1. Introduction
Since their inception in 1984 [
1], organic electrochemical transistors (OECTs) have emerged as a versatile platform for chemical and biological sensing, neuromorphic computing, and neural interfacing, owing to their unique advantages, including mechanical flexibility, outstanding bio-compatibility, high transconductance, large on/off ratio, and low operating voltage [
2]. These characteristics make OECTs particularly well suited for biosensing applications where both sensitivity and real-time signal processing are critical. A typical OECT consists of a poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT: PSS) channel and an ion-rich electrolyte layer. During operation, the application of a positive gate voltage (
) induces an electric field between the gate electrode and the semiconductor channel, promoting the migration of positively charged ions from the electrolyte into the conducting polymer layer. These cations compensate for the sulfonate anions of PSS, resulting in electrochemical doping of the channel. Conversely, when the gate voltage decreases or a negative bias is applied, the cations are expelled from the semiconductor layer, leading to dedoping. Notably, these doping/dedoping processes occur volumetrically throughout the entire channel rather than being confined to an interface, thereby enhancing transconductance to an exceptionally high level. This property enables OECTs to achieve remarkable sensitivity, making them ideal for a wide range of bioelectronic applications. Owing to these advantages, OECTs have been widely employed in the development of high-performance ion sensors [
3,
4] and pH sensors [
5], as well as sensors for oxygen [
6], glucose [
7], and DNA detection [
8]. Their ability to transduce biochemical interactions into highly amplified electrical signals continues to drive innovation in real-time physiological monitoring and lab-on-chip technologies.
In biomedical applications, salivary biomarker monitoring has emerged as a non-invasive, convenient, and effective approach for assessing human health. Saliva-based diagnostics are increasingly recognized for their practicality in detecting early-stage oral diseases [
9,
10,
11], electrolyte imbalances, dehydration, cardiovascular disorders, and metabolic conditions [
12,
13]. Compared to traditional blood tests, saliva sampling offers advantages such as ease of collection, reduced discomfort, and the potential for real-time, on-site health monitoring. Saliva is predominantly composed of water, accounting for approximately 99% of its total volume [
14]. The typical concentration ranges of key electrolytes in human saliva include potassium ions (K
+) at 3.4–21 mM, sodium ions (Na
+) at 0.9–13.7 mM, and calcium ions (Ca
2+) at 0.69–2.45 mM [
15,
16,
17]. In addition to these essential electrolytes, saliva also contains a variety of organic molecules, such as uric acid and glucose, as well as inorganic ions, including chloride and phosphate [
12]. The dynamic composition of saliva makes it an excellent medium for detecting physiological changes, although its complexity poses challenges in sensor design and signal processing [
18]. To address these challenges, prior studies achieved ion detection over wide concentration ranges [
19], and existing research on OECT-based sensors has demonstrated exceptional performance in ion sensing applications (
Supplementary Materials Table S1) [
20,
21,
22,
23,
24]. Despite these advancements, three key limitations hinder the clinical translation of existing OECT-based systems: (1) Miniaturization: While prior studies achieved miniaturization of sensors [
25], integrated systems combining sensors with compact circuits remain rare [
26,
27,
28], limiting portability for wearable applications [
29]. (2) Cross-interference in multi-ion detection: Current OECT sensors primarily focus on single-ion analysis [
3,
4,
5,
6], with limited strategies to address signal overlap in complex biofluids like saliva. (3) Incomplete data processing frameworks: Although machine learning has been proposed for signal enhancement [
30,
31,
32], its integration with real-time hardware for on-device analytics is underexplored.
In this study, we present a multi-ion sensing system based on OECTs, designed for real-time, simultaneous detection and continuous monitoring of Na+, K+, and Ca2+ ions in human saliva. This next-generation biosensing platform integrates hardware miniaturization, high-performance sensing, and advanced data analytics to enhance its practicality for physiological monitoring applications. Several key optimizations have been implemented to improve the system’s performance and usability. First, building upon prior research, the sensor’s form factor has been significantly reduced while preserving its mechanical flexibility, high sensitivity, excellent selectivity, and repeatability within a physiologically relevant detection range. Second, an innovative microcircuit design has been employed to minimize the overall physical dimensions of the system, enhancing its portability and user convenience, making it well suited for wearable and point-of-care applications. Third, machine learning algorithms have been integrated to improve detection accuracy and mitigate cross-interference, thereby increasing the system’s robustness in complex biological environments. Fourth, extending beyond previous studies, the entire system—comprising the detection circuit and a dedicated mobile application—is seamlessly integrated with the trained machine learning models. This enables real-time data processing, allowing test results to be directly analyzed and interpreted through the app, enhancing accessibility and user experience. By combining advanced OECT-based sensing technology with intelligent data analysis, this system demonstrates significant potential for non-invasive, real-time physiological monitoring, paving the way for next-generation personalized health diagnostics and continuous electrolyte assessment.
2. Materials and Methods
2.1. Materials
PEDOT: PSS: The PEDOT: PSS blend was prepared by adding 50 µL of ethylene glycol (Sigma, St. Louis, MO, USA), 10 µL of (3-glycidyloxypropyl) trimethoxysilane (TCI, Shanghai, China) cross-linking agent, and 1 mL of a stock Clevios PH 1000 PEDOT: PSS solution (Heraeus, Hanau, Germany).
Ion-selective membrane (ISM): The polymeric matrix consists of 18.7 mg of poly (vinyl chloride) (26.8 wt%) (Aladdin) and 49 mg of 2-nitrophenyl octyl ether (66.7 wt%) (Aladdin, Shanghai, China) as a plasticizer. Potassium ionophore III (Macklin, Shanghai, China), calcium ionophore II (Macklin), and sodium ionophore X (Macklin), with a concentration of 3 mg (6.5 wt%) in all cases, were used as ionophores. Tetrahydrofuran (100 mg/mL) (Aladdin) was used to dissolve all the components. These ISM cocktails are stored in a refrigerator at 6 °C.
All chemicals below were purchased from Macklin and were used as received: calcium chloride (CaCl2, 96%), sodium chloride (NaCl, 99.5%), potassium chloride (KCl, 99.5%).
2.2. Sensor Array Preparation
The fabrication process of the sensor array is illustrated in
Supplementary Materials Figure S1. First, electrodes (10 nm Ti and 100 nm Au) were deposited on a polyethylene naphthalate (PEN) substrate using thermal evaporation. The channel area had a length of 100 µm and a width of 1000 µm. The gate electrode was circular with a radius of 1500 µm. Then, a patterned layer of PDMS is fabricated above the electrode layer, with PDMS encapsulating the metal wiring while leaving the semiconductor and gate regions exposed. Subsequently, 0.025 µL of the prepared PEDOT: PSS solution was drop-cast between the source and drain electrodes, ensuring full coverage, and the film was then dried on a hotplate at 120 °C for 30 min. The thickness of the PEDOT: PSS film was measured as 600 nm using a stylus profiler. Before functionalization, the channel was conditioned in a 10
−2 M XCl solution (where X corresponds to the cation, either Na
+, K
+, or Ca
2+) for 2 h. The device array was then removed and thoroughly rinsed with deionized water before being dried. A total of 0.8 µL of the ion-selective membrane cocktail was drop-cast onto the region above the semiconductor channel, filling the empty spaces in the PDMS region. The thickness of the ISM film was measured as 10 μm. The sample was then dried at ambient temperature overnight.
2.3. Electrochemical Measurements
To accurately evaluate the sensor performance, a semiconductor analyzer (PDA-FS380, Precision Systems Industrial Limited, Suzhou, China) was used for testing. Before testing, a series of concentration gradients of Na+, K+, and Ca2+ solutions were prepared. Three different types of sensors were sequentially placed in solutions with varying concentrations and types. After 5 s of settling, the transfer characteristics were tested, with a voltage scan range from −0.5 V to 1.4 V. After 10 s of settling, the current–time curve was measured, with a total testing time of 300 s. The source–drain voltage () was fixed at −0.6 V. No gate voltage was applied during the first 5 s to obtain the initial current of the sensor. After testing, the sensor was cleaned, and the solution was replaced for subsequent measurements until all samples were tested.
2.4. Machine Learning
A database was established based on the test data obtained from the OECT sensor array and the dataset contains 264 samples. The input layer of the model comprised 3383 nodes, representing sensor feature data, while the output was configured to simultaneously predict the ionic species and their corresponding concentrations in the analyte solution. For concentration regression, a random forest model was implemented in PyCharm, with parameter optimization performed to enhance its performance. The optimized parameters for each sensor model are as follows: sodium ion sensor model (n_estimators = 14, max_depth = 25); potassium ion sensor model (n_estimators = 46, max_depth = 25); calcium ion sensor model (n_estimators = 40, max_depth = 25). And the dataset was divided into 70% training and 30% test sets. A multi-layer perceptron (MLP) model was used to predict the accuracy of the sensor’s ion type classification. This model included an input layer with 3383 nodes, followed by two hidden layers: the first with 512 neurons using the rectified linear unit (ReLU) activation function and the second with 128 neurons, also employing ReLU. The output layer contained a single node with a Sigmoid activation function to predict the probability of binary classification. The model was trained with the following hyperparameters: a batch size of 4; an initial learning rate of 0.001 utilizing the ReduceLROnPlateau scheduler to monitor the loss and dynamically adjust the learning rate (with a patience of 10 epochs and a factor of 0.5); and a total of 500 epochs. The binary cross-entropy loss (BCELoss) combined with the Adam optimizer was employed to regulate both the learning rate and gradient updates. The dataset was divided into 60% training and 40% testing sets. Finally, the trained model was exported.
2.5. Design and Evaluation of the Circuit Module
The core of the module is an STM32F103RCT6 microcontroller, powered by a battery. The module can output three voltage signals, generated by two Digital-to-Analog Converters (DACs) built into the STM32 and one DAC chip controlled by STM32. These voltages serve as the sensor drain voltage and gate voltage, with a voltage follower to enhance the DAC output current. The current signal is converted to a voltage signal using a trans-impedance amplifier. The microcontroller’s ADC has a 12-bit resolution, with an input voltage range of 0–3.3 V. The circuit uses a 200 Ω feedback resistor and can collect current data up to 16.5 mA with a sampling precision of 4 µA at a sampling frequency of 1000 Hz. The circuit incorporates a Bluetooth module for wireless data transmission, enabling real-time visualization and monitoring through a dedicated mobile application. The data can also be exported in a tabular format.
3. Results and Discussion
3.1. OECT Sensor Array Design
The fabrication process of the OECT sensor array is described in detail in the Materials and Methods section. As illustrated in
Figure 1a, the flexible sensor array comprises three sensing units with a shared gate electrode, enabling a compact design that enhances spatial efficiency and signal consistency for wearable and portable biosensing applications. The selectivity of OECT-based ion sensors is achieved through the incorporation of ion-selective membranes (ISMs). These membranes are engineered to selectively permit the transport of target ions from the surrounding solution into the OECT channel, thereby modulating the sensor’s electrical response [
33]. The ISM-coated OECTs effectively discriminate between different ionic species, ensuring high specificity and minimizing interference from non-target ions. This feature is critical for reliable multi-ion detection in complex biological environments, such as human saliva, where multiple electrolytes coexist at varying concentrations.
The detection mechanism of the OECT sensor is shown in
Figure 1b, where ion carriers in the ISM selectively transport target ions from the regions of high concentration to those of low concentration and create an interface potential. This process generates an interface potential, which in turn affects the equivalent gate voltage of the OECT and consequently alters the channel current [
34,
35,
36]. The potential at the analyte/membrane interface is proportional to the analyte concentration, as described by the Nernst equation [
37,
38]:
where
and
represent the standard electrode potential and the concentration of the analyte ion, respectively.
k is Boltzmann’s constant,
T is the temperature,
n is the valency of the analyte ion, and
e is the elementary charge. As the ion concentration in the solution increases, more ions penetrate the ISM and enter the channel, while simultaneously enhancing the equivalent gate voltage. Both effects contribute to a reduction in channel current. Through the application of different ISM modification, the OECT sensors are capable of detecting Na
+, K
+, and Ca
2+, respectively. The connection between the sensors and the circuit module is implemented using flexible printed circuit (FPC) cables, while the circuit module itself is miniaturized to adapt to a wider range of application scenarios (
Figure 1c).
3.2. Current Response and Optimization
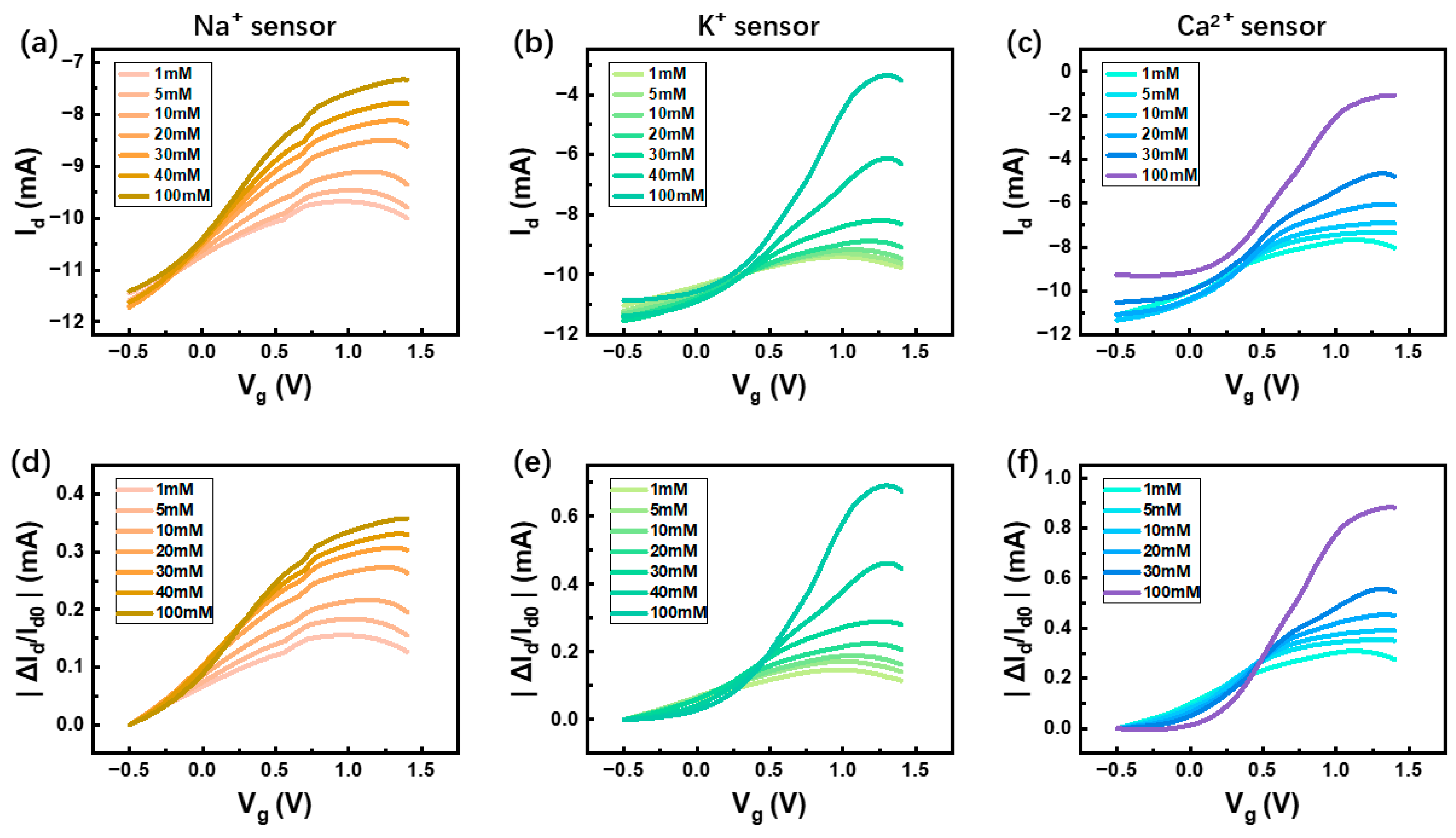
OECT-based sensors function by transducing variations in solution ion concentrations into measurable current responses. To systematically evaluate their performance, the transfer characteristics of the three ion-selective OECTs were analyzed across a range of analyte concentrations, as depicted in
Figure 2a–c. The results demonstrate a consistent trend across all three sensors: as the concentration of target ions in the test environment increases, the drain current (
) decreases, indicative of electrochemical doping effects within the OECT channel. Additionally, an increase in both the on/off ratio and transconductance was observed (
Supplementary Materials Figure S2), reinforcing the device’s high sensitivity to ion fluctuations. Based on the concentration-dependent variations in
, the detection limits (LODs) were calculated (
Supplementary Materials Figure S3). Our sensor exhibits an LOD of 0.181 mM for sodium ions (Na
+), 4.92 μM for potassium ions (K
+), and 0.918 mM for calcium ions (Ca
2+). To address potential challenges associated with baseline drift during long-term measurements, a normalization strategy was implemented. By normalizing the sensor output, we effectively compensated for gradual performance degradation and baseline shifts, ensuring the reliability of the recorded data. Specifically, the relative current variation (
) exhibited a clear correlation with increasing ion concentrations, further enhancing the robustness of the sensing system. This data normalization technique was also integrated into the circuit module, contributing to improved real-time signal processing and accuracy of the overall detection system. Furthermore, a curve-fitting analysis of the device’s transfer characteristics (
Supplementary Materials Figure S4) revealed that the maximum transconductance of the sensor occurs at a gate voltage of 0.8 V, with a peak value of 10 mS. Based on this finding, 0.8 V was selected as the optimal operating voltage for subsequent time-dependent response measurements, ensuring stable and efficient sensor performance.
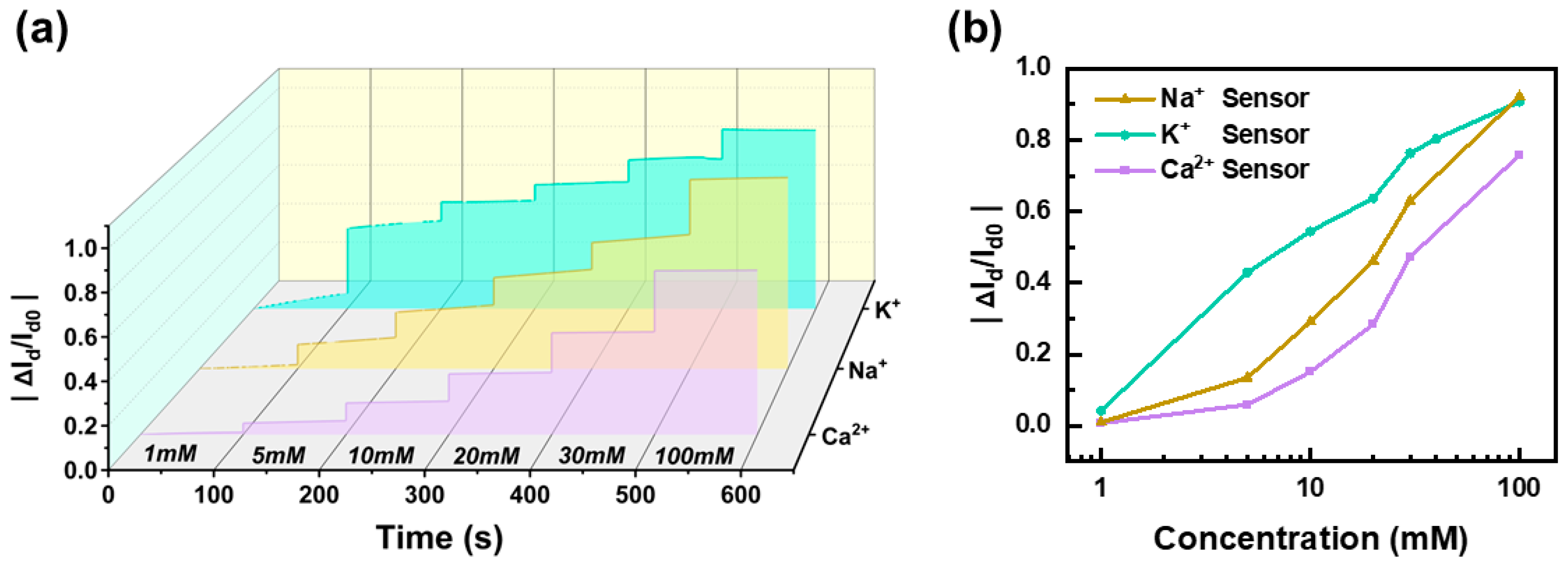
To evaluate the fundamental performance and stability of the OECT-based ion sensors, we conducted extended monitoring experiments across a range of analyte concentrations. The three sensors were immersed in seven concentration gradients (0 mM, 1 mM, 5 mM, 10 mM, 20 mM, 30 mM, and 100 mM) and continuously tested for 100 s at each concentration to assess their response consistency and reliability. Throughout the measurement period, all three sensors exhibited excellent stability, with no significant drift or signal degradation. As illustrated in
Figure 3a, the normalized current variation
increased proportionally with rising ion concentrations, indicating a clear and consistent response pattern. Further analysis in
Figure 3b reveals a linear correlation between the normalized current change and analyte concentration across the measured range, demonstrating the system’s robustness and suitability for quantitative ion detection. Moreover, the sensor array exhibited distinct sensitivity characteristics, highlighting its capability for differential ion detection. The Na
+ sensor demonstrated the highest sensitivity, with a slope of 0.47/dec, followed by the K
+ sensor at 0.43/dec, and the Ca
2+ sensor at 0.38/dec. These results confirm the high selectivity and precision of the OECT-based sensors in detecting physiologically relevant ion concentrations, making them well suited for real-time biofluid monitoring applications.
3.3. Selectivity and Interference Testing
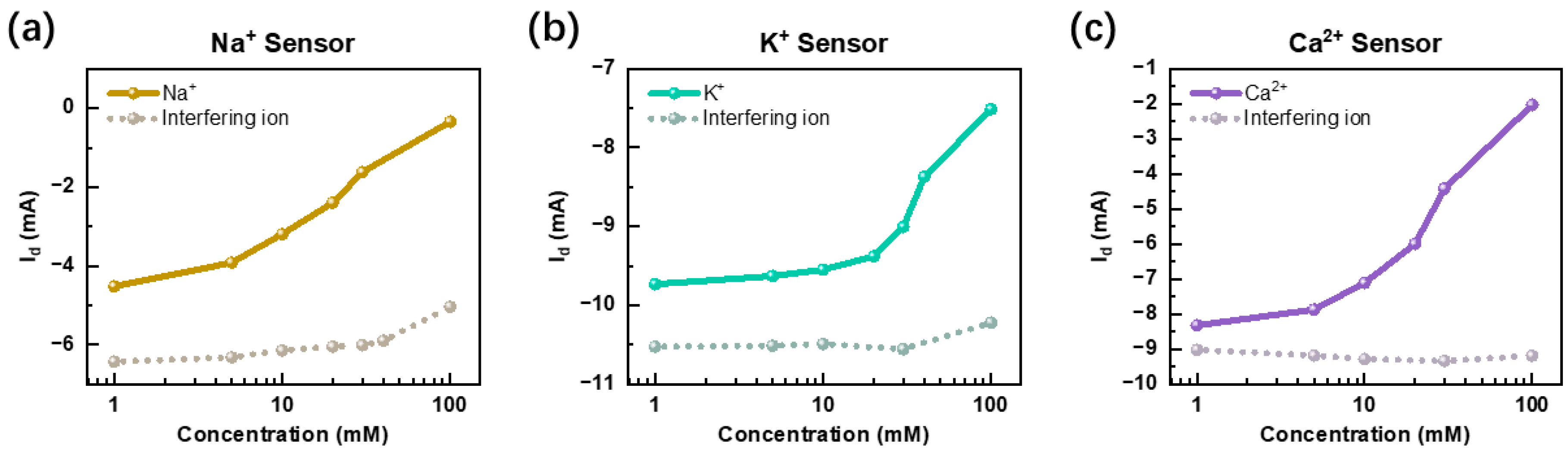
To ensure the reliability of ion detection, it is essential to assess and validate the selectivity of each sensor. As depicted in
Figure 4, we conducted cross-interference experiments to evaluate the specificity of the Na
+, K
+, and Ca
2+ sensors under physiologically relevant conditions. For instance, to assess the selectivity of the Na
+ sensor, we introduced Ca
2+ and K
+ as interfering ions and monitored the sensor’s output response. The results, presented in
Figure 4a, illustrate the comparative current response of the Na
+ sensor in both its target environment and in the presence of interfering ions. The solid line represents the sensor’s response to Na
+ alone, while the dashed line indicates its behavior when subjected to potential cross-interference. By systematically analyzing these cross-interference effects, we establish the selectivity profile of each sensor, ensuring that the system maintains high specificity and accuracy in multi-ion detection. These findings further support the feasibility of using OECT-based sensors for real-time, selective, and interference-resistant monitoring of physiological electrolytes in complex biofluid environments.
The results from all three cross-interference tests demonstrate a clear trend: when the sensor is exposed to its target ion, the drain current (
) decreases as the ion concentration increases, reflecting the expected electrochemical response. In contrast, when the sensor is placed in a non-target solution, the current remains relatively stable, exhibiting minimal variation even with increasing concentrations of interfering ions. This distinct difference in current behavior highlights the high selectivity of the sensor towards its intended analyte. Additionally, as observed in
Figure 4, the current response in a non-target environment is consistently higher than that in the target environment, further reinforcing the sensor’s ability to differentiate between specific ions. To further validate its robustness, multiple rounds of testing were conducted using a variety of interfering ions and two organic substances (
Supplementary Materials Figure S5). These repeated trials confirmed that the sensor maintains strong specificity and stable signal output, even in the presence of multiple interfering species. This consistency underscores the reliability and practicality of the OECT-based ion sensors for real-time, selective monitoring in biologically complex environments, such as human saliva.
3.4. ML-Based Ion Detection
During the fabrication process of OECT-based sensors, unavoidable variations in material properties, deposition conditions, and microstructural uniformity can lead to performance discrepancies across individual sensors. Additionally, external environmental factors, such as temperature fluctuations, humidity levels, and long-term sensor degradation, may introduce signal drift, ultimately affecting the accuracy and reliability of ion detection. These challenges necessitate robust compensation strategies to ensure consistent and precise measurements in real-world applications. To address these non-ideal effects, we integrate machine learning algorithms as an adaptive correction mechanism within our detection system. This approach enhances both sensor stability and data interpretation by systematically learning and compensating for variations in sensor responses. The machine learning workflow consists of three key stages: (1) Binary classification, where real-time sensor data are analyzed to determine whether the current environment contains the target ion; (2) multi-class classification, where the aggregated signals from the entire sensor array are processed to accurately identify specific ion types present in the solution; and (3) regression modeling, which applies data-driven curve fitting to precisely predict the ion concentration in the detected environment. By leveraging data-driven pattern recognition and predictive modeling, the proposed machine learning framework significantly enhances the accuracy, robustness, and adaptability of the OECT-based sensing platform. This intelligent processing capability not only mitigates fabrication inconsistencies and environmental disturbances but also ensures highly reliable, real-time multi-ion monitoring for practical biomedical applications.
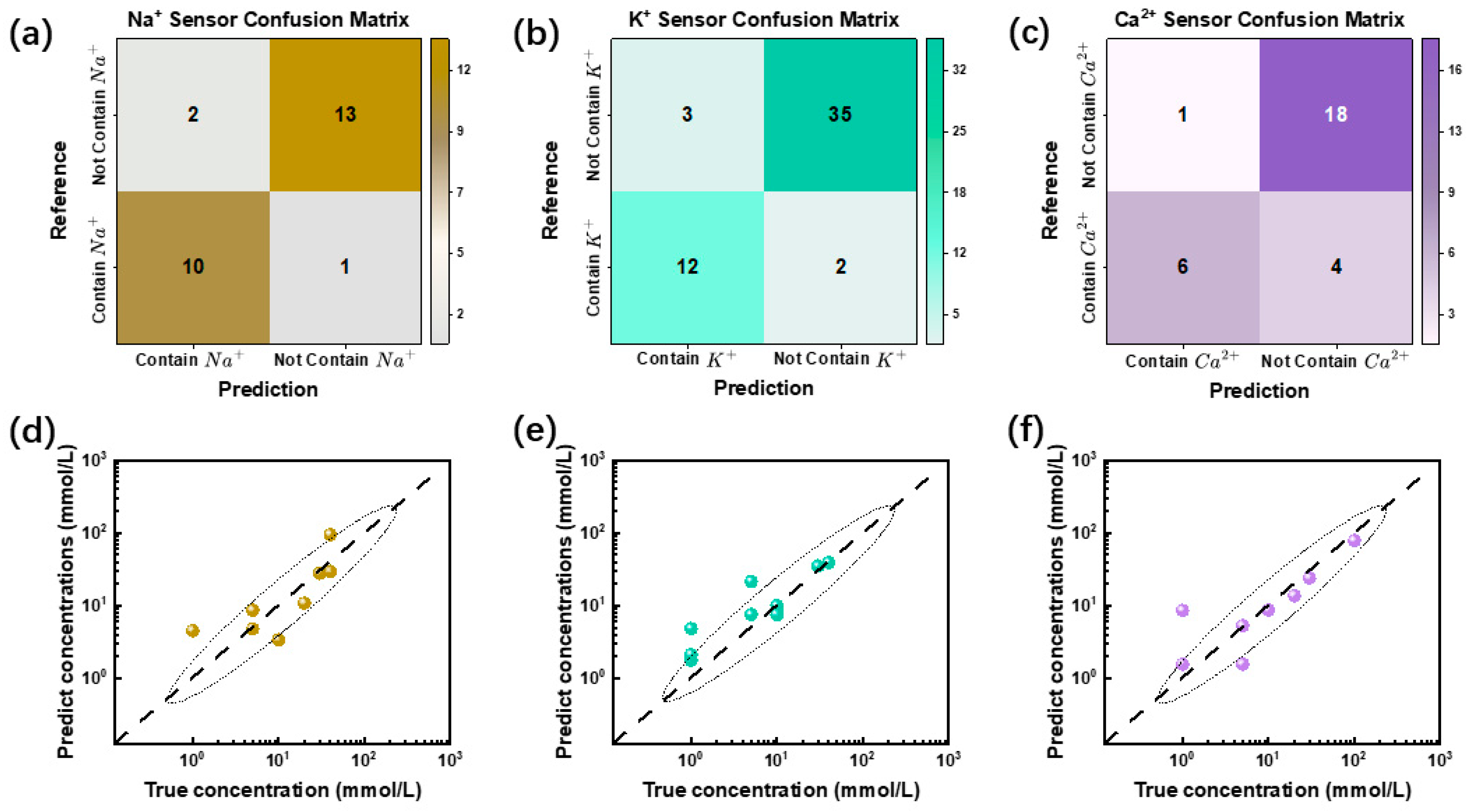
The classification performance of the machine learning model was assessed using a confusion matrix, as shown in
Figure 5a–c. In this matrix, diagonal elements represent correctly classified samples, while off-diagonal elements correspond to misclassified instances. The accuracy rates achieved by the three sensors on the test set were 88.46%, 90.38%, and 82.76%, respectively, indicating strong predictive performance. Notably, the K
+ sensor exhibited the highest classification accuracy at 90.38%, whereas the Ca
2+ sensor demonstrated a slightly lower accuracy of 82.76%, suggesting potential challenges in calcium ion detection. A detailed examination of the confusion matrix reveals sensor-specific misclassification patterns. For instance, the Ca
2+ sensor exhibited a higher incidence of false negatives, meaning that some Ca
2+-containing samples were incorrectly classified as negative cases. This discrepancy may be attributed to two primary factors: insufficient training data or the lower intrinsic sensitivity of the calcium-selective membrane. According to Equation (1), the sensitivity of the calcium-selective membrane is approximately 29.6 mV/dec, which is only half that of the Na
+- and K
+-selective membranes (59.2 mV/dec) [
2,
33]. This lower sensitivity could contribute to the reduced classification performance observed for the Ca
2+ sensor. The sensing system’s performance can be enhanced by modifying the ISM formulation, including the replacement of ionophores, incorporation of anion-exclusion agents, and adjustment of ISM thickness [
39]. Additionally, expanding the training dataset size or employing better-fitting machine learning models can further optimize signal calibration accuracy. Beyond classification, we further employed a random forest regression model to predict the ion concentration based on sensor data. As depicted in
Figure 5d–f, the regression analysis demonstrated a strong correlation between the predicted and actual ion concentrations, the calculated R
2 values for the three sensors were as follows: R
2 = 0.8334(Na
+ sensor), R
2 = 0.9277(K
+ sensor), and R
2 = 0.9554 (Ca
2+ sensor), highlighting the model’s effectiveness in quantitative ion detection. To quantify the regression accuracy, we computed the mean absolute percentage error (MAPE) for the test set, which yielded an average error of 13.8%, and the Mean Absolute Error (MAE) was 6.129, indicating a high degree of predictive reliability. Compared to traditional linear fitting methods (MAPE = 74.5%, MAE = 29.93), the machine learning approach significantly improved prediction accuracy. Overall, these results confirm that the multi-layer perceptron (MLP) model exhibits strong generalization capabilities for binary classification tasks, while the random forest regression approach effectively models ion concentration trends, making this machine-learning-enhanced sensing system highly promising for real-time, selective, and quantitative ion detection.
3.5. Ion Sensing Platform
To enhance the versatility and adaptability of our sensing platform, we integrated three types of OECT sensors onto a flexible polyethylene naphthalate (PEN) substrate, ensuring both mechanical flexibility and durability. These sensors are electrically connected to the circuit board via flexible printed circuit (FPC) cables, as illustrated in
Figure 6. The compact design ensures structural integrity while significantly reducing dimensions, allowing the sensor system to achieve mechanical flexibility with bendable and foldable deformation. Such adaptability is essential for wearable biosensors to maintain stable conformal contact with irregular biological surfaces. Furthermore, the sensor signals are acquired and processed through a custom-designed electronic circuit with compact dimensions of 33 mm × 28 mm, which is only slightly larger than a standard coin. This miniaturized circuit is battery-powered, enabling stable and reliable operation in diverse field applications, including real-time physiological monitoring, point-of-care diagnostics, and remote health assessments. By integrating flexible sensor technology with an ultra-compact and power-efficient circuit system, this platform demonstrates high potential for next-generation, on-demand bioelectronic sensing solutions.
The circuit module serves as the core signal processing unit of the sensing platform, incorporating integrated circuits and peripheral electronic components essential for voltage regulation, signal acquisition, processing, and wireless data transmission via Bluetooth. During operation, the circuit dynamically applies precise gate voltages to the sensor array, ensuring that each sensor operates under optimal electrical conditions for accurate ion detection. The circuit module features a high-precision current resolution of 4 µA and supports a minimum sampling period of 1 µs, meeting the stringent requirements for real-time ion concentration measurements. Additionally, built-in Bluetooth connectivity facilitates seamless wireless data transmission, enabling the real-time calculation and visualization of test results on a custom-developed mobile application. The app displays sensor responses as dynamic time-series curves, allowing for the continuous monitoring of physiological indicator variations and providing users with an intuitive representation of electrolyte fluctuations. Beyond real-time monitoring, the circuit system is designed for data-driven analysis. The acquired test data can be exported directly via the app and fed into a trained machine learning model for automated calibration, improving the accuracy and reliability of ion type classification and concentration estimation. This intelligent processing capability, combined with the compact and wireless-enabled hardware design, enhances the adaptability of the system for personalized health monitoring, point-of-care diagnostics, and remote biofluid analysis.
4. Conclusions
In this study, we have developed a novel OECT-based sensing system for real-time monitoring of physiological indicators, specifically demonstrating the capability for simultaneous detection of Na+, K+, and Ca2+ ions. The proposed OECT sensors exhibit highly linear responses across physiologically relevant ion concentration ranges while maintaining excellent selectivity, with minimal cross-interference from competing ions. To further enhance detection reliability, we integrate machine learning techniques, which effectively mitigate cross-interference effects and significantly improve detection accuracy. This data-driven approach strengthens the robustness of the system, making it well suited for complex biological environments such as human saliva. For practical deployment, we developed a fully integrated system comprising a miniaturized readout circuit and dedicated control software, enabling real-time data acquisition and portable monitoring capabilities. This compact and self-contained platform facilitates on-site physiological assessment, expanding the potential for applications in personalized health monitoring, point-of-care diagnostics, and wearable biosensing technologies. More importantly, by seamlessly integrating the OECT sensor array, micro-detection system, and machine learning-driven data analysis, our system achieves not only high accuracy in multi-ion detection but also enhanced feasibility for real-world implementation. These advancements suggest that the proposed system holds significant promise for clinical applications, paving the way for next-generation, non-invasive electrolyte monitoring solutions with broad implications for medical diagnostics and continuous health tracking.
Prospectively, three strategic directions will drive our future research: First, we will conduct wearable trials to systematically evaluate sensor drift characteristics and develop adaptive recalibration protocols using neural networks. Second, the sensor functionality will be extended to detect additional biomarkers (Cl−, Mg2+, pH) through replaceable ion-selective membranes. Third, we will collaborate with clinical partners to validate the system’s diagnostic efficacy in real disease monitoring (e.g., chronic kidney disease), with particular emphasis on correlating salivary electrolyte profiles with serum reference measurements. These advancements will bridge the gap between laboratory validation and clinical healthcare implementation, potentially enabling a novel paradigm for non-invasive electrolyte balance management.