A Personalized 3D-Printed Smart Splint with Integrated Sensors and IoT-Based Control: A Proof-of-Concept Study for Distal Radius Fracture Management
Abstract
1. Introduction
2. Related Work
2.1. Treatment Options for Distal Radius Fractures
2.2. Analysis of Traditional TCM Small Splint Technique
2.3. Advances in Smart Splints for Wearable Monitoring
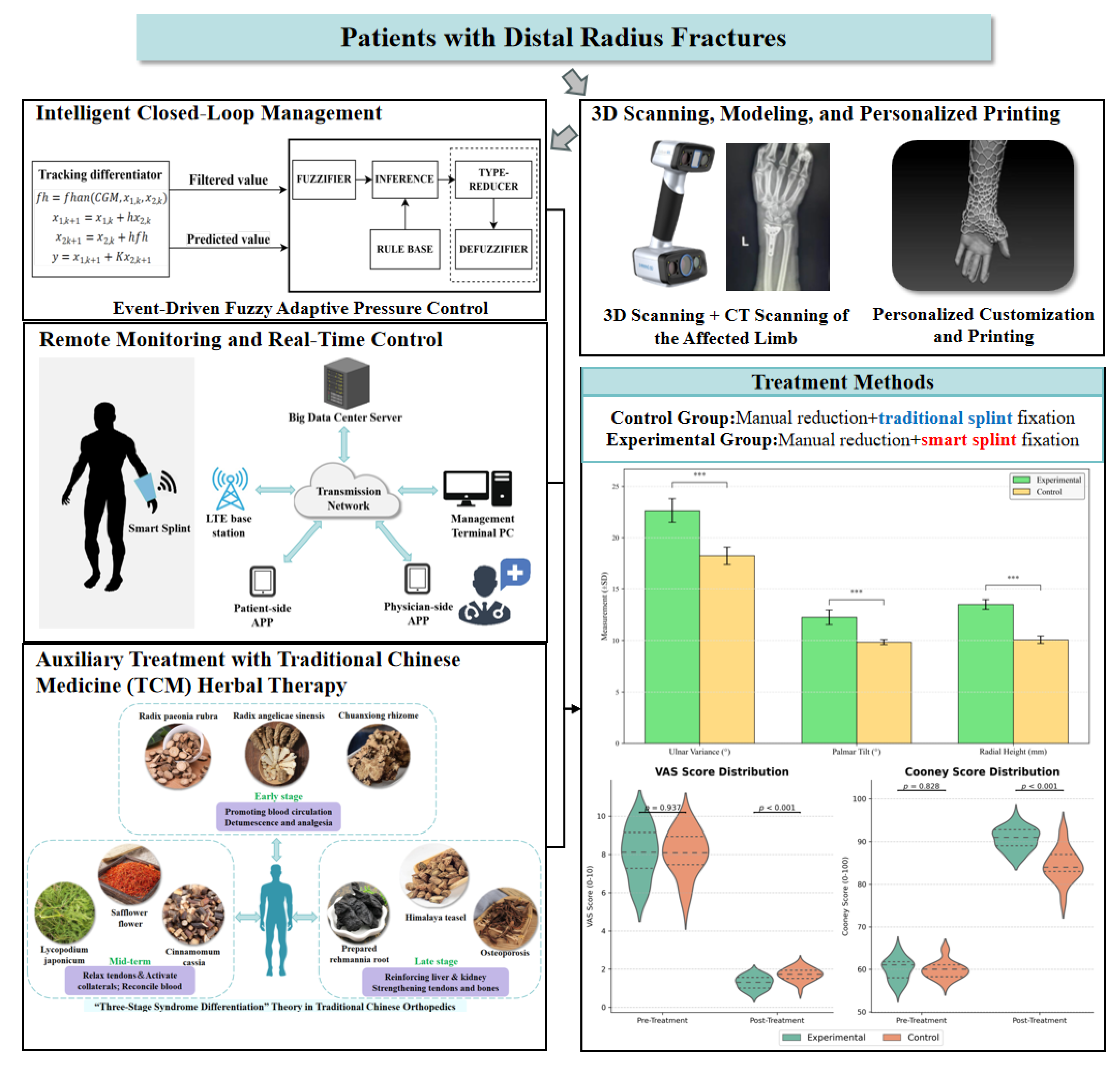
3. Methods
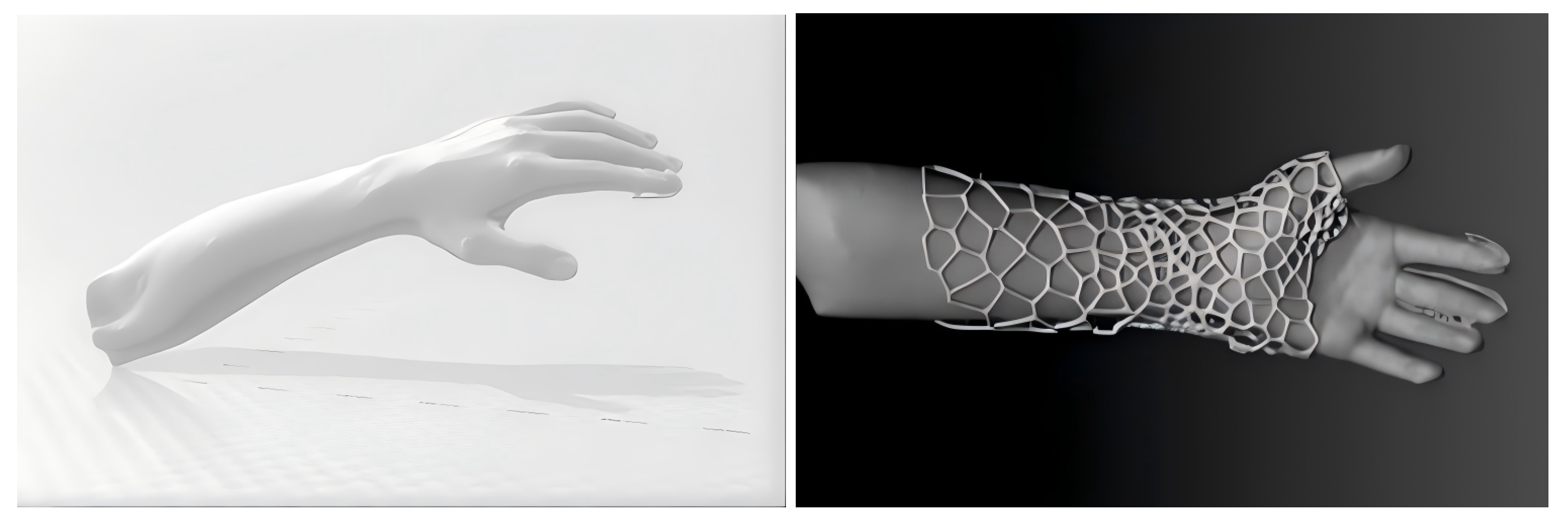
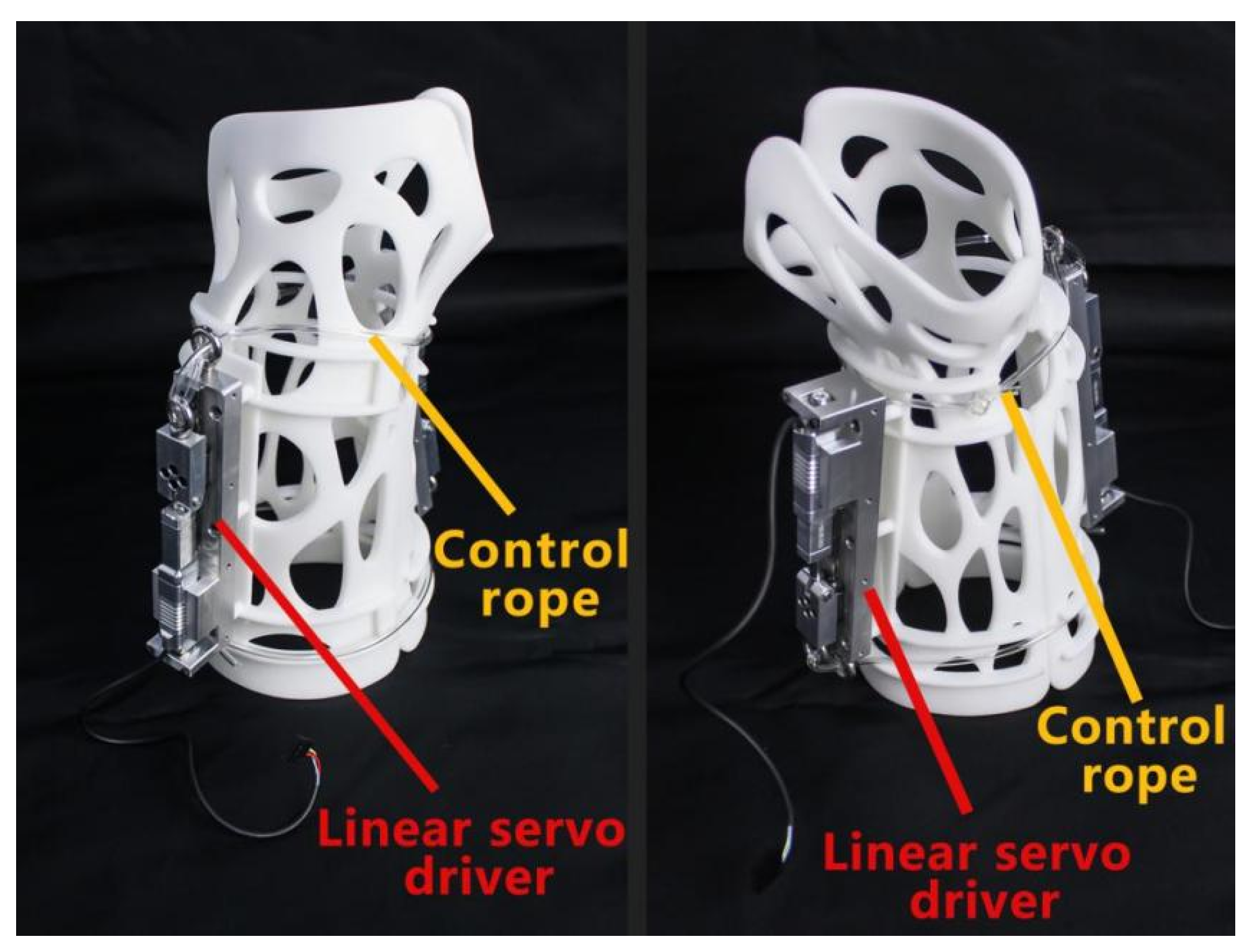
3.1. Personalized External Fixation Device Utilizing 3D Printing
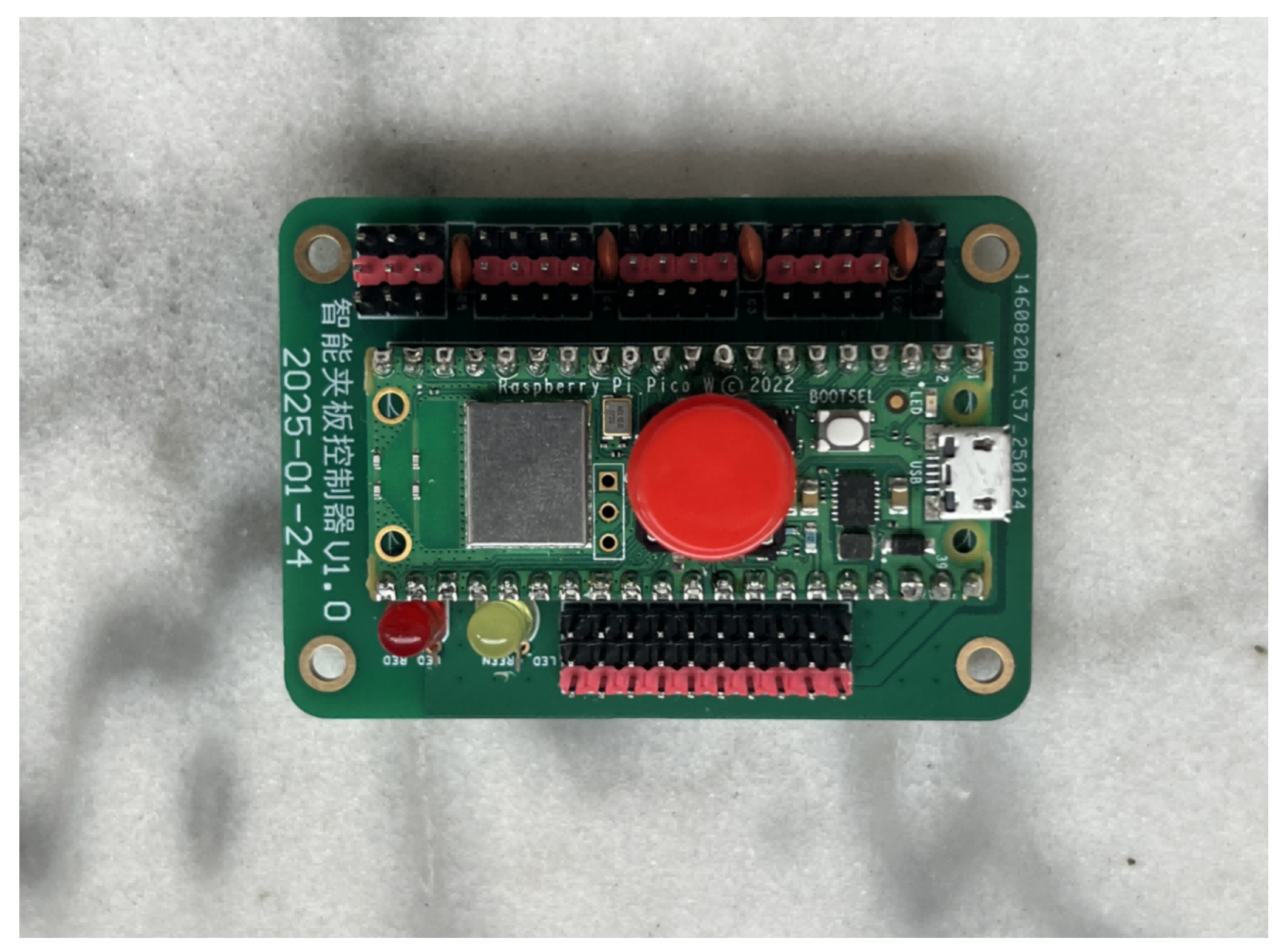
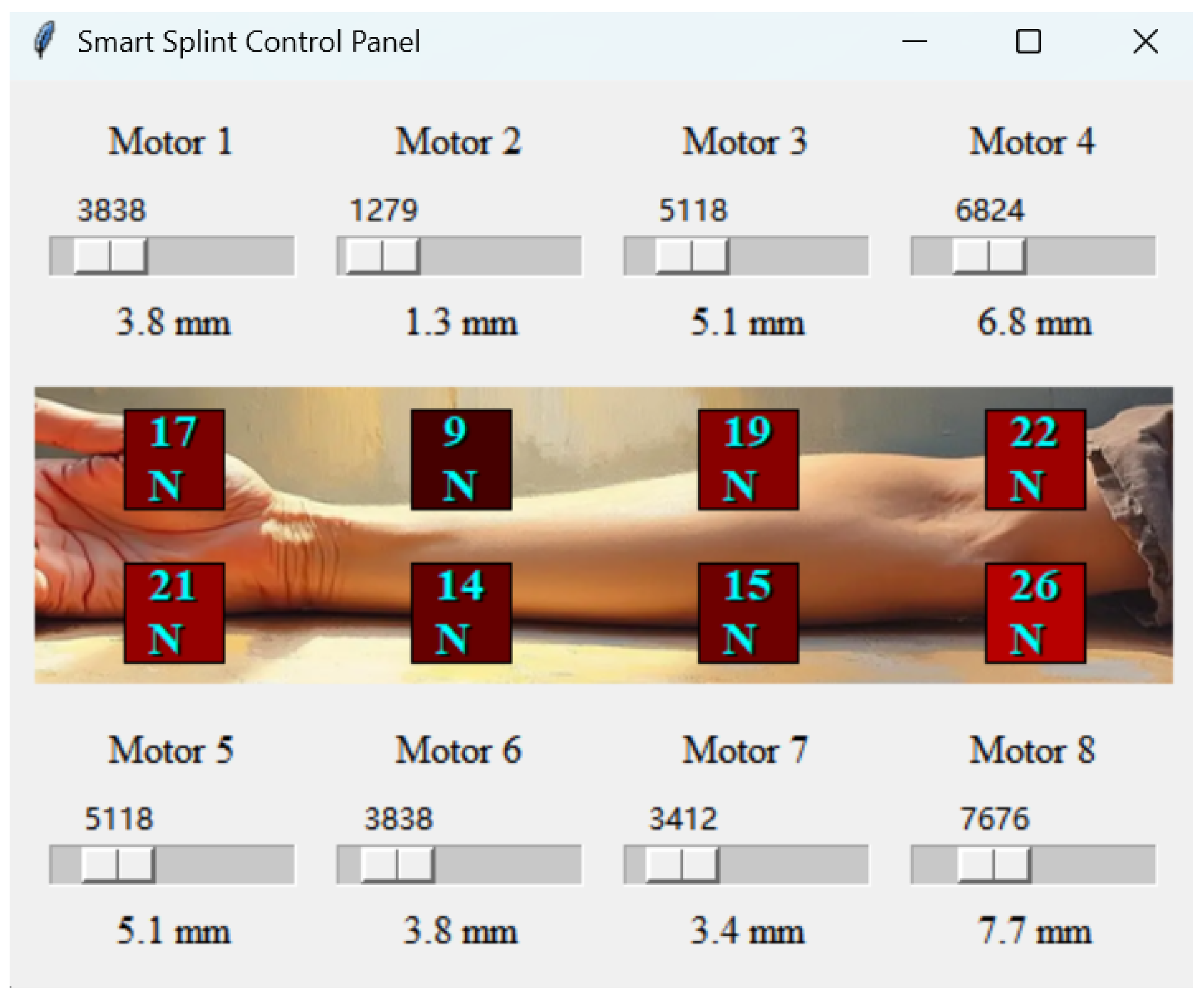
3.2. Advanced Closed-Loop Control System for External Fixation Devices
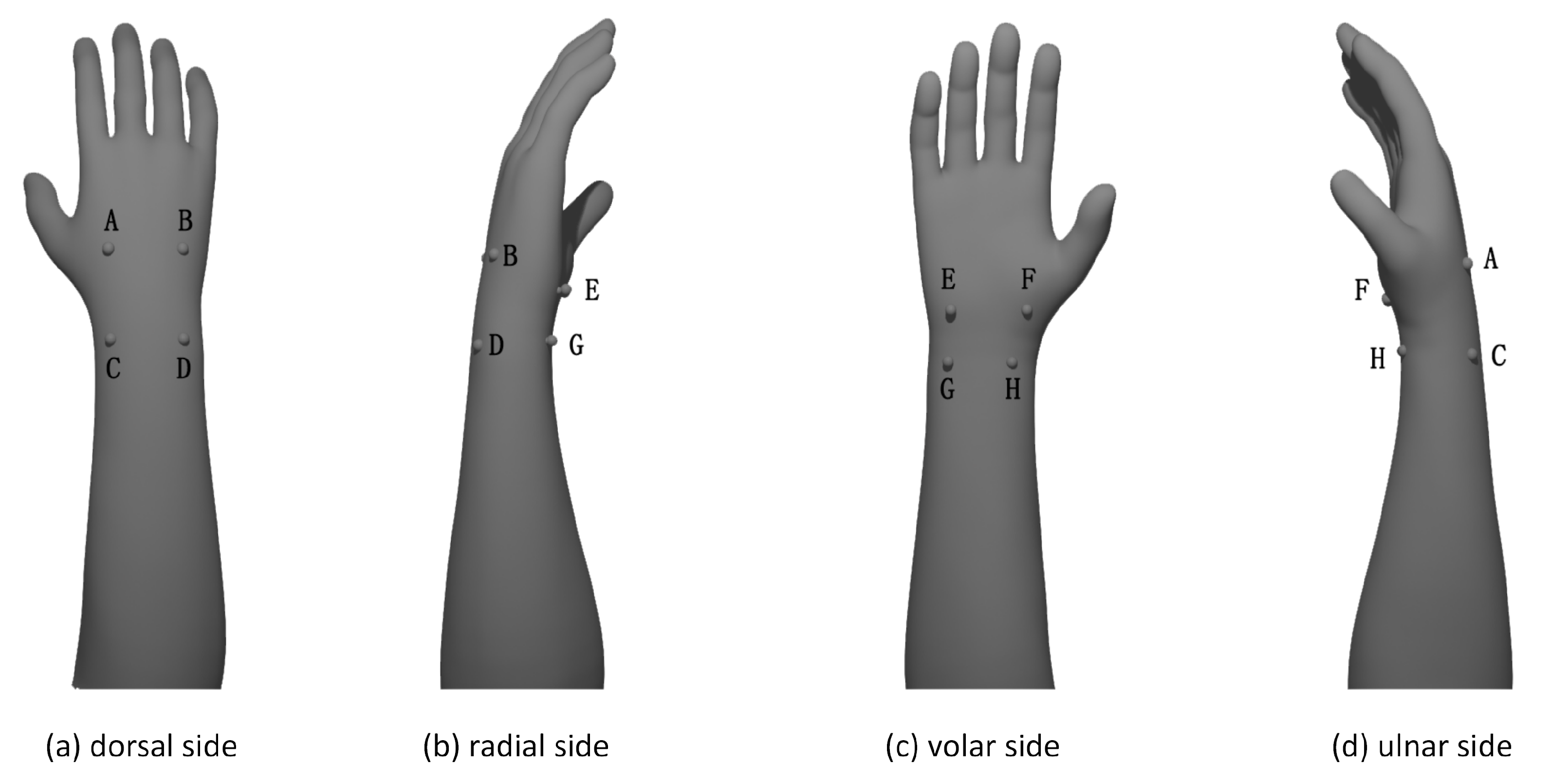
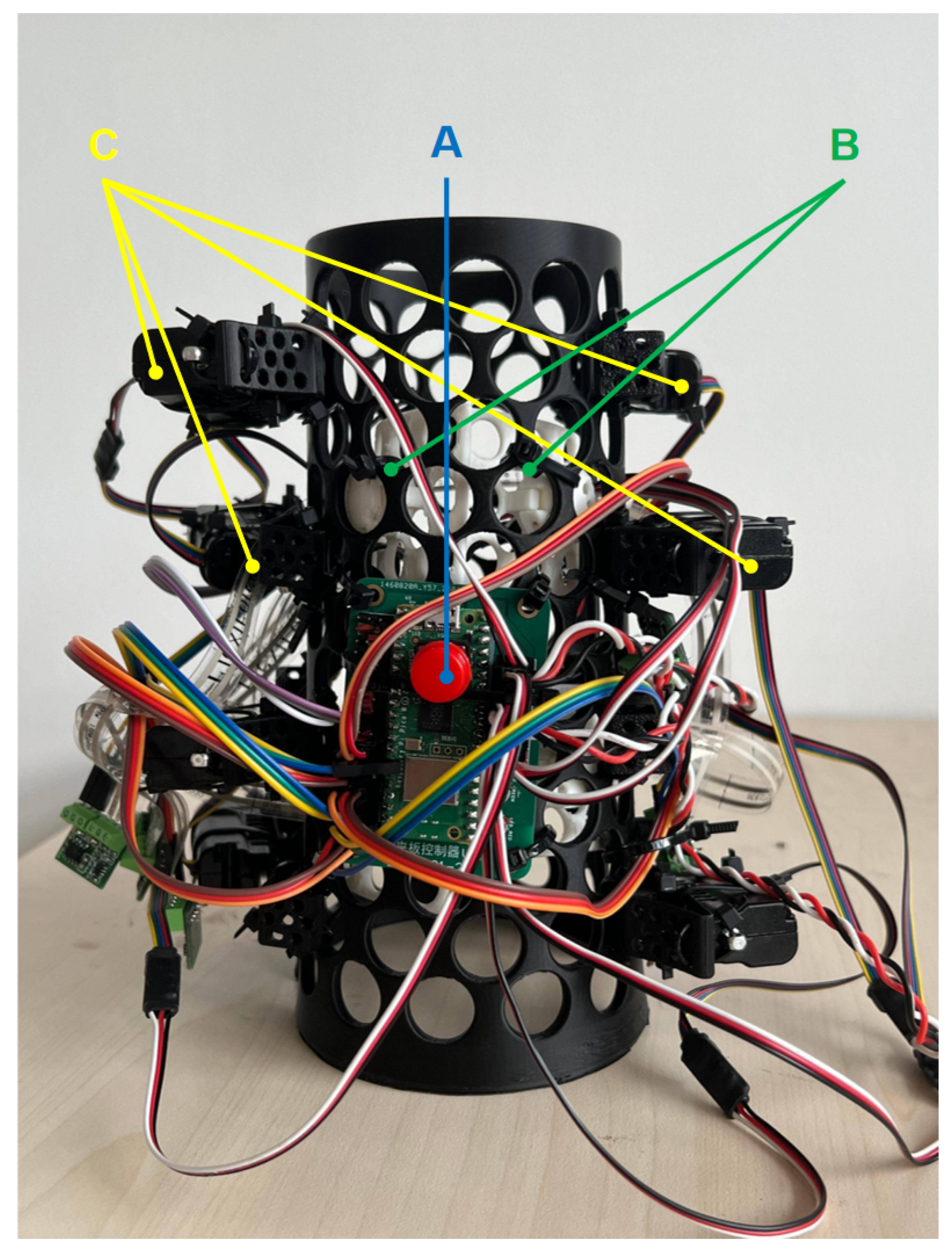
3.2.1. Pressure Sensing System
3.2.2. Automatic Pressure Adjustment System
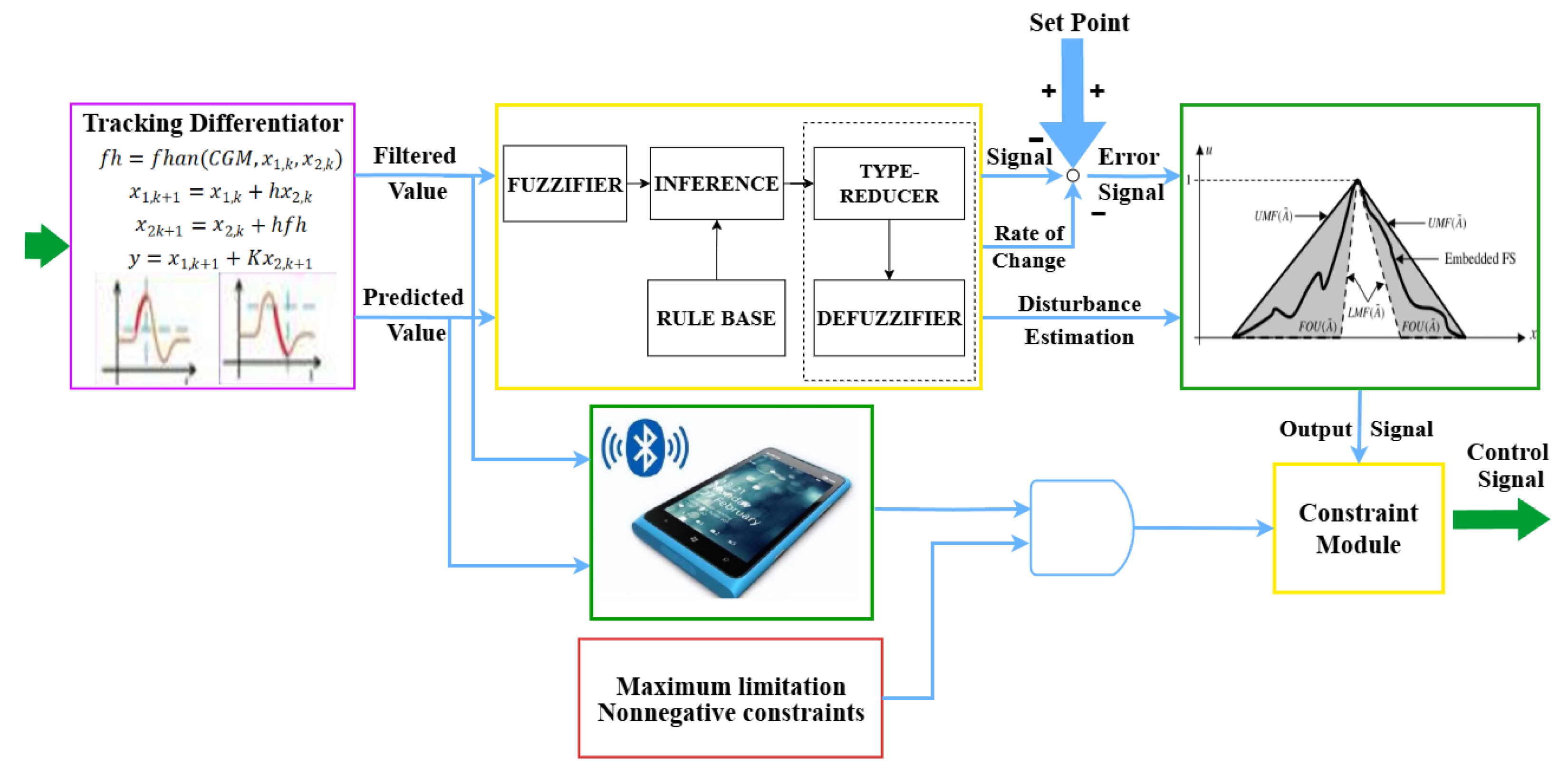
3.3. Optimization of Type-2 Fuzzy Control Algorithm
3.3.1. Determination of the Therapeutic Pressure Range
3.3.2. Membership Function Parameter Optimization
3.3.3. Fuzzy Rule Base Self-Organization Mechanism
3.3.4. Adaptive Type Reduction Algorithm Optimization
3.3.5. Comparative Analysis with Traditional PID Algorithm
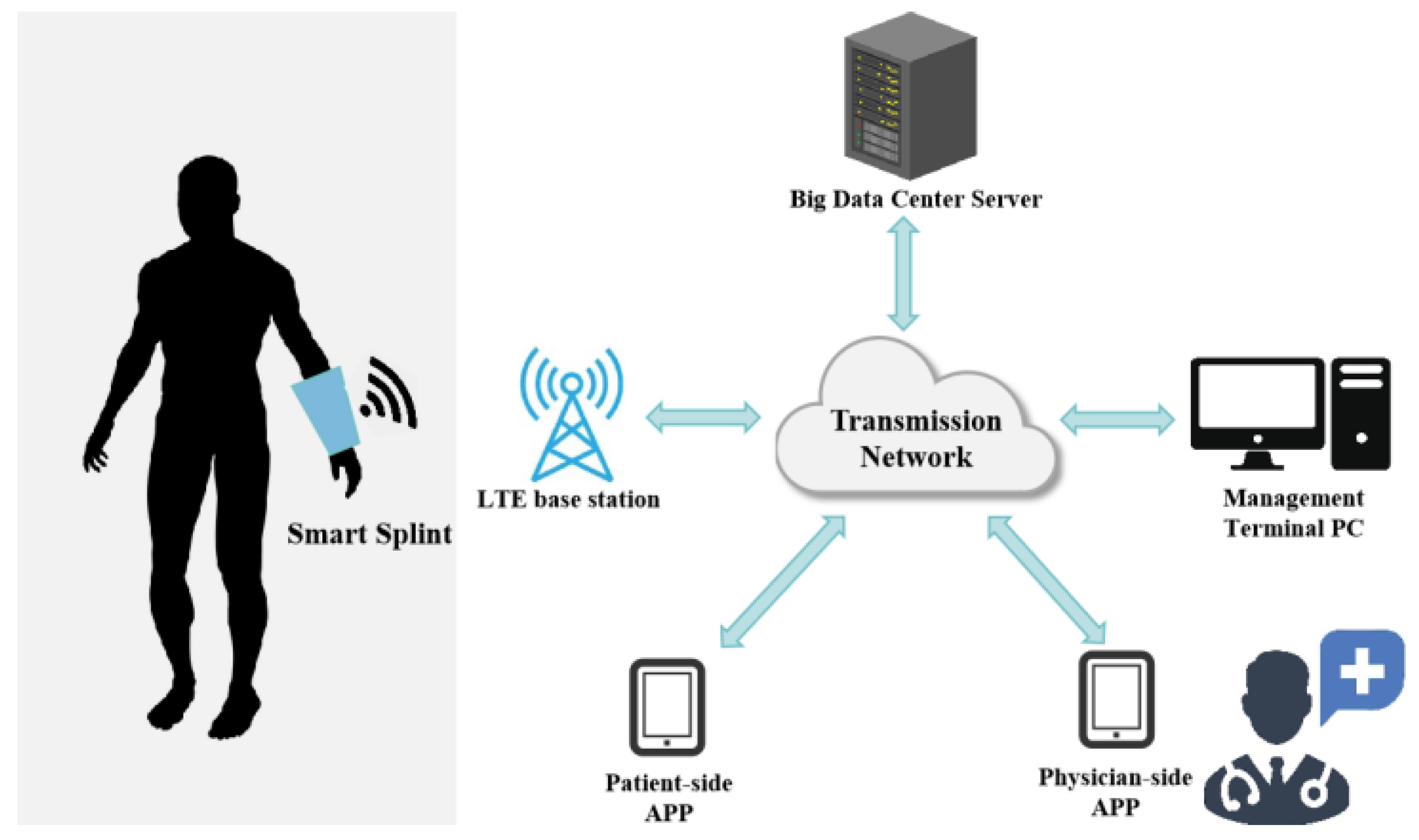
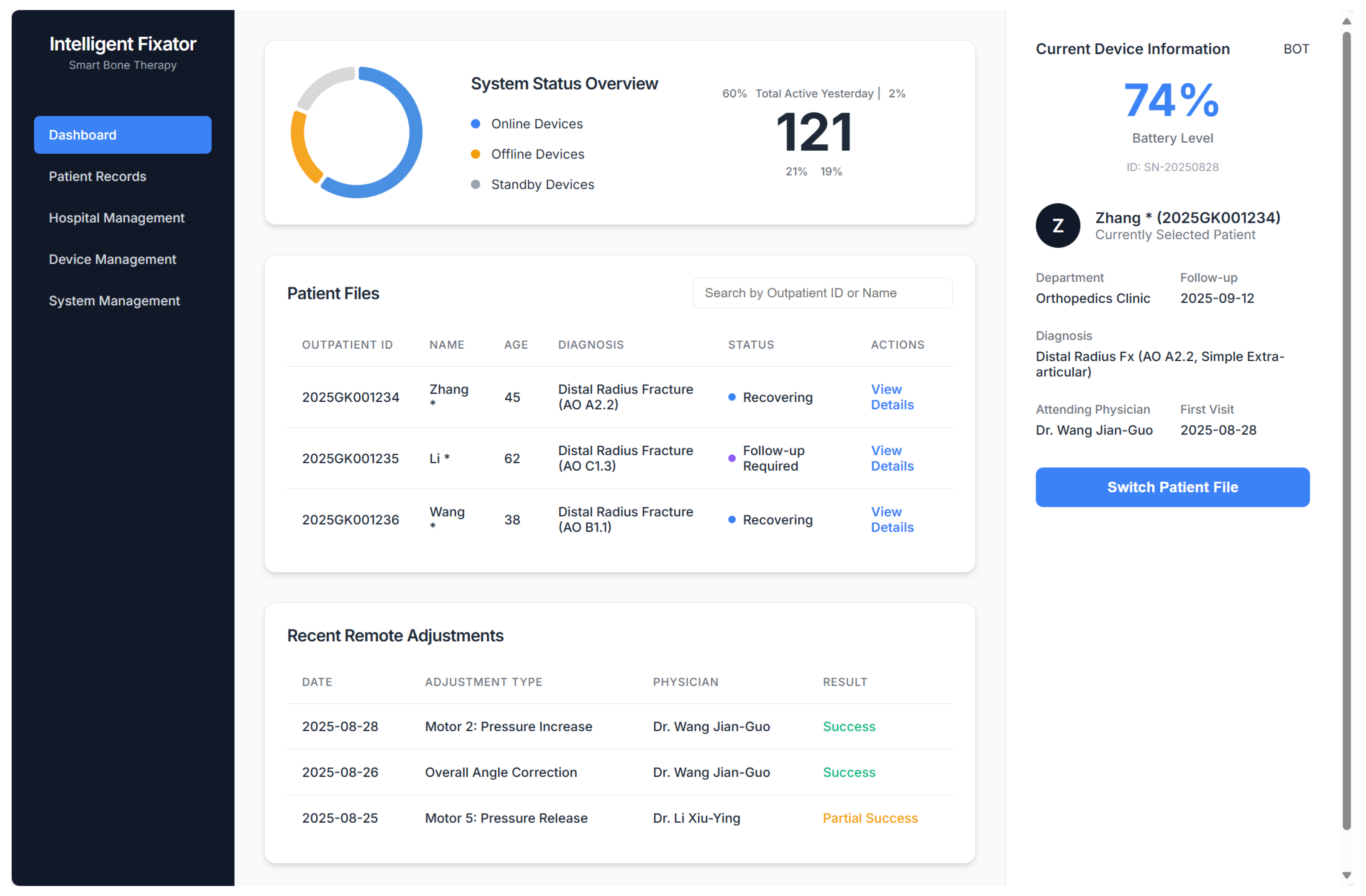
3.4. System for Remote Surveillance and Real-Time Modulation
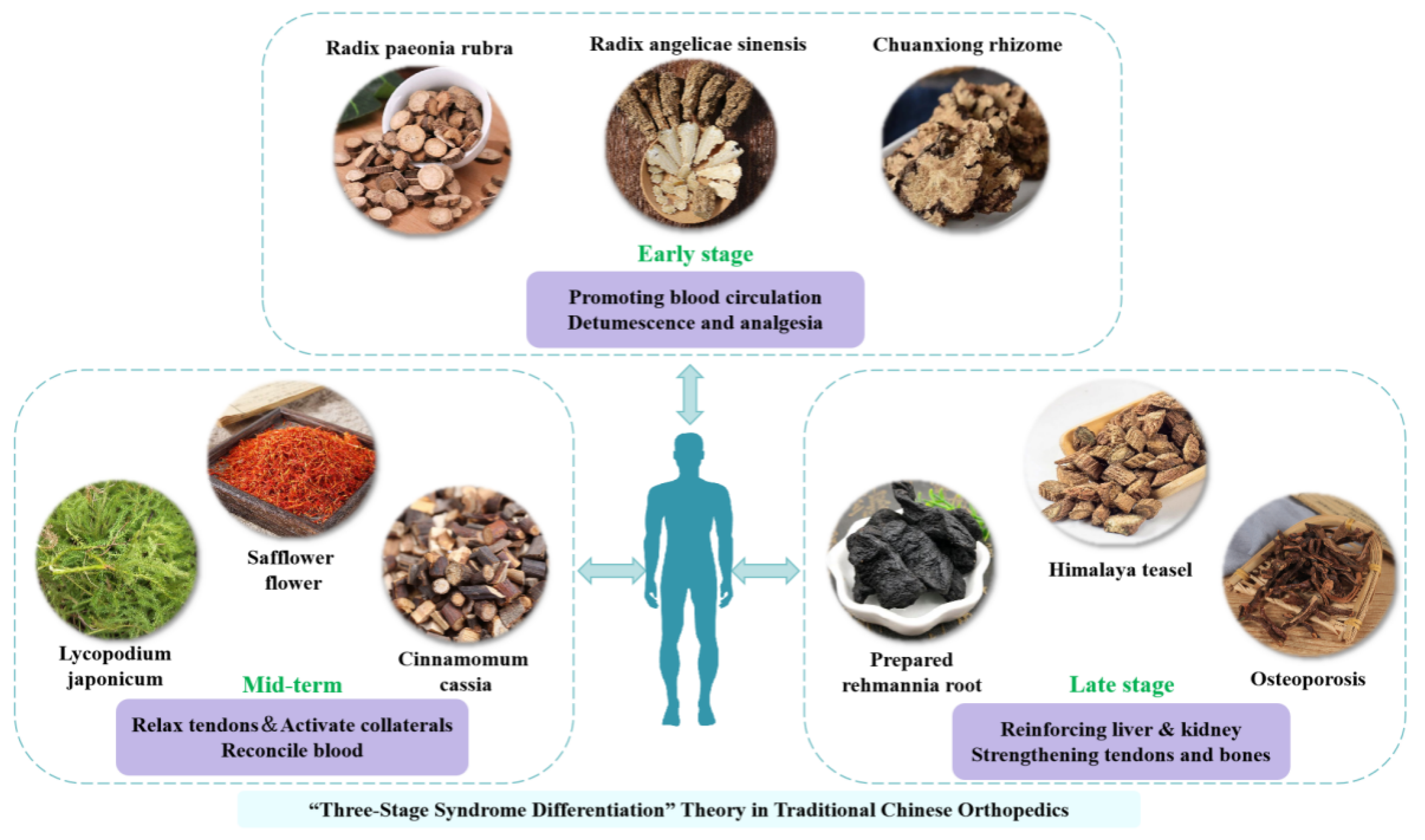
3.5. Design of Herbal Pads in Traditional Chinese Medicine (TCM)
3.6. Clinical Trial Design
3.6.1. Clinical Data
3.6.2. Diagnostic Criteria
3.6.3. Therapeutic Method
Control Group
Experimental Group
3.6.4. Therapeutic Evaluation
Radiographic Evaluation
Visual Analog Scale (VAS)
Cooney Wrist Function Score
Criteria for Clinical Effectiveness
3.6.5. Statistical Analysis
4. Results
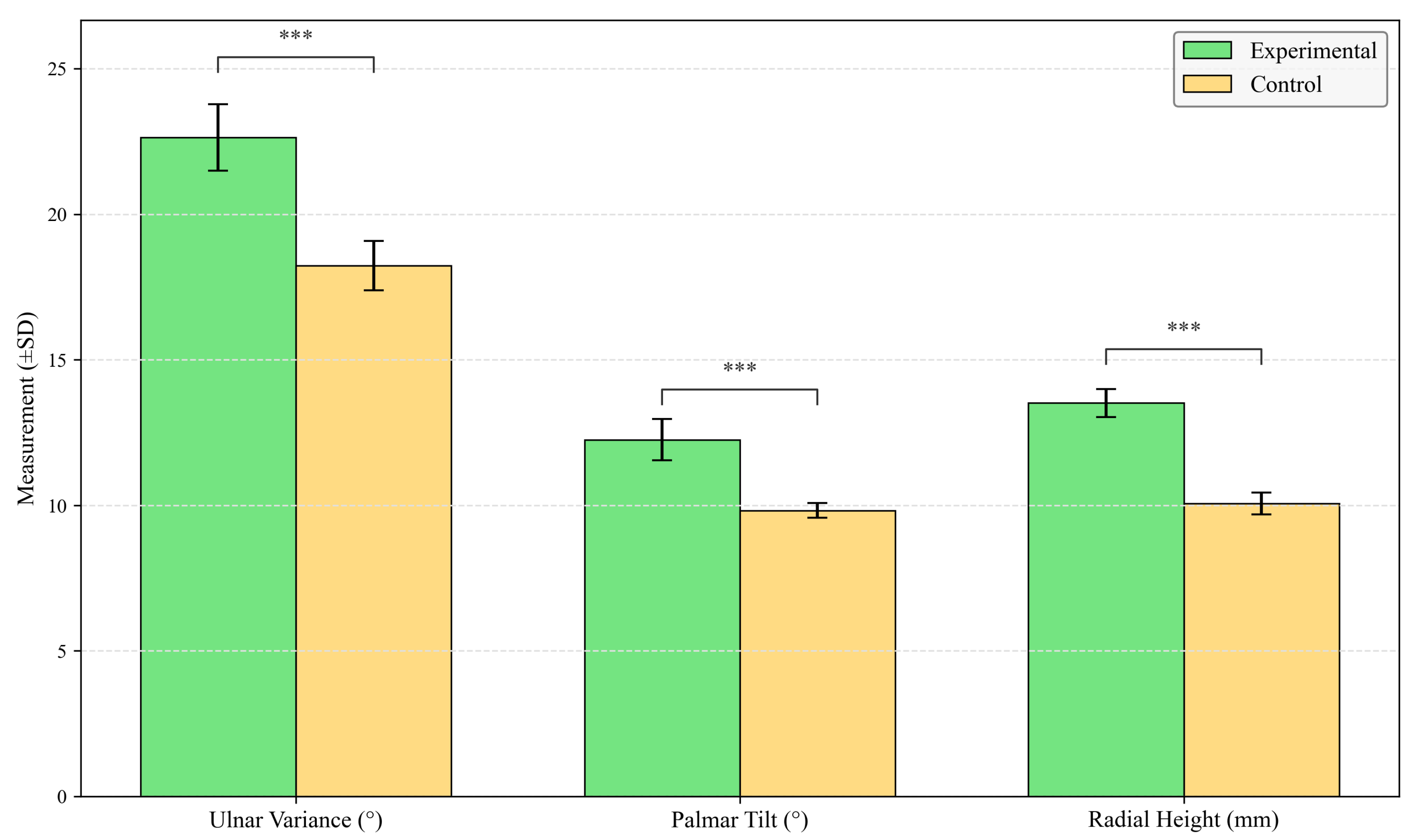
4.1. Radiographic Evaluation
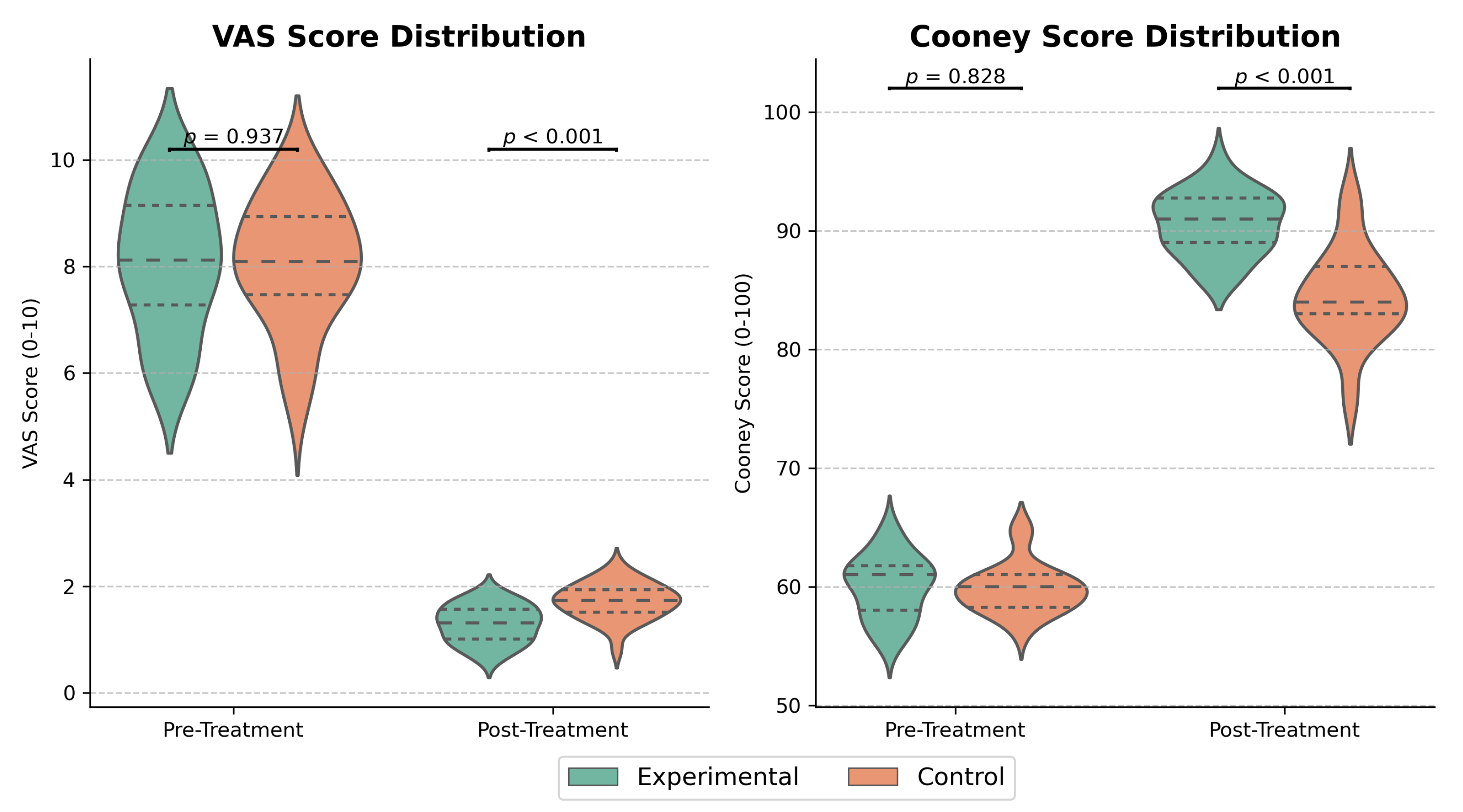
4.2. Evaluation of VAS and Cooney Scores Pre- and Post-Treatment
4.3. Comparison of Clinical Effectiveness
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DRF | Distal Radius Fracture |
| IoT | Internet of Things |
| IT2-FLC | Interval Type-2 Fuzzy Logic Control |
| TCM | Traditional Chinese Medicine |
| CRPS | Complex Regional Pain Syndrome |
| PCB | Printed Circuit Board |
| QPSO | Quantum Particle Swarm Optimization |
| PID | Proportional-Integral-Derivative |
| EMR | Electronic Medical Record |
| PACS | Picture Archiving and Communication System |
| BLE | Bluetooth Low Energy |
| VAS | Visual Analog Scale |
References
- Koo, K.O.; Tan, D.M.; Chong, A.K. Distal radius fractures: An epidemiological review. Orthop. Surg. 2013, 5, 209–213. [Google Scholar] [CrossRef]
- Nellans, K.W.; Kowalski, E.; Chung, K.C. The epidemiology of distal radius fractures. Hand Clin. 2012, 28, 113–125. [Google Scholar] [CrossRef]
- Zhang, X.H.; Xiao, Z.Q.; Wang, A.M.; Zhang, H.L.; Li, H.J.; Huang, S.Q. Comparison study of small splint fixation and plaster slab fixation for the treatment of distal radius fractures. Zhongguo Gu Shang = China J. Orthop. Traumatol 2010, 23, 578–580. [Google Scholar] [PubMed]
- Cooper, C.; Atkinson, E.J.; Jacobsen, S.J.; O’Fallon, W.M.; Melton, L.J., III. Population-based study of survival after osteoporotic fractures. Am. J. Epidemiol. 1993, 137, 1001–1005. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; Oden, A.; Sernbo, I.; Redlund-Johnell, I.; Dawson, A.; Jonsson, B. Long-term risk of osteoporotic fracture in Malmö. Osteoporos. Int. 2000, 11, 669–674. [Google Scholar] [CrossRef]
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Prim. 2016, 2, 16069. [Google Scholar] [CrossRef]
- Hutchinson, T.; Polansky, S.; Feinstein, A. Post-menopausal oestrogens protect against fractures of hip and distal radius: A case-control study. Lancet 1979, 314, 705–709. [Google Scholar] [CrossRef]
- Heinonen, A.; Kannus, P.; Sievänen, H.; Oja, P.; Pasanen, M.; Rinne, M.; Vuori, I. Randomised controlled trial of effect of high-impact exercise on selected risk factors for osteoporotic fractures. Lancet 1996, 348, 1343–1347. [Google Scholar] [CrossRef]
- Riggs, B.L.; Melton, L.J., III. The worldwide problem of osteoporosis: Insights afforded by epidemiology. Bone 1995, 17, S505–S511. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L.; Melton, L.J., III; Robb, R.A.; Camp, J.J.; Atkinson, E.J.; Peterson, J.M.; Khosla, S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J. Bone Miner. Res. 2004, 19, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.S.; Rozell, J.C.; Pulos, N. Distal radius fractures in the elderly. JAAOS 2017, 25, 179–187. [Google Scholar] [CrossRef]
- Mauck, B.M.; Swigler, C.W. Evidence-based review of distal radius fractures. Orthop. Clin. N. Am. 2018, 49, 211–222. [Google Scholar] [CrossRef]
- Mehta, S.P.; MacDermid, J.C.; Richardson, J.; MacIntyre, N.J.; Grewal, R. Baseline pain intensity is a predictor of chronic pain in individuals with distal radius fracture. J. Orthop. Sport. Phys. Ther. 2015, 45, 119–127. [Google Scholar] [CrossRef]
- Jo, Y.H.; Kim, K.; Lee, B.G.; Kim, J.H.; Lee, C.H.; Lee, K.H. Incidence of and risk factors for complex regional pain syndrome type 1 after surgery for distal radius fractures: A population-based study. Sci. Rep. 2019, 9, 4871. [Google Scholar] [CrossRef]
- MacDermid, J.C.; Roth, J.H.; Richards, R.S. Pain and disability reported in the year following a distal radius fracture: A cohort study. BMC Musculoskelet. Disord. 2003, 4, 24. [Google Scholar] [CrossRef]
- van Leerdam, R.H.; Huizing, F.; Termaat, F.; Kleinveld, S.; Rhemrev, S.J.; Krijnen, P.; Schipper, I.B. Patient-reported outcomes after a distal radius fracture in adults: A 3–4 years follow-up. Acta Orthop. 2019, 90, 129–134. [Google Scholar] [CrossRef]
- Hargens, A.R.; Mubarak, S.J.; Owen, C.A.; Garetto, L.P.; Akeson, W.H. Interstitial fluid pressure in muscle and compartment syndromes in man. Microvasc. Res. 1977, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lafontaine, M.; Hardy, D.; Delince, P.H. Stability assessment of distal radius fractures. Injury 1989, 20, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Handoll, H.H.; Cameron, I.D.; Mak, J.C.; Panagoda, C.E.; Finnegan, T.P. Multidisciplinary rehabilitation for older people with hip fractures. Cochrane Database Syst. Rev. 2021, CD007125. [Google Scholar] [CrossRef]
- Tabrizi, J.S.; Behghadami, M.A.; Saadati, M.; Söderhamn, U. Self-care ability of older people living in urban areas of northwestern Iran. Iran. J. Public Health 2018, 47, 1899. [Google Scholar] [PubMed Central]
- Travers, J.L.; Hirschman, K.B.; Naylor, M.D. Older adults’ goals and expectations when using long-term services and supports. J. Appl. Gerontol. 2022, 41, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Espinoza, H.; Araya-Quintanilla, F.; Olguín-Huerta, C.; Gutiérrez-Monclus, R.; Valenzuela-Fuenzalida, J.; Román-Veas, J.; Campos-Jara, C. Effectiveness of surgical versus conservative treatment of distal radius fractures in elderly patients: A systematic review and meta-analysis. Orthop. Traumatol. Surg. Res. 2022, 108, 103323. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, W.; Li, D.; Wu, Y. Morphological characteristics of different types of distal radius die-punch fractures based on three-column theory. J. Orthop. Surg. Res. 2019, 14, 390. [Google Scholar] [CrossRef]
- Song, J.; Yu, A.X.; Li, Z.H. Comparison of conservative and operative treatment for distal radius fracture: A meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2015, 8, 17023. [Google Scholar] [PubMed Central]
- Ba, C.; Ni, X.H.; Zhu, G.P.; Yu, J.L.; Cheng, J.J.; Wu, J.S.; Zhao, Q.M. Clinical effect of open reduction and internal fixation with a steel plate through the triceps approach in the treatment of fractures of the middle and lower 1/3 of the humerus. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7607–7614. [Google Scholar] [CrossRef]
- Uhthoff, H.K.; Poitras, P.; Backman, D.S. Internal plate fixation of fractures: Short history and recent developments. J. Orthop. Sci. 2006, 11, 118–126. [Google Scholar] [CrossRef]
- Yu, G.S.; Lin, Y.B.; Le, L.S.; Zhan, M.F.; Jiang, X.X. Internal fixation vs conservative treatment for displaced distal radius fractures: A meta-analysis of randomized controlled trials. Turk. J. Trauma Emerg. Surg. 2016, 22, 233–240. [Google Scholar] [CrossRef]
- Shauver, M.J.; Chung, K.C. The economic impact of distal radius fractures. Hand Clin. 2011, 27, 137–143. [Google Scholar]
- American Academy of Orthopaedic Surgeons. Management of Distal Radius Fractures Evidence-Based Clinical Practice Guideline. Published 4 December 2020. Available online: https://www.aaos.org/quality/quality-programs/clinical-practice-guidelines/distal-radius-fractures/ (accessed on 1 July 2024).
- Li, L.; Liu, X.; Patel, M.; Zhang, L. Depth camera-based model for studying the effects of muscle loading on distal radius fracture healing. Comput. Biol. Med. 2023, 164, 107292. [Google Scholar] [CrossRef]
- Wei, L.Y.; Zhang, H.W.; Dong, D.H.; Zuo, J.Z.; Li, L.; Wang, G.Q.; Zhang, J. Manual reduction with traditional small splints for distal radius fracture in older patients. J. Acute Dis. 2021, 10, 78–82. [Google Scholar] [CrossRef]
- Chen, Y.J.; Lin, H.; Zhang, X.; Huang, W.; Shi, L.; Wang, D. Application of 3D-printed and patient-specific cast for the treatment of distal radius fractures: Initial experience. 3D Print. Med. 2017, 3, 11. [Google Scholar] [CrossRef]
- Yan, W.; Ding, M.; Kong, B.; Xi, X.; Zhou, M. Lightweight splint design for individualized treatment of distal radius fracture. J. Med. Syst. 2019, 43, 284. [Google Scholar] [CrossRef]
- Blaya, F.; Pedro, P.S.; Silva, J.L.; D’Amato, R.; Heras, E.S.; Juanes, J.A. Design of an orthopedic product by using additive manufacturing technology: The arm splint. J. Med. Syst. 2018, 42, 54. [Google Scholar] [CrossRef]
- Ma, H.; Ruan, B.; Li, J.; Zhang, J.; Wu, C.; Tian, H.; Xi, X. Topology-optimized splints vs casts for distal radius fractures: A randomized clinical trial. JAMA Netw. Open 2024, 7, e2354359. [Google Scholar] [CrossRef]
- Berner, S.H.; Willis, F.B. Dynamic splinting in wrist extension following distal radius fractures. J. Orthop. Surg. Res. 2010, 5, 53. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wang, Y.R.; Gui, J.; Wen, G.W.; Tang, Z.Y.; Liu, P.; Wu, J.H. Shi’s Yi-Qi Bu-Shen Tong-Luo decoction combined with manipulation in the management of wrist stiffness after distal radius fracture: Study protocol clinical trial (SPIRIT Compliant). Medicine 2020, 99, e19308. [Google Scholar] [CrossRef]
- Yuan, Z. Assessment of the safety and efficacy of the Chinese herbal formula (CHF) in fracture treatment. In Proceedings of the 12th International Conference on Biomedical Engineering and Technology; ACM: New York, NY, USA, 2022; pp. 161–168. [Google Scholar] [CrossRef]
- McQueen, M.M.; Gaston, P. Acute compartment syndrome: Who is at risk? J. Bone Jt. Surg. 2000, 82, 200–203. [Google Scholar] [CrossRef]
- Ax, M.; Palola, V.; Ponkilainen, V.; Launonen, A.P.; Mattila, V.M. Duration of sick leave after operated and non-operated distal radial fracture: A Finnish cohort study of 19,995 patients. J. Hand Surg. 2024, 49, 316–321. [Google Scholar] [CrossRef]
- De Agustín Del Burgo, J.M.; Blaya Haro, F.; D’Amato, R.; Juanes Méndez, J.A. Development of a smart splint to monitor different parameters during the treatment process. Sensors 2020, 20, 4207. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Parnandi, A.; Eva, S.; Schambra, H. The use of wearable sensors to assess and treat the upper extremity after stroke: A scoping review. Disabil. Rehabil. 2022, 44, 6119–6138. [Google Scholar] [CrossRef] [PubMed]
- Borchani, W.; Aono, K.; Lajnef, N.; Chakrabartty, S. Monitoring of postoperative bone healing using smart trauma-fixation device with integrated self-powered piezo-floating-gate sensors. IEEE Trans. Biomed. Eng. 2015, 63, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process. International Organization for Standardization—ISO: Geneva, Switzerland, 2018. [CrossRef]



| Change in Error () | NB | NS | ZE | PS | PB | |
|---|---|---|---|---|---|---|
| Error (e) | ||||||
| NB | FL | FL | FL | SL | NC | |
| NS | FL | SL | SL | NC | ST | |
| ZE | SL | SL | NC | ST | ST | |
| PS | SL | NC | ST | ST | FT | |
| PB | NC | ST | FT | FT | FT | |
| Group | Number of Cases | Age (, years) | Gender (n) | |
|---|---|---|---|---|
| Male | Female | |||
| Control | 30 | 49.8 ± 3.7 | 17 | 13 |
| Experimental | 30 | 48.6 ± 3.8 | 18 | 12 |
| t | – | −0.649 | 0.040 | |
| p | – | 0.582 | 1.000 | |
| Parameter | Normal Range | Formula | Definitions of Variables |
|---|---|---|---|
| Ulnar Inclination () | 20°–25° | h: Vertical distance from radial styloid tip to ulnar articular surface; w: Horizontal width of radial metaphysis | |
| Volar Tilt () | 10°–15° | : Dorsal cortex height; : Volar cortex height; L: Longitudinal axis length of radius | |
| Radial Height () | 10–15 mm | : Radial styloid apex coordinates; : Projection point on ulnar articular surface line (Perpendicular distance) |
| Domain | Score Range | Measurement Method | Operational Definition |
|---|---|---|---|
| Daily Activity | 0–25 points | 10-item questionnaire assessing functional tasks | Tasks include: cup holding, key turning, object grasping |
| Grip Strength | 0–25 points | Dynamometer-measured ratio (affected/unaffected limb) | , : Affected limb; : Unaffected |
| Range of Motion | 0–25 points | Flexion/extension arc proportion | , : Measured arc |
| Discomfort Intensity | 0–25 points | Patient-reported VAS (0–100 mm) | , VAS: Visual Analog Scale score |
| Total Score | 0–100 | Sum of all subdomains | Total = GS + ROM + DI + DA |
| Group | Ulnar Inclination (°) | Volar Tilt (°) | Radial Height (mm) |
|---|---|---|---|
| Experimental | |||
| Control | |||
| Mean Difference [95% CI] | 4.40 [3.92, 4.88] | 2.43 [2.15, 2.71] | 3.45 [3.26, 3.64] |
| t | 19.813 | 20.671 | 36.450 |
| Cohen’s d | 1.94 | 1.87 | 2.21 |
| p | <0.001 | <0.001 | <0.001 |
| VAS Score | Cooney Score | |||
|---|---|---|---|---|
| Group | Pre-Treatment | Post-Treatment | Pre-Treatment | Post-Treatment |
| Experimental | ||||
| Control | ||||
| Mean Difference [95% CI] | – | −0.73 [−0.98, −0.48] | – | 8.50 [6.50, 10.50] |
| t/Z | 0.074 | Z = 3.491 | 0.290 | 4.370 |
| Cohen’s d | – | 1.23 | – | 1.97 |
| p-value | 0.941 | 0.744 | ||
| Group | Excellent | Good | Fair | Poor | Excellent/Good Rate |
|---|---|---|---|---|---|
| Experimental | 18 (60.0) | 8 (26.7) | 4 (13.3) | 0 | 26 (86.7) |
| Control | 10 (33.3) | 9 (30.0) | 10 (33.3) | 1 (3.3) | 19 (63.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Tang, H.; Wang, B.; Luo, J.; Liu, X. A Personalized 3D-Printed Smart Splint with Integrated Sensors and IoT-Based Control: A Proof-of-Concept Study for Distal Radius Fracture Management. Electronics 2025, 14, 3542. https://doi.org/10.3390/electronics14173542
Ma Y, Tang H, Wang B, Luo J, Liu X. A Personalized 3D-Printed Smart Splint with Integrated Sensors and IoT-Based Control: A Proof-of-Concept Study for Distal Radius Fracture Management. Electronics. 2025; 14(17):3542. https://doi.org/10.3390/electronics14173542
Chicago/Turabian StyleMa, Yufeng, Haoran Tang, Baojian Wang, Jiashuo Luo, and Xiliang Liu. 2025. "A Personalized 3D-Printed Smart Splint with Integrated Sensors and IoT-Based Control: A Proof-of-Concept Study for Distal Radius Fracture Management" Electronics 14, no. 17: 3542. https://doi.org/10.3390/electronics14173542
APA StyleMa, Y., Tang, H., Wang, B., Luo, J., & Liu, X. (2025). A Personalized 3D-Printed Smart Splint with Integrated Sensors and IoT-Based Control: A Proof-of-Concept Study for Distal Radius Fracture Management. Electronics, 14(17), 3542. https://doi.org/10.3390/electronics14173542






