Design Strategies of Integrated Metal-Oxide Semiconductor-Based Resistive Sensor Systems for Ammonia Detection
Abstract
1. Introduction
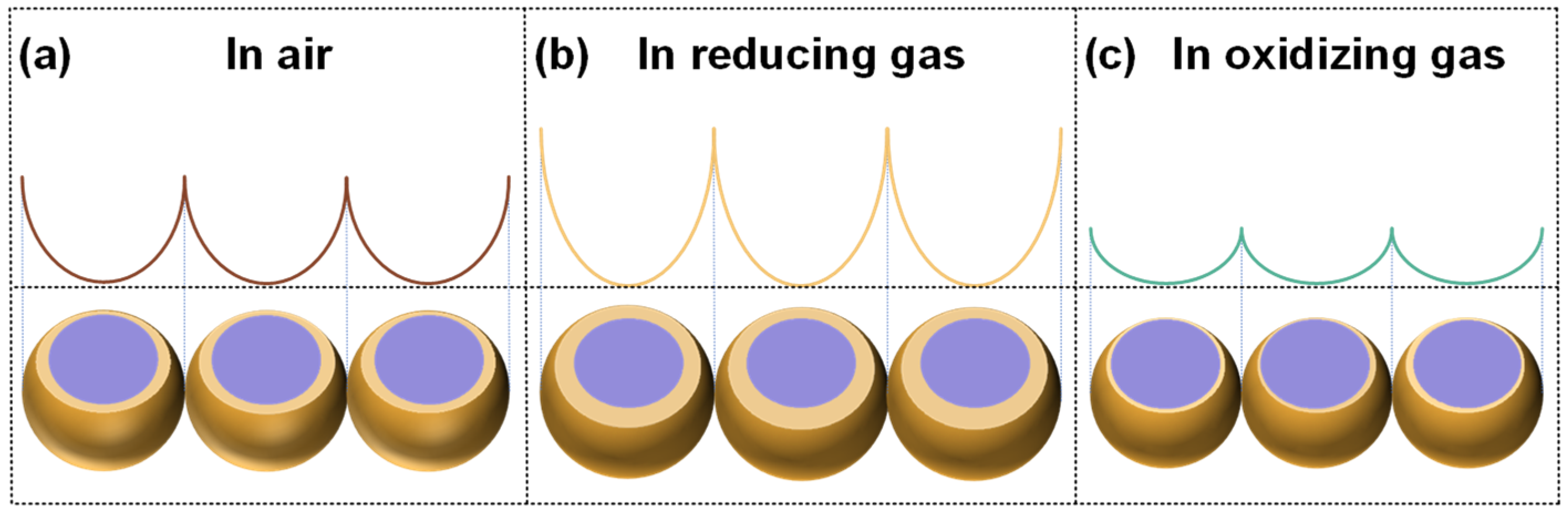
2. Gas Sensing Mechanism of MOS-Based Resistive Sensors
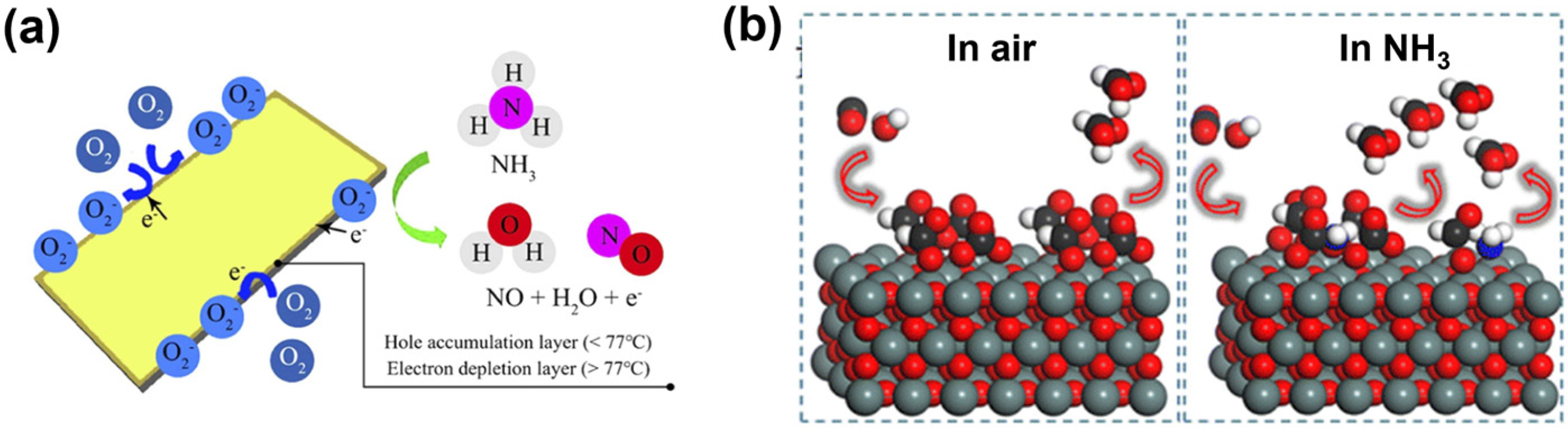
2.1. Resistive Sensor Detection Mechanisms for n-Type Oxide Films
2.2. Resistive Sensor Detection Mechanisms for p-Type Oxide Films
2.3. Material Characteristics of Resistive Sensors
3. Design Strategies for MOS-Based Resistive Sensors
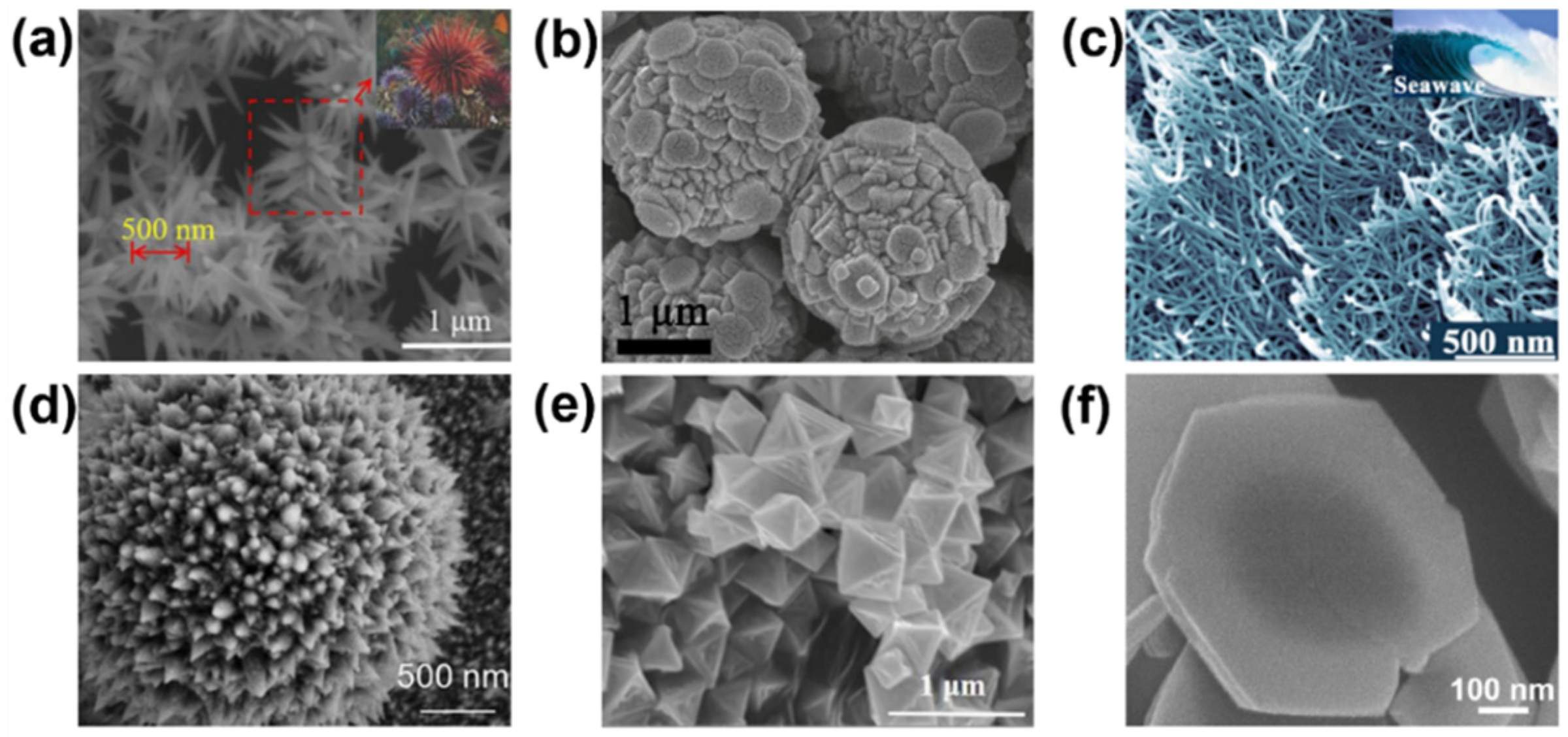
3.1. Single Nanomaterial
3.2. Single-Element Decorated Nanomaterials
3.3. Composite Nanomaterial
4. Design Strategies of Interface Circuits of MOS-Based Resistive Sensors
4.1. CMOS Resistance Sensor Interface Circuits
4.1.1. Circuit Implementation
4.1.2. Circuit Implementation of the Transducer Front-End
4.2. Interface Circuit of the MOS Gas Sensor Array
4.2.1. AFE Circuit
4.2.2. Array Data Acquisition Circuit
4.2.3. Digital System
5. Current Challenges and Opportunities
- (1)
- Sensitivity and selectivity: Improving the sensitivity and selectivity of ammonia sensors remains a major challenge, especially in complex gas environments. We discuss the potential to overcome this challenge through surface modification and nanomaterial design.
- (2)
- Stability and durability: The performance stability and environmental adaptability of ammonia gas sensors in long-term use need to be further improved. We analyze the contribution of material modification and improvements in packaging technology to increase sensor durability.
- (3)
- Low power consumption and miniaturization: With the development of IoT and portable devices, there is an increasing demand for low power consumption and miniaturization of ammonia sensors. This provides opportunities for integrated circuit design and novel material applications.
- (4)
- Multi-functional integration and intelligence: The integration of ammonia sensors with other sensing functions and its intelligent development direction also holds important prospects. We discuss opportunities for sensor system integration and data processing algorithm improvement.
- (1)
- MOS-based resistive gas sensors operating at room temperature need deep research to effectively reduce power consumption of devices without affecting sensing performance.
- (2)
- Due to the poor cross-sensitivity of MOS-based resistive gas sensors, it is necessary to develop new gas recognition strategies to eliminate the influence of interfering gases and improve the selectivity.
- (3)
- Most of the slurry is coated on the electrode using a synthetic material based on drip coating or spray coating, so the device consistency of this method is relatively poor. Novel preparation processes should be developed.
- (4)
- While RV-ADC technology boasts a straightforward design, it does demand a robust ADC with power levels that align with the desired resolution. To ensure top-notch sensitivity and precision, the gain of the programmable transimpedance amplifier (PTA) is fine-tuned, enabling the system to operate seamlessly and automatically.
6. Conclusions
- (1)
- For sensors with single MOS nanomaterials, the performance is mainly improved by structural sensitization that changes the crystal shape and structure, increasing the specific surface area and promoting the exposure of the active site. On this basis, ammonia sensing properties can be further improved by metal modification and composition of composite nanomaterials using the combined effect of electronic, chemical and structure sensitization. The structural properties of MOS nanomaterials enhance the stability and repeatability of the materials, ensuring the reliability of the sensors in complex environments. These properties provide an input reference for the design of the circuit, where higher sensitivity requires a higher resolution and faster response time to ensure that the output signal accurately reflects changes in ammonia concentration.
- (2)
- Noble metal decoration mainly uses its excellent catalytic activity and Schottky junction formation between metal and semiconductor to improve the ammonia sensing properties. Non-noble metals mainly improved ammonia sensing properties by adjusting the lattice defects and changing the crystal phase on the basis of the original nanostructure. The composition of composite nanomaterials can form heterogeneous structures between different nanomaterials and make full use of the unique characteristics of each material and their synergistic effects to greatly improve the ammonia sensing performance, which will be the main research direction of sensitive materials for resistive ammonia sensors in the future. The Schottky junction increases the adsorption activity of the gas molecules by changing the interfacial barriers and improves the selectivity and stability of the sensor for ammonia gas. The enhanced selectivity of this design for specific gases reduces interference from other gases, resulting in more accurate detection results. The noble metal Schottky junction enhances the accuracy of the output signal, allowing the circuit to further optimize the ammonia response through signal processing and filtering methods, while increasing the circuit’s immunity to non-target gases. These features help optimize subsequent circuit design and improve the performance of the signal processing circuit.
- (3)
- The architectural design of interface circuits significantly enhances the flexibility of signal processing while mitigating errors associated with leakage current, series resistance, and system nonlinearity within the digital domain. Furthermore, the precision of the analog reference voltage of the analog-to-digital converter (ADC) in this system does not compromise measurement accuracy. The capability to accurately assess resistance, power dissipation, and ambient temperature of resistive sensors renders this readout architecture particularly suitable for its intended applications.
- (4)
- The programmable interface circuitry of the MOS gas sensor array encompasses sensor configuration, signal acquisition, data processing, and the output of identification results. This interface circuit is characterized by a highly integrated analog front end (AFE) and a high-performance Cortex-M0 processor. Furthermore, the interface circuit is capable of virtualizing additional MOS gas sensors through rapid temperature control and incorporates the k-nearest neighbor classification algorithm, enabling precise identification of gas types during the thermal runaway process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Srirattanapibul, S.; Nakarungsee, P.; Issro, C.; Tang, I.M.; Thongmee, S. Performance of NiO intercalated rGO nanocomposites for NH3 sensing at room temperature. Mater. Sci. Semicond. Process. 2022, 137, 106221. [Google Scholar] [CrossRef]
- Erwiha, G.M.; Ham, J.; Sukor, A.; Wickham, A.; Davis, J.G. Organic Fertilizer Source and Application Method Impact Ammonia Volatilization. Commun. Soil Sci. Plant Anal. 2020, 51, 1469–1482. [Google Scholar] [CrossRef]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-fed fuel cells: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Everitt, H.O.; Liu, J. Light-Induced Thermal Gradients in Ruthenium Catalysts Significantly Enhance Ammonia Production. Nano Lett. 2019, 19, 1706–1711. [Google Scholar] [CrossRef]
- Lima, A.A.S.; Leite, G.D.N.P.; Ochoa, A.A.V.; Santos, C.A.C.D.; Costa, J.A.P.D.; Michima, P.S.A.; Caldas, A.M.A. Absorption Refrigeration Systems Based on Ammonia as Refrigerant Using Different Absorbents: Review and Applications. Energies 2020, 14, 48. [Google Scholar] [CrossRef]
- Lin, X.; Jin, Y.; Yao, J.; Sun, X.; Tian, T.; Li, Z.; Chen, S.; Jiang, J.; Hu, W.; Hao, Y.; et al. Adverse prognosis of nasopharyngeal carcinoma following long-term exposure to multiple air pollutants. Environ. Chem. Lett. 2024, 22, 21–27. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, B.; Erisman, J.W.; Reis, S.; Fang, Y.; Lu, X.; Zhang, X. PM2.5 pollution is substantially affected by ammonia emissions in China. Environ. Pollut. 2016, 218, 86–94. [Google Scholar] [CrossRef]
- Lin, J.-H.; Yang, T.; Zhang, X.; Shiu, B.-C.; Lou, C.-W.; Li, T.-T. Mn-doped ZnO/SnO2-based yarn sensor for ammonia detection. Ceram. Int. 2023, 49, 34431–34439. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, J.; Zhang, Y.; Wang, Y.; Liu, X. Dominating Role of Aligned MoS2/Ni3S2 Nanoarrays Supported on Three-Dimensional Ni Foam with Hydrophilic Interface for Highly Enhanced Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2018, 10, 1752–1760. [Google Scholar] [CrossRef]
- Chen, J.; Cao, J.; Zhou, J.; Zhang, Y.; Li, M.; Wang, W.; Liu, J.; Liu, X. Mechanism of highly enhanced hydrogen storage by two-dimensional 1T′ MoS2. Phys. Chem. Chem. Phys. 2020, 22, 430–436. [Google Scholar] [CrossRef]
- Schwab, J.J.; Li, Y.; Bae, M.-S.; Demerjian, K.L.; Hou, J.; Zhou, X.; Jensen, B.; Pryor, S.C. A Laboratory Intercomparison of Real-Time Gaseous Ammonia Measurement Methods. Environ. Sci. Technol. 2007, 41, 8412–8419. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Nishiura, H.; Honjo, T.; Ishikawa, Y.; Suzuki, K. Continuous Determination of Atmospheric Ammonia by an Automated Gas Chromatographic System. Anal. Chem. 1994, 66, 756–760. [Google Scholar] [CrossRef]
- Martins, M.R.; Sarkis, L.F.; Sant, S.A.C.; Santos, C.A.; Araujo, K.E.; Santos, R.C.; Araujo, E.S.; Alves, B.J.R.; Jantalia, C.P.; Boddey, R.M.; et al. Optimizing the use of open chambers to measure ammonia volatilization in field plots amended with urea. Pedosphere 2021, 31, 243–254. [Google Scholar] [CrossRef]
- Aarya, S.; Kumar, Y.; Chahota, R.K. Recent Advances in Materials, Parameters, Performance and Technology in Ammonia Sensors: A Review. J. Inorg. Organomet. Polym. Mater. 2020, 30, 269–290. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, J.; Li, M.; Chen, J.; Zhang, Y.; Liu, X. Insightful understanding of three-phase interface behaviors in 1T-2H MoS2/CFP electrode for hydrogen evolution improvement. Chin. Chem. Lett. 2022, 33, 3745–3751. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Hua, Z.; Li, Z.; He, X.; Yan, R.; Li, Y.; Zhi, Z.; Tian, C. A low cost and high performance NH3 detection system for a harsh agricultural environment. Sens. Actuators B-Chem. 2022, 361, 131675. [Google Scholar] [CrossRef]
- Seesaard, T.; Goel, N.; Kumar, M.; Wongchoosuk, C. Advances in gas sensors and electronic nose technologies for agricultural cycle applications. Comput. Electron. Agric. 2022, 193, 106673. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, R.; Chen, L.; Wang, D.; Wang, W.; Tan, E.; Meng, X.; Xiu, H.; Wang, L.; Yang, X.; et al. Design strategies and applications of responsive metal-based luminescence probes in the bioanalysis. TrAC-Trends Anal. Chem. 2023, 168, 117338. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, D.; Zhou, J.; Ye, J.; Sun, Y.; Li, X.; Geng, Y.; Wang, J.; Du, Y.; Qian, Z. Visible light-activated room temperature NH3 sensor base on CuPc-loaded ZnO nanorods. Sens. Actuators B-Chem. 2021, 327, 128911. [Google Scholar] [CrossRef]
- Qin, Y.; Gui, H.; Bai, Y.; Liu, S. Enhanced NH3 sensing performance at ppb level derived from Ti3C2Tx-supported ZnTi-LDHs nanocomposite with similar metal-semiconductor heterostructure. Sens. Actuators B-Chem. 2022, 352, 131077. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Chen, D.; Jin, L.; Li, Y.; Zhang, Y.; Xu, L.; Guo, Y.; Lin, F.; Wu, F. Gas Detection Microsystem with MEMS Gas Sensor and Integrated Circuit. IEEE Sens. J. 2018, 18, 6765–6773. [Google Scholar] [CrossRef]
- Fernández-Ramos, M.D.; Capitán-Vallvey, L.F.; Pastrana-Martínez, L.M.; Morales-Torres, S.; Maldonado-Hódar, F.J. Chemoresistive NH3 gas sensor at room temperature based on the carbon gel-TiO2 nanocomposites. Sens. Actuators B Chem. 2022, 368, 132103. [Google Scholar] [CrossRef]
- Cao, J.; Wang, W.; Zhou, J.; Chen, J.; Deng, H.; Zhang, Y.; Liu, X. Controllable gas sensitive performance of 1T’ WS2 monolayer instructed by strain: First-principles simulations. Chem. Phys. Lett. 2020, 758, 137921. [Google Scholar] [CrossRef]
- Wang, W.; Cao, J.; Zhang, R.; Xiu, H.; Zhang, Y. Room-Temperature NO2 Detection by MoS2-Nanoflake-Decorated AuPt/SnO2 Nanotubes. ACS Appl. Nano Mater. 2023, 6, 17941–17951. [Google Scholar] [CrossRef]
- Deng, Z.; Tong, B.; Meng, G.; Liu, H.; Dai, T.; Qi, L.; Wang, S.; Shao, J.; Tao, R.; Fang, X. Insight into the Humidity Dependent Pseudo-n-Type Response of p-CuScO2 toward Ammonia. Inorg. Chem. 2019, 58, 9974–9981. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Nagpal, S. A new model to estimate size distribution from the emission spectra of ZnO nanorods used for highly sensitive ammonia sensors. Pramana-J. Phys. 2022, 96, 83. [Google Scholar] [CrossRef]
- Vasiliev, R.; Kurtina, D.; Udalova, N.; Platonov, V.; Nasriddinov, A.; Shatalova, T.; Novotortsev, R.; Li, X.; Rumyantseva, M. SnS= Nanosheets as a Template for 2D SnO2 Sensitive Material: Nanostructure and Surface Composition Effects. Materials 2022, 15, 8213. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, Q.; Cao, J.; Chen, D.; Zhang, Y.; Liu, J.; Jia, P. Highly Sensitive Gas Sensor for Detection of Air Decomposition Pollutant (CO, NOx): Popular Metal Oxide (ZnO, TiO2)-Doped MoS2 Surface. ACS Appl. Mater. Interfaces 2024, 16, 3674–3684. [Google Scholar] [CrossRef]
- Sivalingam, M.M.; Olmos-Asar, J.A.; Vinoth, E.; Tharmar, T.; Shkir, M.; Said, Z.; Balasubramanian, K. Copper Oxide Nanorod/Reduced Graphene Oxide Composites for NH3 Sensing. ACS Appl. Nano Mater. 2021, 4, 12977–12985. [Google Scholar] [CrossRef]
- Su, P.-G.; Zheng, Y.-X. A room temperature NH3 gas sensor based on a quartz crystal microbalance coated with a rGO-SnO2 composite film. Anal. Methods 2022, 14, 1454–1461. [Google Scholar] [CrossRef]
- Yin, M.; Yao, Y.; Fan, H.; Liu, S. WO3-SnO2 nanosheet composites: Hydrothermal synthesis and gas sensing mechanism. J. Alloys Compd. 2018, 736, 322–331. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, X.; Huang, Y.; Guo, Y.; Gao, C.; Xie, G.; Jiang, Y. Impact of further thermal reduction on few-layer reduced graphene oxide film and its n-p transition for gas sensing. Sens. Actuators B-Chem. 2016, 235, 241–250. [Google Scholar] [CrossRef]
- Choi, Y.R.; Yoon, Y.-G.; Choi, K.S.; Kang, J.H.; Shim, Y.-S.; Kim, Y.H.; Chang, H.J.; Lee, J.-H.; Park, C.R.; Kim, S.Y.; et al. Role of oxygen functional groups in graphene oxide for reversible room-temperature NO2 sensing. Carbon 2015, 91, 178–187. [Google Scholar] [CrossRef]
- Štulík, J.; Polansky, R.; Kuberský, P.; Zabelin, D.; Lyutakov, O.; Kolska, Z.; Švorčík, V. Highly Sensitive Ammonia Sensor Based on Modified Nanostructured Polypyrrole Decorated With MAF-6 to Reduce the Effect of Humidity. IEEE Sens. J. 2023, 23, 1896–1907. [Google Scholar] [CrossRef]
- Singh, R.; Agrohiya, S.; Rawal, I.; Ohlan, A.; Dahiya, S.; Punia, R.; Maan, A.S. Porous polyaniline/flower-like hybrid phase MoS2/phosphorus-doped graphene ternary nanocomposite for efficient room temperature ammonia sensors. Synth. Met. 2024, 307, 117676. [Google Scholar] [CrossRef]
- Xuan, J.; Wang, L.; Zou, Y.; Li, Y.; Zhang, H.; Lu, Q.; Sun, M.; Yin, G.; Zhou, A. Room-temperature gas sensor based on in situ grown, etched and W-doped ZnO nanotubes functionalized with Pt nanoparticles for the detection of low-concentration H2S. J. Alloys Compd. 2022, 922, 166158. [Google Scholar] [CrossRef]
- Zhang, R.; Cao, J.; Wang, W.; Tan, E.; Zhu, R.; Chen, W.; Zhang, Y. Research on design strategies and sensing applications of energy storage system based on renewable methanol fuel. Results Eng. 2023, 20, 101439. [Google Scholar] [CrossRef]
- Gao, Q.; Dai, Y.; Han, B.; Zhu, W.; Li, X.; Li, C. Enhanced gas-sensitivity and ferromagnetism performances by the Ni-doping induced oxygen vacancies in (Mn, Ni) codoped ZnO nanorods. Appl. Surf. Sci. 2019, 490, 178–187. [Google Scholar] [CrossRef]
- Zhang, R.; Cao, J.; Wang, W.; Zhou, J.; Chen, J.; Chen, L.; Chen, W.; Zhang, Y. An improved strategy of passive micro direct methanol fuel cell: Mass transport mechanism optimization dominated by a single hydrophilic layer. Energy 2023, 274, 127276. [Google Scholar] [CrossRef]
- Chen, H.-I.; Hsiao, C.-Y.; Chen, W.-C.; Chang, C.-H.; Chou, T.-C.; Liu, I.P.; Lin, K.-W.; Liu, W.-C. Characteristics of a Pt/NiO thin film-based ammonia gas sensor. Sens. Actuators B-Chem. 2018, 256, 962–967. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Z.; Zhang, R.; Ji, H.; Xing, C.; Meng, F. MoO3/SnO2 Nanocomposite-Based Gas Sensor for Rapid Detection of Ammonia. IEEE Trans. Instrum. Meas. 2021, 70, 9514209. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, J.; Zhang, Y.; Liu, J.; Chen, J.; Li, M.; Wang, W.; Liu, X. Overcoming undesired fuel crossover: Goals of methanol-resistant modification of polymer electrolyte membranes. Renew. Sustain. Energy Rev. 2021, 138, 110660. [Google Scholar] [CrossRef]
- Hizam, S.M.M.; Al-Dhahebi, A.M.; Saheed, M.S.M. Recent Advances in Graphene-Based Nanocomposites for Ammonia Detection. Polymers 2022, 14, 5125. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, T.H.; Park, S.Y.; Eom, T.H.; Cho, I.; Kim, Y.; Kim, C.; Lee, S.A.; Choi, M.-J.; Suh, J.M.; et al. Drastic Gas Sensing Selectivity in 2-Dimensional MoS2 Nanoflakes by Noble Metal Decoration. ACS Nano 2023, 17, 4404–4413. [Google Scholar] [CrossRef]
- Ou, Y.; Zhou, Y.; Guo, Y.; Niu, W.; Wang, Y.; Jiao, M.; Gao, C. 2D/2D Dy2O3 Nanosheet/MoO3 Nanoflake Heterostructures for Humidity-Independent and Sensitive Ammonia Detection. ACS Sens. 2023, 8, 4253–4263. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, X.; Du, B.; Hu, X.; Yang, X.; He, Y.; Zhou, Y.; Zang, Z. Low-operating temperature ammonia sensor based on Cu2O nanoparticles decorated with p-type MoS2 nanosheets. J. Mater. Chem. C 2021, 9, 4838–4846. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Yuan, W.; Li, Z.; Lu, N.; Wang, S.; Wu, Y.; Fan, S.; Hua, Z. Efficient NH3 Detection Based on MOS Sensors Coupled with Catalytic Conversion. ACS Sens. 2020, 5, 1838–1848. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, I.; Husain, A.; Khan, A.; Asiri, A.M. Electrical Conductivity Based Ammonia Sensing Properties of Polypyrrole/MoS2 Nanocomposite. Polymers 2020, 12, 3047. [Google Scholar] [CrossRef]
- Hu, K.; Cai, Y.; Wang, Z.; Zhang, Z.; Xian, J.; Zhang, C. A Review on Metal Oxide Semiconductor-Based Chemo-Resistive Ethylene Sensors for Agricultural Applications. Chemosensors 2024, 12, 13. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Kumar, V.; Mirzaei, A.; Bonyani, M.; Kim, K.-H.; Kim, H.W.; Kim, S.S. Advances in electrospun nanofiber fabrication for polyaniline (PANI)-based chemoresistive sensors for gaseous ammonia. TrAC Trends Anal. Chem. 2020, 129, 115938. [Google Scholar] [CrossRef]
- Szary, M.J. Toward high selectivity of sensor arrays: Enhanced adsorption interaction and selectivity of gas detection (N2, O2, NO, CO, CO2, NO2, SO2, AlH3, NH3, and PH3) on transition metal dichalcogenides (MoS2, MoSe2, and MoTe2). Acta Mater. 2024, 274, 120016. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, B.; Yang, X.; Cao, F.; Xu, H.; Zhou, Y.; Jian, X.; Wang, X. The precise fluorination of ginkgo leaves for enhanced performance of lithium primary batteries. Mater. Lett. 2022, 324, 132812. [Google Scholar] [CrossRef]
- Jian, L.; Peng, R.; He, Y.; Wang, X.; Guo, W. One-step hydrothermal synthesis of urchin-like WO3 with excellent ammonia gas sensing property. Mater. Lett. 2023, 336, 133897. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zhang, X.; Rong, Q.; Hou, N.-N.; Yu, H.-Q. Ammonia sensing by closely packed WO3 microspheres with oxygen vacancies. Chemosphere 2018, 204, 202–209. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, W.; Li, Y. A novel seawave-like hierarchical WO3 nanocomposite and its ammonia gas properties. Mater. Lett. 2019, 248, 86–88. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zhou, H.; Wangyang, Q.; Peng, Y.; Wangyang, P.; Gu, L. Durian-like NiO architectures as an ultra-sensitive sensing materials for ammonia in normal temperature. Ceram. Int. 2019, 45, 1219–1226. [Google Scholar] [CrossRef]
- Zhao, K.; Li, X.; Tang, J.; Yang, H.; Wu, Q.; Wang, X.; Guo, X.; Zeng, D. Effect of exposed facet determined the room-temperature ammonia gas sensing of Cu2O nanoparticles. Appl. Surf. Sci. 2023, 613, 156008. [Google Scholar] [CrossRef]
- Wei, W.; Li, W.; Wang, L. High-selective sensitive NH3 gas sensor: A density functional theory study. Sens. Actuators B-Chem. 2018, 263, 502–507. [Google Scholar] [CrossRef]
- Alwan, A.M.; Abed, H.R.; Yousif, A.A. Effect of the Deposition Temperature on Ammonia Gas Sensing Based on SnO2/Porous Silicon. Plasmonics 2021, 16, 501–509. [Google Scholar] [CrossRef]
- Buyukkose, S. Highly selective and sensitive WO3 nanoflakes based ammonia sensor. Mater. Sci. Semicond. Process. 2020, 110, 104969. [Google Scholar] [CrossRef]
- Jha, R.K.; Singh, V.; Sinha, J.; Avasthi, S.; Bhat, N. CVD Grown Cuprous Oxide Thin Film Based High Performance Chemiresistive Ammonia Gas Sensors. IEEE Sens. J. 2019, 19, 11759–11766. [Google Scholar] [CrossRef]
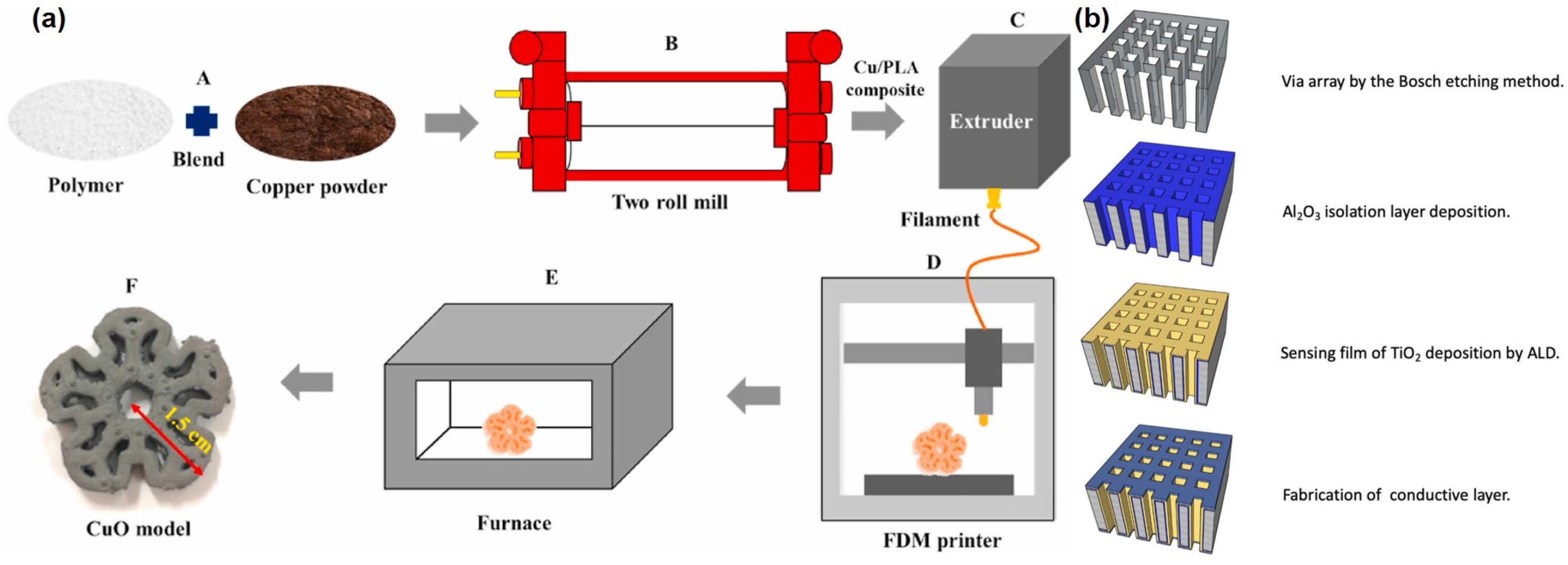
- Chaloeipote, G.; Prathumwan, R.; Subannajui, K.; Wisitsoraat, A.; Wongchoosuk, C. 3D printed CuO semiconducting gas sensor for ammonia detection at room temperature. Mater. Sci. Semicond. Process. 2021, 123, 105546. [Google Scholar] [CrossRef]
- Yeh, Y.-M.; Chang, S.-J.; Wang, P.H.; Hsueh, T.-J. A Room-Temperature TiO=-based Ammonia Gas Sensor with Three-Dimensional Through-Silicon-Via Structure. ECS J. Solid State Sci. Technol. 2022, 11, 067002. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, M.; Zhao, L.; Guo, J.; Zhang, B.; Gu, G.; Cheng, G.; Du, Z. The high-speed ultraviolet photodetector of ZnO nanowire Schottky barrier based on the triboelectric-nanogenerator-powered surface-ionic-gate. Nano Energy 2019, 60, 680–688. [Google Scholar] [CrossRef]
- Choi, Y.M.; Cho, S.-Y.; Jang, D.; Koh, H.-J.; Choi, J.; Kim, C.-H.; Jung, H.-T. Ultrasensitive Detection of VOCs Using a High-Resolution CuO/Cu2O/Ag Nanopattern Sensor. Adv. Funct. Mater. 2019, 29, 1808319. [Google Scholar] [CrossRef]
- Fu, S.; Zheng, Y.; Zhou, X.; Ni, Z.; Xia, S. Visible light promoted degradation of gaseous volatile organic compounds catalyzed by Au supported layered double hydroxides: Influencing factors, kinetics and mechanism. J. Hazard. Mater. 2019, 363, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wang, J.; Liu, B.; Ling, S.; Long, R.; Xiong, Y. Turning Au Nanoclusters Catalytically Active for Visible-Light-Driven CO2 Reduction through Bridging Ligands. J. Am. Chem. Soc. 2018, 140, 16514–16520. [Google Scholar] [CrossRef]
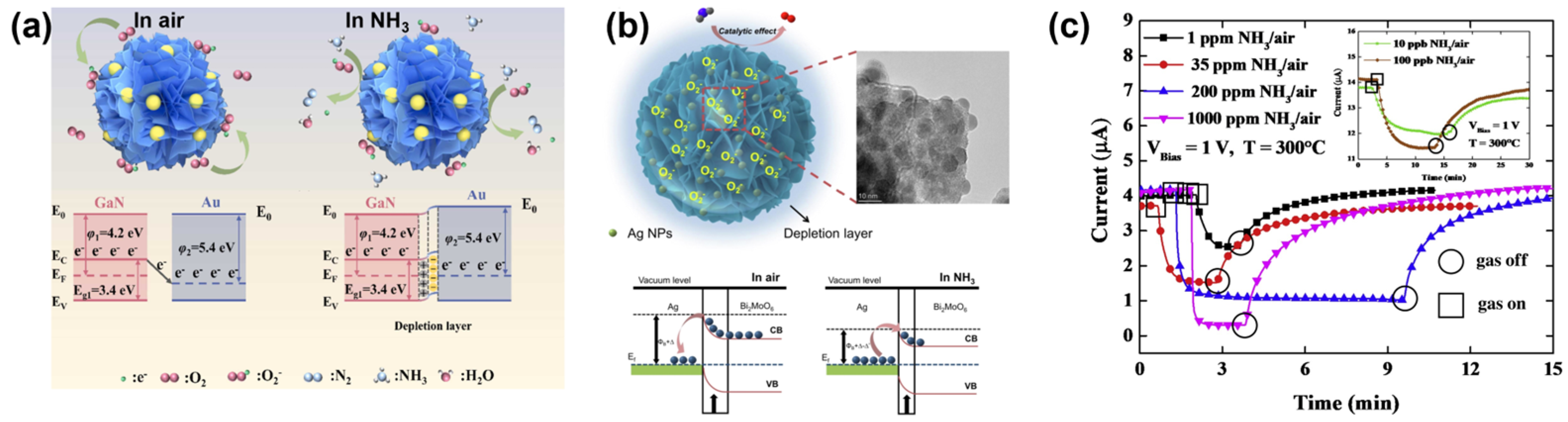
- Han, D.; Chen, Y.; Li, D.; Shi, J.; Wang, H.; He, X.; Zhao, L.; Wang, W.; Sang, S.; Ji, J. Au nanoparticles decorated GaN nanoflowers with enhanced NH3 sensing performance at room temperature. Sens. Actuators B-Chem. 2023, 394, 134320. [Google Scholar] [CrossRef]
- Liu, S.; Qin, Y.; Xie, J. Tuning reactivity of Bi2MoO6 nanosheets sensors toward NH3 via Ag doping and nanoparticle modification. J. Colloid Interface Sci. 2022, 625, 879–889. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, M.; Wen, X.; Ho, H.-P.; Lu, H. Nanostructured ZnO/Ag Film Prepared by Magnetron Sputtering Method for Fast Response of Ammonia Gas Detection. Molecules 2020, 25, 1899. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Tian, X.; Li, Y.; Zeng, Y.; Fan, C.; Wang, M.; Hua, Z. NH3 sensing properties and mechanism of Ru-loaded WO3 nanosheets. J. Mater. Sci.-Mater. Electron. 2018, 29, 11336–11344. [Google Scholar] [CrossRef]
- Dai, Y.-Z.; Liang, S.-Y.; Lv, C.; Wang, G.; Xia, H.; Zhang, T.; Sun, H.-B. Controllably fabricated single microwires from Pd-WO3•xH2O nanoparticles by femtosecond laser for faster response ammonia sensors at room temperature. Sens. Actuators B-Chem. 2020, 316, 128122. [Google Scholar] [CrossRef]
- Garshev, A.V.; Ivanov, V.K.; Krotova, A.A.; Filatova, D.G.; Konstantinovac, E.A.; Naberezhnyi, D.O.; Khmelevsky, N.O.; Marikutsa, A.V.; Kots, P.A.; Smirnov, A.V.; et al. Enhancement of Lewis Acidity of Cr-Doped Nanocrystalline SnO2: Effect on Surface NH3 Oxidation and Sensory Detection Pattern. ChemPhysChem 2019, 20, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, Y.; Song, P.; Yang, Z.; Wang, Q. Metal-organic framework-derived Cr-doped hollow In2O3 nanoboxes with excellent gas-sensing performance toward ammonia. J. Alloys Compd. 2021, 879, 160472. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Chen, J.-L.; Gong, F.-L.; Jin, G.-X.; Xie, K.-F.; Yang, X.-Y.; Zhang, Y.-H. Dual functionalized Ni substitution in shuttle-like In2O3 enabling high sensitivity NH3 detection. Appl. Surf. Sci. 2022, 600, 154158. [Google Scholar] [CrossRef]
- Vorobyeva, N.; Rumyantseva, M.; Platonov, V.; Filatova, D.; Chizhov, A.; Marikutsa, A.; Bozhev, I.; Gaskov, A. Ga2O3(Sn) Oxides for High-Temperature Gas Sensors. Nanomaterials 2021, 11, 2938. [Google Scholar] [CrossRef]
- Varudkar, H.A.; Umadevi, G.; Nagaraju, P.; Dargad, J.S.; Mote, V.D. Fabrication of Al-doped ZnO nanoparticles and their application as a semiconductor-based gas sensor for the detection of ammonia. J. Mater. Sci.-Mater. Electron. 2020, 31, 12579–12585. [Google Scholar] [CrossRef]
- Yao, G.; Yu, J.; Wu, H.; Li, Z.; Zou, W.; Zhu, H.; Huang, Z.; Huang, H.; Tang, Z. P-type Sb doping hierarchical WO3 microspheres for superior close to room temperature ammonia sensor. Sens. Actuators B-Chem. 2022, 359, 131365. [Google Scholar] [CrossRef]
- Zhu, H.; Ji, H.; Yuan, Z.; Shen, Y.; Gao, H.; Meng, F. Highly selective room temperature ammonia gas sensors based on d-band C-SnO2 and response behavior induced by oxidative and reductive role shifts. J. Mater. Chem. A 2023, 11, 10565–10576. [Google Scholar] [CrossRef]
- Sadhanala, H.K.; Nandan, R.; Nanda, K.K. Understanding the ammonia sensing behavior of filter coffee powder derived N-doped carbon nanoparticles using the Freundlich-like isotherm. J. Mater. Chem. A 2016, 4, 8860–8865. [Google Scholar] [CrossRef]
- Liu, M.; Sun, R.-Y.; Ding, Y.-L.; Wang, Q.; Song, P. Au/α-Fe2O3/Ti3C2Tx MXene Nanosheet Heterojunctions for High-Performance NH3 Gas Detection at Room Temperature. ACS Appl. Nano Mater. 2023, 6, 11856–11867. [Google Scholar] [CrossRef]
- Peng, R.; Li, Y.; Liu, T.; Sun, Q.; Si, P.; Zhang, L.; Ci, L. Reduced graphene oxide/SnO2@Au heterostructure for enhanced ammonia gas sensing. Chem. Phys. Lett. 2019, 737, 136829. [Google Scholar] [CrossRef]
- Pang, Z.; Nie, Q.; Lv, P.; Yu, J.; Huang, F.; Wei, Q. Design of flexible PANI-coated CuO-TiO2-SiO2 heterostructure nanofibers with high ammonia sensing response values. Nanotechnology 2017, 28, 225501. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, Y.; Zhang, J.; Meng, F.; Zhang, H. Rose-Like MoO3/MoS2/rGO Low-Temperature Ammonia Sensors Based on Multigas Detection Methods. IEEE Trans. Instrum. Meas. 2021, 70, 9506109. [Google Scholar] [CrossRef]
- Bera, S.; Kundu, S.; Khan, H.; Jana, S. Polyaniline coated graphene hybridized SnO2 nanocomposite: Low temperature solution synthesis, structural property and room temperature ammonia gas sensing. J. Alloys Compd. 2018, 744, 260–270. [Google Scholar] [CrossRef]
- Lu, L.; Liu, M.; Sui, Q.; Zhang, C.; Zou, Y.; Xu, F.; Sun, L.; Xiang, C. MXene/MoS2 nanosheet/polypyrrole for high-sensitivity detection of ammonia gas at room temperature. Mater. Today Commun. 2023, 35, 106239. [Google Scholar] [CrossRef]
- Abdollahi, H.; Samkan, M.; Hashemi, M.M. Facile and fast electrospinning of crystalline ZnO 3D interconnected nanoporous nanofibers for ammonia sensing application. Microsyst. Technol.-Micro-Nanosyst.-Inf. Storage Process. Syst. 2018, 24, 3741–3749. [Google Scholar] [CrossRef]
- He, J.; Liang, B.; Yan, X.; Liu, F.; Wang, J.; Yang, Z.; You, R.; Wang, C.; Sun, P.; Yan, X.; et al. A TPA-DCPP organic semiconductor film-based room temperature NH3 sensor for insight into the sensing properties. Sens. Actuators B-Chem. 2021, 327, 128940. [Google Scholar] [CrossRef]
- Park, P.; Ruffieux, D.; Makinwa, K.A.A. A Thermistor-Based Temperature Sensor for a Real-Time Clock With ±2 ppm Frequency Stability. IEEE J. Solid-State Circuits 2015, 50, 1571–1580. [Google Scholar] [CrossRef]
- Cai, Z.; Veldhoven, R.H.M.V.; Falepin, A.; Suy, H.; Sterckx, E.; Makinwa, K.A.A.; Pertijs, M.A.P. An integrated carbon dioxide sensor based on ratiometric thermal-conductivity measurement. In Proceedings of the 2015 Transducers—2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 622–625. [Google Scholar]
- Damilano, A.; Hayat, H.M.A.; Bonanno, A.; Demarchi, D.; Crepaldi, M. A Flexible Low-Power 130 nm CMOS Read-Out Circuit with Tunable Sensitivity for Commercial Robotic Resistive Pressure Sensors. IEEE Sens. J. 2015, 15, 6650–6658. [Google Scholar] [CrossRef]
- Tadić, N.; Zogović, M.; Gobović, D. A CMOS Controllable Constant-Power Source for Variable Resistive Loads Using Resistive Mirror with Large Load Resistance Dynamic Range. IEEE Sens. J. 2014, 14, 1988–1996. [Google Scholar] [CrossRef]
- Chan, S.S.W.; Chan, P.C.H. A resistance-variation-tolerant constant-power heating circuit for integrated sensor applications. IEEE J. Solid-State Circuits 1999, 34, 432–439. [Google Scholar] [CrossRef]
- Cai, Z.; Veldhoven, R.H.M.V.; Falepin, A.; Suy, H.; Sterckx, E.; Bitterlich, C.; Makinwa, K.A.A.; Pertijs, M.A.P. A Ratiometric Readout Circuit for Thermal-Conductivity-Based Resistive CO2 Sensors. IEEE J. Solid-State Circuits 2016, 51, 2463–2474. [Google Scholar] [CrossRef]
- Cai, Z.; Guerrero, L.E.R.; Louwerse, A.M.R.; Suy, H.; Veldhoven, R.V.; Makinwa, K.A.A.; Pertijs, M.A.P. A CMOS Readout Circuit for Resistive Transducers Based on Algorithmic Resistance and Power Measurement. IEEE Sens. J. 2017, 17, 7917–7927. [Google Scholar] [CrossRef]
- Ge, G.; Zhang, C.; Hoogzaad, G.; Makinwa, K.A.A. A Single-Trim CMOS Bandgap Reference with a 3σ Inaccuracy of ±0.15% from −40 °C to 125 °C. IEEE J. Solid-State Circuits 2011, 46, 2693–2701. [Google Scholar] [CrossRef]
- Shalmany, S.H.; Draxelmayr, D.; Makinwa, K.A.A. A ±36A Integrated Current-Sensing System With a 0.3% Gain Error and a 400 μA Offset From −55 °C to +85 °C. IEEE J. Solid-State Circuits 2017, 52, 1034–1043. [Google Scholar] [CrossRef]
- Saputra, N.; Pertijs, M.A.P.; Makinwa, K.A.A.; Huijsing, J.H. Sigma delta ADC with a dynamic reference for accurate temperature and voltage sensing. In Proceedings of the 2008 IEEE International Symposium on Circuits and Systems (ISCAS), Seattle, WA, USA, 18–21 May 2008; pp. 1208–1211. [Google Scholar]
- Meijer, G.C.M. Thermal sensors based on transistors. Sens. Actuators 1986, 10, 103–125. [Google Scholar] [CrossRef]
- Pertijs, M.A.P.; Makinwa, K.A.A.; Huijsing, J.H. A CMOS smart temperature sensor with a 3σ inaccuracy of ±0.1 °C from −55 °C to 125 °C. IEEE J. Solid-State Circuits 2005, 40, 2805–2815. [Google Scholar] [CrossRef]
- Ren, M.; Xu, H.; Dong, C.; Zhang, Z. Toward a Gas Sensor Interface Circuit—A Review. IEEE Sens. J. 2022, 22, 18253–18265. [Google Scholar] [CrossRef]
- Asri, M.I.A.; Hasan, M.N.; Fuaad, M.R.A.; Yunos, Y.M.; Ali, M.S.M. MEMS Gas Sensors: A Review. IEEE Sens. J. 2021, 21, 18381–18397. [Google Scholar] [CrossRef]
- Gardner, J.W.; Guha, P.K.; Udrea, F.; Covington, J.A. CMOS Interfacing for Integrated Gas Sensors: A Review. IEEE Sens. J. 2010, 10, 1833–1848. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, R. Selective determination of ammonia, ethanol and acetone by reduced graphene oxide based gas sensors at room temperature. Sens. Bio-Sens. Res. 2020, 28, 100336. [Google Scholar] [CrossRef]



| Gas | Material | Conc. (a) [ppm] | Tem (b) [°C] | S (c) | τres (d) [s] | τrec (e) [s] | Ref. (f) |
|---|---|---|---|---|---|---|---|
| NO2 | AuPt/SnO2 | 5 | 23 | 13.16 (h) | 10 | 6 | [24] |
| NO2 | AuPt/SnO2 | 10 | 23 | 13.15 (h) | 20 | 8 | [24] |
| CO | ZnO-MoS2 | (g) | 25 | (g) | (g) | 0.046 | [27] |
| NO2 | SnO2-NiO | (g) | 300 | 36 (h) | 204 | 163 | [28] |
| NH3 | CuO | 50 | (g) | (g) | 3.87 | 6.29 | [29] |
| Material | Response/ Concentration (ppm) | Response Time (s) | Recovery Time (s) | Stability (Days) | Lowest Concentration Detected (ppm) | Operating Temperature | Ref. |
|---|---|---|---|---|---|---|---|
| WO3 | 44.0 (a)/30 | 54 | 60 | - | 49.55 | 300 °C | [54] |
| WO3 | 3.32 (c)/100 | 150 | 210 | - | 1 | 350 °C | [55] |
| WO3 | 77 (j)/300 | - | - | - | 50 | 250 °C | [56] |
| NiO | 40% (i)/50 | 6.3 | 17.2 | - | 15 | Room temperature | [57] |
| Cu2O | 0.35 (g)/100 | - | - | 60 | 25 | Room temperature | [58] |
| Co3O4 | ~1.7 (b)/50 | 126 | 254 | 15 | 10 | Room temperature | [59] |
| Au-Fe3O4 | ~90% (f)/20 | 20 | 70 | 150 | 0.25 | Room temperature | [61] |
| Au-GaN | 86.8% (f)/100 | 41 | 169 | 30 | 72 | Room temperature | [69] |
| Ag-Bi2MoO6 | 37.6 (d)/200 | 5 | 38 | 30 | 50 | Room temperature | [70] |
| Pt-NiO | 1278% (g)/1000 | 15 | 76 | - | 10 | 300 °C | [40] |
| Pd-WO3 | ~1.04 (a)/50 | 1.4 | 3.3 | 16 | 1 | Room temperature | [73] |
| Cr-In2O3 | 11 (a)/10 | 1 | 18 | 16 | 1 | 140 °C | [75] |
| Ni-In2O3 | 2732 (a)/50 | 23 | 10 | 30 | 1 | 140 °C | [76] |
| Sb-WO3 | ~1.22 (b)/5 | 1.22 | 3.2 | 42 | 200 | 35 °C | [79] |
| C-SnO2 | 1996% (e)/100 | 37.5 | 105.5 | 10 | 100 | Room temperature | [80] |
| Au-Fe2O3-Ti3C2Tx | 16.9 (f)/1 | 3 | 2 | 30 | 1 | Room temperature | [82] |
| Au-rGO-SnO2 | 58% (h)/10 | 20 | 41 | 30 | 5 | Room temperature | [83] |
| Mn-ZnO-SnO2 | 13.13 (a)/100 | 64 | 24 | 30 | 10 | Room temperature | [8] |
| CuO-TiO2-SiO2 | 45.67 (g)/100 | - | - | - | 400 | Room temperature | [84] |
| MoO3-MoS2-rGO | 52 (a)/100 | 30 | 4 | 30 | 1 | 200 °C | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Xu, J.; Peng, Z.; Ji, Z.; Gao, Y.; Jia, L.; Xu, Q. Design Strategies of Integrated Metal-Oxide Semiconductor-Based Resistive Sensor Systems for Ammonia Detection. Electronics 2024, 13, 4800. https://doi.org/10.3390/electronics13234800
Yan Y, Xu J, Peng Z, Ji Z, Gao Y, Jia L, Xu Q. Design Strategies of Integrated Metal-Oxide Semiconductor-Based Resistive Sensor Systems for Ammonia Detection. Electronics. 2024; 13(23):4800. https://doi.org/10.3390/electronics13234800
Chicago/Turabian StyleYan, Yingzhan, Jing Xu, Zhilong Peng, Zhe Ji, Yuan Gao, Lu Jia, and Qian Xu. 2024. "Design Strategies of Integrated Metal-Oxide Semiconductor-Based Resistive Sensor Systems for Ammonia Detection" Electronics 13, no. 23: 4800. https://doi.org/10.3390/electronics13234800
APA StyleYan, Y., Xu, J., Peng, Z., Ji, Z., Gao, Y., Jia, L., & Xu, Q. (2024). Design Strategies of Integrated Metal-Oxide Semiconductor-Based Resistive Sensor Systems for Ammonia Detection. Electronics, 13(23), 4800. https://doi.org/10.3390/electronics13234800






