Abstract
Coastal acid-sulfate soils are crucial for producing crops and thus, for food security. However, over time, these soil resources experience degradation, leading to higher agro-input, lower yields, and environmental hazards that finally threaten food security. The optimal use of this fragile resource is only attained by implementing vigorous integrated water–soil–crop management technologies amid the climate change impact. This study aimed to review the distribution, properties, use, and management of acid-sulfate soils in Kalimantan, Indonesia. Acid-sulfate soils cover about 3.5 Mha of the coastal area in Kalimantan and have high acidity, high-risk iron and aluminum toxicity, and low fertility, requiring precise water management, amelioration and fertilizer application, crop variety selection, and rice cultivation technologies. Lime, biochar, organic fertilizer, compost, ash, and fly ash are ameliorants that raise pH, reduce iron and aluminum toxicity, and improve crop yield. Rice cultivation has developed from traditional to modern but needs re-designing to fit local conditions. Depending on the soil nutrient status, rice cultivation requires 80–200 kg ha−1 of urea, 50–150 kg ha−1 of SP36, 50–150 kg ha−1 of KCl, and 125–400 kg ha−1 of NPK compound fertilizer, but is affected by CH4 and CO2 emissions. Good water management impacts the effective implementation of amelioration and fertilizer application technologies. The remaining challenges and future directions for water management, amelioration, fertilizer application, crop varieties, cultivation techniques, land use optimization, climate change adaptation and mitigation, technology adoption and implementation, and resource conservation are outlined. Acid-sulfate soils remain a resource capital that supports food security regionally and nationally in Indonesia.
1. Introduction
Acid-sulfate soils (ASSs) contain a sulfidic layer at a 125 cm depth. In soil taxonomy [1,2], these soils include sulfa or sulfi prefixes in the suborder level, such as Sulfaquents, Sulfaquepts, and Sulfihemists, and sulfic prefixes in the great group levels, such as Sulfic Endoaquents and Sulfic Endoaquepts. ASS has unique characteristics that are controlled by its environmental conditions, such as parent materials, climate, hydro-topography, and climax vegetation, both on-site and upstream. The huge ASS area forms the center for rice production in Indonesia, Vietnam, Thailand, Bangladesh, and China, etc. In Indonesia, ASSs make up about 6.7 Mha [3], distributed mainly in coastal areas and around rivers in the big islands such as Sumatra, Kalimantan, and Papua.
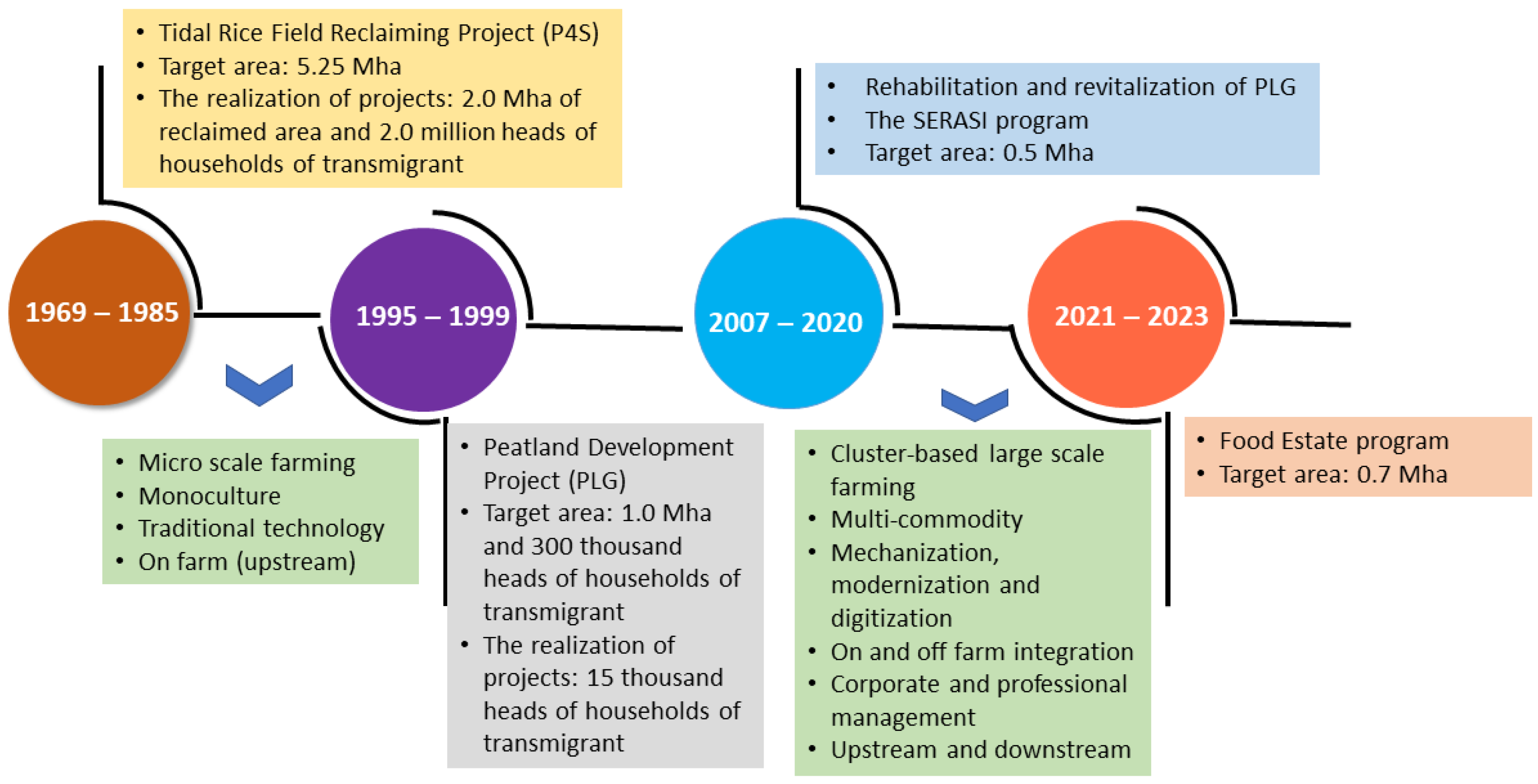
In Indonesia, especially in Kalimantan, research on ASSs has been conducted since 1969, along with national development programs (Figure 1). The Indonesian government launched the Tidal Paddy Field Development Project from 1969 to 1984, where the government converted 0.005–0.2 Mha of tidal lands into paddy fields. Then, the government distributed them to farmers, who got 2.00–2.25 ha of paddy fields per head of household. The government reclaimed around 2.0 Mha of the tidal swamp for agriculture up to 1995, whereas the local people reclaimed 3.0 Mha. In addition, the government reclaimed 1.0 Mha of peatland in Central Kalimantan through the Peatland Development Project (PLG) to increase crop production from 1995 to 1999. The lessons learned from this research and development include (1) the high spatial variation in soil properties requires site-specific management technologies, (2) farmers still implement traditional farming techniques with limited infrastructure; thus, more technological dissemination and infrastructure improvements are needed, (3) production inputs for innovation technology adoption are lacking, and (4) youth interest in ASS-based agriculture is lacking, leading to land being abandoned. Managing ASSs in tidal areas is paramount for optimizing land utilization and crop productivity and reducing environmental hazards from soil and water acidification and iron toxicity.
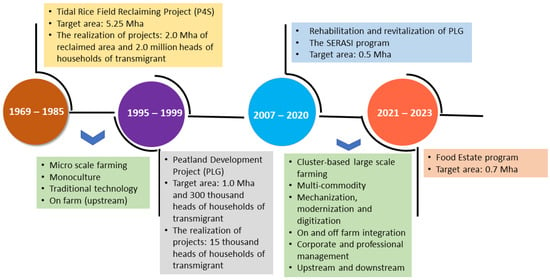
Figure 1.
Progress on research and development on acid-sulfate soils, 1969–2023.
Formulating and implementing robust water and soil management technologies requires a better understanding of ASS characteristics. Mismanagement of these soils leads to land degradation, as indicated by soil pH dropping to 3 or less, and the release of organic acids and metals that are dangerous to water biota and human health [4,5]. During rice cultivation, in particular, these soils emit CH4 under reduced soil conditions (Eh < −250 mV) and CO2 under oxidized conditions. Tidal type influences CH4 and N2O emissions [6], while rice variety controls CH4 emissions [7].
ASS is an essential wetland resource for producing food, feed, fiber, and fuel. Water management, amelioration, fertilizer application, and adaptive crop variety are among the technologies required to increase crop yield while maintaining good environmental quality. In Indonesia, Kalimantan is a crucial ASS-based agriculture production center. Nevertheless, publications elaborating on the state of ASSs in Kalimantan are still limited. Their unique characteristics, distribution, and existing management options are among the remaining questions, and future directions should be outlined accordingly. Therefore, this study aimed to review the distribution, properties, use, and management of ASSs in Kalimantan, Indonesia.
We surveyed articles in reputable journals and proceedings in digital databases (Google Scholar, Web of Science, Scopus, ScienceDirect, Dimensions, and Lens) using the keywords acid-sulfate soils, tanah sulfat masam, and Kalimantan, and collected relevant technical reports from previous projects. Then, we grouped articles into seven themes, i.e., distribution and properties, water management, soil amelioration, fertilizer application, rice varieties, farming system and technology adoption, and greenhouse gas emissions. Considering our experience and the recent information on these themes, we outlined challenges and future directions.
2. Acid-Sulfate Soil Distribution and Selected Properties
2.1. Distribution
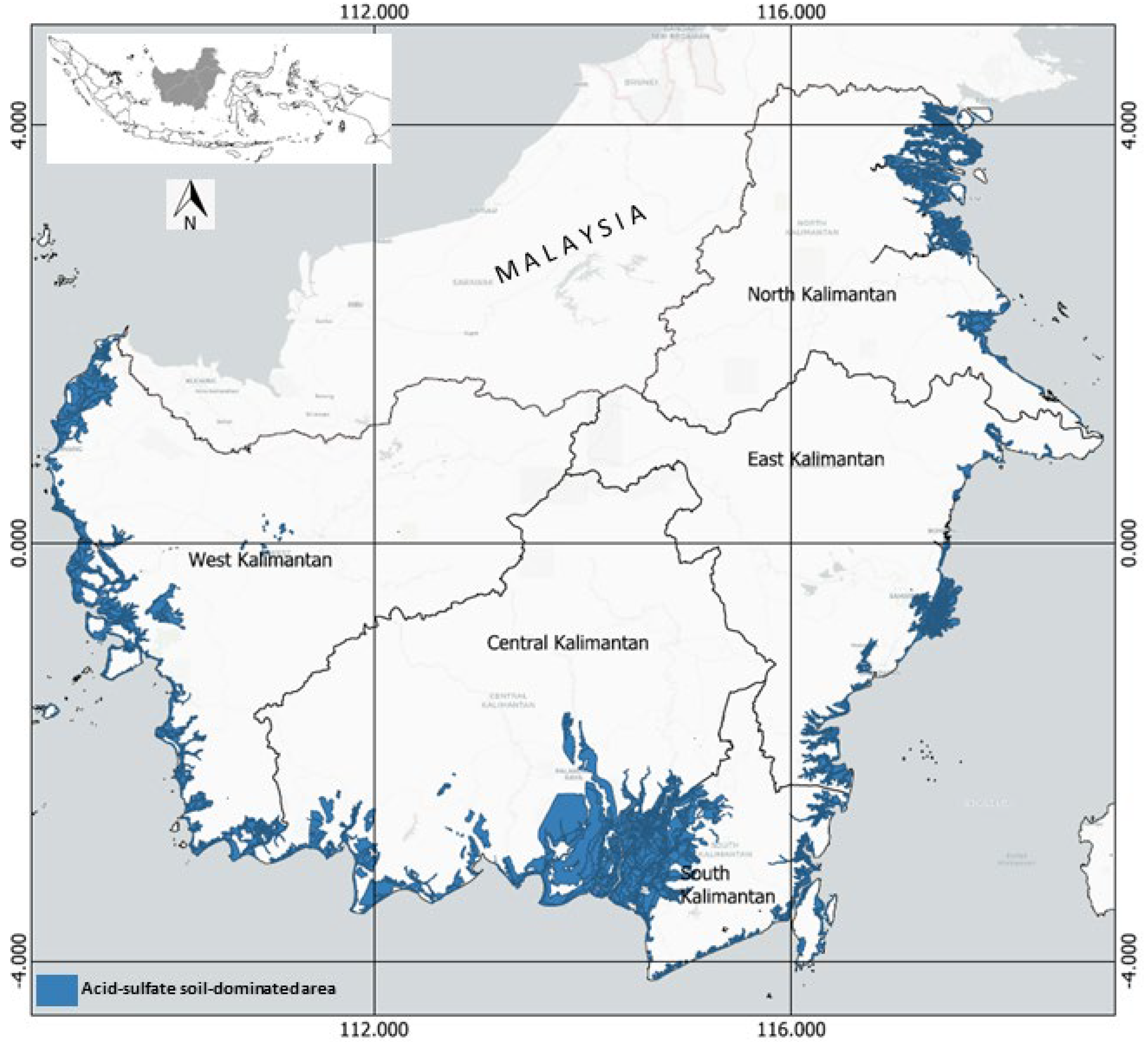
In Kalimantan (Indonesia), the swampland is about 10.0 Mha [8], of which 2.9 Mha is tidal and about 7.1 Mha is inland. Figure 2 shows the indicative distribution of ASSs in the coastal area of Kalimantan, covering about 3.5 Mha. In the West Kalimantan province, this soil is found in the coastal area, from Sambas Regency in the north to Singkawang, Bengkayang, Mempawah, Pontianak, Kuburaya, and Ketapang Regency in the south. ASSs are mainly found in the estuary of the Kapuas River, the longest river in Indonesia (12 km long), and the Bengkayang River (2 km long).
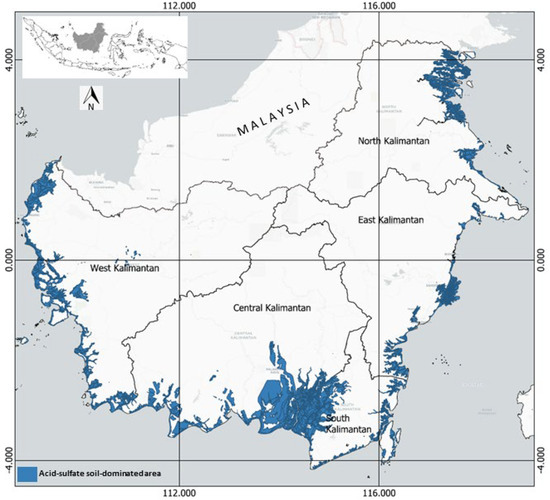
Figure 2.
Indicative distribution of acid-sulfate soils in Kalimantan, Indonesia, plotted using OpenTopomap. Source: [9,10,11,12], re-drawn and updated.
In Central Kalimantan province, ASSs extend along the coastal areas of Kotawaringin Barat Regency, Seruyan Regency, Pulangpisau Regency, and Kapuas Regency. The soils also extend to South Kalimantan province, mainly in Barito Kuala, Barito Selatan, Hulu Sungai Selatan, Tapin, Tanah Laut, Tanah Bumbu, and Kotabaru Regency.
On the eastern coast of Kalimantan, ASSs are found from the coastal area of Paser Regency in the south to Kutai Kertanegara Regency in the north of East Kalimantan province. In North Kalimantan province, these soils are found in the coastal areas of Nunukan, Tana Tidung, and Bulungan Regency.
2.2. Soil Properties
ASSs in Kalimantan are dominated by silt and clay fractions and mostly have a clay texture (the clay fraction is more than 35%). The soils are also categorized as heavy clay soils (clay fraction of more than 60%), for example in Tapin and Nunukan. Table 1 provides the physicochemical properties of selected soils from Kalimantan.

Table 1.
Physicochemical properties of selected profiles of acid-sulfate soils in Kalimantan.
As expected, the pH of the ASSs is low (acidic), ranging from 2.6 to 5.1. The soil pH is very low in the layer containing sulfidic materials (pH 2.6–4.8). Meanwhile, sulfidic materials are found 20 cm below the surface in Kotawaringin Barat and Tana Tidung Regency, and 55 cm below the surface in Seruyan Regency. Sulfidic materials are found near the surface in several sites, such as Nunukan Regency.
Information on the depth of the sulfidic material, pyrite, is crucial in managing this soil for agriculture because pyrite (FeS2) is one of the primary sources of acidity [15,16]. Shamshuddin et al. [17] concluded that the oxidation of 1.0 moles of FeS2 produces 4.0 moles of H2SO4. Several factors affect changes in soil acidity due to pyrite oxidation, i.e., oxygen and ferric (Fe3+) availability, decomposable organic matter, the initial value of soil pH, base cation availability, pyrite content, and the hydrological condition of the land. However, soil moisture and the hydrological condition of the land are the main factors that determine soil acidity [18]. Variations in groundwater levels control pH and Eh. Decreased groundwater level or soil moisture during the dry season or due to drainage of the land leads to the oxidation of pyrite and other ferrous (Fe2+) species [18,19]. Conversely, flooded soil leads to reduced soil conditions, increasing soil pH [18,20].
Soil organic carbon (SOC) content also varies, ranging from 2.14 to 8.40%. In some areas, the soils are covered with peat soils, called peaty ASSs, such as in Kotawaringin Barat, Tapin, and Nunukan Regency (Table 1). This organic matter is less than 50 cm thick, hence excluded from organic soils (Histosols). The peat material contains very high soil organic carbon ranging from 11.84 to 42.17%.
The cation exchange capacity (CEC) of soil is between 15.27 and 83.47 cmol kg−1, while base saturation (BS) varies between locations, ranging from 7% to 67%. High organic carbon and clay are responsible for this relatively high CEC. Variations in BS are closely associated with the variations in exchangeable Ca and exchangeable Mg. Soils from Sambas and Kotawaringin Barat Regency are low in exchangeable Ca and Mg, leading to lower BS. Meanwhile, the base cation content is alleviated and fertilizer application is required to support crop growth.
Nitrogen content varies from low to very high [15,21,22], with total nitrogen ranging from 0.17 to 1.04% (Table 1). Soil pH is essential when determining nutrient availability and toxicity in these soils; low soil pH causes aluminum and iron solubility to increase, and the capacity for fixing phosphorus is large [23]; phosphorus adsorption capacity may reach 800 mg kg−1 [24]. However, flooding in these soils decreases Eh and solubilizes Fe oxides, increasing P availability [25].
During ASS formation, the natural oxidation of sulfide-bearing minerals and sulfuric acid attack clay minerals, resulting in changes to the clay mineral structure. The sulfuric acid lowers pH, which makes nutrients less available; low soil pH causes aluminum and iron solubility to increase, displacing K, Ca, and Mg from the exchange complex. Furthermore, the exchange complex contains aluminum and iron. Therefore, ASS is likely deficient in Ca and K. Soil pH was positively and significantly correlated with exchangeable K, Ca, and Mg content in the soil [26]. However, flooding ASSs increases the availability of K, Ca, and Mg due to the increase in soil pH and the precipitation of aluminum and iron [27,28].
Iron (Fe) is abundant in ASSs. Fe concentrations in flooded ASSs can reach 4700 mg kg−1 [24]. Fe solubility depends on environmental conditions, such as Eh, pH, organic matter, soil moisture, microorganisms, anion presence [17,18,21], and land management systems. Fe solubility is also influenced by its characteristics, such as specific surface area and solubility [29].
Aluminum toxicity is the most critical limiting factor for plant growth in ASSs. A substantial amount of H+ ions are supplied to the soil solution as a result of pyrite oxidation, and the acid reacts with soil minerals, dissolving Al in the soil solution. Al solubility is relatively higher at low pH [30]. Exchangeable Al in ASS ranges from 1.8 to 4.3 cmol kg−1. These levels are toxic to plants and limit the availability of essential nutrient elements such as P, Ca, and Mg [17].
Salinity occurs in soils inundated by seawater daily. These areas are generally covered with mangrove forests and are not used for rice cultivation. During prolonged droughts (such as El Nino), salt concentrations in water increase [31]. The level of seawater increases from the tide to the upper stream, leading to an increase in the coverage of salt-affected soil obstructing crop growth [32]. During the rainy season, salt concentrations return to normal and rice can be planted on land in that area. Haloculture is another system for the sustainable use of saline water for crop production [33].
For rice cultivation, ASS has several constraints because pyrite oxidation increases acidity. In addition, this land has a low content of macro and micronutrients [34] and iron toxicity that can decrease rice yield from 30 to 100% depending on variety tolerance levels, toxicity intensity, and soil fertility status [35]. Managing water conditions is an option for controlling pyrite oxidation. In fact, insights into hydrological characteristics for water management technology are the key to successful crop production in ASS-dominated agricultural land.
3. Hydrological Characteristics and Water Management
Sea tide activities control the hydrological characteristics of ASSs. Pushing spring tide weakens with distance from the estuary (river or primary canal estuary), leading to lower water potency that inundates the ASS. Such a condition is caused by increasing topography upstream and water is pushed by the tide to balance the conditions in the water. The wave decreases moving energy, impacting the irrigation and drainage potency.
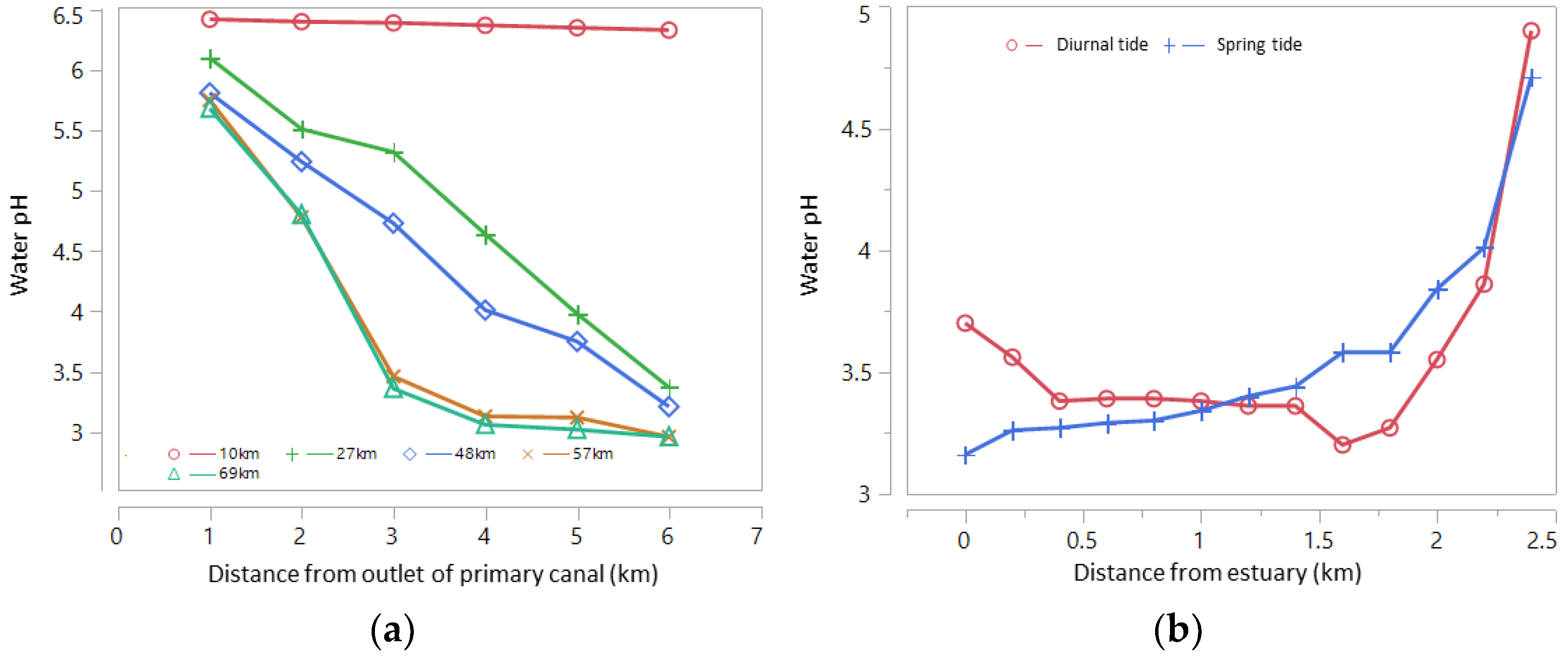
In areas that are routinely inundated by big tides and small tides (Type A), water floods ASSs every day. The potency of pyrite oxidation is very low and hence rarely found in acidic water (water pH < 4.0). In areas inundated only during big tides (Type B), irrigation water is always available; however, some big tides cannot reach the ASSs during small tides in the dry season (DS). Such conditions trigger pyrite oxidation in the soil layer, forming acids from leached soil that accumulate in the quarter/tertiary canal during the early wet season (WS) and move to secondary and primary canals. Some areas are not inundated by spring tides but only seepage below the soil surface (Type C or D); the only water source is rainfall. The potency of pyrite oxidation is high in these areas, primarily during the DS, resulting in acidic soils. Leached acidic compounds (organic acids, Fe, Al) lead to much lower water pH in the canals than in the Type B areas. The influence of the spring tide on water pH is presented in Figure 3, where water acidity increases with distance from the estuary.
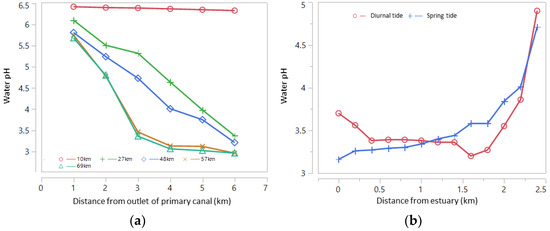
Figure 3.
(a) Water pH at the peak of the spring tide based on distance from river/sea estuary in the primary canal along Barito River, South Kalimantan, measured at distances of 1, 2, 3, 4, 5 and 6 km from the canal estuary, and (b) water pH during the spring tide and the diurnal tide in the secondary canal of the Betaguh wetland irrigation region of Pulang Pisau Regency, Central Kalimantan, as measured on 10 June 2021. Source: primary data from Author/Khairil Anwar.
The depth of the pyrite layer also influences water quality in the ASS area. In the secondary canal (SC) edges with deep pyrite layers and more acidic irrigation water, the water pH will increase upstream of the SC. Mixing tidewater and drainage water in the SC leads to variations in water quality (Figure 3). In the SC, a regular pattern of water pH is more common during the diurnal tide than during the spring tide. The pH of the water in the primary canal (PC) is lower than that of the water in the SC during the diurnal tide; however, the pH of the water in the PC is higher than that of the water in the SC during the spring tide.
The water’s acidity level is an indicator that pyrite has been oxidized. In Type B ASS areas with poor drainage, water acidity negatively correlates with the water’s electrical conductivity (EC), Al3+, Fe2+, Mn2+, SO24−, Ca2+, Mg2+, Na+, and SiO2. Poor drainage in the PC leads to the accumulation of leached ions in the PC water body. Multazam et al. [36] confirmed that water pH is negatively correlated with the EC value. This correlation pattern differs from that in areas with better water circulation, as in the Type A area, where ions easily leach to the edge of the PC.
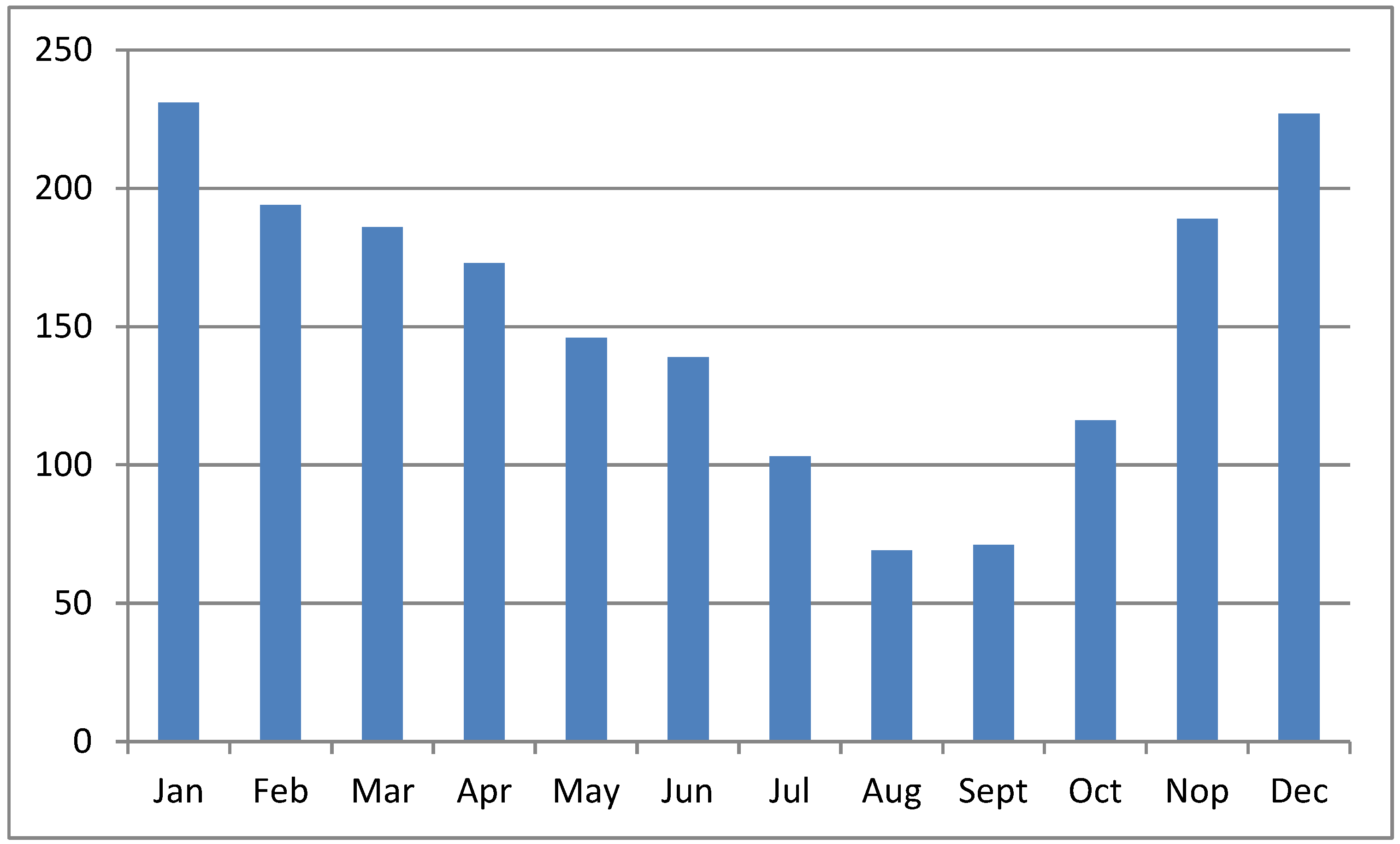
The sea surface is higher during the WS than during the DS [37]; therefore, the potency of tide overflow during the WS is higher than during the DS. Moreover, the volume of water in rivers, tributaries, and other water bodies in the upper region increases the tide overflow potency. Figure 4 shows the monthly rainfall patterns in South Kalimantan province, Indonesia, where the overflow potency is low from July to September. Overflow potency is associated with pyrite oxidation, the leaching of acids from the soil, and acidic compounds in the water canal. Therefore, pyrite oxidation occurs during the DS, and acids leach at the beginning of the WS. As a result, water pH is very low in areas with poor drainage at the start of the rainy season (October–December). Rainfall leaches acidic compounds and toxic ions from the ASSs and increases the acidic content and toxic ions in water bodies downstream [38]. Water pH increased in January along with the increasing rainfall intensity, leading to the dilution of acidic compounds. The fluctuation in standing water levels in the primary canal is influenced by rainfall [36].
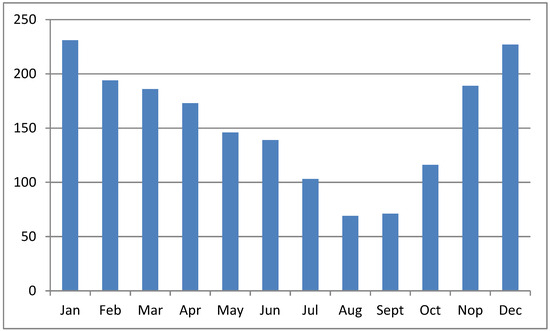
Figure 4.
Monthly average rainfall (in millimeters) in South Kalimantan province, Indonesia. Source: https://dataonline.bmkg.go.id, accessed on 21 January 2024.
Standing water in the tidal region follows the sea tide dynamically, and changes by hour, day, and month depending on the moon’s position relative to the earth and the sun. Such dynamics lead to different potencies in the overflow and drainage over time. Standing water can supply irrigated sources in paddy fields, adjust application time during rice cultivation, leach toxic ions, determine planting time, and select water management technologies [36]. In general, tide overflow supplies good quality water and is used to leach toxic ions into the land by the receding water.
Water management is critical to the sustainable management of ASSs because water management can prevent pyrite oxidation, the leaching of acidic and toxic elements and compounds from the soil, and the supply of water for crops. Water management is based on tidal overflow type, potency, and water table depth (Types A, B, C, and D) [39,40]. Water management can be tailored to the soil, hydrological characteristics, and rice needs and adjusted to the problems in each tidal overflow type.
Water management in ASSs fails because soil and water become more acidic, and crop productivity decreases [41]. This conclusion is supported by the results of research on water acidity [36]. Hence, water management must (i) be tailored to the hydrological characteristics, climate, and soil in each location, (ii) be supported by the government (central and local) and the farmer using the water, and (iii) conducted at macro scales (wetland irrigation regions) and micro-scales (paddy fields).
Type A tidal areas show high inundation during the rainy season, and the areas experience saltwater intrusion during the dry season. Therefore, water infrastructure, including periphery dikes and flapped water gates, is required to prevent overflow and saltwater intrusion (Figure 5a). Type B tidal areas with impeded drainage and poor water quality are a problem because leached acids in waterways cannot go out to river estuaries due to the push from the tide. This condition requires implementing a one-way direction of fork-like canals (macro water management) supported by implementation in paddy fields (micro-scale) for better water circulation. In addition, the primary, secondary, and tertiary canals are shorter than the existing ones and are tailored to each area’s irrigated/drainage potency in such a way that acids move out from the site with the subsided water. A one-way directional system requires a swaying water gate and stop log (overflow) in the paddy fields. A swaying water gate is used to ascertain water circulation during the WS. During the DS, a stop log conserves water and prevents pyrite oxidation (Figure 5b).

Figure 5.
Types of water gates: (a) flapped water gate, (b) swaying water gate, (c) stoplog water gate. Photo by Author/Khairil Anwar.
In Type C tidal areas, acids formed in soils during the DS are leached during the WS; paddy fields are inundated 5–10 cm to prevent pyrite oxidation by using overflow cascades starting from the paddy fields to tertiary and quarter canals (Figure 5c). Lowering inundation and the water table leads to pyrite oxidation, resulting in very acidic soils and water; hence, the water table requires better management.
The ASSs need leaching so that acids and toxic ions can leave the paddy field, but the water needs maintenance to prevent pyrite oxidation [42]. Precise water management, low prices, and economics are key factors in sustainable ASS management [43]. After implementing correct water management, ASSs need amelioration and fertilizers to support crop production [44].
4. Soil Amelioration
Soil amelioration improves soil properties so that the soil is favorable for crop growth and production. Table 2 lists the ameliorants used in ASSs in Kalimantan, including lime, organic fertilizers, biochar, compost, and fly ash. The efficacy of ameliorants on soil properties and rice yield depends on the type and dosage of ameliorant and the soil characteristics.

Table 2.
Contributions of ameliorants to the improvement of the properties of acid-sulfate soils and rice growth and yield in Kalimantan.
Application of lime on ASSs raises soil pH, reduces Al and Fe content, and improves rice growth. Applying magnesium limestone plus biofertilizers increases soil nutrient content, i.e., total N, available P, exchangeable Ca, and exchangeable Mg [46]. Liming and application of organic fertilizer increased rice yields in acid-sulfate paddy soils [45].
Rice-husk biochar was better than ash at improving the chemical properties of ASSs (namely pH, SOC, available P, exchangeable K, exchangeable Na, exchangeable Mg, and CEC) and decreasing Al and Fe content [47]. Applying rice-husk biochar increased soil pH to 5.0 or more and rice yields by 20% [51]. Combining rice-husk biochar (5 Mg ha−1) and chicken manure (0.5 Mg ha−1) increased soil pH and P availability, decreased Fe and iron toxicity, and enhanced rice growth and yield [52]. Combining biochar (from empty fruit bunches of oil palm) increased rice yield from 141 to 472% and decreased Al toxicity [53].
Compost increases available P, whereas biochar is more effective at mitigating GHG by suppressing CO2 emissions [54]. Compost increases soil pH and improves rice growth in ASSs [54]. Applying organic material (compost) to soil improves the pH of ASSs and its effect depends on the Eh and sulfate contents. Organic matter can temporarily replace liming in land management [55]. Adding organic matter can increase P availability in ASSs and P release from the soil [56]. Compost is potent in the arrangement of nematode communities by increasing biodiversity, trophic structure, and metabolic tract in ASS-based paddy fields [57].
Applying biofertilizers (microbes) improves the quality of ASSs. Phosphate-solvent bacteria secrete organic acids, which deactivate Al and Fe through chelation. It also increases soil pH, precipitating Al or Fe as inert hydroxide Al or Fe, decreasing Al and Fe availability [58]. Sulfate-reduction bacteria (Desulfovibrio sp.) are essential in reducing acid-sulfate soils, increasing soil pH and rice yield [52].
Soil amelioration is an essential treatment for ASSs. Ameliorants (lime, biochar, organic fertilizer, compost, ash, and fly ash) and their rates and effects on soil properties have been discussed. Nevertheless, crops still need nutrient input due to the low nutrient content of these soils; thus, fertilizer application is required.
5. Fertilizer Application
The nutrient (N, P, and K) content of ASSs in Kalimantan is generally low to medium, while that of exchangeable Ca is very low to low and the soil pH is 4.5 or lower. Thus, fertilizer application is a priority in soil management and the rate and application depend on the crop, tidal type, and land typology. Field studies assessed dose and production at selected rice production centers, as presented in Table 3.

Table 3.
Rice yields and fertilizers for selected acid-sulfate soils-based tidal paddy fields in Kalimantan.
Levels of N input and N loss in N cycles determine soil nitrogen content variations. Low N content occurs because N is taken up by crops, leached, and volatilized [69]. On average, nutrient loss for every ton of superior rice variety at harvest is about 17.5 kg ha−1 of N, 3.0 kg ha−1 of P, and 17.0 kg ha−1 of K [70]. ASSs with low N status need N fertilizer application. Applying 90 kg ha−1 of N to ASSs containing a total N of 0.25% yielded 4.1 Mg ha−1 of rice while adding 135 kg ha−1 of N showed no increase in yield [71]. Farmers commonly apply urea at more than the recommended rate.
The content of available P in ASSs is low, although the total P content may be high. In the ASSs in South Kalimantan, the available P is very low to medium [72] because Al and Fe fix P [73] in very acidic soil reactions (pH of 2.5–3.9). The effect of P fertilizer application on rice yield depends on P status in the soils; in low P status, fertilizer application significantly increases rice yield. Applying 22.5 kg ha−1 of P2O5 increases rice yield from 3.23 to 4.40 Mg ha−1. Statistically, there were no differences in rice yields between using 22.5 and 45–67.5 kg ha−1 of P2O5 [74].
The availability of K in ASSs is mainly low to very low. For instance, the available K in ASSs in South Kalimantan ranges from 0.09 to 0.25 cmol kg−1, and is categorized as low to very low [72]. K is an essential macronutrient that regulates stomata movement, energy transfer, anion balance, and stress resistance [75]. K is crucial to photosynthesis, carbohydrate distribution, and starch synthesis, leading to higher rice yields [76]. Applying 25 to 37.5 kg ha−1 of K2O under low K status increases grain weight and influences seed quality. However, using a higher K fertilizer rate does not affect yield increase.
Balanced fertilizer application to ASSs has better yield than partial application of only N fertilizer, P fertilizer, or K Fertilizer. Balanced fertilizer can also be applied to local rice varieties. Adding 60 kg ha−1 of N, 60 kg ha−1 of P2O5, and 50 kg ha−1 of K2O to ASSs increased local rice yield by 42%–77% [77]. Application of NPK compound fertilizer and urea are other options for increasing crop production.
In ASSs, liming can increase the effectiveness of the fertilizer. Liming and N, P, and K fertilizer addition increased rice yield from 0.64 Mg ha−1 to 4.24 Mg ha−1. The contributions of lime, N fertilizer, P fertilizer, and K fertilizer to this yield increase were 33.9%, 33.3%, 22.7%, and 10.1%, respectively. For one hectare of this soil, the fertilizer rates for superior varieties are 67.5–135 kg N, 45–70 kg P2O5, 50–75 kg K2O, and 1–3 Mg lime [78].
Results of other studies suggest that increasing crop production in ASSs requires the application of chemical fertilizers (N, P, K), organic fertilizers, and biofertilizers. Biofertilizers contain microbes such as decomposers (Trichoderma sp.), P solvents (Bacillus sp.), and N fixers (Azospirillium sp.). Biofertilizers increase N and P availability, accelerate organic residue decomposition, and promote crop growth. Applying 25 kg ha−1 of Biotara (a biofertilizer), 400 kg ha−1 of NPK compound fertilizer, and in-situ organic matter increased rice yield by 35%–48% [64,74]. Applying 25 kg ha−1 of Biotara and 300 kg ha−1 of NPK compound fertilizer to ASSs increased total N, available P, and available K in Barito Kuala Regency, South Kalimantan province [64].
Organic fertilizer decomposition increased macro and micronutrients in the soil [79]. In tidal paddy soils, applying organic fertilizers, compost from manure, compost from rice straw, and compost from Salvinia sp. increased rice yield by 3.60 Mg ha−1, 3.73 Mg ha−1, and 3.54 Mg ha−1 compared to not applying organic fertilizers (3.15 Mg ha−1) [67]. Adding organic matter increased rice yield and reduced the use of inorganic fertilizers in tidal paddy fields [65]. Thus, for a given rice variety, fertilizer application is site-specific.
6. Adaptive Varieties and Gene Conservation
6.1. Adaptive Varieties
When selecting rice varieties for planting, farmers consider market demand and preference, plant age, high yield, plant height, tolerance to abiotic stress, and resistance to pests and diseases. The local rice variety is adaptive to environmental growth but has a low yield; thus, improving rice varieties for ASSs requires creating a rice variety that is adapted to high soil acidity, iron toxicity, and water stress (flooding and dryness). Iron toxicity limits rice growth and reduces rice yield by 30–60% [80]. The decrease in yield differs in iron-tolerant varieties [81]; the decline is up to 30% for an iron-tolerant variety but 75% for an iron-sensitive one. Iron-tolerant rice varieties absorb and translocate less iron from roots to leaves compared with iron-sensitive varieties.
Improved varieties recommended for acid-sulfate paddy soils in Indonesia include Inpara, Mekongga, and Ciherang. There are nine varieties of Inpara, from Inpara 1 to Inpara 9 [82]. The adaptation test in the tidal paddy field in Barito Kuala Regency (South Kalimantan province) showed that five of nine varieties (Inpara 3, Inpara 4, Inpara 6, Inpara 8, and Inpara 9) were adapted to local conditions. They yielded more than 3 Mg ha−1 of unmilled rice (Table 4). Inpara 4, Inpara 6, Inpara 8, and Inpara 9 may be introduced to farmers as alternatives to Inpara 2 and Inpara 3. Farmers in Barito Kuala Regency (South Kalimantan province) have planted Inpara 2 and Inpara 3 since 2012, while farmers in Hulu Sungai Selatan Regency (South Kalimantan province) have planted Inpara 4. Inpara 2 and Inpara 3 gave a yield of about 4.12–6.20 Mg ha−1 [83,84].

Table 4.
Adaptive and farmers’ preferred rice varieties in Barito Kuala Regency (South Kalimantan province) in the 2016 dry season.
In the tidal paddy fields of Sambas Regency (West Kalimantan province), Inpara yielded 5.43 Mg ha−1 of rice [62], higher than in South Kalimantan province, which yielded 3.09 Mg ha−1 of rice [85]. The yield difference was due to soil fertility and iron toxicity levels. In West Kalimantan, the soil pH was 5.3 and the iron content was 150 mg kg−1 (showing no symptoms of iron toxicity), whereas in South Kalimantan, the soil pH was 4.62 and the iron content was 439 mg kg−1 (showing symptoms of iron toxicity). Low soil fertility and iron toxicity are responsible for the low productivity of superior varieties, ranging from 3.0 to 4.0 Mg ha−1 [85], much lower than the potential yield of about 5.0–7.6 Mg ha−1 [82].
6.2. Conservation of Genes of Local Rice Varieties
Several local tidal rice varieties are available, and some are grown by farmers. Conservation of these local varieties is crucial for safeguarding biodiversity and as materials for improving rice varieties. The indigenous agriculturalists residing in South Kalimantan acknowledged and designated indigenous tidal rice cultivars contingent upon the visual characteristics of the lemma and palea husk coloration. From a genetic standpoint, the dissimilarity in husk coloration could signify genetic or phenotypic adaptability, precisely the capacity of individual genotypes required to generate diverse phenotypes in response to alternative environmental circumstances [86]. Conserving local rice varieties is vital to preventing genetic erosion [87,88,89].
Genebanks, exemplifying ex-situ conservation, guarantee the accessibility, thorough characterization, and documentation of stored materials, thus safeguarding them considerably from external risks [90]. It ensures germplasm preservation when plants are obliterated from their original habitats. Additionally, from the user’s perspective, it can consolidate materials from diverse and dispersed locations into a single site that is readily accessible for utilization [91].
Indonesia is an archipelago distinguished by various climatic conditions, ecological geography, and agricultural practices that sustain extensive rice diversity. With Indonesia’s vast biodiversity, the abundance of genetic resource variability is considerable, encompassing diverse geographical areas. Each specific area in Indonesia possesses numerous distinct genetic resources, often dissimilar to those found in other regions [92]; these are primarily local varieties, and thus immensely different cross islands. Local rice varieties in South Kalimantan exhibit distinctive characteristics. These varieties range in plant height from 105 to 180 cm, with 10–24 tillers. The panicle is prominently exposed and grain threshing is moderate (6%–25%). The leaf angle is horizontal and the flag leaf angle is intermediate and flat, lacking the upright angle in high-yielding varieties. Similarly, the stem angle is generally moderate, falling between upright and open. More than 3300 rice accession numbers are stored in the Indonesian Gene Bank [93].
In the history of rice breeding, numerous studies have shown that rice landraces are the progenitor lines of promising new varieties. The development of IR8 [94], the identification of genes for submergence tolerance [95], and the improvement of rice yield [96] are noteworthy among these studies. IR8 is a hybrid of two landraces, Peta, an active and tall rice variety from Indonesia, and Dee-geo-woo-gen, a Chinese semi-dwarf rice type [94]. In Indonesia, the development of superior rice varieties through cross-breeding began in the 1900s using germplasm originating from various sources. Until 1965, rice breeding was directed at establishing varieties suitable for multiple land conditions, including land with medium and low fertility levels [97]. The most significant increase in production occurred from the 1970s to the 1980s with the introduction of new high-yielding varieties that were more responsive to fertilizers and matured early, for example, IR36, Cisadane, IR64, and IR66, with a growth rate of 3.3% each year [98].
Genetic diversity is also valuable in the gene conservation of natural resources [99]. Several essential genes were discovered and significantly contributed to the rice breeding process. Regarding submergence tolerance, submergence 1 quantitative trait locus (SUB1 QTL) is the origin of the rice landrace FR13A [95]. The narrow leaf 1 (NAL1) allele in the Tropical Japonica rice landrace Daringan is responsible for the substantial enhancement of the yield of contemporary rice varieties [96]. Yustisia et al. [100] reported that the levels of iron and zinc in brown rice varied across five high-yielding varieties (Ciherang, Widas, IR64, Cisokan, and Cimelati) that were cultivated in Inseptisols, with Fe ranging from 10.84 to 19.80 mg kg−1, and Zn ranging from 19.64 to 24.55 mg kg−1. The Widas variety possessed the highest Fe concentration whereas the Cisokan variety had the lowest.
The above information significantly contributed to the development of rice varieties and increased rice production in Indonesia over the years. During the pre-green revolution period, there was a notable enhancement in rice productivity in Indonesia. Notably, the mean yield per hectare, as recorded by FAOStat, went up from 1.76 Mg ha−1 in 1961 to 2.25 Mg ha−1 in 1969, subsequently increasing to 2.38 Mg ha−1 in 1970. This increase can be primarily attributed to the extensive adoption of high-yielding varieties [101]. During the Green Revolution decade, rice varieties with excellent yields and tolerance to numerous pests and plant diseases were released. One of these was IR-64, which has dominated rice fields in Indonesia since its introduction in 1986 due to its high yield and resistance to brown planthoppers. Superior cultivars boosted national rice productivity from 3.96 Mg ha−1 year−1 on average during the 1980–1990 period to 4.35 Mg ha−1 year−1 during the 1990–2000 period, an average gain of 0.23% yearly [101].
In addition to water conditions, soil management, adaptive rice varieties, farming systems, and technology adoption are other essential factors required for successful crop production in coastal acid-sulfate soils. Human resource characteristics and conditions (farmers and other stakeholders) also play crucial roles.
7. Farming Systems and Technology Adoption
In Kalimantan, ASSs predominate tidal paddy fields, mainly in Sambas Regency (West Kalimantan province), Pulangpisau and Kapuas Regency (Central Kalimantan province), and Barito Kuala Regency (South Kalimantan province). These soils are also used in oil palm plantations and swamp forests. Most rice production centers in Indonesia have acid-sulfate paddy soils. Hence, the discussion will focus on the rice cultivation system.
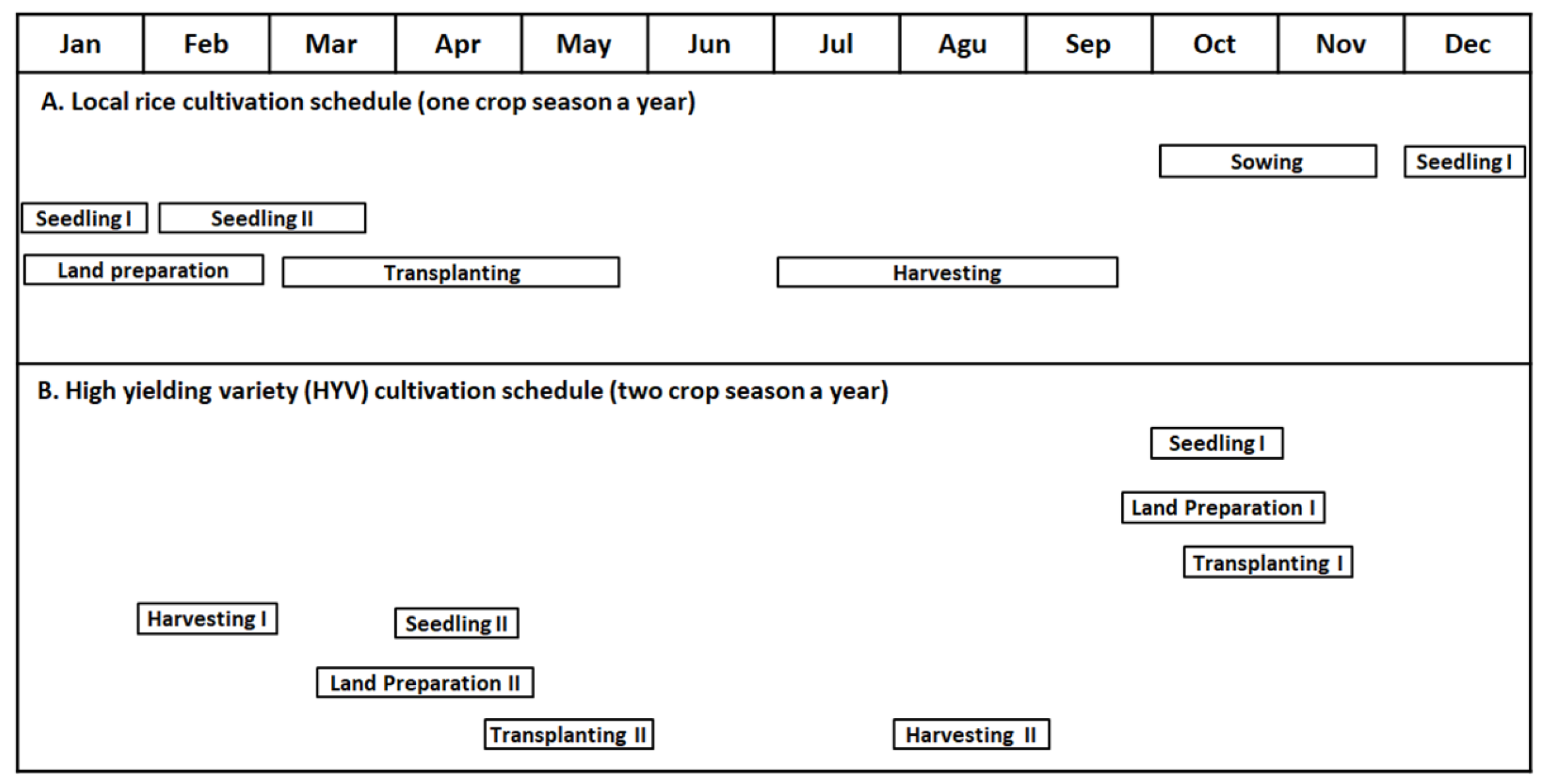
Figure 6 shows the schedules for the cultivation of the local rice variety (A) and the high-yielding rice variety (B) in tidal paddy fields in South Kalimantan province. The local rice is planted once a year, while superior rice is cultivated twice a year, especially in tidal paddy fields with good water management. For local rice, sowing begins from October to November, at the start of the rainy season; from December to January, 30-day-old rice plants are transplanted to the area near the paddy fields as the first seedlings. Then, 45–60-day-old rice plants are transplanted again to the edge of paddy fields as the second seedlings (from February to March). Finally, these plants are transplanted to entire fields (from March to May). For the first seedlings, farmers prepare land from January to February. The farmers harvest the rice from July to September. Seedling adjustment is conducted to tailor inundation in the paddy fields. For high-yielding varieties, farmers cultivate rice twice. The first rice cultivation begins in October, where seedling generation, land preparation, and transplanting are performed from October to November. Then, farmers harvest the rice from February to March. The second rice cultivation period begins in April, where seedling generation, land preparation, and transplanting are performed from March to May. The farmers then harvest rice from August to September. Understanding rice cultivation schedules in ASS-dominated areas is crucial to rice production and farmer-related activities.
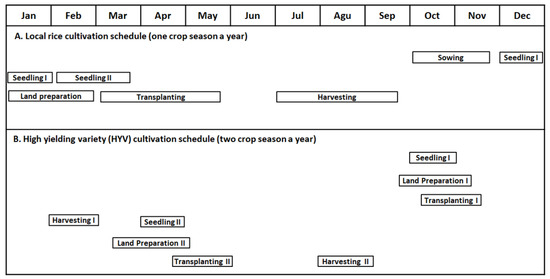
Figure 6.
Schedule for the cultivation of local and superior varieties of rice in South Kalimantan province. Reused with permission from [66]; copyright owned by the MDPI.
Farmers apply several rice cultivation technologies ranging from indigenous knowledge to modern ones. Banjarese farmers, for instance, use indigenous knowledge, termed the tajak-puntal-balik-ampar system, for land preparation. Tajak means cutting paddy stubble and other grasses using a tajak (long sword) (Figure 7a). These stubbles and others are collected in spots, forming a ball-like, mixed organic matter called puntal (Figure 7b) and then stored for about one month. These balls are turned up and down at specific times (balik) so that decomposition occurs evenly. After sufficient composting, the organic matter stack is cut off, separated, and spread evenly on the soil surface (called ampar), as shown in Figure 7c [102]. This system prevents soil acidification, increases the pH from 3.0–3.9 to 5.80–6.20 [103], and the yield of the local rice variety from 2.5 Mg ha−1 to 3.0 Mg ha−1.

Figure 7.
Traditional Banjarese land preparation for rice cultivation: (a) Tajak is used for land clearing, (b) the residue is arranged into Puntal, and (c) compost residues are applied on the land (ampar). Photo by Author/M. Saleh.
Some farmers prefer to grow local varieties rather than superior ones because local varieties tolerate high iron content and very low acidity. The local varieties have photoperiod characteristics, namely, they blossom depending on solar radiation. The generation of seedlings of local varieties follows three steps: taradak, ampak, and lacak [104]. Taradak refers to sowing at the beginning of the wet season. After 40 days, the plants are moved to the second seedling area (ampak). After 40 days in ampak, the plants are transferred to the third seedling area (lacak). The duration of plants in the lacak depends on the inundation level in the planting area.
Other farmers use superior varieties combined with transplanting techniques. Rice transplanting is a common practice of farmers who grow superior varieties, where 25-day-old plants are planted. Farmers who use local varieties grow 60–90-day-old plants after sowing tailored to water inundation [105]. However, the transplanting technique requires more labor and time.
The seedlings can be grown in wet or dry media. In dry seedlings, seeds are distributed in the dry bed, usually in a bund, roadside, or yard, for ease of plant transport. In wet seedlings, seeds are spread in moist beds. The farmer applies 0.1–0.2 kg of dolomite, 5 g of urea, and 5 g of KCl for a one-meter square seedling medium. Each square meter needs 6.25 g of seeds; hence, one ha needs 25 kg. Seeds are submerged for about one night, air-dried, and stored for 24 h before sowing [105]. Sowing is conducted at the beginning of the wet season.
Rice seeds grow well under muddy conditions; hence, the soil tillage method and equipment selected should take into account pyrite depth during land preparation. Land with a pyrite depth of 0.5 m or more offers more opportunity to use the tillage method due to the low risk of pyrite oxidation. However, land with a shallow pyrite layer needs conservation tillage techniques [106]. Meanwhile, the one-way water management system creates better planting conditions because this system can reduce the accumulation of toxic substances.
Besides the tegel system, the Jajar Legowo planting system is used to increase the crop population (Figure 8a). Using jajar legowo 2:1 (Figure 8b), the population increased by 33%, and the yield of superior varieties increased by 43% compared to farmer techniques [107]. The jajar legowo method can be implemented manually or using a transplanter. In flat and 27-cm-deep mud with 3–4 seeds per hole, machinery increases the yield by about 9.89% compared to manual techniques [108]. The challenge of using transplanters is the requirement for a no-flat planting area and mud, resulting in no straight plant strip. Inundation is also a challenge when using a transplanter.

Figure 8.
Rice planting systems and planting distances for optimizing space use, solar radiation, and crop populations: (a) Tegel system, (b) Jajar Legowo 2:1 system, (c) Jajar legowo 3:1 system, and (d) Jajar Legowo 4:1 system. Photo by Author/M. Saleh.
Some farmers use direct seeding, spreading seeds by hand, or using a seedier. Direct seeding requires soil tillage, good water management, a flat planting area, and 0.5–1.0 cm inundation. This technique can save labor and time but requires more seeds—from one-half to twice—compared to the transplanting practice. Other weaknesses include the possibility of seed loss in water due to increasing inundation, loss due to pests (rats, fish), and competition for solar radiation, water, and nutrients by weeds. A pre-growth herbicide is recommended for weed control [109].
A farmer may use a seeder for sowing seeds. Different types of seeders are available. Some farmers manually operate a pipe seeder–a 3-inch-diameter PVC (polyvinyl chloride) pipe with two wheelies. Using this pipe seeder, a farmer distributes seeds evenly following the planting distance set by the equipment. Other seeders include drum seeders, power seeders, and drones. Drones are more effective than drums and power seeders but are not currently used by farmers.
Some farmers use combined rice harvesters to harvest rice, whereas others still use traditional rice harvesters due to the small sizes of their paddy fields. In ASSs, operators should ascertain that paddy fields have good accessibility, no muddy areas, and low inundation so that they can run the combined harvester properly. In addition to the application of machinery, greenhouse gas (GHG) emissions are an emerging issue in rice cultivation on coastal ASSs.
8. Emerging GHG Emission Issues
ASS inundation (due to tides, rainfall, and extreme monsoons) controls soil biochemical processes that impact changes in soil oxidation–reduction (Eh) and influence soil behavior. During flooding, sulfate acid (H2SO4) is released from the sulfuric horizon, leading to deoxygenation and/or water acidification [18,110] and triggering environmental degradation. When sulfate acid reacts with carbonate minerals, H+ converts inorganic carbonate to CO2 because of the dilution of the acid [111]. CO2 production stops if Fe (II) or inorganic carbon over CO2 emission from the soil system results from a biological process, namely soil respiration. Microbe abundance and activity and organic carbon availability determine CO2 emissions [112,113].
Rice cultivation in tidal paddy fields releases CO2, N2O, and CH4 into the atmosphere. CO2 emission is controlled by carbon source availability (organic matter), microbe activity, and soil characteristics (mainly moisture content, pH, and redox potential) [112,113]. The emission of CH4 is correlated with physical properties of soil associated with soil–water movement [6]. Methanogenesis, the process responsible for CH4 production, occurs when 70–80% of the pore spaces are filled with water. Time is needed to reduce molecular oxygen and electron acceptors trapped in the soil pores [114]. Methanogenesis involves methane-producing bacteria in anaerobic zones and methane-oxidizing bacteria in aerobic zones [115,116]. Methane-oxidizing bacteria can oxidize more than 50% of CH4.
The interaction between soil pH and Eh is essential for CH4 emission. The critical determinant of CH4 emission occurs after ASSs are submerged, starting from Eh of −150 mV and pH of 6.5–7.5 [117,118]. During submersion, soil acidity determines the methanogenic microbes. The activity of methanogenic microbes in the soil is controlled by soil Eh, pH, organic matter content, and temperature. The low pH is responsible for the effect of organic matter on CH4 emissions. Microbes mineralize organic matter, resulting in biochemical changes in the soil that decrease redox potential. CH4 production is influenced by soil and nutrient management systems and the rice growth stage [119,120]. The tight requirement for an anoxic condition to produce CH4 shows the importance of precise water management.
Good management practices to minimize CH4 emission during rice cultivation in ASSs include (i) selection of rice variety, (ii) amelioration, (iii) fertilization, and (iv) water management. CH4 emissions vary greatly, depending on water conditions, soil characteristics, cultivation system, rice variety, amelioration, and fertilization (Table 5).

Table 5.
CH4 emission during rice cultivation in acid-sulfate paddy soils of Kalimantan.
N2O emission is closely associated with denitrification, namely, changing N depending on microbe activity, environmental conditions, and N and C sources. Denitrification occurs under anaerobic conditions [124] and is influenced by several factors, including soil aeration, moisture content, NO3 and C availability, and soil pH [125,126,127]. Denitrification is closely associated with organic carbon dissolved in the soil [126]. In ASSs, nitrates oxidized the reduced Fe, resulting in N2O and other N gasses [127].
Submerging accelerates the utilization of electron acceptors (e.g., NH4+, Mn4+, Fe3+, and SO42−). N2O emission occurs due to the addition of N sources (e.g., urea, manure), microbe activity, and environmental conditions supporting the growth of nitrification bacteria (i.e., pH, temperature, and aeration). Denitrification occurs in submerged ASSs; that is, NO3 and/or NO2 are reduced to NO, NP, and N gases, catalyzed by denitrifying microorganisms [128,129].
Measuring GHG emissions during rice cultivation in paddy fields uses the close chamber technique from the International Atomic Energy Agency [130]. In this method, samples are collected with a syringe for laboratory analysis. The closed chamber method provides estimates of GHG fluxes at the observation plot level of less than 1.0 m2 (small scale). The technique is used to study processes that occur in the soil, including microbial activity. GHG emissions from paddy fields are from complex interactions between environmental conditions, soil properties, and management practices. Implementing appropriate land management will minimize GHG emissions.
9. Challenges and Future Directions
Specific and unique tidal land ecosystems need particular care for production or conservation purposes. Disturbing its natural condition changes soil and water properties, and even rice productivity decreases due to mismanagement and degradation. Therefore, the use of tidal land and ASSs for agricultural production should consider the land’s characteristics and crops selected based on suitability. Keys to successful and sustainable agricultural production include soil and water management, adaptive variety selection, amelioration, and fertilizer application tailored to crop needs and soil nutrient status. Based on our experience of more than 30 years in research, development, and offering technical assistance to farmers, we list the remaining challenges and proposed future directions in Table 6.

Table 6.
List of challenges and future directions for successful and sustainable rice production in coastal acid-sulfate paddy soils.
For more efficient implementation, at least four activities are required: (i) re-inventory of soil and water and farmer characteristics, (ii) developing and rehabilitating infrastructure, (iii) developing agribusiness models from upstream to downstream using a holistic and integrated implementation of technology innovation, and (iv) social and institutional engineering.
10. Conclusions
The coastal acid-sulfate soils of Kalimantan cover about 3.5 Mha and remain an invaluable resource for paddy fields and other wetland agriculture. The area has become the national agricultural production center but faces high acidity, a high iron and aluminium toxicity risk, and low soil fertility. Water management for better water circulation is crucial in controlling iron and aluminium toxicity and providing good water quality for crop growth. Water management, soil amelioration, fertilizer application, crop variety selection, and site-specific rice cultivation techniques are keys to high crop production.
Soil ameliorants that raise soil pH, decrease iron and aluminium toxicity and improve crop yield include lime, biochar, organic fertilizer, compost, ash, and fly ash. Fertilizer application for rice requires 80–200 kg ha−1 of Urea, 50–150 kg ha−1 of SP36, 50–150 kg ha−1 of KCL, and 125–400 kg ha−1 of NPK compound fertilizer. The rate and time for ameliorants and fertilizer applications depend on crop variety, soil properties, and soil nutrient status.
Farmers implement traditional or modern rice cultivation technologies, with cultivation schedules adjusted based on rice variety, available resources, water inundation, and water management conditions. Rice varieties are continuously improved to provide tolerant but high-yielding varieties. High CH4 and CO2 emissions challenge rice cultivation but could be minimized by rice variety selection, amelioration, fertilization, and water management.
The remaining challenges are technology development and transfer in water management, soil amelioration, fertilizer application, crop varieties, cultivation technology, land use optimization, climate change adaptation and mitigation, technology adoption and implementation, and resource conservation. Each aspect has specific goals and exit strategies. Four activities are required for effective implementation: (i) re-inventory of soil, water, and farmer characteristics, (ii) developing and rehabilitating infrastructure, (iii) developing agribusiness models from upstream to downstream using holistic and integrated implementation of technology innovation, and (iv) social and institutional engineering.
Author Contributions
Conceptualization, Y.S., E.M., K.A. and I.K.; methodology, Y.S., E.M. and K.A.; software, A.H.; validation, Y.S., I.A.R., M.S., M.N. and A.F.; formal analysis, Y.S., A.F. and A.H.; investigation, Y.S., M.M., I.K., M.A., R.D.N. and I.A.R.; resources, A.N. and M.A.; data curation, Y.S., I.K. and M.S.; writing—original draft preparation, Y.S., E.M., M.N., A.H., S.N., M.M., K.A., A.F., M.S., I.K., I.A.R., M.A., A.N. and R.D.N.; writing—review and editing, Y.S., E.M., M.N., K.A., S.N., M.M., K.A., A.F., M.S., I.K., I.A.R., M.A., A.N. and R.D.N.; visualization, Y.S. and A.H.; supervision, Y.S. and E.M.; project administration, E.M.; funding acquisition, Y.S. and E.M. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by BRIN.
Data Availability Statement
Data are available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
ASSs = acid-sulfate soils; DS = dry season; WS = wet season; PC = primary canal; SC = secondary canal; PVC = polyvinyl chloride; GHG = greenhouse gas.
References
- Soil Survey Staff. Soil Taxonomy. A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Agricultural Handbook 436; Natural Resources Conservation Service, USDA: Washington, DC, USA, 1999. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Noor, M. Lahan Rawa: Sifat Dan Pengelolaan Tanah Bermasalah Sulfat Masam; PT Raja Grafindo Persada: Jakarta, Indonesia, 2004; ISBN 979-3654-28-7. [Google Scholar]
- Sullivan, L. Acid Sulfate Soils and Their Management: A Global Prespective. In Proceedings of the 7th International Acid Sulfate Soils Conference, Vassa, Finland, 26 August–1 September 2012; The Geological Survey of Finland and IUSS: Vassa, Finland, 2012; pp. 127–129. [Google Scholar]
- Powell, B.; Martens, M. A Review of Acid Sulfate Soil Impacts, Actions and Policies That Impact on Water Quality in Great Barrier Reef Catchments, Including a Case Study on Remediation at East Trinity. Mar. Pollut. Bull. 2005, 51, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.; Fatah, L.; Syaifuddin; Abdullah; Affandi, D.N.; Bakar, R.A.; Inubushi, K. Greenhouse Gas Emissions from Peat Soils Cultivated to Rice Field, Oil Palm and Vegetable. J. Trop. Soils 2012, 17, 105–114. [Google Scholar] [CrossRef]
- Su, J.; Hu, C.; Yan, X.; Jin, Y.; Chen, Z.; Guan, Q.; Wang, Y.; Zhong, D.; Jansson, C.; Wang, F.; et al. Expression of Barley SUSIBA2 Transcription Factor Yields High-Starch Low-Methane Rice. Nature 2015, 523, 602–606. [Google Scholar] [CrossRef] [PubMed]
- BBSDLP. Sumber Daya Lahan Pertanian Indonesia: Luas, Penyebaran dan Potensi Ketersediaan, 2015th ed.; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2015; ISBN 978-602-344-083-2. [Google Scholar]
- Hidayat, A.; Hikmatullah; Suparto. Peta Sumberdaya Tanah Tingkat Tinjau Provinsi Kalimantan Barat, Skala 1:250.000; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2010; ISBN 978-602-8977-02-9. [Google Scholar]
- Suparto; Hidayat, H.; Prasodjo, N.; Ponidi. Peta Sumberdaya Tanah Tingkat Tinjau Provinsi Kalimantan Tengah, Skala 1:250.000; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2013; ISBN 978-602-8977-55-4. [Google Scholar]
- Hidayat, A.; Suparto; Hikmatullah. Peta Sumberdaya Tanah Tingkat Tinjau Provinsi Kalimantan Selatan, Skala 1:250.000; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2011; ISBN 978-602-8977-11-1. [Google Scholar]
- Suparto; Hikmatullah; Prasodjo, N.; Ropik; Ponidi; Kuncoro, R.D.; Amalia, L.; Widiastuti, F. Peta Sumberdaya Tanah Tingkat Tinjau Provinsi Kalimantan Timur, Skala 1:250.000; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2014; ISBN 978-602-8977-55-5. [Google Scholar]
- Hikmatullah; Suparto; Tafakresnanto, C.; Sulaeman, Y.; Mulyani, A. Identifikasi Dan Evaluasi Potensi Sumberdaya Lahan Berdasarkan Peta Warisan Untuk Pengembangan Kawasan Komoditas Pertanian Unggulan Di Nusa Tenggara, Kalimantan, Dan Sulawesi; BBSDLP: Bogor, Indonesia, 2013. [Google Scholar]
- Hikmatullah; Suparto; Tafakresnanto, C.; Sulaeman, Y. Identifikasi Dan Evaluasi Potensi Sumberdaya Lahan Untuk Pengembangan Pertanian Di Kalimantan, Sulawesi, Musa Tenggara, Dan Maluku; BBSDLP: Bogor, Indonesia, 2014. [Google Scholar]
- Jayalath, N.; Fitzpatrick, R.W.; Mosley, L.; Marschner, P. Type of Organic Carbon Amendment Influences pH Changes in Acid Sulfate Soils in Flooded and Dry Conditions. J. Soils Sediments 2016, 16, 518–526. [Google Scholar] [CrossRef]
- Jayalath, N.; Mosley, L.; Fitzpatrick, R.; Marschner, P. Addition of Organic Matter Influences pH Changes in Reduced and Oxidised Acid Sulfate Soils. Geoderma 2016, 262, 125–132. [Google Scholar] [CrossRef]
- Shamshuddin, J.; Azura, A.E.; Shazana, M.; Fauziah, C.; Panhwar, Q.; Naher, U. Properties and Management of Acid Sulfate Soils in Southeast Asia for Sustainable Cultivation of Rice, Oil Palm, and Cocoa. Adv. Agron. 2014, 124, 91–142. [Google Scholar] [CrossRef]
- Karimian, N.; Johnston, S.G.; Burton, E.D. Acidity Generation Accompanying Iron and Sulfur Transformations during Drought Simulation of Freshwater Re-Flooded Acid Sulfate Soils. Geoderma 2017, 285, 117–131. [Google Scholar] [CrossRef]
- Johnston, S.G.; Burton, E.D.; Hagan, R.; Aaso, T.; Tuckerman, G. A Revised Method for Determining Existing Acidity in Re-Flooded Acid Sulfate Soils. Appl. Geochem. 2015, 52, 16–22. [Google Scholar] [CrossRef]
- Johnston, S.G.; Burton, E.D.; Aaso, T.; Tuckerman, G. Sulfur, Iron and Carbon Cycling Following Hydrological Restoration of Acidic Freshwater Wetlands. Chem. Geol. 2014, 371, 9–26. [Google Scholar] [CrossRef]
- Anda, M.; Subardja, D. Assessing Soil Properties and Tidal Behaviors as a Strategy to Avoid Environmental Degradation in Developing New Paddy Fields in Tidal Areas. Agric. Ecosyst. Environ. 2013, 181, 90–100. [Google Scholar] [CrossRef]
- Yli-Halla, M.; Virtanen, S.; Regina, K.; Österholm, P.; Ehnvall, B.; Uusi-Kämppä, J. Nitrogen Stocks and Flows in an Acid Sulfate Soil. Environ. Monit. Assess. 2020, 192, 751. [Google Scholar] [CrossRef]
- Zin, K.P.; Lim, L.H.; Mallikarjunaiah, T.H.; Bandara, J.M.R.S. Chemical Properties and Phosphorus Fractions in Profiles of Acid Sulfate Soils of Major Rice Growing Areas in Brunei Darussalam. Geoderma Reg. 2015, 6, 22–30. [Google Scholar] [CrossRef]
- Prade, K.; Ottow, J.C.G.; Jacq, V. Excessive Iron Uptake (Iron Toxicity) by Wetland Rica (Oryza sativa L.) on Acid Sulphate Soil in the Casamance/Senegal. In Selected Papers of the Dakkar Symposium on Acid Sulphate Soils, Dakkar, Senegal, January, 1986; Dost, H., Ed.; ILRI Publication No. 44; International Land Reclamation Institute: Wageningen, The Netherlands, 1986; pp. 150–162. [Google Scholar]
- Wisawapipat, W.; Charoensri, K.; Runglerttrakoolchai, J. Solid-Phase Speciation and Solubility of Phosphorus in an Acid Sulfate Paddy Soil during Soil Reduction and Reoxidation as Affected by Oil Palm Ash and Biochar. J. Agric. Food Chem. 2017, 65, 704–710. [Google Scholar] [CrossRef]
- Dhanya, K.; Gladis, R. Acid Sulfate Soils-Its Characteristics and Nutrient Dynamics. Asian J. Soil Sci. 2017, 12, 221–227. [Google Scholar] [CrossRef]
- Sahrawat, K.L. Soil Fertility in Flooded and Non-Flooded Irrigated Rice Systems. Arch. Agron. Soil Sci. 2012, 58, 423–436. [Google Scholar] [CrossRef]
- Bhaduri, D.; Mandal, A.; Chakraborty, K.; Chatterjee, D.; Dey, R. Interlinked Chemical-Biological Processes in Anoxic Waterlogged Soil—A Review. Indian J. Agric. Sci. 2017, 87, 1587–1599. [Google Scholar] [CrossRef]
- Poggenburg, C.; Mikutta, R.; Schippers, A.; Dohrmann, R.; Guggenberger, G. Impact of Natural Organic Matter Coatings on the Microbial Reduction of Iron Oxides. Geochim. Cosmochim. Acta 2018, 224, 223–248. [Google Scholar] [CrossRef]
- Mosley, L.M.; Fitzpatrick, R.W.; Palmer, D.; Leyden, E.; Shand, P. Changes in Acidity and Metal Geochemistry in Soils, Groundwater, Drain and River Water in the Lower Murray River after a Severe Drought. Sci. Total Environ. 2014, 485, 281–291. [Google Scholar] [CrossRef]
- Kusmiyati, F.; Sumarsono, K.; Karno, K. Pengaruh Perbaikan Tanah Salin Terhadap Karakter Fisiologi Calopogonium Mucunoides. Pastura 2014, 4, 1–6. [Google Scholar]
- Yaduvanshi, N.P.S.; Lal, K.; Swarup, A. Effect of Sodic Water Irrigation with Sulphur through Single Super Phosphate on Yield, Mineral Composition and Soil Properties in Rice-Wheat System. J. Soil Salin. Water Qual. 2015, 7, 35–39. [Google Scholar]
- Pirasteh-Anosheh, H.; Parnian, A.; Spasiano, D.; Race, M.; Ashraf, M. Haloculture: A System to Mitigate the Negative Impacts of Pandemics on the Environment, Society and Economy, Emphasizing COVID-19. Environ. Res. 2021, 198, 111228. [Google Scholar] [CrossRef]
- Panhwar, Q.A.; Naher, U.A.; Shamshuddin, J.; Radziah, O.; Hakeem, K.R. Management of Acid Sulfate Soils for Sustainable Rice Cultivation in Malaysia. In Soil Science: Agricultural and Environmental Prospectives; Hakeem, K., Akhtar, J., Sabir, M., Eds.; Spirnger: Cham, Switzerland, 2016; pp. 91–104. [Google Scholar]
- Mahender, A.; Swamy, B.; Anandan, A.; Ali, J. Tolerance of Iron-Deficient and -Toxic Soil Conditions in Rice. Plants 2019, 8, 31. [Google Scholar] [CrossRef]
- Multazam, Z.; Utami, S.N.H.; Maas, A.; Anwar, K. The Impact of Seasonal Changes on Tidal Water Quality in Acid Sulfate Soils for Rice Cultivation and Water Management Strategies in South Kalimantan, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2022, 1005, 012023. [Google Scholar] [CrossRef]
- Auliyani, D.; Basuki, T.M.; Nugrahanto, E.B.; Widjaja, W.W. Penentuan Nisbah Hantar Sedimen Sub Daerah Aliran Sungai Watujali Dan Silengkong Kabupaten Kebumen. In Proceedings of the Seminar Nasional ke 4 Pengelolaan Pesisir Dan Daerah Aliran Sungai, Yogyakarta, Indonesia, 24 October 2018; Universitas Gajah Mada: Yogyakarta, Indonesia, 2018; pp. 1–8. [Google Scholar]
- Toivonen, J.; Hudd, R.; Nystrand, M.; Osterholm, P. Climatic Effects on Water Quality in Areas with Acid Sulfate Soils with Commensurable Consequences on the Reproduction of Burbot (Lota lota L.). Environ. Geochem. Health 2020, 4, 3141–3156. [Google Scholar] [CrossRef]
- Suriadikarta, D.A.; Sutriadi, M.T. Jenis Jenis Lahan Berpotensi Untuk Pengembangan Pertanian Lahan Rawa. J. Penelit. Dan Pengemb. Pertan. 2007, 26, 115–122. [Google Scholar]
- Ar-Riza, I. Alkasuma Pertanian Lahan Rawa Pasang Surut Dan Strategi Pengembangannya Dalam Era Otonomi Daerah. J. Sumberd. Lahan 2008, 2, 95–104. [Google Scholar]
- Bronswijk, J.J.B.; Groenenberg, J.E.; Ritsema, C.J.; van Wijk, A.L.M.; Nugroho, K. Evaluation of Water Management Strategies for Acid Sulphate Soils Using a Simulation Model: A Case Study in Indonesia. J. Agric. Water Manag. 1995, 27, 125–142. [Google Scholar] [CrossRef]
- Anda, M.; Siswanto, A.B. Properties of Organic and Acid Sulfate Soils and Water of a ‘Reclaimed’ Tidal Backswamp in Central Kalimantan, Indonesia. Geoderma 2009, 149, 54–65. [Google Scholar] [CrossRef]
- Österholm, P.; Virtanen, S.; Rosendahl, R.; Uusi-Kämppä, J.; Ylivainio, K.; Yli-Halla, M. Groundwater Management of Acid Sulfate Soils Using Controlled Drainage, by-Pass Flow Prevention, and Subsurface Irrigation on a Boreal Farmland. Soil Plant Sci. 2015, 65, 110–120. [Google Scholar] [CrossRef]
- Shamshuddin, J.; Muhrizal, S.; Fauziah, I.; Husni, M.H.A. Effects of Adding Organic Materials to an Acid Sulfate Soil on Growth of Cocoa (Theobroma cacao L.). Sci. Total Environ. 2004, 323, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Maftu’ah, E.; Lestari, Y.; Pangaribuan, E.B.; Mayasari, V. Amelioration of Actual Acid Sulfate Soils to Improve Soil Chemical Properties and Rice Yields. IOP Conf. Ser. Earth Environ. Sci. 2021, 648, 012167. [Google Scholar] [CrossRef]
- Panhwar, Q.A.; Naher, U.A.; Shamshuddin, J.; Ismail, M.R. Effects of Biochar and Ground Magnesium Limestone Application, with or without Bio-Fertilizer Addition, on Biochemical Properties of an Acid Sulfate Soil and Rice Yield. Agronomy 2020, 10, 1100. [Google Scholar] [CrossRef]
- Masulili, A.; Utomo, W.H.; Syechfani, M.S. Rice Husk Biochar for Rice Based Cropping System in Acid Soil: 1. The Characteristics of Rice Husk Biochar and Its Influence on the Properties of Acid Sulfate Soils and Rice Growth in West Kalimantan, Indonesia. J. Agric. Sci. 2010, 2, 39–47. [Google Scholar] [CrossRef]
- Santri, J.A.; Maas, A.; Utami, S.N.H.; Yusuf, W.A. Application of Lime and Compost on the Newly Established Field with Acid Sulfate Soil Type in the Belandean Experimental Field, South Kalimantan for Agricultural Cultivation. IOP Conf. Ser. Earth Environ. Sci. 2019, 393, 012002. [Google Scholar] [CrossRef]
- Anwar, K.; Sabiham, S.; Sumawinata, B.; Sapei, A.; Alihamsyah, T. Pengaruh Kompos Jerami Terhadap Kualitas Tanah, Kelarutan Fe2+ Dan SO4 2- Serta Produksi Padi Pada Tanah Sulfat Masam. J. Tanah Dan Iklim 2006, 24, 29–39. [Google Scholar]
- Priatmadi, B.J.; Saidl, A.R.; Septiana, M. Changes in Rice Production in Acidic Tropical Soils as Influenced by Fly-Ash Application. In Proceedings of the International Seminar on the Tropical Natural Resources; Unram Press: Mataram, Indonesia, 2015. [Google Scholar]
- Annisa, W.; Mukhlis, M.; Hairani, A. Biochar-Materials for Remediation on Swamplands: Mechanisms and Effectiveness. J. Sumberd. Lahan 2021, 15, 13–22. [Google Scholar] [CrossRef]
- Mukhlis; Khairullah, I. Effectivity of bioAmeliorant to Increase Rice Productivity in Swamplands. In Strategies and Technologies for the Utilization and Improvement of Rice; IAARD Press: Jakarta, Indonesia, 2020; pp. 277–288. [Google Scholar]
- Bakar, R.A.; Razak, Z.A.; Ahmad, S.H.; Seh-Bardan, B.J.; Tsong, L.C.; Meng, C.P. Influence of Oil Palm Empty Fruit Bunch Biochar on Floodwater pH and Yield Components of Rice Cultivated on Acid Sulphate Soil under Rice Intensification Practices. Plant Prod. Sci. 2015, 18, 491–500. [Google Scholar] [CrossRef]
- Phuong, N.T.K.; Khoi, C.M.; Ritz, K.; Van Sinh, N.; Tarao, M.; Toyota, K. Potential Use of Rice Husk Biochar and Compost to Improve P Availability and Reduce GHG Emissions in Acid Sulfate Soil. Agronomy 2020, 10, 685. [Google Scholar] [CrossRef]
- Michael, P.S.; Fitzpatrick, R.W.; Reid, R. The Role of Organic Matter in Ameliorating Acid Sulfate Soils with Sulfuric Horizons. Geoderma 2015, 225, 42–49. [Google Scholar] [CrossRef]
- Mayakaduwage, S.; Mosley, L.M.; Marschner, P. Phosphorus Pools in Acid Sulfate Soil Are Influenced by pH, Water Content, and Addition of Organic Matter. J. Soil Sci. Plant Nutr. 2021, 21, 1066–1075. [Google Scholar] [CrossRef]
- Sinh, V.N.; Khoi, C.M.; Phuong, N.T.K.; Linh, T.B.; Minh, D.D.; Perry, R.N.; Toyota, K. Impacts of Fallow Conditions, Compost and Silicate Fertilizer on Soil Nematode Community in Salt–Affected Paddy Rice Fields in Acid Sulfate and Alluvial Soils in the Mekong Delta, Vietnam. Agronomy 2021, 11, 425. [Google Scholar] [CrossRef]
- Shamshuddin, J.; Panhwar, Q.A.; Alia, F.J.; Shazana, M.A.R.S.; Radziah, O.; Fauziah, C.I. Formation and Utilisation of Acid Sulfate Soils in Southeast Asia for Sustainable Rice Cultivation. Pertanika J. Trop. Agric. Sci. 2017, 40, 225–246. [Google Scholar]
- Noor, A.; Ningsih, R.D.; Sabur, A. Pengaruh Pemupukan N, P, Dan K Terhadap Hasil Padi Lokal Di Lahan Pasang Surut Kalimantan Selatan. In Proceedings of the Prosiding Seminar Hasil Penelitian Padi; Balai Besar Penelitian Tanaman Padi: Subang, Indonesia, 2010; Volume Buku II, pp. 893–901. [Google Scholar]
- Ningsih, R.D.; Noor, A.; Yasin, M. Pengaruh Keracunan Besi Di Lahan Pasang Surut Terhadap Pertumbuhan Dan Hasil Padi Varietas Inpara. In Proceedings of the Seminar Nasional 2013: Inovasi Teknologi Padi Adaptif Peruabahan Iklim Global Mendukung Surplus 10 Juta Ton Beras; Badan Penelitian dan Pengembangan Pertanian: Jakarta, Indonesia, 2014; pp. 397–404. [Google Scholar]
- Alwi, M.; Raihana, Y. Pengelolaan Hara N, P, Dan K Untuk Meningkatkan Produktivitas Padi Lahan Rawa Pasang Surut. In Proceedings of the Kongres Nasional Perkumpulan Masyarakat Gambut Indonesia (HGI) Ke VII dan Seminar Pengelolaan Lahan Sub-Optimal Secara Berkelanjutan, Bogor, Indonesia, 26–28 October 2016; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2016; pp. 313–325. [Google Scholar]
- Koesrini, K.; Alwi, M.; Saleh, M. Adaptasi Dan Keragaan Hasil Varietas Unggul Padi Di Lahan Rawa Wilayah Perbatasan Kalimantan Barat. J. Penelit. Pertan. Tanam. Pangan 2019, 3, 53–59. [Google Scholar] [CrossRef]
- Raihana, Y.; Alwi, M. Pengelolaan Hara Terpadu Untuk Meningkatkan Produktivitas Padi Lahan Rawa Pasang Surut Sulfat Masam Potensial. In Proceedings of the Seminar Nasional Inovasi Teknologi Pertanian Spesifik Lokasi, Banjarbaru, Indonesia, 20 July 2016; pp. 294–304. [Google Scholar]
- Mukhlis. Uji Keefektivan Pupuk Hayati Biotara Terhadap Tanaman Padi Di Lahan Rawa Sulfat Masam; Laporan Hasil Penelitian; Balai Penelitian Pertanian Lahan Rawa: Banjarbaru, Indonesia, 2011. [Google Scholar]
- Ningsih, R.D.; Napisah, K.; Noor, A. Menghemat Pupuk Kimia Hingga 50% Dengan Menggunakan Pupuk Organik Pada Lahan Pasang Surut. In Proceedings of the Prosiding Balai Besar Penelitian Tanaman Padi; Balai Besar Penelitian Tanaman Padi: Subang, Indonesia, 2017; pp. 321–327. [Google Scholar]
- Sulaeman, Y.; Maftu’ah, E.; Mukhlis, M.; Anwar, K.; Karolinoerita, V.; Wakhid, N.; Saleh, M.; Khairullah, I.; Agustiani, M.; Anggara, A.W.; et al. Tidal Rice Yield Assessment in Central Kalimantan, Indonesia, under Different Cultural Practices. Resources 2022, 11, 116. [Google Scholar] [CrossRef]
- Noor, A.; Lubis, I.; Ghulamahdi, M.; Ningsih, R.; Anwar, K.; Chozin, M.; Wirnas, D. The Response by Selected Rice Genotypes to Organic Ameliorants in Tidal Swampland Which Is Affected by Fe Toxicity. Agron. Res. 2022, 20, 1044–1059. [Google Scholar] [CrossRef]
- Hasbianto, A.; Ningsih, R.D.; Amin, M.; Yasin, M.; Noor, A. Performance of Six New Superior Varieties of Rice on Tidal Swamp-Land in South Kalimantan Province. IOP Conf. Ser. Earth Environ. Sci. 2021, 911, 12030. [Google Scholar] [CrossRef]
- Kurnain, A.; Ifansyah, H. Dinamika Ion Surjan Di Lahan Rawa Pasang Surut. In Proceedings of the Seminar Nasional FKPTPI; Fakultas Pertanian Univaersitas Lambung Mangkurat: Banjarbaru, Indonesia, 2015; pp. 161–163. [Google Scholar]
- Dobermann, A.; Fairhurst, T. Rice: Nutrient Disorders and Nutrient Management, 1st ed.; IRRI: Manila, Philippines, 2000; ISBN 981-04-2742-5. [Google Scholar]
- Gribaldi; Nurlaili; Danial, E. Peningkatan Produktivitas Padi Hibrida Melalui Pemberian Pupuk N Dengan System Ratun Di Lahan Rawa Pasang Surut. J. Agrotek Trop. 2020, 8, 185–192. [Google Scholar] [CrossRef]
- Noor, A.; Ningsih, R.D.; Napisah, K.; Yuliani, N. Tanah Rawa Kalimantan Selatan. In Kesuburan Tanah Rawa; IPB Press: Bogor, Indonesia, 2022; pp. 142–157. [Google Scholar]
- Bahri, S.; Basri, T.H.; Rahmatsyah; Faisal, T.M. Kajian Kecukupan Hara Fosfor Pada Lahan Sulfat Masam Potensial Terhadap Pertumbuhan Dan Produksi Beberapa Varietas Kedelai. J. Agroqua 2021, 19, 1–4. [Google Scholar] [CrossRef]
- Suriadikarta, D.A.; Simanugkalit, R.D.M. Pendahuluan. In Pupuk Organik dan Pupuk Hayati; Badan Penelitian dan Pengembangan Pertanian: Bogor, Indonesia, 2012; pp. 1–10. [Google Scholar]
- Wang, M.Q.; Zheng, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Philip, J.W.; Broadley, M.R.; Gregory, P.J. Managing the Nutrition of Plants and People. Appl. Environ. Soil Sci. 2012, 2012, 1155–11167. [Google Scholar] [CrossRef]
- Nursyamsi, D.; Alwi, M. Ameliorasi Dan Pemupukan Di Lahan Rawa. In Proceedings of the Prosiding Semnas Teknologi Pemupukan dan Pemulihan Lahan Terdegradasi, Bogor, Indonesia, 29–30 July 2012; Balitbangtan-Kementan: Bogor, Indonesia, 2012; pp. 687–700. [Google Scholar]
- Khairullah, I.; Noor, A. Upaya Peningkatan Produktivitas Padi Melalui Pemupukan Di Lahan Pasang Surut Sulfat Masam. J. Pertan. Agros 2018, 20, 123–133. [Google Scholar]
- Juarsah, I. Pemanfaatan Pupuk Organik Untuk Pertanian Organik Dan Lingkungan Berkelanjutan. In Proceedings of the Seminar Nasional Pertanian Organik, Bogor, Indonesia, 18–19 June 2014; Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian: Bogor, Indonesia, 2014; pp. 127–136. [Google Scholar]
- Majerus, V.; Bertin, P.; Lutts, S. Effects of Iron Toxicity on Osmotic Potential, Osmolytes and Polyamines Concentrations in the African Rice (Oryza glaberrima Steud.). Plant Sci. 2007, 173, 96–105. [Google Scholar] [CrossRef]
- Virmani, S.S. Varietal Tolerance of Rice to Iron Toxicity in Liberia. Int. Rice Res. News 1977, 2, 4–5. [Google Scholar]
- Suprihatno, B.; Daradjat, A.A.; Satoto; Baehaki. Deskripsi Varietas Padi; Besar Penelitian Tanaman Padi: Sukamandi, Indonesia, 2010; ISBN 978-979-540-047-9. [Google Scholar]
- Adri, A.; Yardha, Y. Peningkatan Produktivitas Padi Melalui Varietas Unggul Baru Mendukung Swasembada Berkelanjutan Di Provinsi Jambi. J. Agroekotek 2014, 6, 1–11. [Google Scholar]
- Helmi, H. Peningkatan Produktivitas Padi Lahan Rawa Lebak Melalui Penggunaan Varietas Unggul Padi Rawa. J. Pertan. Trop. 2015, 2, 78–92. [Google Scholar] [CrossRef]
- Saleh, M.; Nurzakiah, S. Adaptabilitas Varietas Inpara Di Lahan Rawa Pasang Surut Tipe Luapan Air B Pada Musim Kemarau. J. Agron. Indones. 2017, 45, 117–123. [Google Scholar]
- Fusco, G.; Minelli, A. Phenotypic Plasticity in Development and Evolution: Facts and Concepts. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 547–556. [Google Scholar] [CrossRef]
- Hawkes, J.G.; Maxted, N.; Ford-Lloyd, B.V. The Ex Situ Conservation of Plant Genetic Resources, 1st ed.; Springer: Dordrecht, The Netherlands, 2000; ISBN 978-0-7923-6442-9. [Google Scholar]
- Hairmansis, A.; Aswidinnoor, H.; Trikoesmanitya; Suwarno. Evaluasi Daya Pemulih Kesuburan Padi Lokal Dari Kelompok Tropical Japonica. Bul. Agron. 2005, 33, 1–6. [Google Scholar]
- Sitaresmi, T.; Wening, R.H.; Rakhmi, A.T.; Yunani, N.; Susanto, U. Pemanfaatan Plasma Nutfah Padi Varietas Lokal Dalam Perakitan Varietas Unggul. J. Iptek Tanam. Pangan 2013, 8, 22–30. [Google Scholar]
- Ishaq, M.; Falusi, A. Germplasm Conservation and Its Impact on Crop Improvement in Nigeria. Crop Res. 2008, 36, 285–896. [Google Scholar]
- Ferrer, M.C.; Duldulao, M.D.; Caguiat, X.G.I.; Mananghaya, T.E.; Newingham, M.; Nombrere, J.; Castro, J.; Alfonso, D.O.; Regalario, J.B.; Alvarino, J.B.M.; et al. PhilRice Genebank: Recent Developments in Managing and Sharing the Philippine Rice Germplasm. IOP Conf. Ser. Earth Environ. Sci. 2020, 482, 012010. [Google Scholar] [CrossRef]
- Mulsanti, I.W.; Yunani, N.; Sitaresmi, T. Morphology-Based Genetic Diversity of Early Maturity Rice Germplasm, the Relation to Yield Component. Ecol. Environ. Conserv. 2020, 26, S193–S198. [Google Scholar]
- Prasetiyono, J.; Hidayatun, N.; Tasliah, T. Genetic Diversity Analysis of 53 Indonesian Rice Genotypes Using 6K Single Nucleotide Polymorphism Markers. J. AgroBiogen 2018, 14, 1–10. [Google Scholar] [CrossRef]
- Rabara, R.C.; Ferrer, M.C.; Calayugan, M.I.C.; Duldulao, M.D.; Rabara, J.J. Conservation of Rice Genetic Resources for Food Security. Adv. Food Technol. Nutr. Sci.-Open J. 2015, SE, S51–S56. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Fukao, T.; Ronald, P.; Ismail, A.; Heuer, S.; Mackill, D. Submergence Tolerant Rice: SUB1′s Journey from Landrace to Modern Cultivar. Rice 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Fujita, D.; Trijatmiko, K.R.; Tagle, A.G.; Sapasap, M.V.; Koide, Y.; Sasaki, K.; Tsakirpaloglou, N.; Gannaban, R.B.; Nishimura, T.; Yanagihara, S.; et al. NAL1 Allele from a Rice Landrace Greatly Increases Yield in Modern Indica Cultivars. Proc. Natl. Acad. Sci. USA 2013, 110, 20431–20436. [Google Scholar] [CrossRef]
- Swastika, D.K.S.; Agustian, A.; Suryana, A.; Muslim, C.; Sunarsih; Perdana, R.P. Tinjauan Historis Teknologi Varietas Unggul Dan Program Intensifikasi Dalam Peningkatan Produktivitas Padi Berkelanjutan. Forum Penelit. Agro Ekon. 2021, 39, 103–114. [Google Scholar] [CrossRef]
- Nugraha, Y.; Sitaresmi, T. Upaya Peningkatan Produktivitas Padi Dari Sisi Pendekatan Genetik. J. Iptek Tanam. Pangan 2018, 13, 1–10. [Google Scholar]
- Zhang, P.; Li, J.; Li, X.; Liu, X.; Zhao, X.; Lu, Y. Population Structure and Genetic Diversity in a Rice Core Collection (Oryza sativa L.) Investigated with SSR Markers. PLoS ONE 2011, 6, e27565. [Google Scholar] [CrossRef]
- Yustisia; Tohari; Shiddieq, D.; Subowo, G. Pengkayaan Besi (Fe) Dan Seng (Zn) Dalam Beras Dan Karakter Penentu Varietas Padi Sawah Efisien Pada Tanah Vertisol Dan Inseptisol. Agrotop J. Agric. Sci. 2012, 2, 67–75. [Google Scholar]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. A Primer of Conservation Genetics; Cambridge University Press: Cambridge, UK, 2004; ISBN 978-0-511-81735-9. [Google Scholar]
- Wahyu; Nasrullah. Malacak, Manatak, Maimbul: Kearifan Lokal Petani Dayak Bakumpai Dalam Pengelolaan Padi Di Lahan Rawa Pasang Surut. Komunitas Int. J. Indones. Soc. Cult. 2012, 4, 36–45. [Google Scholar] [CrossRef]
- Djajakirana, G.; Sumawinata, B.; Mulyanto, B. Suwardi The Importance of Organic Matter and Water Management in Sustaining Banjarese Traditional Land Management in Pulau Petak, South Kalimantan. In Proceedings of the International Seminar on Toward Sustainable Agriculture in Humid Tropics Facing 21st Century, Bandar Lampung, Indonesia, 27–28 September 1999; The University of Lampung: Bandar Lampung, Indonesia, 1999; pp. 48–60. [Google Scholar]
- Saleh, M.; Asikin, S. Teknik Pengelolaan Untuk Pertanian. In Lahan Rawa Bongkor: Identifikasi dan Rehabilitasi; Rajawali Pers: Depok, Indonesia, 2022; pp. 55–73. [Google Scholar]
- Saleh, M.; Khairullah, I. Persemaian. In Mengelola Sawah Pasang Surut Bukaan Baru; Sulaeman, Y., Maftu’ah, E., Eds.; UB Press: Malang, Indonesia, 2021; pp. 53–59. ISBN 9786232963160. [Google Scholar]
- Simatupang, R.S.; Nurita, S. Conservation Soil Tillage at Rice Culture in Acid Sulphate Soil. In Proceedings of the International Workshop on Sustainable Management of Lowland for Rice Production; Indonesian Agency for Agricultural Research and Development Ministry of Agriculture: Banjarbaru, Indonesia, 2013; pp. 287–298. [Google Scholar]
- Ningsih, R.D.; Yasin, M.; Noor, A. Rice Productivity on Tidal Swampland in the Agricultural Assisitance Area Program in Barito Kuala Rqency South Kalimantan. IOP Conf. Ser. Earth Environ. Sci. 2020, 484, 012123. [Google Scholar] [CrossRef]
- Umar, S.; Hidayat, A.R.; Pangaribuan, S. Pengujian Mesin Tanam Padi Sistem Jajar Legowo (Jarwo Transplanter) Di Lahan Rawa Pasang Surut. J. Tek. Pertan. Lampung 2017, 6, 63–72. [Google Scholar]
- Pangaribuan, E.E.B.; Simatupang, R.S. Sistem tanam padi sebar langsung di lahan rawa pasang surut. In Inovasi Pengelolaan Lahan Rawa Menuju Pertanian Maju, Mandiri dan Modern; Rajawali Pers: Depok, Indonesia, 2020; pp. 161–183. ISBN 978-623-231-526-6. [Google Scholar]
- Mosley, L.M.; Zammit, B.; Jolley, A.M.; Barnett, L.; Fitzpatrick, R. Monitoring and Assessment of Surface Water Acidification Following Rewetting of Oxidised Acid Sulfate Soils. Environ. Monit. Assess. 2014, 186, 1–18. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, S.; Chen, R.; Bu, X.; Tong, M.; Huang, Q. Oxygenation of Acid Sulfate Soils Stimulates CO2 Emission: Roles of Acidic Dissolution and Hydroxyl Radical Oxidation. Chem. Geol 2020, 533, 119437. [Google Scholar] [CrossRef]
- Šimek, M.; Virtanen, S.; Simojoki, A.; Chroňáková, A.; Elhottová, D.; Krištůfek, V.; Yli-Halla, M. The Microbial Communities and Potential Greenhouse Gas Production in Boreal Acid Sulphate, Non-Acid Sulphate, and Reedy Sulphidic Soils. Sci. Total Environ. 2014, 466, 663–672. [Google Scholar] [CrossRef]
- Kölbl, A.; Bucka, F.; Marschner, P.; Mosley, L.; Fitzpatrick, R.; Schulz, S.; Lueders, T.; Kögel-Knabner, I. Consumption and Alteration of Different Organic Matter Sources during Remediation of a Sandy Sulfuric Soil. Geoderma 2019, 347, 220–232. [Google Scholar] [CrossRef]
- Jain, N.; Pathak, H.; Mitra, S.; Bhatia, A. Emission of Methane from Rice Fields—A Review. J. Sci. Ind. Res. 2004, 63, 101–115. [Google Scholar]
- Horwath, W.R. Greenhouse Gas Emissions from Rice Cropping Systems. In Understanding Greenhouse Gas Emissions from Agricultural Management; Guo, L., Gunasekara, A.S., McConnell, L.L., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1072, pp. 67–89. ISBN 978-0-8412-2654-8. [Google Scholar]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of Greenhouse Gases between Soil and Atmosphere: Interactions of Soil Physical Factors and Biological Processes. Eur. J. Soil Sci. 2003, 54, 779–791. [Google Scholar] [CrossRef]
- Dubey, S.K. Microbial Ecology of Methane Emission in Rice Agroecosystem: A Review. Appl. Ecol. Environ. Res. 2005, 3, 1–27. [Google Scholar] [CrossRef]
- IPCC. Revised 1996 Guideline for National Greenhouse Gas Inventories; UK Meteorological Office: Bracknell, UK, 1996. [Google Scholar]
- Hayashi, K.; Tokida, T.; Kajiura, M.; Yanai, Y.; Yano, M. Cropland Soil–Plant Systems Control Production and Consumption of Methane and Nitrous Oxide and Their Emissions to the Atmosphere. Soil Sci. Plant Nutr. 2015, 61, 2–33. [Google Scholar] [CrossRef]
- Susilawati, H.L.; Pramono, A.; Setyanto, P.; Inubushi, K. Soil Amelioration on Peat and Its Effect on Methane (CH4) Emission and Rice Yield. In Tropical Wetlands—Innovation in Mapping and Management; Sulaeman, Y., Poggio, L., Minasny, B., Nursyamsi, D., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 109–116. ISBN 978-0-367-20964-3. [Google Scholar]
- Abduh, A.M.; Annisa, W. Interaction of Paddy Varieties and Compost with Flux of Methane in Tidal Swampland. J. Tanah Trop. 2016, 21, 179–186. [Google Scholar] [CrossRef]
- Annisa, W.; Nursyamsi, D. Effects of Ameliorants, Fertilizers, and Management Regimes of Acid Sulphate Soils on Rice Yield and Methane Emission. J. Tanah Dan Iklim 2016, 40, 135–145. [Google Scholar]
- Annisa, W. Mukhlis Water Management and Rice Husk Biochar Application to Solve Acid Sulfate Soil Problems to Promote Rice Yield and Reduce Greenhouse Gas Emission. IOP Conf. Ser. Mater. Sci. Eng. 2020, 980, 012067. [Google Scholar] [CrossRef]
- Luo, J.; Tillman, R.W.; Ball, P.R. Factors Regulating Denitrification in a Soil under Pasture. Soil Biol. Biochem. 1999, 31, 913–927. [Google Scholar] [CrossRef]
- Qian, J.H.; Doran, J.W.; Weier, K.L.; Mosier, A.R.; Peterson, T.A.; Power, J.F. Soil Denitrification and Nitrous Oxide Losses under Corn Irrigated with High-Nitrate Groundwater. J. Environ. Qual. 1997, 26, 348–360. [Google Scholar] [CrossRef]
- Zech, W.; Senesi, N.; Guggenberger, G.; Kaiser, K.; Lehmann, J.; Miano, T.M.; Miltner, A.; Schroth, G. Factors Controlling Humification and Mineralization of Soil Organic Matter in the Tropics. Geoderma 1997, 79, 117–161. [Google Scholar] [CrossRef]
- Macdonald, B.; White, I.; Denmead, T. Gas Emissions from the Interaction of Iron, Sulfur and Nitrogen Cycles in Acid Sulfate Soils. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010. Published on DVD, 2010. [Google Scholar]
- Rückauf, U.; Augustin, J.; Russow, R.; Merbach, W. Nitrate Removal from Drained and Reflooded Fen Soils Affected by Soil N Transformation Processes and Plant Uptake. Soil Biol. Biochem. 2004, 36, 77–90. [Google Scholar] [CrossRef]
- Regina, K. Microbial Production of Nitrous Oxide and Nitric Oxide in Boreal Peatlands. Ph.D. Thesis, University of Joensuu, Joensuu, Finland, 1998. [Google Scholar]
- IAEA. Manual on Measurement of Methane and Nitrous Oxide Emission from Agricultural (TECHDOC-674); International Atomic Energy Agency: Vienna, Austria, 1992. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).