Machine Learning First Response to COVID-19: A Systematic Literature Review of Clinical Decision Assistance Approaches during Pandemic Years from 2020 to 2022
Abstract
1. Introduction
2. Research Questions
- RQ1
- Did studies follow open science standards? Specifically, have the data used been published in open access?
- RQ2
- Which ML models have been most frequently proposed and validated?
- RQ3
- Which variables/features are taken into account and which are the most significant risks found?
- RQ4
- Which validation protocols of ML models have been most frequently applied?
- RQ5
- Which performance measures are reported? Which are the performances achieved according to these measures?
3. Methods
Search Strategy
4. Results
4.1. Search Results
Geographical Distribution of the Authors
4.2. RQ 1: Characteristics of Dataset Specifications
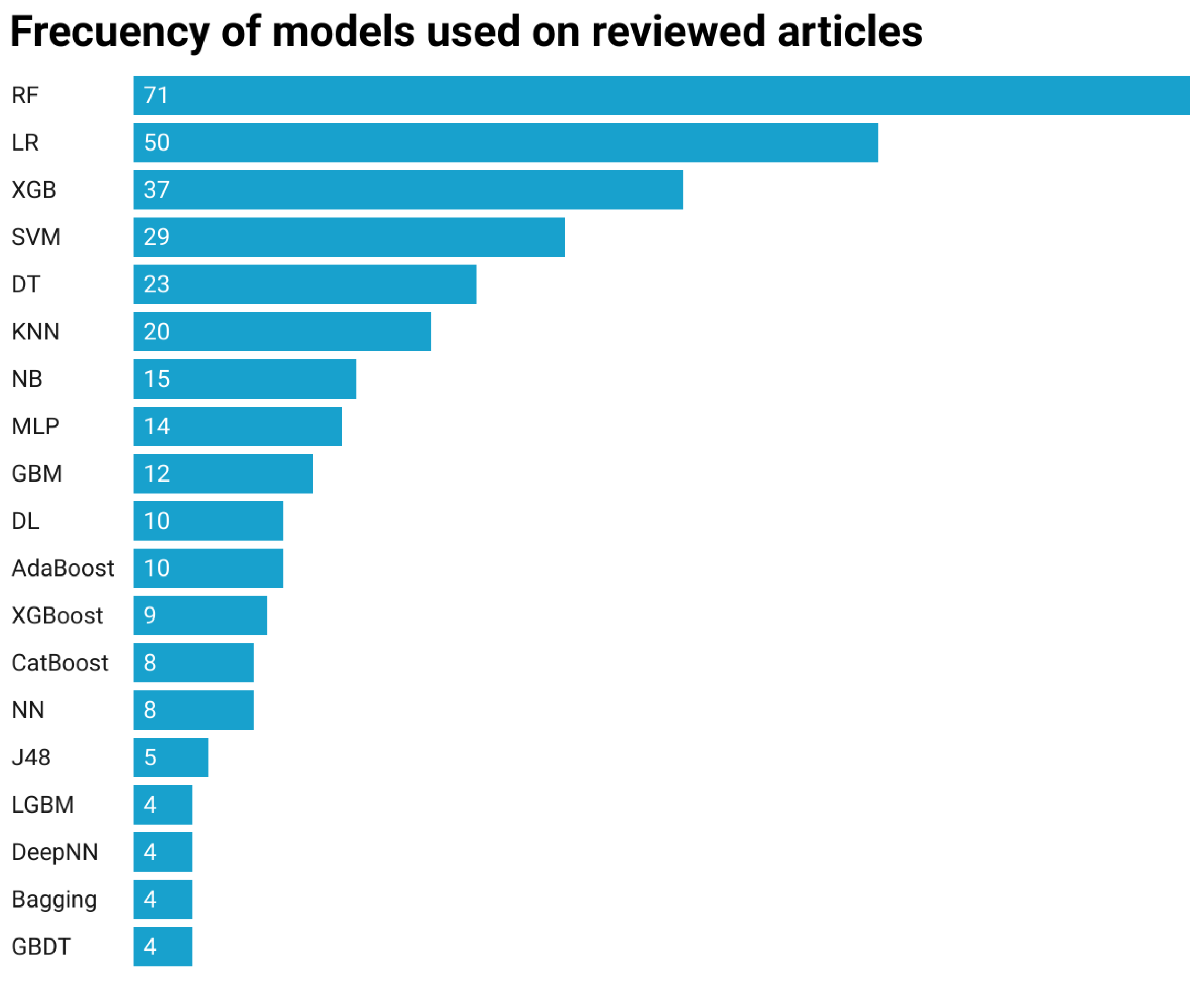
4.3. RQ 2: Machine Learning Algorithms
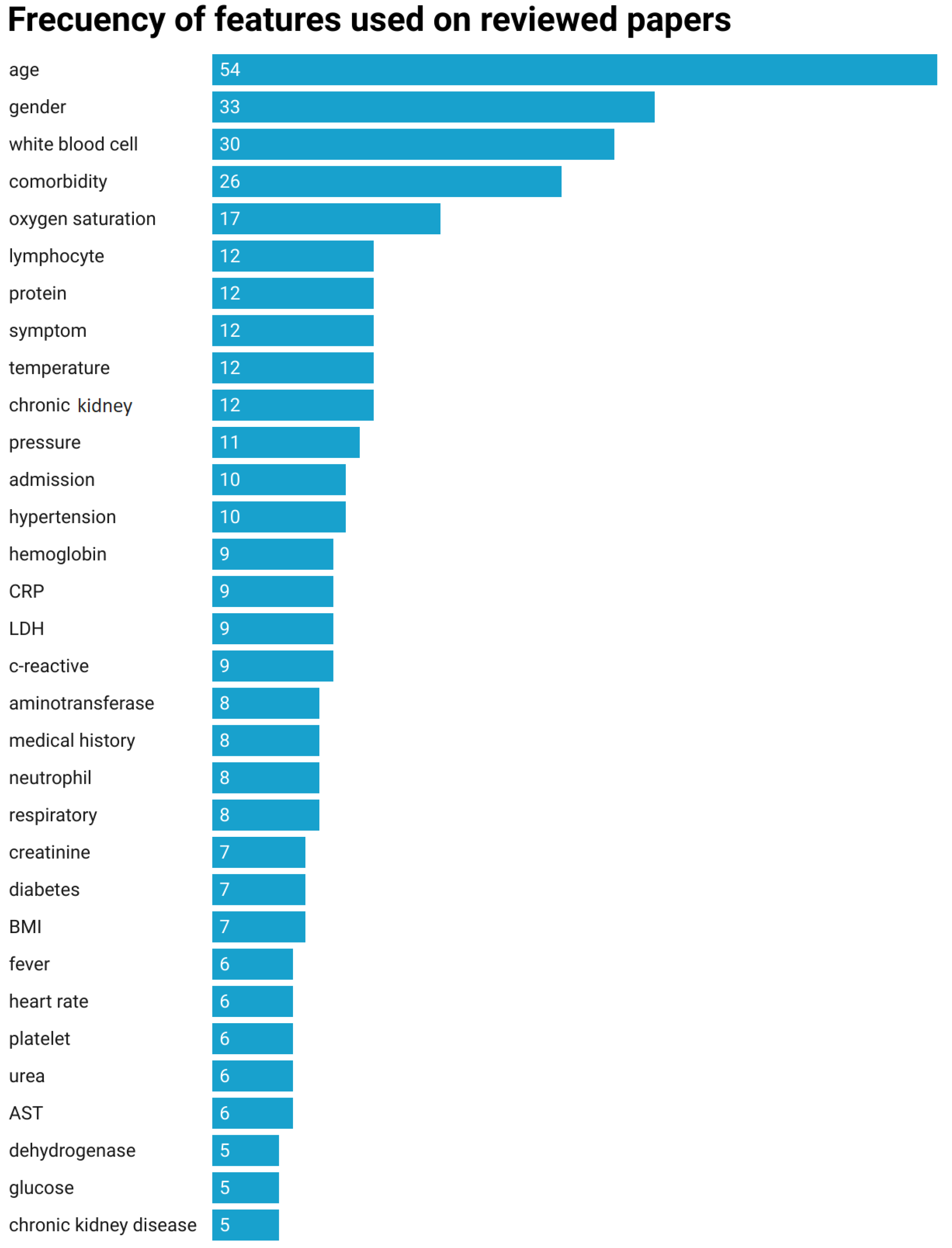
4.4. RQ 3: Features
4.5. RQ 4: Internal and External Validation
4.6. RQ 5: Evaluation Metrics
5. Review of Surveyed Approaches
5.1. Patient Triage Methods
5.2. Prediction of ICU Admission, Progression and Length of Stay
5.3. Mortality Prediction
5.4. Identification of Mortality Risk Factors
5.5. Treatments and Drugs
6. Discussion
- DQ1.
- Are predictive models capable of supporting a COVID-19 outbreak and how?
- DQ2.
- Are there demographic and cultural factors influencing the development of predictive methods to confront or address COVID-19?
- DQ3.
- Are there models with good performance capable of categorizing patients according to severity?
- DQ4.
- Is translation possible from scientific research to clinical practice with the current data on the disease obtained during the pandemic?
7. Insights into ML/AI Advances, Research Directions and Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Acronym | Definition |
| ML | Machine Learning |
| AI | Artificial Intelligence |
| DL | Deep learning |
| ICU | Intensive Care Unit |
| ED | Emergency Department |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| XGB | Extreme Gradient Boosting |
| MLP | Multilayer Perceptron |
| RF | Random Forest |
| LR | Logistic Regression |
| KNN | K-Nearest neighbor |
| GBDT | Gradient Boosting Decision Tree |
| NN | Neural Network |
| DNN | Deep Neural Network |
| DT | Decision Tree |
| NB | Naive Bayes |
| SVM | Support Vector Machine |
| SGD | Stochastic Gradient Descent |
| LGBM | Light GB Machine |
| SGD | Stochastic Gradient Descent |
| ORL | Ordinal Logistic Regression |
| LWL | Locally Weighted Learning |
| AUC | Area Under Curve |
| LDH | lactate dehydrogenase |
| CTSL | Cathepsin L |
| DTI | Drug–target interaction |
| IPD | inpatient mortality probabilities |
| CRP | C-Reactive Protein |
| PCR | Polymerase Chain Reaction |
| PUI | persons under investigation |
| CT | Computerized tomography |
| AST | aspartate transaminase |
| BUN | blood urea nitrogen |
| WBC | white blood cell |
| IGM | gamma interferon-induced monokine |
| SPO2 | Oxygen saturation |
| RR | Respiratory Rate |
| SBP | Systolic Blood pressure |
| DBP | Diastolic Blood Pressure |
| IL-6 | Interleukin 6 |
| LOS | Length of Stay |
References
- Liu, Y.; Gayle, A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, taaa021. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wu, H.Q.; Zhou, D.; Li, K.; Zhang, Y.; Ji, H.; Tong, Z.; Lou, S.; Liu, Z. Application of Big Data and Artificial Intelligence in COVID-19 Prevention, Diagnosis, Treatment and Management Decisions in China. J. Med. Syst. 2021, 45, 84. [Google Scholar] [CrossRef] [PubMed]
- Alafif, T.; Tehame, A.; Bajaba, S.; Barnawi, A.; Zia, S. Machine and Deep Learning towards COVID-19 Diagnosis and Treatment: Survey, Challenges and Future Directions. Int. J. Environ. Res. Public Health 2021, 18, 1117. [Google Scholar] [CrossRef]
- Baby, S.T.; Xavier, S.B.; Kathrine, G.J.W. Prediction of Diabetes and Symptoms of COVID-19 Using Machine Learning Classifiers. In Proceedings of the 2022 International Conference on Applied Artificial Intelligence and Computing (ICAAIC), Salem, India, 9–11 May 2022; pp. 387–393. [Google Scholar] [CrossRef]
- Unberath, M.; Ghobadi, K.; Levin, S.; Hinson, J.; Hager, G.D. Artificial Intelligence-Based Clinical Decision Support for COVID-19-Where Art Thou? Adv. Intell. Syst. 2020, 2, 2000104. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Q.; Inchakalody, V.P.; Merhi, M.; Mestiri, S.; Taib, N.; Moustafa Abo El-Ella, D.; Bedhiafi, T.; Raza, A.; Al-Zaidan, L.; Mohsen, M.O.; et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022, 54, 524–540. [Google Scholar] [CrossRef]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis—A review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, Y.; Huang, Y.M.; Wang, M.; Ling, W.; Sui, Y.; Zhao, H.L. Obesity in patients with COVID-19: A systematic review and meta-analysis. Metabolism 2020, 113, 154378. [Google Scholar] [CrossRef]
- Fernández Villalobos, N.V.; Ott, J.J.; Klett-Tammen, C.J.; Bockey, A.; Vanella, P.; Krause, G.; Lange, B. Effect modification of the association between comorbidities and severe course of COVID-19 disease by age of study participants: A systematic review and meta-analysis. Syst. Rev. 2021, 10, 194. [Google Scholar] [CrossRef]
- Romero Starke, K.; Petereit-Haack, G.; Schubert, M.; Kämpf, D.; Schliebner, A.; Hegewald, J.; Seidler, A. The Age-Related Risk of Severe Outcomes Due to COVID-19 Infection: A Rapid Review, Meta-Analysis and Meta-Regression. Int. J. Environ. Res. Public Health 2020, 17, 5974. [Google Scholar] [CrossRef] [PubMed]
- Romero Starke, K.; Reissig, D.; Petereit-Haack, G.; Schmauder, S.; Nienhaus, A.; Seidler, A. The isolated effect of age on the risk of COVID-19 severe outcomes: A systematic review with meta-analysis. BMJ Glob. Health 2021, 6, e006434. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Kamrowski, C.; Langlois, J.; Rozario, P.; Dircks, I.; Grottodden, K.; Martinez, M.; Tee, W.Z.; Sargeant, K.; LaFleur, C.; et al. A Comprehensive Review of Machine Learning Used to Combat COVID-19. Diagnostics 2022, 12, 1853. [Google Scholar] [CrossRef] [PubMed]
- Abirami, R.S.; Kumar, G.S. Comparative Study Based on Analysis of Coronavirus Disease (COVID-19) Detection and Prediction Using Machine Learning Models. SN Comput. Sci. 2021, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- John, C.C.; Ponnusamy, V.; Krishnan Chandrasekaran, S.; Nandakumar, R. A Survey on Mathematical, Machine Learning and Deep Learning Models for COVID-19 Transmission and Diagnosis. IEEE Rev. Biomed. Eng. 2022, 15, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.M.; Luna, S.A.; Siddique, Z. Machine-Learning-Based Disease Diagnosis: A Comprehensive Review. Healthcare 2022, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Cui, Z.; Ma, X.; Pan, F.; Li, L.; Wang, J.; Sun, P.; Li, H.; Yang, L.; Liang, B. The association of obesity with the progression and outcome of COVID-19: The insight from an artificial-intelligence-based imaging quantitative analysis on computed tomography. Diabetes/Metab. Res. Rev. 2022, 38, e3519. [Google Scholar] [CrossRef]
- Mohanty, S.; Harun Al Rashid, M.; Mridul, M.; Mohanty, C.; Swayamsiddha, S. Application of Artificial Intelligence in COVID-19 drug repurposing. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1027–1031. [Google Scholar] [CrossRef]
- Suri, J.; Puvvula, A.; Majhail, M.; Biswas, M.; Jamthikar, A.; Saba, L.; Faa, G.; Singh, I.; Oberleitner, R.; Turk, M.; et al. Integration of cardiovascular risk assessment with COVID-19 using artificial intelligence. Rev. Cardiovasc. Med. 2020, 21, 541–560. [Google Scholar] [CrossRef]
- Yadollahi, S.; Yadollahi, S.; Zanjani, E.; Khaleghi, F. Application of machine learning and medical imaging in the detection of COVID-19 patients: A review article. J. Family Med. Prim. Care 2022, 11, 2277–2283. [Google Scholar]
- Bhosale, Y.H.; Patnaik, K.S. Application of Deep Learning Techniques in Diagnosis of COVID-19 (Coronavirus): A Systematic Review. Neural Process. Lett. 2022, 55, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ouyang, L.; Bao, F.S.; Li, Q.; Han, L.; Zhang, H.; Zhu, B.; Ge, Y.; Robinson, P.; Xu, M.; et al. A Multimodality Machine Learning Approach to Differentiate Severe and Nonsevere COVID-19: Model Development and Validation. J. Med. Internet Res. 2021, 23, e23948. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, L.; Chen, X.; Zhai, Y.; Zhu, F.; Chen, H.; Wang, Y.; Su, X.; Huang, S.; Tian, L.; et al. A novel artificial intelligence-assisted triage tool to aid in the diagnosis of suspected COVID-19 pneumonia cases in fever clinics. Ann. Transl. Med. 2021, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Bai, Y.; Chen, D.; He, L.; Zhu, J.; Ding, X.; Luo, L.; Ren, Y.; Xing, H.; Jin, X.; et al. Accurate classification of COVID19 patients with different severity via machine learning. Clin. Transl. Med. 2021, 11, e323. [Google Scholar] [CrossRef]
- Wu, P.; Ye, H.; Cai, X.; Li, C.; Li, S.; Chen, M.; Wang, M.; Heidari, A.A.; Chen, M.; Li, J.; et al. An Effective Machine Learning Approach for Identifying Non-Severe and Severe Coronavirus Disease 2019 Patients in a Rural Chinese Population: The Wenzhou Retrospective Study. IEEE Access 2021, 9, 45486–45503. [Google Scholar] [CrossRef]
- Elbaşi, E.; Zreikat, A.; Mathew, S.; Topcu, A. Classification of influenza H1N1 and COVID-19 patient data using machine learning. In Proceedings of the 2021 44th International Conference on Telecommunications and Signal Processing (TSP), Brno, Czech Republic, 26–28 July 2021. [Google Scholar] [CrossRef]
- Marcos, M.; Belhassen-Garcia, M.; Sanchez Puente, A.; Sampedro-Gomez, J.; Azibeiro, R.; Dorado-Díaz, P.I.; Marcano-Millán, E.; Garcia-Vidal, C.; Moreiro-Barroso, M.T.; Cubino-Bóveda, N.; et al. Development of a severity of disease score and classification model by machine learning for hospitalized COVID-19 patients. PLoS ONE 2021, 16, e0240200. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Yao, J.; Chen, A.; Lv, Q.; Zanin, M.; Liu, J.; Wong, S.; Li, Y.; Lu, J.; Liang, H.; et al. Early triage of critically ill COVID-19 patients using deep learning. Nat. Commun. 2020, 11, 3543. [Google Scholar] [CrossRef]
- Soltan, A.; Kouchaki, S.; Zhu, T.; Kiyasseh, D.; Taylor, T.; Hussain, Z.B.; Peto, T.; Brent, A.J.; Eyre, D.W.; Clifton, D.A. Rapid triage for COVID-19 using routine clinical data for patients attending hospital: Development and prospective validation of an artificial intelligence screening test. Lancet Digit. Health 2020, 3, e78–e87. [Google Scholar] [CrossRef]
- Benito-León, J.; Del Castillo, M.; Estirado, A.; Ghosh, R.; Dubey, S.; Serrano, J.I. Using Unsupervised Machine Learning to Identify Age- and Sex-Independent Severity Subgroups Among Patients with COVID-19: Observational Longitudinal Study. J. Med. Internet Res. 2021, 23, e25988. [Google Scholar] [CrossRef]
- Subudhi, S.; Verma, A.; Patel, A. Prognostic machine learning models for COVID-19 to facilitate decision making. Int. J. Clin. Pract. 2020, 74, e13685. [Google Scholar] [CrossRef]
- Sayed, S.; Elkorany, A.; Sayed, S. Applying Different Machine Learning Techniques for Prediction of COVID-19 Severity. IEEE Access 2021, 9, 135697–135707. [Google Scholar] [CrossRef]
- Darapaneni, N.; Gupta, M.; Paduri, A.; Agrawal, R.; Padasali, S.; Kumari, A.; Purushothaman, P. A Novel Machine Learning Based Screening Method For High-Risk COVID-19 Patients Based On Simple Blood Exams. In Proceedings of the 2021 IEEE International IOT, Electronics and Mechatronics Conference (IEMTRONICS), Toronto, ON, Canada, 21–24 April 2021; pp. 1–6. [Google Scholar] [CrossRef]
- La Salvia, M.; Secco, G.; Torti, E.; Florimbi, G.; Guido, L.; Lago, P.; Salinaro, F.; Perlini, S.; Leporati, F. Deep Learning and Lung UltraSound for COVID-19 pneumonia detection and severity classification. Comput. Biol. Med. 2021, 136, 104742. [Google Scholar] [CrossRef]
- Udristoiu, A.L.; Ghenea, A.; Udristoiu, S.; Neaga, M.; Zlatian, O.; Vasile, C.; Popescu, M.; Eugen, T.; Salan, A.; Turcu, A.; et al. COVID-19 and Artificial Intelligence: An Approach to Forecast the Severity of Diagnosis. Life 2021, 11, 1281. [Google Scholar] [CrossRef]
- Quiroz-Juárez, M.A.; torres Gómez, A.; Hoyo-Ulloa, I.; de J. León-Montiel, R.; U’Ren, A.B. Identification of high-risk COVID-19 patients using machine learning. PLoS ONE 2021, 16, e0257234. [Google Scholar] [CrossRef]
- Laatifi, M.; Douzi, S.; Bouklouz, A.; Ezzine, H.; Jaafari, J.; Younes, Z.; Ouahidi, B.; Naciri, M. Machine learning approaches in COVID-19 severity risk prediction in Morocco. J. Big Data 2022, 9, 5. [Google Scholar] [CrossRef]
- Xiong, Y.; Ma, Y.; Ruan, L.; Li, D.; Lu, C.; Huang, L. Comparing different machine learning techniques for predicting COVID-19 severity. Infect. Dis. Poverty 2022, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Moulaei, K.; Shanbehzadeh, M.; Mohammadi-Taghiabad, Z.; Kazemi-Arpanahi, H. Comparing machine learning algorithms for predicting COVID-19 mortality. BMC Med. Inform. Decis. Mak. 2022, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Choi, J.W.; Jiao, Z.; Wang, D.; Wu, J.; Yi, T.; Halsey, K.; Eweje, F.; Tran, L.; Liu, C.; et al. An automated COVID-19 triage pipeline using artificial intelligence based on chest radiographs and clinical data. NPJ Digit. Med. 2022, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Huyut, M. Automatic Detection of Severely and Mildly Infected COVID-19 Patients with Supervised Machine Learning Models. IRBM 2022, 44, 100725. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Kobayashi, D.; Nishioka, E.; Matsuo, H.; Urase, Y.; Onoue, K.; Ishikura, R.; Kitamura, Y.; Sakai, E.; Tomita, M.; et al. Deep learning model for the automatic classification of COVID-19 pneumonia, non-COVID-19 pneumonia and the healthy: A multi-center retrospective study. Sci. Rep. 2022, 12, 8214. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Chen, J.; Feng, H.; Lv, J.; Lu, X.; Ji, M. Early Identification of COVID-19 Progression to Its Severe Form Using Artificial Intelligence. Iran. J. Radiol. 2022, 19, e112562. [Google Scholar] [CrossRef]
- Fu, Y.; Zhong, W.; Liu, T.; Li, J.; Xiao, K.; Ma, X.; Xie, L.; Jiang, J.; Zhou, H.; Liu, R.; et al. Early Prediction Model for Critical Illness of Hospitalized COVID-19 Patients Based on Machine Learning Techniques. Front. Public Health 2022, 10, 880999. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Jo, H.; Lee, H.; Jung, S.Y.; Hwang, H. Machine Learning-Based COVID-19 Patients Triage Algorithm Using Patient-Generated Health Data from Nationwide Multicenter Database. Infect. Dis. Ther. 2022, 11, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, L.; Liu, L.; Sainlaire, M.; Karvar, M.; Kang, M.J.; Pullman, A.; Lipsitz, S.; Massaro, A.; Patil, N.; et al. Predicting Hospitalization of COVID-19 Positive Patients Using Clinician-guided Machine Learning Methods. J. Am. Med. Inform. Assoc. 2022, 29, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, G.W.; Seok, H.; Shin, H.J.; Lee, D.H. Early Triage of COVID-19 patients exploiting Data-Driven Strategies and Machine Learning Techniques. In Proceedings of the 2022 International Conference on Electronics, Information and Communication (ICEIC), Jeju, Republic of Korea, 6–9 February 2022; pp. 234–237. [Google Scholar] [CrossRef]
- Roimi, M.; Gutman, R.; Somer, J.; Arie, A.; Calman, I.; Bar Lavie, Y.; Gelbshtein, U.; Liverant-Taub, S.; Ziv, A.; Eytan, D.; et al. Development and validation of a machine learning model predicting illness trajectory and hospital utilization of COVID-19 patients: A nationwide study. J. Am. Med. Inform. Assoc. 2021, 28, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Migrino, J.; Batangan, A. Using machine learning to create a decision tree model to predict outcomes of COVID-19 cases in the Philippines. West. Pac. Surveill. Response 2021, 12, 56. [Google Scholar] [CrossRef]
- Magunia, H.; Lederer, S.; Verbuecheln, R.; Gilot, B.; Koeppen, M.; Haeberle, H.; Mirakaj, V.; Hofmann, P.; Marx, G.; Bickenbach, J.; et al. Machine learning identifies ICU outcome predictors in a multicenter COVID-19 cohort. Crit. Care 2021, 25, 295. [Google Scholar] [CrossRef]
- Hernández-Pereira, E.; Fontenla-Romero, O.; Bolón-Canedo, V.; Cancela, B.; Guijarro-Berdiñas, B.; Alonso-Betanzos, A. Machine learning techniques to predict different levels of hospital care of COVID-19. Appl. Intell. 2021, 52, 6413–6431. [Google Scholar] [CrossRef]
- Podder, P.; Mondal, M.R. Machine Learning to Predict COVID-19 and ICU Requirement. In Proceedings of the 2020 11th International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 17–19 December 2020. [Google Scholar] [CrossRef]
- Dan, T.; Li, Y.; Zhu, Z.; Chen, X.; Quan, W.; Hu, Y.; Tao, G.; Zhu, L.; Zhu, J.; Jin, Y.; et al. Machine Learning to Predict ICU Admission, ICU Mortality and Survivors’ Length of Stay among COVID-19 Patients: Toward Optimal Allocation of ICU Resources. In Proceedings of the 2020 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Seoul, Republic of Korea, 16–19 December 2020. [Google Scholar] [CrossRef]
- Hossen, M.; Karmoker, D. Predicting the Probability of COVID-19 Recovered in South Asian Countries Based on Healthy Diet Pattern Using a Machine Learning Approach. In Proceedings of the 2020 2nd International Conference on Sustainable Technologies for Industry 4.0 (STI), Dhaka, Bangladesh, 19–20 December 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Mahboub, B.; Albataineh, M.; Alshraideh, H.; Hamoudi, R.; Salameh, L.; Shamayleh, A. Prediction of COVID-19 Hospital Length of Stay and Risk of Death Using Artificial Intelligence-Based Modeling. Front. Med. 2021, 8, 592336. [Google Scholar] [CrossRef]
- Burdick, H.; Lam, C.; Mataraso, S.; Lynn-Palevsky, A.; Braden, G.; Dellinger, R.; McCoy, A.; Vincent, J.L.; Green-Saxena, A.; Barnes, G.; et al. Prediction of respiratory decompensation in COVID-19 patients using machine learning: The READY trial. Comput. Biol. Med. 2020, 124, 103949. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Li, Y.; Xiao, Y.; Han, B.; Su, L.; Su, M.; Li, Y.; Zhang, S.; Jiang, D.; Chen, X.; et al. Prognostic Assessment of COVID-19 in the Intensive Care Unit by Machine Learning Methods: Model Development and Validation. J. Med. Internet Res. 2020, 22, e23128. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Joshi, H.; Tandon, P.; Freeman, R.; Reich, D.; Mazumdar, M.; Kohli-Seth, R.; Levin, M.; Timsina, P.; Kia, A. Using Machine Learning to Predict ICU Transfer in Hospitalized COVID-19 Patients. J. Clin. Med. 2020, 9, 1668. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, S.; Nielsen, M.; Jimenez-Solem, E.; Petersen, T.; Perner, A.; Thorsen-Meyer, H.C.; Igel, C.; Sillesen, M. Using machine learning for predicting intensive care unit resource use during the COVID-19 pandemic in Denmark. Sci. Rep. 2021, 11, 18959. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Hou, Y.; Vasovic, L.V.; Steel, P.; Chadburn, A.; Racine-Brzostek, S.E.; Velu, P.; Cushing, M.M.; Loda, M.; Kaushal, R.; et al. Routine laboratory blood tests predict SARS-CoV-2 infection using machine learning. medRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Saadatmand, S.; Salimifard, K.; Mohammadi, R.; Marzban, M.; Naghibzadeh tahami, A. Predicting the necessity of oxygen therapy in the early stage of COVID-19 using machine learning. Med. Biol. Eng. Comput. 2022, 60, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sollee, J.; Hsieh, C.; Vandal, N.; Shanahan, J.; Choi, J.W.; Tran, L.; Halsey, K.; Iheanacho, F.; Warren, J.; et al. COVID-19 mortality prediction in the intensive care unit with deep learning based on longitudinal chest X-rays and clinical data. Eur. Radiol. 2022, 32, 4446–4456. [Google Scholar] [CrossRef] [PubMed]
- Aslam, N. Explainable Artificial Intelligence Approach for the Early Prediction of Ventilator Support and Mortality in COVID-19 Patients. Computation 2022, 10, 36. [Google Scholar] [CrossRef]
- Afrash, M.R.; Kazemi-Arpanahi, H.; Shanbehzadeh, M.; Nopour, R.; Mirbagheri, E. Predicting hospital readmission risk in patients with COVID-19: A machine learning approach. Inform. Med. Unlocked 2022, 30, 100908. [Google Scholar] [CrossRef] [PubMed]
- Boussen, S.; Cordier, P.Y.; Malet, A.; Simeone, P.; Cataldi, S.; Vaisse, C.; Roche, X.; Castelli, A.; Assal, M.; Pepin, G.; et al. Triage and monitoring of COVID-19 patients in intensive care using unsupervised machine learning. Comput. Biol. Med. 2021, 142, 105192. [Google Scholar] [CrossRef]
- Noy, O.; Coster, D.; Metzger, M.; Atar, I.; Shenhar-Tsarfaty, S.; Berliner, S.; Rahav, G.; Rogowski, O.; Shamir, R. A machine learning model for predicting deterioration of COVID-19 inpatients. Sci. Rep. 2022, 12, 2630. [Google Scholar] [CrossRef]
- Mahmud, S.; Soltanikazemi, E.; Boadu, F.; Dhakal, A.; Cheng, J. Deep Learning Prediction of Severe Health Risks for Pediatric COVID-19 Patients with a Large Feature Set in 2021 BARDA Data Challenge. arXiv 2022, arXiv:2206.01696v2. [Google Scholar]
- Elhazmi, A.; Al-Omari, A.; Sallam, H.; Mufti, H.; Rabie, A.; Alshahrani, M.; Mady, A.; Alghamdi, A.; Altalaq, A.; Azzam, M.; et al. Machine learning decision tree algorithm role for predicting mortality in critically ill adult COVID-19 patients admitted to the ICU. J. Infect. Public Health 2022, 15, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Alabbad, D.; Almuhaideb, A.; Alsunaidi, S.; Alqudaihi, K.; Alamoudi, F.; Alhobaishi, M.; Alaqeel, N.; Alshahrani, M. Machine learning model for predicting the length of stay in the intensive care unit for COVID-19 patients in the eastern province of Saudi Arabia. Inform. Med. Unlocked 2022, 30, 100937. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Nishimura, K.; Ogawa, K.; Miyake, N.; Mizobuchi, T.; Shigeta, K.; Obinata, H.; Takayama, Y.; Tagami, T.; Seike, M.; et al. Machine Learning Prediction for Supplemental Oxygen Requirement in Patients with COVID-19. J. Nippon. Med. Sch. 2021, 89, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Heldt, F.; Vizcaychipi, M.; Peacock, S.; Cinelli, M.; McLachlan, L.; Andreotti, F.; Jovanović, S.; Dürichen, R.; Lipunova, N.; Fletcher, R.; et al. Early risk assessment for COVID-19 patients from emergency department data using machine learning. Sci. Rep. 2021, 11, 4200. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, S.; Esteban-Aizpiri, C.; Lafuente, I.; Barrio, I.; Raúl, Q.L.; Quintana, J.; Uranga, A.; Orive, M.; González, N.; Anton, A.; et al. Machine learning-based model for prediction of clinical deterioration in hospitalized patients by COVID 19. Sci. Rep. 2022, 12, 7097. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Angelotti, G.; Caruso, P.; Zanella, A.; Stomeo, N.; Costantini, E.; Protti, A.; Pesenti, A.; Grasselli, G.; Cecconi, M. Outcome prediction during an ICU surge using a purely data-driven approach: A supervised machine learning case-study in critically ill patients from COVID-19 Lombardy outbreak. Int. J. Med. Inform. 2022, 164, 104807. [Google Scholar] [CrossRef]
- Shanbehzadeh, M.; Yazdani, A.; Shafiee, M.; Kazemi-Arpanahi, H. Predictive modeling for COVID-19 readmission risk using machine learning algorithms. Bmc Med. Inform. Decis. Mak. 2022, 22, 139. [Google Scholar] [CrossRef]
- Etu, E.E.; Monplaisir, L.; Arslanturk, S.; Masoud, S.; Aguwa, C.; Markevych, I.; Miller, J. Prediction of Length of Stay in the Emergency Department for COVID-19 Patients: A Machine Learning Approach. IEEE Access 2022, 10, 42243–42251. [Google Scholar] [CrossRef]
- Metz, J.; Thoral, P.; Chorus, C.; Elbers, P.; van den Bogaard, B. Behavioural artificial intelligence technology for COVID-19 intensivist triage decisions: Making the implicit explicit. Intensive Care Med. 2021, 47, 1327–1328. [Google Scholar] [CrossRef]
- Halasz, G.; Sperti, M.; Villani, M.; Michelucci, U.; Agostoni, P.; Biagi, A.; Rossi, L.; Botti, A.; Mari, C.; Maccarini, M.; et al. A machine learning approach for mortality prediction in COVID-19 pneumonia Development and evaluation of the Piacenza score. J. Med. Internet Res. 2021, 23, e29058. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Dong, D.; Li, L.; Niu, M.; Bai, Y.; Wang, M.; Qiu, X.; Zha, Y.; Tian, J. A Deep Learning Prognosis Model Help Alert for COVID-19 Patients at High-Risk of Death: A Multi-center Study. IEEE J. Biomed. Health Inform. 2020, 24, 3576–3584. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Choubdar, H.; Zabeh, E.; Rieder, M.; Safavi-Naeini, S.; Jobbagy, Z.; Ghorbani, A.; Abedini, A.; Kiani, A.; Khanlarzadeh, V.; et al. A machine learning based exploration of COVID-19 mortality risk. PLoS ONE 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Blagojević, A.; Šušteršič, T.; Lorencin, I.; Baressi Šegota, S.; Andelić, N.; Milovanovic, D.; Baskić, D.; Baskic, D.; Petrović, N.; Sazdanović, P.; et al. Artificial intelligence approach towards assessment of condition of COVID-19 patients—Identification of predictive biomarkers associated with severity of clinical condition and disease progression. Comput. Biol. Med. 2021, 138, 104869. [Google Scholar] [CrossRef]
- Zawbaa, H.; El-Gendy, A.; Saeed, H.; Osama, H.; Ali, A.; Gomaa, D.; Abdelrahman, M.; Harb, H.; Madney, Y.M.; Abdelrahim, M. A study of the possible factors affecting COVID-19 spread, severity and mortality and the effect of social distancing on these factors: Machine learning forecasting model. Int. J. Clin. Pract. 2021, 75, e14116. [Google Scholar] [CrossRef]
- Sotoudeh, H.; Shafaat, O.; Sotoudeh, E.; Tasorian, B.; Sarrami, A.; Didehdar, M.; Tabatabaei, M. Accuracy of Machine Learning Models to Predict Mortality in COVID-19 Infection Using the Clinical and Laboratory Data at the Time of Admission. Cureus 2021, 13, e18768. [Google Scholar] [CrossRef]
- Fidan, H.; Yuksel, M. A comparative study for determining COVID-19 risk levels by unsupervised machine learning methods. Expert Syst. Appl. 2021, 190, 116243. [Google Scholar] [CrossRef]
- Shi, B.; Ye, H.; Zheng, L.; Lyu, J.; Chen, C.; Heidari, A.A.; Hu, Z.; Chen, H.; Wu, P. Evolutionary warning system for COVID-19 severity: Colony predation algorithm enhanced extreme learning machine. Comput. Biol. Med. 2021, 136, 104698. [Google Scholar] [CrossRef]
- Krysko, O.; Kondakova, E.; Vershinina, O.; Galova, E.; Blagonravova, A.; Gorshkova, E.; Bachert, C.; Ivanchenko, M.; Krysko, D.; Vedunova, M. Artificial Intelligence Predicts Severity of COVID-19 Based on Correlation of Exaggerated Monocyte Activation, Excessive Organ Damage and Hyperinflammatory Syndrome: A Prospective Clinical Study. Front. Immunol. 2021, 12, 3298. [Google Scholar] [CrossRef]
- Yaşar, Ş.; Colak, C.; Yologlu, S. Artificial Intelligence-Based Prediction of COVID-19 Severity on the Results of Protein Profiling. Comput. Methods Programs Biomed. 2021, 202, 105996. [Google Scholar] [CrossRef]
- Domínguez-Olmedo, J.L.; Gragera-Martínez, Á.; Mata, J.; Pachón Álvarez, V. Machine Learning Applied to Clinical Laboratory Data in Spain for COVID-19 Outcome Prediction: Model Development and Validation. J. Med. Internet Res. 2021, 23, e26211. [Google Scholar] [CrossRef]
- Garrafa, E.; Vezzoli, M.; Ravanelli, M.; Farina, D.; Borghesi, A.; Calza, S.; Maroldi, R. Early prediction of in-hospital death of COVID-19 patients: A machine-learning model based on age, blood analyses and chest X-ray score. eLife 2021, 10, e70640. [Google Scholar] [CrossRef] [PubMed]
- Baqui, P.; Marra, V.; Alaa, A.; Bica, I.; Ercole, A.; Schaar, M. Comparing COVID-19 risk factors in Brazil using machine learning: The importance of socioeconomic, demographic and structural factors. Sci. Rep. 2021, 11, 15591. [Google Scholar] [CrossRef] [PubMed]
- Di Castelnuovo, A.; Bonaccio, M.; Costanzo, S.; Gialluisi, A.; Antinori, A.; Berselli, N.; Blandi, L.; Bruno, R.; Cauda, R.; Guaraldi, G.; et al. Common cardiovascular risk factors and in-hospital mortality in 3894 patients with COVID-19: Survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1899–1913. [Google Scholar] [CrossRef] [PubMed]
- Tezza, F.; Lorenzoni, G.; Azzolina, D.; Barbar, S.; Leone, L.; Gregori, D. Predicting in-Hospital Mortality of Patients with COVID-19 Using Machine Learning Techniques. J. Pers. Med. 2021, 11, 343. [Google Scholar] [CrossRef]
- Wang, X.; Che, Q.; Ji, X.; Meng, X.; Zhang, L.; Jia, R.; Lyu, H.; Bai, W.; Tan, L.; Gao, Y. Correlation between lung infection severity and clinical laboratory indicators in patients with COVID-19: A cross-sectional study based on machine learning. BMC Infect. Dis. 2021, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Bai, S.; Chen, Q.; Zhou, Y.; Xia, L.; Qin, L.; Gong, S.; Xie, X.; Zhou, C.; Tu, D.; et al. Deep learning for predicting COVID-19 malignant progression. Med. Image Anal. 2021, 72, 102096. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zhang, B.; Fu, M.; Li, M.; Yuan, X.; Zhu, Y.; Peng, J.; Guo, H.; Lu, Y. Clinical and inflammatory features based machine learning model for fatal risk prediction of hospitalized COVID-19 patients: Results from a retrospective cohort study. Ann. Med. 2021, 53, 257–266. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Balan, J.; Walsh, J.; Wu, Y.; Minnich, S.; Piazza, A.; Osborne, C.; Oliver, G.; Lesko, J.; Bates, K.; et al. COVID-19 mortality prediction from deep learning in a large multistate EHR and LIS dataset: Algorithm development and validation (Preprint). J. Med. Internet Res. 2021, 23, e30157. [Google Scholar] [CrossRef]
- Schöning, V.; Liakoni, E.; Baumgartner, C.; Exadaktylos, A.; Hautz, W.; Atkinson, A.; Hammann, F. Development and validation of a prognostic COVID-19 severity assessment (COSA) score and machine learning models for patient triage at a tertiary hospital. J. Transl. Med. 2021, 19, 56. [Google Scholar] [CrossRef]
- Zhu, J.; Ge, P.; Jiang, C.; Zhang, Y.; Li, X.; Zhao, Z.; Zhang, L.; Duong, T. Deep-learning artificial intelligence analysis of clinical variables predicts mortality in COVID-19 patients. J. Am. Coll. Emerg. Physicians Open 2020, 1, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, Y.; Zhu, T.; Fan, M.; Xu, S.; Qiu, W.; Chen, C.; Li, L.; Wang, Y.; Yan, J.; et al. Development and external evaluation of predictions models for mortality of COVID-19 patients using machine learning method. Neural Comput. Appl. 2021, 35, 13037–13046. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, J.C.; Feng, Y.Z.; Cheng, Z.Y.; Rezazadegan, D.; Chen, P.K.; Lin, Q.T.; Qian, L.; Liu, X.F.; Berkovsky, S.; Coiera, E.; et al. Development and Validation of a Machine Learning Approach for Automated Severity Assessment of COVID-19 Based on Clinical and Imaging Data: Retrospective Study. JMIR Med. Inform. 2021, 9, e24572. [Google Scholar] [CrossRef] [PubMed]
- de Fátima, A.; Stremel, D.; Fachi, M.; Surek, M.; Wiens, A.; Stumpf Tonin, F.; Pontarolo, R. Diagnosis and prediction of COVID-19 severity: Can biochemical tests and machine learning be used as prognostic indicators? Comput. Biol. Med. 2021, 134, 104531. [Google Scholar] [CrossRef]
- Booth, A.; Abels, E.; McCaffrey, P. Development of a prognostic model for mortality in COVID-19 infection using machine learning. Mod. Pathol. 2020, 34, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Q.; Liu, T.; Luo, P.; Zhou, Y.; Liu, M.; Xiong, B.; Zhou, F. Development and Validation of Predictors for the Survival of Patients with COVID-19 Based on Machine Learning. Front. Med. 2021, 8, 683431. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Z.; Jiang, Y.; Shi, O.; Zhang, X.; Xu, K.; Suo, C.; Wang, Q.; Song, Y.; Yu, K.; et al. Early prediction of mortality risk among patients with severe COVID-19, using machine learning. Int. J. Epidemiol. 2020, 49, 1918–1929. [Google Scholar] [CrossRef]
- Zhou, K.; Ss, T.; Li, L.; Zang, Z.; Wang, J.; Li, J.; Liang, J.; Zhang, F.; Zhang, Q.; Ge, W.; et al. Eleven Routine Clinical Features Predict COVID-19 Severity Uncovered by Machine Learning of Longitudinal Measurements. Comput. Struct. Biotechnol. J. 2021, 19, 3640–3649. [Google Scholar] [CrossRef]
- Shi, W.; Peng, X.; Liu, T.; Cheng, Z.; Lu, H.; Yang, S.; Zhang, J.; Wang, M.; Gao, Y.; Shi, Y.; et al. A deep learning-based quantitative computed tomography model for predicting the severity of COVID-19: A retrospective study of 196 patients. Ann. Transl. Med. 2021, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, E.; Malchiodi, D.; Trucco, G.; Frasca, M.; Cappelletti, L.; Fontana, T.; Esposito, A.; Avola, E.; Jachetti, A.; Reese, J.; et al. Explainable Machine Learning for Early Assessment of COVID-19 Risk Prediction in Emergency Departments. IEEE Access 2020, 8, 196299–196325. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hua, M.; Zhu, F. Machine Learning Algorithms are Superior to Conventional Regression Models in Predicting Risk Stratification of COVID-19 Patients. Risk Manag. Healthc. Policy 2021, 14, 3159–3166. [Google Scholar] [CrossRef]
- Aznar-Gimeno, R.; Esteban, L.; Lezaun, G.; del Hoyo-Alonso, R.; Abadia-Gallego, D.; Paño-Pardo, J.; Esquillor-Rodrigo, M.; Lanas, A.; Serrano, M.T. A Clinical Decision Web to Predict ICU Admission or Death for Patients Hospitalised with COVID-19 Using Machine Learning Algorithms. Int. J. Environ. Res. Public Health 2021, 18, 8677. [Google Scholar] [CrossRef]
- Dabbah, M.; Reed, A.; Booth, A.; Yassaee, A.; Despotovic, A.; Klasmer, B.; Binning, E.; Aral, M.; Plans, D.; Morelli, D.; et al. Machine learning approach to dynamic risk modeling of mortality in COVID-19: A UK Biobank study. Sci. Rep. 2021, 11, 16936. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Chung, H.; Kang, W.S.; Park, C.; Kim, D.W.; Kim, S.E.; Chung, C.R.; Ko, R.E.; Lee, H.; Seo, J.H.; et al. An Artificial Intelligence Model to Predict the Mortality of COVID-19 Patients at Hospital Admission Time Using Routine Blood Samples: Development and Validation of an Ensemble Model. J. Med. Internet Res. 2020, 22, e25442. [Google Scholar] [CrossRef]
- Sánchez-Montañés, M.; Rodriguez, P.; Serrano-López, A.; Olivas, E.; Alakhdar-Mohmara, Y. Machine Learning for Mortality Analysis in Patients with COVID-19. Int. J. Environ. Res. Public Health 2020, 17, 8386. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Kher, V.; Desai, B.; Lei, X.; Cen, S.; Nanda, N.; Gholamrezanezhad, A.; Duddalwar, V.; Varghese, B.; Oberai, A. Machine learning based predictors for COVID-19 disease severity. Sci. Rep. 2021, 11, 4673. [Google Scholar] [CrossRef]
- Aktar, S.; Talukder, A.; Ahamad, M.; Kamal, A.; Khan, J.; Hossain, N.; Azad, A.K.M.; Quinn, J.; Summers, M.; Liaw, S.T.; et al. Machine Learning Approaches to Identify Patient Comorbidities and Symptoms That Increased Risk of Mortality in COVID-19. Diagnostics 2021, 11, 1383. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cai, G.; Fang, W.; Li, H.Y.; Wang, S.Y.; Chen, L.; Yu, Y.; Liu, D.; Xu, S.; Cui, P.F.; et al. Machine Learning Based Early Warning System Enables Accurate Mortality Risk Prediction for COVID-19. Nat. Commun. 2020, 11, 5033. [Google Scholar] [CrossRef]
- Yu, L.; Halalau, A.; Dalal, B.; Abbas, A.; Ivascu, F.; Amin, M.; Nair, G. Machine learning methods to predict mechanical ventilation and mortality in patients with COVID-19. PLoS ONE 2021, 16, e0249285. [Google Scholar] [CrossRef]
- Shiri, I.; Salimi, Y.; Pakbin, M.; Hajianfar, G.; Haddadi Avval, A.; Sanaat, A.; Mostafaei, S.; Akhavanallaf, A.; Saberi Manesh, A.; Mansouri, Z.; et al. COVID-19 prognostic modeling using CT radiomic features and machine learning algorithms: Analysis of a multi-institutional dataset of 14,339 patients. Comput. Biol. Med. 2022, 145, 105467. [Google Scholar] [CrossRef]
- Lipták, P.; Banovcin, P.; Rosoľanka, R.; Prokopič, M.; Kocan, I.; Žiačiková, I.; Uhrik, P.; Grendár, M.; Hyrdel, R. A machine learning approach for identification of gastrointestinal predictors for the risk of COVID-19 related hospitalization. PeerJ 2022, 10, e13124. [Google Scholar] [CrossRef]
- Yarbakhsh, R.; Mortazavi, S.; Mortazavi, S.; Parsaei, H.; Rad, D. Artificial intelligence effectively predicts the COVID-19 death rate in different UK cities. J. Intell. Fuzzy Syst. 2022, 43, 1853–1857. [Google Scholar] [CrossRef]
- Mohammad, R.; Aljabri, M.; Aboulnour, M.; Mirza, S.; Alshobaiki, A. Classifying the Mortality of People with Underlying Health Conditions Affected by COVID-19 Using Machine Learning Techniques. Appl. Comput. Intell. Soft Comput. 2022, 2022, 3783058. [Google Scholar] [CrossRef]
- Baik, S.M.; Lee, M.; Hong, K.S.; Park, D.J. Development of Machine-Learning Model to Predict COVID-19 Mortality: Application of Ensemble Model and Regarding Feature Impacts. Diagnostics 2022, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.K.; Huang, R.X.; Tulu, T.; Liu, J.D.; Vodencarevic, A.; Wong, C.W.; Chan, K.h. Identifying Predictors of COVID-19 Mortality Using Machine Learning. Life 2022, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Azizi, Z.; Shiba, Y.; Alipour, P.; Maleki, F.; Raparelli, V.; Norris, C.; Forghani, R.; Pilote, L.; Emam, K. Importance of sex and gender factors for COVID-19 infection and hospitalisation: A sex-stratified analysis using machine learning in UK Biobank data. BMJ Open 2022, 12, e050450. [Google Scholar] [CrossRef] [PubMed]
- Vezzoli, M.; Inciardi, R.; Oriecuia, C.; Paris, S.; Murillo, N.; Agostoni, P.; Ameri, P.; Bellasi, A.; Camporotondo, R.; Canale, C.; et al. Machine learning for prediction of in-hospital mortality in coronavirus disease 2019 patients: Results from an Italian multicenter study. J. Cardiovasc. Med. 2022, 23, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Reina Reina, A.; Barrera, J.; Valdivieso, B.; Gas Lopez, M.E.; Maté, A.; Trujillo, J. Machine learning model from a Spanish cohort for prediction of SARS-CoV-2 mortality risk and critical patients. Sci. Rep. 2022, 12, 5723. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Singh, H.; Mavuduru, R.; Pattanaik, S.; Rana, P. Quantifying prognosis severity of COVID-19 patients from deep learning based analysis of CT chest images. Multimed. Tools Appl. 2022, 81, 18129–18153. [Google Scholar] [CrossRef]
- Chen, L.; Mei, Z.; Guo, W.; Ding, S.; Huang, T.; Cai, Y.D. Recognition of Immune Cell Markers of COVID-19 Severity with Machine Learning Methods. BioMed Res. Int. 2022, 2022, 6089242. [Google Scholar] [CrossRef]
- Mazloumi, R.; Abazari, S.R.; Nafarieh, F.; Aghsami, A.; Jolai, F. Statistical analysis of blood characteristics of COVID-19 patientsand their survival or death prediction using machine learningalgorithms. Neural Comput. Appl. 2022, 34, 14729–14743. [Google Scholar] [CrossRef]
- Agrawal, S.; Patil, N. Machine Learning based COVID-19 Mortality Prediction using Common Patient Data. In Proceedings of the 2022 IEEE 7th International Conference for Convergence in Technology (I2CT), Mumbai, India, 7–9 April 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Laino, M.; Generali, E.; Tommasini, T.; Angelotti, G.; Aghemo, A.; Desai, A.; Morandini, P.; Stefanini, G.; Lleo, A.; Voza, A.; et al. An Individualized Algorithm to Predict Mortality in COVID-19 Pneumonia: A Machine Learning Based Study. Arch. Med. Sci. 2022, 18, 587. [Google Scholar] [CrossRef] [PubMed]
- Bertram, M.G.; Sundin, J.; Roche, D.G.; Sánchez-Tójar, A.; Thoré, E.S.J.; Brodin, T. Open science. Curr. Biol. 2023, 33, R792–R797. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Ishak, M.K.; Bhatti, M.K.L. A Machine Learning Approach for Early COVID-19 Symptoms Identification. Comput. Mater. Contin. 2022, 70, 3803–3820. [Google Scholar] [CrossRef]
- Tadepalli, S.; Thulasiram, R. COVID-19 Early Symptom Prediction Using Blockchain and Machine Learning. In Blockchain and Applications: 3rd International Congress; Springer: Berlin/Heidelberg, Germany, 2022; pp. 243–251. [Google Scholar] [CrossRef]
- Silva, C.; Junior, A.; Lopes, R. Predictive Analysis of COVID-19 Symptoms in Social Networks through Machine Learning. Electronics 2022, 11, 580. [Google Scholar] [CrossRef]
- Chen, Z.; Li, M.; Wang, R.; Sun, W.; Liu, J.; Li, H.; Wang, T.; Lian, Y.; Zhang, J.; Wang, X. Diagnosis of COVID-19 via Acoustic Analysis and Artificial Intelligence by Monitoring Breath Sounds on Smartphones. J. Biomed. Inform. 2022, 130, 104078. [Google Scholar] [CrossRef]
- Lee, D.; Wang, C.; McAlister, F.; Ma, S.; Chu, A.; Rochon, P.; Kaul, P.; Austin, P.; Wang, X.; Kalmady, S.; et al. Factors associated with SARS-CoV-2 test positivity in long-term care homes: A population-based cohort analysis using machine learning. Lancet Reg. Health Am. 2022, 6, 100146. [Google Scholar] [CrossRef] [PubMed]
- Gorji, F.; Shafiekhani, S.; Namdar, P.; Abdollahzade, S.; Rafiei, S. Machine learning-based COVID-19 diagnosis by demographic characteristics and clinical data. Adv. Respir. Med. 2022, 90, 171–183. [Google Scholar] [CrossRef]
- Sharma, D.; Subramanian, M.; Malyadri, P.; Reddy, B.; Sharma, D.; Tahreem, M. Classification of COVID -19 by Using Supervised Optimized Machine Learning Technique. Mater. Today Proc. 2021, 56, 2058–2062. [Google Scholar] [CrossRef]
- Chadaga, K.; Chakraborty, C.; Prabhu, S.; Umakanth, S.; Bhat, V.; Sampathila, N. Clinical and Laboratory Approach to Diagnose COVID-19 Using Machine Learning. Interdiscip. Sci. Comput. Life Sci. 2022, 14, 452–470. [Google Scholar] [CrossRef]
- Thimoteo, L.; Vellasco, M.; Amaral, J.; Figueiredo, K.; Yokoyama, C.; Marques, E. Explainable Artificial Intelligence for COVID-19 Diagnosis Through Blood Test Variables. J. Control Autom. Electr. Syst. 2022, 33, 625–644. [Google Scholar] [CrossRef]
- Sridhar, A.; Chen, Z.H.; Mayfield, J.; Fohner, A.; Arvanitis, P.; Atkinson, S.; Braunschweig, F.; Chatterjee, N.; Zamponi, A.; Johnson, G.; et al. Identifying Risk of Adverse Outcomes in COVID-19 Patients via Artificial Intelligence-Powered Analysis of 12-Lead Intake Electrocardiogram. Cardiovasc. Digit. Health J. 2021, 3, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Kapoor, A.; Mahajan, G.; Kapur, A. Use of Artificial Intelligence to Triage Patients with Flu-Like Symptoms Using Imaging in Non- COVID-19 Hospitals during COVID-19 Pandemic: An Ongoing 8-Month Experience. Indian J. Radiol. Imaging 2022, 31, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Cabras, S. A Bayesian-Deep Learning Model for Estimating COVID-19 Evolution in Spain. Mathematics 2021, 9, 2921. [Google Scholar] [CrossRef]
- Soltan, A.; Yang, J.; Pattanshetty, R.; Novak, A.; Yang, Y.; Rohanian, O.; Beer, S.; Soltan, M.; Thickett, D.; Fairhead, R.; et al. Real-world evaluation of rapid and laboratory-free COVID-19 triage for emergency care: External validation and pilot deployment of artificial intelligence driven screening. Lancet Digit. Health 2022, 4, e266–e278. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, D.; Kim, J.H.; Kim, D.; Ha, B.; Seog, W.; Lee, Y.K.; Lim, D.; Hong, S.; Park, M.J.; et al. An Easy-to-Use Machine Learning Model to Predict the Prognosis of Patients With COVID-19: Retrospective Cohort Study. J. Med. Internet Res. 2020, 22, e24225. [Google Scholar] [CrossRef]
- Mueller, Y.; Schrama, T.; Ruijten, R.; Schreurs, M.; Grashof, D.; van de Werken, H.; Jona Lasinio, G.; Alvarez-de la Sierra, D.; Kiernan, C.; Eiro, M.; et al. Stratification of hospitalized COVID-19 patients into clinical severity progression groups by immuno-phenotyping and machine learning. Nat. Commun. 2022, 13, 915. [Google Scholar] [CrossRef]
- Hou, W.; Zhao, Z.; Chen, A.; Li, H.; Duong, T. Machining learning predicts the need for escalated care and mortality in COVID-19 patients from clinical variables. Int. J. Med. Sci. 2021, 18, 1739–1745. [Google Scholar] [CrossRef]
- Campbell, T.W.; Wilson, M.P.; Roder, H.; MaWhinney, S.; Georgantas, R.W.; Maguire, L.K.; Roder, J.; Erlandson, K.M. Predicting prognosis in COVID-19 patients using machine learning and readily available clinical data. Int. J. Med. Inform. 2021, 155, 104594. [Google Scholar] [CrossRef]
- Vaid, A.; Somani, S.; Russak, A.; Freitas, J.; Chaudhry, F.; Paranjpe, I.; Johnson, K.; Lee, S.; Miotto, R.; Richter, F.; et al. Machine Learning to Predict Mortality and Critical Events in a Cohort of Patients With COVID-19 in New York City: Model Development and Validation. J. Med. Internet Res. 2020, 22, e24018. [Google Scholar] [CrossRef]
- Kar, S.; Chawla, R.; Haranath, S.; Ramasubban, S.; Ramakrishnan, N.; Vaishya, R.; Sibal, A.; Reddy, S. Multivariable mortality risk prediction using machine learning for COVID-19 patients at admission (AICOVID). Sci. Rep. 2021, 11, 12801. [Google Scholar] [CrossRef]
- Xu, W.; Sun, N.N.; Gao, H.N.; Chen, Z.Y.; Yang, Y.; Bin, J.; Tang, L.L. Risk factors analysis of COVID-19 patients with ARDS and prediction based on machine learning. Sci. Rep. 2021, 11, 2933. [Google Scholar] [CrossRef]
- Rahman, T.; Khandakar, A.; Haque, M.; Ibtehaz, N.; Kashem, S.; Islam, M.; Al-Maadeed, S.; Zughaier, S.; Doi, S.; Chowdhury, M. Development and Validation of an Early Scoring System for Prediction of Disease Severity in COVID-19 Using Complete Blood Count Parameters. IEEE Access 2021, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Ko, H.; Kang, W.S.; Kim, K.W.; Lee, H.; Park, C.; Song, H.O.; Choi, T.Y.; Seo, J.H.; Lee, J. Prediction and Feature Importance Analysis for Severity of COVID-19 in South Korea Using Artificial Intelligence: Model Development and Validation. J. Med. Internet Res. 2021, 23, e27060. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Al-Ishaq, F.; Al-Mohannadi, F.; Mubarak, R.; Al-Hitmi, M.; Islam, K.; Khandakar, A.; Ait Hssain, A.; Al-Madeed, S.; Zughaier, S.; et al. Mortality Prediction Utilizing Blood Biomarkers to Predict the Severity of COVID-19 Using Machine Learning Technique. Diagnostics 2021, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, K.; Goldstein, D.; Szymanski, J.; Bellin, E.; Stahl, L.; Yagi, Y.; Saada, M.; Simone, K.; Reyes, M.; Billett, H. Using Automated-Machine Learning to Predict COVID-19 Patient Mortality (Preprint). J. Med. Internet Res. 2020, 23, e23458. [Google Scholar] [CrossRef] [PubMed]
- Munera, N.; Garcia-Gallo, E.; Gonzalez, Á.; Zea, J.; Fuentes, Y.; Serrano, C.; Ruiz-Cuartas, A.; Rodríguez, A.; Reyes, L. A novel model to predict severe COVID-19 and mortality using an artificial intelligence algorithm to interpret chest X-Rays and clinical variables. ERJ Open Res. 2022, 8, 00010–2022. [Google Scholar] [CrossRef] [PubMed]
- Cihan, P. The machine learning approach for predicting the number of intensive care, intubated patients and death: The COVID-19 pandemic in Turkey. Sigma J. Eng. Nat. Sci. 2022, 40, 85–94. [Google Scholar] [CrossRef]
- Izquierdo, J.L.; Ancochea, J.; Soriano, J.B. Clinical Characteristics and Prognostic Factors for Intensive Care Unit Admission of Patients With COVID-19: Retrospective Study Using Machine Learning and Natural Language Processing. J. Med. Internet Res. 2020, 22, e21801. [Google Scholar] [CrossRef] [PubMed]
- Nino, G.; Linguraru, M.G. Developing artificial intelligence technology for pediatric pulmonology: Lessons from COVID-19. Pediatr. Pulmonol. 2022, 57, 1588. [Google Scholar] [CrossRef]
- Subudhi, S.; Verma, A.; Patel, A.; Hardin, C.; Khandekar, M.; Lee, H.; Mcevoy, D.; Stylianopoulos, T.; Munn, L.; Dutta, S.; et al. Comparing machine learning algorithms for predicting ICU admission and mortality in COVID-19. npj Digit. Med. 2021, 4, 87. [Google Scholar] [CrossRef]
- Shamout, F.; Shen, Y.; Wu, N.; Kaku, A.; Park, J.; Makino, T.; Jastrzębski, S.; Witowski, J.; Wang, D.; Zhang, B.; et al. An artificial intelligence system for predicting the deterioration of COVID-19 patients in the emergency department. npj Digit. Med. 2021, 4, 80. [Google Scholar] [CrossRef]
- Arévalo, J.; Gómez, J.; Casas, J.; Antón-Santos, J.; Melero-Bermejo, J.; López-Carmona, M.; Palacios, L.; Sanz-Cánovas, J.; Pesqueira-Fontán, P.; Peña-Fernández, A.; et al. The importance of association of comorbidities on COVID-19 outcomes: A machine learning approach. Curr. Med. Res. Opin. 2022, 38, 501–510. [Google Scholar] [CrossRef]
- Kalabarige, L.R.; Maringanti, H. Symptom Based COVID-19 Test Recommendation System Using Machine Learning Technique. Intell. Decis. Technol. 2022, 16, 181–191. [Google Scholar] [CrossRef]
- Li, X.; Ge, P.; Zhu, J.; Li, H.; Graham, J.; Singer, A.; Richman, P.; Duong, T. Deep learning prediction of likelihood of ICU admission and mortality in COVID-19 patients using clinical variables. PeerJ 2020, 8, e10337. [Google Scholar] [CrossRef]
- Arvind, V.; Kim, J.; Cho, B.; Geng, E.; Cho, S. Development of a machine learning algorithm to predict intubation among hospitalized patients with COVID-19. J. Crit. Care 2021, 62, 25–30. [Google Scholar] [CrossRef]
- Famiglini, L.; Campagner, A.; Carobene, A.; Cabitza, F. A robust and parsimonious machine learning method to predict ICU admission of COVID-19 patients. Med. Biol. Eng. Comput. 2022. [CrossRef] [PubMed]
- Bolourani, S.; Brenner, M.; Wang, P.; McGinn, T.; Hirsch, J.S.; Barnaby, D.; Zanos, T.P. A Machine Learning Prediction Model of Respiratory Failure Within 48 Hours of Patient Admission for COVID-19: Model Development and Validation. J. Med. Internet Res. 2021, 23, e24246. [Google Scholar] [CrossRef]
- Izadi, Z.; Gianfrancesco, M.; Hyrich, K.; Strangfeld, A.; Gossec, L.; Carmona, L.; Mateus, E.; Lawson-Tovey, S.; Trupin, L.; Rush, S.; et al. Machine learning algorithms to predict COVID-19 acute respiratory distress syndrome in patients with rheumatic diseases: Results from the global rheumatology alliance provider registry. Ann. Rheum. Dis. 2021, 80, 175–176. [Google Scholar] [CrossRef]
- Bouhamed, H.; Hamdi, M.; Gargouri, R. COVID-19 Patients’ Hospital Occupancy Prediction During the Recent Omicron Wave via some Recurrent Deep Learning Architectures. Int. J. Comput. Commun. Control 2022, 17, 4697. [Google Scholar] [CrossRef]
- Laino, M.; Ammirabile, A.; Lofino, L.; Lundon, D.; Chiti, A.; Francone, M.; Savevski, V. Prognostic findings for ICU admission in patients with COVID-19 pneumonia: Baseline and follow-up chest CT and the added value of artificial intelligence. Emerg. Radiol. 2022, 29, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.P.P.; Kitamura, F.; Prado, G.; Kuriki, P.; Garcia, M. Machine learning model for predicting severity prognosis in patients infected with COVID-19: Study protocol from COVID-AI Brasil. PLoS ONE 2021, 16, e0245384. [Google Scholar] [CrossRef]
- van de Leur, R.; Bleijendaal, H.; Taha, K.; Mast, T.; Gho, J.; Linschoten, M.; Rees, B.; Henkens, M.; Heymans, S.; Sturkenboom, N.; et al. Electrocardiogram-based mortality prediction in patients with COVID-19 using machine learning. Neth. Heart J. 2022, 30, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Maria, A.; Dimitrios, V.; Ioanna, M.; Charalampos, M.; Gerasimos, M.; Constantinos, K. Clinical Decision Making and Outcome Prediction for COVID-19 Patients Using Machine Learning. In Pervasive Computing Technologies for Healthcare, Proceedings of the 15th EAI International Conference, Pervasive Health 2021, Virtual Event, 6–8 December 2021; Lewy, H., Barkan, R., Eds.; Springer: Cham, Switzerland, 2022; pp. 3–14. [Google Scholar]
- Huang, F.; Chen, L.; Guo, W.; Zhou, X.; Feng, K.; Huang, T.; Cai, Y. Identifying COVID-19 Severity-Related SARS-CoV-2 Mutation Using a Machine Learning Method. Life 2022, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Boddu, R.S.K.; Karmakar, P.; Bhaumik, A.; Nassa, V.K.; Vandana; Bhattacharya, S. Analyzing the impact of Machine learning and Artificial intelligence and its Effect on Management of lung cancer detection in COVID-19 pandemic. Mater. Today Proc. 2021, 56, 2213–2216. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; D’Silva, K.; Li, M.; Hsu, T.; DiIorio, M.; Fu, X.; Cook, C.; Prisco, L.; Martin, L.; Vanni, K.; et al. Assessing the Severity of COVID-19 Lung Injury in Rheumatic Diseases versus the General Population Using Deep Learning-Derived Chest Radiograph Scores. Arthritis Care Res. 2022, 75, 657–666. [Google Scholar] [CrossRef]
- Gadipudi, P.; Teja, P.; Yelamancheli, C.; Thaniserikaran, A.; Mohiuddin, R.; Joy, A. Detection of pneumonia progression in lungs of individuals affected with COVID-19 severely using deep learning techniques. In Proceedings of the 2022 3rd International Conference for Emerging Technology (INCET), Belgaum, India, 27–29 May 2022; pp. 1–5. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, S.; Wang, S.; Yu, X.; Wang, S.J.; Yao, L.; Pan, Y.; Zhang, Y.D. Diagnosis of COVID-19 pneumonia via a novel deep learning architecture. J. Comput. Sci. Technol. 2022, 37, 0679. [Google Scholar] [CrossRef]
- Aftab, M.; Amin, R.; Koundal, D.; Aldabbas, H.; Alouffi, B.; Iqbal, Z. Classification of COVID-19 and Influenza Patients Using Deep Learning. Contrast Media Mol. Imaging 2022, 2022, 8549707. [Google Scholar] [CrossRef]
- Jingxin, L.; Mengchao, Z.; Yuchen, L.; Jinglei, C.; Yutong, Z.; Zhong, Z.; Lihui, Z. COVID-19 lesion detection and segmentation—A deep learning method. Methods 2021, 202, 62–69. [Google Scholar] [CrossRef]
- Shahin, O.; M. Abd El Aziz, R.; Taloba, A. Detection and classification of COVID-19 in CT-lungs screening using machine learning techniques. J. Interdiscip. Math. 2022, 25, 791–813. [Google Scholar] [CrossRef]
- Aswathy, A.; Anand, H.; Chandra, V. COVID-19 severity detection using machine learning techniques from CT-images. Evol. Intell. 2022, 16, 1423–1431. [Google Scholar] [CrossRef]
- Shahin, O.; Alshammari, H.; Taloba, A.; M. Abd El Aziz, R. Machine Learning Approach for Autonomous Detection and Classification of COVID-19 Virus. Comput. Electr. Eng. 2022, 101, 108055. [Google Scholar] [CrossRef]
- Chieregato, M.; Frangiamore, F.; Morassi, M.; Baresi, C.; Nici, S.; Bassetti, C.; Bnà, C.; Galelli, M. A hybrid machine learning/deep learning COVID-19 severity predictive model from CT images and clinical data. Sci. Rep. 2022, 12, 4329. [Google Scholar] [CrossRef]
- Venkataramana, L.; Prasad, D.; Shunmuganathan, S.; Mithumary, C.; Karthikeyan, R.; Monika, N. Classification of COVID-19 from tuberculosis and pneumonia using deep learning techniques. Med. Biol. Eng. Comput. 2022, 60, 2681–2691. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, A.L.; Hareendran, A.; Vinod Chandra, S.S. COVID-19 diagnosis and severity detection from CT-images using transfer learning and back propagation neural network. J. Infect. Public Health 2021, 14, 1435–1445. [Google Scholar] [CrossRef]
- Purkayastha, S.; Xiao, Y.; Jiao, Z.; Thepumnoeysuk, R.; Halsey, K.; Wu, J.; Tran, L.; Hsieh, B.; Choi, J.W.; Wang, D.; et al. Machine Learning-Based Prediction of COVID-19 Severity and Progression to Critical Illness Using CT Imaging and Clinical Data. Korean J. Radiol. 2021, 22, 1213. [Google Scholar] [CrossRef]
- Gazzah, S.; Bayi, R.; Kaloun, S.; Bencharef, O. A Deep Learning to Distinguish COVID-19 from Others Pneumonia Cases. Intell. Autom. Soft Comput. 2021, 31, 677–693. [Google Scholar] [CrossRef]
- Elkamouny, M.; Ghantous, M. Pneumonia Classification for COVID-19 Based on Machine Learning. In Proceedings of the 2022 2nd International Mobile, Intelligent and Ubiquitous Computing Conference (MIUCC), Cairo, Egypt, 8–9 May 2022; pp. 135–140. [Google Scholar] [CrossRef]
- Liu, Q.; Pang, B.; Li, H.; Zhang, B.; Liu, Y.; Lai, L.; Le, W.; Li, J.; Xia, T.; Zhang, X.; et al. Machine learning models for predicting critical illness risk in hospitalized patients with COVID-19 pneumonia. J. Thorac. Dis. 2021, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Ye, Q.; Ding, W.; Jiang, Y.; Wang, M.; Niu, Z.; Zhou, X.; Gao, Y.; Wang, C.; Menpes-Smith, W.; et al. Can Clinical Symptoms and Laboratory Results Predict CT Abnormality? Initial Findings Using Novel Machine Learning Techniques in Children With COVID-19 Infections. Front. Med. 2021, 8, 699984. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Cui, Z.; Pan, F.; Li, L.; Li, L.; Liang, B.; Yang, L.; Zheng, C. Glycemic status affects the severity of coronavirus disease 2019 in patients with diabetes mellitus: An observational study of CT radiological manifestations using an artificial intelligence algorithm. Acta Diabetol. 2021, 58, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Vepa, A.; Saleem, A.; Rakhshan, K.; Daneshkhah, A.; Sedighi, T.; Shohaimi, S.; Omar, A.; Salari, N.; Chatrabgoun, O.; Dharmaraj, D.; et al. Using Machine Learning Algorithms to Develop a Clinical Decision-Making Tool for COVID-19 Inpatients. Int. J. Environ. Res. Public Health 2021, 18, 6228. [Google Scholar] [CrossRef]
- Guzmán-Torres, J.A.; Alonso-Guzmán, E.M.; Domínguez-Mota, F.J.; Tinoco-Guerrero, G. Estimation of the main conditions in (SARS-CoV-2) COVID-19 patients that increase the risk of death using Machine learning, the case of Mexico. Results Phys. 2021, 27, 104483. [Google Scholar] [CrossRef]
- Iacolare, B.; Perrone, V.; Sangiorgi, D.; Ghigi, A.; Giacomini, E.; Nappi, C.; Paoli, D.; Ancona, D.; Andretta, M.; Barbieri, A.; et al. POSA310 Artificial Intelligence Applied on Administrative Big Data to Predict the Severity of SARS-CoV-2 Infection. Value Health 2022, 25, S199–S200. [Google Scholar] [CrossRef]
- Ho, D. Addressing COVID-19 Drug Development with Artificial Intelligence. Adv. Intell. Syst. 2020, 2, 2000070. [Google Scholar] [CrossRef]
- Arora, G.; Joshi, J.; Mandal, R.; Shrivastava, N.; Virmani, R.; Sethi, T. Artificial Intelligence in Surveillance, Diagnosis, Drug Discovery and Vaccine Development against COVID-19. Pathogens 2021, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Vera, D.; Sinclair, D. Can artificial intelligence identify effective COVID-19 therapies? Embo Mol. Med. 2020, 12, e12817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, T.; Xi, H.; Juhas, M.; Li, J. Deep Learning Driven Drug Discovery: Tackling Severe Acute Respiratory Syndrome Coronavirus 2. Front. Microbiol. 2021, 12, 739684. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Kronberg, R.; Stachura, P.; Ostermann, P.; Müller, L.; Schaal, H.; Bhatia, S.; Kather, J.; Borkhardt, A.; Pandyra, A.; et al. Deep Transfer Learning Approach for Automatic Recognition of Drug Toxicity and Inhibition of SARS-CoV-2. Viruses 2021, 13, 610. [Google Scholar] [CrossRef]
- Majumdar, S.; Nandi, S.; Ghosal, S.; Ghosh, B.; Mallik, W.; Dutta Roy, N.; Biswas, A.; Mukherjee, S.; Pal, S.; Bhattacharyya, N. Deep Learning-Based Potential Ligand Prediction Framework for COVID-19 with Drug–Target Interaction Model. Cogn. Comput. 2021. [Google Scholar] [CrossRef]
- Mikkili, I.; Peele, A.; Vekateswarulu, T.; Vidya Prabhakar, K.; Macamdas, D.; Sreerama, K. Potential of artificial intelligence to accelerate diagnosis and drug discovery for COVID-19. PeerJ 2021, 9, e12073. [Google Scholar] [CrossRef]
- Gawriljuk, V.; Zin, P.P.; Puhl, A.; Zorn, K.; Foil, D.; Lane, T.; Hurst, B.; Almeida Tavella, T.; Costa, F.; Lakshmanane, P.; et al. Machine Learning Models Identify Inhibitors of SARS-CoV-2. J. Chem. Inf. Model. 2021, 61, 4224–4235. [Google Scholar] [CrossRef]
- Suvarna, K.; Biswas, D.; Pai, M.G.; Acharjee, A.; Bankar, R.; Palanivel, V.; Salkar, A.; Verma, A.; Mukherjee, A.; Choudhury, M.; et al. Proteomics and Machine Learning Approaches Reveal a Set of Prognositc Markers for COVID-19 Severity With Drug Repurposing Potential. Front. Physiol. 2021, 12, 652799. [Google Scholar] [CrossRef]
- Han, L.; Shan, G.; Chu, B.; Wang, H.; Wang, Z.; Gao, S.; Zhou, W. Accelerating drug repurposing for COVID-19 treatment by modeling mechanisms of action using cell image features and machine learning. Cogn. Neurodyn. 2021, 17, 803–811. [Google Scholar] [CrossRef]
- Aghdam, R.; Habibi, M.; Taheri, G. Using informative features in machine learning based method for COVID-19 drug repurposing. J. Cheminform. 2021, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Prashar, D.; Rashid, M.; Shafiq, M.; Khan, R.; Pruncu, C.; Siddiqui, S.; Saravana Kumar, M. Deep Learning Approach for Discovery of in Silico Drugs for Combating COVID-19. J. Healthc. Eng. 2021, 2021, 6668985. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Qiu, Y.; Zeng, J.; Xie, L.; Zhang, P. A deep learning framework for high-throughput mechanism-driven phenotype compound screening and its application to COVID-19 drug repurposing. Nat. Mach. Intell. 2021, 3, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Auwul, M.; Rahman, M.R.; Gov, E.; Shahjaman, M.; Moni, M.A. Bioinformatics and machine learning approach identifies potential drug targets and pathways in COVID-19. Briefings Bioinform. 2021, 22, bbab120. [Google Scholar] [CrossRef] [PubMed]
- Bhati, A.; Wan, S.; Alfè, D.; Clyde, A.; Bode, M.; Tan, L.; Titov, M.; Merzky, A.; Turilli, M.; Jha, S.; et al. Pandemic Drugs at Pandemic Speed: Infrastructure for Accelerating COVID-19 Drug Discovery with Hybrid Machine Learning- and Physics-based Simulations on High Performance Computers. Interface Focus 2021, 11, 20210018. [Google Scholar] [CrossRef]
- Abdel-Basset, M.; Hawash, H.; Elhoseny, M.; Chakrabortty, R.; Ryan, M. DeepH-DTA: Deep Learning for Predicting Drug-Target Interactions: A Case Study of COVID-19 Drug Repurposing. IEEE Access 2020, 8, 170433–170451. [Google Scholar] [CrossRef]
- Nguyen, T.; Pham, D.H.; Hiep, D.; Phuong-Thao, T.; Quang, D.; Ngo, S.T. Searching and designing potential inhibitors for SARS-CoV-2 Mpro from natural sources using atomistic and deep-learning calculations. RSC Adv. 2021, 11, 38495–38504. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, F.; Tang, J.; Nussinov, R.; Cheng, F. Artificial intelligence in COVID-19 drug repurposing. Lancet. Digit. Health 2020, 2, e667–e676. [Google Scholar] [CrossRef]
- Kolitz, S.; Kim, J.; Zhang, J.; Cha, Y.; Battula, S.; Kusko, R.; Krishnan, R.; Zeskind, B.; Kaufman, H. 477 Deep learning to drive COVID-19 rapid drug repurposing. J. Immunother. Cancer 2020, 8, A509. [Google Scholar] [CrossRef]
- Loucera, C.; Esteban-Medina, M.; Rian, K.; Marín Falco, M.; Dopazo, J.; Peña-Chilet, M. Drug repurposing for COVID-19 using machine learning and mechanistic models of signal transduction circuits related to SARS-CoV-2 infection. Signal Transduct. Target. Ther. 2020, 5, 290. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Nath, P.; Chatterjee, M.; Das, N.; Kalita, D.; Roy, P.; Satapathi, S. Repurposing therapeutics for COVID-19: Rapid prediction of commercially available drugs through machine learning and docking. PLoS ONE 2020, 15, e0241543. [Google Scholar] [CrossRef] [PubMed]
- El-Behery, H.; Attia, A.F.; El-Fishawy, N.; Torkey, H. Efficient machine learning model for predicting drug-target interactions with case study for COVID-19. Comput. Biol. Chem. 2021, 93, 107536. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Thakur, A.; Mukhopadhyay, A.; Kamboj, S.; Rastogi, A.; Gautam, S.; Jassal, H.; Kumar, M. Prediction of repurposed drugs for Coronaviruses using artificial intelligence and machine learning. Comput. Struct. Biotechnol. J. 2021, 19, 3133–3148. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Stokes, J.; Eastman, R.; Itkin, Z.; Zakharov, A.; Collins, J.; Jaakkola, T.; Barzilay, R. Deep learning identifies synergistic drug combinations for treating COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105070118. [Google Scholar] [CrossRef] [PubMed]
- Burdick, H.; Lam, C.; Mataraso, S.; Siefkas, A.; Braden, G.; Dellinger, R.; McCoy, A.; Vincent, J.L.; Green-Saxena, A.; Barnes, G.; et al. Is Machine Learning a Better Way to Identify COVID-19 Patients Who Might Benefit from Hydroxychloroquine Treatment?—The IDENTIFY Trial. J. Clin. Med. 2020, 9, 3834. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Song, X.; Ma, T.; Pan, Q.; Zhou, Y.; Hou, Y.; Zhang, Z.; Li, K.; Karypis, G.; Cheng, F. Repurpose Open Data to Discover Therapeutics for COVID-19 using Deep Learning. J. Proteome Res. 2020, 19, 4624–4636. [Google Scholar] [CrossRef]
- Su, X.; Hu, L.; You, Z.; Hu, P.; Wang, L.; Zhao, B. A deep learning method for repurposing antiviral drugs against new viruses via multi-view nonnegative matrix factorization and its application to SARS-CoV-2. Briefings Bioinform. 2021, 23, bbab526. [Google Scholar] [CrossRef]
- Santos, S.; Torres, M.; Sánchez, M.; Cernuzzi, L.; Paccanaro, A. Machine Learning and Network Medicine approaches for Drug Repositioning for COVID-19. Patterns 2021, 3, 100396. [Google Scholar] [CrossRef]
- Beck, B.R.; Shin, B.; Choi, Y.; Park, S.; Kang, K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput. Struct. Biotechnol. J. 2020, 18, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Bung, N.; Krishnan, S.; Bulusu, G.; Roy, A. De novo design of new chemical entities for SARS-CoV-2 using artificial intelligence. Future Med. Chem. 2021, 13, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.Y.; Peng, T.T.; Yeh, T.K.; Huang, W.Z.; Chang, S.E.; Wu, S.H.; Hung, H.C.; Hsu, T.A.; Lee, S.J.; Song, J.S.; et al. Artificial intelligence approach fighting COVID-19 with repurposing drugs. Biomed. J. 2020, 43, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, Q.; Sun, J.; Tan, S.; Tang, Y.H.; Zhao, M.; Li, Y.Y.; Cao, X.; Zhao, J.C.; Yang, J.K. Potential Drug Discovery for COVID-19 Treatment Targeting Cathepsin L Using a Deep Learning-Based Strategy. Comput. Struct. Biotechnol. J. 2022, 20, 2442–2454. [Google Scholar] [CrossRef] [PubMed]
- Mekni, N.; Coronnello, C.; Langer, T.; De Rosa, M.; Perricone, U. Support Vector Machine as a Supervised Learning for the Prioritization of Novel Potential SARS-CoV-2 Main Protease Inhibitors. Int. J. Mol. Sci. 2021, 22, 7714. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, J.; Ray, A. Predicting novel drugs for SARS-CoV-2 using machine learning from a >10 million chemical space. Heliyon 2020, 6, e04639. [Google Scholar] [CrossRef]
- Pinto, G.P.; Vavra, O.; Marques, S.M.; Filipovic, J.; Bednar, D.; Damborsky, J. Screening of world approved drugs against highly dynamical spike glycoprotein of SARS-CoV-2 using CaverDock and machine learning. Comput. Struct. Biotechnol. J. 2021, 19, 3187–3197. [Google Scholar] [CrossRef] [PubMed]
- Zame, W.; Bica, I.; Shen, C.; Curth, A.; Lee, H.S.; Bailey, S.; Weatherall, J.; Wright, D.; Bretz, F.; Schaar, M. Machine Learning for Clinical Trials in the Era of COVID-19. Stat. Biopharm. Res. 2020, 12, 506–517. [Google Scholar] [CrossRef]
- da Motta, O.J.; Silva, E.; Siqueira-Batista, R. Bioethical aspects of artificial intelligence: COVID-19 & end of life. Rev. Assoc. Méd. Bras. 2020, 66, 5–6. [Google Scholar] [CrossRef]
- Jung, C.; Excoffier, J.B.; Raphaël-Rousseau, M.; Salaün-Penquer, N.; Ortala, M.; Chouaid, C. Evolution of hospitalized patient characteristics through the first three COVID-19 waves in Paris area using machine learning analysis. PLoS ONE 2022, 17, e0263266. [Google Scholar] [CrossRef] [PubMed]
- Asif, S.; Saba, T.; Alghanim, A. Exploring Prediction of COVID-19 and its Severity using Machine Learning. In Proceedings of the 2022 Fifth International Conference of Women in Data Science at Prince Sultan University (WiDS PSU), Riyadh, Saudi Arabia, 28–29 March 2022; pp. 117–122. [Google Scholar] [CrossRef]
- Cortes, U.; Cortes, A.; Garcia-Gasulla, D.; Perez-Arnal, R.; Avarez-Napagao, S.; Alvarez, E. The ethical use of high-performance computing and artificial intelligence: Fighting COVID-19 at Barcelona Supercomputing Center. AI Ethics 2022, 2, 325–340. [Google Scholar] [CrossRef] [PubMed]

| Ref. | Region | Model | Features | DS | DA | DSS | Tr | Te | AC | F1 | Se | AUC | Sp | R | Validation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [23] | Wuhan, China | RF | age, hypertension, cardiovascular disease, gender and diabetes for the clinical features modality and dimerized plasmin fragment D, | N/A | N/A | Private | 290 | 72 | 0.97 | 0.97 | 0.99 | 0.97 | 0.94 | N/A | N/A |
| [24] | Beijing | Lasso regression | Age, Temperature, HR, fever classification; headache, interleukin-6; systolic blood pressure; monocyte ratio; platelet count; diastolic blood pressure | N/A | Random oversampling | Private | 105 | 27 | N/A | 0.57 | N/A | 0.84 | 0.727 | 1.00 | 10-fold CV |
| [25] | N/A | XGBoost | Top 60 important features consisting of 19 proteins, 11 metabolites, 7 lipids, and 23 mRNAs | N/A | N/A | Private | 108 | 27 | N/A | N/A | N/A | 0.93 | N/A | N/A | 5-fold CV |
| [26] | China | RF and SVM | 28 features (age, gender, white blood cell, neutrophil percentage, lymphocyte percentage, monocyte percentage, …) | N/A | Bootstrap resampling | N/A | 40 | 11 | 0.9 | N/A | 0.88 | N/A | 0.9 | N/A | 10-fold CV |
| [27] | N/A | BN, NB, MLP, LWL and RF | age and gender, blood or tissue sample results, the period of the illness, symptoms and lab results, and risk factors | N/A | N/A | N/A | 880 | 587 | 0.99 (MLP) | N/A | N/A | N/A | N/A | 0.99 (MLP) | N/A |
| [28] | Salamanca, Spain | RF, xgboost and LR | demographic variables, comorbidities, clinical characteristics, physical examination parameters and biochemical parameters available at hospital admission | N/A | N/A | N/A | 734 | 184 | N/A | N/A | 0.9 | 0.83 | 0.52 | N/A | 10-stratified fold CV scheme with 10 repetitions |
| [29] | China | DL | clinicians to estimate an individual COVID-19 patient risk and make decisions based on availability of resources for critical patients and patient overload | N/A | N/A | N/A | 752 | 188 | N/A | N/A | 0.95 | 0.894 | 0.95 | 0.44 | N/A |
| [30] | Oxford | LR, RF and XGBoost | presentation blood tests, blood gas testing, vital signs, and results of PCR testing for respiratory viruses | N/A | N/A | N/A | 303 | 77 | N/A | N/A | 0.774 | 0.939 | 0.948 | N/A | 10-fold CV |
| [31] | Madrid, Spain | x-means clustering | 10 Vital signs, 29 laboratory tests and 168 ICD-10 codes | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| [32] | China | RF, GB, SVM, NB, KNN, LR | Demographic data, comorbidities, outpatient medications, vital signs and laboratory values | N/A | N/A | N/A | 80% | 20% | 0.9 | N/A | N/A | N/A | N/A | N/A | N/A |
| [33] | N/A | XGBoost | 24 features (after PCA) | N/A | N/A | Private | 102 | 25 | 0.97 | 0.96 | N/A | 1 | N/A | 0.95 | EV |
| [33,34] | Brazil | N/A | features of routine blood analysis | N/A | smote | N/A | N/A | N/A | 0.91 | 0.87 | 0.83 | 0.74 | 0.91 | 0.83 | EV |
| [35] | Pavia | ResNet50 | age, SBP, DBP, RT, SPO2, temperature, hemogoblin, white blood cell, lymphocytes, Platelets, C-reactive protein and Lactate dehydrogenase | N/A | Image noise, Colour jittering, flip, centre cropping | N/A | 337 | 45 | 0.9872 | 0.9922 | N/A | 0.9997 | N/A | 98.62 | 15% dataset |
| [36] | N/A | Ada Boost, RF, XGBoost, CatBoost | age, sex, respiratory parameters (SPO2, RR), cardiovascular parameters, body temperature, symptoms, associated comorbidities, full blood count, biochemical parameters | N/A | brightness changes, contrast adjustment and parallel shifting | Private | 380 | 95 | 0.9859 | N/A | 0.9793 | N/A | 0.9897 | N/A | 5-fold CV |
| [37] | Mexico | NN, LR, SVM, KNN | APACHE II score, white blood cell count, time from symptoms to admission, SPO2 and blood lymphocytes count | N/A | N/A | Public | 301,421 | 64,590 | 0.81–0.931 | N/A | 0.83–0.961 | N/A | 0.8–0.92 | N/A | 15% of dataset |
| [38] | Cheikh Zaid Hospital, Morocco | X_GBoost, AdaBoost, RF and ExtraTrees | Sex. Age. Platelet, Lymphocyte, PLR, ALT, AST, LDH, D-dimers, C_reactive protein, Weight, Comorbidities | N/A | N/A | Private | 225 | 97 | 1 | N/A | 1 | 1 | 1 | N/A | N/A |
| [39] | JinYinTan Hospital, China | RF, SVM, LR | chest computed tomography, fever, malignant tumor, HR, SBP, hemoglobin concentration, neutrophil-to-lymphocyte ratio | N/A | N/A | Private | N/A | N/A | 0.845–0.885 | N/A | 0.923–0.967 | 0.928–0.970 | 0.695–0.79 | N/A | 10-fold CV |
| [40] | N/A | RF, XGBoost, KNN, MLP, LR, J48, NB | Gender, age, length of hospitalization, Smoking, ICU admission, hypertension, pneumonia, diabetes, cardiac disease, symptoms, BUN, WBC, C-reactive protein, hypersensitive troponin, glucose, erythrocyte sedimentation rate, creatinine, alkaline phosphatase | N/A | SMOTE | N/A | 1350 | 150 | 0.9503 | N/A | 0.907 | 0.9902 | 0.951 | N/A | 10-fold CV |
| [41] | N/A | DL | X-rays, radiology reports and RT-PCR data | N/A | N/A | private | 11,599 | 800 | 0.77 | N/A | 0.683 | 0.925 | 0.966 | N/A | internally (Brown-April) and externally (External and Xiangya-February) |
| [42] | Turkey | LR, SVM, Voted Perceptron, KNN, K star, LWL, NB, SGD, DT, Hoeffding DT, RF | age, data, lymphocytes count (LYM), neutrophils count (NEU), white blood cells (WBC), mean corpuscular volume (MCV), mean platelet volume (MPV) and erythrocyte distribution width (RDW), eosinophils count (EOS), monocytes count (MONO), red blood cells count (RBC), hematocrit, hemoglobin and (mean corpuscolar hemoglobin con- centration (MCVC) | N/A | N/A | private | 3362 | 840 | 0.8762–0.9786 | 0.9271–0.988 | 0.9107–0.9920 | 0.8810–0.9786 | 0.8762–0.9786 | N/A | 10-fold CV |
| [43] | N/A | EfficientNet | CXR images | N/A | N/A | public/ private | 455 | 150 | 0.8667 | 0.7865–0.9174 | N/A | 0.95 | N/A | 0.7–1 | IV |
| [44] | China | LR | Age, Sex, Comorbidity, primary symptons, outcomes, laboratory indicators | lung images segmentation | N/A | N/A | 628 | 158 | N/A | N/A | 0.833 | 0.732 | 0.781 | N/A | 5-fold CV |
| [45] | China | LR | CK-MB, neutrophils, PCT, α-HBDH, D-dimer, LDH, glucose, PT, APTT, RDW (SD and CV), fibrinogen and AST | N/A | N/A | private | N/A | N/A | N/A | N/A | N/A | 0.83 | N/A | N/A | EV |
| [46] | South Korea | XGB | body temperature, pulse rate, RR, blood pressure, any symptoms, and past medical history | N/A | N/A | private | 119,576 | 29,895 | 0.923 | 0.861 | N/A | 0.95 | 0.933 | 0.807 | N/A |
| [47] | UK | RF | Age, Gender, BMI, Smoking Status, SPO2, Temperature, comorbidities, Albumin, White Blood Count, Blood Urea Nitrogen, Lymphocyte Count | N/A | N/A | private | 1196 | 299 | 0.76 | 0.67 | 0.78 | 0.83 | 0.75 | N/A | 5-fold CV |
| [48] | Korea | LGBM, ORL | AGE and SEX and BMI, HR, temperature, SBP, DBP, chronic cardiac disease, asthma, chronic obstructive pulmonary disease, hemoglobin, platelets, WBC | N/A | N/A | private | 3,940 | 1688 | 0.85–0.88 | 0.49–0.57 | N/A | N/A | N/A | 0.44–0.56 | 10-fold CV |
| [49] | Israel | Multistate Cox regression | Age, sex, patient being in 1 to 3 clinical states | N/A | N/A | N/A | 297 | 33 | N/A | N/A | N/A | 0.88 | N/A | N/A | 8-fold CV repeated 8 times |
| [50] | Philippines | DT | Sex, Age, Region, | N/A | Random undersampling | N/A | 197,164 | N/A | 0.8142 | 0.1674 | 0.8165 | 0.876 | 0.8141 | N/A | 5-fold CV |
| Ref. | Region | Model | Features | DS | DA | DSS | Tr | Te | AC | F1 | Se | AUC | Sp | R | Validation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [51] | Germany | Explainable Boosting Machine | 49 variables | N/A | N/A | N/A | 949 | 237 | 0.73 | N/A | N/A | 0.69 | N/A | N/A | 5-fold CV |
| [52] | Galicia, Spain | MLP. DeepNetwork, RF, AB, SVM, KNN, LR | Age, gender, diabetes, hypertrophy, hydrocele, pneumonia, frequent urination, therapeutic advice, white blood cells, heart failure | N/A | Smote, adasyn | N/A | 110,454 | N/A | N/A | N/A | N/A | 0.761 | N/A | N/A | 10-fold CV |
| [53] | Brazil | RF, XGB, LR | 67 attributes | N/A | N/A | N/A | 5644 | N/A | 0.94 | 0.91 | N/A | 0.9 | 0.95 | 0.92 | 10-fold CV |
| [54] | Wuhan, China | N/A | 194 variables | N/A | N/A | N/A | 586 | 147 | N/A | N/A | >0.571 | >0.622 | >0.353 | N/A | 3-fold CV |
| [55] | South Asia | RF, KNN, SVM | Alcoholic beverages, animal products, cereals excluding beer, meat, vegetal products | N/A | N/A | N/A | N/A | N/A | >0.77 | N/A | N/A | N/A | N/A | N/A | N/A |
| [56] | Dubai, UAE | DT | Age, gender, nationality, blood group, BMI | N/A | N/A | N/A | 1513 | 504 | 0.96 | N/A | 0.965 | N/A | 0.878 | N/A | 10-fold CV |
| [57] | USA | XGBoost | Age, gender, acute diagnoses | N/A | N/A | N/A | 2313 | N/A | N/A | N/A | 0.9 | N/A | 0.58 | N/A | N/A |
| [58] | Wuhan, China | XGBoost | lymphocyte percentage, prothrombin time, lactate dehydrogenase, total bilirubin, eosinophil percentage | N/A | N/A | N/A | 98 | 25 | N/A | N/A | 0.8 | 0.92 | 0.9 | N/A | 5-fold CV |
| [59] | NY, USA | RF | 31 variables | N/A | N/A | N/A | 1375 | 612 | N/A | 0.762 | 0.728 | 0.79 | 0.763 | N/A | 10-fold CV |
| [60] | Denmark | RF | Age, sex, BMI, comorbidities, smoking, lab tests and temporal features | N/A | N/A | N/A | 42,526 | N/A | N/A | N/A | N/A | 0.995 | N/A | N/A | N/A |
| [61] | NY, USA | LR, DT, RF, GBDT | RT-PCR results, routine laboratory testing results and patient demographic information | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0.761 | 0.854 | 0.808 | N/A | 5-fold CV |
| [62] | Iran | LR, NN, C5.0, RF, XGBoost | demographic characteristics, patient’s background, disease symptoms and a target variable | N/A | N/A | N/A | 318 | 80 | 0.7901–0.852 | N/A | 0.9091–0.9273 | N/A | 0.5385–0.7308 | N/A | 10-fold CV |
| [63] | Philadelphia, PA and Providence, RI, USA | RF | demographic, clinical and laboratory variables taken on admission to the ICU including age, sex, temperature, SpO2, WBC, absolute lymphocyte count, serum creatinine concentration, CRP and comorbidities | chest X-rays were segmented | N/A | Private | 546 | 108 | 0.727 | 0.707 | 0.714 | 0.732 | 0.746 | N/A | 5-fold CV |
| [64] | Kingdom of Saudi Arabia | DL | The dataset contains demographic features, laboratory results from complete blood count CBC and radiological findings and comorbidity | Chest X-ray segmentation | SMOTE | Private | 1210 | 303 | 0.904 | N/A | 0.86 | 0.875 | 0.84 | N/A | 10-fold CV |
| [65] | N/A | XGBoost | Sex, Marital status, Age, number of admissions, type of admissions, hospitalization data, comorbidities | N/A | N/A | N/A | 5212 | 579 | 0.917 | 0.918 | 0.916 | 0.91 | 0.913 | N/A | 10-fold CV |
| [66] | N/A | Gaussian mixture mode | breathing frequency (BF) and (SpO2) | N/A | N/A | N/A | N/A | N/A | 0.878 | N/A | N/A | 0.94 | N/A | N/A | N/A |
| [67] | Tel-Aviv Sourasky Medical Center, Israel | CatBoost | demographics, background disease, vital signs and lab measurements | N/A | N/A | Private | 20,029 | 5007 | 0.8 | N/A | N/A | 0.76 | N/A | 0.7 | 20-fold CV and EV on data from a different hospital |
| [68] | USA | DL | condition, procedure, measurement, observation, drug, devices, payer, visit, health providers, medical sites and personal information | N/A | N/A | Public | 9545 | 200 | N/A | N/A | N/A | 0.9 | N/A | N/A | 10 times by randomly splitting training and validation data |
| [69] | Saudi Arabia | J48 | demographics, comorbidities, signs and symptoms of COVID-19 illness, laboratory values, mechanical ventilation | N/A | N/A | Private | 1101 | 367 | 0.731 | N/A | N/A | 0.7542 | N/A | N/A | 10-fold CV |
| [70] | Saudi Arabia | RF | Age, gender, Weight, career, Heart disease, Hypertension, Diabetes mellitus, Stroke, Vascular disease, lymphocyte absolute value | N/A | N/A | private | 115 | - | 0.9416 | 0.9414 | N/A | N/A | N/A | 0.9416 | 29 samples for validation |
| [71] | Tokyo, Japan | LR | Age, gender, comorbidities, smoing status, temperature, RR, SBP, DBP, HR, SpO2 | N/A | N/A | Private | N/A | N/A | 0.9 | 0.695 | N/A | 0.875 | 0.976 | 0.513 | No validation |
| [65] | N/A | XGBoost | Sex, Marital status, Age, number of admissions, type of admissions, hospitalization data, comorbidities | N/A | N/A | N/A | 5212 | 579 | 0.917 | 0.918 | 0.916 | 0.91 | 0.913 | N/A | 10-fold CV |
| [72] | London, UK | LR, RF and XGBoost | 64 clinical features | N/A | N/A | private | 299 | 145 | N/A | 0.42–0.60 | N/A | 0.76–0.87 | N/A | 0.48 | 3-fold CV |
| [73] | Basque Country, Spain | CatBoost | pressure of oxygen, platelets, lymphocytes, monocytes and eosinophils and the highest values for CRP, age, procalcitonin, urea, LDH | N/A | N/A | Private | N/A | N/A | N/A | N/A | 0.99 | N/A | 0.95 | N/A | EV |
| [74] | Lombardy; Italy | LR | age, gender, home medications, comorbidities and ventilation parameters from the first 24 h in ICU | N/A | SMOTE | Private | N/A | N/A | 0.7–0.76 | 0.71–0.75 | N/A | 0.77–0.8 | N/A | 0.72–0.75 | 10-fold CV |
| [75] | Iran | water wave optimization (WWO) | Demographic data, clinical manifestation, comorbidities, laboratory test, Radiological factors and Treatment | N/A | SMOTE | Private | N/A | N/A | 0.9705 | 0.9795 | N/A | 0.9729 | 0.9259 | 0.9869 | 10-fold CV |
| [76] | Detroit | GBoost | 60 features | N/A | SMOTE | Private | 2641 | 660 | 0.85 | N/A | 0.88 | 0.93 | N/A | 0.92 | 10-fold CV |
| [77] | Netherlands | behavioural AI technology | Clinical frailty score, age, cognitive comorbidity, admission capacity | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0.95 | N/A | N/A | N/A |
| Ref. | Region | Model | Features | DS | DA | DSS | Tr | Te | AC | F1 | Se | AUC | Sp | R | Validation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [78] | Italy | naïve Bayes | age, mean corpuscular hemoglobin concentration, PaO2/FiO2 ratio, temperature, previous stroke and gender | N/A | N/A | private | 596 | 256 | 0.67 | 0.67 | 0.88 | 0.78 | 0.55 | N/A | EV |
| [79] | China | 3D densely connected CNN | clinical information and image features | automatic lung segmentation | N/A | private | 246 | 120 | 0.88 | N/A | 1 | 0.95 | 0.81 | N/A | N/A |
| [80] | Iran | SVM | SPO2 and age and laboratory biomarkers | N/A | N/A | private | N/A | N/A | N/A | N/A | 0.81 | 0.92 | 0.91 | N/A | 10% hold out CV |
| [81] | Kragujevac, Serbia and Rijeka, Croatia | XGBoost | demographic data, symptons, blood analysis | N/A | N/A | private | N/A | N/A | 0.94 | 0.96 | 0.97 | N/A | 0.93 | 0.95 | 10-fold CV |
| [82] | Nine countries | MLP | Average age, Average weather temperature, BCG vaccination, Malaria treatment | N/A | N/A | public | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| [83] | Slovenia | NB+NN | demographic, medical history and clinical and laboratory findings | N/A | N/A | public | 520 | 94 | 0.8 | N/A | 0.8 | 0.86 | 0.8 | N/A | 10-fold CV |
| [84] | Turkey | K-means, gray relational, fuzzy c-means, hierarchical clustering | number of cases, population density, average age, air pollution | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Calinski-Harabasz index, Dunn index |
| [85] | Shangai | LR | age, LDH, CRP | N/A | N/A | private | N/A | N/A | 0.847 | N/A | 0.861 | 0.9 | 0.8 | N/A | 10-fold CV |
| [86] | Nizhniy Novgorod, Russia | LR | LDH, IL-6, monokine induced by gamma interferon (MIG), D-dimer, fibrinogen and glucose | N/A | N/A | N/A | N/A | N/A | 0.83 | N/A | N/A | N/A | N/A | N/A | leave-one-out CV approach |
| [87] | N/A | GBT | 370 variables | N/A | N/A | public | 84 | 9 | 0.9698 | 0.9375 | 90.91 | N/A | 0.9798 | N/A | 10-fold CV |
| [88] | HM hospitals, Spain | DT, KNN, LR, MLP, Gaussian NB, RF and SVM | Age, Sex and Outcome, Clinical laboratory values | N/A | N/A | N/A | N/A | N/A | 0.94 | 0.76 | N/A | 0.97 | N/A | N/A | 10-fold CV |
| [89] | Italy | 30 models | LDH, D-dimer, neutr/lymph, neutrophils, fibrinogen, CRP, Brescia chest X-ray, lymphocytes, ferritin, monocytes | N/A | SMOTE | N/A | 1947 | 835 | N/A | N/A | >0.8 | >0.52 | >0.26 | N/A | 10-fold CV |
| [90] | Brazil | XGBoost | clinical data, socio-geographic data and structural hospital- level | N/A | N/A | private | 184,889 | 46,223 | N/A | N/A | 0.8 | 0.813 | 0.8 | N/A | 10-fold CV |
| [91] | Italy | RF | patients’ demographics, laboratory test results, historical data | N/A | N/A | N/A | 2725 | 1169 | 0.834 | 0.904 | 0.952 | N/A | 0.308 | N/A | 10-fold CV |
| [92] | Veneto region, Italy | Recursive partition tree, SVM, GBM, RF | Gender, Age, Smoking, Duration of hospitalization, comorbidities | N/A | N/A | N/A | 341 | N/A | N/A | N/A | >0.33 | >0.64 | >0.52 | N/A | N/A |
| [93] | China | DL | demographic, clinical characteristics and laboratory findings | DL automatic segmentation system | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| [94] | China | MLP | age, sex, CT scans, hypertension, fever, RR, WBC, | N/A | random horizontal flips and random rotations | N/A | 832 | 208 | 0.877 | N/A | 0.891 | 0.920 | 0.873 | N/A | EV |
| [95] | Wuhan, China | XGBoost | disease severity, age, CRP, LDH, ferritin and IL-10 | N/A | N/A | N/A | 689 | 295 | >0.9 | 0.993 | >0.85 | 0.921 | N/A | 0.993 | IV, EV |
| [96] | MN, USA | 18 ML algorithms | 54 variables | N/A | N/A | N/A | 11,807 | 2372 | 0.78 | N/A | N/A | 0.8938 | N/A | 0.85 | 10-fold CV |
| [97] | Bern, Switzerland | COSA score, LR, DT, RF, AdaBoost, SVM, KNN, MLP | demographic data, medical history and laboratory values | N/A | N/A | N/A | 159 | 39 | N/A | N/A | 1 | 0.94 | 1 | N/A | N/A |
| [98] | Wuhan, China | DNN | Demographics, comorbidities, vital signs, symptoms and laboratory tests | N/A | N/A | N/A | 154 | 27 | N/A | N/A | N/A | 0.968 | N/A | N/A | 5-fold CV |
| [99] | Wuhan, China | Gradient boosting DT (GBDT) | Demographic, clinical, laboratory, radiological characteristics, and treatment and outcomes data | N/A | N/A | N/A | 2339 | 585 | 0.889 | N/A | 0.899 | 0.941 | 0.889 | N/A | 5-fold CV |
| [100] | Hubei Province, China | LR, XGB, NN | demographics, signs, symptoms, comorbidities and blood test results | lung segmentation (R231CovidWeb) | SMOTE | N/A | 346 | 116 | N/A | 0.668 | 0.845 | 0.960 | 0.929 | N/A | 5-fold CV |
| [101] | Sao Paulo, Brazil | Artificial NNs, DT and KNN | N/A | N/A | N/A | public | 390 | 167 | 0.98 | N/A | 0.97 | N/A | 0.99 | N/A | leave-one-out CV |
| [102] | Texas, USA | SVM | c-reactive protein, blood urea nitrogen, serum calcium, serum albumin, and lactic acid | N/A | N/A | N/A | 318 | 80 | N/A | N/A | 0.91 | 0.93 | 0.91 | N/A | N/A |
| [103] | Leishenshan, China | LR | albumin, saturation of pulse oxygen at admission, alanine aminotransferase and percentage of neutrophils | N/A | N/A | N/A | 1507 | 503 | 0.98 | 0.98 | N/A | N/A | N/A | 0.98 | N/A |
| [104] | Wuhan, China | RF | Age, high-sensitivity C-reactive protein level, lymphocyte count and d-dimer level | N/A | N/A | public | N/A | N/A | N/A | N/A | 0.914 | 0.922 | 0.760 | N/A | 10-fold CV |
| [105] | Taizhou, China | SVM | oxygenation index, basophil counts, AST, gender, Mg, SPO2, body temperature and days of symptom | N/A | N/A | private | 228 | 130 | 0.98 | N/A | 0.9 | 0.98 | 0.99 | N/A | 10-fold CV |
| [106] | Wenzhou, China | ECPA-KELM | AGE, Alanine aminotransferase, Albumin, Globulin ratio, AST and LDH | N/A | N/A | private | 35 | 16 | 0.905 | N/A | 0.922 | N/A | 0.896 | N/A | 10-fold CV |
| [107] | urban multi-center health system | RF | saturation values, laboratory values, comorbidities, radiological values | N/A | N/A | private | 210 | 91 | 0.74 to 0.68 | 0.62 to 0.55 | 0.72 to 0.66 | 0.81 to 0.76 | 0.76 to 0.71 | N/A | 10-fold CV |
| [108] | Wuxi Fifth People’s Hospital, China | XGBoost | CRP, age, neutrophil count (Neuc), hemoglobin, neutrophils, and platelet distribution width | N/A | N/A | private | N/A | N/A | 0.919 | N/A | 0.966 | 0.978 | 0.909 | N/A | N/A |
| [109] | Aragon, Spain | MLP, RF, XGBoost | 165 variables, demographics, comorbidity, chronic drugs, vital signs and laboratory data | N/A | N/A | N/A | 2717 | 453 | N/A | N/A | 0.71 | 0.821 | 0.78 | N/A | EV with 12.5% of data |
| [110] | UK | RF | detailed anthropometry, acute renal failure, urinary tract infection and pneumonias | N/A | N/A | public | N/A | N/A | N/A | N/A | N/A | 0.91 | N/A | N/A | leave-one-out (LOO) CV |
| [111] | Wuhan, China | EDRnet | 28 biomakers, age and gender | N/A | N/A | N/A | 375 | 106 | 0.92 | N/A | 1 | N/A | 0.91 | N/A | 10-repetition 10-fold stratified CV |
| [112] | HM Hospitals, Madrid, Spain | LR, DT, RF, NB | 29 variables | N/A | N/A | public | N/A | N/A | N/A | N/A | 0.69–0.87 | 0.77–0.9 | 0.76–0.83 | N/A | 10-fold CV |
| [113] | USA | RF | CRP, Procalcitonin, d-Dimer, SpO2 and RR | N/A | N/A | private | 127 | 85 | N/A | N/A | 0.73 | 0.8 | 0.74 | N/A | 5-fold CV |
| [114] | 141 countries | RF, DT, GBM, XGB, SVM and LGBM | age, sex, travel history and the commonly occurring comorbidities | N/A | N/A | public | 914 | 229 | 0.83–0.9 | 0.88–0.93 | N/A | 0.76–0.89 | N/A | 0.91–0.94 | Not done |
| [115] | China | LR, SVM, GBDT, NN, KNN and RF | sex, sputum, BUN, RR, D dimer, comorbidities, age, fever, albumin [ALB], SpO2, lymphocyte and chronic kidney disease | N/A | N/A | private | 2160 | 360 | 0.871–0.955 | 0.46–0.69 | 0.316–0.607 | N/A | 0.946–0.996 | N/A | IV |
| [116] | Michigan, USA | XGBoost and CatBoost | Age, Sex, Race, BMI; comorbidities, vital signs, LOS and treatment data | N/A | N/A | private | 810 | 202 | 0.803 | N/A | N/A | 0.89 | N/A | N/A | k-fold CV |
| [117] | Iran | LR, RF, AdaBoost, NB, MLP | CT image and Clinical outcomes | lung segmentations performed by DL | N/A | private | 9612 | 4119 | N/A | N/A | 0.83 | N/A | 0.72 | N/A | 10 strategies |
| [118] | Martin, Slovakia | RF | age, SPO2, CRP, gamma glutamyltransferase, AST, Bilirubin | N/A | N/A | N/A | N/A | N/A | N/A | N/A | >0.9 | 0.799 | >0.9 | N/A | Out-Of-Bag |
| [119] | UK | RF regressor and xGboost | Hypertension, diabetes, coronary heart disease, obesity, asthma | N/A | N/A | public | N/A | N/A | N/A | N/A | N/A | N/A | R2=0.88 | N/A | CV |
| [120] | UK | SVM, RF, J48, NB, LR, Bagging | demographic, ethnic, socioeconomic and clinical risk factors | N/A | SMOTE | public | 408 | 174 | 0.7402–0.8355 | N/A | N/A | N/A | N/A | N/A | CV |
| [121] | Mokdong Hospital, South Korea | XGBoost, LGBM, RF, KNN, SVM, DL and Ensemble model | sex, age, medical history, vital signs, chief complaints, review of systems, mortality and laboratory data | N/A | N/A | private | N/A | N/A | 0.85 | 0.77 | N/A | 0.8811 | N/A | 0.75 | 5-fold CV |
| [122] | UK | DNN, RF, XGB, SVM | Age, Death, Gender, Height, Weight, BMI, smoker, vascular problems, alcohol consumption | N/A | SMOTE | public | 9521 | 2975 | N/A | N/A | N/A | 0.81-0.86 | N/A | N/A | 5-fold CV |
| [123] | UK | GBDT | Gender, comorbidities, BMI, vit D, haemoglobin A1c, high-density lipoprotein and low-density lipoprotein | N/A | N/A | public | 3157 | 1353 | 0.56 | N/A | N/A | 0.57 | 0.56 | 0.56 | 10-fold CV |
| [124] | Italia | RF | age, SPO2, PaO2/FiO2, creatinine clearance and elevated troponin | N/A | N/A | Private | 561 | 140 | N/A | N/A | 0.88 | 0.78 | N/A | N/A | N/A |
| [125] | Valencia, Spain | SVM, LR, KNN, DT, NB, RF, MLP, GP, AdaBoost | data from the EHR system, including demographics, comorbidities and outpatient data | N/A | N/A | Private | 20,183 | 5046 | 0.8416 | 0.6762 | 0.8333 | 0.871 | 0.8429 | N/A | 5-fold CV |
| [126] | N/A | Siamese NN (SNN) | gender, age, location, offset time period, severity of COVID-19 and radiology | N/A | N/A | Private | 490 | 140 | 0.876 | 0.871 | N/A | 0.951 | N/A | N/A | 5-fold CV |
| [127] | Mariland, USA | DT, RF | B cell, CD4+ T cell, CD8+ T cell, monocytes, and NK cell | N/A | SMOTE | Public | N/A | N/A | N/A | 0.76–0.91 | N/A | N/A | N/A | N/A | 10-fold CV |
| [128] | Wuhan, China | KNN, DT, LR, SVM, RF, SGD, bagging, AdaBoost | Age, Gender admission to ICU, LD, CRP, Lymphocyte, Leukocytes | N/A | SMOTENC | N/A | 80% | 20% | 0.7697–0.9163 | N/A | 0.82-0.98 | N/A | 0.72–0.85 | N/A | 10-fold CV |
| [129] | N/A | CatBoost | chest Xray images and patient meta-data (age and sex) | N/A | N/A | Public | 95 | 41 | 0.93 | 0.93 | N/A | 0.95 | N/A | 0.93 | N/A |
| [130] | Milan, Lombardy, Italy | LR | age, comorbidities, vital signs and comorbidities | N/A | SMOTE | private | 760 | 327 | 0.841610 | 0.827546 | 0.789 | N/A | 0.972 | 0.823369 | CV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badiola-Zabala, G.; Lopez-Guede, J.M.; Estevez, J.; Graña, M. Machine Learning First Response to COVID-19: A Systematic Literature Review of Clinical Decision Assistance Approaches during Pandemic Years from 2020 to 2022. Electronics 2024, 13, 1005. https://doi.org/10.3390/electronics13061005
Badiola-Zabala G, Lopez-Guede JM, Estevez J, Graña M. Machine Learning First Response to COVID-19: A Systematic Literature Review of Clinical Decision Assistance Approaches during Pandemic Years from 2020 to 2022. Electronics. 2024; 13(6):1005. https://doi.org/10.3390/electronics13061005
Chicago/Turabian StyleBadiola-Zabala, Goizalde, Jose Manuel Lopez-Guede, Julian Estevez, and Manuel Graña. 2024. "Machine Learning First Response to COVID-19: A Systematic Literature Review of Clinical Decision Assistance Approaches during Pandemic Years from 2020 to 2022" Electronics 13, no. 6: 1005. https://doi.org/10.3390/electronics13061005
APA StyleBadiola-Zabala, G., Lopez-Guede, J. M., Estevez, J., & Graña, M. (2024). Machine Learning First Response to COVID-19: A Systematic Literature Review of Clinical Decision Assistance Approaches during Pandemic Years from 2020 to 2022. Electronics, 13(6), 1005. https://doi.org/10.3390/electronics13061005







