Performance of a Pd-Zn Cathode Electrode in a H2 Fueled Single PEM Fuel Cell
Abstract
:1. Introduction
2. Experimental Details
2.1. Synthesis of the Electrocatalytic Powders
2.2. Physicochemical Characterization
2.3. MEA Preparation
2.3.1. Electrodes Preparation
2.3.2. Electrolyte Membrane Purification and Activation
2.3.3. Assembling the Electrodes with the Membrane
2.4. PEMFC Operation
2.4.1. Experimental Apparatus
2.4.2. Experimental Procedure
3. Results and Discussion
3.1. Physicochemical Characteristics
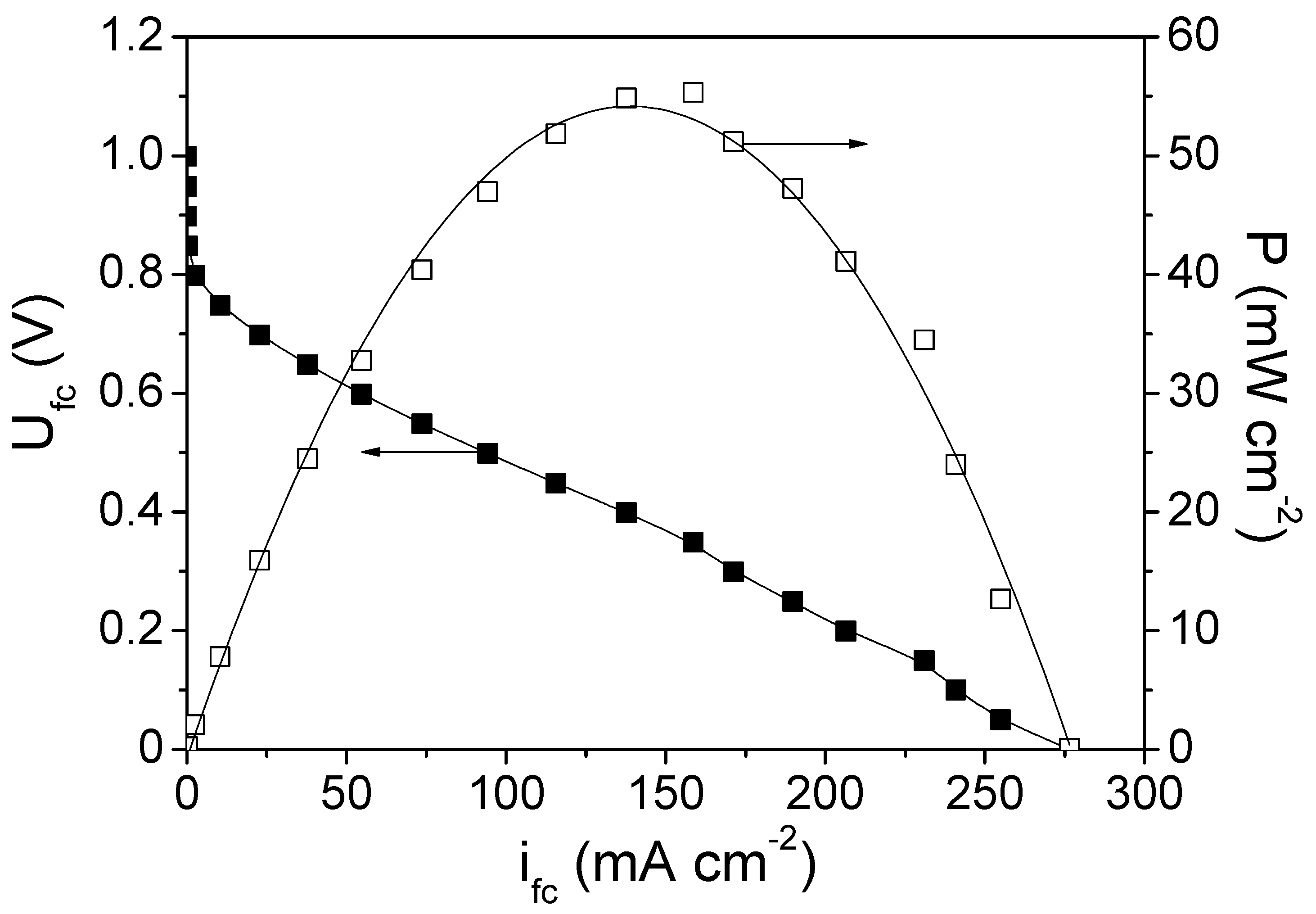
3.2. PEMFC Electrochemical Measurements
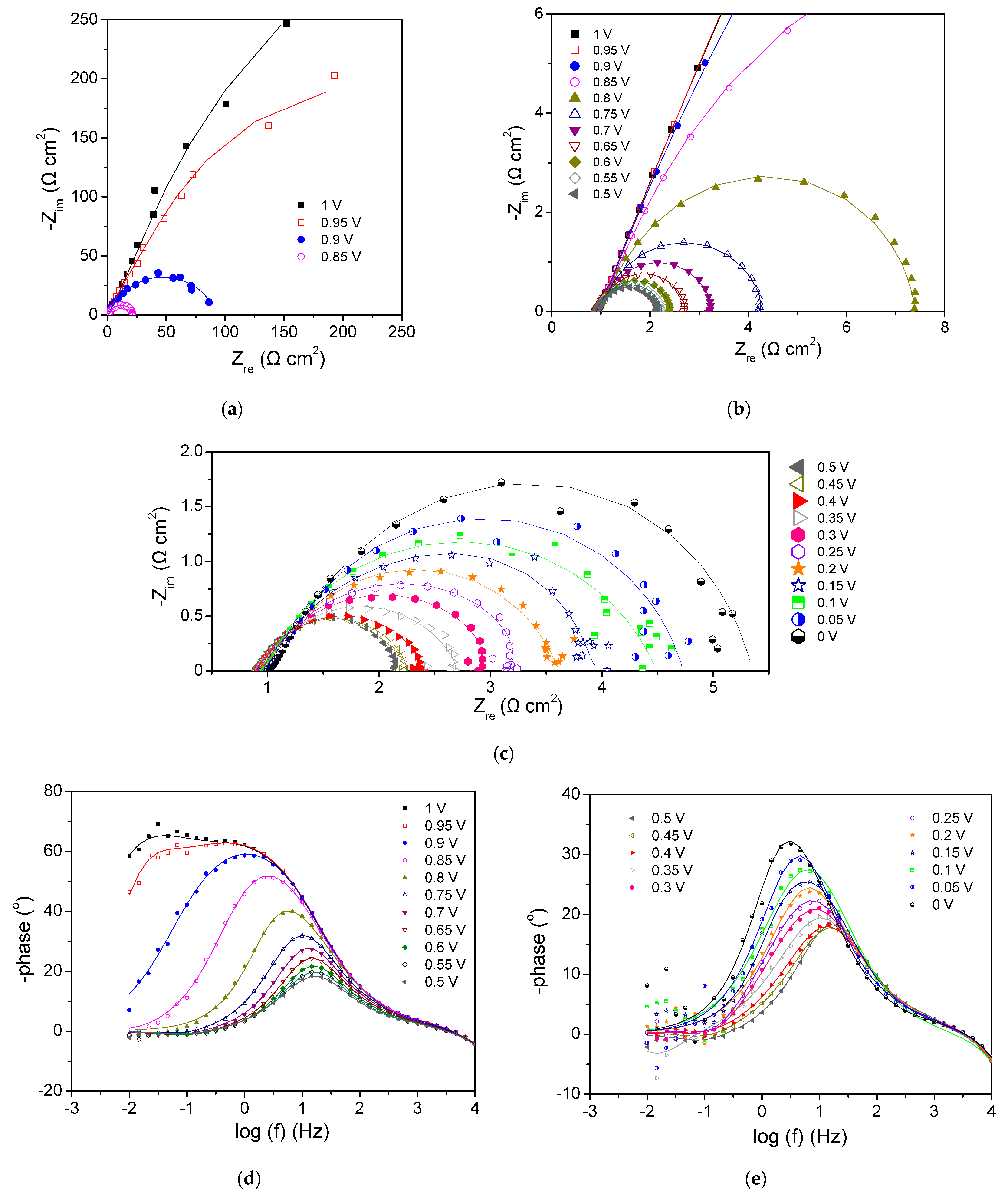
3.2.1. Cathode 29Pt—Anode Pt-Ru (Commercial)
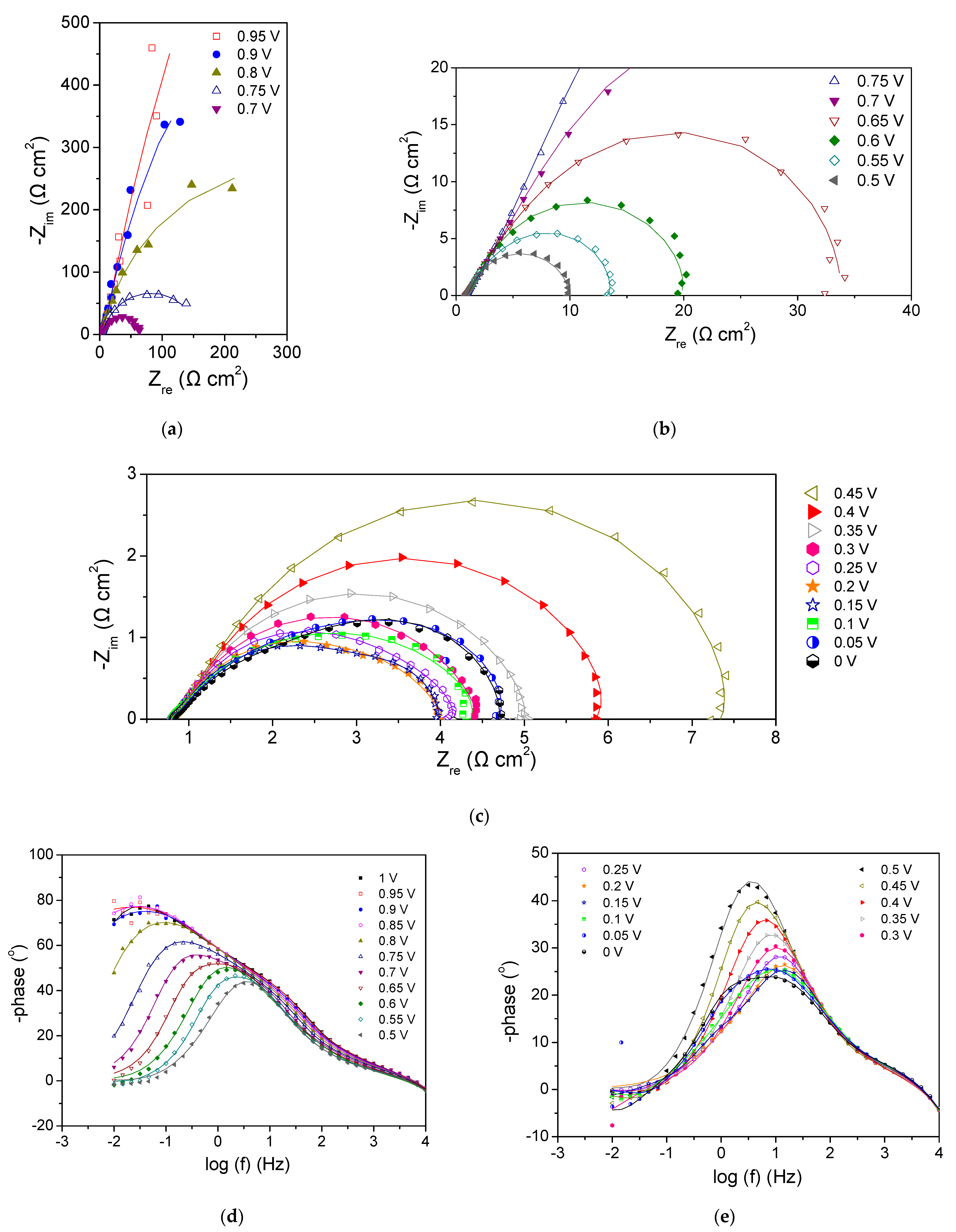
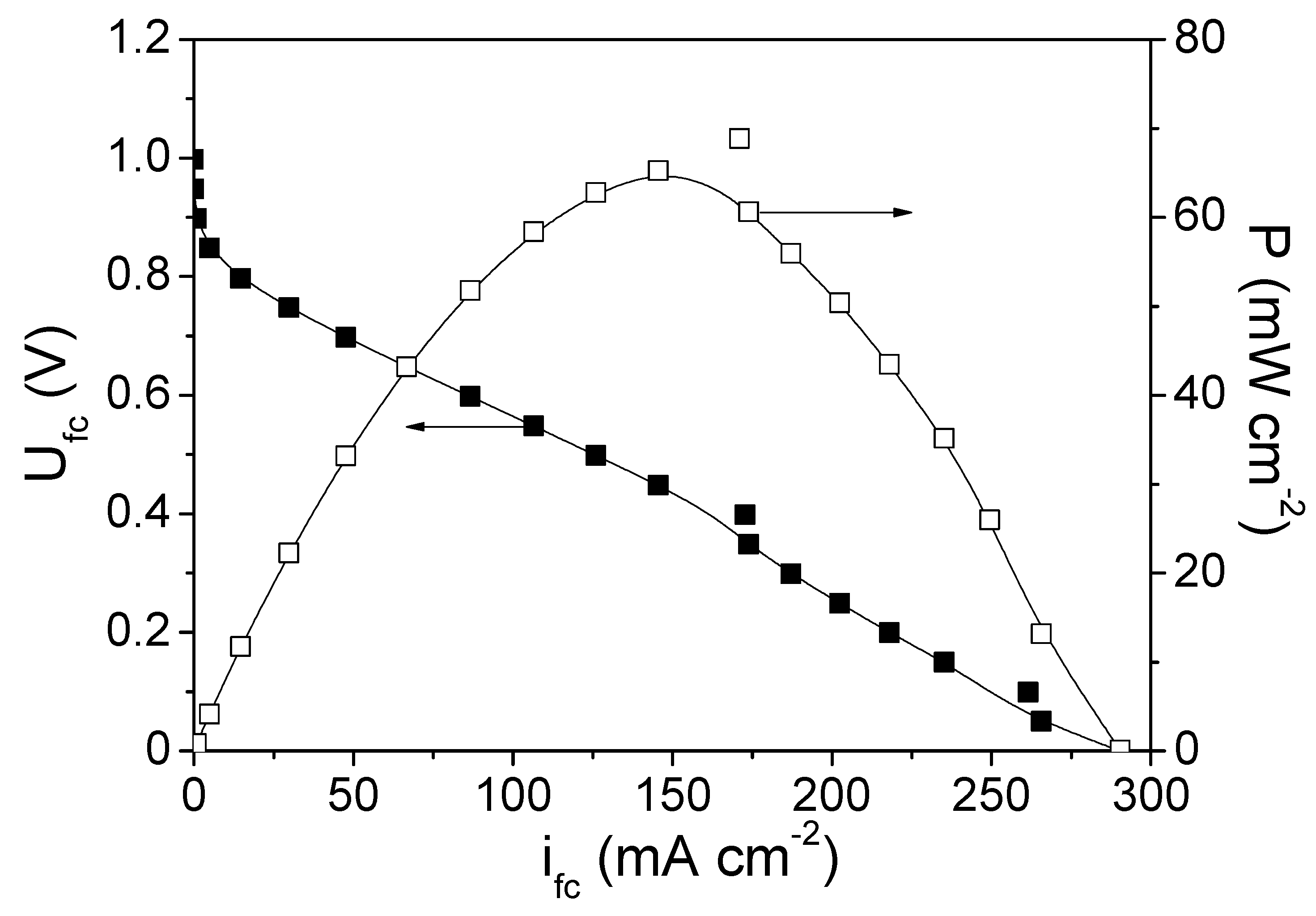
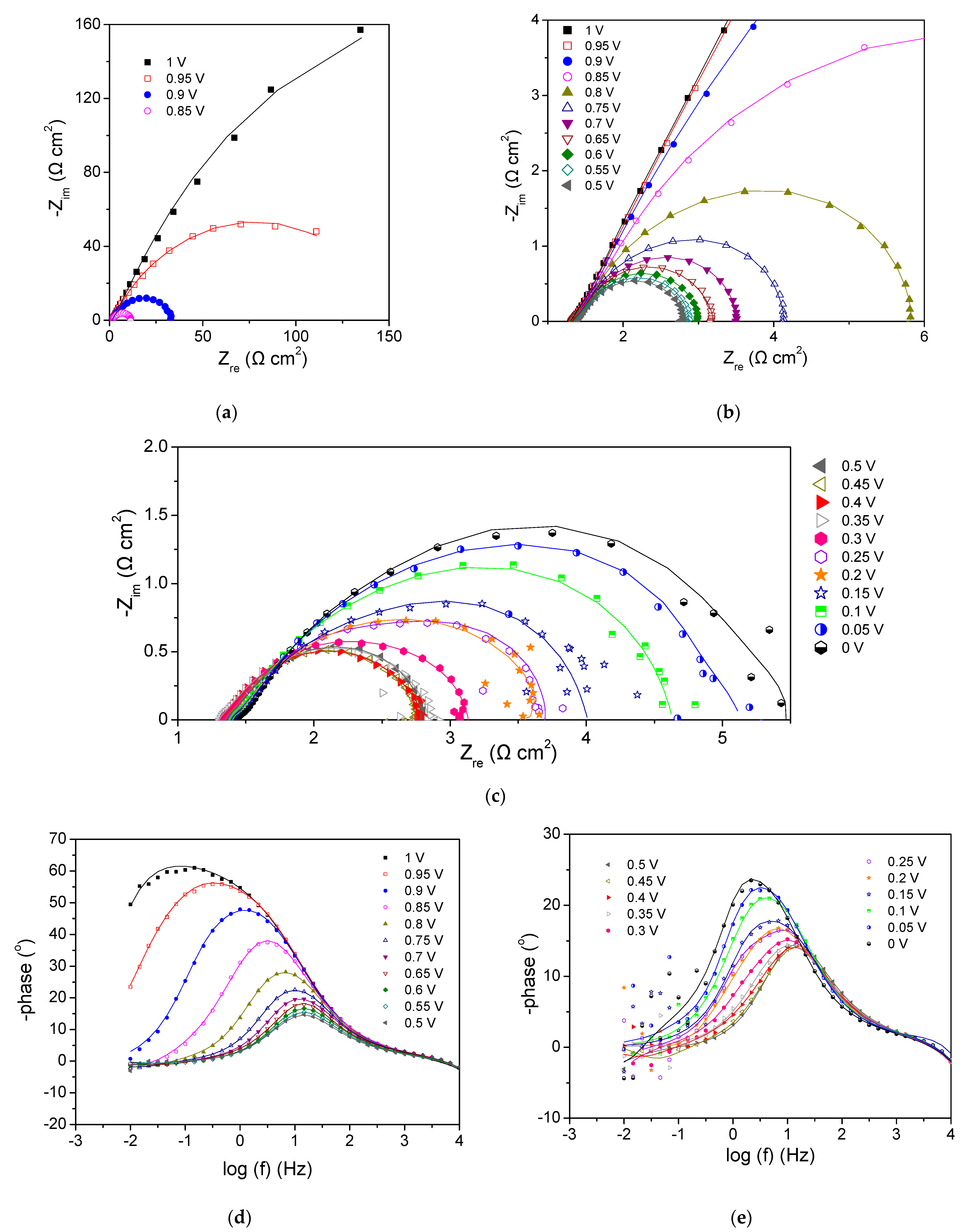
3.2.2. Cathode 22Pd-7Zn—Anode Pt-Ru (Commercial)
3.2.3. Cathode 29Pt (TKK)—Anode Pt-Ru (Commercial)
3.2.4. Comparison of the Tested PEMFCs with the Different Cathode Electrodes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Polani, S.; Luo, F.; Ott, S.; Strasser, P.; Dionigi, F. Advancements in cathode catalyst and cathode layer design for proton exchange membrane fuel cells. Nat. Commun. 2021, 12, 5984. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Zheng, Z.; Pahon, E.; Péra, M.-C.; Gao, F. A review on lifetime prediction of proton exchange membrane fuel cells system. J. Power Sources 2022, 529, 231256. [Google Scholar] [CrossRef]
- Miller, E.L.; Thompson, S.T.; Randolph, K.; Hulvey, Z.; Rustagi, N.; Satyapal, S. US Department of Energy hydrogen and fuel cell technologies perspectives. MRS Bull. 2020, 45, 57–64. [Google Scholar] [CrossRef]
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive Review on Fuel Cell Technology for Stationary Applications as Sustainable and Efficient Poly-Generation Energy Systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Shabani, B. Proton exchange membrane fuel cells heat recovery opportunities for combined heating/cooling and power applications. Energy Convers. Manag. 2020, 204, 112328. [Google Scholar] [CrossRef]
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Barbir, F. Fuel Cell Applications. In PEM Fuel Cells: Theory and Practice, 2nd ed.; Elsevier/Academic Press: Waltham, MA, USA, 2013; pp. 373–434. [Google Scholar]
- Fuel Cells and Hydrogen 2 Joint Undertaking (FCH 2 JU). 2019 Annual Work Plan and Budget (FCH 2 JU, 2018). Available online: https://ec.europa.eu/research/participants/data/ref/h2020/other/wp/jtis/h2020-wp19-fch_en.pdf (accessed on 3 July 2022).
- U.S. Department of Energy. Automotive Fuel Cell Targets and Status. DOE Hydrogen and Fuel Cells Program Record #20005. 31 August 2020. Available online: https://www.hydrogen.energy.gov/pdfs/20005-automotive-fuel-cell-targets-status.pdf (accessed on 3 July 2022).
- Xu, Z.; Qiu, D.; Yi, P.; Peng, L.; Lai, X. Towards mass applications: A review on the challenges and developments in metallic bipolar plates for PEMFC. Prog. Nat. Sci. Mater. Int. 2020, 30, 815–824. [Google Scholar] [CrossRef]
- DOE Technical Targets for Polymer Electrolyte Membrane Fuel Cell Components. 2019. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-polymer-electrolyte-membrane-fuel-cell-components (accessed on 3 July 2022).
- Al-Zoubi, T.; Zhou, Y.; Yin, X.; Janicek, B.; Sun, C.; Schulz, C.E.; Zhang, X.; Gewirth, A.A.; Huang, P.; Zelenay, P.; et al. Preparation of Nonprecious Metal Electrocatalysts for the Reduction of Oxygen Using a Low-Temperature Sacrificial Metal. J. Am. Chem. Soc. 2020, 142, 5477–5481. [Google Scholar] [CrossRef]
- Wan, X.; Liu, X.; Li, Y.; Yu, R.; Zheng, L.; Yan, W.; Wang, H.; Xu, M.; Shui, J. Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2019, 2, 259–268. [Google Scholar] [CrossRef]
- Chen, M.-X.; Zhu, M.; Zuo, M.; Chu, S.-Q.; Zhang, J.; Wu, Y.; Liang, H.-W.; Feng, X. Identification of Catalytic Sites for Oxygen Reduction in Metal/Nitrogen-Doped Carbons with Encapsulated Metal Nanoparticles. Angew. Chem. Int. Ed. 2020, 59, 1627–1633. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, J.; Wang, F.; Dai, L. Efficient Oxygen Reduction Reaction (ORR) Catalysts Based on Single Iron Atoms Dispersed on a Hierarchically Structured Porous Carbon Framework. Angew. Chem. Int. Ed. 2018, 57, 9038–9043. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, D.; Xu, H.; Zeng, X.; Wan, X.; Shui, J.; Xiang, Z.; Cao, D. Unveiling the high-activity origin of single-atom iron catalysts for oxygen reduction reaction. Proc. Natl. Acad. Sci. USA 2018, 115, 6626–6631. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Cullen, D.A.; Pan, Y.-T.; Hwang, S.; Wang, M.; Feng, Z.; Wang, J.; Engelhard, M.H.; Zhang, H.; He, Y.; et al. Nitrogen-Coordinated Single Cobalt Atom Catalysts for Oxygen Reduction in Proton Exchange Membrane Fuel Cells. Adv. Mater. 2018, 30, 1706758. [Google Scholar] [CrossRef]
- Hu, B.-C.; Wu, Z.-Y.; Chu, S.-Q.; Zhu, H.-W.; Liang, H.-W.; Zhang, J.; Yu, S.-H. SiO2-protected shell mediated templating synthesis of Fe–N-doped carbon nanofibers and their enhanced oxygen reduction reaction performance. Energy Environ. Sci. 2018, 11, 2208–2215. [Google Scholar] [CrossRef]
- Zhang, H.; Hwang, S.; Wang, M.; Feng, Z.; Karakalos, S.; Luo, L.; Qiao, Z.; Xie, X.; Wang, C.; Su, D.; et al. Single Atomic Iron Catalysts for Oxygen Reduction in Acidic Media: Particle Size Control and Thermal Activation. J. Am. Chem. Soc. 2017, 139, 14143–14149. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Zheng, L.; Shui, J. The Solid-Phase Synthesis of an Fe-N-C Electrocatalyst for High-Power Proton-Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2018, 57, 1204–1208. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Y.-G.; Chen, W.; Xu, R.; Zheng, L.; Zhang, J.; Luo, J.; Shen, R.-A.; Zhu, Y.; Cheong, W.-C.; et al. Hollow N-Doped Carbon Spheres with Isolated Cobalt Single Atomic Sites: Superior Electrocatalysts for Oxygen Reduction. J. Am. Chem. Soc. 2017, 139, 17269–17272. [Google Scholar] [CrossRef]
- Shen, H.; Gracia-Espino, E.; Ma, J.; Zang, K.; Luo, J.; Wang, L.; Gao, S.; Mamat, X.; Hu, G.; Wagberg, T.; et al. Synergistic Effects between Atomically Dispersed Fe−N−C and C−S−C for the Oxygen Reduction Reaction in Acidic Media. Angew. Chem. Int. Ed. 2017, 56, 13800–13804. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zamani, P.; Choi, J.-Y.; Hassan, F.M.; Jiang, G.; Higgins, D.C.; Zhang, Y.; Hoque, M.A.; Chen, Z. In Situ Polymer Graphenization Ingrained with Nanoporosity in a Nitrogenous Electrocatalyst Boosting the Performance of Polymer-Electrolyte-Membrane Fuel Cells. Adv. Mater. 2017, 29, 1604456. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Core–Shell Compositional Fine Structures of Dealloyed PtxNi1–x Nanoparticles and Their Impact on Oxygen Reduction Catalysis. Nano Lett. 2012, 12, 5423–5430. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef]
- Han, B.; Carlton, C.E.; Kongkanand, A.; Kukreja, R.S.; Theobald, B.R.; Gan, L.; O’Malley, R.; Strasser, P.; Wagner, F.T.; Shao-Horn, Y. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells. Energy Environ. Sci. 2015, 8, 258–266. [Google Scholar] [CrossRef]
- Escudero-Escribano, M.; Malacrida, P.; Hansen, M.H.; Vej-Hansen, U.G.; Velázquez-Palenzuela, A.; Tripkovic, V.; Schiøtz, J.; Rossmeisl, J.; Stephens, I.E.L.; Chorkendorff, I. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 2016, 352, 73–76. [Google Scholar] [CrossRef]
- Schwämmlein, J.N.; Harzer, G.S.; Pfändner, P.; Blankenship, A.; El-Sayed, H.A.; Gasteiger, H.A. Activity and Stability of Carbon Supported PtxY Alloys for the ORR Determined by RDE and Single-Cell PEMFC Measurements. J. Electrochem. Soc. 2018, 165, J3173–J3185. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Mavrikakis, M.; Hammer, B.; Nørskov, J.K. Effect of strain on the reactivity of metal surfaces. Phys. Rev. Lett. 1998, 81, 2819–2822. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef]
- Hoster, H.E.; Alves, O.B.; Koper, M.T.M. Tuning Adsorption via Strain and Vertical Ligand Effects. ChemPhysChem 2010, 11, 1518–1524. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals. J. Chem. Phys. 2004, 120, 10240–10246. [Google Scholar] [CrossRef]
- Liu, P.; Nørskov, J.K. Ligand and ensemble effects in adsorption on alloy surfaces. Phys. Chem. Chem. Phys. 2001, 3, 3814–3818. [Google Scholar] [CrossRef]
- Antolini, E.; Zignani, S.C.; Santos, S.F.; Gonzalez, E.R. Palladium-based electrodes: A way to reduce platinum content in polymer electrolyte membrane fuel cells. Electrochim. Acta 2011, 56, 2299–2305. [Google Scholar] [CrossRef]
- Fernández, J.L.; Walsh, D.A.; Bard, A.J. Thermodynamic Guidelines for the Design of Bimetallic Catalysts for Oxygen Electroreduction and Rapid Screening by Scanning Electrochemical Microscopy. M−Co (M: Pd, Ag, Au). J. Am. Chem. Soc. 2005, 127, 357–365. [Google Scholar] [CrossRef]
- Shao, M.H.; Huang, T.; Liu, P.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Adzic, R.R. Palladium Monolayer and Palladium Alloy Electrocatalysts for Oxygen Reduction. Langmuir 2006, 22, 10409–10415. [Google Scholar] [CrossRef]
- Shao, M.-H.; Sasaki, K.; Adzic, R.R. Pd−Fe Nanoparticles as Electrocatalysts for Oxygen Reduction. J. Am. Chem. Soc. 2006, 128, 3526–3527. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, Q.; Chen, H.; Shao, M. Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 2016, 29, 198–219. [Google Scholar] [CrossRef]
- Bampos, G.; Bebelis, S.; Kondarides, D.I.; Verykios, X. Comparison of the Activity of Pd–M (M: Ag, Co, Cu, Fe, Ni, Zn) Bimetallic Electrocatalysts for Oxygen Reduction Reaction. Top. Catal. 2017, 60, 1260–1273. [Google Scholar] [CrossRef]
- Bampos, G.; Kondarides, D.I.; Bebelis, S. Pd–Zn/C bimetallic electrocatalysts for oxygen reduction reaction. J. Appl. Electrochem. 2018, 48, 675–689. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, Q.-A.; Wang, Y.-J.; Zhang, F.; Li, W.; Li, A.; Zhang, L.; Zhang, J. Recent progress in the use of electrochemical impedance spectroscopy for the measurement, monitoring, diagnosis and optimization of proton exchange membrane fuel cell performance. J. Power Sources 2020, 468, 228361. [Google Scholar] [CrossRef]
- Cruz-Manzo, S.; Chen, R.; Rama, P. Inductive Effect on the Fuel Cell Cathode Impedance Spectrum at High Frequencies. J. Fuel Cell Sci. Technol. 2012, 9, 051002. [Google Scholar] [CrossRef]
- Pivac, I.; Barbir, F. Inductive phenomena at low frequencies in impedance spectra of proton exchange membrane fuel cells—A review. J. Power Sources 2016, 326, 112–119. [Google Scholar] [CrossRef]
- Schiefer, A.; Heinzmann, M.; Weber, A. Inductive Low-Frequency Processes in PEMFC-Impedance Spectra. Fuel Cells 2020, 20, 499–506. [Google Scholar] [CrossRef]
- Bampos, G.; Sygellou, L.; Bebelis, S. Oxygen reduction reaction activity of Pd-based bimetallic electrocatalysts in alkaline medium. Catal. Today 2020, 355, 685–697. [Google Scholar] [CrossRef]
- Mench, M.M. Performance Characterization of Fuel Cell Systems. In Fuel Cell Engines; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 121–190. [Google Scholar]
- Arif, M.; Cheung, S.C.P.; Andrews, J. A systematic approach for matching simulated and experimental polarization curves for a PEM fuel cell. Int. J. Hydrogen Energy 2020, 45, 2206–2223. [Google Scholar] [CrossRef]
- Fischer, A.; Jindra, J.; Wendt, H. Porosity and catalyst utilization of thin layer cathodes in air operated PEM-fuel cells. J. Appl. Electrochem. 1998, 28, 277–282. [Google Scholar] [CrossRef]
- Asghari, S.; Mokmeli, A.; Samavati, M. Study of PEM fuel cell performance by electrochemical impedance spectroscopy. Int. J. Hydrogen Energy 2010, 35, 9283–9290. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, J.C.; Blanco, M.; Wang, H.; Zhang, J.; Wilkinson, D.P. AC impedance diagnosis of a 500 W PEM fuel cell stack: Part I: Stack impedance. J. Power Sources 2006, 161, 900–928. [Google Scholar] [CrossRef]
- Yuan, X.; Sun, J.C.; Wang, H.; Zhang, J. AC impedance diagnosis of a 500W PEM fuel cell stack: Part II: Individual cell impedance. J. Power Sources 2006, 161, 929–937. [Google Scholar] [CrossRef]
- Martens, S.; Asen, L.; Ercolano, G.; Dionigi, F.; Zalitis, C.; Hawkins, A.; Bonastre, A.M.; Seidl, L.; Knoll, A.C.; Sharman, J.; et al. A comparison of rotating disc electrode, floating electrode technique and membrane electrode assembly measurements for catalyst testing. J. Power Sources 2018, 392, 274–284. [Google Scholar] [CrossRef]
- Mohideen, M.M.; Radhamani, A.V.; Ramakrishna, S.; Wei, Y.; Liu, Y. Recent insights on iron based nanostructured electrocatalyst and current status of proton exchange membrane fuel cell for sustainable transport. J. Energy Chem. 2022, 69, 466–489. [Google Scholar] [CrossRef]

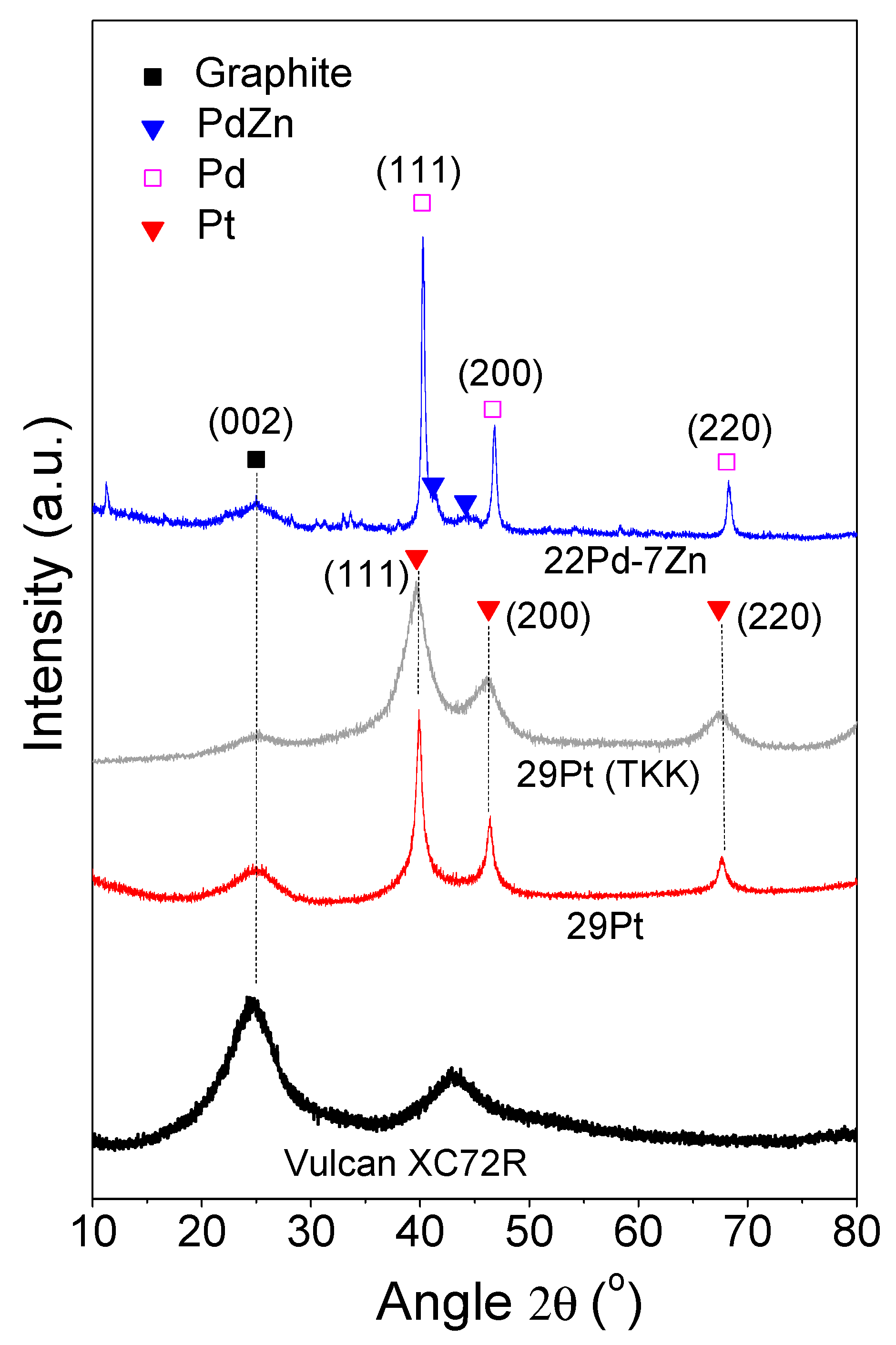


| Notation | Electrocatalyst Composition | Specific Surface Area (m2 g−1) | Particle Size (nm) |
|---|---|---|---|
| 22Pd—7Zn | 21.7 wt.% Pd—7.3 wt.% Zn/C | 68 1 | 8.4 ± 2.0 1 |
| 29Pt | 29 wt.% Pt/C | 88 1 | 10.0 ± 0.5 1 |
| 29Pt (TKK) | 29 wt.% Pt/C | 84 1 | 2.0 ± 0.1 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bampos, G.; Bebelis, S. Performance of a Pd-Zn Cathode Electrode in a H2 Fueled Single PEM Fuel Cell. Electronics 2022, 11, 2776. https://doi.org/10.3390/electronics11172776
Bampos G, Bebelis S. Performance of a Pd-Zn Cathode Electrode in a H2 Fueled Single PEM Fuel Cell. Electronics. 2022; 11(17):2776. https://doi.org/10.3390/electronics11172776
Chicago/Turabian StyleBampos, Georgios, and Symeon Bebelis. 2022. "Performance of a Pd-Zn Cathode Electrode in a H2 Fueled Single PEM Fuel Cell" Electronics 11, no. 17: 2776. https://doi.org/10.3390/electronics11172776
APA StyleBampos, G., & Bebelis, S. (2022). Performance of a Pd-Zn Cathode Electrode in a H2 Fueled Single PEM Fuel Cell. Electronics, 11(17), 2776. https://doi.org/10.3390/electronics11172776







