Fluoride Removal from Aqueous Medium Using Biochar Produced from Coffee Ground
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining the Precursor Material (Coffee Grounds)
2.2. BC Production
2.3. Physicochemical Characterization of BC
2.4. Evaluation of the Fluoride Adsorption Process
2.4.1. Influence of pH and Adsorbent Dosage on Adsorption
2.4.2. Influence of Initial Fluorine Concentration on Adsorption Kinetics
2.4.3. Evaluation of Adsorption Capacity
2.4.4. Effect of Temperature on the Adsorption Process and Determination of Thermodynamic Parameters
2.5. Preparation of Solutions and Physico-Chemical Analysis
3. Results
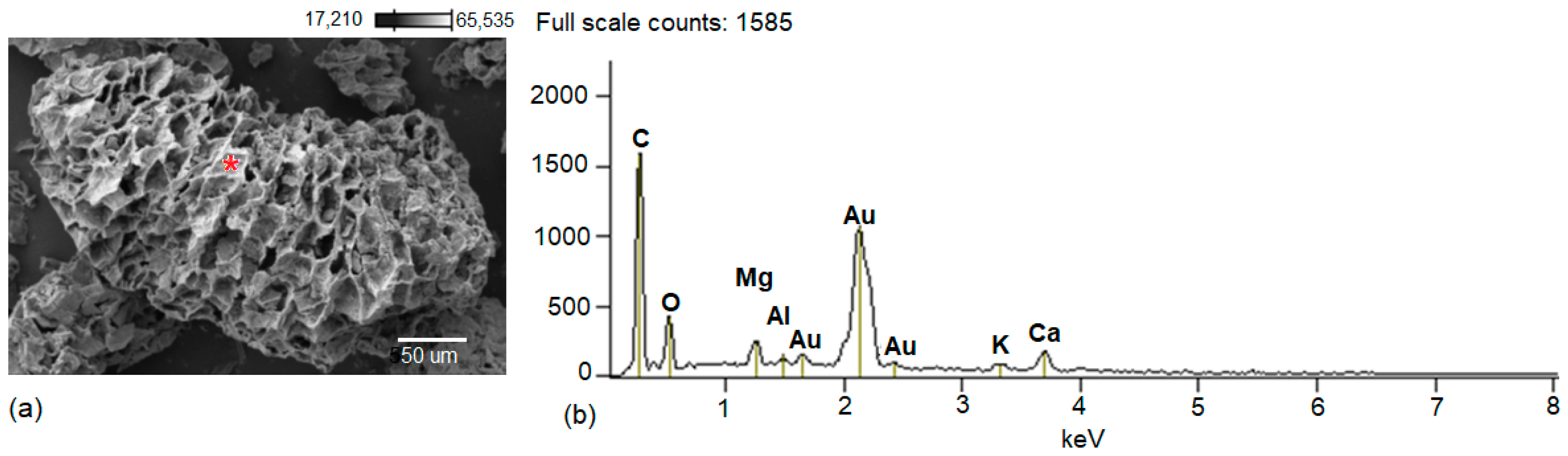
3.1. Physicochemical Characterization of BC
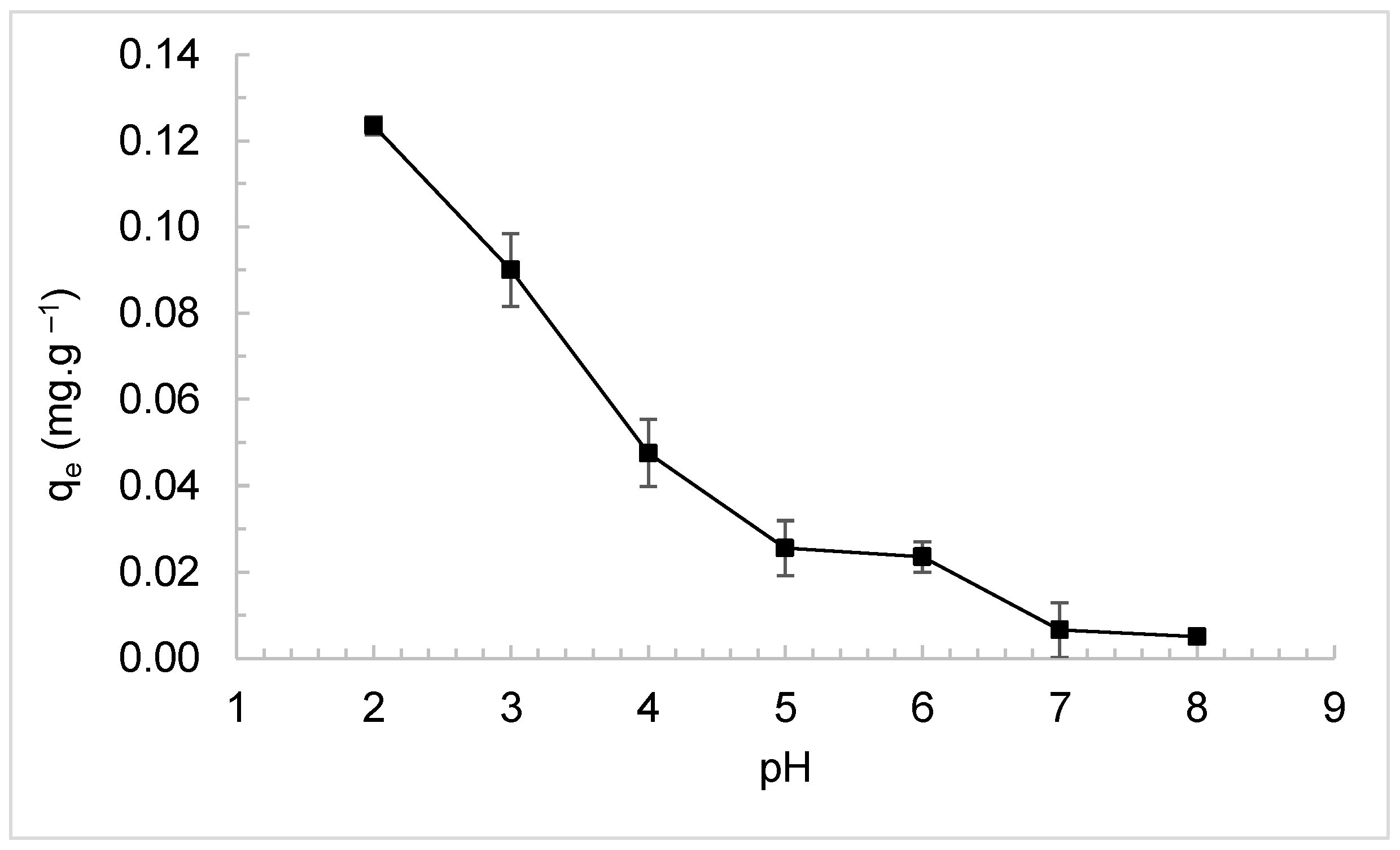
3.2. Effect of pH on Adsorption
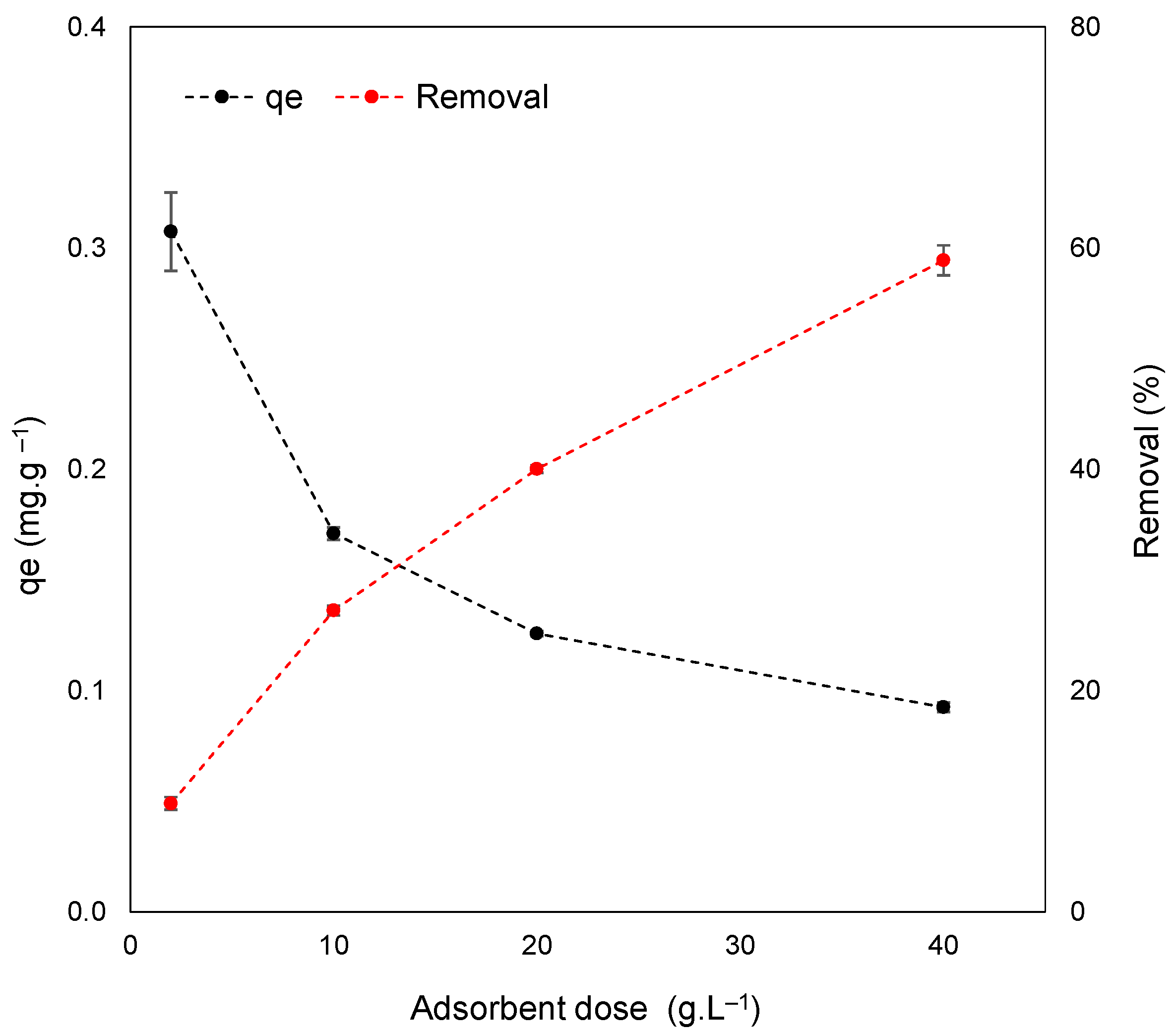
3.3. Effect of Adsorbent Dosage
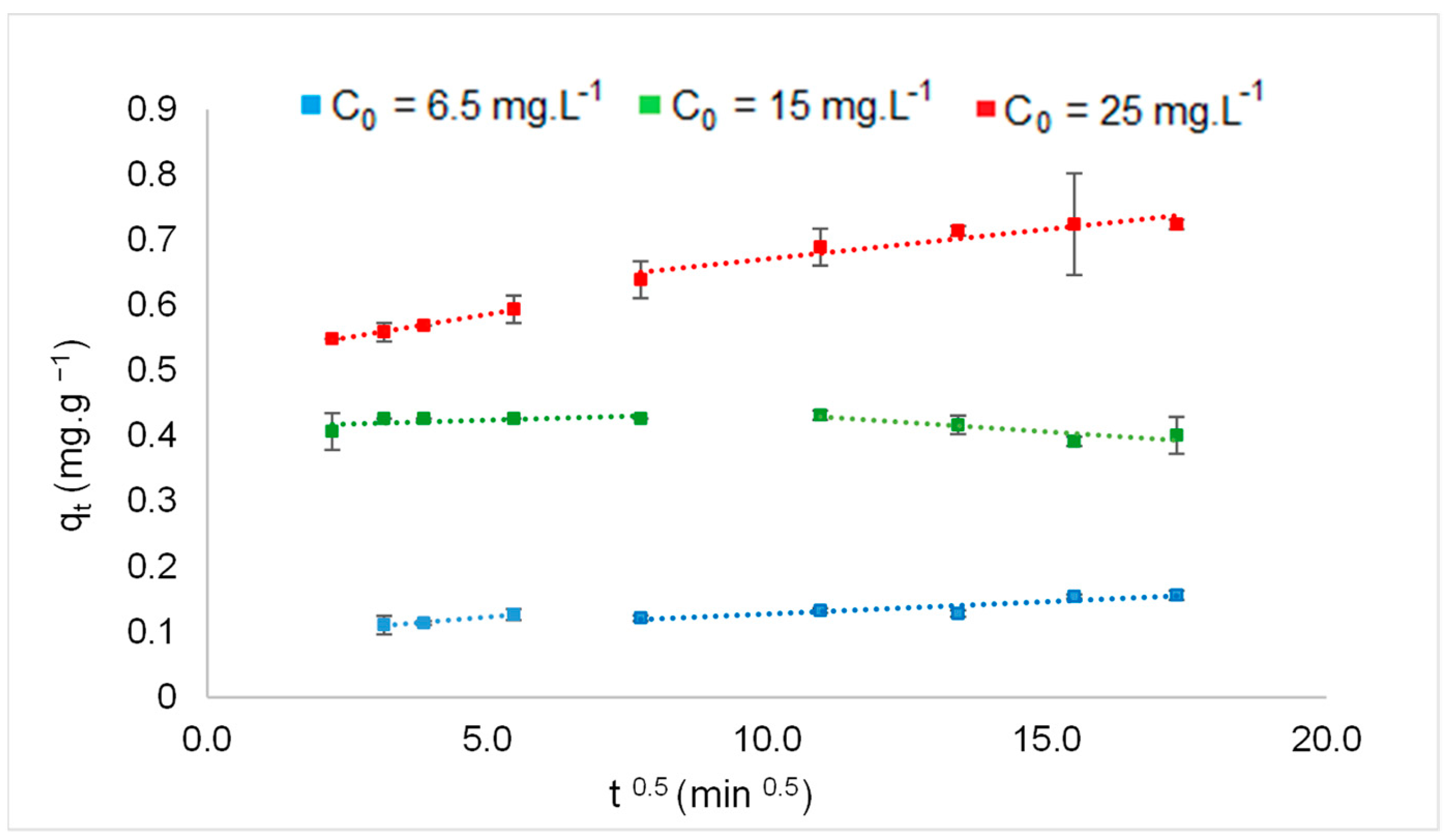
3.4. Influence of Contact Time for Different Concentrations of F−
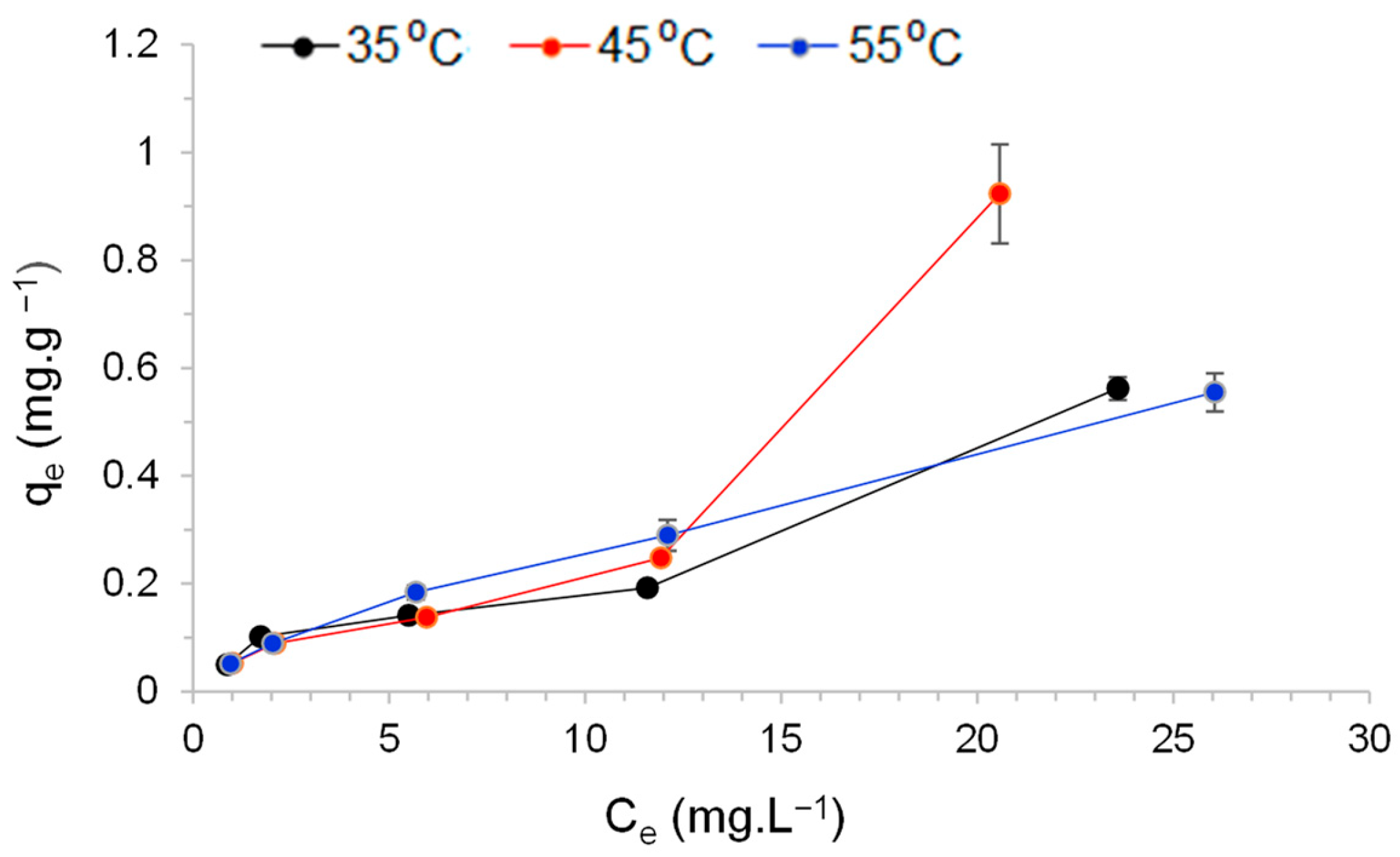
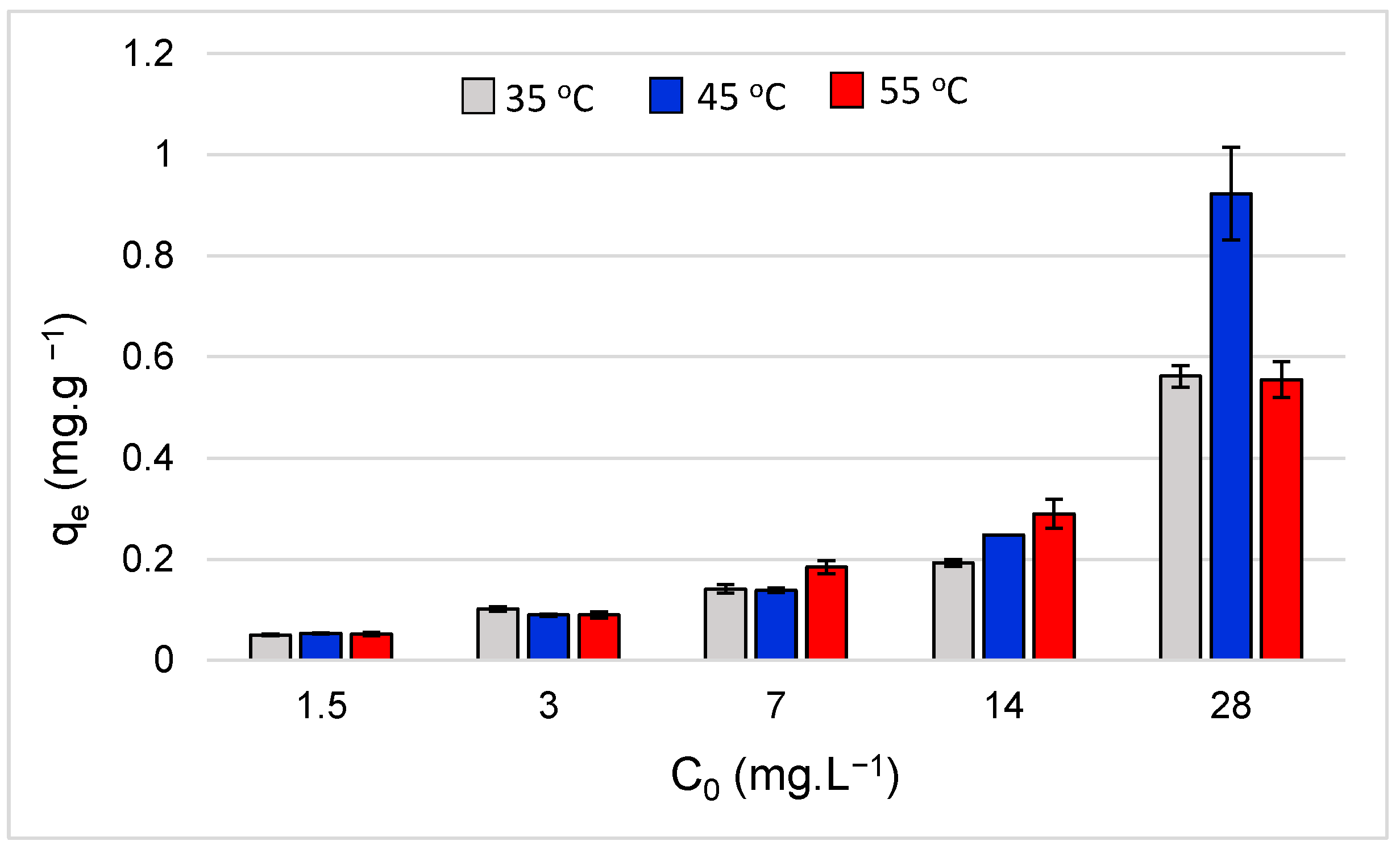
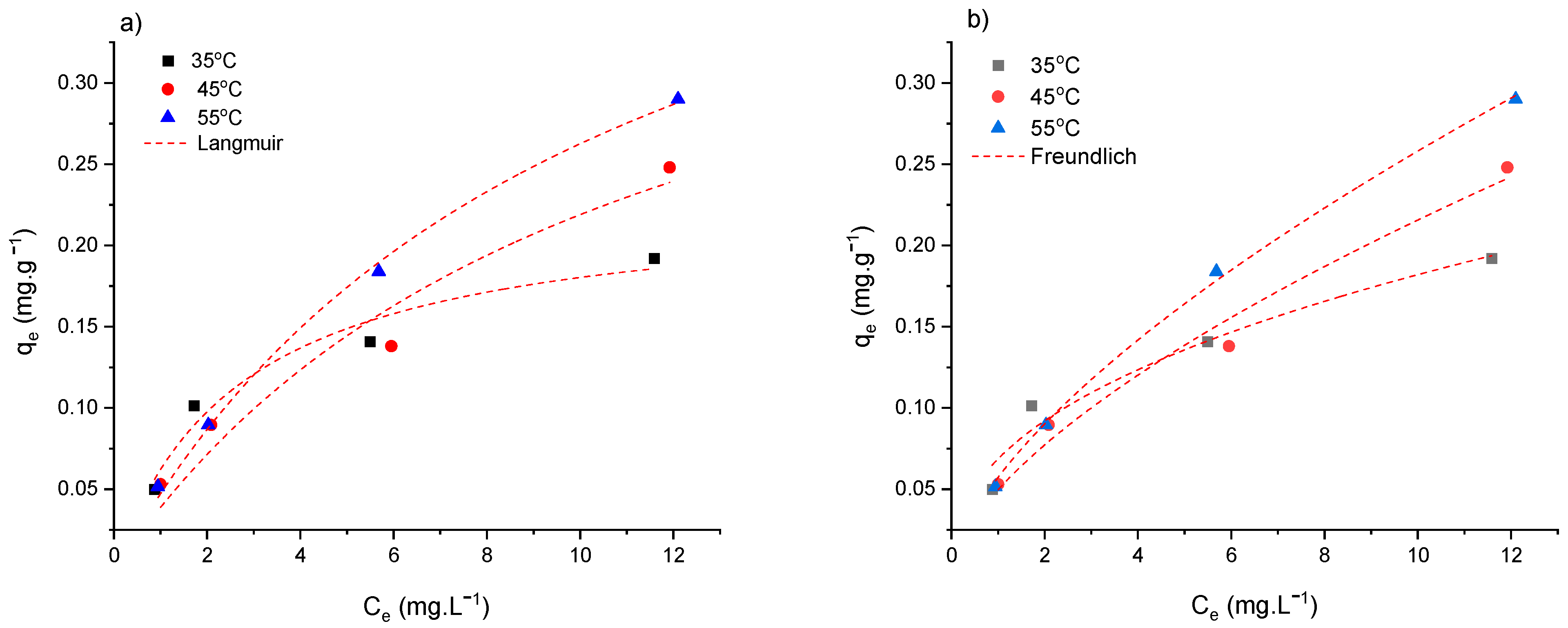
3.5. Influence of Temperature on Adsorption Capacity
3.6. Adsorption Thermodynamics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jullien, S. Prophylaxis of Caries with Fluoride for Children under Five Years. BMC Pediatrics 2021, 351 (Suppl. 1), 351. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Flora, S. Fluoride in Drinking Water and Skeletal Fluorosis: A review of the global impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef] [PubMed]
- WHO—World Heath Organization. Fluoride in Drinking-Water. In Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Raichur, A.M.; Basu, M.J. Adsorption of Fluoride onto Mixed Rare Earth Oxides. Sep. Purif. Technol. 2001, 24, 121–127. [Google Scholar] [CrossRef]
- Ezaki, S.; Pérez-Aguilar, A.; Hypolito, R.; Shinzato, M.C. Anomalias de Flúor nas Águas Subterrâneas do Estado de São Paulo. Rev. Inst. Geológico 2016, 37, 65–98. [Google Scholar] [CrossRef]
- Colombani, N.; Giuseppe, D.D.; Kebede, S.; Mastrocicco, M. Assessment of the Anthropogenic Fluoride Export in Addis Ababa Urban Environment (Ethiopia). J. Geochem. Explor. 2018, 190, 390–399. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A Review of Emerging Adsorbents and Current Demand for Defluoridation of Water: Bright future in water sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global Analysis and Prediction of Fluoride in Groundwater. Nat. Commun. 2022, 13, 4232. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of fluoride removal from water environment by adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Sadhu, M.; Bhattacharya, P.; Vithanage, M.; Sudhakar, P.P. Adsorptive Removal of Fluoride Using Biochar—A Potential Application in Drinking Water Treatment. Sep. Purif. Technol. 2022, 278, 119106. [Google Scholar] [CrossRef]
- Waghmare, S.S.; Arfin, T. Fluoride Removal from Water by various techniques: Review. Int. J. Innov. Sci. Eng. Technol. 2015, 2, 560–571. Available online: https://ijiset.com/vol2/v2s9/IJISET_V2_I9_67.pdf (accessed on 7 December 2022).
- Khandare, R.V.; Desai, S.B.; Bhujbal, S.S.; Watharkar, A.D.; Biradar, S.P.; Pawar, P.K.; Govindwar, S.P. Phytoremediation of fluoride with garden ornamental Nerium oleander, Portulaca oleracea, and Pogonatherum crinitum. Environ. Sci. Pollut. Res. 2017, 24, 6833–6839. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.; Liang, X.; Goh, H.H.; Othman, M.H.D.; Chong, K.-K.; Chew, K.W. Remediation Technologies for Contaminated Groundwater Due to Arsenic (As), Mercury (Hg), and/or Fluoride (F): A critical review and way forward to contribute to carbon neutrality. Sep. Purif. Technol. 2023, 314, 123474. [Google Scholar] [CrossRef]
- Gao, S.-L.; Li, L.-Q.; Huang, J.; Xu, Z.-L.; Tang, Y.-J. Fine-Tuning of Fully-Aromatic Polyamide Membrane for Fluorinated Water Purification. J. Appl. Polym. Sci. 2023, 140, e53967. [Google Scholar] [CrossRef]
- Gmar, S.; Sayadi, I.B.S.; Helali, N.; Tlili, M.; Amor, M.B. Desalination and Defluoridation of Tap Water by Electrodialysis. Environ. Process. 2015, 2, 209–222. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, J.; Alcalá, L.; Megido, L.; Castrillón, L. Removal of Fluoride from Coke Wastewater by Aluminum Doped Chelating Ion-Exchange Resins: A tertiary treatment. Environ. Sci. Pollut. Res. 2022, 29, 8705–8715. [Google Scholar] [CrossRef]
- Islam, S.M.D.-U. Electrochemical Remediation of Arsenic and Fluoride from Water: A review of the current state and future prospects. Environ. Technol. Innov. 2023, 31, 103148. [Google Scholar] [CrossRef]
- Jagtap, S.; Yenkie, M.K.; Labhsetwar, N.; Rayalu, S. Fluoride in Drinking Water and Defluoridation of Water. Chem. Rev. 2020, 112, 2454–2466. [Google Scholar] [CrossRef]
- Cao, H.; Wu, X.; Syed-Hassan, S.S.A.; Zhang, S.; Mood, S.H.; Milan, Y.J.; Garcia-Perez, M. Characteristics and mechanisms of phosphorous adsorption by rape straw-derived biochar functionalized with calcium from eggshell. Bioresour. Technol. 2020, 318, 124063. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef]
- Thompson, K.A.; Shimabuku, K.K.; Kearns, J.P.; Knappe, D.R.U.; Summers, R.S.; Cook, S.M. Environmental Comparison of Biochar and Activated Carbon for Tertiary Wastewater Treatment. Environ. Sci. Technol. 2016, 50, 11253–11262. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, D.; Luo, L.; He, Q.; Cai, F.; Cheng, S.; Chen, Y. Engineering Porous Biochar for Capacitive Fluorine Removal. Sep. Purif. Technol. 2021, 257, 117932. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kim, J.Y.; Choi, Y.G. Synthesis of Bone Char from Cattle Bones and its Application for Fluoride Removal from the Contaminated Water. Groundw. Sustain. Dev. 2019, 8, 324–331. [Google Scholar] [CrossRef]
- Goswami, R.; Kumar, M. Removal of Fluoride from Aqueous Solution Using Nanoscale Rice Husk Biochar. Groundw. Sustain. Dev. 2018, 7, 446–451. [Google Scholar] [CrossRef]
- George, L.Y.; Ma, L.; Zhang, W.; Yao, G. Parametric Modelling and Analysis to Optimize Adsorption of Atrazine by MgO/Fe3O4-Synthesized Porous Carbons in Water Environment. Environ. Sci. Eur. 2023, 35, 21. [Google Scholar] [CrossRef]
- Roy, S.; Sengupta, S.; Manna, S.; Das, P. Chemically Reduced Tea Waste Biochar and its Application in Treatment of Fluoride Containing Wastewater: Batch and optimization using response surface methodology. Process Saf. Environ. Prot. 2018, 116, 553–563. [Google Scholar] [CrossRef]
- Yang, L.; He, L.; Xue, J.; Wu, L.; Ma, Y.; Li, H.; Peng, P.; Li, M.; Zhang, Z. Highly efficient nickel (II) removal by sewage sludge biochar supported α-Fe2O3 and α-FeOOH: Sorption characteristics and mechanisms. PLoS ONE 2019, 14, e0218114. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.-X. Modified-biochar adsorbents (MBAs) for heavy-metal ions adsorption: A critical review. J. Environ. Chem. Eng. 2022, 10, 107393. [Google Scholar] [CrossRef]
- Kumar, P.; Prajapati, A.K.; Dixit, S.; Yadav, V.L. Adsorption of Fluoride from Aqueous Solution Using Biochar Prepared from Waste Peanut Hull. Mater. Res. Express 2019, 6, 125553. [Google Scholar] [CrossRef]
- Tang, J.; Xiang, B.; Li, Y.; Tan, T.; Zhu, Y. Adsorption Characteristics and Charge Transfer Kinetics of Fluoride in Water by Different Adsorbents. Front. Chem. 2022, 10, 917511. [Google Scholar] [CrossRef]
- Song, J.; Yang, W.; Han, X.; Jiang, S.; Zhang, C.; Pan, W.; Jian, S.; Hu, J. Performance of Rod-Shaped Ce Metal-Organic Frameworks for Defluoridation. Molecules 2023, 28, 3492. [Google Scholar] [CrossRef]
- Hu, J.; Song, J.; Han, X.; Wen, Q.; Yang, W.; Pan, W.; Jian, S.; Jiang, S. Fabrication of Ce-La-MOFs for defluoridation in aquatic systems: A kinetics, thermodynamics and mechanisms study. Sep. Purif. Technol. 2023, 314, 123562. [Google Scholar] [CrossRef]
- Jian, S.; Chen, Y.; Shi, F.; Liu, Y.; Jiang, W.; Hu, J.; Han, X.-U.; Jiang, S.; Yang, W. Template-Free Synthesis of Magnetic La-Mn-Fe Tri-Metal Oxide Nano-fibers for Efficient Fluoride Remediation: Kinetics, Isotherms, Thermodynamics and Reusability. Polymers 2022, 14, 5417. [Google Scholar] [CrossRef]
- Jian, S.; Shi, F.; Hu, R.; Liu, Y.; Chen, Y.; Jiang, W.; Yuan, X.; Hu, J.; Zhang, K.; Jiang, S.; et al. Electrospun magnetic La2O3–CeO2–Fe3O4 composite nanofibers for removal of fluoride from aqueous solution. Compos. Commun. 2022, 33, 101194. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Y.; Chen, Z.; Song, N.; Li, X.; Ren, D.; Zhang, S. Evaluation of Fluoride and Cadmium Adsorption Modification of Corn Stalk by Aluminum Trichloride. Appl. Surf. Sci. 2021, 543, 148727. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Bianco, F.; Marcińczyk, M.; Race, M.; Papirio, S.; Esposito, G.; Oleszczuk, P. Low temperature–produced and VFA–coated biochar enhances phenanthrene adsorption and mitigates toxicity in marine sediments. Sep. Purif. Technol. 2022, 296, 121414. [Google Scholar] [CrossRef]
- Shin, J.; Kwak, J.; Kim, S.; Son, C.; Lee, Y.-G.; Kim, J.; Bae, S.; Park, Y.; Lee, S.-H.; Chon, K. Highly selective recovery of phosphate ions using a novel carbonaceous adsorbent synthesized via co-pyrolysis of spent coffee grounds and steel slags: A potential phosphatic fertilizer. Chem. Eng. J. 2023, 451, 138978. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel Insights into the Adsorption of Organic Contaminants by Biochar: A review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef]
- Keerthanan, S.; Rajapaksha, S.M.; Trakal, L.; Vithanage, M. Caffeine removal by Gliricidia sepium biochar: Influence of pyrolysis temperature and physicochemical properties. Environ. Res. 2020, 189, 109865. [Google Scholar] [CrossRef]
- Shakya, A.; Vithanage, M.; Agarwal, T. Influence of Pyrolysis Temperature on Biochar Properties and Cr(VI) Adsorption from Water with Groundnut Shell Biochars: Mechanistic approach. Environ. Res. 2022, 215, 114243. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Kim, K.; Jeong, T.-U.; Ahn, K.-H. Influence of pyrolysis temperature on characteristics and phosphate adsorption capability of biochar derived from waste-marine macroalgae (Undaria pinnatifida roots). Bioresour. Technol. 2016, 200, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.R.; Torres, E.; Zalazar, D.; Zhang, H.; Rodriguez, R.; Mazza, G. Influence of pyrolysis temperature and bio-waste composition on biochar characteristics. Renew. Energy 2020, 155, 837–847. [Google Scholar] [CrossRef]
- Angin, D. Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef]
- Ogata, F.; Tominaga, H.; Yabutani, H.; Kawasaki, N. Removal of Fluoride Ions from Water by Adsorption onto Carbonaceous Materials Produced from Coffee Grounds. J. Oleo Sci. 2011, 60, 619–625. [Google Scholar] [CrossRef]
- Hussain, N.; Chantrapromma, S.; Suwunwong, T.; Phoungthong, K. Cadmium (II) Removal from Aqueous Solution Using Magnetic Spent Coffee Ground Biochar: Kinetics, isotherm and thermodynamic adsorption. Mater. Res. Express 2020, 7, 085503. [Google Scholar] [CrossRef]
- Shin, H.; Tiwari, D.; Kim, D.-J. Phosphate Adsorption/Desorption Kinetics and P Bioavailability of Mg-Biochar from Ground Coffee Waste. J. Water Process Eng. 2020, 37, 101484. [Google Scholar] [CrossRef]
- Mcnutt, J.; He, S.Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Cherdchoo, W.; Nithettham, S.; Charoenpanich, J. Removal of Cr(VI) from Synthetic Wastewater by Adsorption Onto Coffee Ground and Mixed Waste Tea. Chemosphere 2019, 221, 758–767. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Morsy, H. Biochar of Spent Coffee Grounds as Per Se and impregnated with TiO2: Promising waste-derived adsorbents for balofloxacin. Molecules 2021, 26, 2295. [Google Scholar] [CrossRef]
- Shin, J.; Lee, S.-H.; Kim, S.; Ochir, D.; Park, Y.; Kim, J.; Lee, Y.-G.; Chon, K. Effects of Physicochemical Properties of Biochar Derived from Spent Coffee Grounds and commercial activated carbon on adsorption behavior and mechanisms of strontium ions (Sr2+). Environ. Sci. Pollut. Res. Int. 2021, 28, 40632. [Google Scholar] [CrossRef]
- Kwak, J.; Lee, S.-H.; Shin, J.; Lee, Y.-G.; Kim, S.; Son, C.; Ren, X.; Shin, J.-K.; Park, Y.; Chon, K. Synthesis and applications of bismuth-impregnated biochars originated from spent coffee grounds for efficient adsorption of radioactive iodine: A mechanism study. Environ. Pollut. 2022, 313, 120138. [Google Scholar] [CrossRef]
- Shin, J.; Kwak, J.; Kim, S.; Son, C.; Kang, B.; Lee, Y.-G.; Chon, K. Enhanced selectivity and recovery of phosphate and nitrate ions onto coffee ground waste biochars via co-precipitation of Mg/Al layered double hydroxides: A potential slow-release fertilizer. Environ. Res. 2023, 231, 116266. [Google Scholar] [CrossRef]
- Alves, A.C.F.; Antero, R.V.P.; Oliveira, S.B.; Ojala, S.A.; Scalize, P.S. Activated Carbon Produced from Waste Coffee Grounds for an Effective Removal of Bisphenol-A in Aqueous Medium. Environ. Sci. Pollut. Res. 2019, 26, 24850–24862. [Google Scholar] [CrossRef]
- APHA—American Public Health Association. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Fito, J.; Said, H.; Feleke, S.; Worku, A. Fluoride Removal from Aqueous Solution onto Activated Carbon of Catha edulis through the Adsorption Treatment Technology. Environ. Syst. Res. 2019, 8, 25. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Souissi-Najar, S.; Ouederni, A. Comments on “Comments on ‘Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2017, 154, 269–275. [Google Scholar] [CrossRef]
- Webber, W.J., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–42. [Google Scholar] [CrossRef]
- Sen Gupta, S.; Bhattacharyya, K.G. Kinetics of Adsorption of Metal Ions on Inorganic Materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef]
- Nascimento, R.F.; Lima, A.C.A.; Vidal, C.B.; Melo, D.Q.; Raulino, G.S.C. Adsorção: Aspectos Teóricos e Aplicações Ambientais, 2nd ed.; Imprensa Universitária da Universidade Federal do Ceará (UFC): Fortaleza, Brazil, 2020; Available online: https://repositorio.ufc.br/handle/riufc/53271 (accessed on 19 May 2023).
- Almeida, E.; Silva, E.S.C.; Pinto, B.C.; Lima, A.S.; Oliveira, M.A.; Bezerra, A.C.S.; Souza, T.S.F.; Rodrigues, C.G.; Machado, A.R.T. Equilíbro de Adsorção do Corente Rodamina B em Carvão Ativado Obtido dos Resíduos do Coco Verde. J. Eng. Exact Sci. 2017, 3, 1051–1058. [Google Scholar] [CrossRef]
- Lee, K.-T.; Du, J.-T.; Chen, W.-H.; Ubando, A.T.; Lee, K.T. Green Additive to Upgrade Biochar from Spent Coffee Grounds by Torrefaction for Pollution Mitigation. Environ. Pollut. 2021, 285, 117244. [Google Scholar] [CrossRef]
- Singh, Y.D.; Mahanta, P.; Bora, U. Comprehensive characterization of lignocellulosic biomass through proximate, ultimate and compositional analysis for bioenergy production. Renew. Energy 2017, 103, 490–500. [Google Scholar] [CrossRef]
- Nowicki, P. Effect of heat treatment on the physicochemical properties of nitrogen-enriched activated carbons. J. Therm. Anal. Calorim. 2016, 125, 1017–1024. [Google Scholar] [CrossRef]
- Marsh, H.; Rodriguez-Reinoso, F. Activated Carbon, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- IUPAC—International Union of Pure and Applied Chemistry; Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015. Available online: https://www.posmat.cefetmg.br/wp-content/uploads/sites/120/2017/11/IUPAC_Recommendations_gas_sorption_2015.pdf (accessed on 19 May 2023). [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Process; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- IUPAC—International Union of Pure and Applied Chemistry; Sing, K.S.W.; Evere, D.H.; Haul, R.A.W.; Moscou, L.; Pieroti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 57, 603–619. Available online: https://www.degruyter.com/document/doi/10.1351/pac198254112201/html (accessed on 19 May 2023). [CrossRef]
- Clark, J. Atomic and Ionic Radius. Available online: https://www.chemguide.co.uk/atoms/properties/atradius.html (accessed on 20 April 2023).
- Lee, K.-T.; Cheng, C.-L.; Lee, D.-S.; Chen, W.-H.; Vo, D.-V.N.; Ding, L.; Lam, S.S. Spent coffee grounds biochar from torrefaction as a potential adsorbent for spilled diesel oil recovery and as an alternative fuel. Energy 2022, 239, 122467. [Google Scholar] [CrossRef]
- Güzel, F.; Sayğili, H.; Akkaya-Sayğili, G.; Koyuncu, F.; Yilmaz, C. Optimal oxida-tion with nitric acid of biochar derived from pyrolysis of weeds and its application in removal of hazardous dye methylene blue from aqueous solution. J. Clean. Prod. 2017, 144, 260–265. [Google Scholar] [CrossRef]
- Taskin, E.; Castro Bueno, C.; Allegretta, I.; Terzano, R.; Rosa, A.H.; Loffredo, E. Multianalytical Characterization of Biochar and Hydrochar Produced from Waste Biomasses for Environmental and Agricultural Applications. Chemosphere 2019, 233, 422–430. [Google Scholar] [CrossRef]
- Mukherjee, A.; Boruggada, V.B.; Dynes, J.J.; Niu, C.; Dalai, A.K. Carbon Dioxide Capture from Flue Gas in Biochar Produced from Spent Coffee Grounds: Effect of surface chemistry and porous structure. J. Environ. Chem. Eng. 2021, 9, 106049. [Google Scholar] [CrossRef]
- Sha, Q.; Xie, H.; Liu, W.; Yang, D.; He, Y.; Yang, C.; Wang, N.; Ge, C. Removal of Fluoride Using Platanus Acerifoli Leaves Biochar—An Efficient and Low-Cost Application in Wastewater Treatment. Environ. Technol. 2021, 44, 93–107. [Google Scholar] [CrossRef]
- Hettithanthri, O.; Rajapaksha, A.U.; Nanayakkara, N.; Vithanage, M. Temperature Influence on Layered Double Hydroxide Tailored Corncob Biochar and its Application for Fluoride Removal in Aqueous Media. Environ. Pollut. 2023, 320, 121054. [Google Scholar] [CrossRef]
- Araga, R.; Kali, S.; Sharma, C.S. Coconut-Shell-Derived Carbon/Carbon Nanotube Composite for Fluoride Adsorption from Aqueous Solution. Clean Soil Air Water 2019, 47, 1800286. [Google Scholar] [CrossRef]
- Tala, W.; Chantara, S. Use of Spent Coffee Ground Biochar as Ambient PAHs Sorbent and Novel Extraction Method for GC-MS Analysis. Environ. Sci. Pollut. Res. Int. 2019, 26, 13025–13040. [Google Scholar] [CrossRef]
- Oh, T.-K.; Choi, B.; Shinogi, Y.; Chikushi, J. Effect of pH Conditions on Actual and Apparent Fluoride Adsorption by Biochar in Aqueous Phase. Water Air Soil Pollut. 2012, 223, 3729–3738. [Google Scholar] [CrossRef]
- Daifullah, A.A.M.; Yakout, S.M.; Elreefy, S.A. Adsorption of Fluoride in Aqueous Solutions Using KMnO4-Modified Activated Carbon Derived from Steam Pyrolysis of Rice Straw. J. Hazard. Mater. 2007, 147, 633–643. [Google Scholar] [CrossRef]
- Ma, S.; Jing, F.; Sohi, S.P.; Chen, J. New Insights into Contrasting Mechanisms for PAE Adsorption on Millimeter, Micron- and Nano-Scale Biochar. Environ. Sci. Pollut. Res. Int. 2019, 26, 18636–18650. [Google Scholar] [CrossRef]
- Sivaraj, R.; Namasivayam, C.; Kadirvelu, K. Orange Peel as na Adsorbent in the Removal of Acid Violet 17 (Acid Dye) form Aqueous Solutions. Waste Manag. 2001, 21, 105–110. [Google Scholar] [CrossRef]
- Mohan, D.; Kumar, S.; Srivastava, A. Fluoride Removal from Ground Water Using Magnetic and Nonmagnetic Corn Stover Biochars. Ecol. Eng. 2014, 73, 798–808. [Google Scholar] [CrossRef]
- Ho, Y.S.; Mckay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Alagumuthu, G.; Rajan, M. Equilibrium and Kinetics of Adsorption of Fluoride onto Zirconium Impregnated Cashew Nut Shell Carbon. Chem. Eng. J. 2010, 158, 451–457. [Google Scholar] [CrossRef]
- Huang, X.; An, G.; Zhu, S.; Wang, L.; Ma, F. Can Cd Translocation in Oryza sativa L. be Attenuated by Arbuscular Mycorrhizal Fungi in the Presence of EDTA? Environ. Sci. Pollut. Res. Int. 2018, 25, 9380–9390. [Google Scholar] [CrossRef]
- Heier, D.; Blackstock, T.; Stack, K.; Richardson, D.; Lewis, T. Adsorption of Wood Extractives and Model Compounds onto Bentonite. Physicochem. Eng. Asp. 2015, 482, 213–221. [Google Scholar] [CrossRef]
- Chunhui, L.; Jin, T.; Puli, Z.; Bin, Z.; Duo, B.; Xuebin, L. Simultaneous Removal of Fluoride and Arsenic in Geothermal Water in Tibet Using Modified Yak Dung Biochar as an Adsorbent. R. Soc. Open Sci. 2018, 5, 181266. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M.; Alkan, M.; Demirbas, Ö.; Özdemir, Y.; Özmetin, C. Adsorption Kinetics of Maxilon Blue GRL Onto Sepiolite from Aqueous Solutions. Chem. Eng. J. 2006, 124, 89–101. [Google Scholar] [CrossRef]
- Braun, J.C.A.; Schommer, V.A.; Lima, F.O.; Dervanoski, A. Utilização de Adsorvente Natural para Remoção de Ferro(II) de Solução Aquosa—Tratamento Cinético e Equilíbrio Termodinâmico. Rev. CIATEC–UPF 2020, 12, 21–35. [Google Scholar]
- Gomide, R. Operações Unitárias—1° Volume: Operações com Sistemas Sólidos Granulares; Edição do autor: São Paulo, Brazil, 1983. [Google Scholar]
- Nascimento, R.F.; Lima, A.C.A.; Vidal, C.B.; Melo, D.Q.; Raulino, G.S.C. Adsorção: Aspectos Teóricos e Aplicações Ambientais; Imprensa Universitária da Universidade Federal do Ceará (UFC): Fortaleza, Brazil, 2014. [Google Scholar]
- Chiavola, A.; D’amato, E.; Di Marcantonio, C. Comparison of Adsorptive Removal of Fluoride from Water by Different Adsorbents under Laboratory and Real Conditions. Water 2022, 14, 1423. [Google Scholar] [CrossRef]
- Bibi, S.; Farooqi, A.; Yasmin, A.; Kamran, M.A.; Niazi, N.K. Arsenic and Fluoride Removal by Potato Peel and Rice Husk (PPRH) Ash in Aqueous Environment. Int. J. Phytoremed. 2017, 19, 1029–1036. [Google Scholar] [CrossRef]






| Materials | C (%) | N (%) | H (%) |
|---|---|---|---|
| Coffee grounds | 52.69 | 7.42 | 2.11 |
| BC | 63.13 | 3.01 | 4.87 |
| Fluorine Concentration (mg·L−1) | qeexp (mg·g−1) | Pseudo-First-Order | Pseudo-Second-Order |
|---|---|---|---|
| 6.5 | 0.156 | qe = 0.137 k1 = 0.144 R2 = 0.99283 | qe = 0.143 k2 = 1.958 R2 = 0.94666 |
| 15 | 0.426 | qe = 0.421 k1 = 0.691 R2 = 0.99283 | qe =0.420 k2 =44.931 R2 = 0.99091 |
| 25 | 0.721 | qe = 0.655 k1 = 0.300 R2 = 0.90627 | qe = 0.686 k2 = 0.771 R2 = 0.95027 |
| 6.5 | 0.156 | qe = 0.137 k1 = 0.144 R2 = 0.99283 | qe = 0.143 k2 = 1.958 R2 = 0.94666 |
| Temperature (°C) | Langmuir | Freundlich | ||
|---|---|---|---|---|
| Parameters | Value | Parameters | Value | |
| 35 | qm KL R2 | 0.22863 0.37304 0.96437 | KF n R2 | 0.06871 0.42304 0.96843 |
| 45 | qm KL R2 | 0.45222 0.09386 0.94872 | KF n R2 | 0.04965 0.63778 0.97935 |
| 55 | qm KL R2 | 0.53203 0.09747 0.99636 | KF n R2 | 0.05735 0.65319 0.99843 |
| Adsorbent | T (K) | Kd | ∆G (kJ·mol−1) | ∆S [kJ·(molK)−1] | ∆H (kJ·mol−1) |
|---|---|---|---|---|---|
| BC | 308.15 | 0.8367 | 0.456344241 | −0.36355 | 11.466 |
| 318.15 | 0.0613 | 7.379929863 | |||
| 328.15 | 0.0619 | 7.586640694 |
| Adsorbent | qm (mg·g−1) | Reference |
|---|---|---|
| Biochar produced from coffee grounds | 0.53 | This study |
| Commercial granular activated carbon (GAC) | 0.54 | [93] |
| Chemically modified rice straw BC (KMnO4) | 18.9 | [80] |
| Raw sawdust and sugarcane bagasse BC | 1.73 and 1.15 | [7] |
| Rice husk ash | 2.91 | [94] |
| Chemically modified yak dung BC (FeCl2) | 4.85 | [88] |
| Chemically modified tea waste BC (H2SO4 + NaNO3 + KMnO4) | 24.56 | [26] |
| Cattle bone BC | 11.98 | [23] |
| Unmodified peanut shell BC | 3.66 | [30] |
| Chemically modified corn cob BC (MgCl2·6H2O e FeCl3·6H2O) | 7.24 | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, H.V.R.; Scalize, P.S.; Teran, F.J.C.; Cuba, R.M.F. Fluoride Removal from Aqueous Medium Using Biochar Produced from Coffee Ground. Resources 2023, 12, 84. https://doi.org/10.3390/resources12070084
dos Santos HVR, Scalize PS, Teran FJC, Cuba RMF. Fluoride Removal from Aqueous Medium Using Biochar Produced from Coffee Ground. Resources. 2023; 12(7):84. https://doi.org/10.3390/resources12070084
Chicago/Turabian Styledos Santos, Hellem Victoria Ribeiro, Paulo Sérgio Scalize, Francisco Javier Cuba Teran, and Renata Medici Frayne Cuba. 2023. "Fluoride Removal from Aqueous Medium Using Biochar Produced from Coffee Ground" Resources 12, no. 7: 84. https://doi.org/10.3390/resources12070084
APA Styledos Santos, H. V. R., Scalize, P. S., Teran, F. J. C., & Cuba, R. M. F. (2023). Fluoride Removal from Aqueous Medium Using Biochar Produced from Coffee Ground. Resources, 12(7), 84. https://doi.org/10.3390/resources12070084










