Abstract
Type 1 Diabetes Mellitus (DM1) is a condition of the metabolism typified by persistent hyperglycemia as a result of insufficient pancreatic insulin synthesis. This requires patients to be aware of their blood glucose level oscillations every day to deduce a pattern and anticipate future glycemia, and hence, decide the amount of insulin that must be exogenously injected to maintain glycemia within the target range. This approach often suffers from a relatively high imprecision, which can be dangerous. Nevertheless, current developments in Information and Communication Technologies (ICT) and innovative sensors for biological signals that might enable a continuous, complete assessment of the patient’s health provide a fresh viewpoint on treating DM1. With this, we observe that current biomonitoring devices and Continuous Glucose Monitoring (CGM) units can easily obtain data that allow us to know at all times the state of glycemia and other variables that influence its oscillations. A complete review has been made of the variables that influence glycemia in a T1DM patient and that can be measured by the above means. The communications systems necessary to transfer the information collected to a more powerful computational environment, which can adequately handle the amounts of data collected, have also been described. From this point, intelligent data analysis extracts knowledge from the data and allows predictions to be made in order to anticipate risk situations. With all of the above, it is necessary to build a holistic proposal that allows the complete and smart management of T1DM. This approach evaluates a potential shortage of such suggestions and the obstacles that future intelligent IoMT-DM1 management systems must surmount. Lastly, we provide an outline of a comprehensive IoMT-based proposal for DM1 management that aims to address the limits of prior studies while also using the disruptive technologies highlighted before.
1. Introduction
The primary characteristic of Type 1 Diabetes Mellitus (DM1) is elevated glycemia levels in the bloodstream caused by the body’s incapacity to segregate or utilize insulin or both. Glucose homeostasis is a closed-loop mechanism capable of regulating blood glucose levels in a healthy adult. The pancreas supplies the cells with insulin, stimulated by high glucose levels, which subsequently reduces hyperglycemia by acting as a potent hormone [].
Due to the autoimmune nature of DM1, the pancreas’ critical insulin-producing cells are destroyed, making it impossible to achieve such control. In light of this, DM1 could be considered the most challenging form of diabetes to be controlled. Individuals with DM1 are unable to create insulin, necessitating the use of an insulin pump or exogenous insulin injections to manage glucose levels. Furthermore, in order to prevent hyperglycemia, using capillary glucometers, diabetics must monitor their glucose levels throughout the day to infer how much insulin is required in combination with their meals and physical activity []. Maintaining healthy levels can be accomplished by continuous infusion, skin permeation, or many injections given throughout the day.
Intelligent Data Analysis (IDA) facilitates blood glucose level modeling and may improve the capacity of persons diagnosed with diabetes to monitor their status and accomplish the abovementioned maintenance. The development of IDA occurred 50 years after the initial efforts to create an Artificial Pancreas (AP) []. Researchers believe that these APs, which are still under development, would consist of a Continuous Glucose Monitoring (CGM) device focused on monitoring an individual’s glucose levels in real time, followed by injecting the necessary insulin, guided by a mathematical model capable of simulating the ideal glycemic balance [,]. Using a control algorithm, a computer-assisted system was developed to mimic the movements of a pancreas; however, the currently projected pioneering advances are likely to be heralded as something of a sea shift.
Alternatives to AP and CGM devices may also be accessible, as other biometrics researchers are examining the viability of 24 h patient monitoring, allowing crucial health data to be gathered to support the development of effective glycemia treatment. This is accomplished by using specific variables that can be examined consistently, such as heart rate, temperature, rest quality, and physical activity, in accordance with the primary sensors contained in commercially available intelligent watches (an appropriate biomonitoring process was proposed in []). However, there are drawbacks to such benefits since managing such a wealth of data requires dependable Information and Communication Technology (ICT) capable of obtaining and processing meaningful data for further use. The pervasiveness of the Internet of Medical Things (IoMT) offers a highly suitable technical framework for this approach as it enables the collection of vast quantities of data using various sensors linked to a person with diabetes, transmitting this information to a smartphone via Bluetooth []. Unfortunately, smartphones currently lack the processing capacity required to construct a glucose model, and thus this job must be performed on a powerful server.
The use of IoT presents substantial promise for the development of sophisticated and dependable model systems to improve the monitorization of DM1. Extensive data analysis provides the opportunity to harness the vast data stores generated by IoT links to identify characteristics that facilitate the acquisition of important information that may lead to a better interpretation of blood sugar discrepancies. Meanwhile, cloud computing enables the use of computationally demanding intelligent data approaches that demonstrate the fluctuation of glucose levels.
In this paper, we want to offer a vision of IoMT to the management of T1DM. To do so, we review the potential of IoT in the healthcare environment. To this end, in Section 2, we have reviewed the fundamentals of IoMT approaches. With this background, IoMT platforms for the specific management of chronic diseases are studied in Section 3. Section 4 and Section 5 analyze the main wearable devices that can be used in a T1DM-IoMT environment as a source of information, with a special emphasis on the Continuous Glucose Monitoring device. With these devices, it will be possible to collect the biomedical variables described in Section 6, which will allow a diabetic patient to be fully monitored. In the following sections, solutions for IoMT management of T1DM are sought: connectivity options are reviewed in Section 7, intelligent data analysis is in Section 8, and a complete description is proposed in Section 9. Section 10 offers some conclusions and challenges to be met.
The main contribution of the paper is to compile the necessary elements to make a proper management of T1DM using an IoMT environment. It takes into account the solutions that, in this sense, have been given in other approaches to health care and reviews the specific biomonitoring devices that are useful for T1DM, reviewing the influence of the biomedical variables measured. After this, taking into account the forms of data transmission and artificial intelligence, a holistic solution is arrived at, and future challenges to be solved are proposed.
2. Internet of Medical Things (IoMT)
Interconnected medical equipment for healthcare monitoring is known as the Internet of Medical Things (IoMT). Healthcare IoT devices use automated inter-facial sensors and ML to facilitate human-free healthcare supervision []. Using medical devices, IoMT technology lets patients and doctors remotely collect, evaluate, and transfer medical data via wearable, in-home, tailored, real-time health monitoring. Health monitoring using wireless health indicators based on IoMT technology reduces unnecessary hospital visits and health costs. In the ambient-aided environment, the IoMT architecture alerts caregivers to abnormalities in the patient’s health. IoMT networking nodes and sensing devices collaborate, integrating intelligent platforms, while caretakers’ healthcare apps record patient information and health problems. For example, a local monitoring agent can inform a caretaker of the client’s odd position, and medical staff can monitor the patient’s status and help reach the hospital if necessary []. Based on the severity of the patient’s condition, they may notify hospital personnel.
IoMT has already been widely studied. Moosavi et al. [] evaluated healthcare IoT end-to-end security solutions. Since healthcare IoT sensors have minimal memory, computing power, and bandwidth, the authors recommend updating cryptographic key generation algorithms. They employ a three-layer design incorporating key generation by merging ECG signal interpulse interval sequences with pseudo-random numbers, the reciprocal authentication of machines and users via a smart gateway, as well as secure end-to-end transmission between endpoints via handover techniques.
Talpur et al. [] created an energy-optimized algorithm for a smartphone-based chronic heart disease monitoring system. In their approach, Bluetooth connects body sensors to smartphones, which assemble and transmit data to distant database servers. Priority warnings are quickly transmitted to the database server for analysis and transmission to relevant parties, which is energy-intensive. However, the harshness of the data determines smartphone energy usage.
Ali et al. [] developed an approach to use the IoT to identify depression signs and provide appropriate help—a seldom discussed issue. They employed a Web of Items (WoI) framework to virtualize real-world objects and combine heterogeneous devices connected to resources to construct IoT services and deploy and operate them. They suggest a microservices paradigm for system functions such as Preference Learner and Recommendation Analyzer. The user-updated real-world knowledge module generates a suggestion based on user input and preferences. In contrast, the semantic ontology module integrates and links virtual objects and provides an ontology network with nodes for objects and branches for their attributes.
Romero et al. [] utilize IoT to diagnose and monitor Parkinson’s disease, an elderly neurological illness that produces uncontrollable tremors. They suggest using body sensors to identify better and quantify tremors and facilitate clinical evaluation. This is beneficial to the patient since a brief hospital visit may not disclose the whole nature of the condition, but IoT data collection and evaluation from home will.
Bajaj et al. [] investigated applications in terms of cardiology. Their heart attack detection system tracks heart rate and temperature, and the technique can be used to measure excess Fatty Acid Binding Protein (FABP3) before cardiac arrest. A microcontroller sends sensor data to the cloud through the Internet.
Hemalatha et al. [] present a unique approach to identifying and calculating chronic coughs using an ECG, chest belt, oximeter, accelerometer, etc. A battery-powered neck-mounted MEMS vibration sensor sends data to a medically supervised cloud-based health platform through a smartphone.
Matar et al. [] suggest body pressure distribution on the mattress as a modern posture monitoring method for remote medical monitoring. Computer workstations analyze body pressure distribution data from pressure sensor mattresses. A diagnostic analysis follows. This approach is employed in sleep research and anesthetic surgery. The suggested technique leverages supervised learning SVM.
Magsi et al. [] examined how 5G and IoT interact. The 5G can support IoT healthcare diagnosis and treatment with high data, speed, and battery life. The authors also discussed 5G-based sensor node design for patient medical control and longstanding connections.
Fan et al. [] studied cloud-based RFID systems—a cutting-edge healthcare technology. However, faulty cloud servers can transmit confidential medical data across public wireless networks, putting patient data at risk of leaks. Using quadratic residuals and a pseudo-random number creator, a lightweight authentication technique addresses confidentiality and security concerns while tagging anonymity, endurance to tag tracking assaults, reciprocal authentication, and de-synchronization guarantee privacy.
Onasanya et al. [] proposed an IoT-based cancer therapy and detection system powered by cloud/business analytics. The research focused on the use of IoT-WSN to improve cancer therapy. WSN and smart connected devices hereby enable data transmission/exchange by linking a number of geographically dispersed autonomous sensors to the network fabric based on geographical routing from source to destination. Business analytics/cloud services generate patient data streams for informed decision making.
Having reviewed the possibilities of IoMT in a general way, we have seen that it can be applied from hospital stays to mental illness monitoring, electrocardiogram monitoring, as well as other applications. However, in the next section, we are going to focus on chronic diseases with continuous needs for the patient due to their similarity with T1DM.
3. IoMT-Based Methods for Chronic Diseases and DM1
According to the scientific literature, IoMT can be used to control specific diseases that need constant monitoring, such as DM1.
Telemedicine, i.e., remote monitoring, uses a wireless Body Area Network (BAN) of wearable computer devices []. Telemedicine requires BANs to reflect all applications over, inside, and close to the body, as well as communications. BANs are ideal for monitoring patients’ physiological signs in medical settings. A Wireless Body Area Network (WBAN) is specially conceived as a wireless sensor network that uses several networks and wireless tools for distant monitoring in a variety of situations. The authors of [] introduced a BAN for epileptic patients, with the device monitoring for epileptic attacks 24/7. Thus, such a report might recommend that DM1 patients estimate glucose progression and inform health specialists via the Internet or smartphones if emergencies arise. Like in [], an appropriate information-exchange platform might fulfill this concept.
BANs need a body gateway as well as a network hub, which is generally a smartphone. The authors of [] highlighted the diabetes control potential of it. This approach registers food, insulin, location, and activity data on the phone to identify glucose trends and provide insulin dosage recommendations. Today, technology makes it simple to carry more accurate biological sensors that can be linked. Smartphones can act as gateways and information management tools. The authors of [] reviewed smartphone diabetes care apps and showed that applications are generally limited, concentrating on one aspect of management (e.g., monitoring physical activity, glycemia control).
However, it should be reiterated that the AP approach is impossible without a CGM device []. This technique can measure the amount, tendency, frequency, and length of glucose changes in diabetic patients, revolutionizing diabetes management. This field is constantly evolving. Abbott Laboratories’ 2014 flash glucose monitoring device, Freestyle Libre, was revolutionary owing to its accuracy and cost []. These gadgets have expanding connection capabilities.
In [], a smartphone-based type 2 diabetes healthcare platform is described, addressing the information management issue. This proposal collects physical activity, heart rate, and food intake data and displays exercise objectives, moods, and diets. However, the suggested system employs unpleasant sensors and does not detect glycemia. It also cannot handle dangerous circumstances. The hypoglycemia risk makes this an essential concern in diabetes treatment. Alarm-based Mobile Diabetes Management (ADMAN) was suggested in []. Remote monitoring helps elderly patients regulate their diabetes. This approach has an emergency mechanism but does not monitor most aspects or anticipate future glycemia levels. Sanofi’s (Paris, FranceiBGSTAR®) Diabetes Manager app and Dexcom Inc.’s G5 Mobile CGM System are other smartphone data management examples (San Diego, CA, USA).
Other previous work [] recommends using the IoT for diabetes control. Smartphones can remotely gather, monitor, and provide patient and medical caregiver input. While it is a comprehensive proposal, certain aspects are not monitored, and CGM is omitted. The model also advises patients to change their insulin dose, but this cannot be adequately performed without 24 h monitoring. The authors of [] suggest cloud computing for diabetes management. The cloud makes information-sharing easier, but emergency monitoring and management are lacking.
COMMODITY12 [] is a personal-health system for diabetics. Wearable and portable devices collect important bodily indicators, which are analyzed by expert algorithms. They can direct patient and health professional input. This is an intriguing idea that combines heart rate and physical activity, but biosensors would add more information. Furthermore, while expert knowledge interprets the data, other machine learning algorithms could forecast glycemia or make autonomous judgments. This system does not anticipate these tasks, even to prevent hypoglycemia. Finally, CGM should be included in the monitoring, which is lacking in this approach. The work of [] is similarly weak: Although a BAN was developed, integrating aspects such as body temperature and an online display that provides information could generate further knowledge.
Finally, the acceptability of telemedicine must also be addressed. Continuous monitoring, capturing of personal data, and thorough patient characterization may compromise patient privacy, resulting in user rejection. A compilation of these difficulties can be found in []. In this study, age, sex, and telemedicine experience affect acceptability.
The above shows that a BAN is needed to gather data on glycemia, insulin, heart rate, physical activity, and sleep hours when constructing a complete DM1 management system. Smartphones may potentially act as body gateways and deliver more information via software apps. In the end, IoT and cloud computing provide distant administration, and the system has to ensure the possibility of auto-adjustment, decision assistance, and patient guidance through machine learning algorithms or deep learning processes.
As we have seen, in T1DM, the use of a BAN is highly convenient since it is the structure that allows combining permanent monitoring and continuous data collection and channeling it to more computationally powerful environments, thus satisfying the management of a large amount of information.
With this in mind, we will study the following issues in the following sections:
- Biomonitoring devices, in order to obtain data that allow us to know at all times the state of glycemia and other variables that influence its oscillations;
- Variables that can be monitored, starting with glycemia but adding others of relevance, according to the biomedical monitoring devices studied;
- Communication systems needed to transfer the information collected to a more powerful computational environment that can manage the information collected;
- Intelligent data analysis that extracts knowledge from the data and allows predictions to be made in order to anticipate risk situations;
- With all of the above, it is necessary to build a holistic proposal that allows the complete and smart management of the T1DM.
4. Continuous Glucose Monitoring (CGM)
The first step in diabetes monitoring is choosing a CGM. An AP is not feasible without a CGM device. This approach could predict the amount, tendency, frequency, and length of glucose swings in diabetic patients revolutionizing diabetes management []. For example, CGM can conduct one glucose data point per minute (i.e., 1440 values/24 h), compared to the three to ten per day achieved with fingerstick or capillary blood glucose monitoring. This sampling frequency is enough for an input of a hypothetical system for glycemic management. However, Kovatchev et al. [] note that CGM has significant drawbacks, as could be random noise and a transitory failure of sensitivity.
The delay between CGM data gathering and patient glucose levels is a common concern. The CGM device estimates blood glucose based on interstitial liquid under the skin, which is deferred by a few min and represents a drawback. A recent study has indicated that this time lag is 5–10 min []. Basu’s [] tests showed a 6 min delay. Mathematical approaches can be used to correct this delay’s inaccuracy.
MARD and a Clarke error grid can be employed to assess the accuracy of such devices []. MARD is calculated from the average difference between the CGM device and the actual value. MARD lowers the average error and increases device accuracy. MARD is 6–8% for the most accurate CGM systems. Table 1 presents the metrics for the significant CGM devices.

Table 1.
Current CGM sensors.
Dexcom may be the most accurate CGM. The G6 variant allows smartphone glucose readings without the Dexcom receiver. Dexcom G6’s sophisticated algorithm achieves a 9.9% MARD. The manufacturers say the sensor lasts 10 days, but consumers can reset it.
Medtronic Guardian Sensor 3 CGM sensors can be used with insulin pumps or individually. The sensor has a 7-day lifespan with a MARD of 8.7–10.5%, similar to the Dexcom devices.
Abbott offers the Freestyle Libre Pro []. Previous versions merely recorded and sent glucose data. Since Freestyle Libre 2, it has been possible to set alerts for low and high glucose levels, making it a safe CGM device. The arm-mounted sensor has a MARD of 9.4% and a 14-day lifespan, but it cannot be reset. Its non-calibration is a benefit.
All CGM devices utilize various algorithms to reduce the delay between the bloodstream and interstitial fluid glucose levels when predicting the following result. This delay, significantly when glucose levels change quickly, makes anticipated values unreliable. CGM’s various advancements prevent this. However, the primary issues are denoising, improving raw data correctness, and reducing error due to rectification latency [].
An adaptive self-tunable Bayesian smoother [] might determine the CGM signal-to-noise ratio in real time for the denoising process. Palerm and Bequette [] and Mahmoudi et al. [] also provide denoising methods.
The improvement module presented in [] uses a stochastic deconvolution-based recalibration technique to rescale CGM data utilizing a simple linear regressor whose parameters are calculated with each new SMBG value. This stage may also use enhancement/recalibration methods from Barcelo-Rico et al. [] (adaptive adjustment) and Kirchsteiger et al. [] (LMI-based approach).
A simple yet successful forecaster based on an autoregressive model of grade one may be utilized in the prediction stage []. Its primary feature is real-time parameter estimation utilizing a recursive least squares implementation and a forgetting factor to weight previously obtained data correctly. This has been investigated by Zecchin et al. [] (neural network), Zarkogianni et al. [] (physical activity), and Georga et al. [] (regression models).
5. DM1 Monitoring System Incorporating Commercial Smart Devices
This sector has seen significant technical improvements. New technologies on the market have transformed monitoring. Smartphones are the keystone of these novel intelligent devices and have unmatched versatility. They enable the following in particular:
- Maintaining the software responsible for system dynamics, glucose prediction, solution optimization, and process control;
- Performing CGM and gathering insulin pump data using extensive connectivity, smartphones can now interact through 4G, Bluetooth, Wi-Fi, NFC, Ant+, and more, giving users many options;
- Sending data to the cloud for storage or computation (cloud computing);
- Forwarding emergency calls to protect the patient;
- Updating software as needed.
Smartphones provide several monitoring options. Today, accelerometers, gyroscopes, and pedometers quantify physical activity. Some can assess ambient temperature and heart rate using a camera flash (albeit with a high error). These traits are commonly utilized recreationally. Other, more precise sensors are detailed below.
Place et al. [] tracked patients’ progress from various locations to investigate the possibilities of GPS. Other studies have examined the usage of smartphones to transmit emergency calls using GPS locations [].
Smartphones may not be certified as medical equipment (class III, high risk). To prevent conflicts with other phone apps, the controller app must be dependable and should prevent battery discharge and connection issues. Rigla lists these benefits and downsides [].
The field of biometrics, which studies quantifiable biological traits, has been miniaturized and improved by advances in electronics. Continuously measuring vital signals, such as heart rate, exercise, and others, is now achievable, and these measures affect the blood glucose balance. Smart bands and other health and sports wearables now do it simple to collect a whole day’s worth of data, providing much helpful information []. These technologies can gather and show data in a user-friendly manner, making them useful in health monitoring. Bluetooth connectivity gives them much potential; however, they are limited by their size, battery life, and professional usage. Despite these drawbacks [], they may deliver accurate data. These devices may characterize patients based on a wide variety of physiological characteristics. For example, the patient can wear various medical gadgets to monitor their temperature, blood pressure, etc.
Innovative devices such as accelerometers, electrocardiograms, thermistors, and others have been tried in standalone trials but not in DM1 managing systems. Some of them have been utilized in conjunction with CGM to examine the link between blood glucose and other characteristics or to predict glycemia levels. A 24 h concurrent monitoring of all aspects has not been performed, nor has a study of the kinds of variables, their connections, or the levels of effect on blood glucose; there has also been no valuable collection of features useful for modeling glycemia progression.
6. Variables Related to DM1 in an IoMT Context
This section outlines some of the biosignals related to DM1. These features can be monitored continuously with biosensors (such as smart bands) in an IoT context []. Most diabetes management systems only include glycemia, insulin, and meal estimations; however, it appears appropriate to include other factors that might affect glucose levels as far as they can be measured or estimated. The majority of studies use glycemia, insulin, and meals as significant variables [,], but some use only previous glycemia data (such as in autoregressive model approaches) or just add insulin to glycemia and use an autoregressive with exogenous terms model []. In recent years, several studies have included various factors, including exercise, both in silico and in vivo [], heart rate, temperature, etc. Figure 1 synthesizes the variables that are described below.
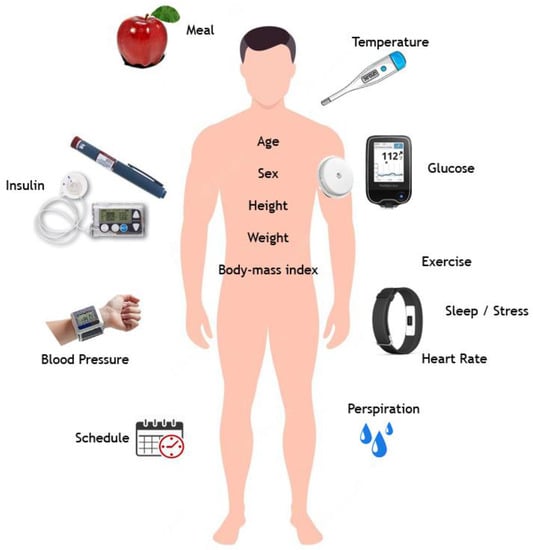
Figure 1.
Variables related to DM1 in an IoMT context.
A complete list of DM1 control system variables is examined in the following. Some of these have been addressed in the academic literature, while others are newly introduced in this article.
- Glycaemia: One key variable is the blood glucose level. Traditional capillary blood monitoring provides discrete glucose measurements. New CGM systems have adequate precision and sample frequency [] to provide input for a continuous control algorithm.
- Insulin: Diabetics must decide how much insulin to inject. This is the crucial variable that controls the hypoglycemic response, and hence it may need to be added. Three insulin concepts must hereby be considered:
- Instantaneous insulin input: Recent dosages, particularly fast-acting insulin (boluses). This insulin typically lasts two and a half hours, peaking at 90 min.
- Basal insulin: This compensates for glucagon’s delayed glucose release from liver glycogen over 24 h [].
- Accumulated insulin: Some scientific literature considers the body’s present insulin quantity (which is active). This includes basal insulin and fast insulin, which may operate slowly yet noticeably over many hours. Another study suggests an adequate limit of five to eight hours [,].
- Exercise: Muscles need glucose to work. Because exercise momentarily makes cellular barriers more permeable, glucose enters cells more efficiently, and insulin is burned up more quickly [], potentially causing hypoglycemia and insulin reduction. Exercise reduces glycogen, which is progressively released into the circulation throughout the day, and more quickly during hypoglycemia. Thus, recent, intense, and accumulated exercise should be distinguished. Exercise increases blood glucose and insulin needs, and the impact may continue for up to 48 h [,]. Regular exercise also balances blood sugar and decreases insulin needs [].
- Meals: Food affects blood glucose levels. Glucose is mostly digested and absorbed, quickly boosting blood sugar. Processing software can model this interaction []. Three features of meals should be noted: Notification of ingestion, carbohydrate measuring, and how quickly and how much the blood glucose level rises depending on the quantity of carbs (glycemic index). Diabetics are routinely educated in meal and carbohydrate counting using standardized tables. However, it is difficult and inefficient to quantify the precise quantities ingested (patients are often confused since it is a subjective judgment). Bell et al. [] evaluated how fast various meals are absorbed and reflected in glycemia. In that investigation, CGM predicted fat absorption at five hours, protein at three hours, albeit less noticeably, and carbohydrates at two hours. Meals are a combination of these three components, and thus the maximum overall contribution will vary. Accumulated intakes build glycogen stores, which makes the final hypoglycemia response rapid yet raises glucose levels owing to the gradual and continuous glucagon dumping and glucose release (mainly from the glycogen accumulated in the liver). It should also be remembered that one macronutrient might impede the absorption of another. For example, fat slows glucose absorption [] and may reduce insulin sensitivity [], although fiber can delay glucose absorption [].
- Stress and sleep quality: Stress hormones cause hyperglycemia []. Several factors cause adrenaline and cortisol release. On the other hand, poor sleep quality or a lack of sleep may change glucose metabolism and insulin resistance, resulting in hyperglycemic consequences [].
- Heart rate: This may rise for several reasons, such as exercise. Stress can also cause such an increase []. The heart rate may also indicate hypoglycemia [], as has been found for hyperglycemia [].
- Temperature: Hypoglycemia lowers the body temperature []. Long-term patients with recurrent glucose decrease typically have no symptoms until it is too late. Hyperthermia, which typically indicates an illness, causes hyperglycemia []. Hypothermia may also cause hyperglycemia [].
- Perspiration: Hypoglycemia causes sweating. Long-term patients may not experience this symptom, like in a fever [].
- Blood pressure: Metabolic syndrome, which causes insulin resistance and kidney failure in type 1 long-term diabetics, produces arterial hypertension. Thus, elevated blood pressure may indicate poor glycemic management [] and cause heart, eye, kidney, and blood vessel problems.
- Schedule (time): Patients’ daily schedules are similar because people tend to follow a set of conventions and habits. Diabetics can typically recognize a weekly pattern, which helps them manage their diabetes. Working, eating, exercising, and injecting insulin are often performed simultaneously. Thus, knowing the hour and day of the week should facilitate system prediction. The schedule helps identify basal insulin evolution [].
- Age, sex, height, weight, and BMI might also be used to customize solutions.
Other patient characteristics, settings, and concurrent (chronic or transitory) disorders might impact glucose levels. This topic has been subject to some debate. In certain situations for women, such as pregnancy [], additional attention should be given to enhance gestation and delivery. Menstruation, menopause [], and osteoporosis [] are some of the other issues that diabetic women must consider.
In another vein, [] discussed the impact of mental illness, emphasizing its relevance. Other concurrent disorders, particularly those related to diabetes, must also be considered a “variable” when describing a diabetic patient. In this sense, cardiovascular and renal impairment [], diabetic foot, retinopathy [], and others have underlined the need to recognize these simultaneous situations and implement a coordinated strategy. Although the causes of these concurrent conditions are wide-ranging and need to be explored on a case-by-case basis, a detailed characterization such as the present study might offer more accurate information and allow tailored diabetes treatment. Thus, only variables that can be assessed non-invasively are considered.
7. Connectivity and the Communications Environment
New continuous biometric sensors can produce substantial amounts of patient data. The implemented clinical gadgets, platform management, and medical institutions’ computerized records must be compatible with encouraging data exchange. The biosensors must also be able to communicate data clearly. Health Level 7 (known as HL7 []), the essential standard for health data transmission across healthcare provider software applications, is an acknowledged requirement. It specifies real-time clinical data transfer across medical apps. Figure 2 depicts the connectivity ways explained in this section.
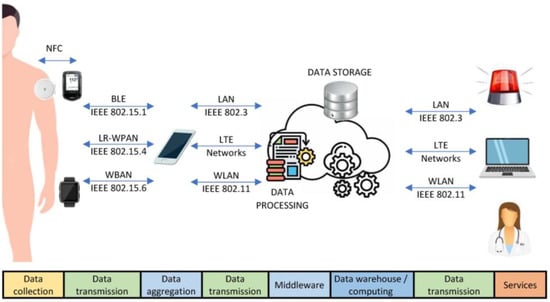
Figure 2.
Connectivity ways for DM1 management.
Mobile Access to Health Information (MAHI) [] initially links diabetic patients and medical professionals through Bluetooth to a glucometer. However, asynchronous communication prevents real-time advice and warnings.
The mobility of wireless sensor networks makes their role in data management unique []. This aspect, coupled with their low power consumption, facilitates the use of low-power BANs. Smartphones can use Bluetooth Low Energy (BLE), Near-Field Communication (NFC), and Wi-Fi wireless connections. In-bedroom patient health monitoring is one example of such an application [].
Data capture is the initial step in an IoT healthcare system. WBANs and environmental sensors collect physiological and ambient information. WBANs, a key IoT healthcare technology, need dependable sensors to function properly []. IEEE 802.15.6, a WBAN reference standard, provides low-power, short-range, and highly reliable wireless communication, both outside and within the body. Based on the application, IEEE 802.15.6 specifies three layers: Narrowband (NB), Ultra-wideband (UWB), and Human Body Communications (HBC). CSMA/CA, TDMA, and unscheduled access are provided by the Medium Access Control (MAC) layer. IEEE 802.15.6 [] classifies WBAN applications as medical or non-medical. The work of [] classifies IEEE 802.15.6 medical applications into three groups, namely wearable WBAN, implanted WBAN, and medical device remote control. The standard specifies 10 kbps to 10 Mbps bit speeds, and nodes must be mobile as well as removable within 3 s. Medical applications should have a latency below 125 ms, and non-medical ones should have one below 250 ms. The Packet Error Rate (PER) for a 256-octet payload for 95% of the best-performing connections should be below 10% for in-body and on-body nodes.
The literature suggests WBAN and WPAN protocols for numerous applications. ZigBee, BLE, and Wi-Fi have experienced the most widespread adoption. BLE, RFID [], ZigBee [], and IPv6 via time-slotted channel hopping can be used to send sensor data to a central unit for collection and analysis. IEEE 802.11ah, often known as Wi-Fi HaLow, is a new wireless networking standard that improves coverage and efficiency for IoT and M2M applications []. Long-range technology such as LP-WAN or LTE [] conveys process data to send to the patient or doctor’s office for additional analysis.
As mentioned above, various new sensor types have emerged, creating a broad spectrum of IoT communications needs. As a result, the transmission of this data must be continuous, low-consumption, high-payload, and have adequate bandwidth. This issue has already received some attention. For instance, [] examined smartphone-based health monitoring focusing on energy savings. They found that Zigbee and Bluetooth connections save energy. However, the study did not investigate a specific illness, and no anticipated conditions were examined. Low-power WBANs with sensors such as blood pressure devices and scales to control weight use ZigBee and Bluetooth []. The Abbot Freestyle Libre, a glycemia glucose monitoring system that has grown in popularity over the last two years, uses NFC to measure glycemia.
Continua Alliance (C.A.) has established dependable and secure protocols for communications, such as the Bluetooth Health Device Profile (HDP) [], to enable device interoperability. IEEE 1073 standards establish this ecosystem’s protocols and compatibility. However, C.A. Electrocardiogram (ECG) sensors are too sophisticated for ZigBee. Considerable research has been conducted on 6LoWPAN and NFC. Bluetooth, ZigBee, and NFC can all provide a suitable IoT ecosystem, as will be discussed later.
A communications infrastructure with high-capacity channels is needed to link IoT devices to the cloud for data processing. Thus, today’s connections provide several methods to send information. Wi-fi transmitters can be used to expand the connections of medical equipment. If a patient’s home or hospital does not have Wi-Fi, a smartphone with a 4G connection is needed to upload the data to the cloud. Each device may also connect independently via SIM cards.
BLE can also be used to communicate between devices, and this communication technique is the focus of the proposal in this paper’s architecture. BLE was created for the IoT to transmit data without connection, making pairing and transmission quicker (3 ms). BLE can collect data and facilitate remote administration. In discrete transfer, sensors can be waiting and auto-enable to communicate, thus conserving energy.
Alternative links are equally intriguing. ZigBee is an IEEE 802.15.4-based standard for a suite of high-level communication protocols that establish personal area networks using tiny, low-power digital radios for home automation, medical device data collecting, and other low-power, low-bandwidth wireless applications. It connects more than 66,000 nodes and uses less energy since the nodes can be inactive when not in use. Nevertheless, since 24/7 monitoring is required, that number of nodes is unnecessary, and the sensors could hardly be disabled.
BLE was released in 2006 as “Wibree” and incorporated into the core Bluetooth standard in 2010 with the Bluetooth Core Specification Version 4.0. Smartphones, laptops, and smartwatches include BLE modules, making it a standard low-power wireless technology for monitoring and the IoT. Unlike standard Bluetooth, BLE transmits brief burst packets, which stream data continuously. Bluetooth 5.0 has medical-optimized software []. Increased transmission power and a coded physical layer improve the data rate, interoperability, compatibility, and coverage in this standard. Bluetooth 5.1 supports high-precision, interoperable location systems.
Time-critical smart healthcare applications demand minimal data transmission latency. Thus, these systems need traffic prioritization and QoS algorithms []. Communication technology crashes may also create data loss, potentially leading to life-threatening circumstances. Thus, medical gadget safety and optimum functioning need rigorous processes. New standard adjustments IEEE 802.15.4e, IEEE 802.15.4m, IEEE 802.15.4g, and many others include efficient transmission techniques to decrease interference and multipath fading and improve reliability.
NFC, which allows two electrical gadgets to communicate by touching, is currently employed in Abbott Freestyle Libre CGM devices. Another option is ANT+, a wireless technology that promotes data interoperability and open access.
Due to connectivity incompatibility, smart diabetes monitoring system devices may not connect properly, as was examined by []. Proprietary software/protocols and company policies may also hinder the creation of an integrated system. The Nightscout Project [], a “do-it-yourself” project, permits real-time entrance to CGM data via personal websites, smartband viewers, or smartphone apps. It was created by DM1 parents and is supported by volunteers.
8. Intelligent Data Analysis in IoT Platforms for DM1 Management and Modeling Methods
The data collected, whether glycemia or other biomedical variables, once transferred by means of appropriate communication systems to a server in the cloud, should allow the extraction of knowledge that allows anticipating risk situations or refining the doses of insulin or other drugs based on the patient’s previous and current (and future) condition. To this end, the advancement of artificial intelligence and machine learning techniques allows this task to be carried out.
The research highlights many ways to use the IoT paradigm to manage and evaluate biosensor data. As noted, some suggested models apply intelligent analysis to ordinary devices such as smartphones []. However, advanced machine learning approaches cannot be implemented without cloud computing and a consideration of the IoT elements. Consequently, current proposals advise creating a holistic, ICT-driven platform to take advantage of this definitive shift, which has led to complete diabetes intensive care and IDA integration. Machine learning is necessary for glycemia modeling, dose data security, and DM1 manipulation by care providers. The flow of data is explained in Figure 3. In it, we can observe how the data obtained by the sensors are transmitted to the computing layer, where a glycemia prediction is performed using machine learning strategies. After an optimization process, insulin dosage is obtained and then recirculated as an input.
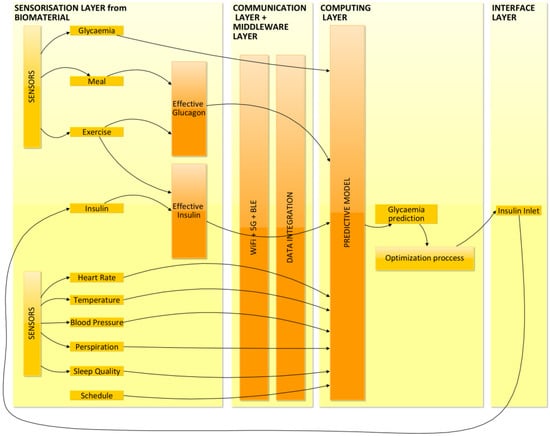
Figure 3.
Flow of data to the predictive model.
Machine learning algorithms at this moment evaluate real-time data processing to provide a healthcare platform for DM1 management utilizing BLE sensors [] and other connectivity ways previously described.
The algorithm will be specifically proposed to simulate blood sugar dynamics and must transform the huge number of new variables observed in an IoT context into knowledge. If the model is correct, insulin doses may be adjusted. This approach of obtaining a comprehensive and validated patient status with the novel devices outlined above will effect a more exact depiction of glucose level variations and improved diabetes treatment.
An Artificial Neural Network (ANN) replicates the nervous system to detect and differentiate items. ANN models may predict complicated, with several dimensions, highly nonlinear, and time-variant glucose metabolism component interactions []. This work will use scientifically published ANNs to develop the modeling core, which will forecast glycemia.
Each internal node in a Multi-Layer Perceptron (MLP) implements a logistic activation function that combines the weighted inputs from the preceding layer to create an output value. The model’s parameters—the weights of the links among nodes on succeeding tiers—are learned quickly utilizing Back Propagation (BP) of error. As shown in [], an MLP with BP can predict glucose levels with a Prediction Horizon (PH) of up to 45 min. The researchers in [,] use Neuro Solutions software and BP to estimate blood glucose at various PHs up to 180 min. The online glucose prediction from CGM data in [] uses an MLP with BP. The neural network predicts glucose using CGM sensor measurements from the past 20 min. The vector of glucose measurements reaches 45 min with an RMSE of 27 mg/dL.
The literature has examined the BP learning algorithm. Regularization prevents hypoglycemia. Bayesian Regularized Neural Networks (BRNNs) were employed in diabetes management. BRNNs [] are more resilient than BP nets and can remove cross-validation. Bayesian regularization turns a nonlinear regression into a ridge regression via an arithmetic process. Evidence processes provide Bayesian criteria for halting training, making overtraining impossible, although overfitting is problematic [].
Gaussian Processes (GP) with Radial Basis Function Kernels (RBF) [] are infrequently employed; however, several methods have shown promising results. A recent study on autonomous insulin administration to reduce hypoglycemia investigated GP [].
SVMs use dot products to process instances. A kernel function can efficiently calculate dot products between feature vectors without iterating across all the variables. SVM learners use the kernel function to create a hyperplane that divides positive and negative samples and maximizes their margin. The max-margin optimization criterion makes SVMs resistant to overfitting and excellent at generalization. The convex optimization formulation of the SVM guarantees a global optimum, whereas the MLP approach may only be a local optimum. The authors of [] use electrocardiograms to predict hypoglycemia (ECG). They predict 75% of low glucose levels using EEG data and Fuzzy SVM. Another SVM-based hypoglycemia detection method includes a smartwatch, heart-rate monitoring, galvanic skin reaction, and skin and ambient temperatures. However, the dataset size and type hamper the outcomes.
Deep learning is another approach. Although some deep learning models have recently demonstrated their ability to forecast glucose levels with an accuracy of RMSE = 9.38 ± 0.71 mg/dL over a 30 min horizon, we do not have enough information to apply this method. Nonetheless, it is a practical approach to keep in mind due to the options it presents [].
Based on the preceding, the prior methodologies would make appropriate modeling cores for glycemia dynamics estimation systems.
9. IoMT Structure for Complete DM1 Management
With all of the above, a platform is proposed that integrates the contributions that have been studied, i.e., biosensors, forms of communication, and intelligent data analysis, which are necessary to form a suitable management platform for IoMT-based T1DM.
In summary, an IoT platform for diabetes management is structured in layers with diverse technology challenges. Figure 4 presents the structure of an integrative diabetes administration platform:
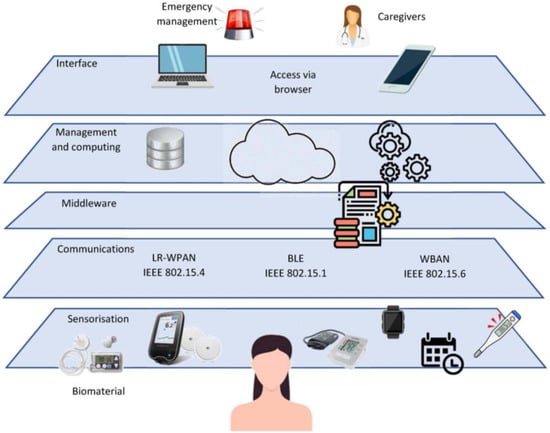
Figure 4.
IoMT structure for DM1 management.
- Biomaterial: Patient layer. This layer generates data and activates the scheme’s outputs (via medical decisions). Skin, blood, movement, and other bodily changes are measured as variables;
- Sensorization: Numerous IoT-connected sensors provide input data. Remotely configuring and controlling sensors through the Internet permits many monitoring applications and technical frameworks;
- Communications: Data permeate to the following layer. All sensors must have many communication channels to connect conveniently. Wi-fi, 5G, ZigBee (or 6LowPAN), and BLE connections must be accessible to allow direct access to the sensors, typically via a smart gateway device (smartphone or tablet). A small LAN connects all the components and a smartphone (or the cloud);
- Middleware. A middleware mediator is required to handle heterogeneous data sources and the smooth integration of gadgets and networks contained by our architecture’s sensors and communication tier. Thus, the middleware layer converts data from several sources into a common language.
The glucose levels ontology undoubtedly arises a user-centric methodology, taking into consideration all the data about the circumstances, the equipment, and the patient.
- Management and computing: This layer analyzes data, predicts glucose, and selects the best treatment. The data processing and modeling cores are located here. Local smartphone and cloud-pervasive computing are needed to avert difficulties due to Internet outages or battery malfunction. Data are transferred via LAN to a smartphone or, if possible, straight from the sensors to the cloud. Thus, ubiquitous computing provides a robust, secure solution;
- Interface layer: Browser-based system access, allowing a smartphone or computer, local or distant, to modify the settings for the patient or healthcare professionals. The preceding layer must have predicted blood glucose and optimized the insulin input, which may be provided to the patient for information or for approval or modification. Statistics, data, and glycemia control system status are available;
- Outputs. The platform must meet the standards using all acquired data and resources to achieve holistic management.
This yields the following:
- Therapeutic choices: As a result of the data treatment, artificial intelligence, and optimization procedures, the platform must be ready to propose a glycemia forecast as continuous information for the diabetic person or remote medical staff to forecast and prevent unwanted situations, safely adopt autonomous therapeutic choices to avoid hyperglycemia or maintain euglycemia in well-known circumstances and provide therapeutic advice;
- Emergency management: A complete supervision approach must be prepared for dangerous situations. When hypoglycemia develops, insulin administration should be halted, and the patient’s consciousness should be confirmed by asking and waiting. The platform should contact emergency responders with a description and GPS position if the user loses consciousness;
- Clinical data sharing for caregivers: Healthcare researchers may benefit from convenient, real-time communication of filtered and processed data, and the platform should be prepared to include experts’ comments. Remote data transmission will also allow parents of diabetic children and carers of old persons with diabetes to regulate and receive automated alerts in abnormal scenarios;
- The network of this structure. A gateway (smartphone) linked to the Internet through 5G or home Wi-Fi. Each patient’s smartphone will be unique. The data warehouse, processing center, and modeling core may be placed online. Internet access permits system administration through a web browser.
The connection of patient devices must be dependable. Smartphones capture sensor data. BLE, 6LoWPAN, NFC, and others may be used to communicate data and connect several biometric meters.
BLE is even more energy efficient than 6LoWPAN. Due to the decrease from 16 to 3 advertising channels, a more straightforward protocol simplifies scanning and requires a standby period before data transfer []. In addition, the connection is quicker and automated while scanning, so connection and transmission are accessible within 3 ms. The BLE top current is below 17.5 mA, so coin cell batteries are suitable as electric supplies, which is significant for compactness.
Bluetooth, NFC, or other technologies may be used to link skin sensors.
10. IoT-Based Diabetes Management Obstacles to Be Overcome
After reviewing the scientific literature, several aspects should be enhanced to provide a comprehensive ICT-based DM1 management solution.
First, CGM’s potential as a diabetes management phenomenon is still being realized. Until recently, this approach was underdeveloped. CGM devices still have certain issues, including accuracy, decalibration, and short usability. CGM data lag behind the patient’s blood glucose level, which is still causing some problems. As the CGM device estimates blood glucose based on interstitial liquid glucose beneath the skin, there is a delay of a few minutes, which represents a drawback.
In addition, past research lacks comprehensive patient monitoring, which is surprising. Studies have shown that additional factors may affect glycemia trends in addition to glycemia, insulin, and meals. In diabetes care, accelerometers are used to track physical activity, which is commonly believed to be a factor. A realistic diabetes management system is now starting to include the heart rate, although this component is generally underestimated and solely linked to low blood sugar levels. Other factors, such as exhaled breathing [] or polarimetry to observe ocular aqueous fluid fluctuations [], have been explored, although this research is still in the early stages. While other factors that make intuitive sense have been subject to some research, they have been explored in a supervised setting under laboratory situations of temperature, sweat, etc., and in isolation. Finally, schedule and weekday effects are well-known, but the research has not examined these.
A consistent manner of handling information is essential, and HL7 has set a precedent. ICT advances are currently underutilized. However, Wi-Fi, ZigBee, 6LowPAN, and BLE provide several communication routes that must be explored and described.
Due to their variety, expanding power, connection, and mobility, smartphones are now the most adaptable devices []. Their connection allows us to create the software model of the system dynamics, anticipate glucose, optimize the solution, regulate the procedure, and manage information between CGM and an insulin pump. Other features include GPS-forwarding emergency calls and software updates. It should be noted that the use of smartphones as medicinal equipment is disputed (class III, high risk), and to prevent conflict with other phone apps, the controller app must be dependable. Battery drain and connection loss must also be avoided.
Biometrics, which studies quantifiable biological traits, has been miniaturized and improved by advances in electronics. It is now possible to continuously measure heart rate, exercise, and other factors. This study discusses their usage in DM1 management.
Modeling approaches based on artificial intelligence can forecast glycemia, actuation, and the Kowalski route [], which defines the steps to create an AP. Unfortunately, such strategies have not been investigated. Thus, the new task domains are vast and promising. Predicting patient progression using a predictive model would be ideal as it might be able to predict risks and aid decision making. Unfortunately, machine learning models require clinical data, which should be collected at several sites to calibrate the models for various patient populations. The model must also adjust to patient health and routine changes to minimize inaccurate forecasts and unfavorable outcomes. Be that as it may, an early stage of the creation of an IoT-based solution would aid with data collecting, even permitting a test setting with patients under supervision, reducing hazards.
After examining the current telemedicine platform suggestions, a scheme that can handle new ICT breakthroughs while maximizing their potential is needed. Some systems link patients and medical professionals, but they do not optimize the solution, take into consideration a substandard feature set, or handle emergency management.
To synthesize the prior concepts, the following difficulties can be summarized for an IoT DM1 management system:
- Sensor integration. New sensors for diabetes treatment and general health information have been developed based on technological advances. As these devices have different ways of sending data, a platform that can handle them all is needed;
- Glycemia pattern prediction. Patterns help us forecast. The most intriguing skill in this work is glycemia prediction. Hypoglycemia and hyperglycemia may be predicted with reasonable accuracy. Thus, machine learning applications are expanding, and ANNs can predict glycemia and recommend insulin dosages [];
- Optimization. After the preceding phase, an insulin dose or health advice can be given. Global diabetes management can recommend insulin injections with the correct dose, but the user must provide their consent to prevent mistakes. The platform must also recognize suboptimal scenarios such as inactive lifestyles and a lack of rest. Cloud computing can handle computerized model complexity and optimization without limits [];
- Big data management. CGM sends data every 1–5 min. A heart-rate monitor and other sensors should record data in minutes. This generates massive data sets at short intervals. Thus, big data analysis is advisable [,]. This pertains to data sets that are so huge or complicated that typical data-processing applications fail. This area is facing issues in terms of evaluation, capture, search, sharing, storing, transmission, visualization, and data privacy. Big data generally means using predictive analytics or other sophisticated approaches to extract value from data, and healthcare uses this resource to generate meaningful knowledge of data [];
- Emergency. There are cases in which the IoT platform must take over, such as strong hypoglycemia, that may cause coma or death. In the instance of a diabetic using an infusion pump, stopping the pump and alerting emergency services and medical professionals may reduce the effects of hypoglycemia. The devices will also record insulin doses, meals, and physical activity over the previous hours;
- Accessible patient–caregiver–medical worker communication. In an information-rich environment, relevant knowledge must be shared with all diabetes management stakeholders;
- To examine data and procedures and modify settings, the patient’s smartphone, the Internet, or another smartphone (helpful for examining infants or elderly/impaired patients) must be accessible and comfortable for users [];
- User-friendly. User-centeredness is essential in health care. To benefit from this effort, patients need a cross-platform interface. When providing information to persons with special needs, such as the elderly, disabled, or children, the user experience must be pleasant, which can be achieved through customization;
- Privacy, security, integrity. This work uses ICT frameworks and processes personal data []. Thus, control methods are needed to handle sensitive information properly [,].
11. Conclusions
To create an ICT-based DM1 management system, numerous factors should be improved after analyzing the scientific literature. Studies have demonstrated that factors other than glycemia, insulin, and meals can alter glycemia patterns. Accelerometers track physical activity, which is thought to affect diabetes. Others, such as heart rate, exhaled breathing, temperature, sweat, and polarimetry, are also being investigated. Schedule and weekday effects are also well-known. Electronics have miniaturized and improved biometrics. Continuous heart rate, exercise, and other measurements are possible. This study covers DM1 management.
In addition, ICT advances such as Wi-Fi, ZigBee, 6LowPAN, and BLE offer multiple communication paths, and joining machine learning algorithms lets us develop software models of system dynamics, predict glucose, optimize the solution, and manage CGM-insulin pump information.
Machine learning algorithms need continuous biological data to calibrate the models and forecast glycemia for different patients. To avoid erroneous forecasts and undesirable outcomes, the model must adapt to patient health and routine. Fortunately, early development of an IoT-based solution would help collect data and allow supervised patient testing, decreasing risks.
In conclusion, the primary contribution that this study makes is a compilation of the components that are required to carry out effective management of T1DM within an IoMT context. It takes into account the options that have been provided in this sense by other methods of health care and re-examines the particular biomonitoring devices that are helpful for T1DM, assessing the influence of the biological variables that are assessed. After this, a comprehensive solution is found by taking into account the various modes of data transmission and the various forms of AI technology, and then future problems that need to be handled are presented.
Our perspective on the world continues to evolve toward new frontiers as a result of technological advancement. In many ways, life has become simpler due to the many innovations that might be deemed revolutionary. As two prominent examples, CGM systems and insulin pumps have enabled significant progress in the quest for a comprehensive and definitive method of diabetes regulation. However, the entire potential of this new technology has not yet been realized, and the research should generate new approaches in the future. This study evaluated glycemia modeling architectures in order to determine the function that IoT may play in the management of DM1.
Author Contributions
Conceptualization, I.R.-R. and J.-V.R.; methodology, I.R.-R.; software, M.C.-V.; validation, I.R.-R., J.-V.R. and M.C.-V.; formal analysis, I.R.-R. and J.-V.R.; investigation, I.R.-R. and M.C.-V.; resources, J.-V.R. and I.R.-R.; data curation, I.R.-R. and M.C.-V.; writing—original draft preparation, J.-V.R. and M.C.-V.; visualization, I.R.-R. and M.C.-V.; supervision, I.R.-R. and J.-V.R.; funding acquisition, I.R.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Ignacio Rodríguez-Rodríguez would like to thank Plan Andaluz de Investigación, Desarrollo e Innovación (PAIDI), Junta de Andalucía, Spain. María Campo-Valera is grateful for postdoctoral program Margarita Salas—Spanish Ministry of Universities (financed by European Union—NextGenerationEU).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haller, M.J.; Atkinson, M.A.; Schatz, D. Type 1 diabetes mellitus: Etiology, presentation, and management. Pediatr. Clin. 2005, 52, 1553–1578. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef]
- Albisser, A.M.; Leibel, B.S.; Ewart, T.G.; Davidovac, Z.; Botz, C.K.; Zingg, W. An artificial endocrine pancreas. Diabetes 1974, 23, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Peyser, T.; Dassau, E.; Breton, M.; Skyler, J.S. The artificial pancreas: Current status and future prospects in the management of diabetes. Ann. N. Y. Acad. Sci. 2014, 1311, 102–123. [Google Scholar] [CrossRef]
- Cobelli, C.; Renard, E.; Kovatchev, B. Artificial pancreas: Past, present, future. Diabetes 2011, 60, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, I.; Rodríguez, J.-V.; Zamora-Izquierdo, M.-Á. Variables to be monitored via biomedical sensors for complete type 1 diabetes mellitus management: An extension of the “on-board” concept. J. Diabetes Res. 2018, 2018, 4826984. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, I.; Zamora-Izquierdo, M.-Á.; Rodríguez, J.-V. Towards an ICT-based platform for type 1 diabetes mellitus management. Appl. Sci. 2018, 8, 511. [Google Scholar] [CrossRef]
- Karagiannis, D.; Mitsis, K.; Nikita, K.S. Development of a Low-Power IoMT Portable Pillbox for Medication Adherence Improvement and Remote Treatment Adjustment. Sensors 2022, 22, 5818. [Google Scholar] [CrossRef]
- Kaushal, C.; Islam, M.K.; Singla, A.; Al Amin, M. An IoMT-Based Smart Remote Monitoring System for Healthcare. In IoT-Enabled Smart Healthcare Systems, Services and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 177–198. [Google Scholar]
- Movassaghi, S.; Abolhasan, M.; Lipman, J.; Smith, D.; Jamalipour, A. Wireless body area networks: A survey. IEEE Commun. Surv. Tutor. 2014, 16, 1658–1686. [Google Scholar] [CrossRef]
- Talpur, M.S.H.; Bhuiyan, M.Z.A.; Wang, G. Energy-efficient healthcare monitoring with smartphones and IoT technologies. Int. J. High Perform. Comput. Netw. 2015, 8, 186–194. [Google Scholar] [CrossRef]
- Ali, S.; Kibria, M.G.; Jarwar, M.A.; Kumar, S.; Chong, I. Microservices model in WoO based IoT platform for depressive disorder assistance. In Proceedings of the 2017 International Conference on Information and Communication Technology Convergence (ICTC), Jeju, Republic of Korea, 18–20 October 2017. [Google Scholar]
- Romero, L.E.; Chatterjee, P.; Armentano, R.L. An IoT approach for integration of computational intelligence and wearable sensors for Parkinson’s disease diagnosis and monitoring. Health Technol. 2016, 6, 167–172. [Google Scholar] [CrossRef]
- Bajaj, A.; Bhatnagar, M.; Chauhan, A. Recent trends in internet of medical things: A review. In Advances in Machine Learning and Computational Intelligence; Springer: Berlin/Heidelberg, Germany, 2021; pp. 645–656. [Google Scholar]
- Hemalatha, M.P.; Vidhyalakshmi, M.R. A study on chronic cough detection using IoT and machine learning. Int. J. Res. Arts Sci. 2019, 5, 151–160. [Google Scholar]
- Matar, G.; Lina, J.-M.; Carrier, J.; Riley, A.; Kaddoum, G. Internet of Things in sleep monitoring: An application for posture recognition using supervised learning. In Proceedings of the 2016 IEEE 18th International Conference on e-Health Networking, Applications and Services (Healthcom), Munich, Germany, 14–16 September 2016. [Google Scholar]
- Magsi, H.; Sodhro, A.H.; Chachar, F.A.; Abro, S.A.K.; Sodhro, G.H.; Pirbhulal, S. Evolution of 5G in Internet of medical things. In Proceedings of the 2018 International Conference on Computing, Mathematics and Engineering Technologies (iCoMET), Sukkur, Pakistan, 3–4 March 2018. [Google Scholar]
- Fan, K.; Luo, Q.; Li, H.; Yang, Y. Cloud-based lightweight RFID mutual authentication protocol. In Proceedings of the 2017 IEEE Second International Conference on Data Science in Cyberspace (DSC), Shenzhen, China, 26–29 June 2017. [Google Scholar]
- Onasanya, A.; Elshakankiri, M. IoT implementation for cancer care and business analytics/cloud services in healthcare systems. In Proceedings of the10th International Conference on Utility and Cloud Computing, Austin, TX, USA, 5–8 December 2017. [Google Scholar]
- Gardašević, G.; Fotouhi, H.; Tomasic, I.; Vahabi, M.; Björkman, M.; Lindén, M. A heterogeneous IoT-based architecture for remote monitoring of physiological and environmental parameters. In Proceedings of the International Conference on IoT Technologies for HealthCare, Angers, France, 24–25 October 2017. [Google Scholar]
- Broens, T.; Van Halteren, A.; Van Sinderen, M.; Wac, K. Towards an application framework for context-aware m-health applications. Int. J. Internet Protoc. Technol. 2007, 2, 109–116. [Google Scholar] [CrossRef]
- Yuce, M.R. Implementation of wireless body area networks for healthcare systems. Sens. Actuators A: Phys. 2010, 162, 116–129. [Google Scholar] [CrossRef]
- Preuveneers, D.; Berbers, Y. Mobile phones assisting with health self-care: A diabetes case study. In Proceedings of the 10th International Conference on Human Computer Interaction with Mobile Devices and Services, Amsterdam, The Netherlands, 2–5 September 2008. [Google Scholar]
- Sieverdes, J.C.; Treiber, F.; Jenkins, C.; Hermayer, K. Improving diabetes management with mobile health technology. Am. J. Med. Sci. 2013, 345, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Cappon, G.; Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Wearable continuous glucose monitoring sensors: A revolution in diabetes treatment. Electronics 2017, 6, 65. [Google Scholar] [CrossRef]
- Leelarathna, L.; Wilmot, E.G. Flash forward: A review of flash glucose monitoring. Diabet. Med. 2018, 35, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Nachman, L.; Baxi, A.; Bhattacharya, S.; Darera, V.; Deshpande, P.; Kodalapura, N.; Mageshkumar, V.; Rath, S.; Shahabdeen, J.; Acharya, R. Jog falls: A pervasive healthcare platform for diabetes management. In Proceedings of the International Conference on Pervasive Computing, Helsinki, Finland, 17–20 May 2010. [Google Scholar]
- Al Kukhun, D.; Soukkarieh, B.; Sèdes, F. ADMAN: An alarm-based mobile diabetes management system for mobile geriatric teams. In Proceedings of the East European Conference on Advances in Databases and Information Systems, Poitiers, France, 8–11 September 2015. [Google Scholar]
- Al-Taee, M.A.; Al-Nuaimy, W.; Al-Ataby, A.; Muhsin, Z.J.; Abood, S.N. Mobile health platform for diabetes management based on the Internet-of-Things. In Proceedings of the 2015 IEEE Jordan conference on applied electrical engineering and computing technologies (AEECT), Amman, Jordan, 3–5 November 2015. [Google Scholar]
- Hsu, W.C.; Lau, K.H.K.; Huang, R.; Ghiloni, S.; Le, H.; Gilroy, S.; Abrahamson, M.; Moore, J. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol. Ther. 2016, 18, 59–67. [Google Scholar] [CrossRef]
- Kafalı, Ö.; Bromuri, S.; Sindlar, M.; van der Weide, T.; Pelaez, E.A.; Schaechtle, U.; Alves, B.; Zufferey, D.; Rodriguez-Villegas, E.; Schumacher, M.I.; et al. Commodity 12: A smart e-health environment for diabetes management. J. Ambient. Intell. Smart Environ. 2013, 5, 479–502. [Google Scholar] [CrossRef]
- Vivekanandan, S.; Devanand, M. Remote monitoring for diabetes disorder: Pilot study using InDiaTel prototype. Eur. Res. Telemed./La Rech. Eur. En Télémédecine 2015, 4, 63–69. [Google Scholar] [CrossRef]
- Lanzola, G.; Losiouk, E.; Del Favero, S.; Facchinetti, A.; Galderisi, A.; Quaglini, S.; Magni, L.; Cobelli, C. Remote blood glucose monitoring in mHealth scenarios: A review. Sensors 2016, 16, 1983. [Google Scholar] [CrossRef]
- Kovatchev, B.P.; Renard, E.; Cobelli, C.; Zisser, H.C.; Keith-Hynes, P.; Anderson, S.M.; Brown, S.A.; Chernavvsky, D.R.; Breton, M.D.; Farret, A.; et al. Feasibility of outpatient fully integrated closed-loop control: First studies of wearable artificial pancreas. Diabetes Care 2013, 36, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Wientjes, K.J.C.; Schoonen, A.J.M. Determination of time delay between blood and interstitial adipose tissue glucose concentration change by microdialysis in healthy volunteers. Int. J. Artif. Organs 2001, 24, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Dube, S.; Veettil, S.; Slama, M.; Kudva, Y.C.; Peyser, T.; Carter, R.E.; Cobelli, C.; Basu, R. Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J. Diabetes Sci. Technol. 2014, 9, 63–68. [Google Scholar] [CrossRef]
- Cox, D.J.; Clarke, W.L.; Gonder-Frederick, L.; Pohl, S.; Hoover, C.; Snyder, A.; Zimbelman, L.; Carter, W.R.; Bobbitt, S.; Pennebaker, J. Accuracy of perceiving blood glucose in IDDM. Diabetes Care 1985, 8, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, A. Continuous glucose monitoring sensors: Past, present and future algorithmic challenges. Sensors 2016, 16, 2093. [Google Scholar] [CrossRef]
- Facchinetti, A.; Sparacino, G.; Cobelli, C. Online denoising method to handle intraindividual variability of signal-to-noise ratio in continuous glucose monitoring. IEEE Trans. Biomed. Eng. 2011, 58, 2664–2671. [Google Scholar] [CrossRef]
- Palerm, C.C.; Bequette, B.W. Hypoglycemia Detection and Prediction Using Continuous Glucose Monitoring—A Study on Hypoglycemic Clamp Data; SAGE Publications: Los Angeles, CA, USA, 2007. [Google Scholar]
- Mahmoudi, Z.; Johansen, M.D.; Christiansen, J.S.; Hejlesen, O.K. A multistep algorithm for processing and calibration of microdialysis continuous glucose monitoring data. Diabetes Technol. Ther. 2013, 15, 825–835. [Google Scholar] [CrossRef]
- Guerra, S.; Facchinetti, A.; Sparacino, G.; De Nicolao, G.; Cobelli, C. Enhancing the accuracy of subcutaneous glucose sensors: A real-time deconvolution-based approach. IEEE Trans. Biomed. Eng. 2012, 59, 1658–1669. [Google Scholar] [CrossRef]
- Barcelo-Rico, F.; Diez, J.-L.; Rossetti, P.; Vehi, J.; Bondia, J. Adaptive calibration algorithm for plasma glucose estimation in continuous glucose monitoring. IEEE J. Biomed. Health Inform. 2013, 17, 530–538. [Google Scholar] [CrossRef]
- Kirchsteiger, H.; Zaccarian, L.; Renard, E.; del Re, L. LMI-based approaches for the calibration of continuous glucose measurement sensors. IEEE J. Biomed. Health Inform. 2014, 19, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Sparacino, G.; Zanderigo, F.; Corazza, S.; Maran, A.; Facchinetti, A.; Cobelli, C. Glucose concentration can be predicted ahead in time from continuous glucose monitoring sensor time-series. IEEE Trans. Biomed. Eng. 2007, 54, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Zecchin, C.; Facchinetti, A.; Sparacino, G.; Cobelli, C. Jump neural network for online short-time prediction of blood glucose from continuous monitoring sensors and meal information. Comput. Methods Programs Biomed. 2014, 113, 144–152. [Google Scholar] [CrossRef]
- Zarkogianni, K.; Mitsis, K.; Litsa, E.; Arredondo, M.-T.; Fico, G.; Fioravanti, A.; Nikita, K.S. Comparative assessment of glucose prediction models for patients with type 1 diabetes mellitus applying sensors for glucose and physical activity monitoring. Med. Biol. Eng. Comput. 2015, 53, 1333–1343. [Google Scholar] [CrossRef]
- Georga, E.I.; Protopappas, V.C.; Polyzos, D.; Fotiadis, D.I. Evaluation of short-term predictors of glucose concentration in type 1 diabetes combining feature ranking with regression models. Med. Biol. Eng. Comput. 2015, 53, 1305–1318. [Google Scholar] [CrossRef]
- Place, J.; Robert, A.; Brahim, N.B.; Keith-Hynes, P.; Farret, A.; Pelletier, M.-J.; Buckingham, B.; Breton, M.; Kovatchev, B.; Renard, E. DiAs Web Monitoring: A Real-Time Remote Monitoring System Designed for Artificial Pancreas Outpatient Trials; SAGE Publications Sage CA: Los Angeles, CA, USA, 2013. [Google Scholar]
- Dassau, E.; Jovanovic, L.; Doyle, F.J., III; Zisser, H.C. Enhanced 911/global position system wizard: A telemedicine application for the prevention of severe hypoglycemia—Monitor, alert, and locate. J. Diabetes Sci. Technol. 2009, 3, 1501–1506. [Google Scholar] [CrossRef]
- Rigla, M. Smart telemedicine support for continuous glucose monitoring: The embryo of a future global agent for diabetes care. J. Diabetes Sci. Technol. 2011, 5, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Schumacher, M. Sensor monitoring of physical activity to improve glucose management in diabetic patients: A review. Sensors 2016, 16, 589. [Google Scholar] [CrossRef] [PubMed]
- Case, M.A.; Burwick, H.A.; Volpp, K.G.; Patel, M.S. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA 2015, 313, 625–626. [Google Scholar] [CrossRef]
- Marling, C.; Xia, L.; Bunescu, R.; Schwartz, F. Machine learning experiments with noninvasive sensors for hypoglycemia detection. In Proceedings of the IJCAI Workshop on Knowledge Discovery in Healthcare Data, New York, NY, USA, 10 July 2016. [Google Scholar]
- Kirchsteiger, H.; Johansson, R.; Renard, E.; Re, L. Continuous-time interval model identification of blood glucose dynamics for type 1 diabetes. Int. J. Control 2014, 87, 1454–1466. [Google Scholar] [CrossRef]
- Bondia, J.; Vehi, J. Physiology-based interval models: A framework for glucose prediction under intra-patient variability. In Prediction Methods for Blood Glucose Concentration; Springer: Berlin/Heidelberg, Germany, 2016; pp. 159–181. [Google Scholar]
- Estrada, G.C.; del Re, L.; Renard, E. Nonlinear gain in online prediction of blood glucose profile in type 1 diabetic patients. In Proceedings of the 49th IEEE Conference on Decision and Control (CDC), Atlanta, GA, USA, 15–17 December 2010. [Google Scholar]
- Garg, S.K.; Weinzimer, S.A.; Tamborlane, W.V.; Buckingham, B.A.; Bode, B.W.; Bailey, T.S.; Brazg, R.L.; Ilany, J.; Slover, R.H.; Anderson, S.M.; et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol. Ther. 2017, 19, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, D. Continuous glucose monitoring: A review of successes, challenges, and opportunities. Diabetes Technol. Ther. 2016, 18, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Nordisk, N. Novo Nordisk Receives Complete Response Letter in the US for Tresiba® and Ryzodeg®; Press Release. 2013. Available online: https://www.fiercebiotech.com/biotech/novo-nordisk-receives-complete-response-letter-us-for-tresiba%C2%AE-and-ryzodeg%C2%AE (accessed on 30 December 2022).
- Patek, S.D.; Magni, L.; Dassau, E.; Karvetski, C.; Toffanin, C.; De Nicolao, G.; Del Favero, S.; Breton, M.; Man, C.D.; Renard, E.; et al. Modular closed-loop control of diabetes. IEEE Trans. Biomed. Eng. 2012, 59, 2986–2999. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, C.; Dassau, E.; Zisser, H.; Grosman, B.; Percival, M.W.; Jovanovič, L.; Doyle, F.J., III. Safety constraints in an artificial pancreatic β cell: An implementation of model predictive control with insulin on board. J. Diabetes Sci. Technol. 2009, 3, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Borghouts, L.B.; Keizer, H.A. Exercise and insulin sensitivity: A review. Int. J. Sport. Med. 2000, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Devlin, J.T.; Hirshman, M.; Horton, E.D.; Horton, E.S. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes 1987, 36, 434–439. [Google Scholar] [CrossRef]
- King, D.S.; Baldus, P.J.; Sharp, R.L.; Kesl, L.D.; Feltmeyer, T.L.; Riddle, M.S. Time course for exercise-induced alterations in insulin action and glucose tolerance in middle-aged people. J. Appl. Physiol. 1995, 78, 17–22. [Google Scholar] [CrossRef]
- Zecchin, C.; Facchinetti, A.; Sparacino, G.; Man, C.D.; Manohar, C.; Levine, J.A.; Basu, A.; Kudva, Y.C.; Cobelli, C. Physical activity measured by physical activity monitoring system correlates with glucose trends reconstructed from continuous glucose monitoring. Diabetes Technol. Ther. 2013, 15, 836–844. [Google Scholar] [CrossRef]
- Man, C.D.; Rizza, R.A.; Cobelli, C. Meal simulation model of the glucose-insulin system. IEEE Trans. Biomed. Eng. 2007, 54, 1740–1749. [Google Scholar]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: Implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef]
- Hung, T.; Sievenpiper, J.L.; Marchie, A.; Kendall, C.W.C.; Jenkins, D.J.A. Fat versus carbohydrate in insulin resistance, obesity, diabetes and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C. The influence of dietary fat on insulin resistance. Curr. Diabetes Rep. 2002, 2, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Kiehm, T.G.; Anderson, J.W.; Ward, K. Beneficial effects of a high carbohydrate, high fiber diet on hyperglycemic diabetic men. Am. J. Clin. Nutr. 1976, 29, 895–899. [Google Scholar] [CrossRef]
- Housiaux, M.; Dorchy, H.; Luminet, O. Influence of an emotional level of conscience on the glycemic equilibrium in type 1 diabetic children and adolescents. Diabetes Metab. 2008, 34, A33. [Google Scholar]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Taelman, J.; Vandeput, S.; Spaepen, A.; Huffel, S.V. Influence of mental stress on heart rate and heart rate variability. In Proceedings of the 4th European Conference of the International Federation for Medical and Biological Engineering, Antwerp, Belgium, 23–27 November 2009. [Google Scholar]
- Alexakis, C.; Nyongesa, H.O.; Saatchi, R.; Harris, N.D.; Davies, C.; Emery, C.; Ireland, R.H.; Heller, S.R. Feature extraction and classification of electrocardiogram (ECG) signals related to hypoglycaemia. In Proceedings of the Computers in Cardiology, Thessaloniki, Greece, 21–24 September 2003. [Google Scholar]
- Buñag, R.D.; Tomita, T.A.T.S.U.O.; Sasaki, S.U.S.U.M.U. Chronic sucrose ingestion induces mild hypertension and tachycardia in rats. Hypertension 1983, 5, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.; Ellegaard, S.; Wex, S. Detection of nocturnal hypoglycemia in insulin-treated diabetics by a skin temperature-skin conductance meter. Acta Med. Scand. 1986, 220, 213–217. [Google Scholar] [CrossRef]
- Feldman, J.; Gellhorn, E. The influence of fever on the vago-insulin and sympathetico-adrenal systems. Endocrinology 1941, 29, 141–143. [Google Scholar] [CrossRef]
- Melhuish, T. Linking hypothermia and hyperglycemia. Nurs. Manag. 2009, 40, 42–45. [Google Scholar] [CrossRef]
- McAulay, V.; Deary, I.J.; Frier, B.M. Symptoms of hypoglycaemia in people with diabetes. Diabet. Med. 2001, 18, 690–705. [Google Scholar] [CrossRef]
- Lurbe, A.; Redon, J.; Pascual, J.M.; Tacons, J.; Alvarez, V.; Batlle, D.C. Altered blood pressure during sleep in normotensive subjects with type I diabetes. Hypertension 1993, 21, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Guerci, B.; Meyer, L.; Delbachian, I.; Kolopp, M.; Ziegler, O.; Drouin, P. Blood glucose control on Sunday in IDDM patients: Intensified conventional insulin therapy versus continuous subcutaneous insulin infusion. Diabetes Res. Clin. Pract. 1998, 40, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Kekäläinen, P.; Juuti, M.; Walle, T.; Laatikainen, T. Pregnancy planning in type 1 diabetic women improves glycemic control and pregnancy outcomes. J. Matern.-Fetal Neonatal Med. 2016, 29, 2252–2258. [Google Scholar] [CrossRef]
- Stuenkel, C.A.; Davis, S.R.; Gompel, A.; Lumsden, M.A.; Murad, M.H.; Pinkerton, J.V.; Santen, R.J. Treatment of symptoms of the menopause: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 3975–4011. [Google Scholar] [CrossRef] [PubMed]
- Siddapur, P.R.; Patil, A.B.; Borde, V.S. Comparison of bone mineral density, T-scores and serum zinc between diabetic and non diabetic postmenopausal women with osteoporosis. J. Lab. Physicians 2015, 7, 043–048. [Google Scholar] [CrossRef]
- Larsen, J.R.; Siersma, V.D.; Davidsen, A.S.; Waldorff, F.B.; Reventlow, S.; de Fine Olivarius, N. The excess mortality of patients with diabetes and concurrent psychiatric illness is markedly reduced by structured personal diabetes care: A 19-year follow up of the randomized controlled study Diabetes Care in General Practice (DCGP). Gen. Hosp. Psychiatry 2016, 38, 42–52. [Google Scholar] [CrossRef]
- Tong, L.; Adler, S. Glycemic control of type 2 diabetes mellitus across stages of renal impairment: Information for primary care providers. Postgrad. Med. 2018, 130, 381–393. [Google Scholar] [CrossRef]
- Chew, E.Y. There Is Level 1 Evidence for Intensive Glycemic Control for Reducing the Progression of Diabetic Retinopathy in Persons with Type 2 Diabetes; Springer: Berlin/Heidelberg, Germany, 2015; Volume 49, pp. 1–3. [Google Scholar]
- Dolin, R.H.; Alschuler, L.; Boyer, S.; Beebe, C.; Behlen, F.M.; Biron, P.V.; Shabo, A. HL7 clinical document architecture, release 2. J. Am. Med. Inform. Assoc. 2006, 13, 30–39. [Google Scholar] [CrossRef]
- Mamykina, L.; Mynatt, E.D.; Kaufman, D.R. Investigating health management practices of individuals with diabetes. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Montréal, QC, Canada, 22–27 April 2006. [Google Scholar]
- Lim, H.B.; Teo, Y.M.; Mukherjee, P.; Lam, V.T.; Wong, W.F.; See, S. Sensor grid: Integration of wireless sensor networks and the grid. In Proceedings of the IEEE Conference on Local Computer Networks 30th Anniversary (LCN’05) l, Sydney, NSW, Austrilia, 17 November 2005. [Google Scholar]
- Choi, J.M.; Choi, B.H.; Seo, J.W.; Sohn, R.H.; Ryu, M.S.; Yi, W.; Park, K.S. A system for ubiquitous health monitoring in the bedroom via a Bluetooth network and wireless LAN. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004. [Google Scholar]
- Wu, T.; Wu, F.; Redoute, J.-M.; Yuce, M.R. An autonomous wireless body area network implementation towards IoT connected healthcare applications. IEEE Access 2017, 5, 11413–11422. [Google Scholar] [CrossRef]
- IE EE Std 802.15. 6; IEEE standard for local and metropolitan area networks part 15.6: Wireless body area networks. IEEE: New York, NY, USA, 2012.
- Liu, X.; Yin, J.; Liu, Y.; Zhang, S.; Guo, S.; Wang, K. Vital signs monitoring with RFID: Opportunities and challenges. IEEE Netw. 2019, 33, 126–132. [Google Scholar] [CrossRef]
- Elsts, A.; Fafoutis, X.; Woznowski, P.; Tonkin, E.; Oikonomou, G.; Piechocki, R.; Craddock, I. Enabling healthcare in smart homes: The SPHERE IoT network infrastructure. IEEE Commun. Mag. 2018, 56, 164–170. [Google Scholar] [CrossRef]
- Raza, U.; Kulkarni, P.; Sooriyabandara, M. Low power wide area networks: An overview. IEEE Commun. Surv. Tutor. 2017, 19, 855–873. [Google Scholar] [CrossRef]
- Hadi, M.S.; Lawey, A.Q.; El-Gorashi, T.E.H.; Elmirghani, J.M.H. Patient-centric cellular networks optimization using big data analytics. IEEE Access 2019, 7, 49279–49296. [Google Scholar] [CrossRef]
- Omre, A.H.; Keeping, S. Bluetooth low energy: Wireless connectivity for medical monitoring. J. Diabetes Sci. Technol. 2010, 4, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Carroll, R.; Cnossen, R.; Schnell, M.; Simons, D. Continua: An interoperable personal healthcare ecosystem. IEEE Pervasive Comput. 2007, 6, 90–94. [Google Scholar] [CrossRef]
- Sathyaseelan, M.P.; Chakravarthi, M.K.; Sathyaseelan, A.P.; Sudipta, S. IoT based covid de-escalation system using bluetooth low level energy. In Proceedings of the 2021 6th International Conference on Inventive Computation Technologies (ICICT), Coimbatore, India, 20–22 January 2021. [Google Scholar]
- Cai, G.; Fang, Y.; Wen, J.; Han, G.; Yang, X. QoS-aware buffer-aided relaying implant WBAN for healthcare IoT: Opportunities and challenges. IEEE Netw. 2019, 33, 96–103. [Google Scholar] [CrossRef]
- Doyle-Delgado, K.; Chamberlain, J.J. Use of diabetes-related applications and digital health tools by people with diabetes and their health care providers. Clin. Diabetes 2020, 38, 449–461. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, I.; Rodríguez, J.-V.; Chatzigiannakis, I.; Zamora Izquierdo, M.A. On the possibility of predicting glycaemia ‘on the fly’with constrained IoT devices in type 1 diabetes mellitus patients. Sensors 2019, 19, 4538. [Google Scholar] [CrossRef]
- Alfian, G.; Syafrudin, M.; Ijaz, M.F.; Syaekhoni, M.A.; Fitriyani, N.L.; Rhee, J. A personalized healthcare monitoring system for diabetic patients by utilizing BLE-based sensors and real-time data processing. Sensors 2018, 18, 2183. [Google Scholar] [CrossRef]
- Cappon, G.; Vettoretti, M.; Marturano, F.; Facchinetti, A.; Sparacino, G. A neural-network-based approach to personalize insulin bolus calculation using continuous glucose monitoring. J. Diabetes Sci. Technol. 2018, 12, 265–272. [Google Scholar] [CrossRef]
- Pappada, S.M.; Cameron, B.D.; Rosman, P.M. Development of a neural network for prediction of glucose concentration in type 1 diabetes patients. J. Diabetes Sci. Technol. 2008, 2, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Pappada, S.M.; Cameron, B.D.; Rosman, P.M.; Bourey, R.E.; Papadimos, T.J.; Olorunto, W.; Borst, M.J. Neural network-based real-time prediction of glucose in patients with insulin-dependent diabetes. Diabetes Technol. Ther. 2011, 13, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gandı, C.; Facchinetti, A.; Sparacino, G.; Cobelli, C.; Gómez, E.J.; Rigla, M.; de Leiva, A.; Hernando, M.E. Artificial neural network algorithm for online glucose prediction from continuous glucose monitoring. Diabetes Technol. Ther. 2010, 12, 81–88. [Google Scholar] [CrossRef]
- Burden, F.; Winkler, D. Bayesian regularization of neural networks. In Artificial Neural Networks; Springer: Berlin/Heidelberg, Germany, 2008; pp. 23–42. [Google Scholar]
- Nguyen, H.T.; Ghevondian, N.; Jones, T.W. Detection of nocturnal hypoglycemic episodes (natural occurrence) in children with type 1 diabetes using an optimal Bayesian neural network algorithm. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008. [Google Scholar]
- Williams, C.K.I.; Barber, D. Bayesian classification with Gaussian processes. IEEE Trans. Pattern Anal. Mach. Intell. 1998, 20, 1342–1351. [Google Scholar] [CrossRef]
- Ortmann, L.; Shi, D.; Dassau, E.; Doyle, F.J.; Misgeld, B.J.E.; Leonhardt, S. Automated insulin delivery for type 1 diabetes mellitus patients using Gaussian process-based model predictive control. In Proceedings of the 2019 American Control Conference (ACC), Philadelphia, PA, USA, 10–12 July 2019. [Google Scholar]
- Nuryani, N.; Ling, S.S.H.; Nguyen, H.T. Electrocardiographic signals and swarm-based support vector machine for hypoglycemia detection. Ann. Biomed. Eng. 2012, 40, 934–945. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar]
- Darroudi, S.M.; Gomez, C.; Crowcroft, J. Bluetooth low energy mesh networks: A standards perspective. IEEE Commun. Mag. 2020, 58, 95–101. [Google Scholar] [CrossRef]
- Minh, T.D.C.; Oliver, S.R.; Ngo, J.; Flores, R.; Midyett, J.; Meinardi, S.; Carlson, M.K.; Rowland, F.S.; Blake, D.R.; Galassetti, P.R. Noninvasive measurement of plasma glucose from exhaled breath in healthy and type 1 diabetic subjects. Am. J. Physiol.-Endocrinol. Metab. 2011, 300, E1166–E1175. [Google Scholar] [CrossRef]
- Baba, J.S.; Cameron, B.D.; Cote, G.L. Effect of temperature, pH, and corneal birefringence on polarimetric glucose monitoring in the eye. J. Biomed. Opt. 2002, 7, 321–328. [Google Scholar] [CrossRef]
- Kowalski, A.J. Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: A roadmap to better diabetes outcomes. Diabetes Technol. Ther. 2009, 11, S-113–S-119. [Google Scholar] [CrossRef]
- Angrisani, L.; Annuzzi, G.; Arpaia, P.; Bozzetto, L.; Cataldo, A.; Corrado, A.; De Benedetto, E.; Di Capua, V.; Prevete, R.; Vallefuoco, E. Neural Network-Based Prediction and Monitoring of Blood Glucose Response to Nutritional Factors in Type-1 Diabetes. In Proceedings of the 2022 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Ottawa, ON, Canada, 16–19 May 2022. [Google Scholar]
- Dow, D.E.; Urrea, M.; Qin, I.; Pham, T. Cloud Recording for Diabetes Regulation of Blood Glucose Concentrations. In Proceedings of the 2018 Joint 10th International Conference on Soft Computing and Intelligent Systems (SCIS) and 19th International Symposium on Advanced Intelligent Systems (ISIS), Toyama, Japan, 5–8 December 2018. [Google Scholar]
- Assunção, M.D.; Calheiros, R.N.; Bianchi, S.; Netto, M.A.S.; Buyya, R. Big Data computing and clouds: Trends and future directions. J. Parallel Distrib. Comput. 2015, 79, 3–15. [Google Scholar] [CrossRef]
- Hashem, I.A.T.; Yaqoob, I.; Anuar, N.B.; Mokhtar, S.; Gani, A.; Khan, S.U. The rise of “big data” on cloud computing: Review and open research issues. Inf. Syst. 2015, 47, 98–115. [Google Scholar] [CrossRef]
- Marr, B. How big data is changing healthcare. Forbes/Tech 2015. Available online: https://www.forbes.com/sites/bernardmarr/2015/04/21/how-big-data-is-changing-healthcare/?sh=54ce3a052873 (accessed on 30 December 2022).
- Rho, M.J.; Kim, H.S.; Chung, K.; Choi, I.Y. Factors influencing the acceptance of telemedicine for diabetes management. Clust. Comput. 2015, 18, 321–331. [Google Scholar] [CrossRef]
- Azbeg, K.; Ouchetto, O.; Andaloussi, S.J.; Fetjah, L.; Sekkaki, A. Blockchain and IoT for security and privacy: A platform for diabetes self-management. In Proceedings of the 2018 4th International Conference on Cloud Computing Technologies and Applications (Cloudtech), Brussels, Belgium, 26–28 November 2018. [Google Scholar]
- Moosavi, S.R.; Gia, T.N.; Nigussie, E.; Rahmani, A.M.; Virtanen, S.; Tenhunen, H.; Isoaho, J. End-to-end security scheme for mobility enabled healthcare Internet of Things. Future Gener. Comput. Syst. 2016, 64, 108–124. [Google Scholar] [CrossRef]
- Vayena, E.; Dzenowagis, J.; Brownstein, J.S.; Sheikh, A. Policy implications of big data in the health sector. Bull. World Health Organ. 2018, 96, 66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).