Scientific studies provide a fundamental overview of the principles behind neurorehabilitation, motor control, and neuroplasticity [
7,
8]. Functional recovery is based on the repeated, intensive, and variable execution of specific exercises capable of stimulating cortical reorganisation and the formation of new synaptic connections [
9]. To this end, traditional rehabilitation protocols involve a combination of passive and active techniques, strengthening exercises, occupational therapy, functional electrical stimulation, and increasingly, the use of advanced technologies such as robotics and virtual reality [
10,
11]. Among the various rehabilitation exercises employed, gripping and manipulating a latex ball with the affected hand plays a clinically relevant role [
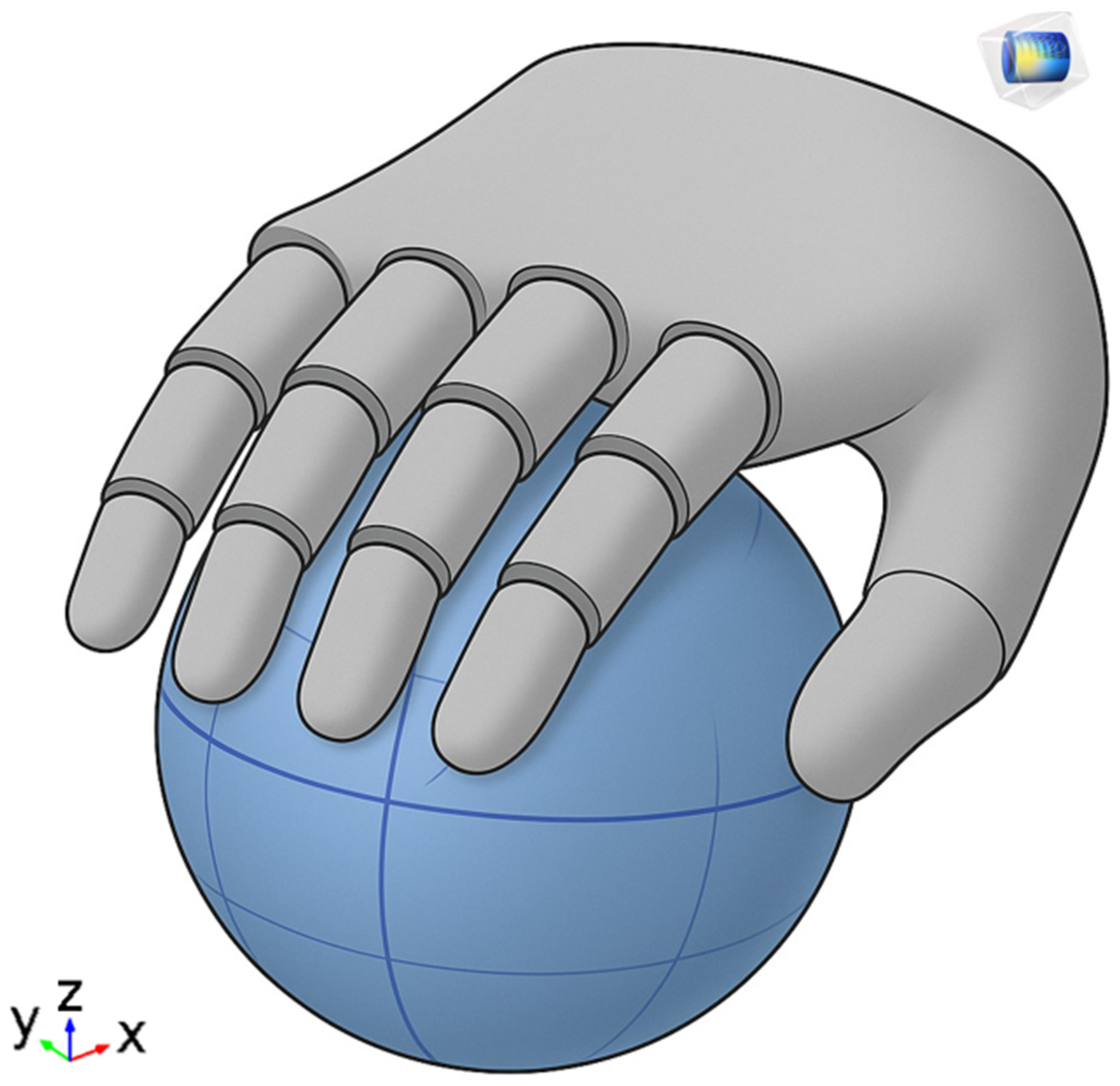
12]. The selection of this exercises for this study is motivated by both its clinical significance and its technical suitability for sensor-based analysis. Gripping tasks provide a robust foundation for biomechanical modelling due to their well-defined contact interactions, and they are frequently included in standardized rehabilitation protocols. This makes them not only a realistic target for monitoring but also a generalizable proxy for evaluating hand motor function recovery.
This simple yet highly effective task stimulates fine motor control, improving grip strength and enhancing proprioceptive feedback. Patients are typically asked to perform repetitive compression movements, as well as coordinated movements such as rolling, rotating, or transferring the ball, which activate multiple muscle groups and joints in a functional and controlled manner [
13]. This exercise is often included in both the early and advanced stages of rehabilitation and serves as an important reference for assessing the patient’s progress over time [
14]. An important role in the rehabilitation process is played by the physiotherapist, who not only guides and adapts exercises based on the patient’s functional level but also takes care of the continuous monitoring of progress through validated scales (e.g., Fugl-Meyer Assessment and Box and Block Test) and clinical observations [
15]. However, in home settings or environments without direct supervision, this monitoring function becomes complex, with the risk of losing therapeutic effectiveness or the emergence of harmful compensatory movements [
16,
17,
18]. In recent years, home-based neurological rehabilitation has undergone a radical transformation thanks to the increasing integration of advanced digital technologies, capable of overcoming many of the limitations of traditional therapeutic models [
19,
20]. Wearable devices, artificial intelligence (AI), and telemonitoring systems have emerged as strategic tools capable of supporting motor recovery in a continuous, personalized, and high-intensity manner, even outside structured clinical settings [
21,
22]. Wearable devices integrated with inertial and force sensors allow for the continuous monitoring of upper limb movements, providing real-time feedback and guidance [
23,
24]. The integration of virtual and augmented reality (VR/AR) in rehabilitation has enabled immersive, engaging environments that improve motor learning and increase patient adherence [
25,
26,
27]. Adaptive rehabilitation scenarios using gamification and AI-driven personalization have shown improved clinical outcomes by increasing engagement and adjusting difficulty levels to patient progress [
28,
29,
30,
31,
32]. The adoption of telemonitoring platforms also allows therapists to access their patients’ clinical and kinematic data in real-time or asynchronously, facilitating remote follow-up, timely modification of the rehabilitation plan, and proactive management of the care pathway [
33,
34]. This approach also allows overcoming geographical barriers and offering quality treatments to patients in remote areas or with mobility difficulties, contributing to greater equity in access to care. The reduction in distances represents only one important aspect of the application on patients. In fact, studies have highlighted the effectiveness of these tools in promoting functional recovery post-stroke or in other chronic neurological conditions. The improvements achieved are tested in terms of strength, dexterity, coordination, and independence in daily activities [
35]. The quantitative data collected in the conducted studies allowed for an objective analysis of rehabilitative progress, overcoming the limits of subjective evaluations and increasing precision in defining therapeutic goals [
36]. Despite their potential, current systems face challenges in usability, interoperability, and the need for large-scale validation [
37]. The combination of wearable devices, virtual reality, and artificial intelligence today represents one of the most promising avenues for revolutionising upper-limb home rehabilitation, making it more intensive, personalised, accessible, and sustainable. The technological approach does not aim to replace the therapist but to enhance their capabilities, extending their effectiveness even in unsupervised contexts. This study addresses these challenges by presenting an integrated system that combines physical modelling, AI-based analysis, and wearable technology to support adaptive and personalized home rehabilitation (see
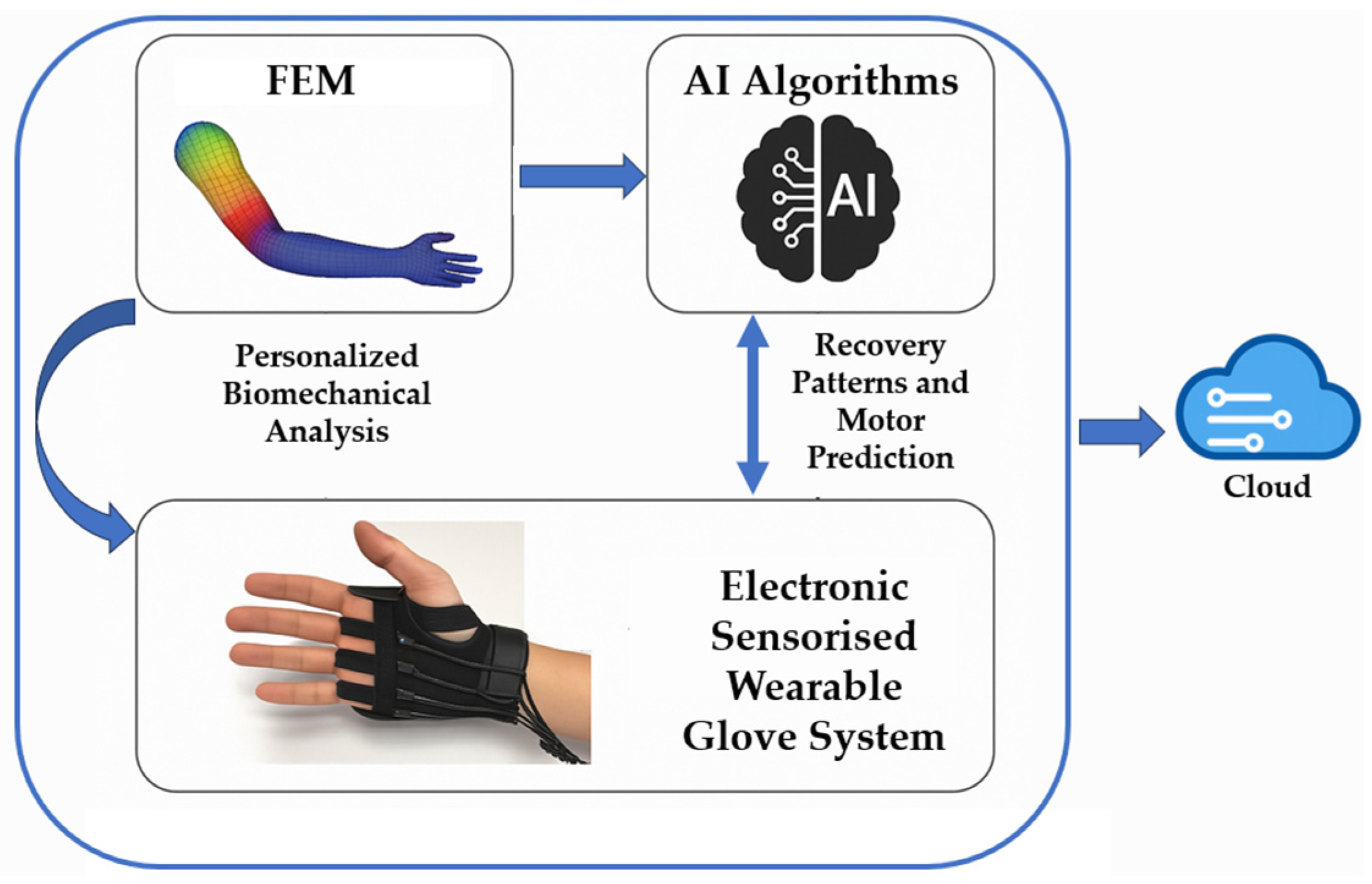
Section 2 for system details). The synergistic integration of biomechanical modelling, machine learning algorithms, and embedded electronic systems represents a challenge to be implemented for continuous monitoring and prediction of motor patterns. The use of finite element modelling (FEM) in the rehabilitation field can offer a detailed representation of joint and muscular dynamics, allowing for more precise adaptation of therapeutic protocols to individual morphological and functional characteristics [
38]. This research fits into this scenario with the aim of developing and validating a hybrid intelligent system that combines the following: physical modelling (FEM) of the upper limbs for personalized biomechanical analysis, artificial intelligence algorithms for the automatic identification of recovery patterns and the prediction of motor evolution, and an integrated electronic sensorised system for the collection and transmission of real-time data. This approach aims to overcome the limitations of current solutions, offering a truly adaptive system, accessible even in home settings, and capable of providing advanced decision support to the physiotherapist. Specifically, the use of FEM modelling allows for the high-detail simulation of the mechanical properties and physiological dynamics of the musculoskeletal structures of the arm and hand. Thanks to FEM, it is possible to construct personalized three-dimensional models derived from individual anatomical data (for example, from MRI or 3D scans) and capable of accurately representing the tensions, forces, deformations, and joint interactions during the execution of an exercise [
39,
40]. In a rehabilitative context, these models can be used to simulate the biomechanical load exerted during specific therapeutic movements, identify areas subject to overload or at risk of postural compensations, or optimize the selection and intensity of exercises to be assigned to the patient. Such a level of detail allows for a deep personalization of protocols, based not only on the general functional level but also on the actual biomechanical characteristics of the individual user, with the aim of maximizing effectiveness and minimizing risks. At the same time, the application of artificial intelligence (AI) algorithms allows for the analysis of large volumes of motor data collected during rehabilitation sessions. Such algorithms are capable of recognizing rehabilitative patterns, classifying motor trajectories based on their quality (fluency, accuracy, and amplitude), and above all, predicting the functional evolution of the patient based on historical data [
41]. The integration of AI enables the automatic evaluation of daily motor performance, the early detection of stagnations or regressions, and the dynamic personalization of the protocol, adapted in real-time to the emerging needs of the patient. This predictive capability offers the physiotherapist quantitative and up-to-date decision support, which can be used to recalibrate the therapeutic intervention more precisely and promptly, optimizing clinical outcomes and maintaining high patient motivation. The third pillar of the proposed study is represented by an embedded platform integrated into a wearable glove, equipped with Inertial Measurement Unit (IMU) sensors, flexion and pressure sensors, and wireless transmission modules. These systems continuously collect data during the execution of exercises, without the need for direct supervision or complex equipment. The raw data are pre-processed locally (edge computing) to reduce latency and then sent to a cloud platform for analysis via AI. The main advantage of this architecture is the ability to operate in real-time and in home environments, without requiring specialized infrastructure, ensuring patient autonomy, monitoring treatment adherence, and detecting any deviations from the prescribed therapeutic protocol. The integration of these three components into a hybrid intelligent system represents a qualitative leap compared to traditional approaches. The combination of a detailed biomechanical model (FEM), an AI-based predictive analytical engine, and an embedded sensorised interface allows not only the ability to observe and evaluate but also to anticipate, adapt, and optimize every aspect of home rehabilitation. This paper is organised as follows:
Section 2 provides an overview of the implemented system.
Section 3 describes the system components:
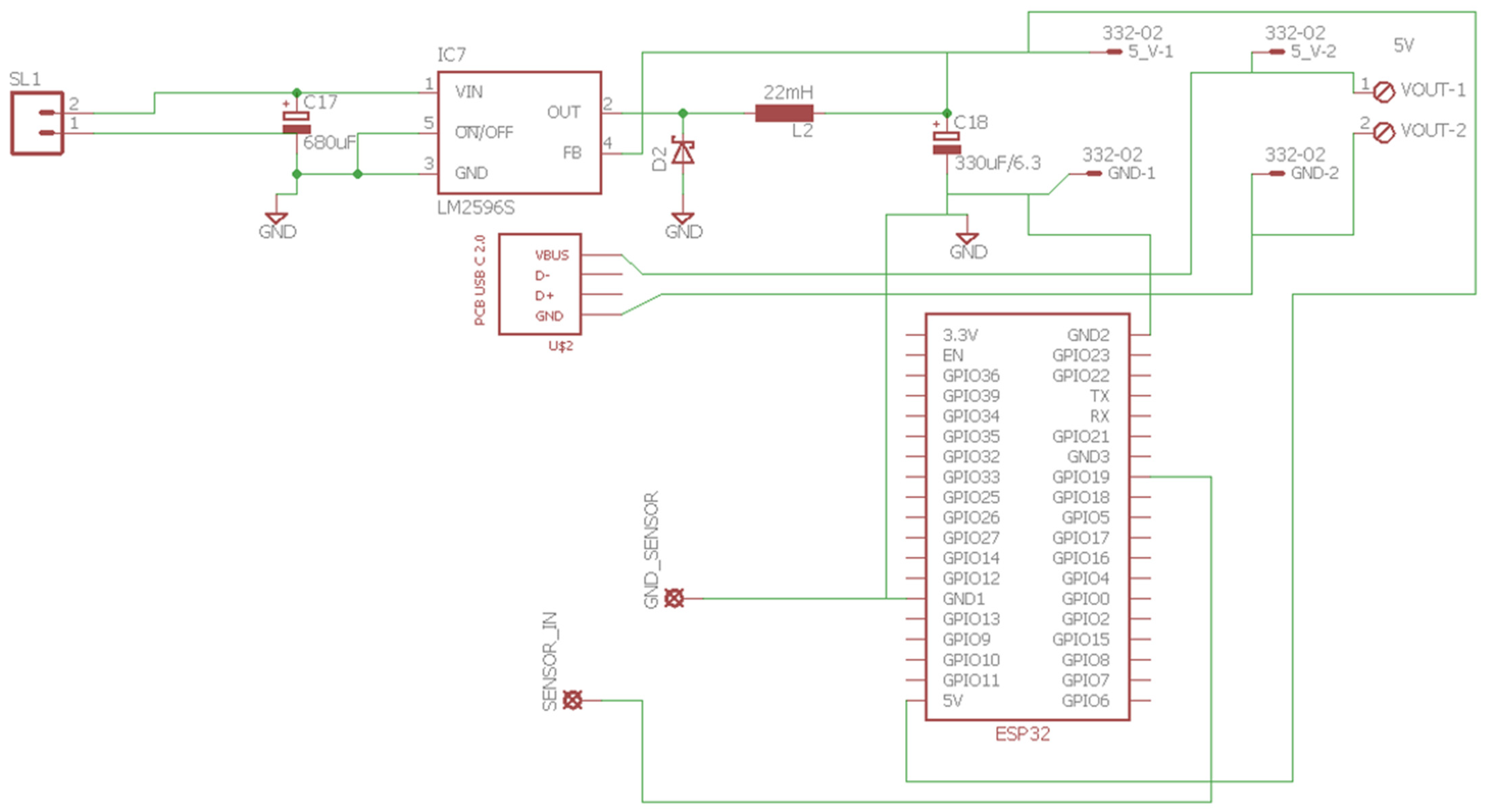
Section 3.1, the COMSOL Multiphysics model;
Section 3.2, the integrated electronic and acquisition system; and
Section 3.3, the computational model.
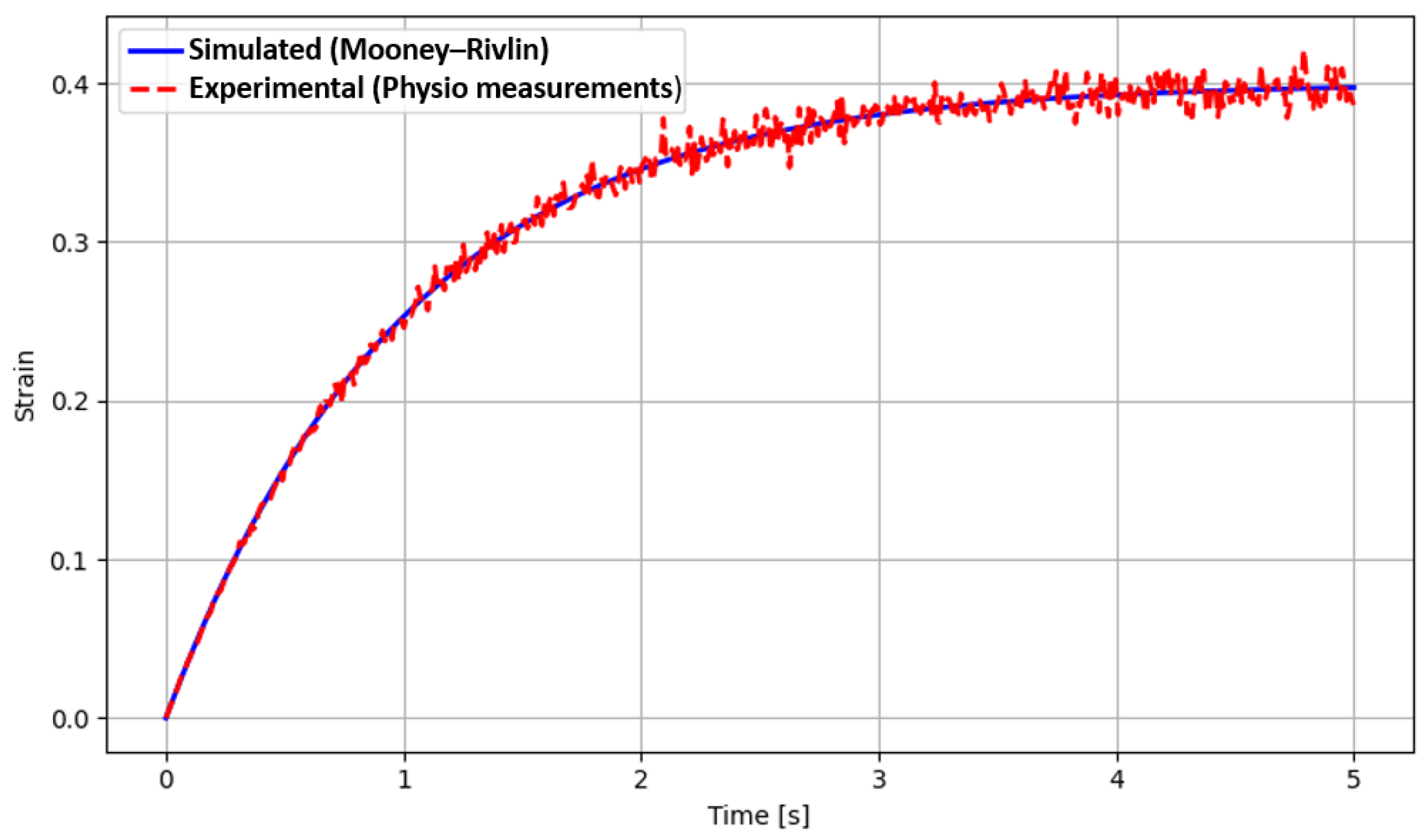
Section 4 presents the experimental results, followed by conclusions and future developments.