28 | 4.29 | 4.46 | 3.65 | 3.61 | 3.64 | 3.99 | 3.78 | 4.04 | 4.02 |
| 2 | 3.59 | 3.68 | 3.55 | 3.67 | 3.52 | 3.62 | 3.46 | 3.58 | 3.51 | 3.43 | 3.55 | 3.73 | 3.37 | 3.64 | 3.50 | 3.78 |
| 3 | 3.60 | 3.77 | 3.49 | 3.68 | 3.64 | 3.77 | 3.59 | 3.38 | 4.09 | 4.30 | 5.98 | 4.13 | 3.56 | 3.59 | 3.58 | 3.55 |
| 4 | 10.88 | 5.03 | 3.66 | 3.53 | 14.71 | 4.66 | 3.71 | 3.74 | 24.09 | 14.22 | 8.81 | 7.30 | 33.62 | 14.62 | 6.44 | 5.92 |
| 5 | 10.90 | 10.67 | 9.44 | 6.46 | 13.12 | 13.77 | 10.84 | 5.93 | 12.27 | 11.84 | 11.56 | 6.84 | 15.97 | 16.84 | 14.86 | 9.11 |
| 6 | 33.18 | 35.37 | 42.77 | 35.95 | 19.56 | 20.15 | 22.53 | 22.78 | 22.92 | 20.10 | 30.23 | 26.20 | 16.37 | 19.56 | 23.17 | 19.28 |
| 7 | 5.10 | 3.79 | 3.60 | 3.60 | 5.67 | 5.16 | 4.63 | 5.08 | 9.38 | 4.84 | 4.90 | 4.48 | 4.77 | 4.38 | 4.29 | 4.48 |
| 8 | 5.90 | 5.38 | 7.45 | 6.28 | 9.90 | 9.56 | 9.87 | 9.40 | 10.62 | 11.30 | 13.64 | 11.47 | 13.00 | 12.07 | 12.29 | 11.85 |
| 9 | 8.75 | 3.75 | 3.93 | 3.82 | 8.57 | 4.20 | 4.08 | 4.29 | 16.97 | 9.63 | 9.08 | 6.58 | 14.71 | 9.32 | 10.61 | 8.56 |
| 10 | 12.01 | 11.14 | 11.19 | 7.67 | 9.11 | 9.13 | 10.18 | 5.53 | 13.12 | 13.64 | 12.02 | 8.08 | 10.23 | 11.56 | 10.29 | 4.75 |
| 11 | 14.69 | 15 | 14.30 | 3.76 | 16.5 | 14.23 | 15.69 | 4.08 | 21.05 | 22.72 | 19.91 | 3.81 | 21.19 | 21.80 | 21 | 4.32 |
| 12 | 3.41 | 3.40 | 3.33 | 3.45 | 4.73 | 4.43 | 4.28 | 4.12 | 3.29 | 3.12 | 3.26 | 3.20 | 4.22 | 4.20 | 4.27 | 4.37 |
| 13 | 12.48 | 12.57 | 11.12 | 12.53 | 5.79 | 5.13 | 5.46 | 5.03 | 26.68 | 24.98 | 27.62 | 30.26 | 30.43 | 27.92 | 31.92 | 29.50 |
| 14 | 11.75 | 5.34 | 4.97 | 5 | 10.17 | 5.28 | 5.06 | 4.60 | 30.31 | 20.48 | 16.79 | 14.36 | 26.62 | 17.36 | 14.67 | 12.58 |
| 15 | 10.64 | 4.96 | 6.24 | 4.17 | 6.17 | 4.61 | 4.42 | 3.62 | 12.77 | 9.08 | 9.26 | 6.71 | 9.87 | 6.63 | 6.10 | 4.90 |
| 16 | 4.47 | 5.38 | 4.40 | 5.16 | 4.45 | 1.83 | 3.86 | 4.49 | 4.08 | 4.76 | 4.42 | 4.47 | 4.74 | 5.00 | 4.53 | 5.08 |
| 17 | 4.48 | 3.29 | 4.13 | 4.11 | 6.08 | 4.60 | 4.98 | 4.77 | 3.93 | 3.67 | 4.37 | 4.14 | 4.63 | 3.96 | 4.56 | 4.43 |
| 18 | 3.93 | 3.60 | 3.83 | 3.71 | 3.85 | 3.94 | 4.00 | 4.11 | 4.37 | 4.35 | 3.95 | 3.89 | 12.13 | 4.99 | 4.50 | 4.23 |
| 19 | 29.06 | 25.61 | 22.97 | 23.56 | 27.52 | 26.15 | 23.95 | 19.20 | 25.80 | 24.18 | 23.77 | 23.27 | 31.07 | 30.56 | 32.01 | 28.95 |
| 20 | 3.76 | 3.64 | 3.90 | 3.98 | 3.49 | 3.44 | 3.53 | 3.51 | 3.26 | 3.33 | 3.43 | 3.59 | 3.43 | 3.64 | 3.47 | 3.70 |
| 21 | 4.18 | 3.90 | 3.64 | 3.42 | 3.82 | 3.74 | 3.62 | 3.68 | 3.94 | 3.72 | 4.19 | 4.49 | 3.27 | 3.60 | 4.22 | 3.55 |
| 22 | 15.78 | 15.60 | 16.50 | 8.50 | 11.87 | 11.21 | 11.55 | 5.69 | 16.12 | 10.35 | 10.58 | 7.28 | 15.78 | 9.31 | 8.15 | 6.50 |
| 23 | 7.69 | 8.46 | 8.05 | 6.59 | 29.20 | 20.66 | 22.97 | 17.14 | 34.10 | 42.09 | 40.04 | 43.69 | 11.81 | 14.04 | 13.25 | 12.89 |
| 24 | 23.45 | 21.19 | 22.19 | 20.79 | 26.00 | 30.90 | 27.13 | 22.67 | 31.54 | 38.26 | 27.56 | 23.73 | 12.70 | 17.17 | 7.55 | 5.97 |
| 25 | 12.40 | 10.78 | 9.91 | 11.16 | 20.90 | 21.24 | 22.98 | 24.01 | 20.71 | 21.64 | 21.60 | 23.24 | 22.03 | 15.39 | 13.75 | 14.51 |
| Subject ID | SEX | AGE | GROUP | LSX | LDX | MSX | MDX | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||||
| 1 | F | 51 | LBP | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P |
| 2 | F | 40 | HEALTHY (GOLD STANDARD) | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P |
| 3 | F | 34 | HEALTHY | P | P | P | P | P | P | P | P | P | P | N | P | P | P | P | P |
| 4 | M | 57 | LBP | N | P | P | P | N | P | P | P | N | N | N | N | N | N | N | N |
| 5 | M | 30 | LBP | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 6 | M | 31 | HEALTHY | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 7 | M | 35 | HEALTHY | P | P | P | P | P | P | P | P | N | P | P | P | P | P | P | P |
| 8 | M | 25 | HEALTHY | N | P | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 9 | M | 58 | LBP | N | P | P | P | N | P | P | P | N | N | N | N | N | N | N | N |
| 10 | F | 52 | LBP | N | N | N | N | N | N | N | P | N | N | N | N | N | N | N | P |
| 11 | F | 46 | LBP | N | N | N | P | N | N | N | P | N | N | N | P | N | N | N | P |
| 12 | F | 40 | HEALTHY | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P |
| 13 | M | 49 | LBP | N | N | N | N | N | P | P | P | N | N | N | N | N | N | N | N |
| 14 | F | 49 | LBP | N | P | P | P | N | P | P | P | N | N | N | N | N | N | N | N |
| 15 | F | 51 | LBP | N | P | N | P | N | P | P | P | N | N | N | N | N | N | N | P |
| 16 | F | 60 | HEALTHY | P | P | P | P | P | N | P | P | P | P | P | P | P | P | P | P |
| 17 | F | 36 | HEALTHY | P | P | P | P | N | P | P | P | P | P | P | P | P | P | P | P |
| 18 | M | 22 | HEALTHY | P | P | P | P | P | P | P | P | P | P | P | P | N | P | P | P |
| 19 | M | 52 | LBP | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 20 | F | 22 | HEALTHY | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P |
| 21 | M | 60 | HEALTHY | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P | P |
| 22 | F | 51 | HEALTHY | N | N | N | N | N | N | N | P | N | N | N | N | N | N | N | N |
| 23 | M | 60 | LBP | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 24 | M | 61 | LBP | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| 25 | M | 52 | HEALTHY | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| GROUP | SAMPLES | RMS (MEAN) | RMS (STD) |
|---|---|---|---|
| POSITIVE | 190 | 4.09 | 0.58 |
| NEGATIVE | 210 | 16.68 | 8.60 |
| TP | TN | FP | FN | ACCURACY | SENSITIVITY | SPECIFICITY |
|---|---|---|---|---|---|---|
| 167 | 182 | 23 | 28 | 87% | 86% | 89% |
| TP | TN | FP | FN | ACCURACY | SENSITIVITY | SPECIFICITY |
|---|---|---|---|---|---|---|
| 9 | 11 | 1 | 4 | 80% | 69% | 92% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paoletti, M.; Belli, A.; Palma, L.; Pierleoni, P. Electromyography Pattern Likelihood Analysis for Flexion-Relaxation Phenomenon Evaluation. Electronics 2020, 9, 2046. https://doi.org/10.3390/electronics9122046
Paoletti M, Belli A, Palma L, Pierleoni P. Electromyography Pattern Likelihood Analysis for Flexion-Relaxation Phenomenon Evaluation. Electronics. 2020; 9(12):2046. https://doi.org/10.3390/electronics9122046
Chicago/Turabian StylePaoletti, Michele, Alberto Belli, Lorenzo Palma, and Paola Pierleoni. 2020. "Electromyography Pattern Likelihood Analysis for Flexion-Relaxation Phenomenon Evaluation" Electronics 9, no. 12: 2046. https://doi.org/10.3390/electronics9122046
APA StylePaoletti, M., Belli, A., Palma, L., & Pierleoni, P. (2020). Electromyography Pattern Likelihood Analysis for Flexion-Relaxation Phenomenon Evaluation. Electronics, 9(12), 2046. https://doi.org/10.3390/electronics9122046








