Aquatic-Derived Biomaterials for a Sustainable Future: A European Opportunity
Abstract
1. Introduction
2. Fish Market and Waste Management: Crustaceans, Cephalopods, and Bivalves
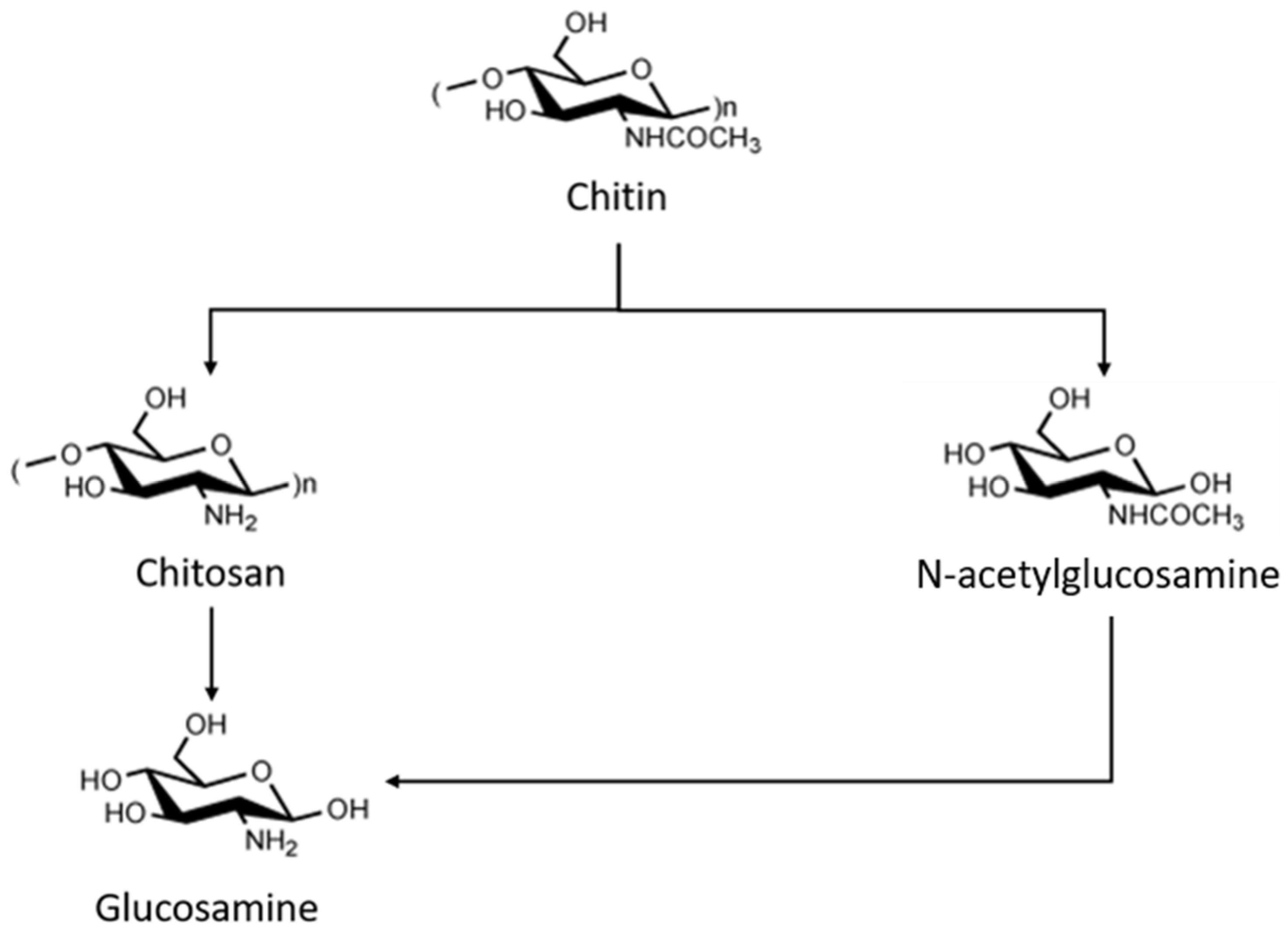
3. The Chitin Industry Case Study
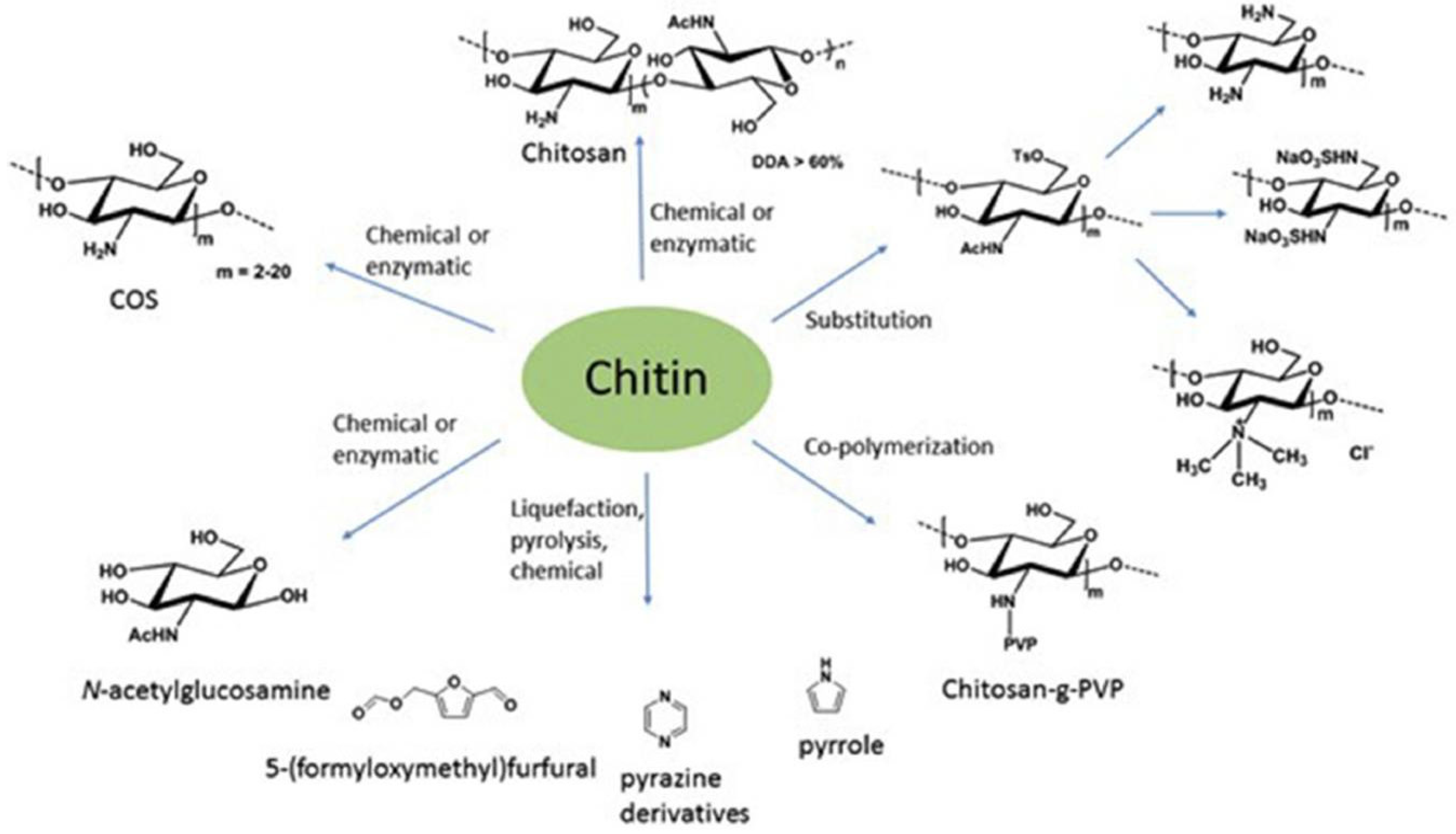
4. Chitin: A European Opportunity
5. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kerton, F.M.; Liu, Y.; Omari, K.W.; Hawboldt, K. Green chemistry and the ocean-based biorefinery. Green Chem. 2013, 15, 860–871. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2012, 12, 539–554. [Google Scholar] [CrossRef]
- Thomsen, M.; Seghetta, M.; Mikkelsen, M.H.; Gyldenkærne, S.; Becker, T.; Caro, D.; Frederiksen, P. Comparative life cycle assessment of biowaste to resource management systems—A Danish case study. J. Clean. Prod. 2017, 142, 4050–4058. [Google Scholar] [CrossRef]
- Lopes, C.; Antelo, L.T.; Franco-Uria, A.; Alonso, A.A.; Perez-Martin, R. Valorisation of fish by-products against waste management treatments—Comparison of environmental impacts. Waste Manag. 2015, 46, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Clean. Prod. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- Ciuta, S.; Antognoni, S.; Rada, E.C.; Ragazzi, M.; Badea, A.; Cioca, L.I. Respirometric index and biogas potential of different foods and agricultural discarded biomass. Sustainability 2016, 8, 1311. [Google Scholar] [CrossRef]
- Rivero, C.P.; Hu, Y.; Kwan, T.H.; Webb, C.; Theodoropoulos, C.; Daoud, W.; Lin, C.S.K. 1-Bioplastics from solid waste. In Current Developments in Biotechnology and Bioengineering, Solid Waste Management, 1st ed.; Wong, J.W.C., Tyagi, R.D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–26. [Google Scholar]
- Brockhaus, S.; Petersen, M.; Kersten, W. A crossroads for bioplastics: Exploring product developers’ challenges to move beyond petroleum-based plastics. J. Clean. Prod. 2016, 127, 84–95. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Remzi Becer, C. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Nisticò, R.; Evon, P.; Labonne, L.; Vaca-Medina, G.; Montoneri, E.; Vaca-Garcia, C.; Negre, M. Post-harvest tomato plants and urban food wastes for manufacturing plastic films. J. Clean. Prod. 2017, 167, 68–74. [Google Scholar] [CrossRef]
- Zia, K.M.; Noreen, A.; Zuber, M.; Tabasum, S.; Mujahid, M. Recent developments and future prospects on bio-based polyesters derived from renewable resources: A review. Int. J. Biol. Macromol. 2016, 82, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Producing PHAs in the bioeconomy—Towards a sustainable bioplastics. Sustain. Prod. Consum. 2017, 9, 58–70. [Google Scholar] [CrossRef]
- Franzoso, F.; Vaca-Garcia, C.; Rouilly, A.; Evon, P.; Montoneri, E.; Persico, P.; Mendichi, R.; Nisticò, R.; Francavilla, M. Extruded versus solvent cast blends of poly(vinyl alcohol-co-ethylene) and biopolymers isolated from municipal biowaste. J. Appl. Polym. Sci. 2016, 133, 43009–43025. [Google Scholar] [CrossRef]
- Nisticò, R.; Evon, P.; Labonne, L.; Vaca-Medina, G.; Montoneri, E.; Francavilla, M.; Vaca-Garcia, C.; Magnacca, G.; Franzoso, F.; Negre, M. Extruded poly(ethylene–co–vinyl alcohol) composite films containing biopolymers isolated from municipal biowaste. Chem. Sel. 2016, 1, 2354–2365. [Google Scholar] [CrossRef]
- Wang, C.; Kelley, S.S.; Venditti, R.A. Lignin-based thermoplastic materials. ChemSusChem 2016, 9, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Bastioli, C.; Bellotti, V.; Del Giudice, L.; Gilli, G. Mater-Bi: Properties and biodegradability. J. Environ. Polym. Degrad. 1993, 1, 181–191. [Google Scholar] [CrossRef]
- Bastioli, C. Properties and applications of Mater-Bi starch-based materials. Polym. Degrad. Stab. 1998, 59, 263–272. [Google Scholar] [CrossRef]
- Borchani, K.E.; Carrot, C.; Jaziri, M. Biocomposites of Alfa fibers dispersed in the Mater-Bi® type bioplastic: Morphology, mechanical and thermal properties. Compos. Part A Appl. Sci. Manuf. 2015, 78, 371–379. [Google Scholar] [CrossRef]
- Lopez, J.P.; Vilaseca, F.; Barberà, L.; Bayer, R.J.; Pelach, M.A.; Mutjé, P. Processing and properties of biodegradable composites based on Mater-Bi® and hemp core fibres. Resour. Conserv. Recycl. 2012, 59, 38–42. [Google Scholar] [CrossRef]
- Chen, Z.; Wan, C. Biological valorization strategies for converting lignin into fuels and chemicals. Renew. Sust. Energ. Rev. 2017, 73, 610–621. [Google Scholar] [CrossRef]
- Chen, P.; Xie, Q.; Addy, M.; Zhou, W.; Liu, Y.; Wang, Y.; Cheng, Y.; Li, K.; Ruan, R. Utilization of municipal solid and liquid wastes for bioenergy and bioproducts production. Bioresour. Technol. 2016, 215, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Dahunsi, S.O.; Oranusi, S.; Efeovbokhan, V.E. Cleaner energy for cleaner production: Modeling and optimization of biogas generation from Carica papayas (Pawpaw) fruit peels. J. Clean. Prod. 2017, 156, 19–29. [Google Scholar] [CrossRef]
- Senghor, A.; Dioh, R.M.N.; Müller, C.; Yourn, I. Cereal crops for biogas production: A review of possible impact of elevated CO2. Renew. Sust. Energ. Rev. 2017, 71, 548–554. [Google Scholar] [CrossRef]
- Yu, K.L.; Show, P.L.; Ong, H.C.; Ling, T.C.; Lan, J.C.-W.; Chen, W.-H.; Chang, J.-S. Microalgae from wasyewater treatment to biochar—Feedstock preparation and conversion technologies. Energ. Convers. Manag. 2017, 150, 1–13. [Google Scholar] [CrossRef]
- Jung, S.-J.; Kim, S.-H.; Chung, I.-M. Comparison of lignin, cellulose, and hemicelluloses contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenergy 2015, 83, 322–327. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Zanariah Ngah, C.W. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sust. Energ. Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Schmidt, L.M.; Mthembu, L.D.; Reddy, P.; Deenadayalu, N.; Kaltschmitt, M.; Smirnova, I. Levulinic acid production integrated into a sugarcane bagasse based biorefiney using thermal-enzymatic pretreatment. Ind. Crops Prod. 2017, 99, 172–178. [Google Scholar] [CrossRef]
- Beauchet, R.; Monteul-Rivera, F.; Lavoie, J.M. Conversion of lignin to aromatic-based chemicals (L-chems) and biofuels (L-fuels). Bioresour. Technol. 2012, 121, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.; Cruz, I.B.; Jorge, R.F.; Malcata, F.X.; Pintado, M.E.; Castro, P.M.L. Valorisation of natural extracts from marine source focused on marine by-products: A review. Food Res. Int. 2010, 43, 2221–2233. [Google Scholar] [CrossRef]
- Biodegradable waste. Available online: http://ec.europa.eu/environment/waste/compost/index.htm (accessed on 11 October 2017).
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Avetta, P.; Nisticò, R.; Faga, M.G.; D’Angelo, D.; Aimo Boot, E.; Lamberti, R.; Martorana, S.; Calza, P.; Fabbri, D.; Magnacca, G. Hernia-repair prosthetic devices functionalised with chitosan and ciprofloxacin coating: Controlled release and antibacterial activity. J. Mater. Chem. B 2014, 2, 5287–5294. [Google Scholar] [CrossRef]
- Nagaoka, I.; Igarashi, M.; Hua, J.; Ju, Y.; Yomogida, S.; Sakamoto, K. Recent aspects of the anti-inflammatory actions of glucosamine. Carbohydr. Polym. 2011, 84, 825–830. [Google Scholar] [CrossRef]
- Waste. Review of Waste policy and Legislation. Available online: http://ec.europa.eu/environment/waste/target_review.htm (accessed on 11 October 2017).
- European Market Observatory for Fisheries and Aquaculture Products (EUMOFA), European Union. The EU Fish Market, 2016 ed.; Directorate-General for Maritime Affairs and Fisheries of the European Commission: Brussels, Belgium, 2016; ISBN 978-92-79-69443-1. [Google Scholar] [CrossRef]
- European Commissioner for Environment, Maritime Affairs and Fisheries, European Union. Facts and Figures on the Common Fisheries Policy, Basic Statistical Data, 2016 ed.; Publications Office of the European Union: Luxembourg City, Luxembourg, 2016; ISBN 978-92-79-60972-5. [Google Scholar] [CrossRef]
- Eumofa.eu. Ad Hoc Queries. Available online: https://www.eumofa.eu/it/ad-hoc-queries1 (accessed on 2 May 2017).
- Seafish.org. Crustacea Processing Waste Management. Available online: http://www.seafish.org/b2b/info.asp?p=102 (accessed on 29 April 2017).
- O’Keefe, D.M.; Owens, J.M.; Chynoweth, D.P. Anaerobic composting of crab-picking wastes for byproduct recovery. Bioresour. Technol. 1996, 58, 265–272. [Google Scholar] [CrossRef]
- Hu, Z.; Lane, R.; Wen, Z. Composting clam processing wastes in a laboratory scale in-vessel system. Waste Manag. 2009, 29, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, O. Glucosamine derivatives sulfo disaccharides co-working with Klotho. J. Nutr. Food Sci. 2015, 5, 416. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Al Sagheer, F.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitin, 1st ed.; Pergamon Press Ltd.: Oxford, MS, USA, 1977; ISBN 9781483159461. [Google Scholar]
- Signini, R.; Campana-Filho, S.P. On the preparation and characterization of chitosan hydrochloride. Polym. Bull. 1999, 42, 159–166. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of the degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Saito, Y.; Putaux, J.-L.; Okano, T.; Gaill, F.; Chanzy, H. Structural aspects of the swelling of β-chitin in HCl and its conversion into α-chitin. Macromolecules 1997, 30, 3867–3873. [Google Scholar] [CrossRef]
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R. Magnetic materials and water treatments for a sustainable future. Res. Chem. Intermed. 2017, 43, 6911–6949. [Google Scholar] [CrossRef]
- Nisticò, R.; Franzoso, F.; Cesano, F.; Scarano, D.; Magnacca, G.; Parolo, M.E.; Carlos, L. Chitosan-derived iron oxide systems for magnetically guided and efficient water purification processes from polycyclic aromatic hydrocarbons. ACS Sustain. Chem. Eng. 2017, 5, 793–801. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Nisticò, R.; Celi, L.R.; Bianco Prevot, A.; Carlos, L.; Magnacca, G.; Zanzo, E.; Martin, M. Sustainable magnet-responsive nanomaterials for the removal of arsenic from contaminated water. J. Hazard. Mater. 2018, 342, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Falcon, A.B.; Cabrera, J.C.; Costales, D.; Ramírez, M.A.; Cabrera, G.; Toledo, V.; Martinez-Tellez, M.A. The effect of size and acetylation degree of chitosan derivatives on tobacco plant protection against Phytophthora parasitica nicotianae. World J. Microbiol. Biotechnol. 2008, 24, 103–112. [Google Scholar] [CrossRef]
- Fernandez, J.G.; Ingber, D.E. Manufacturing of large-scale functional objects using biodegradable chitosan bioplastic. Macromol. Mater. Eng. 2014, 299, 932–938. [Google Scholar] [CrossRef]
- Fernando, L.A.T.; Poblete, M.R.S.; Ongkiko, A.G.M.; Diaz, L.J.L. Chitin extraction and synthesis of chitin-based polymer films from Philippine Blue Swimming Crab (Portunus pelagicus) shells. Procedia Chem. 2016, 19, 462–468. [Google Scholar] [CrossRef]
- Magnacca, G.; Guerretta, F.; Vizintin, A.; Benzi, P.; Valsania, M.C.; Nisticò, R. Preparation, characterization and environmental/electrochemical energy storage testing of low-cost biochar from natural chitin obtained via pyrolysis at mild conditions. Appl. Surf. Sci. 2018, 427, 883–893. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Bonfaroni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Caramella, C. Chitosan and its salts for mucosal and transmucosal delivery. Expert Opin. Drug Deliv. 2009, 6, 923–939. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R.; Faga, M.G.; Gautier, G.; Magnacca, G.; D’Angelo, D.; Ciancio, E.; Piacenza, G.; Lamberti, R.; Martorana, S. Physico-chemical characterization of functionalized polypropylenic fibers for prosthetic applications. Appl. Surf. Sci. 2012, 258, 7889–7896. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, S.-B.; Wang, Y.-G.; Zhang, S.-H.; Yu, Z.-F.; Tang, T.-T. Bacterial inhibition potential of quaternised chitosan-coated VICRYL absorbable suture: An in vitro and in vivo study. J. Orthop. Transl. 2017, 8, 49–61. [Google Scholar] [CrossRef]
- Alay, E.; Duran, K.; Korlu, A. A sample of work on green manufacturing in textile industry. Sustain. Chem. Pharm. 2016, 3, 39–46. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Montemayor, M.I.; Fraguas, J.; del Pilar González, M.; Anxo Murado, M. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef] [PubMed]
- Khanafari, A.; Marandi, R.; Sanatei, S. Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. Iran. J. Environ. Health Sci. Eng. 2008, 5, 1–24. [Google Scholar]
- Bustos, R.O.; Healy, M.G. Microbial deproteinization of waste prawn shell. In Proceedings of the Second International Symposium on Environmental Biotechnology, Brighton, UK, 4–6 July 1994; pp. 15–25. [Google Scholar]
- Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Kurita, K.; Sannan, T.; Iwakura, Y. Studies on chitin, 4: Evidence for formation of block and random copolymers of N-acetyl-D-glucosamine and D-glucosamine by hetero- and homogeneous hydrolyses. Makromol. Chem. 1977, 178, 3197–3202. [Google Scholar] [CrossRef]
- Chang, K.L.B.; Tsai, G.; Lee, J.; Fu, W.R. Heterogeneous N-deacetylation of chitin in alkaline solution. Carbohydr. Res. 1977, 303, 327–332. [Google Scholar] [CrossRef]
- Sannan, T.; Kurita, K.; Iwakura, Y. Studies on chitin, 2. Effect of deacetylation on solubility. Makromol. Chem. 1976, 177, 3589–3600. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Sandanamsamy, S.; Kamaruzaman, S.F.S.; Ahmad, F. Chitosan extracted from mud crab (Scylla olivicea) shells: Physicochemical and antioxidant properties. J. Food Sci. Technol. 2015, 52, 4266–4275. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.Y.; Man, Y.B.; Wong, M.H. Use of food waste, fish waste and food processing waste for China’s aquaculture industry: Needs and challenge. Sci. Total Environ. 2018, 613–614, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.; Camara, A.; Piqué, R.; Dioh, E.; Guèye, M.; Diadhiou, H.D.; Faye, M.; Carré, M. Shellfishing and shell midden construction in the Saloum Delta, Senegal. J. Anthropol. Archaeol. 2016, 41, 19–32. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Z.; Li, X.; Xu, H.; Li, X. Recycling of shell wastes into nanosized calcium carbonate powders with different phase compositions. J. Clean. Prod. 2015, 92, 223–229. [Google Scholar] [CrossRef]
- Rudnik, E. Compostable polymer materials: Definitions, structures, and methods of preparation. In Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications; Ebnesajja, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 213–264. ISBN 9781455728343. [Google Scholar]
- Fiori, L.; Volpe, M.; Lucian, M.; Anesi, A.; Manfrini, M.; Guella, G. From fish waste to omega-3 concentrates in a biorefinery concept. Waste Biomass Valor. 2017, 8, 2609–2620. [Google Scholar] [CrossRef]
- Yoon, J.H. Enzymatic synthesis of chitooligosaccharides in organic cosolvents. Enzyme Microb. Technol. 2005, 37, 663–668. [Google Scholar] [CrossRef]
- Sashiwa, H.; Fujishima, S.; Yamano, N.; Kawasaki, N.; Nakayama, A.; Muraki, E.; Aiba, S.-I. Production of N-acetyl-D-glucosamine from β-chitin by enzymatic hydrolysis. Chem. Lett. 2001, 31, 308–309. [Google Scholar] [CrossRef]
- Choi, C.; Nam, J.-P.; Nah, J.-W. Application of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Jardine, A.; Sayed, S. Challenges in the valorization of chitinous biomass within the biorefinery concept. Curr. Opin. Green Sustain. Chem. 2016, 2, 34–39. [Google Scholar] [CrossRef]
- Gao, X.; Chen, X.; Zhang, J.; Guo, W.; Jin, F.; Yan, N. Transformation of chitin and waste shrimp shells into acetic acid and pyrrole. ACS Sustain. Chem. Eng. 2016, 4, 3912–3920. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, N. Formic acid-mediated liquefaction of chitin. Green Chem. 2016, 18, 5050–5058. [Google Scholar] [CrossRef]
- Chen, X.; Chew, S.L.; Kerton, F.M.; Yan, N. Direct conversion of chitin into a N-containing furan derivative. Green Chem. 2014, 16, 2204–2212. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Kerton, F.M.; Yan, N. Conversion of chitin and N-acetyl-D-glucosamine into a N-containing furan derivative in ionic liquids. RSC Adv. 2015, 5, 20073–20080. [Google Scholar] [CrossRef]
- Omari, K.W.; Besaw, J.E.; Kerton, F.M. Hydrolysis of chitosan to yield levulinic acid and 5-hydroxymethylfurfural in water under microwave irradiation. Green Chem. 2012, 14, 1480–1487. [Google Scholar] [CrossRef]
- Deepthi, S.; Venatesan, J.; Kim, S.-K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93B, 1338–1353. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, H.; Chen, J.; Li, S.; Huang, J.; Zhou, C. Chitosan-chitin nanocrystal composite scaffolds for tissue engineering. Carbohydr. Polym. 2016, 152, 832–840. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, W.-R.; Zhang, Y.; Li, W.; Hu, J.; Zheng, F.; Wu, Y. Liquefied chitin/polyvinyl alcohol based blend membranes: Preparation and characterization and antibacterial activity. Carbohydr. Polym. 2018, 180, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Chitin and Chitosan Derivatives Market Trends. Available online: http://www.strategyr.com/MarketResearch/Chitin_and_Chitosan_Derivatives_Market_Trends.asp (accessed on 12 October 2017).

| EU Countries | Cephalopods | Bivalves | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cuttlefish (Sepia) | Octopus (Octopus) | Squid (Loligo) | other Cephalopods | Clam (Chamelea) | Scallop (Pecten) | Mussel (Mytilus) | Oyster (Ostrea) | other Mussels | |
| Austria | 326.50 | 252.90 | 804.10 | 459.00 | 78.10 | 140.50 | 458.10 | 125.80 | 465.00 |
| Belgium | 4039.52 | 1903.04 | 6874.84 | 2141.20 | 1066.50 | 7621.05 | 24,229.30 | 1981.80 | 3979.90 |
| Bulgaria | 21.60 | 165.40 | 482.60 | 110.40 | 551.70 | 75.50 | 661.60 | 10.80 | 157.10 |
| Croatia | 491.70 | 513.80 | 9403.50 | 294.40 | 65.80 | 242.80 | 263.70 | 389.40 | 4.00 |
| Cyprus | 159.10 | 1049.60 | 1479.60 | 45.80 | 7.70 | 15.90 | 142.50 | 50.70 | 184.80 |
| Czech Rep. | 60.00 | 274.60 | 270.30 | 81.60 | 30.00 | 66.80 | 197.40 | 79.40 | 73.20 |
| Denmark | 253.40 | 821.20 | 1593.60 | 2308.03 | 739.44 | 2323.15 | 44,916.67 | 376.34 | 8081.50 |
| Estonia | 6.40 | 31.30 | 113.00 | 319.70 | 2.50 | 23.50 | 44.80 | 5.90 | 93.10 |
| Finland | 8.20 | 26.50 | 66.60 | 140.50 | 6.50 | 40.20 | 170.70 | 23.00 | 81.50 |
| France | 19,871.01 | 6078.84 | 25,254.52 | 13,563.03 | 10,130.50 | 33,630.63 | 46,808.53 | 19,034.92 | 15,153.20 |
| Germany | 1980.60 | 3107.20 | 9078.43 | 6772.60 | 742.46 | 2430.20 | 36,001.00 | 826.90 | 4461.90 |
| Greece | 1396.70 | 7206.00 | 20,552.40 | 530.70 | 3567.10 | 54.50 | 11,907.80 | 148.30 | 1327.10 |
| Hungary | 27.70 | 34.50 | 168.70 | 70.80 | 58.00 | 14.20 | 323.20 | 10.10 | 44.20 |
| Ireland | 207.94 | 24.18 | 658.98 | 33.00 | 2626.31 | 2336.88 | 10,428.30 | 7592.60 | 167.50 |
| Italy | 24,058.39 | 63,884.13 | 10,1342.40 | 4723.30 | 19,315.10 | 8248.27 | 46,364.11 | 6368.30 | 11,612.40 |
| Latvia | 4.70 | 54.90 | 181.90 | 151.10 | 3.20 | 27.10 | 244.60 | 67.90 | 76.80 |
| Lithuania | 16.50 | 52.40 | 772.70 | 428.10 | 41.60 | 9.10 | 112.60 | 22.50 | 62.80 |
| Luxembourg | 35.10 | 156.00 | 276.30 | 31.70 | 83.60 | 519.20 | 760.00 | 144.80 | 24.90 |
| Malta | 61.90 | 289.10 | 374.80 | 83.80 | 77.40 | 4.80 | 120.30 | 8.50 | 100.70 |
| Netherlands | 2053.70 | 1836.00 | 11,303.70 | 647.80 | 9017.70 | 9804.00 | 79,811.50 | 3022.20 | 5009.40 |
| Poland | 66.00 | 410.20 | 337.50 | 894.40 | 183.90 | 263.70 | 340.00 | 72.20 | 433.80 |
| Portugal | 6210.88 | 47,626.83 | 35,539.20 | 2187.90 | 23,877.50 | 334.84 | 4789.46 | 618.53 | 3599.10 |
| Romania | 106.50 | 243.40 | 452.40 | 517.10 | 41.90 | 154.60 | 255.10 | 22.50 | 136.40 |
| Slovakia | 11.00 | 11.80 | 30.40 | 16.10 | 16.70 | 0.00 | 46.20 | 0.00 | 8.90 |
| Slovenia | 79.20 | 417.60 | 4580.30 | 138.50 | 32.10 | 77.20 | 165.40 | 11.40 | 218.80 |
| Spain | 46,058.15 | 105,421.65 | 232,400.60 | 21,039.85 | 48,100.90 | 8772.11 | 52,654.28 | 4996.70 | 19,424.00 |
| Sweden | 86.44 | 89.23 | 364.25 | 79.05 | 178.70 | 715.82 | 770.00 | 331.40 | 858.80 |
| UK | 10,810.69 | 1663.77 | 10,436.04 | 2503.30 | 7419.30 | 49,297.37 | 3911.52 | 2020.19 | 4212.90 |
| EU-28 | 118,509.53 | 243,646.07 | 475,193.67 | 60,312.76 | 128,062.21 | 127,243.91 | 366,898.67 | 48,363.07 | 80,053.70 |
| EU Countries | Crustaceans | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| European Lobster (Homarus) | Norway Lobster (Nephrops) | Red Lobster (Palinurus) | European Crayfish (Astacus) | King/Striped Prawn (Aristaeomorpha/Penaeus) | Brown Crab (Cancer) | Common Shrimp (Cragon) | other Shrimps | other Crustaceans | |
| Austria | 58.70 | 27.80 | 75.10 | 28.60 | 1528.90 | 162.10 | 425.40 | 4232.00 | 95.50 |
| Belgium | 4708.83 | 1326.39 | 2476.60 | 685.80 | 43,419.20 | 4343.36 | 4061.26 | 38,863.30 | 1646.75 |
| Bulgaria | 6.90 | 0.10 | 39.00 | 0.60 | 150.20 | 72.60 | 18.80 | 5072.20 | 3.30 |
| Croatia | 21.80 | 618.90 | 110.80 | n.d. | 162.60 | 13.00 | 11.00 | 843.00 | 54.40 |
| Cyprus | 20.30 | 1.80 | 11.40 | 1.10 | 938.20 | 36.40 | 0.20 | 399.60 | 9.20 |
| Czech Rep. | 16.90 | 8.80 | 2.90 | 7.40 | 1177.10 | 23.80 | 10.50 | 1028.70 | 174.30 |
| Denmark | 511.20 | 7906.02 | 110.20 | 8.23 | 8368.00 | 3582.66 | 3735.48 | 138,614.75 | 6139.00 |
| Estonia | 7.90 | n.d. | 32.60 | 0.30 | 179.90 | 13.30 | 6.10 | 6163.30 | 14.00 |
| Finland | 33.90 | 2.20 | 57.60 | 39.50 | 267.80 | 64.50 | 26.00 | 2043.40 | 234.10 |
| France | 7195.34 | 10,411.29 | 3795.68 | 855.60 | 86,436.32 | 21,205.36 | 2896.47 | 32,947.31 | 2626.93 |
| Germany | 1302.10 | 492.81 | 280.40 | 302.90 | 33,181.90 | 3042.54 | 17,310.56 | 32,613.30 | 1075.60 |
| Greece | 160.90 | 33.10 | 206.40 | 8.30 | 2623.60 | 1046.50 | 175.70 | 6532.30 | 326.90 |
| Hungary | 6.60 | 5.40 | 34.50 | 2.20 | 236.20 | 42.50 | 0.40 | 356.10 | 25.40 |
| Ireland | 1074.44 | 11,167.50 | 617.74 | 5.60 | 751.90 | 255,462.28 | 366.53 | 4759.93 | 2047.90 |
| Italy | 4481.49 | 10,223.54 | 1242.90 | 6154.30 | 33,482.08 | 4161.79 | 392.46 | 41,823.06 | 2610.00 |
| Latvia | 2.10 | 2.10 | 11.80 | 0.10 | 382.90 | 17.90 | 7.80 | 1740.30 | 35.70 |
| Lithuania | 3.80 | 1.20 | 71.50 | 1.00 | 176.60 | 296.40 | 0.30 | 1298.70 | 46.20 |
| Luxembourg | 102.20 | 48.70 | 12.10 | 14.90 | 230.70 | 79.10 | 260.50 | 925.20 | 276.90 |
| Malta | 11.00 | 0.40 | 2.70 | n.d. | 162.00 | 5.40 | 0.10 | 347.30 | 7.50 |
| Netherlands | 1951.60 | 3949.20 | 732.70 | 494.70 | 46,250.40 | 6887.50 | 31,297.90 | 60,528.40 | 5171.80 |
| Poland | 15.10 | 2.10 | 11.20 | 11.40 | 1865.40 | 81.20 | 617.60 | 5194.90 | 54.20 |
| Portugal | 204.17 | 577.34 | 950.62 | 49.10 | 15,896.01 | 4392.39 | 42.90 | 25,022.90 | 1555.26 |
| Romania | 15.50 | 4.50 | 7.50 | 109.50 | 487.70 | 10.40 | 2.10 | 793.00 | 3.90 |
| Slovakia | 1.00 | 0.10 | 0.80 | 0.00 | 35.30 | 3.50 | 1.00 | 130.40 | 0.70 |
| Slovenia | 20.50 | 168.20 | 10.60 | 6.60 | 90.40 | 4.50 | 2.30 | 453.90 | 61.70 |
| Spain | 5139.42 | 7086.95 | 4249.79 | 1997.30 | 102,764.99 | 14,562.77 | 763.40 | 108,968.87 | 6879.25 |
| Sweden | 686.53 | 1222.40 | 355.40 | 323.60 | 1884.00 | 1156.96 | 191.80 | 30,564.16 | 3204.70 |
| UK | 8891.67 | 40,729.50 | 1034.34 | 760.30 | 33,911.21 | 45,273.32 | 2367.55 | 57,478.61 | 2183.80 |
| EU-28 | 36,651.88 | 96,018.33 | 16,544.86 | 11,868.93 | 417,041.50 | 366,044.02 | 64,992.11 | 609,738.89 | 36,564.90 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisticò, R. Aquatic-Derived Biomaterials for a Sustainable Future: A European Opportunity. Resources 2017, 6, 65. https://doi.org/10.3390/resources6040065
Nisticò R. Aquatic-Derived Biomaterials for a Sustainable Future: A European Opportunity. Resources. 2017; 6(4):65. https://doi.org/10.3390/resources6040065
Chicago/Turabian StyleNisticò, Roberto. 2017. "Aquatic-Derived Biomaterials for a Sustainable Future: A European Opportunity" Resources 6, no. 4: 65. https://doi.org/10.3390/resources6040065
APA StyleNisticò, R. (2017). Aquatic-Derived Biomaterials for a Sustainable Future: A European Opportunity. Resources, 6(4), 65. https://doi.org/10.3390/resources6040065





