Quantitative Assessment of Organic and Inorganic Contaminants in Charcoal
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
- Potentially very highly harmful elements: As, Cd, Cu, Hg, and Pb.
- Potentially highly harmful elements: Zn, Ba, Cr, Mn, and Mo.
- Elements representing a lesser degree of a hazard: Co, Ni, Sn, and Te.
- Elements representing a low degree of a hazard: Ag, Bi, Ce, Se, Sr, and Zr.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gowlett, J.A.J.; Harris, J.W.K.; Walton, D.; Wood, B.A. Early archaeological sites, hominid remains and traces of fire from Chesowanja, Kenya. Nature 1981, 294, 125–129. [Google Scholar] [CrossRef]
- Carmody, R.N.; Wrangham, R.W. The energetic significance of cooking. J. Hum. Evol. 2009, 57, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Maddin, R.; Muhly James, D.; Wheeler Tamara, S. How the Iron Age Began. Sci. Am. A Div. Nat. Am. 1977, 237, 122–131. [Google Scholar] [CrossRef]
- Gottesman, A. Inventor of Weber Kettle Barbecue. Chicago Tribune. 2004. Available online: https://www.chicagotribune.com/news/ct-xpm-2004-05-26-0405260116-story.html (accessed on 28 June 2021).
- Plaza-Bolaños, P.; Frenich, A.G.; Vidal, J.L.M. Polycyclic aromatic hydrocarbons in food and beverages. Analytical methods and trends. J. Chromatogr. A 2010, 1217, 6303–6326. [Google Scholar] [CrossRef] [PubMed]
- Advances in Smoking of Foods. In Proceedings of the Advances in Smoking of Foods, Warsaw, Poland, 8–10 September 1976; Elsevier BV: Amsterdam, The Netherlands, 1978.
- Więk, A.; Tkacz, K.; Żywica, R. Content of Polycyclic Aromatic Hydrocarbons (Pahs) in Grilled Meat Products Depending on Fat Content in Raw Material. Zywnosc Nauka Technol. Jakosc Food Sci. Technol. Qual 2013, 2, 39. [Google Scholar] [CrossRef]
- Alhamdow, A.; Gustavsson, P.; Rylander, L.; Jakobsson, K.; Tinnerberg, H.; Broberg, K. Chimney sweeps in Sweden: A questionnaire-based assessment of long-term changes in work conditions, and current eye and airway symptoms. Int. Arch. Occup. Environ. Health 2016, 90, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhang, G.; Liao, X. Negative role of biochars in the dissipation and vegetable uptake of polycyclic aromatic hydrocarbons (PAHs) in an agricultural soil: Cautions for application of biochars to remediate PAHs-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 213, 112075. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Godlewska, P.; Reible, D.D.; Kraska, P. Bioaccessibility of polycyclic aromatic hydrocarbons in activated carbon or biochar amended vegetated (Salix viminalis) soil. Environ. Pollut. 2017, 227, 406–413. [Google Scholar] [CrossRef]
- Lyu, H.; He, H.; Tang, J.; Hecker, M.; Liu, Q.; Jones, P.; Codling, G.; Giesy, J.P. Effect of pyrolysis temperature on potential toxicity of biochar if applied to the environment. Environ. Pollut. 2016, 218, 1–7. [Google Scholar] [CrossRef]
- Mu, L.; Peng, L.; Liu, X.; Song, C.; Bai, H.; Zhang, J.; Hu, N.; He, Q.; Li, F. Characteristics of polycyclic aromatic hydrocarbons and their gas/particle partitioning from fugitive emissions in coke plants. Atmos. Environ. 2014, 83, 202–210. [Google Scholar] [CrossRef]
- Sharma, R.K.; Hajaligol, M.R. Effect of pyrolysis conditions on the formation of polycyclic aromatic hydrocarbons (PAHs) from polyphenolic compounds. J. Anal. Appl. Pyrolysis 2003, 66, 123–144. [Google Scholar] [CrossRef]
- Kruge, A.M. Determination of thermal maturity and organic matter type by principal components analysis of the distributions of polycyclic aromatic compounds. Int. J. Coal Geol. 2000, 43, 27–51. [Google Scholar] [CrossRef]
- Marynowski, L.; Kubik, R.; Uhl, D.; Simoneit, B.R. Molecular composition of fossil charcoal and relationship with incomplete combustion of wood. Org. Geochem. 2014, 77, 22–31. [Google Scholar] [CrossRef]
- Qi, H.; Li, W.-L.; Zhu, N.-Z.; Ma, W.-L.; Liu, L.-Y.; Zhang, F.; Li, Y.-F. Concentrations and sources of polycyclic aromatic hydrocarbons in indoor dust in China. Sci. Total Environ. 2014, 491–492, 100–107. [Google Scholar] [CrossRef]
- Szatyłowicz, E.; Skoczko, I. Evaluation of the PAH Content in Soot from Solid Fuels Combustion in Low Power Boilers. Energies 2019, 12, 4254. [Google Scholar] [CrossRef] [Green Version]
- Chaemsai, S.; Kunanopparat, T.; Srichumpuang, J.; Nopharatana, M.; Tangduangdee, C.; Siriwattanayotin, S. Reduction of the polycyclic aromatic hydrocarbon (PAH) content of charcoal smoke during grilling by charcoal preparation using high carbonisation and a preheating step. Food Addit. Contam. Part A 2016, 33, 385–390. [Google Scholar] [CrossRef]
- Barbosa, J.M.; Ré-Poppi, N.; Santiago-Silva, M. Polycyclic aromatic hydrocarbons from wood pyrolyis in charcoal production furnaces. Environ. Res. 2006, 101, 304–311. [Google Scholar] [CrossRef]
- Sparrevik, M.; Chris, A.; Martinsen, V.; Jubaedah; Cornelissen, G. Emissions of gases and particles from charcoal/biochar production in rural areas using medium-sized traditional and improved “retort” kilns. Biomass Bioenergy 2015, 72, 65–73. [Google Scholar] [CrossRef]
- Walker, C.H.; Hopkin, S.P.; Sibly, R.M.; Peakall, D.B. Podstawy Ekotoksykologii; WN PWN: Warszawa, Poland, 2002; ISBN 83-01-13802-5. [Google Scholar]
- Susaya, J.; Kim, K.-H.; Ahn, J.-W.; Jung, M.-C.; Kang, C.-H. BBQ charcoal combustion as an important source of trace metal exposure to humans. J. Hazard. Mater. 2010, 176, 932–937. [Google Scholar] [CrossRef]
- PN-EN ISO 16967:2015-06, Solid Biofuels—Determination of Main Elements. Available online: https://sklep.pkn.pl/pn-en-iso-16967-2015-06e.html (accessed on 28 June 2021).
- PN-EN ISO 17225-1:2014-07, Solid Biofuels—Fuel Specifications and Grades—Part 1: General Requirements. Available online: https://sklep.pkn.pl/pn-en-iso-17225-1-2014-07e.html (accessed on 28 June 2021).
- New Jersey Department of Health and Senior Services, Hazardous Substance Fact Sheet. Available online: https://web.doh.state.nj.us/rtkhsfs/factsheets.aspx (accessed on 28 June 2021).
- Klavina, K.; Blumberga, D. A comparison of different charcoal production technology outputs. In Proceedings of the International Scientific and Practical Conference, Environment, Rezekne, Latvia, 18–20 June 2015; Volume II, pp. 137–140. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Herath, H.M.S.K. Polycyclic aromatic hydrocarbons (PAHs) in biochar—Their formation, occurrence and analysis: A review. Org. Geochem. 2017, 114, 1–11. [Google Scholar] [CrossRef]
- Simoneit, B.R.T. Biomarker PAHs in the environment. In The Handbook of Environmental Chemistry 3, Part I, PAHs and Related Compounds; Chapter 5; Neilson, A.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 176–221. ISBN 978-3-642-08286-3. [Google Scholar]
- Yunker, M.B.; Macdonald, R.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Charriau, A.; Bodineau, L.; Ouddane, B.; Fischer, J.-C. Polycyclic aromatic hydrocarbons and n-alkanes in sediments of the Upper Scheldt River Basin: Contamination levels and source apportionment. J. Environ. Monit. 2009, 11, 1086–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, S.K.; Kim, K.-H.; Kang, C.-H.; Jung, M.C.; Yoon, H. BBQ charcoal as an important source of mercury emission. J. Hazard. Mater. 2009, 162, 536–538. [Google Scholar] [CrossRef]
- Oskarsson, A. Barium. In Handbook on the Toxicology of Metals 2015; Academic Press: Cambridge, MA, USA, 2014; pp. 625–634. [Google Scholar]
- Kraszkiewicz, A. Zawartość Wybranych Metali Ciężkich w Drewnie Robinii Akacjowej, Problemy Inżynierii Rolniczej nr 2/2010. 2010. Available online: https://www.itp.edu.pl/old/wydawnictwo/pir/zeszyt_68_2010/Kraszkiewicz_Zawartosc%20wybranych%20metali.pdf (accessed on 28 June 2021).
- Nisbet, I.C.; Lagoy, P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Zhang, D.; An, T.; Qiao, M.; Loganathan, B.; Zeng, X.; Sheng, G.; Fu, J. Source identification and health risk of polycyclic aromatic hydrocarbons associated with electronic dismantling in Guiyu town, South China. J. Hazard. Mater. 2011, 192, 1–7. [Google Scholar] [CrossRef]
- Bourotte, C.; Forti, M.-C.; Taniguchi, S.; Bícego, M.C.; Lotufo, P. A wintertime study of PAHs in fine and coarse aerosols in São Paulo city, Brazil. Atmos. Environ. 2005, 39, 3799–3811. [Google Scholar] [CrossRef]
- Sienra, M.D.R.; Rosazza, N.G.; Préndez, M. Polycyclic aromatic hydrocarbons and their molecular diagnostic ratios in urban atmospheric respirable particulate matter. Atmos. Res. 2005, 75, 267–281. [Google Scholar] [CrossRef]
- Barrán-Berdón, A.L.; García González, V.; Pedraza Aboytes, G.; Rodea-Palomares, I.; Carrillo-Chávez, A.; Gómez-Ruiz, H.; Verduzco Cuéllar, B. Polycyclic aromatic hydrocarbons in soils from a brick manufacturing location in central Mexico. Rev. Int. Contam. Ambient. 2012, 28, 277–288. [Google Scholar]
- Wei, H.; Guangbin, L.; Yong, T.; Qin, Z. Emission of polycyclic aromatic hydrocarbons from different types of motor vehicles’ exhaust. Environ. Earth Sci. 2015, 74, 5557–5564. [Google Scholar] [CrossRef]
- Tsai, P.-J.; Shieh, H.-Y.; Lee, W.-J.; Lai, S.-O. Health-risk assessment for workers exposed to polycyclic aromatic hydrocarbons (PAHs) in a carbon black manufacturing industry. Sci. Total Environ. 2001, 278, 137–150. [Google Scholar] [CrossRef]
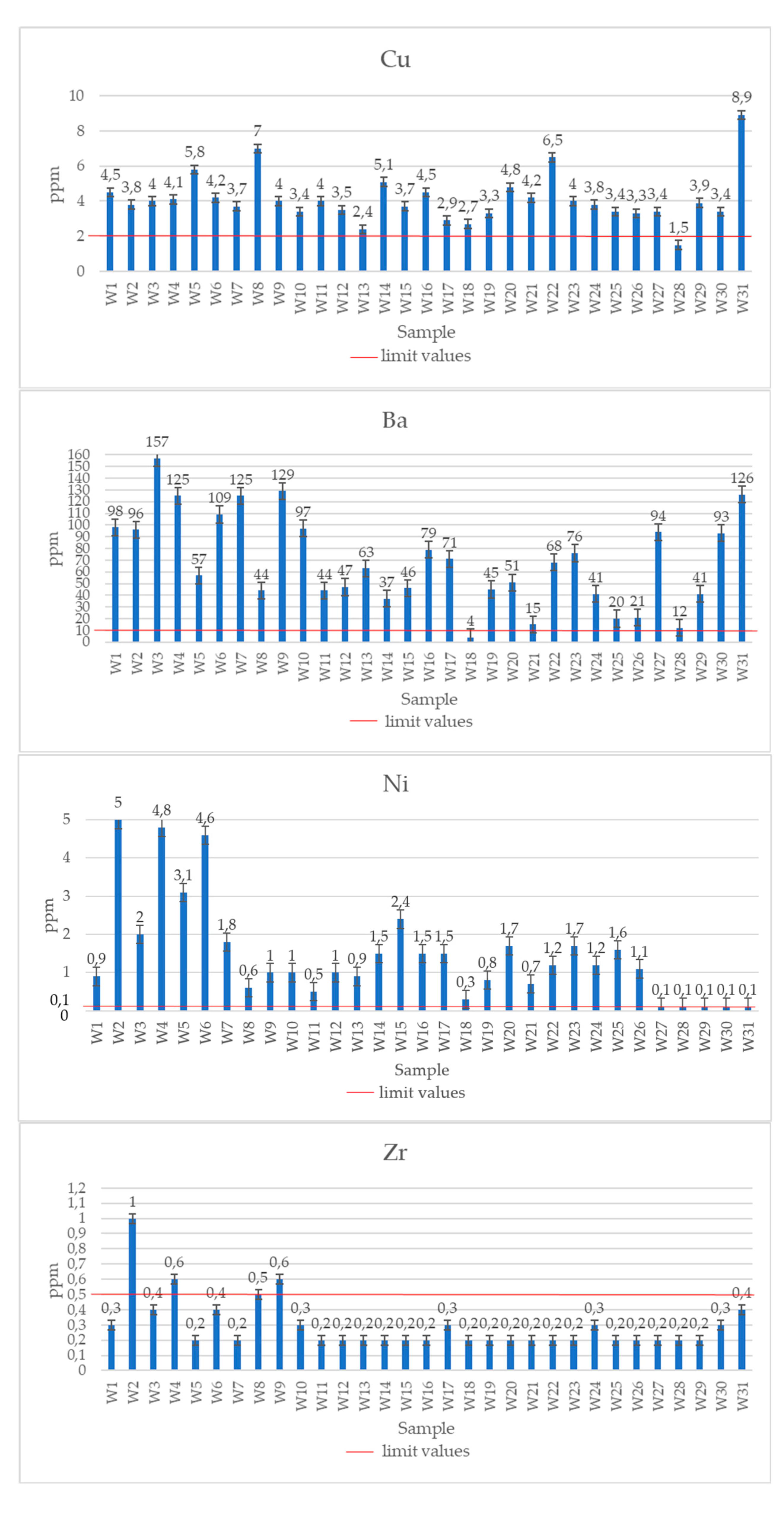
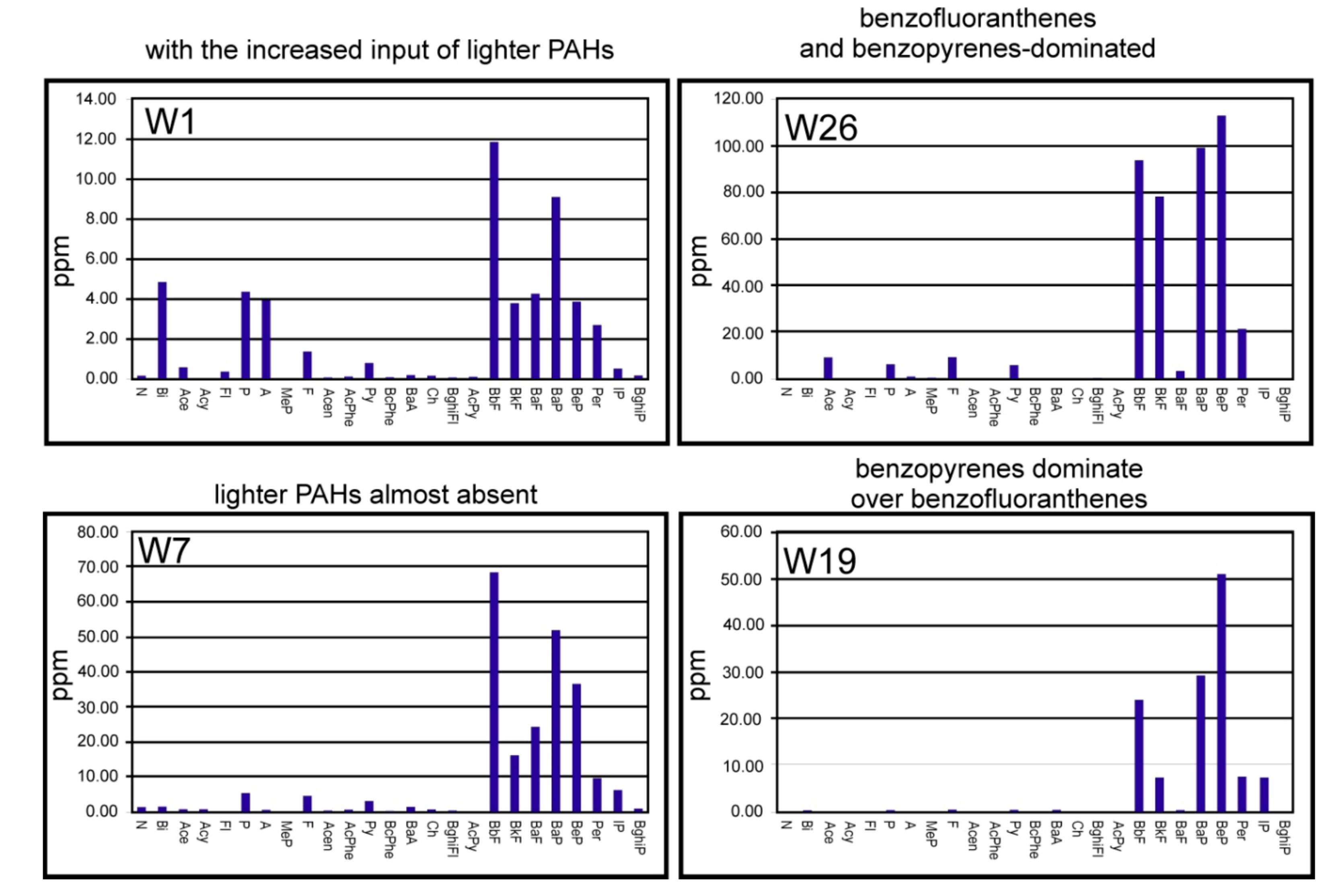
| Symbol | Cr | Mn | Co | Ni | Cu | Zn | As | Se | Sr | Zr | Mo | Ag | Cd | Sn | Te | Ba | Ce | Hg | Pb | Bi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | 1.0 | 83.0 | 1.5 | 0.5 | 2.0 | 10.0 | 0.2 | 0.3 | 5.0 | 0.5 | 24.0 | 20.0 | 0.1 | 0.1 | 1.0 | 10.0 | 10.0 | 0.02 | 2.0 | 0.1 |
| σ | 4.9 | 121.5 | 0.85 | 4.95 | 4.75 | 47.5 | 0.95 | 3.82 | - | - | 40 | 3 | 0.22 | 0.1 | 11.5 | 10.5 | - | 0.02 | 4.75 | 2.49 |
| Sample | Production Method | Location Country/Voivodeship or Region | Type of Wood Used for the Production | ||
|---|---|---|---|---|---|
| Generator | Annealed | Earth Pits | |||
| 1 | 2 | 3 | 4 | 5 | 6 |
| W1 | X | Poland /West Pomeranian Voivodeship | hornbeam, beech, ash | ||
| W2 | X | Poland/West Pomeranian Voivodeship | beech, birch | ||
| W3 | X | Poland/Lublin Voivodeship | hornbeam, beech, ash, oak | ||
| W4 | no data | ||||
| W5 | X | Poland/Podlaskie Voivodeship | beech | ||
| W6 | no data | ||||
| W7 | X | Poland/Podlaskie Voivodeship | hornbeam, beech, ash, oak | ||
| W8 | X | Poland/Mazovian Voivodeship | hornbeam, beech, ash, oak | ||
| W9 | X | Poland/Podlaskie Voivodeship | beech, oak | ||
| W10 | X | Poland/Podlaskie Voivodeship | beech | ||
| W11 | X | Poland/Greater Poland Voivodeship | beech, oak, ash, hornbeam, | ||
| W12 | X | Poland/Lesser Poland | beech, hornbeam, oak, birch | ||
| W13 | no data | ||||
| W14 | X | Poland/Podkarpackie Voivodeship | beech, hornbeam | ||
| W15 | X | Poland/Greater Poland Voivodeship | birch, hornbeam, oak, beech | ||
| W16 | X | Poland/West Pomeranian Voivodeship | hornbeam, beech, ash, oak | ||
| W17 | no data | ||||
| W18 | X | Poland/West Pomeranian Voivodeship | beech, ash | ||
| W19 | X | Poland/Greater Poland Voivodeship | beech, hornbeam, oak, birch | ||
| W20 | X | Poland/Mazovian Voivodeship | no data | ||
| W21 | x | Poland/Lesser Poland | beech, hornbeam, oak, birch | ||
| W22 | X | Poland/Greater Poland Voivodeship | beech, hornbeam, oak, birch | ||
| W23 | X | Poland/Mazovian Voivodeship | mixed deciduous wood | ||
| W24 | X | Poland/Kuyavian-Pomeranian Voivodeship | mixed deciduous wood | ||
| W25 | X | Poland/Silesian Voivodeship | beech, hornbeam, birch | ||
| W26 | X | Poland/Mazovian Voivodeship | beech, hornbeam, oak, birch | ||
| W27 | X | Poland/Kuyavian-Pomeranian Voivodeship | no data | ||
| W28 | X | Poland/Pomeranian Voivodeship | beech, hornbeam, oak, birch | ||
| W29 | X | Germany/Mannheim | beech, hornbeam, oak, birch | ||
| W30 | X | Poland/Lesser Poland | hornbeam, birch | ||
| W31 | X | Poland/Lower Silesian Voivodeship | beech, hornbeam, oak, birch | ||
| Sample | Symbol | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | Cr | Mn | Co | Ni | Cu | Zn | As | Se | Sr | Zr | Mo | Ag | Cd | Sn | Te | Ba | Ce | Hg | Pb | Bi | |
| W1 | 1 | 32.0 | 0.3 | 0.9 | 4.5 | 7.6 | <0.2 | 0.4 | 260 | 0.3 | 1.24 | <0.02 | 0.02 | <0.1 | 0.88 | 98 | 1.73 | <0.01 | 1.58 | <0.04 | |
| W2 | <1 | 124.0 | 0.2 | 5.0 | 3.8 | 7.7 | <0.2 | <0.3 | 47 | 1.0 | 0.24 | <0.02 | 0.02 | <0.1 | 0.20 | 96 | 0.29 | <0.01 | 0.36 | <0.04 | |
| W3 | <1 | 240.0 | 0.2 | 2.0 | 4.0 | 6.0 | <0.2 | <0.3 | 25 | 0.4 | 0.10 | <0.02 | 0.02 | <0.1 | 0.16 | 157 | 0.10 | <0.01 | 0.26 | <0.04 | |
| W4 | 1 | 218.0 | 0.2 | 4.8 | 4.1 | 31.8 | 0.2 | 0.3 | 91 | 0.6 | 0.08 | 0.02 | 0.09 | <0.1 | 0.30 | 125 | 0.06 | 0.02 | 0.92 | <0.04 | |
| W5 | <1 | 717.0 | 0.2 | 3.1 | 5.8 | 6.6 | <0.2 | <0.3 | 18 | <0.2 | 0.09 | 0.24 | 0.03 | <0.1 | 0.09 | 57 | 0.13 | <0.01 | 0.27 | <0.04 | |
| W6 | 1 | 231.0 | 0.2 | 4.6 | 4.2 | 26.0 | <0.2 | <0.3 | 83 | 0.4 | 0.08 | <0.02 | 0.07 | <0.1 | 0.18 | 109 | 0.09 | <0.01 | 0.81 | <0.04 | |
| W7 | <1 | 273.0 | 0.2 | 1.8 | 3.7 | 8.4 | 0.3 | <0.3 | 32 | <0.2 | 0.06 | 0.33 | 0.05 | <0.1 | 0.13 | 125 | 0.08 | <0.01 | 0.38 | <0.04 | |
| W8 | <1 | 127.0 | <0.2 | 0.6 | 7.0 | 47.3 | <0.2 | <0.3 | 17 | 0.5 | 0.07 | 0.82 | 0.20 | <0.1 | 0.05 | 44 | 0.06 | <0.01 | 1.50 | <0.04 | |
| W9 | <1 | 220.0 | <0.2 | 1.0 | 4.0 | 8.0 | 0.3 | <0.3 | 127 | 0.6 | 0.81 | <0.02 | 0.09 | <0.1 | 0.58 | 129 | 0.59 | <0.01 | 0.69 | <0.04 | |
| W10 | <1 | 1817.0 | 0.5 | 1.0 | 3.4 | 41.9 | 0.3 | <0.3 | 27 | 0.3 | 0.05 | <0.02 | 0.23 | <0.1 | 0.11 | 97 | 0.08 | <0.01 | 1.98 | <0.04 | |
| W11 | <1 | 127.0 | <0.2 | 0.5 | 4.0 | 4.5 | <0.2 | <0.3 | 10 | <0.2 | 0.05 | <0.02 | 0.03 | <0.1 | 0.12 | 44 | 0.03 | <0.01 | 0.36 | <0.04 | |
| W12 | <1 | 366.0 | <0.2 | 1.0 | 3.5 | 23.1 | 0.2 | <0.3 | 16 | 0.2 | 0.09 | 0.34 | 0.09 | <0.1 | 0.18 | 47 | 0.18 | <0.01 | 1.65 | <0.04 | |
| W13 | <1 | 183.0 | 0.2 | 0.9 | 2.4 | 11.3 | 0.2 | <0.3 | 47 | <0.2 | 0.09 | <0.02 | 0.09 | <0.1 | 0.29 | 63 | 0.09 | <0.01 | 2.0 | <0.04 | |
| W14 | <1 | 76.0 | <0.2 | 1.5 | 5.1 | 3.5 | <0.2 | <0.3 | 34 | 0.2 | 0.05 | <0.02 | 0.04 | <0.1 | 0.20 | 37 | 0.09 | <0.01 | 0.23 | <0.04 | |
| W15 | <1 | 272.0 | 0.4 | 2.4 | 3.7 | 9.0 | <0.2 | <0.3 | 44 | <0.2 | 0.07 | 0.21 | 0.04 | <0.1 | 0.14 | 46 | 0.17 | <0.01 | 0.36 | <0.04 | |
| W16 | <1 | 293.0 | 0.2 | 1.5 | 4.5 | 60.2 | 0.5 | <0.3 | 25 | <0.2 | 0.05 | <0.02 | 0.02 | <0.1 | 0.12 | 79 | 0.11 | <0.01 | 0.65 | <0.04 | |
| W17 | <1 | 169.0 | <0.2 | 1.5 | 2.9 | 21.6 | <0.2 | 0.3 | 8 | 0.3 | 0.09 | <0.02 | 0.15 | <0.1 | 0.16 | 71 | 0.93 | <0.01 | 0.97 | <0.04 | |
| W18 | <1 | 8.0 | <0.2 | 0.3 | 2.7 | 9.6 | <0.2 | <0.3 | 53 | 0.2 | 0.05 | <0.02 | 0.02 | <0.1 | 0.10 | 4 | 0.02 | <0.01 | 0.19 | <0.04 | |
| W19 | <1 | 179.0 | <0.2 | 0.8 | 3.3 | 15.3 | <0.2 | <0.3 | 38 | <0.2 | 0.15 | <0.02 | 0.04 | <0.1 | 0.14 | 45 | 0.10 | <0.01 | 0.24 | <0.04 | |
| W20 | <1 | 145.0 | <0.2 | 1.7 | 4.8 | 5.4 | <0.2 | <0.3 | 14 | 0.2 | 0.05 | <0.02 | 0.08 | <0.1 | 0.10 | 51 | 0.06 | <0.01 | 0.31 | <0.04 | |
| W21 | <1 | 50.0 | <0.2 | 0.7 | 4.2 | 4.9 | 0.3 | <0.3 | 65 | <0.2 | 0.12 | <0.02 | 0.05 | <0.1 | 0.16 | 15 | 0.05 | <0.01 | 0.23 | <0.04 | |
| W22 | <1 | 338.0 | 0.4 | 1.2 | 6.5 | 53.2 | <0.2 | <0.3 | 54 | <0.2 | 0.13 | <0.02 | 0.03 | <0.1 | 0.33 | 68 | 0.20 | <0.01 | 0.46 | <0.04 | |
| W23 | <1 | 349.0 | <0.2 | 1.7 | 4.0 | 8.4 | <0.2 | 0.4 | 37 | <0.2 | 0.09 | <0.02 | 0.03 | <0.1 | 0.24 | 76 | 0.12 | <0.01 | 0.29 | <0.04 | |
| W24 | <1 | 318.0 | 0.3 | 1.2 | 3.8 | 13.5 | 0.2 | 0.4 | 9 | 0.3 | 0.05 | 0.23 | 0.10 | <0.1 | 0.05 | 41 | 0.19 | <0.01 | 0.37 | <0.04 | |
| W25 | <1 | 28.0 | <0.2 | 1.6 | 3.4 | 5.1 | <0.2 | <0.3 | 14 | 0.2 | 0.06 | <0.02 | 0.03 | <0.1 | 0.07 | 20 | 0.07 | <0.01 | 0.16 | <0.04 | |
| W26 | <1 | 113.0 | <0.2 | 1.1 | 3.3 | 4.2 | 0.3 | <0.3 | 16 | <0.2 | 0.05 | <0.02 | 0.04 | <0.1 | 0.10 | 21 | 0.10 | <0.01 | 0.35 | <0.04 | |
| W27 | <1 | 359.0 | <0.2 | 1.4 | 3.4 | 9.0 | <0.2 | 0.3 | 25 | <0.2 | 0.05 | <0.02 | 0.06 | <0.1 | 0.19 | 94 | 0.06 | <0.01 | 0.41 | <0.04 | |
| W28 | <1 | 4.0 | <0.2 | 0.4 | 1.5 | 3.7 | <0.2 | <0.3 | 16 | <0.2 | 0.05 | <0.02 | 0.03 | <0.1 | 0.05 | 12 | 0.03 | <0.01 | 0.13 | <0.04 | |
| W29 | <1 | 141.0 | <0.2 | 1.9 | 3.9 | 4.8 | <0.2 | <0.3 | 17 | <0.2 | 0.05 | 0.28 | 0.06 | <0.1 | 0.09 | 41 | 0.11 | <0.01 | 0.27 | <0.04 | |
| W30 | <1 | 117.0 | 0.3 | 1.0 | 3.4 | 41.9 | <0.2 | 0.4 | 27 | 0.3 | 0.05 | <0.02 | 0.13 | <0.1 | 0.38 | 93 | 0.22 | <0.01 | 1.98 | <0.04 | |
| W31 | <1 | 747.0 | 0.2 | 1.4 | 8.9 | 26.7 | <0.2 | 0.4 | 175 | 0.4 | 0.39 | 0.21 | 0.03 | <0.1 | 0.24 | 126 | 0.92 | <0.01 | 1.46 | <0.04 | |
| * MDL | 1 | 1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.2 | 0.3 | 1 | 0.2 | 0.05 | 0.02 | 0.02 | 0.1 | 0.05 | 1 | 0.02 | 0.01 | 0.02 | 0.04 | |
| Sample | Symbol | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | As | Cd | Cu | Hg | Pb | Zn | Σ | Ba | Cr | Mn | Mo | Σ | |
| W1 | <0.2 | 0.02 | 4.5 | <0.01 | 1.58 | 7.6 | 13.91 | 98 | 1 | 32.0 | 1.24 | 132.24 | |
| W2 | <0.2 | 0.02 | 3.8 | <0.01 | 0.36 | 7.7 | 12.09 | 96 | <1 | 124.0 | 0.24 | 221.24 | |
| W3 | <0.2 | 0.02 | 4.0 | <0.01 | 0.26 | 6.0 | 26 | 157 | <1 | 240.0 | 0.10 | 353.48 | |
| W4 | 0.2 | 0.09 | 4.1 | 0.02 | 0.92 | 31.8 | 37.13 | 125 | 1 | 218.0 | 0.08 | 344.08 | |
| W5 | <0.2 | 0.03 | 5.8 | <0.01 | 0.27 | 6.6 | 12.91 | 57 | <1 | 717.0 | 0.09 | 775.09 | |
| W6 | <0.2 | 0.07 | 4.2 | <0.01 | 0.81 | 26.0 | 50.04 | 109 | 1 | 231.0 | 0.08 | 1119.17 | |
| W7 | 0.3 | 0.05 | 3.7 | <0.01 | 0.38 | 8.4 | 12.84 | 125 | <1 | 273.0 | 0.06 | 399.06 | |
| W8 | <0.2 | 0.20 | 7.0 | <0.01 | 1.50 | 47.3 | 56.21 | 44 | <1 | 127.0 | 0.07 | 172.07 | |
| W9 | 0.3 | 0.09 | 4.0 | <0.01 | 0.69 | 8.0 | 69.05 | 129 | <1 | 220.0 | 0.81 | 571.13 | |
| W10 | 0.3 | 0.23 | 3.4 | <0.01 | 1.98 | 41.9 | 47.82 | 97 | <1 | 1817.0 | 0.05 | 1915.05 | |
| W11 | <0.2 | 0.03 | 4.0 | <0.01 | 0.36 | 4.5 | 9.1 | 44 | <1 | 127.0 | 0.05 | 172.05 | |
| W12 | 0.2 | 0.09 | 3.5 | <0.01 | 1.65 | 23.1 | 56.92 | 47 | <1 | 366.0 | 0.09 | 2087.1 | |
| W13 | 0.2 | 0.09 | 2.4 | <0.01 | 2.0 | 11.3 | 16 | 63 | <1 | 183.0 | 0.09 | 247.09 | |
| W14 | <0.2 | 0.04 | 5.1 | <0.01 | 0.23 | 3.5 | 9.08 | 37 | <1 | 76.0 | 0.05 | 114.05 | |
| W15 | <0.2 | 0.04 | 3.7 | <0.01 | 0.36 | 9.0 | 25.08 | 46 | <1 | 272.0 | 0.07 | 361.14 | |
| W16 | 0.5 | 0.02 | 4.5 | <0.01 | 0.65 | 60.2 | 65.88 | 79 | <1 | 293.0 | 0.05 | 373.05 | |
| W17 | <0.2 | 0.15 | 2.9 | <0.01 | 0.97 | 21.6 | 25.83 | 71 | <1 | 169.0 | 0.09 | 241.09 | |
| W18 | <0.2 | 0.02 | 2.7 | <0.01 | 0.19 | 9.6 | 91.71 | 4 | <1 | 8.0 | 0.05 | 614.14 | |
| W19 | <0.2 | 0.04 | 3.3 | <0.01 | 0.24 | 15.3 | 19.09 | 45 | <1 | 179.0 | 0.15 | 225.15 | |
| W20 | <0.2 | 0.08 | 4.8 | <0.01 | 0.31 | 5.4 | 10.8 | 51 | <1 | 145.0 | 0.05 | 197.05 | |
| W21 | 0.3 | 0.05 | 4.2 | <0.01 | 0.23 | 4.9 | 29.89 | 15 | <1 | 50.0 | 0.12 | 422.2 | |
| W22 | <0.2 | 0.03 | 6.5 | <0.01 | 0.46 | 53.2 | 60.4 | 68 | <1 | 338.0 | 0.13 | 407.13 | |
| W23 | <0.2 | 0.03 | 4.0 | <0.01 | 0.29 | 8.4 | 12.93 | 76 | <1 | 349.0 | 0.09 | 426.09 | |
| W24 | 0.2 | 0.10 | 3.8 | <0.01 | 0.37 | 13.5 | 73.33 | 41 | <1 | 318.0 | 0.05 | 833.22 | |
| W25 | <0.2 | 0.03 | 3.4 | <0.01 | 0.16 | 5.1 | 8.9 | 20 | <1 | 28.0 | 0.06 | 49.06 | |
| W26 | 0.3 | 0.04 | 3.3 | <0.01 | 0.35 | 4.2 | 8.2 | 21 | <1 | 113.0 | 0.05 | 135.05 | |
| W27 | <0.2 | 0.06 | 3.4 | <0.01 | 0.41 | 9.0 | 17.1 | 94 | <1 | 359.0 | 0.05 | 184.11 | |
| W28 | <0.2 | 0.03 | 1.5 | <0.01 | 0.13 | 3.7 | 5.57 | 12 | <1 | 4.0 | 0.05 | 17.05 | |
| W29 | <0.2 | 0.06 | 3.9 | <0.01 | 0.27 | 4.8 | 9.24 | 41 | <1 | 141.0 | 0.05 | 183.05 | |
| W30 | <0.2 | 0.13 | 3.4 | <0.01 | 1.98 | 41.9 | 14.81 | 93 | <1 | 117.0 | 0.05 | 200.1 | |
| W31 | <0.2 | 0.03 | 8.9 | <0.01 | 1.46 | 26.7 | 37.3 | 126 | <1 | 747.0 | 0.39 | 874.39 | |
| Sample | Symbol | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | Co | Ni | Sn | Te | Σ | Ag | Bi | Ce | Se | Sr | Zr | Σ | |
| W1 | 0.3 | 0.9 | <0.1 | 0.88 | 2.18 | <0.2 | <0.04 | 1.73 | 0.4 | 260 | 0.3 | 48.83 | |
| W2 | 0.2 | 5.0 | <0.1 | 0.20 | 5.5 | <0.2 | <0.04 | 0.29 | <0.3 | 47 | 1.0 | 311.5 | |
| W3 | 0.2 | 2.0 | <0.1 | 0.16 | 7.68 | <0.2 | <0.04 | 0.10 | <0.3 | 25 | 0.4 | 92.2 | |
| W4 | 0.2 | 4.8 | <0.1 | 0.30 | 5.4 | 0.2 | <0.04 | 0.06 | 0.3 | 91 | 0.6 | 18.91 | |
| W5 | 0.2 | 3.1 | <0.1 | 0.09 | 3.49 | 0.24 | <0.04 | 0.13 | <0.3 | 18 | <0.2 | 111.11 | |
| W6 | 0.2 | 4.6 | <0.1 | 0.18 | 8.89 | <0.2 | <0.04 | 0.09 | <0.3 | 83 | 0.4 | 32.95 | |
| W7 | 0.2 | 1.8 | <0.1 | 0.13 | 2.23 | 0.33 | <0.04 | 0.08 | <0.3 | 32 | <0.2 | 18.72 | |
| W8 | <0.2 | 0.6 | <0.1 | 0.05 | 0.95 | 0.82 | <0.04 | 0.06 | <0.3 | 17 | 0.5 | 51.67 | |
| W9 | <0.2 | 1.0 | <0.1 | 0.58 | 3.18 | <0.2 | <0.04 | 0.59 | <0.3 | 127 | 0.6 | 27.92 | |
| W10 | 0.5 | 1.0 | <0.1 | 0.11 | 1.71 | <0.2 | <0.04 | 0.08 | <0.3 | 27 | 0.3 | 10.77 | |
| W11 | <0.2 | 0.5 | <0.1 | 0.12 | 0.92 | <0.2 | <0.04 | 0.03 | <0.3 | 10 | <0.2 | 38.69 | |
| W12 | <0.2 | 1.0 | <0.1 | 0.18 | 2.63 | 0.34 | <0.04 | 0.18 | <0.3 | 16 | 0.2 | 47.83 | |
| W13 | 0.2 | 0.9 | <0.1 | 0.29 | 1.49 | <0.2 | <0.04 | 0.09 | <0.3 | 47 | <0.2 | 34.83 | |
| W14 | <0.2 | 1.5 | <0.1 | 0.20 | 2 | <0.2 | <0.04 | 0.09 | <0.3 | 34 | 0.2 | 82.66 | |
| W15 | 0.4 | 2.4 | <0.1 | 0.14 | 3.49 | 0.21 | <0.04 | 0.17 | <0.3 | 44 | <0.2 | 25.85 | |
| W16 | 0.2 | 1.5 | <0.1 | 0.12 | 1.92 | <0.2 | <0.04 | 0.11 | <0.3 | 25 | <0.2 | 9.77 | |
| W17 | <0.2 | 1.5 | <0.1 | 0.16 | 1.96 | <0.2 | <0.04 | 0.93 | 0.3 | 8 | 0.3 | 35.62 | |
| W18 | <0.2 | 0.3 | <0.1 | 0.10 | 3.88 | <0.2 | <0.04 | 0.02 | <0.3 | 53 | 0.2 | 38.84 | |
| W19 | <0.2 | 0.8 | <0.1 | 0.14 | 1.24 | <0.2 | <0.04 | 0.10 | <0.3 | 38 | <0.2 | 14.8 | |
| W20 | <0.2 | 1.7 | <0.1 | 0.10 | 2.1 | <0.2 | <0.04 | 0.06 | <0.3 | 14 | 0.2 | 53.64 | |
| W21 | <0.2 | 0.7 | <0.1 | 0.16 | 3.34 | <0.2 | <0.04 | 0.05 | <0.3 | 65 | <0.2 | 54.94 | |
| W22 | 0.4 | 1.2 | <0.1 | 0.33 | 2.03 | <0.2 | <0.04 | 0.20 | <0.3 | 54 | <0.2 | 37.96 | |
| W23 | <0.2 | 1.7 | <0.1 | 0.24 | 2.24 | <0.2 | <0.04 | 0.12 | 0.4 | 37 | <0.2 | 92.9 | |
| W24 | 0.3 | 1.2 | <0.1 | 0.05 | 4.27 | 0.23 | <0.04 | 0.19 | 0.4 | 9 | 0.3 | 14.81 | |
| W25 | <0.2 | 1.6 | <0.1 | 0.07 | 1.97 | <0.2 | <0.04 | 0.07 | <0.3 | 14 | 0.2 | 16.84 | |
| W26 | <0.2 | 1.1 | <0.1 | 0.10 | 1.5 | <0.2 | <0.04 | 0.10 | <0.3 | 16 | <0.2 | 31.65 | |
| W27 | <0.2 | 1.4 | <0.1 | 0.19 | 3.47 | <0.2 | <0.04 | 0.06 | 0.3 | 25 | <0.2 | 16.77 | |
| W28 | <0.2 | 0.4 | <0.1 | 0.05 | 0.75 | <0.2 | <0.04 | 0.03 | <0.3 | 16 | <0.2 | 17.93 | |
| W29 | <0.2 | 1.9 | <0.1 | 0.09 | 2.29 | 0.28 | <0.04 | 0.11 | <0.3 | 17 | <0.2 | 34.7 | |
| W30 | 0.3 | 1.0 | <0.1 | 0.38 | 3.04 | <0.2 | <0.04 | 0.22 | 0.4 | 27 | 0.3 | 176.87 | |
| W31 | 0.2 | 1.4 | <0.1 | 0.24 | 1.94 | 0.21 | <0.04 | 0.92 | 0.3 | 175 | 0.4 | 262.67 | |
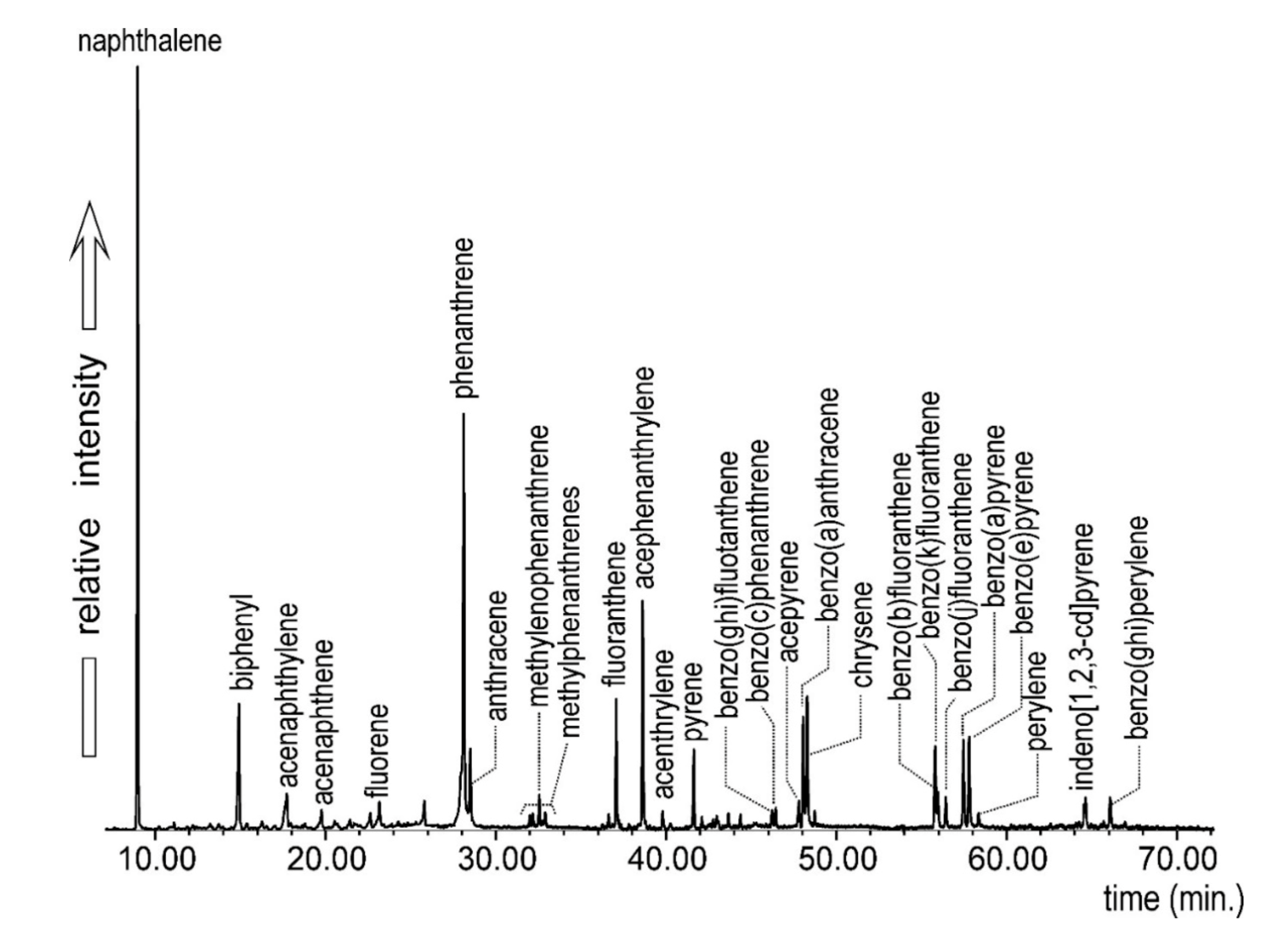
| Sample | N | Bi | Ace | Acy | F | P | A | MeP | Fl | Acen | AcPhe | Py | BcPhe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | 0.16 | 4.84 | 0.58 | - | 0.34 | 4.34 | 3.93 | - | 1.39 | 0.07 | 0.14 | 0.80 | 0.07 |
| W2 | 0.04 | 1.55 | - | - | 0.23 | 0.71 | 0.07 | - | 0.40 | 0.01 | 0.05 | 0.20 | 0.02 |
| W3 | 0.40 | 0.13 | - | - | 0.13 | 0.65 | 0.10 | - | 0.40 | - | - | 0.30 | - |
| W4 | 0.09 | - | - | - | - | 0.53 | 0.06 | - | 0.25 | 0.02 | 0.04 | 0.20 | 0.01 |
| W5 | 0.47 | 8.93 | 0.15 | 0.85 | 0.20 | 1.25 | 0.16 | 0.19 | 1.00 | 0.06 | 0.09 | 0.63 | 0.06 |
| W6 | 29.21 | 12.08 | 0.12 | - | - | 20.89 | 1.50 | - | 36.89 | 1.45 | 2.38 | 26.33 | 1.30 |
| W7 | 1.43 | 1.56 | 0.64 | 0.92 | - | 5.45 | 0.70 | - | 4.66 | 0.49 | 0.54 | 3.37 | 0.27 |
| W8 | 21.96 | 6.56 | 0.28 | - | 1.51 | 19.09 | 3.05 | 5.13 | 16.84 | 0.62 | 0.67 | 11.96 | 0.66 |
| W9 | 0.19 | 0.67 | 0.04 | 0.07 | - | 5.00 | 0.99 | - | 2.13 | 0.09 | 0.17 | 1.77 | 0.10 |
| W10 | 12.45 | 2.53 | 0.05 | 1.40 | - | 2.49 | 0.30 | - | 3.22 | - | 0.17 | 1.65 | 0.15 |
| W11 | - | - | - | - | - | 0.13 | 0.03 | - | 0.23 | - | 0.01 | 0.06 | - |
| W12 | 6.78 | 3.09 | 0.19 | 2.17 | 0.18 | 7.01 | 0.94 | 0.86 | 5.51 | 0.22 | 0.38 | 3.59 | 0.25 |
| W13 | 1.79 | 1.59 | - | - | 0.14 | 3.00 | 0.44 | 0.15 | 2.49 | 0.17 | 0.21 | 1.97 | 0.21 |
| W14 | 7.92 | 3.34 | 0.28 | 4.01 | 1.91 | 11.00 | 1.47 | 2.23 | 12.48 | 0.47 | 0.87 | 7.95 | 0.45 |
| W15 | 50.20 | 9.29 | 0.14 | 0.74 | 0.05 | 32.46 | 5.43 | 3.44 | 19.79 | 0.82 | 0.92 | 16.63 | 2.03 |
| W16 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| W17 | - | 0.20 | - | - | - | 0.23 | 0.08 | - | 0.23 | - | 0.03 | 0.14 | - |
| W18 | - | 0.22 | - | - | - | 0.71 | 0.19 | - | 24.95 | - | 0.05 | 0.36 | 0.07 |
| W19 | - | 0.41 | 0.08 | - | - | 0.59 | 0.11 | - | 0.54 | - | 0.04 | 0.50 | 0.16 |
| W20 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| W21 | 0.85 | - | - | - | 0.20 | 4.93 | 1.21 | 0.83 | 4.27 | - | - | 3.17 | 0.31 |
| W22 | 0.86 | 1.52 | - | - | 0.14 | 0.60 | 0.10 | - | 0.45 | - | - | 0.36 | 0.08 |
| W23 | - | - | - | - | - | 0.72 | 0.14 | - | 0.48 | - | - | 0.30 | 0.03 |
| W24 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| W25 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| W26 | - | - | 9.37 | - | 0.13 | 6.35 | 1.09 | 0.55 | 9.26 | - | 0.31 | 5.68 | - |
| W27 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| W28 | - | - | 0.06 | - | - | 0.52 | 0.13 | - | 0.77 | - | - | 0.46 | - |
| W29 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| W30 | - | - | 0.44 | - | 0.22 | 1.80 | 0.31 | - | 1.80 | - | - | 1.26 | - |
| W31 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Sample | BaA | Ch | BghiFl | AcPy | BbF | BkF | BaF | BaP | BeP | Pe | IP | BghiP | |
| W1 | 0.18 | 0.15 | 0.04 | 0.10 | 11.82 | 3.77 | 4.29 | 9.10 | 3.86 | 2.70 | 0.53 | 0.17 | |
| W2 | 0.11 | 0.02 | - | - | 3.75 | 2.13 | 0.12 | 7.99 | 3.46 | 0.74 | - | - | |
| W3 | - | - | - | - | 4.35 | 2.22 | 0.07 | 2.07 | 1.71 | - | - | - | |
| W4 | 0.11 | 0.01 | 0.03 | 0.05 | 3.69 | 1.10 | 0.06 | 4.55 | 2.77 | 2.22 | - | - | |
| W5 | 0.20 | 0.03 | 0.03 | 0.03 | 7.99 | 3.82 | 0.25 | 3.36 | 4.41 | 3.87 | - | - | |
| W6 | 4.27 | 2.38 | 2.65 | 1.34 | 436.61 | 105.04 | - | 166.29 | 223.62 | 48.16 | 30.88 | 3.08 | |
| W7 | 1.35 | 0.65 | 0.30 | - | 68.46 | 15.93 | 1.38 | 51.46 | 36.62 | 9.51 | 6.29 | 0.83 | |
| W8 | 4.23 | 1.41 | 0.85 | 0.16 | 206.13 | 51.88 | 3.79 | 109.38 | 120.13 | 41.67 | 10.17 | 1.75 | |
| W9 | 0.52 | 0.28 | 0.09 | 0.13 | 17.97 | 6.20 | 0.68 | 21.99 | 17.52 | 3.49 | - | - | |
| W10 | 0.23 | 0.09 | 0.18 | 0.06 | 23.69 | 10.10 | 0.62 | 23.65 | 20.08 | 6.90 | 1.17 | 0.18 | |
| W11 | - | - | - | - | 3.22 | 1.34 | 0.03 | 5.05 | 3.04 | 1.92 | - | - | |
| W12 | 1.03 | 0.50 | 0.38 | 0.27 | 47.56 | 6.43 | 0.97 | 36.84 | 29.28 | 5.52 | 3.46 | 0.32 | |
| W13 | 1.10 | 0.58 | 0.12 | 0.10 | 53.07 | 8.62 | 1.07 | 38.63 | 37.74 | 8.80 | 7.70 | 0.71 | |
| W14 | 1.88 | 0.72 | 0.68 | 0.25 | 118.37 | 21.36 | 2.14 | 73.62 | 81.47 | 24.65 | 8.43 | 0.99 | |
| W15 | 17.67 | 6.04 | 0.75 | - | 928.50 | 335.67 | 24.53 | 892.60 | 1037.38 | 140.52 | 23.35 | 5.16 | |
| W16 | - | - | - | - | - | - | - | - | - | - | - | - | |
| W17 | - | - | - | - | 5.09 | 2.28 | 0.06 | 2.03 | 2.18 | - | - | - | |
| W18 | 0.15 | 0.09 | - | - | 44.25 | 28.35 | 0.89 | 35.53 | 50.92 | 5.78 | - | - | |
| W19 | 0.46 | 0.13 | 0.26 | 0.07 | 25.85 | 7.79 | 0.64 | 27.42 | 50.99 | 7.41 | 7.69 | - | |
| W20 | - | - | - | - | - | - | - | - | - | - | - | - | |
| W21 | 1.87 | 0.69 | 0.24 | - | 60.50 | 38.64 | 2.09 | 64.04 | 59.01 | 14.65 | 0.89 | 0.07 | |
| W22 | 0.56 | 0.09 | - | - | 10.14 | 4.08 | 0.22 | 14.98 | 28.74 | 8.77 | 1.35 | 0.30 | |
| W23 | 0.09 | 0.02 | - | - | 7.85 | 0.00 | 0.12 | 6.32 | 1.98 | 2.63 | - | - | |
| W24 | - | - | - | - | - | - | - | - | 0.00 | 0.00 | - | - | |
| W25 | - | - | - | - | - | - | - | - | 0.00 | 0.00 | - | - | |
| W26 | - | - | 0.45 | - | 93.48 | 78.41 | 3.25 | 99.26 | 113.43 | 21.14 | - | - | |
| W27 | - | - | - | - | - | - | 0.00 | - | 0.00 | - | - | - | |
| W28 | - | - | - | - | 3.70 | 5.19 | 0.45 | 7.32 | 4.92 | 4.78 | - | - | |
| W29 | - | - | - | - | - | - | - | - | 0.00 | 0.00 | - | - | |
| W30 | - | - | 0.12 | - | 14.65 | 8.69 | 1.13 | 35.31 | 14.44 | 8.62 | - | - | |
| W31 | - | - | - | - | - | - | - | - | - | - | - | - |
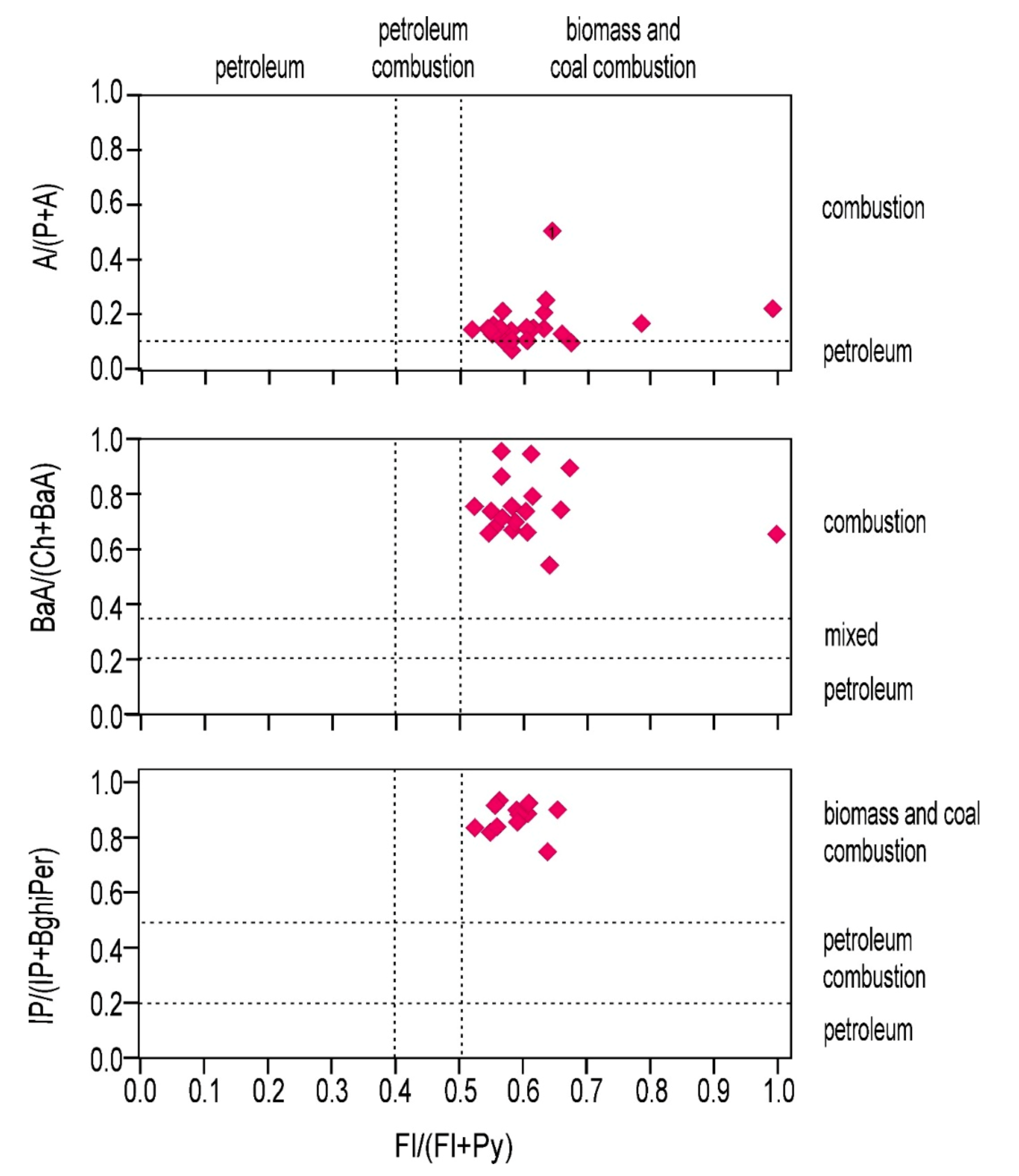
| Sample | P/A | A/(A + P) | Fl/(Fl + Py) | Fl/Py | Fl/(Fl + P) | BaA/(BaA + Ch) | BaP/BghiP | IP/BghiP | IP/(IP + BghiP) | BaA/BaP | Py/BaP |
|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | 1.10 | 0.48 | 0.64 | 1.74 | 0.24 | 0.55 | 52.52 | 3.05 | 0.75 | 0.02 | 0.09 |
| W2 | 10.48 | 0.09 | 0.67 | 2.04 | 0.36 | 0.86 | - | - | - | 0.01 | 0.02 |
| W3 | 6.17 | 0.14 | 0.58 | 1.36 | 0.38 | - | - | - | - | 0.00 | 0.14 |
| W4 | 9.01 | 0.10 | 0.56 | 1.25 | 0.32 | 0.95 | - | - | - | 0.02 | 0.04 |
| W5 | 7.96 | 0.11 | 0.61 | 1.58 | 0.44 | 0.89 | - | - | - | 0.06 | 0.19 |
| W6 | 13.95 | 0.07 | 0.58 | 1.40 | 0.64 | 0.64 | 54.04 | 10.03 | 0.91 | 0.03 | 0.16 |
| W7 | 7.83 | 0.11 | 0.58 | 1.38 | 0.46 | 0.68 | 61.77 | 7.55 | 0.88 | 0.03 | 0.07 |
| W8 | 6.26 | 0.14 | 0.58 | 1.41 | 0.47 | 0.75 | 62.36 | 5.80 | 0.85 | 0.04 | 0.11 |
| W9 | 5.06 | 0.16 | 0.55 | 1.21 | 0.30 | 0.65 | - | - | - | 0.02 | 0.08 |
| W10 | 8.38 | 0.11 | 0.66 | 1.95 | 0.56 | 0.73 | 130.97 | 6.47 | 0.87 | 0.01 | 0.07 |
| W11 | 4.71 | 0.18 | 0.78 | 3.61 | 0.64 | - | - | - | - | 0.00 | 0.01 |
| W12 | 7.44 | 0.12 | 0.61 | 1.54 | 0.44 | 0.67 | 115.20 | 10.81 | 0.92 | 0.03 | 0.10 |
| W13 | 6.79 | 0.13 | 0.56 | 1.26 | 0.45 | 0.66 | 54.28 | 10.82 | 0.92 | 0.03 | 0.05 |
| W14 | 7.46 | 0.12 | 0.61 | 1.57 | 0.53 | 0.72 | 74.01 | 8.47 | 0.89 | 0.03 | 0.11 |
| W15 | 5.98 | 0.14 | 0.54 | 1.19 | 0.38 | 0.75 | 172.93 | 4.52 | 0.82 | 0.02 | 0.02 |
| W16 | - | - | - | - | - | - | - | - | - | - | - |
| W17 | 2.89 | 0.26 | 0.63 | 1.70 | 0.50 | - | - | - | - | 0.00 | 0.07 |
| W18 | 3.78 | 0.21 | 0.99 | 69.87 | 0.97 | 0.64 | - | - | - | 0.00 | 0.01 |
| W19 | 5.60 | 0.15 | 0.52 | 1.08 | 0.48 | 0.78 | - | - | 1.00 | 0.02 | 0.02 |
| W20 | - | - | - | - | - | - | - | - | - | - | - |
| W21 | 4.08 | 0.20 | 0.57 | 1.35 | 0.46 | 0.73 | 955.55 | 13.31 | 0.93 | 0.03 | 0.05 |
| W22 | 6.15 | 0.14 | 0.56 | 1.25 | 0.43 | 0.86 | 49.82 | 4.49 | 0.82 | 0.04 | 0.02 |
| W23 | 5.16 | 0.16 | 0.61 | 1.58 | 0.40 | 0.79 | - | - | - | 0.01 | 0.05 |
| W24 | - | - | - | - | - | - | - | - | - | - | - |
| W25 | - | - | - | - | - | - | - | - | - | - | - |
| W26 | 5.80 | 0.15 | 0.62 | 1.63 | 0.59 | - | - | - | - | 0.00 | 0.06 |
| W27 | - | - | - | - | - | - | - | - | - | - | - |
| W28 | 3.95 | 0.20 | 0.62 | 1.66 | 0.59 | - | - | - | - | 0.00 | 0.06 |
| W29 | - | - | - | - | - | - | - | - | - | - | - |
| W30 | 5.91 | 0.14 | 0.59 | 1.43 | 0.50 | - | - | - | - | 0.00 | 0.04 |
| W31 | - | - | - | - | - | - | - | - | - | - | - |
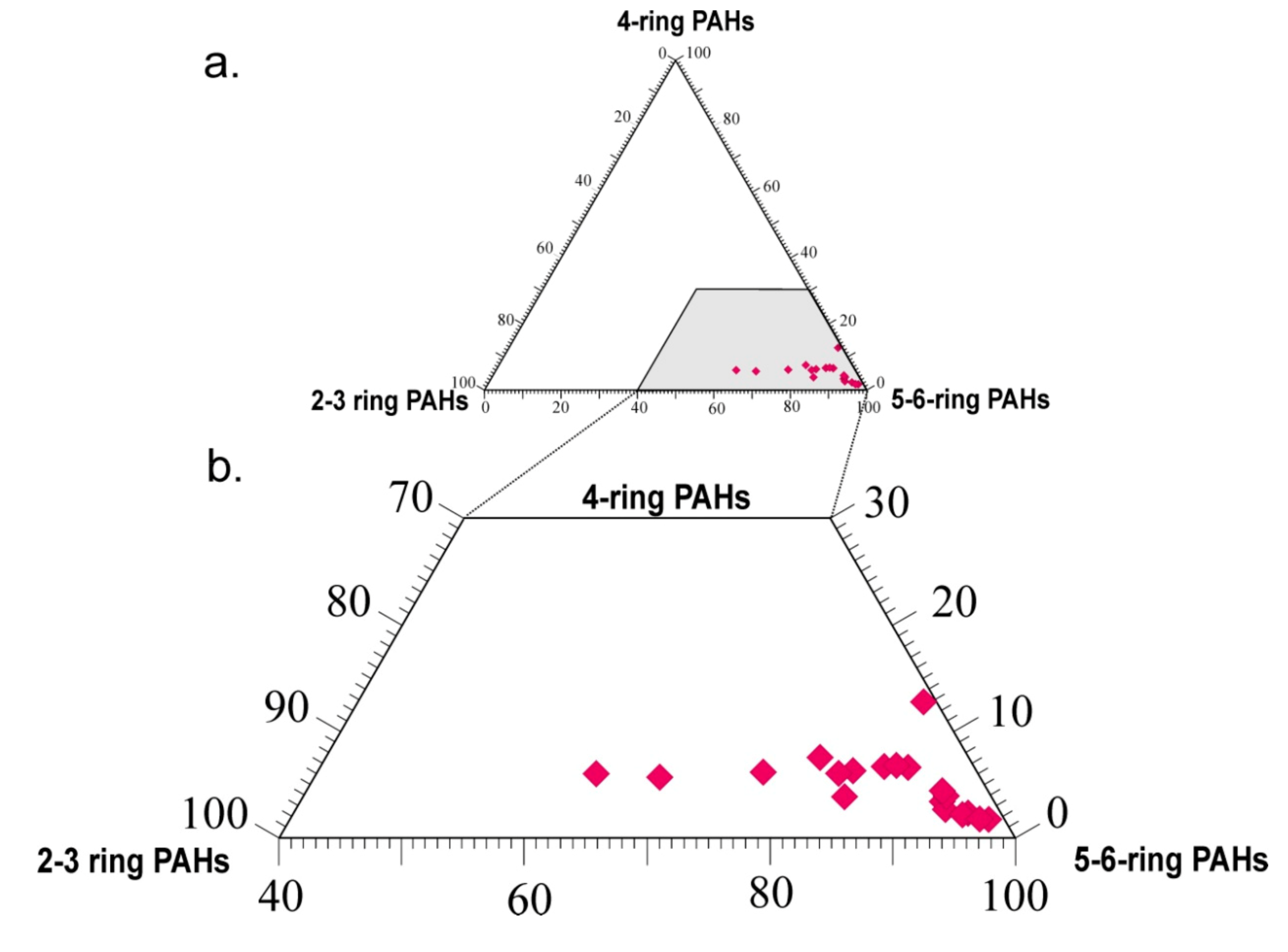
| Sample | ∑Bf/∑Bp (1) | PAH Sum (2) | TEQ (3) | ∑PAHcarc/∑PAHtot (4) | BaPE (5) | BaPE/BaP (6) | MEQ (7) | L/H (8) |
|---|---|---|---|---|---|---|---|---|
| W1 | 1.53 | 53.37 | 10.79 | 0.59 | 10.35 | 1.14 | 10.24 | 0.45 |
| W2 | 0.52 | 21.61 | 8.59 | 0.87 | 8.41 | 1.05 | 8.52 | 0.18 |
| W3 | 1.76 | 12.55 | 2.73 | 0.83 | 2.53 | 1.22 | 2.63 | 0.20 |
| W4 | 0.66 | 15.79 | 5.04 | 0.77 | 4.89 | 1.08 | 4.83 | 0.08 |
| W5 | 1.55 | 38.03 | 4.57 | 0.68 | 4.20 | 1.25 | 4.32 | 0.58 |
| W6 | 1.39 | 1156.47 | 224.43 | 0.82 | 208.78 | 1.26 | 202.78 | 0.13 |
| W7 | 0.97 | 212.81 | 60.77 | 0.82 | 58.45 | 1.14 | 57.57 | 0.10 |
| W8 | 1.14 | 639.88 | 136.91 | 0.78 | 129.56 | 1.18 | 125.90 | 0.16 |
| W9 | 0.63 | 80.08 | 24.48 | 0.81 | 23.71 | 1.08 | 23.55 | 0.16 |
| W10 | 0.79 | 111.34 | 27.21 | 0.71 | 26.23 | 1.11 | 26.57 | 0.28 |
| W11 | 0.57 | 15.06 | 5.51 | 0.84 | 5.37 | 1.06 | 5.38 | 0.03 |
| W12 | 0.83 | 163.71 | 42.76 | 0.76 | 41.15 | 1.12 | 39.59 | 0.23 |
| W13 | 0.82 | 170.40 | 45.77 | 0.82 | 44.06 | 1.14 | 43.32 | 0.08 |
| W14 | 0.91 | 388.91 | 88.79 | 0.77 | 84.79 | 1.15 | 81.79 | 0.16 |
| W15 | 0.67 | 3554.11 | 1023.87 | 0.90 | 987.11 | 1.11 | 984.98 | 0.04 |
| W16 | - | - | - | - | - | - | - | - |
| W17 | 1.77 | 12.55 | 2.77 | 0.94 | 2.54 | 1.25 | 2.60 | 0.08 |
| W18 | 0.85 | 192.50 | 42.83 | 0.83 | 40.62 | 1.14 | 42.62 | 0.16 |
| W19 | 0.44 | 131.15 | 31.61 | 0.86 | 30.42 | 1.11 | 31.76 | 0.02 |
| W20 | - | - | - | - | - | - | - | - |
| W21 | 0.82 | 258.47 | 74.27 | 0.86 | 71.20 | 1.11 | 74.02 | 0.06 |
| W22 | 0.33 | 73.34 | 16.63 | 0.81 | 16.30 | 1.09 | 16.48 | 0.06 |
| W23 | 0.96 | 20.68 | 7.12 | 0.78 | 6.87 | 1.09 | 6.32 | 0.09 |
| W24 | - | - | - | - | - | - | - | - |
| W25 | - | - | - | - | - | - | - | - |
| W26 | 0.82 | 442.16 | 116.49 | 0.87 | 111.29 | 1.12 | 118.86 | 0.08 |
| W27 | - | - | - | - | - | - | - | - |
| W28 | 0.76 | 28.31 | 8.21 | 0.75 | 7.94 | 1.09 | 8.62 | 0.07 |
| W29 | - | - | - | - | - | - | - | - |
| W30 | 0.49 | 88.79 | 37.65 | 0.82 | 36.94 | 1.05 | 37.48 | 0.07 |
| W31 | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelonek, Z.; Fabiańska, M.; Jelonek, I. Quantitative Assessment of Organic and Inorganic Contaminants in Charcoal. Resources 2021, 10, 69. https://doi.org/10.3390/resources10070069
Jelonek Z, Fabiańska M, Jelonek I. Quantitative Assessment of Organic and Inorganic Contaminants in Charcoal. Resources. 2021; 10(7):69. https://doi.org/10.3390/resources10070069
Chicago/Turabian StyleJelonek, Zbigniew, Monika Fabiańska, and Iwona Jelonek. 2021. "Quantitative Assessment of Organic and Inorganic Contaminants in Charcoal" Resources 10, no. 7: 69. https://doi.org/10.3390/resources10070069
APA StyleJelonek, Z., Fabiańska, M., & Jelonek, I. (2021). Quantitative Assessment of Organic and Inorganic Contaminants in Charcoal. Resources, 10(7), 69. https://doi.org/10.3390/resources10070069