1. Introduction
Production of crude benzene at coke-chemical enterprises in Ukraine is carried out using the traditional absorption–desorption method, with the participation of wash oil, which circulates in the system [
1]. The content of benzene hydrocarbons (benzene, toluene, xylene homologues, etc.) in coke oven gas is quite low, so for their recovery, absorption oils of various origins are most often used [
2]. The variety of equipment design for the benzene hydrocarbon recovery process is due to the desire to reduce the disadvantages of the existing process, primarily to reduce the cost of crude benzene production, including by saving wash oil [
3].
Wash oil, used in the process of recovering benzene hydrocarbons, acts as a non-selective absorbent. It absorbs aromatic hydrocarbons with varying molecular weights, as well as sulphur- and nitrogen-containing compounds, water aerosols, and tars from coke gas. To desorb benzene hydrocarbons, the wash oil is heated to 145–165 °C and subjected to steam stripping. As a result, benzene hydrocarbons are removed from the oil, but some light wash oil fractions are also lost in the process. Due to the degradation of oil quality, a portion of it is continually removed for regeneration (distillation under more severe conditions), and the deficit is compensated by introducing fresh oil into the cycle. The principle scheme of circulation of wash oil for coke oven gas treatment using a typical absorption-desorption method is shown in
Figure 1.
Cyclic temperature changes, contact with coke gas impurities, evaporation of light components, and loss of light fractions during stripping intensify polymerisation and oxidation processes that lead to oil degradation [
4]. This results in an increase in density, viscosity, an increase in the loss of light fractions, an increase in the content of mechanical impurities, an increase in the coke number, and the appearance of acidic components [
5]. To prevent deterioration of oil quality, a part of it (1–2 wt.%) is redistilled with the return of the light fraction into circulation and withdrawal of the so-called polymers [
6]. Due to the high cost of coal wash oil and the different efficiency of coal tar rectification, the choice of fresh oil requires scientific substantiation on the basis of detailed analysis of oil quality changes during operation.
Until recently, coke-chemical enterprises of Ukraine used wash oil for domestic production, which is obtained as a result of the fractionation of coal tar on one- or two-column units with separation of the fraction boiling in the range of 230–300 °C. Unlike foreign producers, such oil is denser, contains more naphthalene and high-boiling components, and the boiling end temperature is higher [
7]. Due to the current deficit of domestically produced oil, enterprises now use both types of fresh wash oil, conventionally called HO and LO.
The purpose of this study was to determine the changes in different types of wash oils that occur during operation in industrial conditions. This would help to determine the possibilities for slowing down the degradation of oils to select the appropriate type of fresh oil, taking into account the specifics of the benzene plant operation and the quality of coke oven gas.
Many studies have documented changes in oil quality during the operation of a benzene hydrocarbon recovery unit from coke oven gas [
8,
9]. First of all, fluctuations in the fractional composition of absorbents should be mentioned. Oil thickening is associated with the loss of cuts up to 270–280 °C, with the largest loss occurring in the cuts up to 260 °C. It is believed that it is the fraction of wash oil (270–300 °C) that is most susceptible to polymerisation [
10].
During the operation of wash oil, significant changes in the component composition also occur, which are the result of both the loss of components with coke oven gas and crude benzene and the formation of new substances due to chemical reactions or contamination [
11]. This indicates the complex nature of the processes occurring in the wash oil during operation, including evaporation, chemical transformations, oxidation, and the formation of new compounds.
Resin formation results in the accumulation of sulphur- and oxygen-containing products, as evidenced by the elemental composition of oil and polymers of the benzene unit [
4].
To improve the quality of the wash oil as well as to obtain valuable components [
12], β-methylnaphthalene [
13], fluorene [
14], isoquinoline [
15], nitrogen-containing substances [
16], indoles [
17], heterocyclic nitrogen-containing substances [
18], etc., are removed from the wash oil. The life cycle of the wash oil is affected by the quality of the coke oven gas to be cleaned, namely the presence of resin-forming components, the presence of contaminants (resinous substances, coal and coke dust), oxygen concentrations, the presence of acid gases (H
2S, HCN), the presence of ammonia, phenols. As a result of the relevant reactions in the oil, the condensation of aromatic hydrocarbons is accelerated in the presence of sulfur [
5]. For example, the condensation of diphenylmethane begins at 160 °C, phenol at 180 °C, acenaphthene, fluorene, and biphenyl at 190 °C [
19]. Equipment corrosion products (metals and their oxides) accelerate thickening processes and significantly increase oil viscosity [
20].
The presence of certain chemicals can either accelerate or slow down these processes, which must be taken into account when using a wash oil. The presence of phenols and xylenes slows down polymerisation, while pyridines increase resin yields and softening points. Metals, such as iron and brass, and contaminants, such as iron oxide, significantly increase the viscosity of the oil.
The degradation processes of absorbent oil are complex, the nature of which is not fully understood and requires research and development of measures taking into account the specifics of the absorbent’s operation. It is of interest to determine the effect of the fractional and component composition of oils on the specific consumption of oils during operation and to establish differences in the mechanisms of oil degradation.
Despite numerous studies on the quality of wash oils, the degradation processes occurring during their operation in benzene units remain insufficiently explored. This is due to the complex interplay of polymerisation, oxidation, condensation, and coke formation reactions. Moreover, there is a notable lack of comparative data on the component composition of oils from different manufacturers. Even more critically, the literature provides almost no insights into the comparison between the component compositions of fresh and operating oils, leaving a significant gap in understanding the changes these oils undergo during operation.
The limited understanding of the degradation mechanisms of wash oils in benzene units, combined with the lack of comparative data on the component composition of oils from different manufacturers and their transformation during operation, highlights an urgent need for further research. This study aims to address this gap by systematically analysing changes in the component composition and properties of fresh and operating oils, providing valuable insights into the pathways and nature of oil degradation. Understanding these processes is essential for optimising oil performance and improving the efficiency of coke plants. The practical value of such studies lies in guiding the selection of fresh oil under current operating conditions.
2. Materials and Methods
Samples of wash oils from two benzene units were selected for the study: one unit used LO from an imported trader (DEZA, a.c. Czech Republic, Valašské Meziříčí), and the other used HO from a Ukrainian producer (Zaporizhkoks, Ukraine, Zaporizhzhia). These oil types differ in viscosity, density, and volatility, affecting specific oil consumption in benzene units and attracting both practical and scientific research interest.
The oil samples were taken from different units; the principal difference between the units using the HO is the presence of a highly efficient structured packing in the scrubber, which allows the capture of benzene hydrocarbons in one unit. Mainly for this reason, the capture ratio at this unit is slightly higher; in other parameters, the operating conditions did not differ much from each other. The quality indicators of oils were determined according to the methods described in [
21].
The characteristics of the samples are presented in
Table 1.
By analysing the component composition of the oil according to the chromatography-mass spectrometry results, the mean molecular weight (MW) of the chromatographed part of the oil can be calculated:
where C
i—mass fraction, wt.%; M
i—component molecular weight (Da).
The concentration ratio was calculated to evaluate the change in the component composition of the fresh oil during operation and to assess its consistency equation:
where C
if—is the concentration of the component in the fresh oil (mass fraction, wt.%), and C
io is the concentration of the component in the operating oil (mass fraction, wt.%).
An estimation of the absorption of coke oven gas components by the operating wash oil, which is also present in the fresh oil, was carried out using known dependencies [
22]:
where C
Igas—equilibrium concentration of indene in coke oven gas, g × m
−3; C
Ioil—equilibrium indene concentration in oil, wt.%; C
Ngas—equilibrium concentration of naphthalene in coke oven gas, g × m
−3; C
Noil—equilibrium concentration of naphthalene in oil, wt.%; T—temperature, K; 13.50 and 4770.0—empiric constants [
22].
To identify the substances in the mixture, the sample weights were dissolved in an organic solvent (dichloromethane), and aliquots were chromatographed. For the chemical analysis of the provided samples, the gas chromatography/mass spectrometry method was used. An Agilent 7890A GC System 5975C Inert gas chromatograph with a mass-selective detector (Agilent Technologies, Santa Clara, CA, USA) was used as a measuring instrument. The separation of the mixture components was carried out on an HP-5MS (5 wt.% Diphenyl) capillary column (30 m × 0.25 mm × 0.25 µm). The method provides measurements of the components in wash oil (mass fraction, wt.%) with a relative total measurement error of ±δ, not exceeding 25%, at a confidence level of
p = 0.95 for the entire range of measurements. Identification of the compounds was conducted by comparing the obtained mass spectra with reference spectra from the NIST 08 electronic library. Characterisation of the spectrometry method is presented in
Table 2.
The dynamic viscosity of the oils was determined using a Brookfield LV DV2T (Brookfield Engineering Laboratories, Inc., Middleboro, Massachusetts, (USA)—low viscosity digital rotational viscometer with a thermal cell at variable shear rates. Viscous flow tests were performed across a shear rate range of 1.2 to 250 s⁻1 using Searle geometry. The spindle was selected to achieve maximum torque, and the “ULA” spindle was used for measurements with a spindle multiplier constant of 0.64 and a shear rate constant of 1.223. The method involves recording the torque resistance of the inner cylinder as it rotates through the fluid under test at varying shear rates, allowing for the calculation of shear stress (N × m−2) and dynamic viscosity (mPa × s). At least five replicates of each test were performed on fresh samples. The viscometer and thermocell were supplied pre-calibrated. An AutoZero operation was performed immediately prior to measurement, setting the zero reading for the measurement system. For any two parallel determinations, consisting of a series of three measurements carried out within a short time interval, the results did not differ by more than 3.5% from the average value at a confidence level of p = 0.95. The arithmetic means of parallel determinations from the same sample, at a confidence level of p = 0.95, did not differ by more than 14.5% from the mean results of measurements conducted in different laboratories.
4. Discussion
4.1. Trends in the Fractional Composition of Oils
The results of the fractional composition determination indicate that the change in temperature differences after operation suggests that the HO becomes less volatile while the LO becomes slightly more volatile. A slight increase in density and minor shift in distillation characteristics suggest it maintains a relatively stable composition with minimal degradation.
In fresh oil samples, the phenol content is low and ranges from 0.8–0.89 wt.%, which corresponds to oil grades “A2” and “B”, respectively, according to [
21]. In the process of fresh oil from both producers being operated, there is an accumulation of total phenols, which are delivered to the benzene compartment with coke oven gas. This fact is confirmed by earlier observations at other enterprises [
23]. This fact also testifies to the inexpediency of deep extraction of phenols from wash oil.
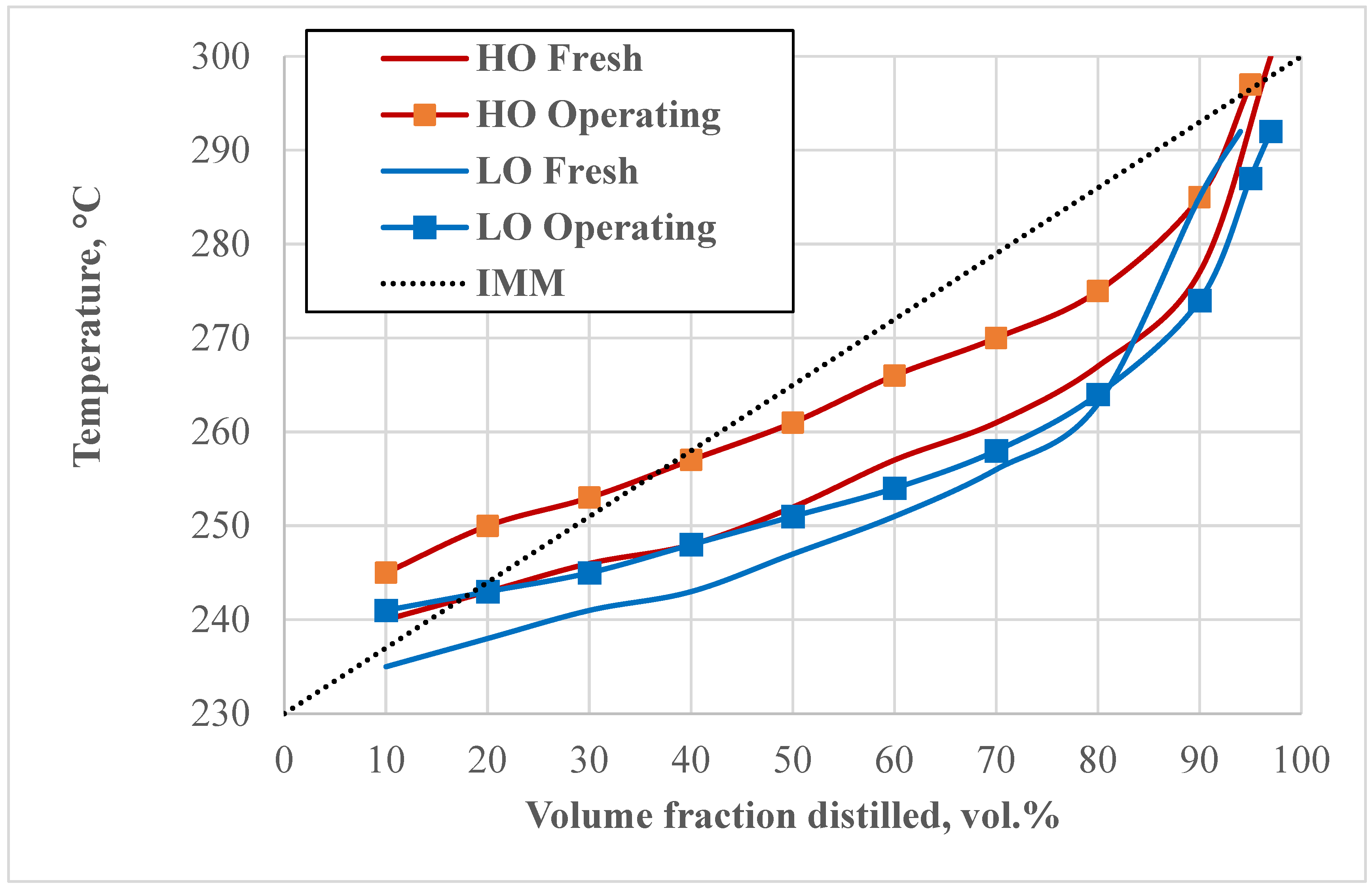
Table 2 and
Figure 2 summarise the results of oil distillation indices. Swentoslawski [
24] suggested that 5 vol.%, 50 vol.% and 95 vol.% of the volume of the distilled sample should be recorded, and the temperatures ta, tb and tc are noted, respectively. The length of the segments tb-ta and tc-tb characterises the nature of the distilled mixture; if tb-ta = tc-tb, the distilled mixture is polyaseotropic with uniformly increasing boiling temperatures (ideal oil). Both samples of fresh oils are characterised by an upper asymmetry, i.e., most of the oil consists of fractions boiling above the 50 vol.% distillation temperature. For HO, the upper asymmetry is more pronounced, and the fractions are more evenly distributed by boiling point. In the LO, 40 vol.% of the volume boils in the range 240–250 °C, which indicates a high content of methyl homologues of naphthalene. LO maintains a relatively stable composition up to 80 vol.% distillation; the slight increase in boiling points beyond 80 vol.% suggests certain changes in heavier components. The HO undergoes substantial changes, with increased boiling points throughout, suggesting a need for careful management and possibly more frequent maintenance or replacement.
Thus, the oil distillation curves indicate the enrichment of oils with high-boiling components, with HO undergoing significant changes, requiring freshening to prevent increased viscosity and sludge formation.
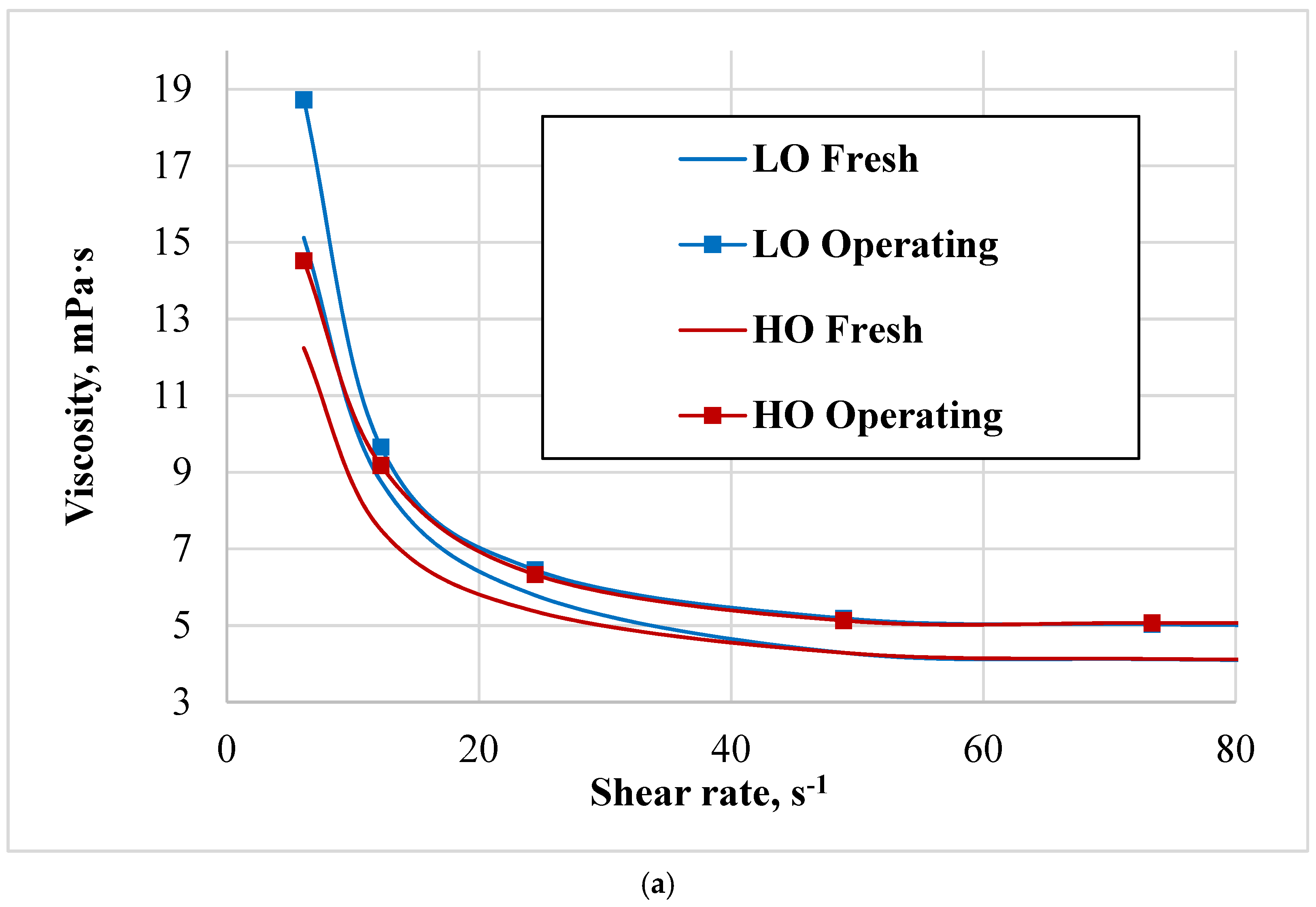
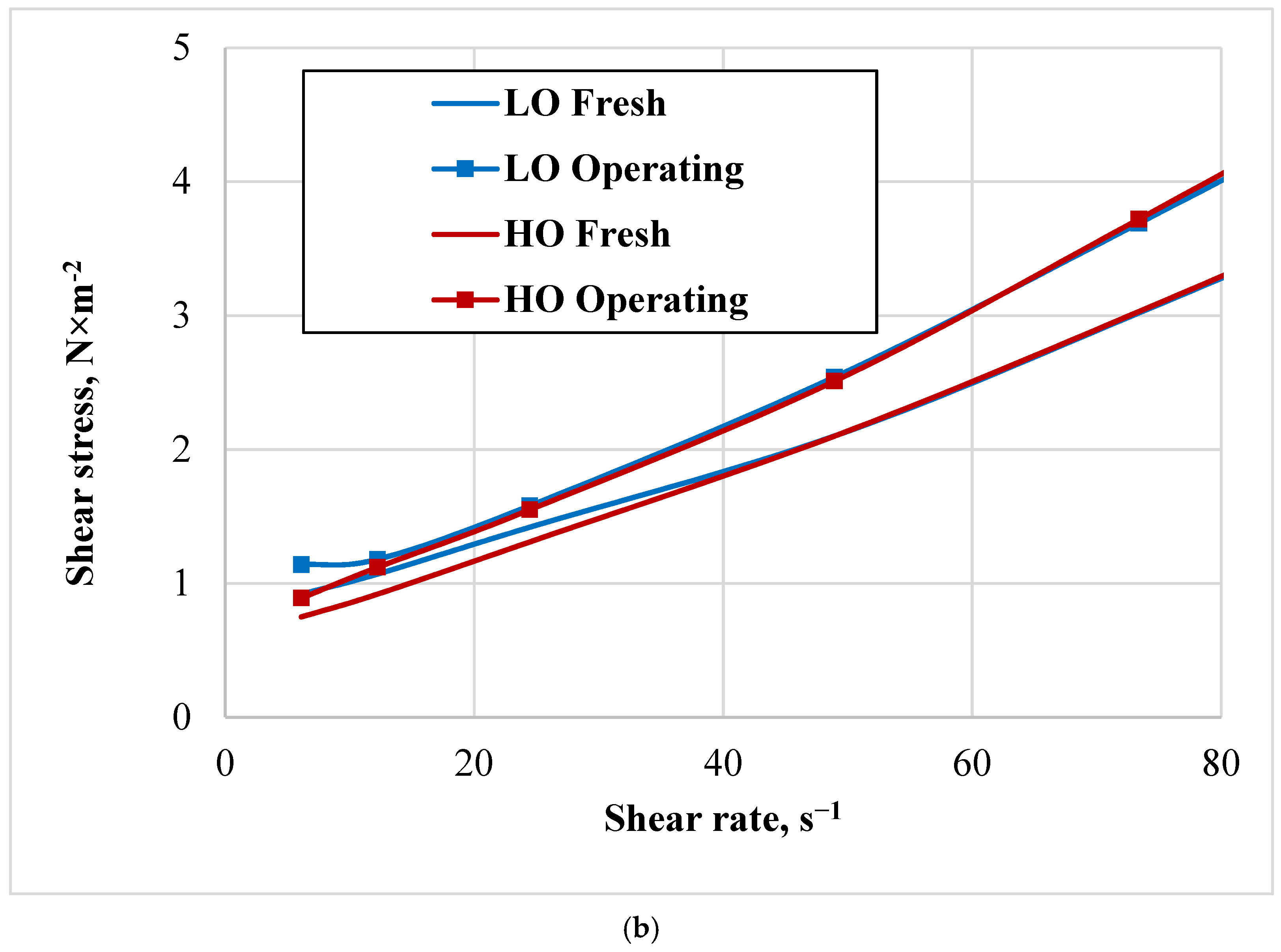
4.2. Viscosity Characterisation
The character of the viscosity curves indicates a relatively small absolute increase in the viscosity of the working oil, which is associated with satisfactory operating conditions of the regenerators of the analysed benzene units. This increase in viscosity in operating oil could be due to factors such as contamination, oxidation, or thermal degradation occurring during use. HO shows a more consistent and slightly higher percentage increase in viscosity compared to LO, suggesting it may be more susceptible to changes under operational conditions.
Both HO and LO exhibit shear-thinning behaviour, becoming less viscous at higher shear rates, which is typical for coal tar [
25]. The shear-thinning behaviour is more pronounced in the operating state for both oils. The choice between HO and LO will depend on the specific requirements of the application, including the expected shear rates and the tolerance for viscosity changes due to operational conditions.
The curves of fresh and working oils diverge with increasing shear rates, with working oils exhibiting higher shear stress at equivalent shear rates. This suggests that the viscosity of the oils increases after use, indicating thickening or increased resistance to flow under the same conditions.
The widening gap between the fresh and working oil curves suggests that operating conditions have a slightly more pronounced effect on heavy oil viscosity at higher shear rates, possibly due to changes in molecular interactions or minor oxidation during operation.
Thus, HO demonstrates a steadier and somewhat greater rise in viscosity compared to LO, indicating that it might be more sensitive to variations during operation.
4.3. Findings from Chromatography and Mass Spectrometry
Both fresh oil samples have approximately the same value of average molecular weight calculated from the chromatographed portion of the oil (
Table 4).
The average molecular mass of HO during operation increased by 3 Da., which agrees with the available ideas about molecular mass increase during operation. But for LO, the decrease of molecular mass did not occur because the absorbent was saturated with naphthalene from 6.8 to 12.4 wt.% and other easily boiling components, which even led to a decrease of average molecular mass by 3 Da.
Another indicator characterising the initial properties of oils and the directions of their degradation is, in our opinion, the sum of alkyl derivatives of aromatic hydrocarbons. LO is characterised by a higher total concentration of alkyl derivatives, and HO contains a higher total concentration of non-substituted nuclear aromatic hydrocarbons. In the process of oil operation, there is a decrease in the content of alkyl derivatives, which may be due to some loss of alkyl groups as a result of thickening reactions and detachment of side groups.
From the array of obtained data on component concentrations for the two groups of oils, those identified in both fresh and operational oils were selected. In this sample, a concentration ratio was calculated to assess the change in the component composition of the fresh oil during operation and to assess constancy (
Table 5).
The operational efficiency of benzene units primarily depends on the stability of the wash oil. This stability is characterised by the concentration coefficient K ≈ 1, indicating minimal oil degradation and reducing the need for frequent replacement with fresh oil.
The data analysis showed that 34.6 wt.% of the components of fresh HO have a concentration ratio in the range of 0.9–1.1, while for LO, 41.1 wt.% of all components of fresh oil have the same range of concentration ratio. This means that the LO maintains a relatively stable composition.
The change in the component composition also reflects the loss of low-boiling substances of the oil during its evaporation into coke oven gas and during its distillation for recovery of benzene hydrocarbons.
The maximum value of K
max is observed for indene, which is explained by its absorption into the oil from coke oven gas, which is confirmed by the data on the existence of equilibrium between the indene content in the gas and in the oil [
22].
Special attention should be paid to the increase of naphthalene content at operation of LO from 6.8 to 12.4 wt.% (K = 1.8) with a practically unchanged value of K for HO. LO maintains a relatively stable composition. This is due to the fact that when LO with low naphthalene content is brought into contact with coke gas poorly cleaned from naphthalene, the content of this component in the oil increases. Therefore, the limitation of naphthalene content in fresh oil at unsatisfactory gas purification does not make sense, as there is an equilibrium (4).
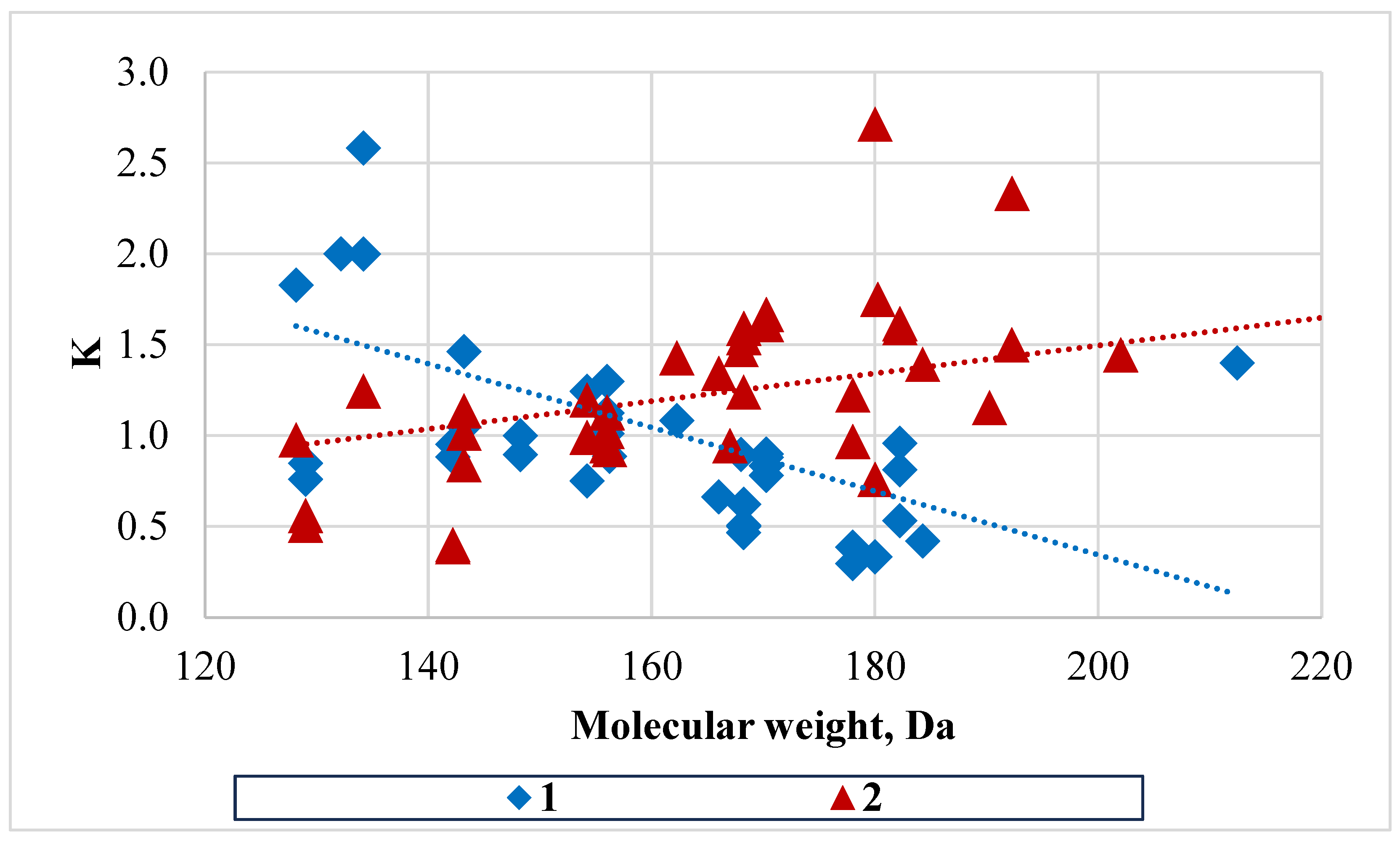
The directionality of the processes leading to changes in the component composition of oils during operation was estimated by the dependence of the concentration ratio on the molecular composition of components (
Figure 4).
As can be seen from the above data, the direction of change in the concentration of oil components during their operation differs. For LO, the character of the distribution of components by molecular weight is as follows: for low-boiling components, the concentration ratio is reduced. Probably, it is connected with the processes of oil enrichment with low-boiling components (first of all, naphthalene by 5.6 wt.%; indene by 1.3%, quinoline homologues by 0.5 wt.%), which leads to dilution of high-boiling components in the operating oil. For HO, there is a tendency to lose low-boiling components (especially 1- and 2-methylnaphthalenes) and to increase the concentration of high-boiling components (especially methyl homologues of dibenzofuran, fluorene, anthracene and phenanthrene). Earlier data obtained by us on the analysis of HO through the gas-chromatographic method show that the content of 2- and 1-methylnaphthalenes always decreases by 3–9 wt.% in the operating oil in comparison with fresh oils. These data are also confirmed for HO and analysed in this work by gas-chromatography/mass spectrometry. When the benzene unit is run on fresh LO oil, only a slight decrease in the concentration of mono-methylnaphthalenes was recorded. Another difference is the reduction of biphenyl content in the operating oils when replenishing the operating cycle with fresh HO, which was also previously established by gas chromatographic methods (by 2–4 wt.%).
Individual low molecular weight components with concentration ratio K > 1, indene, styrene and coumarone as components of the coke oven gas are absorbed into the operating oil. Naphthalene may enter the oil in the form of vapours or sublimates. A “companion” of naphthalene is thionaphthene, which has a similar boiling point but a different crystallisation temperature. The ratio of naphthalene to thionaphthene in fresh oils (
Table 3) is 19 ÷ 22, and the same ratio is characteristic of the naphthalene fraction [
26]. Only for the operating oil, which was obtained on the basis of LO (fresh), the ratio of naphthalene to thionaphthene is 13, which suggests the naphthalene supply with coke gas in the form of vapour or sublimate.
The lowest temperature of crystallisation has anthracene and acenaphthene, which are precipitated on the working surfaces of the equipment. This also serves as a possible explanation for the decrease in the concentration ratio when working with heavier oils. In addition, the component composition of the oil also changes in the process of oil polymerisation, which is illustrated by a decrease in the concentration of acenaphthene. In [
26], acenaphthene is characterised as the least heat-resistant component of wash oil, having a high yield of non-volatile residue on heating.
4.4. Oxidative Processes in Operating Oils
It is also necessary to pay attention to the newly appeared components in the operating oil, the content of which is small, but they are indicators of oil degradation in the process of its operation (
Table 6).
In the newly “appeared” oxygen-containing components, 1-acenaphthenone is identified, which is found, for example, in the products of atmospheric oxidation of acenaphthene [
27], which proves the susceptibility of acenaphthene contained in coal oil to oxidation under the conditions of operation of the benzene unit of a coke-chemical plant. Probably, therefore, 2,2-dimethylacenaphthylene-1-one is also found as a result of similar oxidation of the corresponding methyl derivative of acenaphthene.
A mechanism for the oxidation of ethylbenzene to yield acetophenone is known [
28], which is also identified in the operating oil obtained from the exploitation of LO (fresh). In the operating oil replenished with HO 9-hydroxyfluorene, significant amounts of fluorene were detected, which can be formed as a result of fluorene oxidation [
29]. The occurrence of such processes indicates the oxidation of aromatic rings with the possible formation of alcohols or ketones.
Among the oxidation products, 3-methylacetophenone, which is obtained by oxidation of the corresponding aromatic alcohols, has been identified [
30].
The formation of benzofurans and indenes in the context of oil processing can be explained by the oxidation and rearrangement of existing PAHs. The disappearance of 2,4-dimethylphenol in HO and 2,4,6-trimethylphenol in LO, along with the corresponding appearance of methyl derivatives of benzofurans, such as 2-methylbenzofuran and 7-methylbenzofuran, supports the possibility of a new synthesis of benzofurans from phenols [
31], where various phenols were transformed into different benzofurans with good yields. Benzofurans typically form from naphthalene derivatives through oxidative pathways, while indenes can arise from the oxidation and cyclisation of larger PAHs like phenanthrene. These transformations are well-documented in the literature, providing a solid foundation for understanding the chemical processes involved [
32].
Analysis of the oxygen-containing components in samples of operating oils shows that LO’s oxidation products contain oxidation products of alkyl groups and oxidation products of non-substituted nuclear hydrocarbons in oxidation products of HO. Moreover, the total concentration of identified oxidation products in the operating oil obtained from HO is higher. These compounds could form through cyclisation and condensation reactions of phenolic compounds or through oxidation and rearrangement of polycyclic aromatic hydrocarbons.
When comparing data on the content of oil components, components that “disappear” from fresh oil are also found. Some of the original components may have polymerised under the influence of heat and the presence of oxygen, leading to the formation of heavier and more complex compounds, which may not be easily detectable or were not listed among the identified components. High temperatures facilitate condensation reactions, where smaller molecules combine to form larger, more complex structures. This can explain the appearance of new polycyclic compounds. The presence of ≈1 vol.% oxygen in coke oven gas likely initiated oxidation reactions, leading to the formation of oxygenated compounds (e.g., 9-hydroxyfluorene) and the disappearance of certain hydrocarbons. Phenols and thiophenes are particularly prone to oxidation, forming quinones, sulfones, or other oxidised species.
The differences between the operating of LO and HO indicate variations in the secondary reactions during the operating conditions. The presence of oxygen and different conditions might have led to the formation of different sets of oxidation products, with the LO showing more evidence of substituted benzenes and complex nitrogenous compounds. Both oils follow similar primary pathways of oxidation and polymerisation but diverge in secondary reaction products.
The research focused on determining the optimal temperature regimes for the absorption and distillation processes, ensuring the efficient removal of waste oil during distillation. The technological parameters were optimised to achieve the lowest possible specific consumption of wash oil. However, any regime violations leading to increased wash oil consumption would result in altered oil compositions, with the oil components reflecting the technological regime violations more significantly than the degradation processes identified earlier.
It is of great scientific and practical interest to further research the influence of coke oven gas composition and its impurities on the degradation rate of oils from different producers. Future research will focus on extending the analysis under consistent operational conditions to evaluate the lifecycle performance of both light and heavy wash oils. This ongoing work will provide deeper insights into their long-term stability, degradation patterns, and overall performance in continuous industrial use.
5. Conclusions
An analysis of the processes occurring during the operation of wash oils from different manufacturers has shown that, regardless of the oil type, there is a 0.3 wt.% accumulation of total phenols in the operating oils. This indicates that deep extraction of phenols from the 230–300 °C fraction of coal tar is unnecessary.
The different behaviours of the two types of fresh oils compared during operation determine the difference in the fractional composition of the operating oils, with the HO becoming less volatile after operation and the LO becoming slightly more volatile. It is connected with more intensive absorption of low-boiling components of coke gas at a lower intensity of LO degradation. At the same time, the average molecular weight of HO increases by 3 Da. during operation, while the same indicator shows a slight decrease for LO.
Operating oil derived from HO undergoes a slightly higher viscosity increase (by a factor of 1.25). Both HO and LO exhibit shear-thinning behaviour, which becomes more pronounced in the operating state for both oils.
Light oil is characterised by a higher total concentration of alkyl derivatives (48 wt.% compared to 44 wt.% for HO). In contrast to LO, HO shows a significant decrease in alkyl derivatives of aromatic hydrocarbons during operation, which may be associated with the intensive thickening reactions and the detachment of side groups.
The direction of change in the concentration of the same identified components in fresh and operating oils during their exploitation differs. For higher boiling components, the concentration ratio of components from LO (fresh) to operating oil decreases, which is connected with the processes of oil enrichment with naphthalene and indene of coke oven gas. HO is characterised by losses of 1- and 2- methylnaphthalenes and an increase in the concentration of methylhomologues of dibenzofuran, fluorene, anthracene and phenanthrene.
In the oxidation products of LO, alkyl groups are identified, while in the oxidation products of HO, non-substituted hydrocarbons are observed. The total concentration of identified oxidation products in the operating oil derived from HO is 1.7 times higher.
The above conclusions allow us to characterise LO (fresh) as an absorbent that is more resistant to degradation than heavier wash oil; however, to reduce the losses of LO, it is necessary to observe an appropriate temperature regime of absorption.