A 1.8 V Low-Power Low-Noise High Tunable Gain TIA for CMOS Integrated Optoelectronic Biomedical Applications
Abstract
:1. Introduction
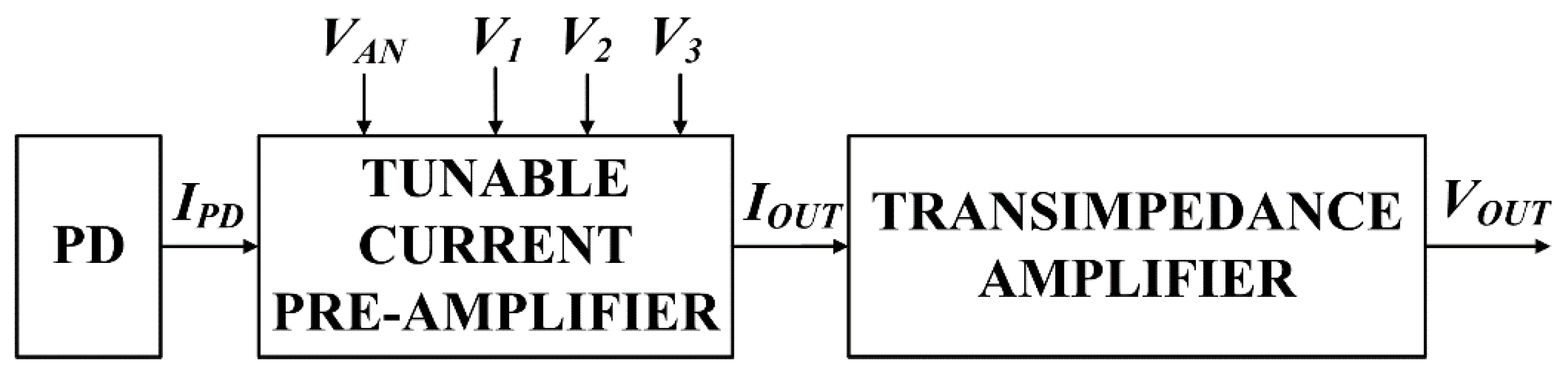
2. Circuit Design and Theory
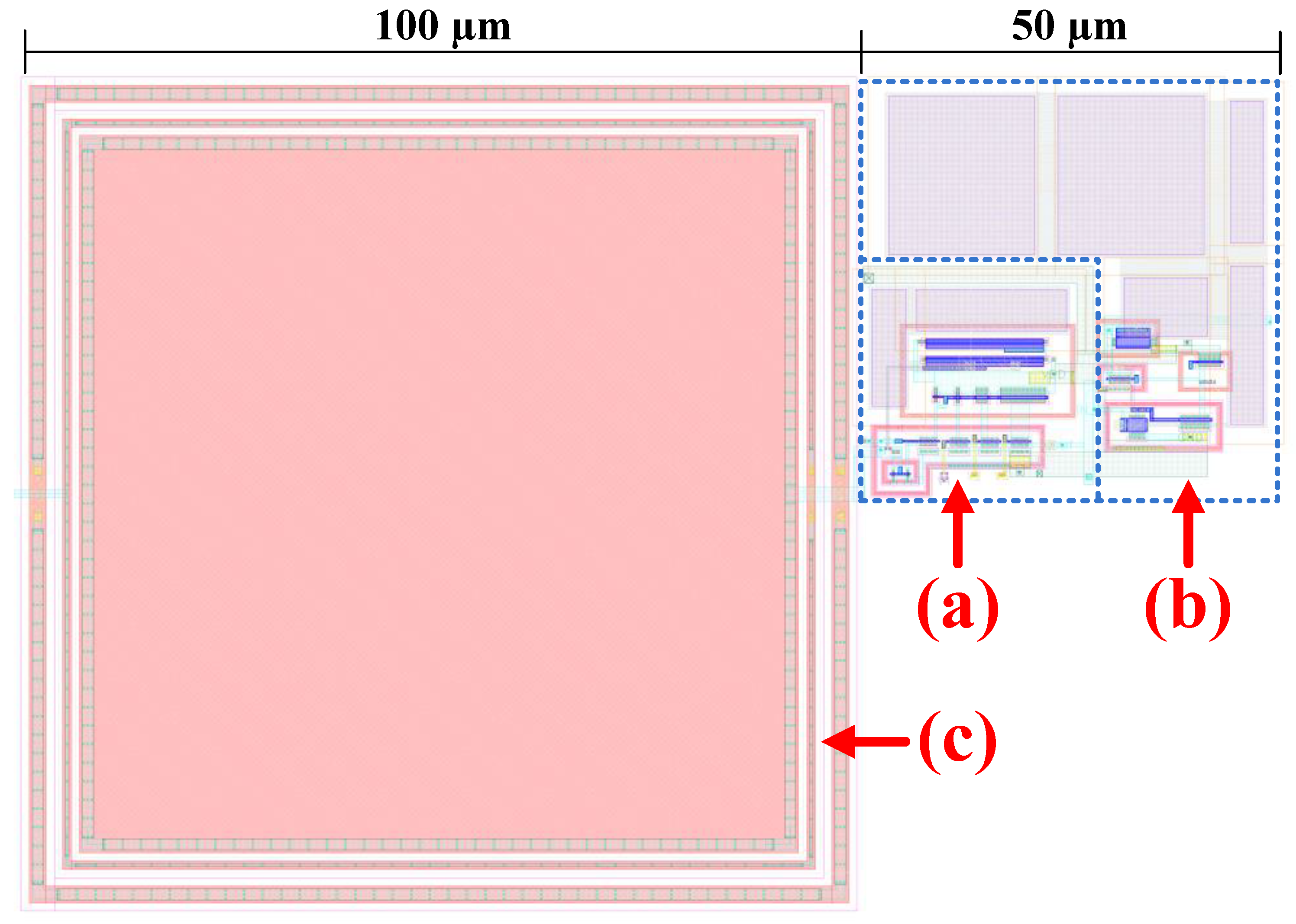
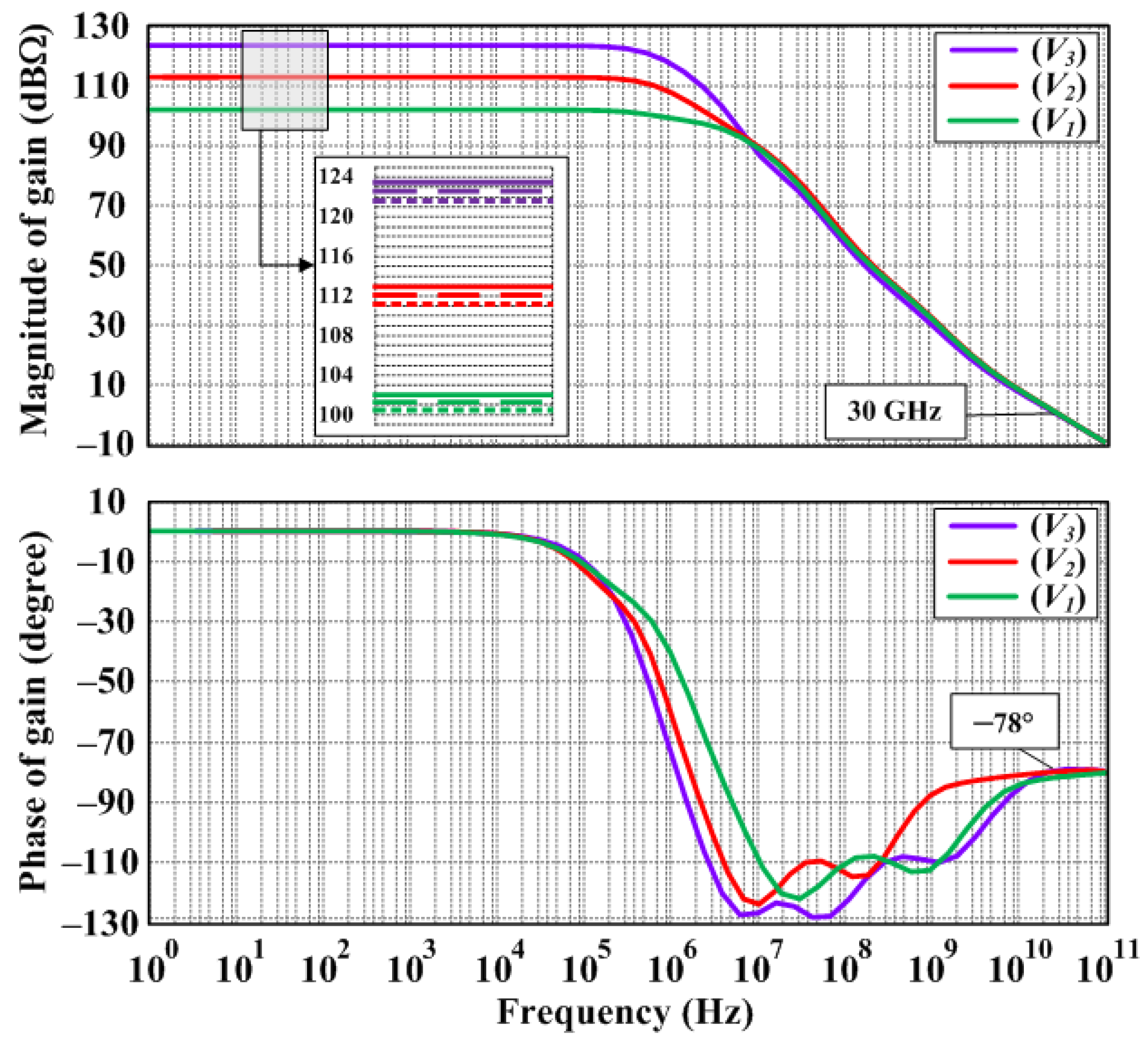
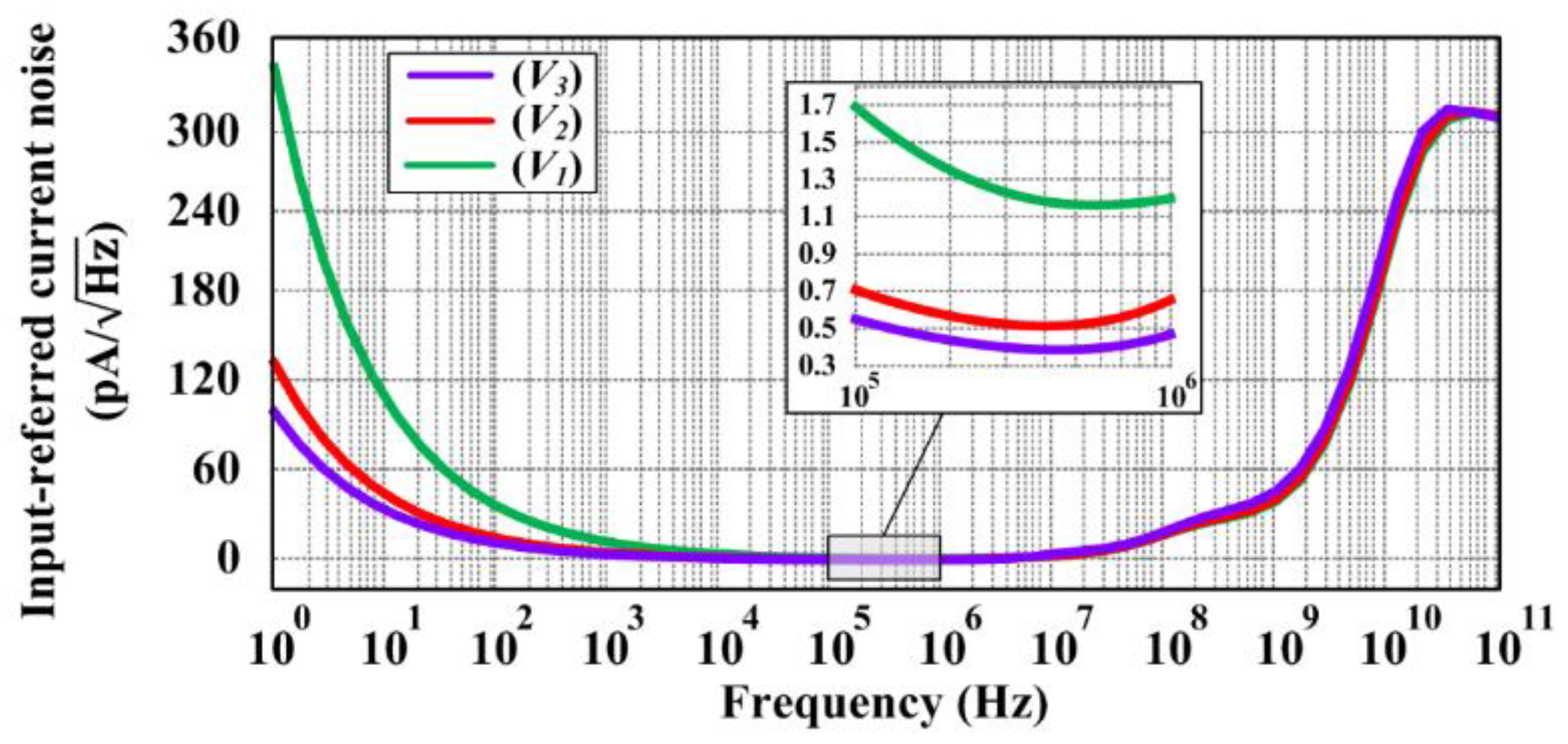
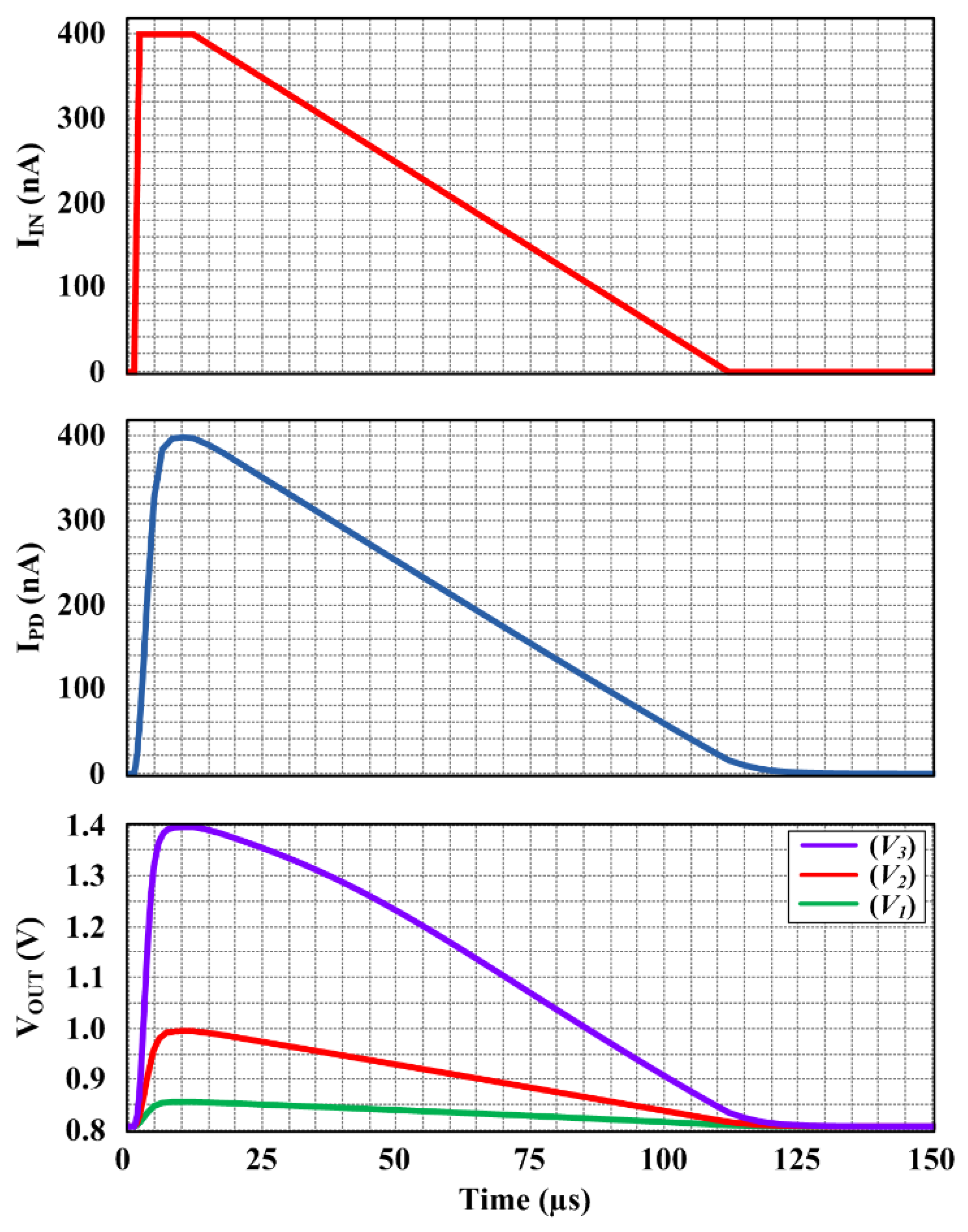
3. Circuit Simulation Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iqbal, S.M.A.; Mahgoub, I.; Du, E.; Leavitt, M.A.; Asghar, W. Advances in healthcare wearable devices. NPJ Flex. Electron. 2021, 5, 1–14. [Google Scholar] [CrossRef]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.-K.; Jung, J. Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardona, M.; Solanki, V.K.; García Cena, C.E. Internet of Medical Things—Paradigm of Wearable Devices; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9780367272630. [Google Scholar]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A.; Ka´zmierczak, A.; Piramidowicz, R. State-of-the-Art Optical Devices for Biomedical Sensing Applications—A Review. Electronics 2021, 10, 973. [Google Scholar] [CrossRef]
- Hinrichs, H.; Scholz, M.; Baum, A.K.; Kam, J.W.; Knight, R.T.; Heinze, H.J. Comparison between a wireless dry electrode EEG system with a conventional wired wet electrode EEG system for clinical applications. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Dufour, S.; De Koninck, Y. Optrodes for combined optogenetics and electrophysiology in live animal. Neurophotonics 2015, 2, 031205. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.U.; Kim, B.H.; Lee, J.Y.; Lee, Y.; Xie, Z.; Ibler, E.M.; Lee, K.; Banks, A.; Jeong, J.Y.; Kim, J.; et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 2019, 363, eaau0780. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Konijnenburg, M.; van Wegberg, R.; Xu, Y.; Ha, H.; Sijbers, W.; Stanzione, S.; Biswas, B.; Breeschoten, A.; Vis, P.; et al. A 769 w battery-powered single-chip soc with ble for multi-modal vital sign monitoring health patches. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 1506–1517. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Mahmood, M.; Kwon, S.; Zavanelli, N.; Kim, H.S.; Rim, Y.S.; Epps, F.; Yeo, W. Fully integrated, stretchable, wireless skin-conformal bioelectronics for continuous stress monitoring in daily life. Adv. Sci. 2020, 7, 2000810. [Google Scholar] [CrossRef]
- Costanzo, I.; Sen, D.; Adegite, J.; Rao, P.M.; Guler, U. A Noninvasive Miniaturized Transcutaneous Oxygen Monitor. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 474–485. [Google Scholar] [CrossRef]
- Jia, Y.; Guler, U.; Lai, Y.; Gong, Y.; Weber, A.; Li, W.; Ghovanloo, M. A Trimodal Wireless Implantable Neural Interface System-on-Chip. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 1207–1217. [Google Scholar] [CrossRef]
- Moreno, J.C.; Bueno, L.; Pons, J.L. Wearable robot technologies. In Wearable Robots: Biomechatronic Exoskeleton; Pons, L.J., Ed.; Wiley: West Sussex, UK, 2008; ISBN 978-0-470-51294-4. [Google Scholar]
- Porkodi, S.; Kesavaraja, D. Healthcare Robots Enabled with IoT and Artificial Intelligence for Elderly Patients. In AI and IoT-Based Intelligent Automation in Robotics; Dubey, A.K., Kumar, A., Kumar, S.R., Gayathri, N., Das, P., Eds.; John Wiley & Sons Inc. and Scrivener Publishing LLC: Salem, MA, USA, 2021; pp. 87–108. ISBN 9781119711209. [Google Scholar]
- Loeppke, R.R.; Hohn, T.; Baase, C.; Bunn, W.B.; Burton, W.N.; Eisenberg, B.S.; Ennis, T.; Fabius, R.; Hawkins, R.J.; Hudson, T.W.; et al. Integrating health and safety in the workplace: How closely aligning health and safety strategies can yield measurable benefits. J. Occup. Environ. Med. 2015, 57, 585–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarra, A.; Di Patrizio Stanchieri, G.; De Marcellis, A.; Bordi, F.; Postorino, P.; Palange, E. Laser Transmission Spectroscopy Based on Tunable-Gain Dual-Channel Dual-Phase LIA for Biological Nanoparticles Characterization. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Lee, N.-E. Recent Advancements in Development of Wearable Gas Sensors. Adv. Mater. Technol. 2021, 6, 2000883. [Google Scholar] [CrossRef]
- Kumar, S.; Vanita. A Review of Transimpedance Amplifiers Used in Biomedical Applications. In Proceedings of the IEEE 5th International Conference on Computing Methodologies and Communication (ICCMC), Erode, India, 8–10 April 2021. [Google Scholar]
- Atef, A.; Atef, M.; Khaled, E.E.M.; Abbas, M. CMOS Transimpedance Amplifiers for Biomedical Ap-plications: A Comparative Study. IEEE Circuits Syst. Mag. 2020, 20, 12–31. [Google Scholar] [CrossRef]
- Lu, Z.; Yeo, K.S.; Lim, W.M.; Do, M.A.; Boon, C.C. Design of a cmos broadband transimpedance amplifier with active feedback. IEEE Trans. Very Large Scale Integr. (VLSI) Syst. 2010, 18, 461–472. [Google Scholar] [CrossRef]
- Aflatouni, F.; Hashemi, H. A 1.8 mW Wideband 57 dBΩ Transimpedance Amplifier in 0.13 μm CMOS. In Proceedings of the IEEE Radio Frequency Integrated Circuits Symposium, Boston, MA, USA, 7–9 June 2009. [Google Scholar] [CrossRef]
- De Marcellis, A.; Di Patrizio Stanchieri, G.; Palange, E.; Faccio, M.; Constandinou, T.G. An Ultra-Wideband-Inspired System-on-Chip for an Optical Bidirectional Transcutaneous Biotelemetry. In Proceedings of the IEEE Biomedical Circuits and Systems Conference (BioCAS), Cleveland, OH, USA, 17–19 October 2018. [Google Scholar] [CrossRef]
- Lin, B.; Atef, M.; Wang, G. 14.85 µW Analog Front-End for Photoplethysmography Acquisition with 142-dBΩ Gain and 64.2-pArms Noise. Sensors 2019, 19, 512. [Google Scholar] [CrossRef] [Green Version]
- Atef, M.; Wang, M.; Wang, G. A Fully Integrated High-Sensitivity Wide Dynamic Range PPG Sensor with an Integrated Photodiode and an Automatic Dimming Control LED Driver. IEEE Sens. J. 2018, 18, 652–659. [Google Scholar] [CrossRef]
- Konijnenburg, M.; Stanzione, S.; Yan, L.; Jee, D.-W.; Pettine, J.; van Wegberg, R.; Kim, H.; Van Liempd, C.; Fish, R.; Schuessler, J.; et al. A Multi(bio)sensor Acquisition System with Integrated Processor, Power Management, 8 × 8 LED Drivers, and Simultaneously Synchronized ECG, BIO-Z, GSR, and Two PPG Readouts. IEEE J. Solid State Circuits 2016, 51, 2584–2595. [Google Scholar] [CrossRef]
- Kang, E.; Tan, M.; An, J.-S.; Chang, Z.-Y.; Vince, P.; Sénégond, N.; Mateo, T.; Meynier, C.; Pertijs, M.A.P. A Variable-Gain Low-Noise Transimpedance Amplifier for Miniature Ultrasound Probes. IEEE J. Solid State Circuits 2020, 55, 3157–3168. [Google Scholar] [CrossRef]
- Mathew, M.; Hart, B.L.; Hayatleh, K. Design of a low-current shunt-feedback transimpedance amplifier with inherent loop-stability. Analog. Integr. Circuits Signal Processing 2019, 99, 539–545. [Google Scholar] [CrossRef]
- Abu-Taha, J. High Gain, Widebandwidth and Low PowerTransimpedance Amplifier Using DTMOS Transistor. In Proceedings of the IEEE 7th Palestinian International Conference on Electrical and Computer Engineering (PICECE), Gaza, Palestine, 26–27 March 2019. [Google Scholar] [CrossRef]
- Lee, S.B.S.; Yeo, K.S. A 4 GHz Single-to-Differential Cross-Coupled Variable-Gain Transimpedance Amplifier for Optical Communication. Electronics 2021, 10, 3042. [Google Scholar] [CrossRef]
- Lee, S.B.S.; Liu, H.; Yeo, K.S.; Yu, X.P. Design of differential variable-gain transimpedance amplifier in 0.18-µm SiGe BiCMOS. Electronics 2020, 9, 1058. [Google Scholar] [CrossRef]
- Royo, G.; Martinez-Perez, A.D.; Sanchez-Azqueta, C.; Aldea, C.; Celma, S. A Highly Linear Low-Noise Transimpedance Amplifier for Indoor Fiber-Wireless Remote Antenna Units. Electronics 2019, 8, 437. [Google Scholar] [CrossRef] [Green Version]
- Van Der Horst, M.J.; Linnenbank, A.C.; Serdijn, W.A.; Long, J.R. Systematic Design of a Transimpedance Amplifier with Specified Electromagnetic Out-of-Band Interference Behavior. IEEE Trans. Circuits Syst. I Regul. Pap. 2010, 57, 530–538. [Google Scholar] [CrossRef]
- Valagiannopoulos, C.A.; Tsitsas, N.L. Integral equation analysis of a low-profile receiving planar microstrip antenna with a cloaking superstrate. Radio Sci. 2012, 47, 1–12. [Google Scholar] [CrossRef]
- Kassis, M. Moments-Based Techniques for Complex Microelectronic Systems. Ph.D. Thesis, McGill University, Montréal, QC, Canada, 2020. [Google Scholar]
- Fikioris, G.; Valagiannopoulos, C.A. Input admittances arising from explicit solutions to integral equations for infinite-length dipole antennas. Prog. Electromagn. Res. 2005, 55, 285–306. [Google Scholar] [CrossRef] [Green Version]
- Ziabakhsh, S.; Alavi-Rad, H.; Ahandani, M.A.; Yagoub, M.C.E. Design and optimization of a fully differential CMOS variable-gain lna with differential evolution algorithm for wlan applications. J. Circuits Syst. Comput. 2014, 23, 1450124. [Google Scholar] [CrossRef]
- Valencia-Ponce, M.A.; Tlelo-Cuautle, E.; De La Fraga, L.G. On the Sizing of CMOS Operational Amplifiers by Applying Many-Objective Optimization Algorithms. Electronics 2021, 10, 3148. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [Green Version]
- Esipova, T.V.; Karagodov, A.; Miller, J.; Wilson, D.F.; Busch, T.M.; Vinogradov, S.A. Two New “Protected” Oxyphors for Biological Oximetry: Properties and Application in Tumor Imaging. Anal. Chem. 2011, 83, 8756–8765. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Dai, S.; Wang, L. Optical aptasensors for quantitative detection of small biomolecules: A review. Biosens. Bioelectron. 2014, 59, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Yan, Y.-Z.; Park, Y.; Lee, T.; Peng, D.; Liu, Y.; Ha, C.-S.; Kim, K.C. Phosporescence-Based Flexible and Transparent Optical Temperature-Sensing Skin Capabale of Operating in Extreme Environments. ACS Appl. Polym. Mater. 2021, 3, 2461–2469. [Google Scholar] [CrossRef]
- Zhao, E.; Chen, S. Materials with aggregation-induced emission characteristics for applications in diagnosis, therag-nosis, disease mechanism study and personalized medicine. Mater. Chem. Front. 2021, 5, 3322–3343. [Google Scholar] [CrossRef]


| Transistor | Size W/L (µm/µm) |
|---|---|
| M1, M2, M4 | 0.22/0.18 |
| M5 | 0.28/0.36 |
| M3, M6, M8, M10 | 2.2/0.18 |
| M7 | 1.4/0.36 |
| M9 | 5.4/0.36 |
| M11, M12 | 0.22/14 |
| M0T | 2/1.6 |
| M1T | 3.6/0.36 |
| M4T, M2T | 2.65/0.18 |
| M3T | 1/4 |
| CL | 0.7 pF |
| Component | Value |
| C1 | 0.3 pF |
| C2 | 1.8 pF |
| CJ | 8 pF |
| RSH | 100 MΩ |
| RS | 50 Ω |
| [Ref] Year | [22] 2019 | [23] 2018 | [24] 2016 | [25] 2020 | [26] 2019 | [This Work] 2021 |
|---|---|---|---|---|---|---|
| CMOS Technology (µm) | 0.35 | 0.35 | 0.18 | 0.18 | 0.35 | 0.18 |
| Supply Voltage (V) | 2.0–3.7 | 2.7–4 | 1.2–1.8 | ±0.9/1.8 | 3.3 | 1.8 |
| Si Area (mm2) | 0.64 | 1.68 + 0.74 (PD) | 37.7 | 0.12 | N.A. | 0.002 + 0.01 (PD) |
| Achieved Results | Measured | Measured | Measured | Measured | Simulated | Simulated |
| Circuit Operating Mode | Continuous | Pulsed | Pulsed | N.A. | Sinusoidal | Pulsed |
| Transimpedance Gain (dBΩ) | 135–142 | 144 | 84–127 | 107 | 120 | 102–124 |
| Bandwidth (Hz) | 10 | 50 | 64 | 7 M | 8.2 M | 1.15 M (@ 102 dBΩ) 555 k (@ 124 dBΩ) |
| Power Consumption (µW) | 15 | 3360 | 135 | 5200 | 330 | 33.1 (@ 102 dBΩ) 36.1 (@ 124 dBΩ) |
| ) | 42 | 79 | 27 | 1.7 | 3.5 | 0.39 (@ 124 dBΩ) 1.2 (@ 102 dBΩ) |
| FOM (Ω × GHz/mW) | 8.4 | 0.24 | 1.06 | 301 | 24.8k | 24.4k (@ 124 dBΩ) 4.4k (@ 102 dBΩ) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Patrizio Stanchieri, G.; De Marcellis, A.; Battisti, G.; Faccio, M.; Palange, E.; Guler, U. A 1.8 V Low-Power Low-Noise High Tunable Gain TIA for CMOS Integrated Optoelectronic Biomedical Applications. Electronics 2022, 11, 1271. https://doi.org/10.3390/electronics11081271
Di Patrizio Stanchieri G, De Marcellis A, Battisti G, Faccio M, Palange E, Guler U. A 1.8 V Low-Power Low-Noise High Tunable Gain TIA for CMOS Integrated Optoelectronic Biomedical Applications. Electronics. 2022; 11(8):1271. https://doi.org/10.3390/electronics11081271
Chicago/Turabian StyleDi Patrizio Stanchieri, Guido, Andrea De Marcellis, Graziano Battisti, Marco Faccio, Elia Palange, and Ulkuhan Guler. 2022. "A 1.8 V Low-Power Low-Noise High Tunable Gain TIA for CMOS Integrated Optoelectronic Biomedical Applications" Electronics 11, no. 8: 1271. https://doi.org/10.3390/electronics11081271
APA StyleDi Patrizio Stanchieri, G., De Marcellis, A., Battisti, G., Faccio, M., Palange, E., & Guler, U. (2022). A 1.8 V Low-Power Low-Noise High Tunable Gain TIA for CMOS Integrated Optoelectronic Biomedical Applications. Electronics, 11(8), 1271. https://doi.org/10.3390/electronics11081271









