Polymer-Driven Fuel Conditioning: A Novel Approach to Improving the Stability and Environmental Performance of Marine Fuels
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Moisture Reduction Procedure Results
3.2. Thermographic Analysis Results
3.2.1. TGA Thermographic Analysis Results
3.2.2. DSC Analysis Results
4. Conclusions—Future Research
- Resource efficiency—achieving moisture reduction under ambient conditions with minimal energy input compared to heat-based drying.
- Compliance and governance—supporting adherence to international and EU fuel-quality and emission standards.
- Economic benefits—reducing corrosion, maintenance costs, and service interruptions across the fuel supply chain.
- Circularity and life cycle performance—enabling polymer reuse and integration with existing filtration infrastructure.
- System resilience—stabilizing biodiesel blends and maintaining fuel integrity during storage and transport.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Bashir, H.; Bibi, I.; Jafar, A.; Niazi, N.K.; Rasheed, F.; Ghafoor, N.; Saleem, A.; Hussain, M.M.; Farooqi, Z.U.R. Role of Biofuels in Building Circular Bioeconomy. In Biofuels in Circular Economy; Springer Nature: Singapore, 2023; pp. 59–71. [Google Scholar]
- Fazal, M.; Haseeb, A.; Masjuki, H. Biodiesel feasibility study: An evaluation of material compatibility; performance; emission and engine durability. Renew. Sustain. Energy Rev. 2011, 15, 1314–1324. [Google Scholar] [CrossRef]
- Gui, M.; Lee, K.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Lamarque, J.-F.; Bond, T.C.; Eyring, V.; Granier, C.; Heil, A.; Klimont, Z.; Lee, D.; Liousse, C.; Mieville, A.; Owen, B.; et al. Historical (1850–2000) gridded anthropogenic and biomass burning emissions of reactive gases and aerosols: Methodology and application. Atmos. Chem. Phys. 2010, 10, 7017–7039. [Google Scholar] [CrossRef]
- Fontaras, G.; Skoulou, V.; Zanakis, G.; Zabaniotou, A.; Samaras, Z. Integrated environmental assessment of energy crops for biofuel and energy production in Greece. Renew. Energy 2012, 43, 201–209. [Google Scholar] [CrossRef]
- Zafeiriou, E.; Mallidis, I.; Galanopoulos, K.; Arabatzis, G. Greenhouse gas emissions and economic performance in EU agriculture: An empirical study in a non-linear framework. Sustainability 2018, 10, 3837. [Google Scholar] [CrossRef]
- Zafeiriou, E.; Arabatzis, G.; Tampakis, S.; Soutsas, K. The impact of energy prices on the volatility of ethanol prices and the role of gasoline emissions. Renew. Sustain. Energy Rev. 2014, 33, 87–95. [Google Scholar] [CrossRef]
- Drosos, D.; Skordoulis, M.; Arabatzis, G.; Tsotsolas, N.; Galatsidas, S. measuring industrial customer satisfaction: The case of the natural gas market in greece. Sustainability 2019, 11, 1905. [Google Scholar] [CrossRef]
- Tampakis, S.; Arabatzis, G.; Tsantopoulos, G.; Rerras, I. Citizens’ views on electricity use, savings and production from renewable energy sources: A case study from a Greek island. Renew. Sustain. Energy Rev. 2017, 79, 39–49. [Google Scholar] [CrossRef]
- Giannarakis, G.; Zafeiriou, E.; Arabatzis, G.; Partalidou, X. Determinants of corporate climate change disclosure for European firms. Corp. Soc. Responsib. Environ. Manag. 2018, 25, 281–294. [Google Scholar] [CrossRef]
- International Maritime Organization (IMO). IMO 2020—Sulphur Cap for Marine Fuels. 2020. Available online: https://www.imo.org/en/MediaCentre/HotTopics/Pages/Sulphur-2020.aspx (accessed on 25 June 2025).
- Zhao, G.; Yu, B.; An, R.; Wu, Y.; Zhao, Z. Energy system transformations and carbon emission mitigation for China to achieve global 2 °C climate target. J. Environ. Manag. 2021, 292, 112721. [Google Scholar] [CrossRef] [PubMed]
- Hazrat, M.A.; Rasul, M.G.; Khan, M.M.K.; Mofijur, M.; Ahmed, S.F.; Ong, H.C.; Vo, D.-V.N.; Show, P.L. Techniques to improve the stability of biodiesel: A review. Environ. Chem. Lett. 2021, 19, 2209–2236. [Google Scholar] [CrossRef]
- Issa, M.; Ilinca, A. Petrodiesel and biodiesel fuels for marine applications. In Petrodiesel Fuels; CRC Press: Boca Raton, FL, USA, 2021; pp. 1015–1033. [Google Scholar]
- Usta, N.; Aydoğan, B.; Çon, A.; Uğuzdoğan, E.; Özkal, S. Properties and quality verification of biodiesel produced from tobacco seed oil. Energy Convers. Manag. 2011, 52, 2031–2039. [Google Scholar] [CrossRef]
- Zhu, Y.; Taylor, D.; Wang, Z. The role of renewable energy in reducing residential fossil energy-related CO2 emissions: Evidence from rural China. J. Clean. Prod. 2022, 366, 132891. [Google Scholar] [CrossRef]
- Zhang, C.; Bengio, S.; Hardt, M.; Recht, B.; Vinyals, O. Understanding deep learning (still) requires rethinking generalization. Commun. ACM 2021, 64, 107–115. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; He, F.; Lucey, B. Impact of climate policy uncertainty on traditional energy and green markets: Evidence from time-varying granger tests. Renew. Sustain. Energy Rev. 2023, 173, 113058. [Google Scholar] [CrossRef]
- Kurihara, T.; Isogai, A. Properties of poly(acrylamide)/TEMPO-oxidized cellulose nanofibril composite films. Cellulose 2013, 21, 291–299. [Google Scholar] [CrossRef]
- Voronova, M.I.; Surov, O.V.; Afineevskii, A.V.; Zakharov, A.G. Properties of polyacrylamide composites reinforced by cellulose nanocrystals. Heliyon 2020, 6, e05529. [Google Scholar] [CrossRef]
- Borba, P.C.S.; Gonçalves, A.R.; Costa, R.S.; Cumplido, M.A.; Martins, F.R. Integrating renewable energy for power security under water stress scenarios due to climate change: Strategies and opportunities. Energy 2025, 326, 136169. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, W.; Xiong, X.; Wu, S.; Niu, Z.; Cheng, P.; Du, H.; Hou, Y. Stable carbon isotopic characteristics of fossil fuels in China. Sci. Total Environ. 2022, 805, 150240. [Google Scholar] [CrossRef]
- ISO 8217:2017; Petroleum Products—Specifications of Marine Fuels. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://dan-bunkering.com/media/fjljsr0p/iso_8217_2017.pdf (accessed on 14 October 2025).
- ISO 12937:2000; Petroleum Products—Determination of Water—Coulometric Karl Fischer Titration Method. International Organization for Standardization: Geneva, Switzerland, 2000. Available online: https://cdn.standards.iteh.ai/samples/2730/d1f8ee11083a4fbc84aad409e4bcf40f/ISO-12937-2000.pdf (accessed on 16 October 2025).
- Liu, H.; Zhang, Z.; Zhang, T.; Wang, L. Revisiting China’s provincial energy efficiency and its influencing factors. Energy 2020, 208, 118361. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.; Khan, Y.; He, C.; Chen, J.; Alsagr, N.; Song, H.; Khan, N. Environmental regulations, political risk and consumption-based carbon emissions: Evidence from OECD economies. J. Environ. Manag. 2022, 320, 115893. [Google Scholar] [CrossRef]
- ISO 12185:2024; Crude Petroleum, Petroleum Products and Related Products—Determination of Density—Laboratory Density Meter with an Oscillating U-Tube Sensor. International Organization for Standardization: Geneva, Switzerland, 2024. Available online: https://cdn.standards.iteh.ai/samples/82592/0a5a0b12615741c4843dbab8592827f6/ISO-12185-2024.pdf (accessed on 12 September 2025).
- ISO 3405:2019; Petroleum and Related Products from Natural or Synthetic Sources—Determination of Distillation Characteristics at Atmospheric Pressure. International Organization for Standardization: Geneva, Switzerland, 2019. Available online: https://standards.iteh.ai/catalog/standards/sist/99b7749a-ed74-463a-9773-000d3e24bdbf/iso-3405-2019 (accessed on 17 October 2025).
- ISO 20846:2019; Petroleum Products—Determination of Sulfur Content of Automotive Fuels—Ultraviolet Fluorescence Method. International Organization for Standardization: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/70824.html (accessed on 17 October 2025).
- ISO 2719:2016; Determination of Flash Point—Pensky-Martens Closed Cup Method. International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/64847.html (accessed on 17 October 2025).
- ISO 3104:2023; Petroleum Products—Transparent and Opaque Liquids—Determination of Kinematic Viscosity and Calculation Of Dynamic Viscosity. International Organization for Standardization: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/83060.html (accessed on 17 October 2025).
- ISO 4264:2018; Petroleum Products—Calculation of Cetane Index of Middle-Distillate Fuels by the Four-Variable Equation. International Organization for Standardization: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/66112.html (accessed on 17 October 2025).
- ISO 5165:2020; Petroleum Products—Determination of the Ignition Quality of Diesel Fuels—Cetane Engine Method. International Organization for Standardization: Geneva, Switzerland, 2020. Available online: https://www.iso.org/standard/70907.html (accessed on 17 October 2025).
- Zhang, H.; Chen, L.; Li, Y.; Hu, Y.; Li, H.; Xu, C.C.; Yang, S. Functionalized organic–inorganic hybrid porous coordination polymer-based catalysts for biodiesel production via trans/esterification. Green Chem. 2022, 24, 7763–7786. [Google Scholar] [CrossRef]
- Kurniawan, R.; Feinnudin, A. Assessing the implementation of the energy management system in the first ISO 50001 building in Indonesia. Indones. J. Energy 2021, 4, 129–139. [Google Scholar] [CrossRef]
- Bozbay, R.; Teke, Ş.; Ersoy, K.K.; Orakdogen, N. Tuning physicomechanical properties of PEG-interpenetrated anionically modified semi-IPN cryogels functionalized with carboxylate groups. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 693, 134060. [Google Scholar] [CrossRef]
- Ji, S.-Y.; Jung, H.-B.; Kim, M.-K.; Lim, J.-H.; Kim, J.-Y.; Ryu, J.; Jeong, D.-Y. Enhanced energy storage performance of polymer/ceramic/metal composites by increase of thermal conductivity and coulomb-blockade effect. ACS Appl. Mater. Interfaces 2021, 13, 27343–27352. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Elhaj, M.E. Polyacrylamide-Based Solutions: A Comprehensive Review on Nanomaterial Integration, Supramolecular Design, and Sustainable Approaches for Integrated Reservoir Management. Polymers 2025, 17, 2202. [Google Scholar] [CrossRef]
- Ajjarapu, V.M.K. Comparative Analysis of Polyacrylamide (PAM) and Sodium Polyacrylate (PAAS) Applica-tions in Water Treatment and Oil Industry Processes. Int. J. Water. Wastewater Treat. 2025, 10, 9. [Google Scholar] [CrossRef]
- Ryu, J.H.; Han, N.K.; Lee, J.S.; Jeong, Y.G. Microstructure, thermal and mechanical properties of composite films based on carboxymethylated nanocellulose and polyacrylamide. Carbohydr. Polym. 2019, 211, 84–90. [Google Scholar] [CrossRef]
- Herth, G.; Schornick, G.; Buchholz, F. Polyacrylamides and Poly(Acrylic Acids). In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2015; pp. 1–16. [Google Scholar]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide Degradation and Its Implications in Environmental Systems. NPJ Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Tsanaktsidis, C.G.; Favvas, E.P.; Scaltsoyiannes, A.A.; Christidis, S.G.; Katsidi, E.X.; Scaltsoyiannes, A.V. Natural resins and their application in antifouling fuel technology: Part I: Improving the physicochemical properties of diesel fuel using natural resin polymer as a removable additive. Fuel Process. Technol. 2013, 114, 135–143. [Google Scholar] [CrossRef]
- Favvas, E.P.; Tsanaktsidis, C.G.; Christidis, S.G.; Tzilantonis, G.T. H2O removal from diesel and JP8 fuels: A comparison study between synthetic and natural dehydration agents. J. Eng. Sci. Technol. Rev. 2014, 4, 104–108. [Google Scholar] [CrossRef]
- Roschat, W.; Kacha, M.; Yoosuk, B.; Sudyoadsuk, T.; Promarak, V. Biodiesel production based on heterogeneous process catalyzed by solid waste coral fragment. Fuel 2012, 98, 194–202. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.; Díaz, J.D.M.; Polo, M.S.; Utrilla, J.R. Selection of heterogeneous catalysts for biodiesel production from animal fat. Fuel 2012, 94, 418–425. [Google Scholar] [CrossRef]
- Sagin, S.V.; Sagin, S.S.; Fomin, O.; Gaichenia, O.; Zablotskyi, Y.; Píštěk, V.; Kučera, P. Use of biofuels in marine diesel engines for sustainable and safe maritime transport. Renew. Energy 2024, 224, 120221. [Google Scholar] [CrossRef]
- Medjahed, L.; Bousbaa, H.; Benramdane, M.; Naima, K.; Ameur, H.; Ozsahin, D.U.; Ahmad, H. Integrated valorization of waste cooking oil into biodiesel: Optimizing upstream processes and blend performance for sustainable energy. Model. Earth Syst. Environ. 2025, 11, 389. [Google Scholar] [CrossRef]
- McCormick, R.; Moriarty, K. Biodiesel Handling and Use Guide (No. NREL/TP-4A00-86939; CRD-15-00593); National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2023. [Google Scholar]
- Bórawski, P.; Wyszomierski, R.; Bełdycka-Bórawska, A.; Mickiewicz, B.; Kalinowska, B.; Dunn, J.W.; Rokicki, T. Development of renewable energy sources in the European Union in the context of sustainable development policy. Energies 2022, 15, 1545. [Google Scholar] [CrossRef]
- Kang, H.; Li, G.; Gao, J. Development of bio-diesel to achieve Sustainable Development Goal 7. Front. Energy Res. 2023, 10, 1057336. [Google Scholar] [CrossRef]
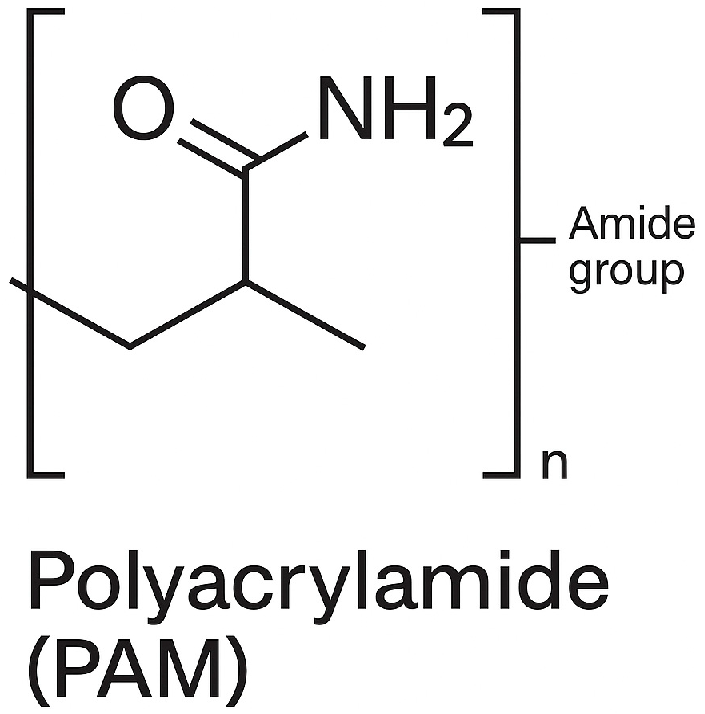

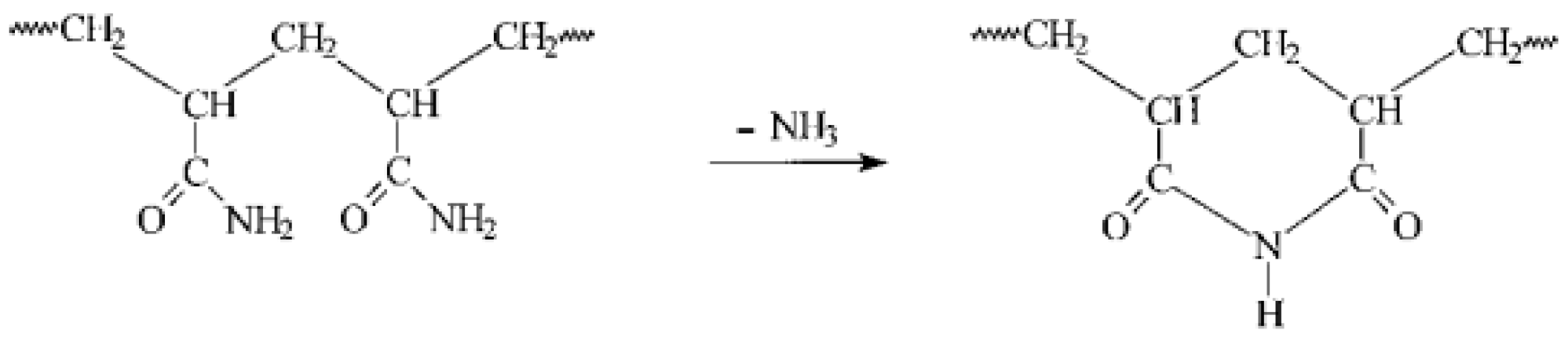

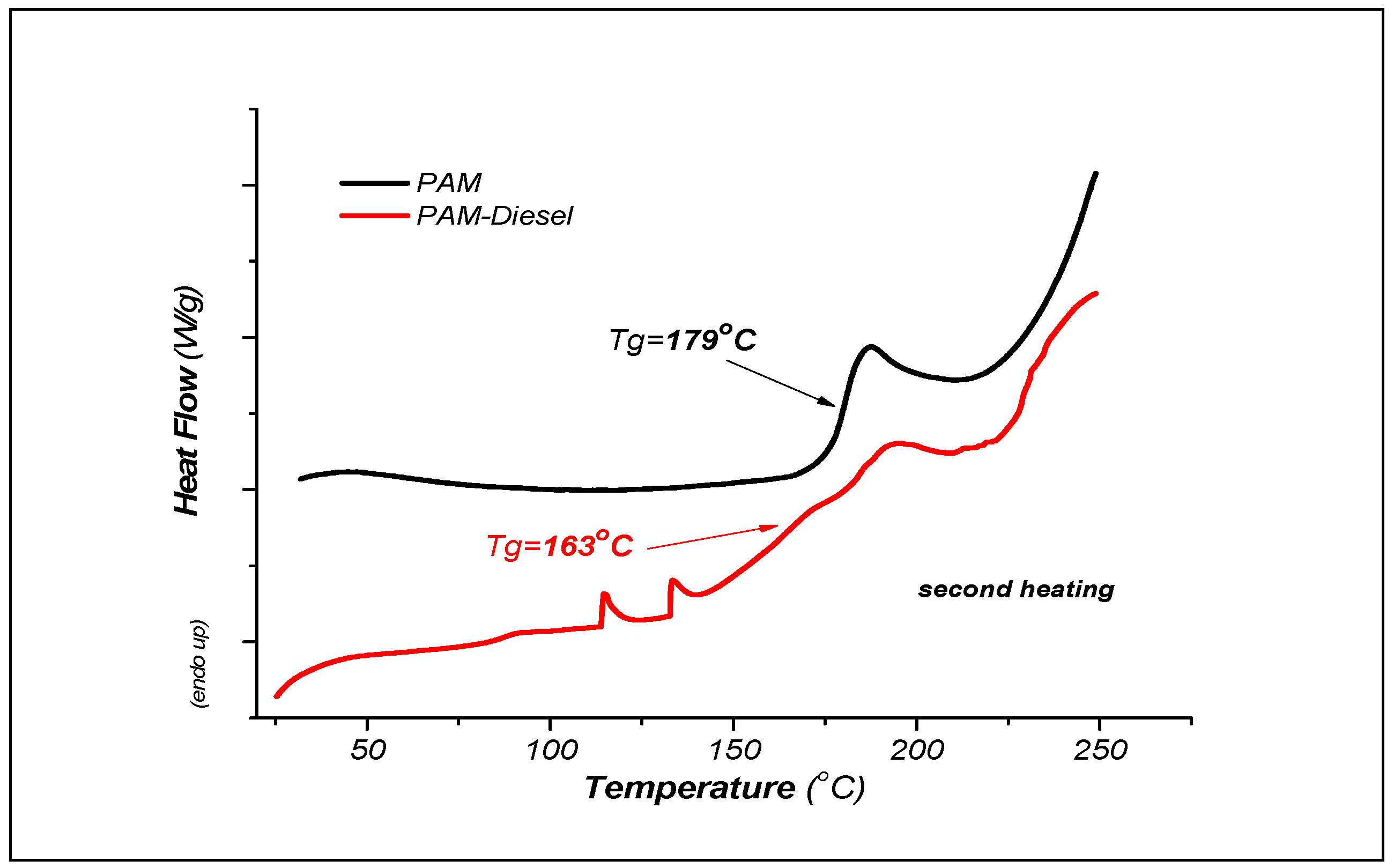
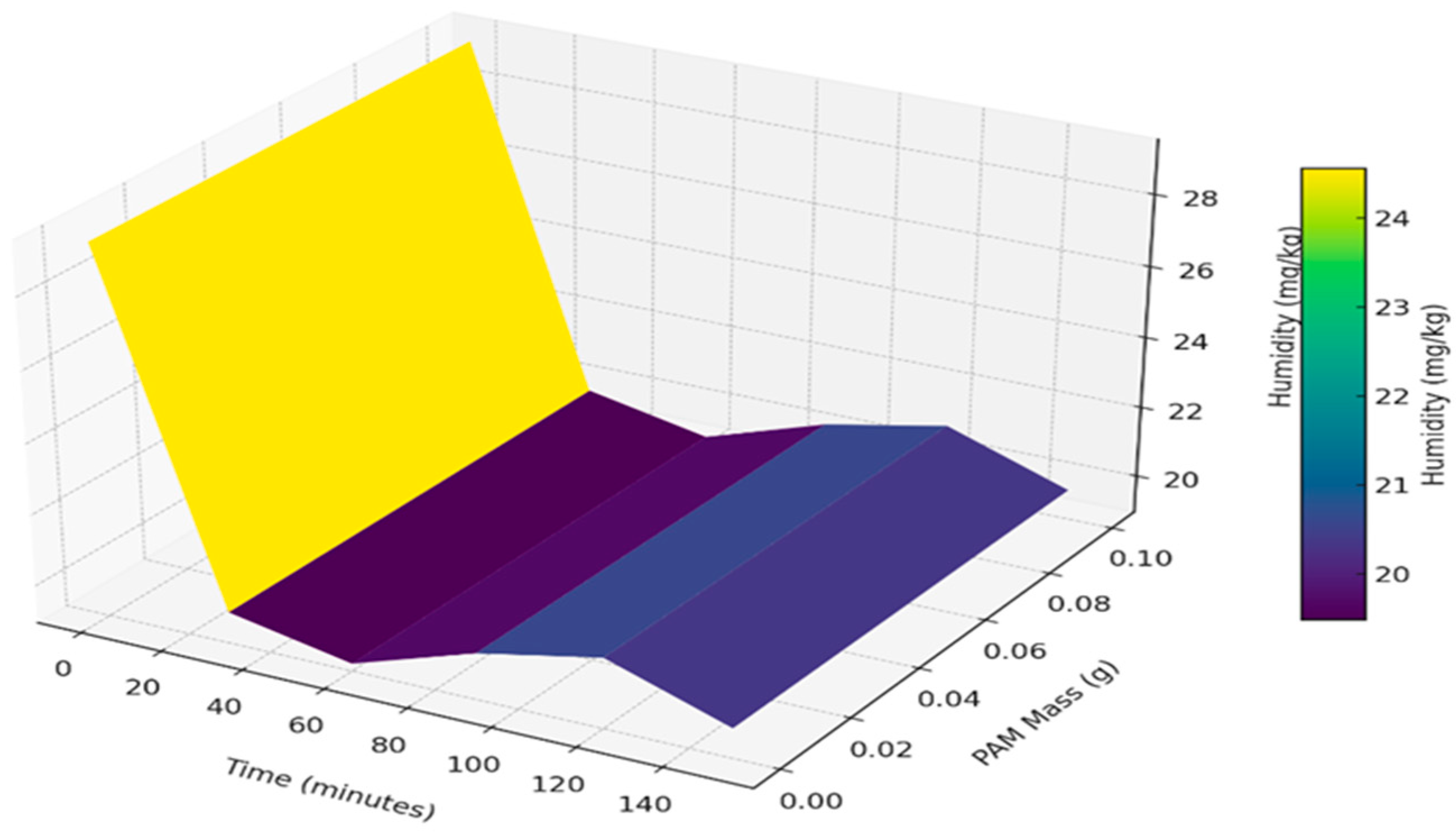
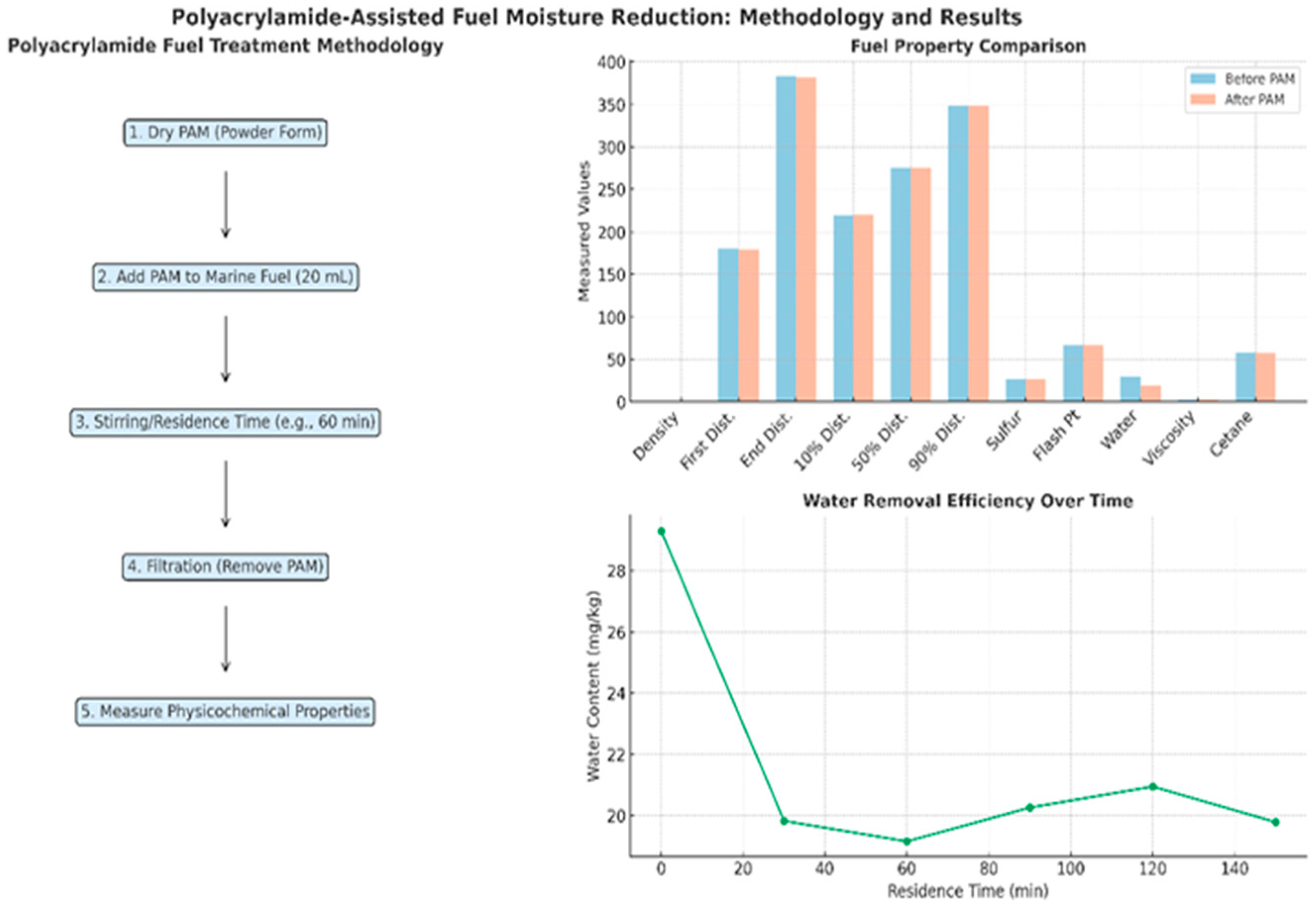
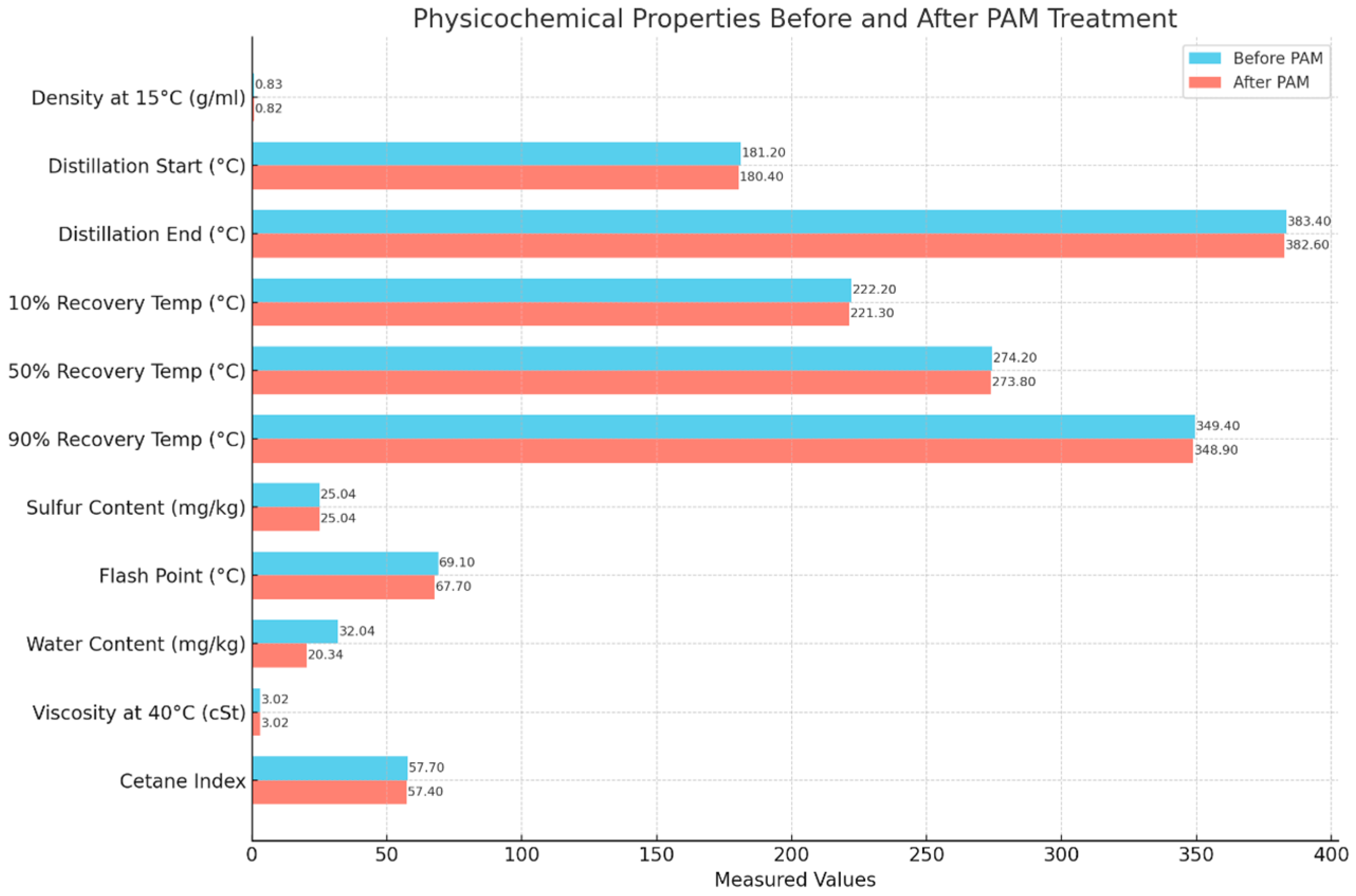
| Parameter | Units | Limits | Methods | (MGO) |
|---|---|---|---|---|
| Density at 15 °C | gr/mL | max 0.8900 | ISO 12185:2024 [28] | 0.828 |
| Distillation | ISO 3405:2019 [29] | |||
| first drop of distillation | % v/v | Reported | 180.2 | |
| end of distillation | % v/v | Reported | 382.4 | |
| Distillation temperature 10% recovery | °C | Reported | 220.2 | |
| Distillation temperature 50% recovery | °C | - | 275.2 | |
| Distillation temperature 90% recovery | °C | - | 348.4 | |
| Sulfur Content | mg/kg | max 10,000 | ISO 20846:2019 [30] | 26.00 |
| Flash Point | °C | min 60 | ISO 2719:2016 [31] | 67.1 |
| Water Content | mg/kg | max 200.0 | ISO 12937 [25] | 29.3 |
| Color/appearance | - | Black | VISUAL | Black |
| Kinematic Viscosity at 40 °C | cSt | 2.0–6.0 | ISO 3104:2023 [32] | 3.017 |
| Cetane Index | - | min 40 | ISO 4264:2018 [33] | 57.7 |
| Parameters | Units | Limits | Methods | Biodiesel |
|---|---|---|---|---|
| Density at 15 °C | gr/mL | 0.860–0.900 | ISO 12185:2024 [28] | 0.881 |
| Flash Point | °C | min 60 | ISO 2719:2016 [31] | 175.1 |
| Water Content | mg/kg | max 500.0 | ISO 12937 [25] | 250.5 |
| Cetane Index | mg/kg | min 51 | ISO 5165 [34] | 51.5 |
| Kinematic Viscosity at 40 °C | cSt | 2.0–6.0 | ISO 3104:2020 [32] | 3.017 |
| Sulfur Content | mg/kg | 10max | EN ISO 20846 [30] | 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzilantonis, G.; Zafeiriou, E.; Stimoniaris, A.; Kanapitsas, A.; Tsanaktsidis, C. Polymer-Driven Fuel Conditioning: A Novel Approach to Improving the Stability and Environmental Performance of Marine Fuels. Resources 2025, 14, 167. https://doi.org/10.3390/resources14110167
Tzilantonis G, Zafeiriou E, Stimoniaris A, Kanapitsas A, Tsanaktsidis C. Polymer-Driven Fuel Conditioning: A Novel Approach to Improving the Stability and Environmental Performance of Marine Fuels. Resources. 2025; 14(11):167. https://doi.org/10.3390/resources14110167
Chicago/Turabian StyleTzilantonis, George, Eleni Zafeiriou, Adam Stimoniaris, Athanasios Kanapitsas, and Constantinos Tsanaktsidis. 2025. "Polymer-Driven Fuel Conditioning: A Novel Approach to Improving the Stability and Environmental Performance of Marine Fuels" Resources 14, no. 11: 167. https://doi.org/10.3390/resources14110167
APA StyleTzilantonis, G., Zafeiriou, E., Stimoniaris, A., Kanapitsas, A., & Tsanaktsidis, C. (2025). Polymer-Driven Fuel Conditioning: A Novel Approach to Improving the Stability and Environmental Performance of Marine Fuels. Resources, 14(11), 167. https://doi.org/10.3390/resources14110167







