The Potential Use of Oyster Shell Waste in New Value-Added By-Product
Abstract
1. Introduction
2. Materials and Methods
Technical Analysis
3. Results and Discussion
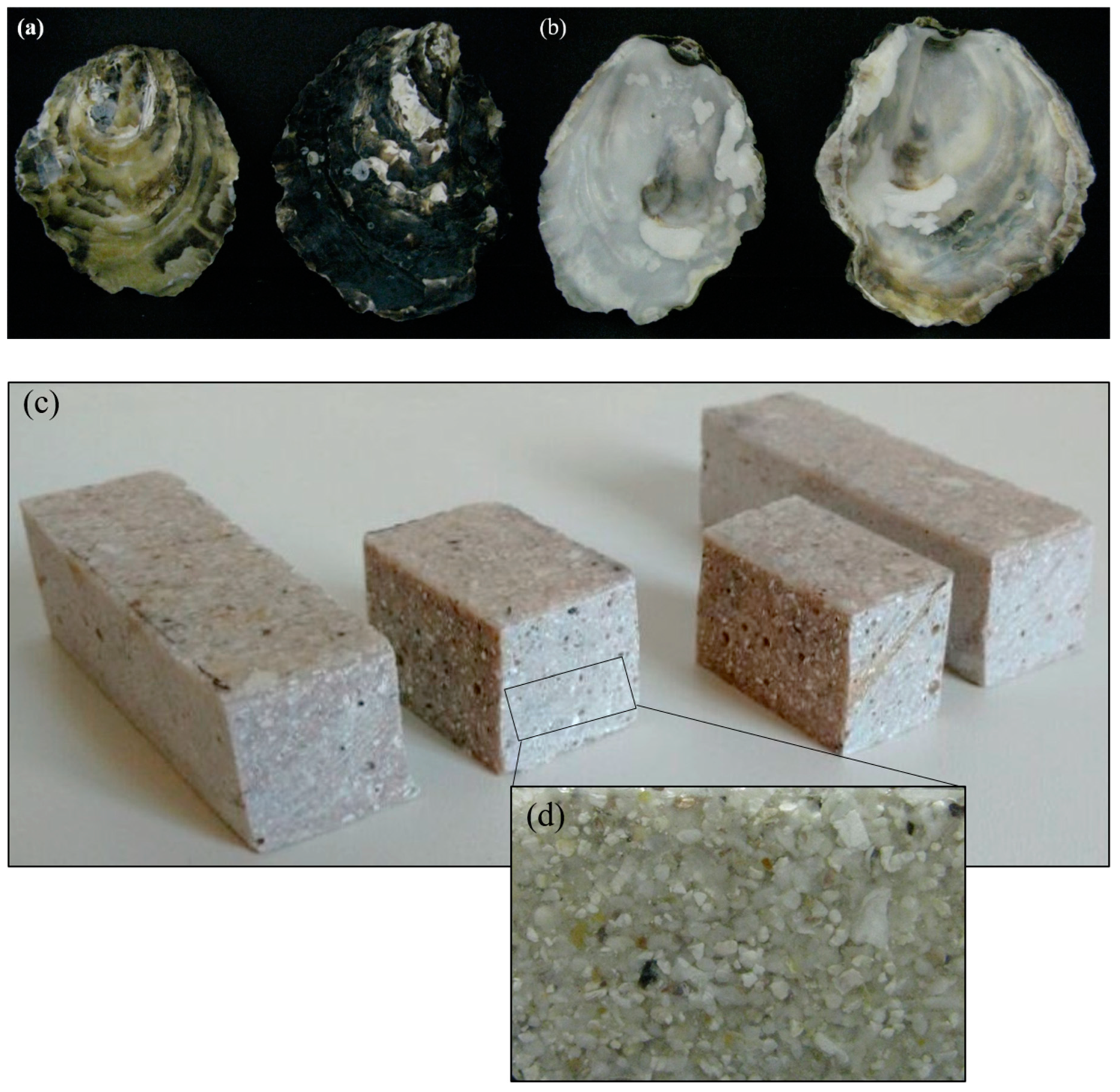
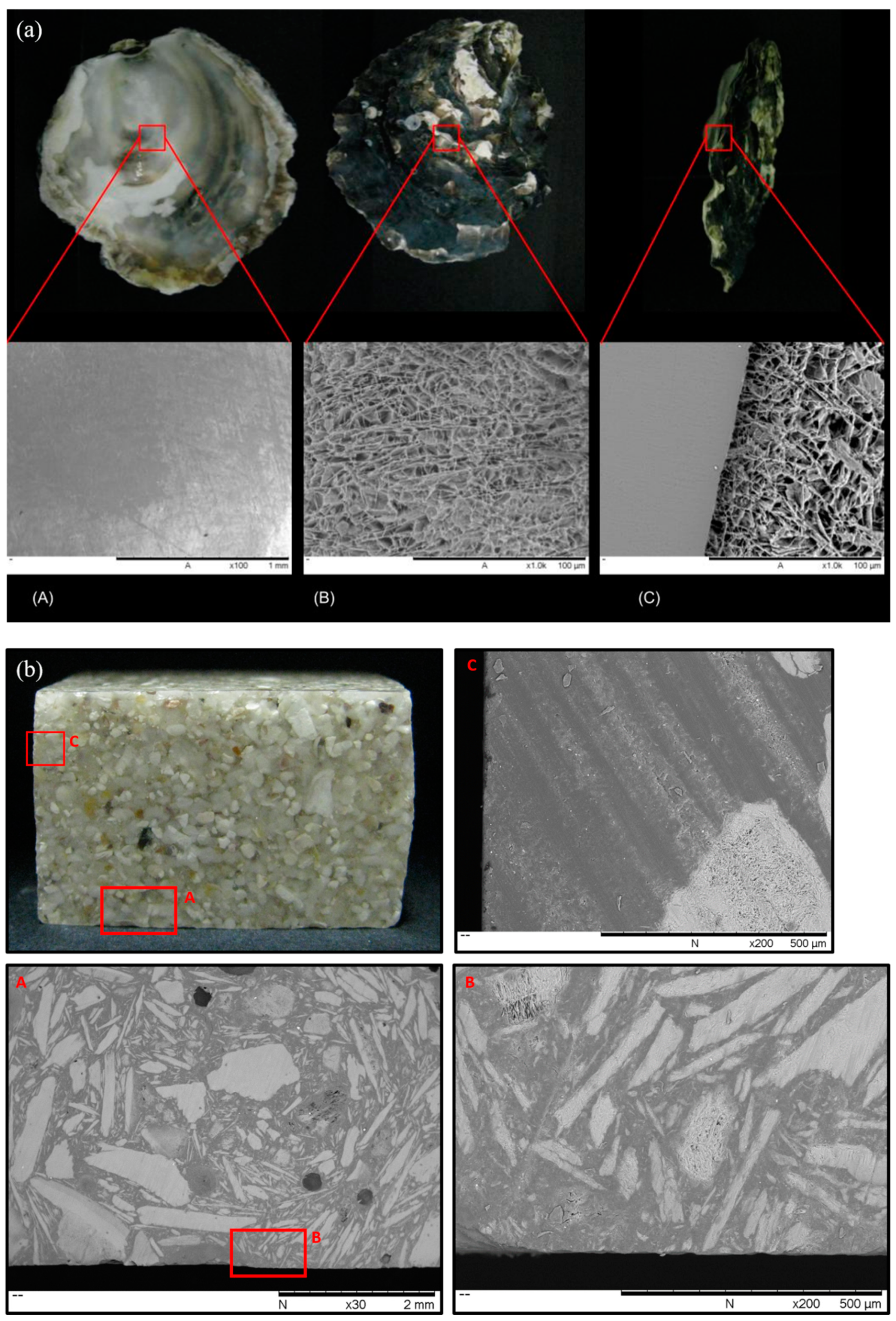
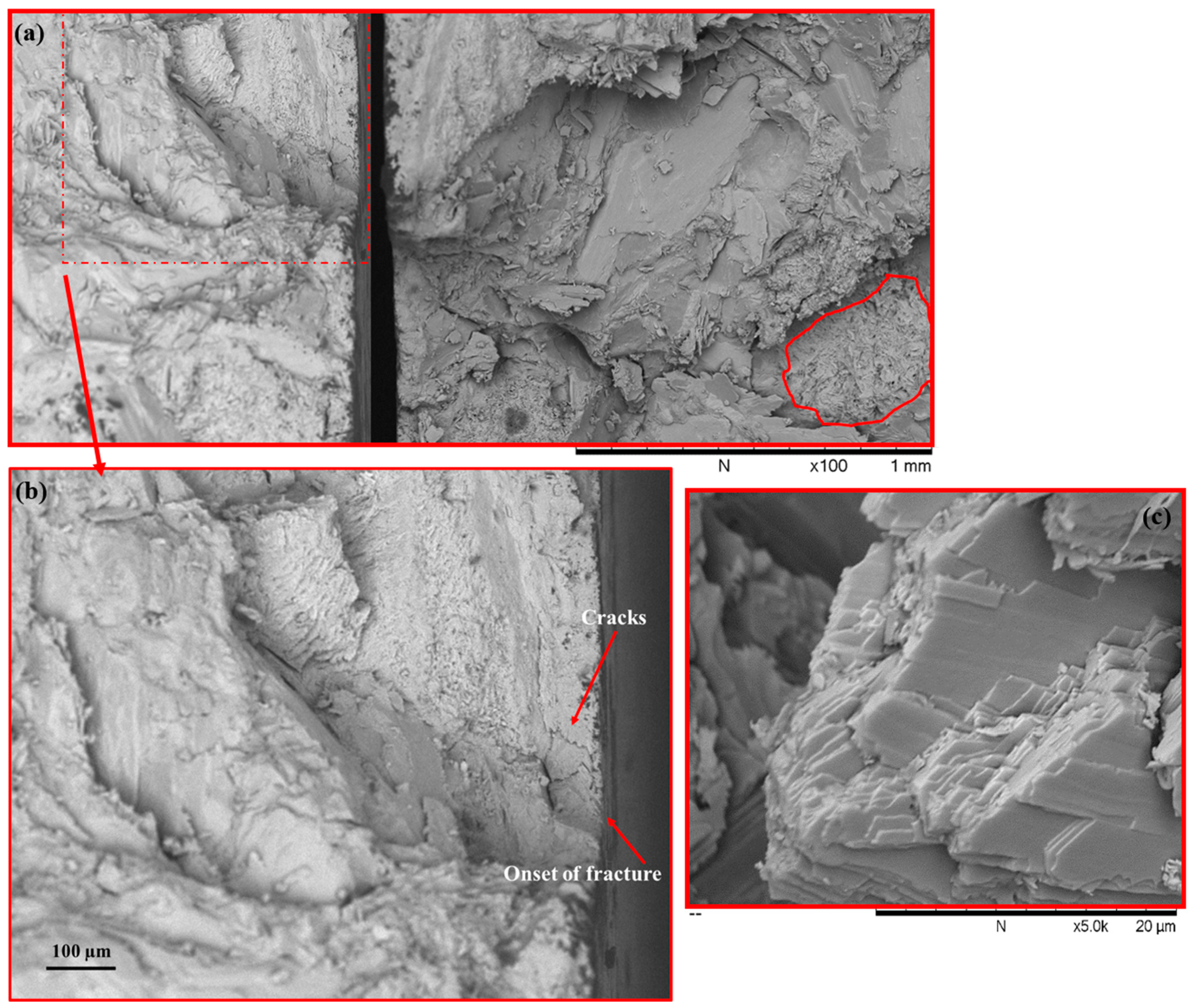
3.1. Characterisation of the Oyster Shells and Artificial Stone Samples
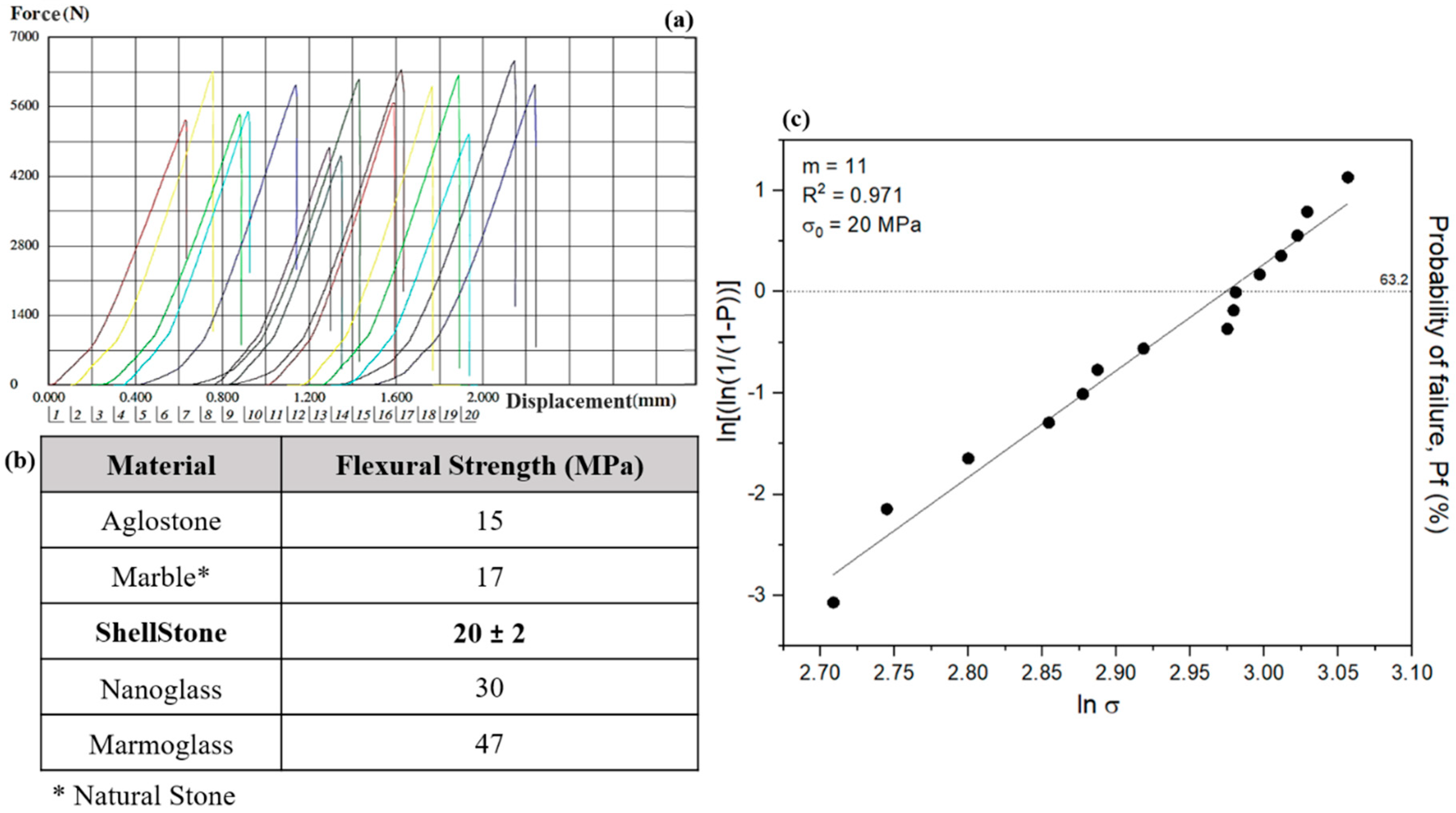
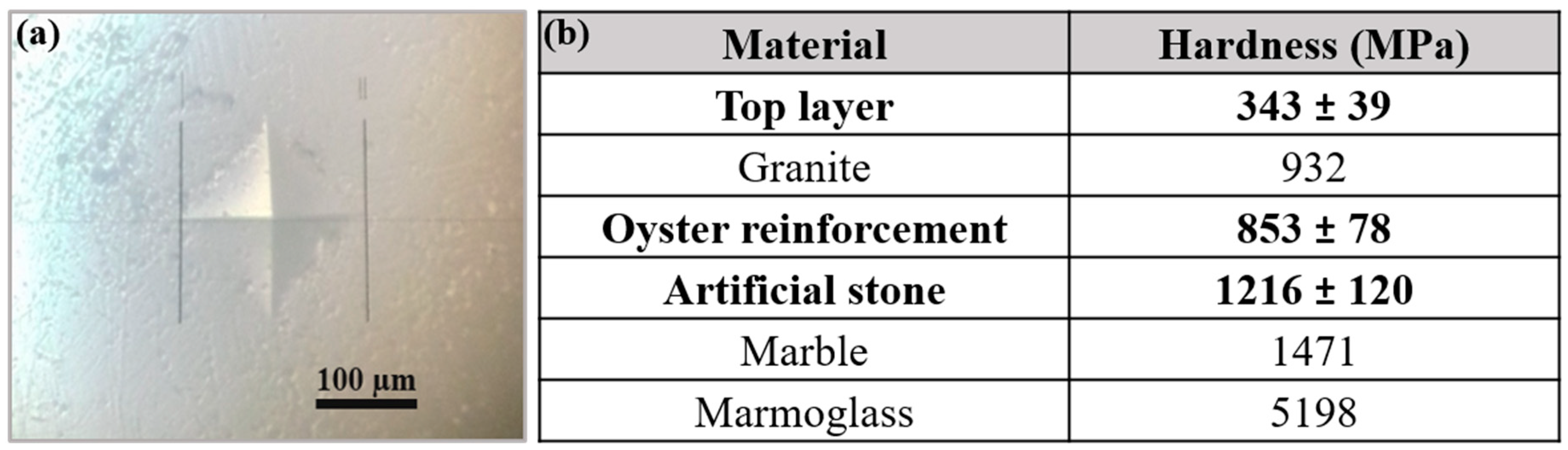
3.2. Mechanical Properties of the Commercial Artificial Stone Samples
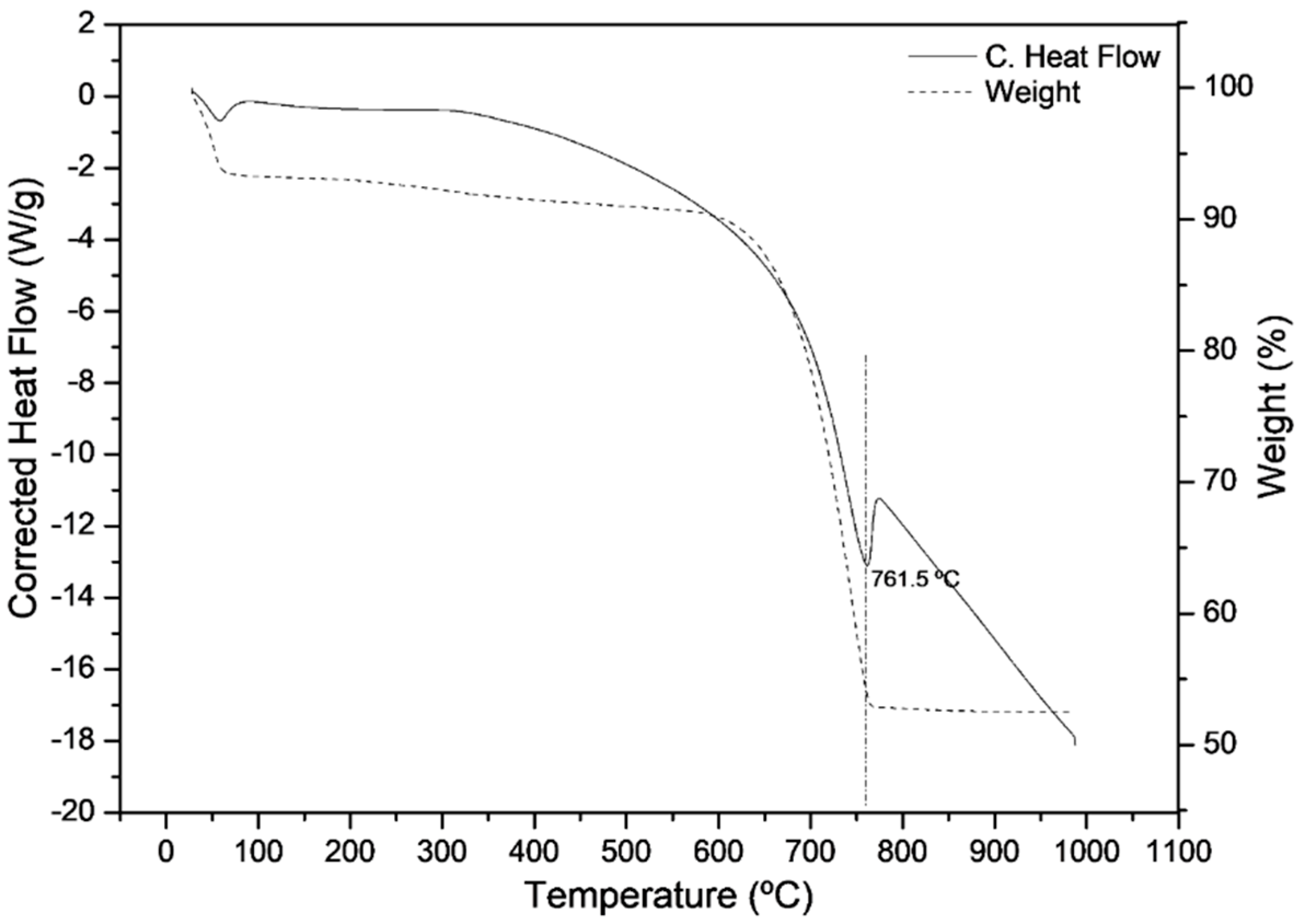
3.3. Transformation of Oyster Powder into Calcium Oxide
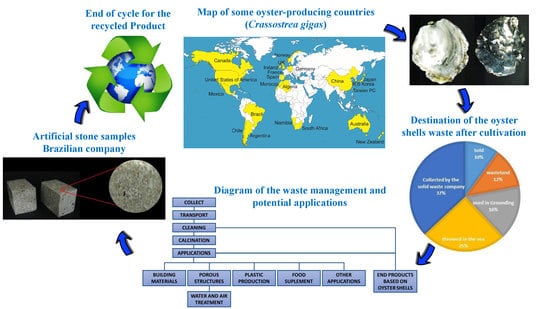
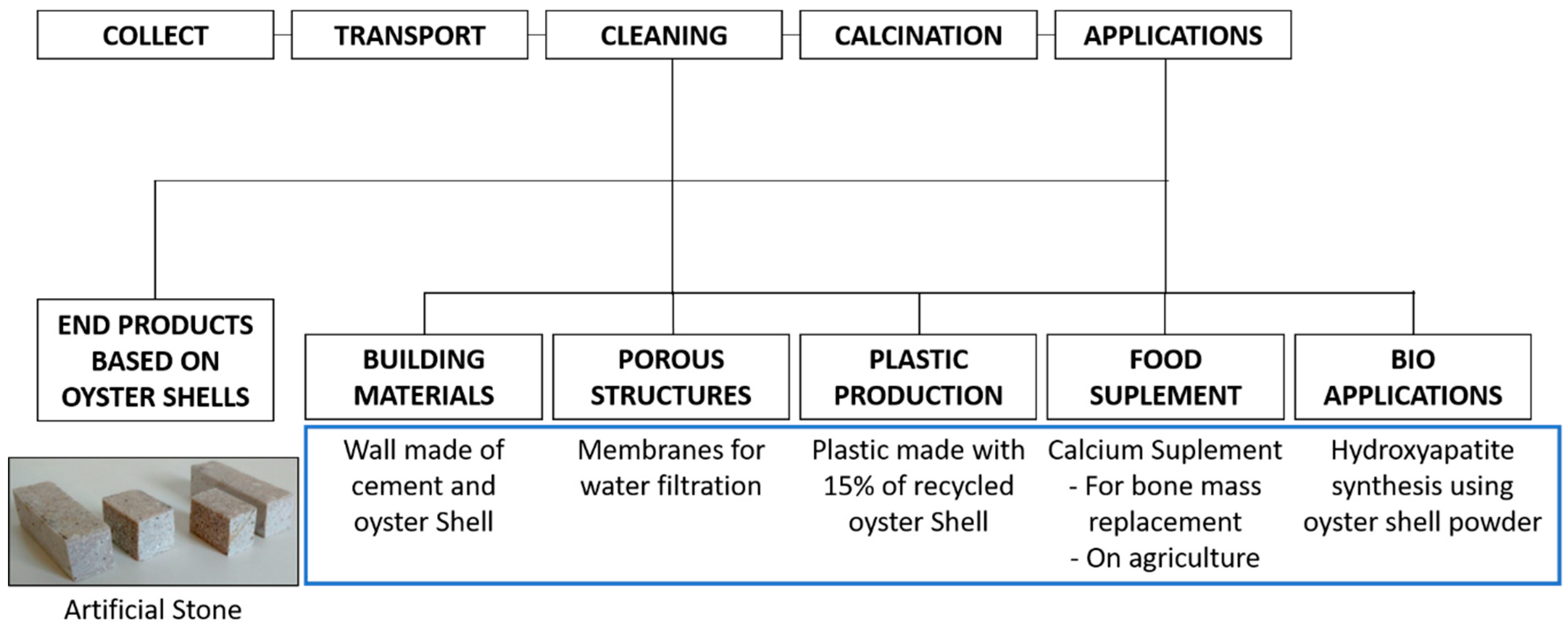
4. Current and Potential Applications for Oyster Shell Wastes
4.1. Calcium Carbonate and Calcium Oxide Source
4.1.1. Building Materials
4.1.2. Water and Air Treatment
4.1.3. Plastic Production
4.1.4. Food Supplement
4.1.5. Other Applications
5. Conclusions
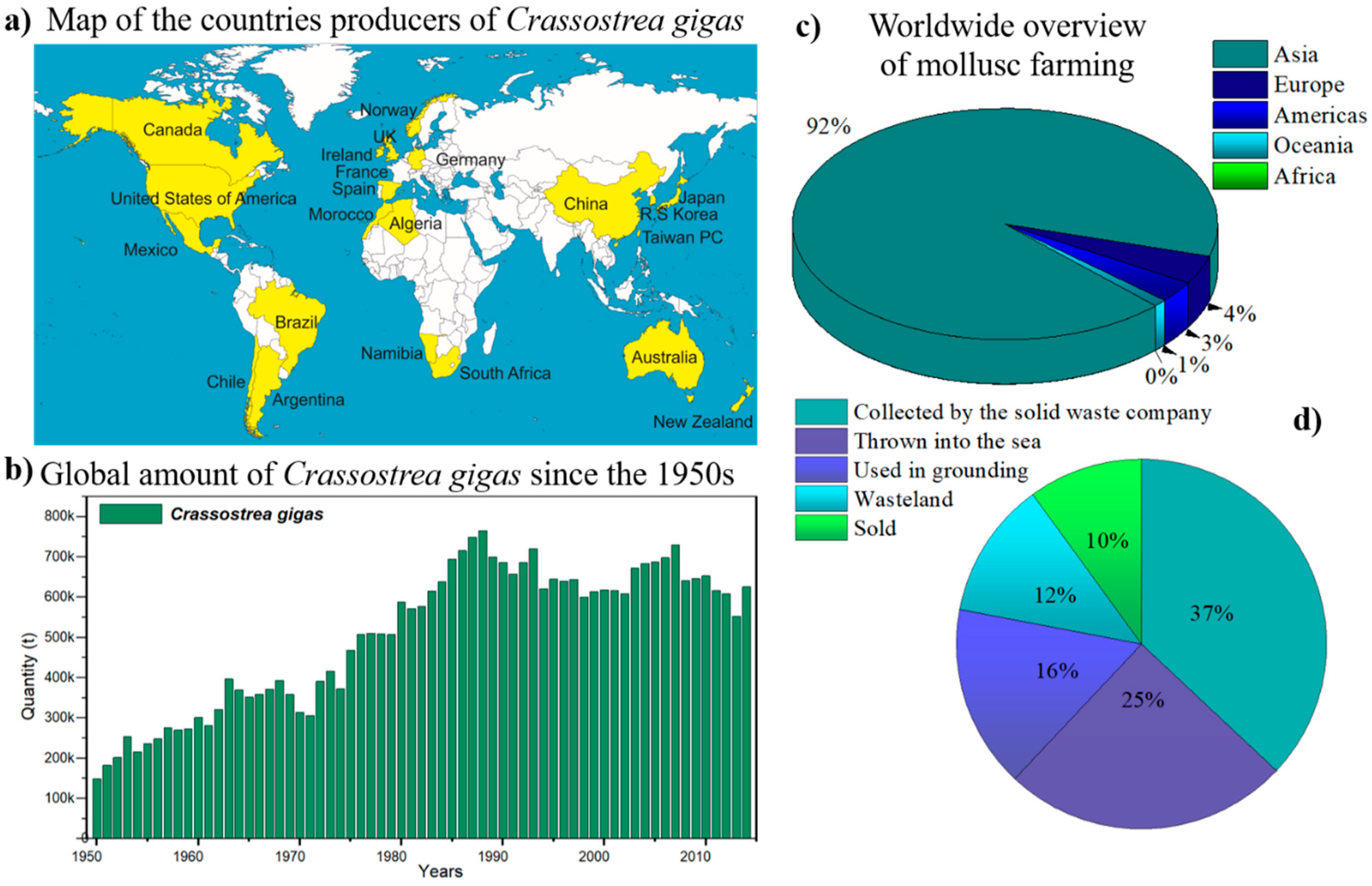
- The production of molluscs, particularly the Pacific oyster (Crassostrea gigas), generates thousands of tons of waste each year.
- The careless disposal of this massive amount of waste impacts the soil, water, and air quality and represents an environmental and public health problem.
- Governments should focus their attention and resources on general efforts to reduce environmental damage and special programs for waste treatment.
- This product can be used for tabletops and workbenches, for home decoration, for laboratory benches, for industrial kitchens, amongst other potential uses.
- In comparing the mechanical results of the new artificial stone with the other natural and artificial stones, like granite, marble, and Aglostone, it is concluded that the new artificial stone exhibits higher mechanical properties. However, other strengthening solutions must be tested in order to position the new artificial stone at the same level of mechanical resistance as Nanoglass and Marmoglass.
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Barros, M.C.; Bello, P.M.; Bao, M.; Torrado, J.J. From waste to commodity: Transforming shells into high purity calcium carbonate. J. Clean. Prod. 2009, 17, 400–407. [Google Scholar] [CrossRef]
- Ruviaro, C.F.; Gianezini, M.; Brandão, F.S.; Winck, C.A.; Dewes, H. Life cycle assessment in Brazilian agriculture facing worldwide trends. J. Clean. Prod. 2012, 28, 9–24. [Google Scholar] [CrossRef]
- Montibeller Filho, G. Maricultura e meio ambiente: A experiência da Escócia como alerta para o Brasil. In Textos de Economia—Maricultura e meio-ambiente; UFSC: Florianopolis, Brazil, 2008; Volume 8, pp. 193–206. (In Portuguese) [Google Scholar]
- De Alvarenga, R.A.F.; Galindro, B.M.; Helpa, C.F.; Soares, S.R. The recycling of oyster shells: An environmental analysis using Life Cycle Assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; ISBN 9789251091852. [Google Scholar]
- Paris, J.M.; Roessler, J.G.; Ferraro, C.C.; DeFord, H.D.; Townsend, T.G. A review of waste products utilized as supplements to Portland cement in concrete. J. Clean. Prod. 2016, 121, 1–18. [Google Scholar] [CrossRef]
- Samuel-Fitwi, B.; Wuertz, S.; Schroeder, J.P.; Schulz, C. Sustainability assessment tools to support aquaculture development. J. Clean. Prod. 2012, 32, 183–192. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2010. [Google Scholar]
- FAO Food and Agriculture Organization of the United Nations, for a World Without Hunger. Available online: http://www.fao.org/fishery/culturedspecies/Crassostrea_gigas/en (accessed on 26 December 2018).
- Petrielli, F.A.S. Viabilidade técnica e econômica da utilização comercial das conchas de ostras descartadas na localidade do Ribeirão da Ilha, Florianópolis, Santa Catarina. 2008. Available online: https://repositorio.ufsc.br/handle/123456789/91343 (accessed on 26 December 2018). (In Portuguese).
- Sandra, E. Shumway Shellfish Aquaculture and the Environment; Shumway, S.E., Ed.; John Wiley & Sons, Inc., Publication: Hoboken, NJ, USA, 2011; ISBN 9780813814131. [Google Scholar]
- Hamester, M.R.R.; Becker, D. Obtenção de carbonato de cálcio a partir de conchas de mariscos. In Proceedings of the 19th Congresso Brasileiro de Engenharia e Ciência dos Materiais—CBECiMat, Campos do Jordão, SP, Brazil, 21–25 November 2010. (In Portuguese). [Google Scholar]
- Yang, E.-I.; Yi, S.-T.; Leem, Y.-M. Effect of oyster shell substituted for fine aggregate on concrete characteristics: Part I. Fundamental properties. Cem. Concr. Res. 2005, 35, 2175–2182. [Google Scholar] [CrossRef]
- Yoon, G.-L.; Kim, B.-T.; Kim, B.-O.; Han, S.-H. Chemical-mechanical characteristics of crushed oyster-shell. Waste Manag. 2003, 23, 825–834. [Google Scholar] [CrossRef]
- Silva, D. Resíduo sólido da malacocultura: Caracterização e potencialidade de utilização de conchas de ostras (Crassostrea gigas) e mexilhão (Perna perna). Available online: https://repositorio.ufsc.br/handle/123456789/90191 (accessed on 26 December 2018). (In Portuguese).
- Santos, A.; Costa, S. Síntese Informativa da Maricultura 2014. Empres. Pesqui. Agropecuária e Extensão Rural St. Catarina (Epagri) 2015, 48, 1–8. (In Portuguese) [Google Scholar]
- Anglostone a. 2018. Available online: http://www.dimagran.com.br/produtos/aglostone.html (accessed on 12 November 2018).
- Anglostone b. 2018. Available online: http://www.alicante.com.br/aglostone-2/especificacoes-tecnicas/ (accessed on 12 November 2018).
- Gomes Ribeiro, C.E.; Sanchez Rodriguez, R.J.; de Carvalho, E.A. Microstructure and mechanical properties of artificial marble. Constr. Build. Mater. 2017, 149, 149–155. [Google Scholar] [CrossRef]
- Adi Kristiawan, S.; Bekti Prakoso, A. Flexural Behaviour of Patch-Repair Material Made from Unsaturated Polyester Resin (UPR)-Mortar. Mater. Sci. Forum 2016, 857, 426–430. [Google Scholar] [CrossRef]
- Nanoglass. 2018. Available online: http://www.countertopresource.com/nano-crystallized-glass-for-countertops/ (accessed on 12 November 2018).
- Marmoglass a. 2018. Available online: https://casaeconstrucao.org/pedras/marmoglass/ (accessed on 12 November 2018).
- Marmoglass b. 2018. Available online: http://www.alicante.com.br/marmoglass-3/sobre-o-marmoglass/ (accessed on 12 November 2018).
- Quinn, J.B.; Quinn, G.D. A practical and systematic review of Weibull statistics for reporting strengths of dental materials. Dent. Mater. 2011, 26, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Lüthy, H.; Filser, F.; Loeffel, O.; Schumacher, M.; Gauckler, L.J.; Hammerle, C.H.F. Strength and reliability of four-unit all-ceramic posterior bridges. Dent. Mater. 2005, 21, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Ozkol, E.; Watjen, A.M.; Bermejo, R.; Deluca, M.; Ebert, J.; Danzer, R.; Telle, R. Mechanical characterisation of miniaturised direct inkjet printed 3Y-TZP specimens for microelectronic applications. J. Eur. Ceram. Soc. 2010, 30, 3145–3152. [Google Scholar] [CrossRef]
- Danzer, R.; Harrer, W.; Supancic, P.; Lube, T.; Wang, Z.; Börger, A. The ball on three balls test—Strength and failure analysis of different materials. J. Eur. Ceram. Soc. 2007, 27, 1481–1485. [Google Scholar] [CrossRef]
- Kakisawa, H.; Sumitomo, T. The toughening mechanism of nacre and structural materials inspired by nacre. Sci. Technol. Adv. Mater. 2011, 12. [Google Scholar] [CrossRef]
- Mahshuri, Y.; Amalina, M.A. Hardness and compressive properties of calcium carbonate derived from clam shell filled unsaturated polyester composites. Mater. Res. Innov. 2014, 18, 291–294. [Google Scholar] [CrossRef]
- Sultana, R.; Akter, R.; Alam, Z. Preparation and characterization of sand reinforced polyester composites. Int. J. Eng. Technol. 2013, 13, 111–118. [Google Scholar]
- Liang, Y.; Zhao, Q.; Li, X.; Zhang, Z.; Ren, L. Study of the microstructure and mechanical properties of white clam shell. Micron 2016, 87, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Beniash, E.; Ivanina, A.; Lieb, N.S.; Kurochkin, I.; Sokolova, I.M. Elevated level of carbon dioxide affects metabolism and shell formation in oysters Crassostrea virginica. Mar. Ecol. Prog. Ser. 2010, 419, 95–108. [Google Scholar] [CrossRef]
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.F.; Bekhit, A.E.A. Marine shells: Potential opportunities for extraction of functional and health-promoting materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116. [Google Scholar] [CrossRef]
- Morris, J.P.; Wang, Y.; Backeljau, T.; Chapelle, G. Biomimetic and bio-inspired uses of mollusc shells. Mar. Genom. 2016, 27, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.B.; Fernandes, V.K.; Maia, B.G.O.; Arcaro, S.; de Oliveira, A.P.N. Vitrocrystalline foams produced from glass and oyster shell wastes. Ceram. Int. 2017, 43, 6730–6737. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Boutouil, M.; Sebaibi, N.; Leleyter, L.; Baraud, F. Valorization of seashell by-products in pervious concrete pavers. Constr. Build. Mater. 2013, 49, 151–160. [Google Scholar] [CrossRef]
- Lee, C.W.; Kwon, H.B.; Jeon, H.P.; Koopman, B. A new recycling material for removing phosphorus from water. J. Clean. Prod. 2009, 17, 683–687. [Google Scholar] [CrossRef]
- Jung, J.; Lee, J.-J.; Lee, G.; Yoo, K.; Sho, B.-H. Reuse of waste shells as a SO2/NOx removal sorbent. In Material Recycling—Trends and Perspectives; InTech: Rijeka, Croatia, 2012; Volume 13, pp. 301–322. ISBN 978-953-51-0327-1. [Google Scholar]
- Souza, R.G.; SantAnna, F.S.P.; Fredel, M.C.; Alarcon, O.E. Emprego das Conchas Residuais da Maricultura na Fabricação de Revestimento Cerâmico Autoclavado. Cerâmica Ind. 2014, 19, 27–30. (In Portuguese) [Google Scholar] [CrossRef]
- Shell Stucco. 2018. Available online: https://carrollsbuildingmaterials.com/landscape-products/florida-shell/how-to-create-tabby-shell-stucco/ (accessed on 12 November 2018).
- Jung, S.; Heo, N.S.; Kim, E.J.; Oh, S.Y.; Lee, H.U.; Kim, I.T.; Hur, J.; Lee, G.-W.; Lee, Y.-C.; Huh, Y.S. Feasibility test of waste oyster shell powder for water treatment. Process Saf. Environ. Prot. 2016, 102, 129–139. [Google Scholar] [CrossRef]
- Tribord. 2018. Available online: https://www.livingcircular.veolia.com/en/industry/decathlon-surfing-recycled-oyster-shells (accessed on 12 November 2018).
- Wu, S.C.; Hsu, H.C.; Hsu, S.K.; Tseng, C.P.; Ho, W.F. Preparation and characterization of hydroxyapatite synthesized from oyster shell powders. Adv. Powder Technol. 2017, 28, 1154–1158. [Google Scholar] [CrossRef]
- Teixeira, L.B.; de Oliveira, A.P.N. Desenvolvimento de Espumas Vítreas Obtidas a Partir de Resíduos; Universidade Federal de Santa Catarina, UFSC: Florianópolis, Brazil, 2016. (In Portuguese) [Google Scholar]
- Bocchese, D. Eliminação da Matéria Orgânica de Conchas de Ostras por Processo Biológico. Master’s Thesis, UFSC, Florianopolis, Brazil, June 2008. (In Portuguese). [Google Scholar]
- Boicko, A.L.; Hotza, D.; Sant’anna, F.S.P. In Utilização de conchas da ostra Crasosotrea Gigas como carga para produtos de policloreto de vinila (PVC). In Proceedings of the Congresso Brasileiro de Ciência e Tecnologia em Resíduos e Desenvolvimento Sustentável, ICTR, Florianópolis, Brazil, 2004; Available online: https://www.ipen.br/biblioteca/cd/ictr/2004/ARQUIVOS%20PDF/14/14-081.pdf (accessed on 26 December 2018). (In Portuguese).
- Souza, R.G.; Alarcon, O.E. Estudo de pozolana autoclavada baseada em óxido de cálcio derivado da concha da ostra Crassostrea gigas; Universidade Federal de Santa Catarina, UFSC: Florianópolis, Brazil, 2008. (In Portuguese) [Google Scholar]
- Chiou, I.J.; Chen, C.H.; Li, Y.H. Using oyster-shell foamed bricks to neutralize the acidity of recycled rainwater. Constr. Build. Mater. 2014, 64, 480–487. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Kuo, W.-T.; Lin, C.-C.; Po-Yo, C. Study of the material properties of fly ash added to oyster cement mortar. Constr. Build. Mater. 2013, 41, 532–537. [Google Scholar] [CrossRef]
- Binag, N.D. Powdered shell wastes as partial substitute for masonry cement mortar in binder, tiles and bricks production. Int. J. Eng. Res. Technol. 2016, 5, 70–77. [Google Scholar]
- Elliott Richardson, A.; Fuller, T. Sea shells used as partial aggregate replacement in concrete. Struct. Surv. 2013, 31, 347–354. [Google Scholar] [CrossRef]
- Chierighini, D.; Bridi, R.; Rocha, A.A.; Lapa, K.R. Possibilidades do Uso das Conchas de Moluscos. In Proceedings of the 3rd International Workshop Advances in Cleaner Production, São Paulo, Brazil, 18–20 May 2011; p. 5. (In Portuguese). [Google Scholar]
- Rodrigues, A. Viabilidade do uso de conchas de mariscos como corretivo de solos. In Proceedings of the III Congresso Brasileiro de Gestão Ambiental, Goiânia, Brazil, 19–22 November 2012; pp. 1–7. (In Portuguese). [Google Scholar]
- Asaoka, S.; Yamamoto, T.; Kondo, S.; Hayakawa, S. Removal of hydrogen sulfide using crushed oyster shell from pore water to remediate organically enriched coastal marine sediments. Bioresour. Technol. 2009, 100, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-B.; Lee, C.-W.; Jun, B.-S.; Yun, J.; Weon, S.-Y.; Koopman, B. Recycling waste oyster shells for eutrophication control. Resour. Conserv. Recycl. 2004, 41, 75–82. [Google Scholar] [CrossRef]
- Fujita, T.; Fukase, M.; Miyamoto, H.; Matsumoto, T.; Ohue, T. Increase of bone mineral density by calcium supplement with oyster shell electrolysate. Bone Miner. 1990, 11, 85–91. [Google Scholar] [CrossRef]
- Chong, M.H.; Chun, B.C.; Chung, Y.-C.; Cho, B.G. Fire-retardant plastic material from oyster-shell powder and recycled polyethylene. J. Appl. Polym. Sci. 2006, 99, 1583–1589. [Google Scholar] [CrossRef]
- Rujitanapanich, S.; Kumpapan, P.; Wanjanoi, P. Synthesis of Hydroxyapatite from Oyster Shell via Precipitation. Energy Procedia 2014, 56, 112–117. [Google Scholar] [CrossRef]
- Xing, R.; Qin, Y.; Guan, X.; Liu, S.; Yu, H.; Li, P. Comparison of antifungal activities of scallop shell, oyster shell and their pyrolyzed products. Egypt. J. Aquat. Res. 2013, 39, 83–90. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Lee, C.W.; Yun, J.H. Development of vermicast from sludge and powdered oyster shell. J. Clean. Prod. 2009, 17, 708–711. [Google Scholar] [CrossRef]
| Technical Analysis | Objectives |
|---|---|
| Scanning Electronic Microscope | Inspection of the oyster shell parts, microstructure of the composite material, and the type of fracture of the artificial stone. |
| Energy dispersive X-ray spectroscopy | Confirm the chemical elements of the sample, as Ca, C, and O, without contaminations. |
| 4-point bending flexural test | Determine the flexural strength of the product samples. Use of the statistical analysis of Weibull modulus on the flexural results. |
| Microhardness test | Determine the hardness of the composite, as well as the hardness of each component. |
| Differential thermal analysis and thermogravimetry | Determine the calcination temperature of the residues and the amount of CaO generated in this process. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
H. Silva, T.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The Potential Use of Oyster Shell Waste in New Value-Added By-Product. Resources 2019, 8, 13. https://doi.org/10.3390/resources8010013
H. Silva T, Mesquita-Guimarães J, Henriques B, Silva FS, Fredel MC. The Potential Use of Oyster Shell Waste in New Value-Added By-Product. Resources. 2019; 8(1):13. https://doi.org/10.3390/resources8010013
Chicago/Turabian StyleH. Silva, Thamyres, Joana Mesquita-Guimarães, Bruno Henriques, Filipe S. Silva, and Márcio C. Fredel. 2019. "The Potential Use of Oyster Shell Waste in New Value-Added By-Product" Resources 8, no. 1: 13. https://doi.org/10.3390/resources8010013
APA StyleH. Silva, T., Mesquita-Guimarães, J., Henriques, B., Silva, F. S., & Fredel, M. C. (2019). The Potential Use of Oyster Shell Waste in New Value-Added By-Product. Resources, 8(1), 13. https://doi.org/10.3390/resources8010013