Pretreatment of Lignocellulosic Biomass with 1-Ethyl-3-methylimidazolium Acetate for Its Eventual Valorization by Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Samples
2.2. Ionic Liquid Pretreatment
2.3. Anaerobic Digestion Test
2.4. Analytical Methods
3. Results
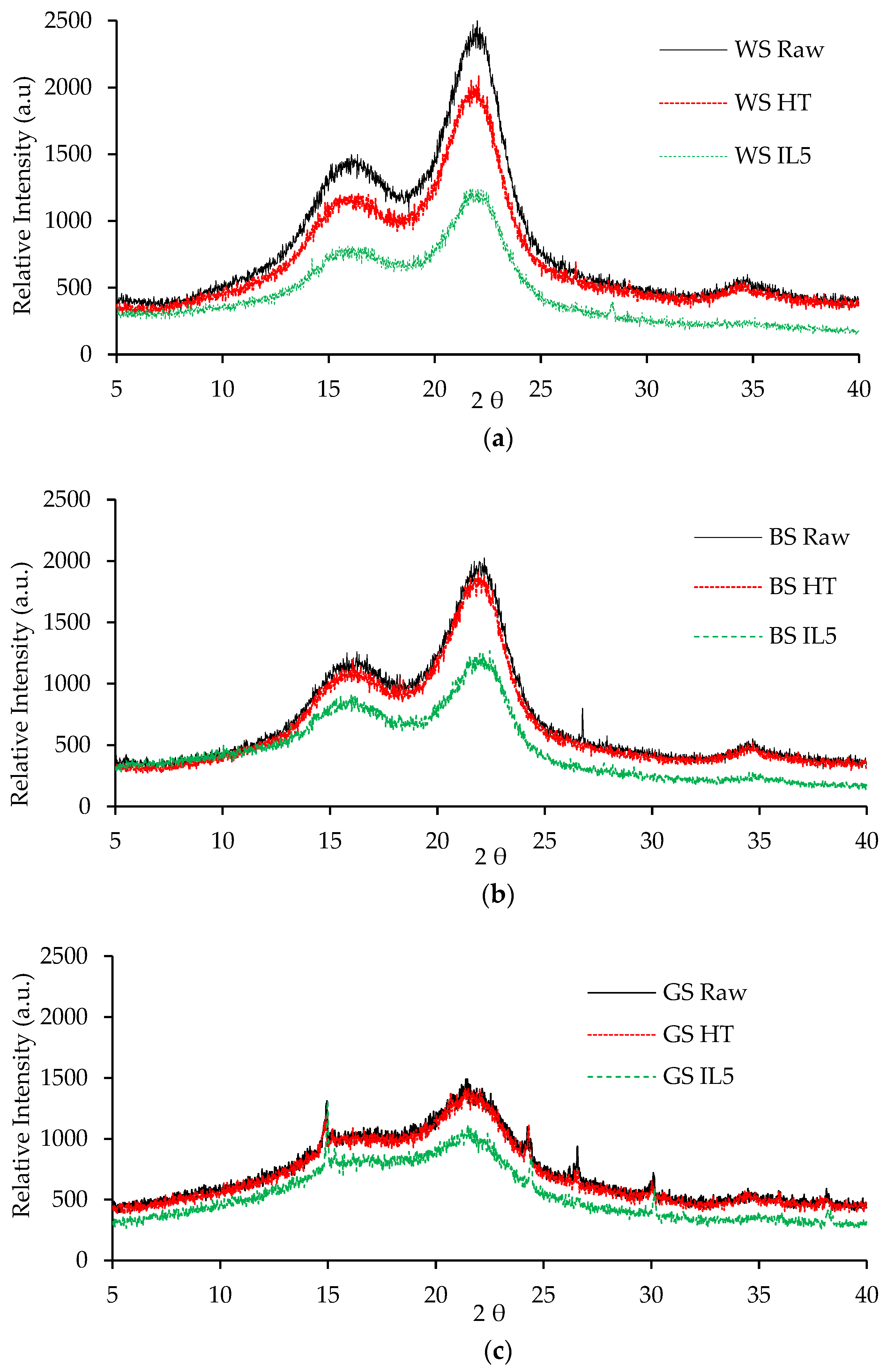
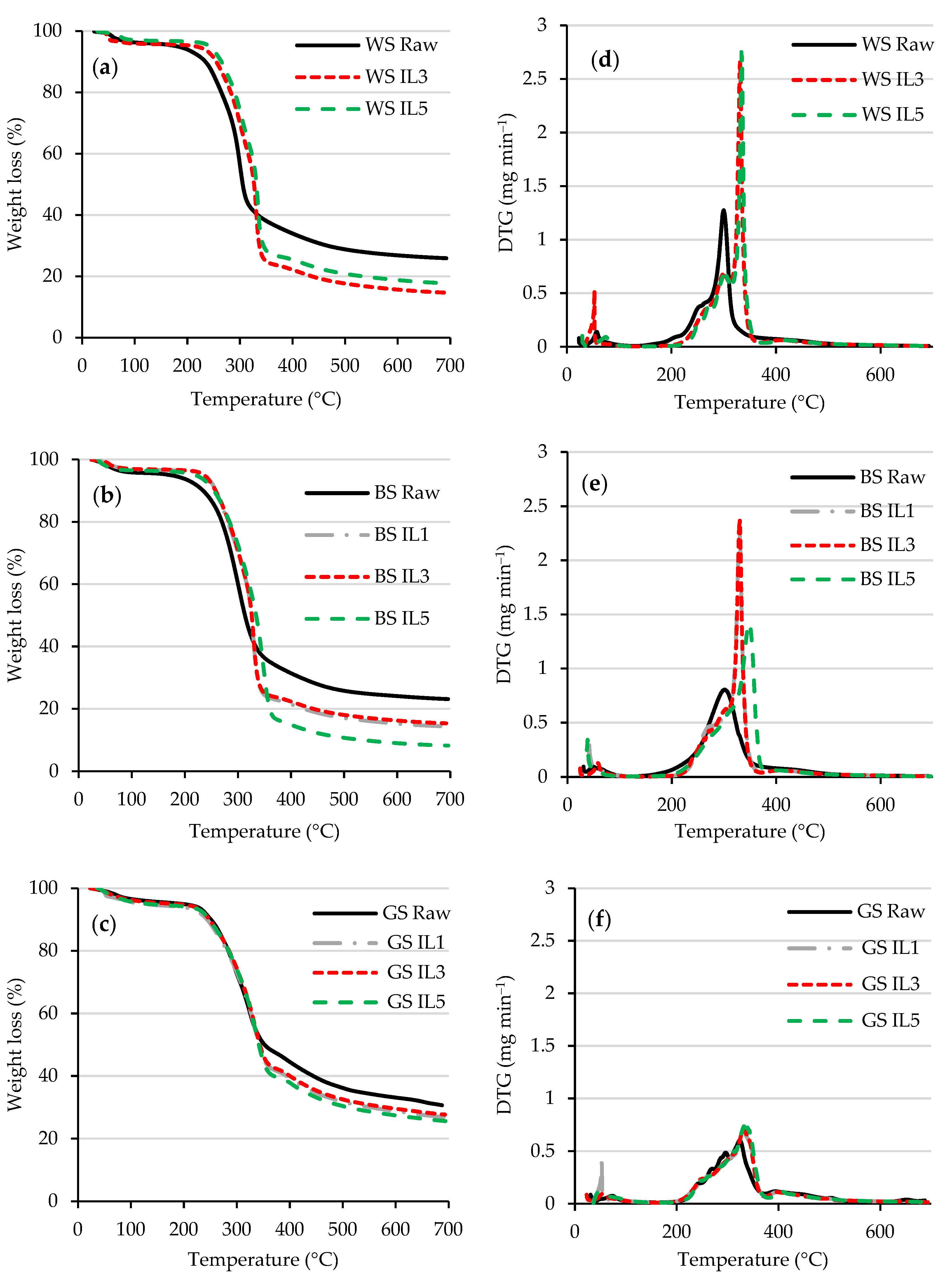
3.1. Characterization of Raw and Pretreated Biomass
3.2. Fermentable Reducing Sugar in the Supernatant Solution
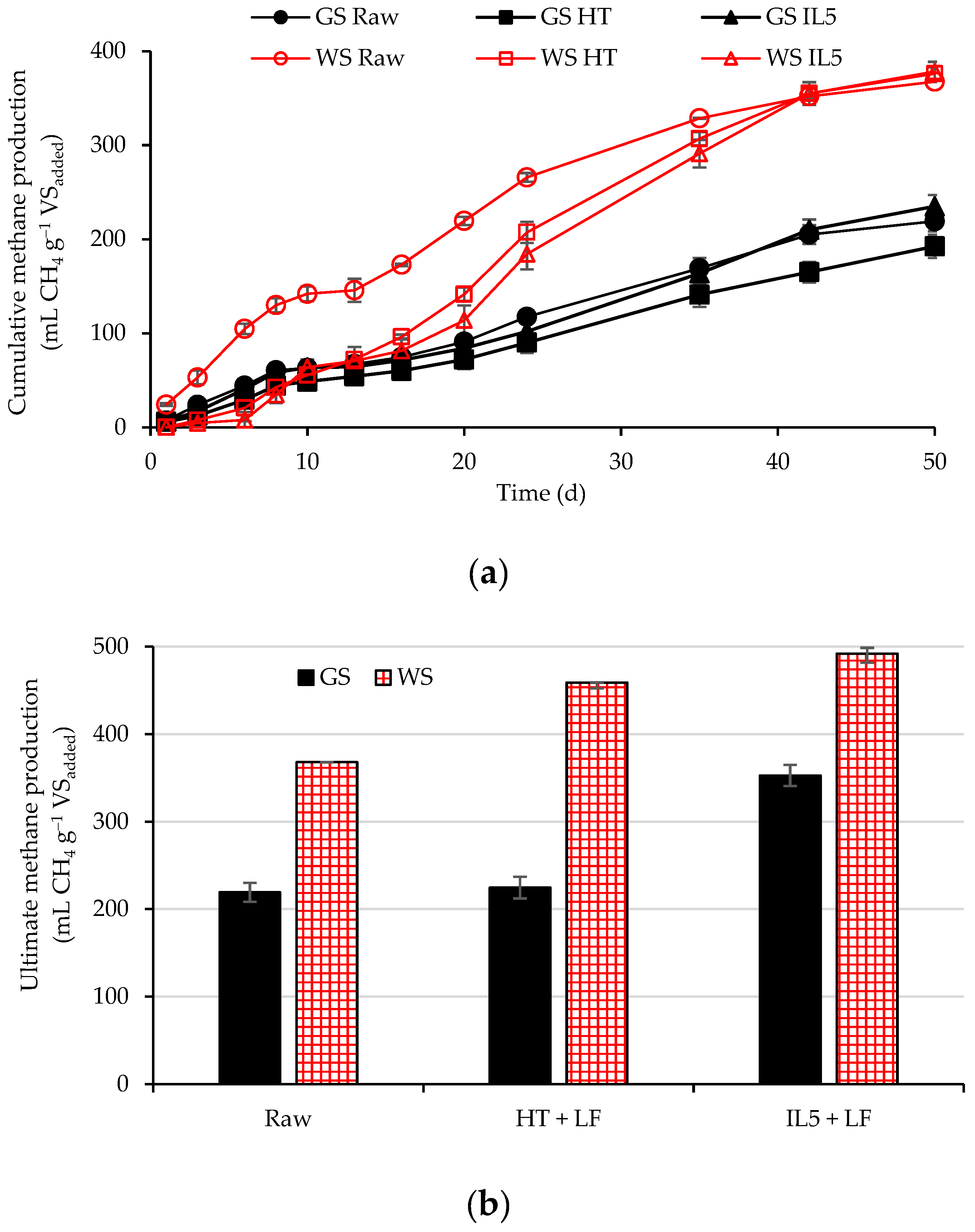
3.3. Anaerobic Digestion Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eurostat. Smarter, Greener, More Inclusive? Indicators to Support the Europe 2020 Strategy; Publications Office of the European Union: Luxembourg, 2019; pp. 2–112. [Google Scholar] [CrossRef]
- European Commission. Mapping and Analyses of the Current and Future (2020–2030) of Heating/Cooling Fuel Deployment (Fossil/Renewables); European Commission: Brussel, Belgium, 2018. [Google Scholar]
- Hoo, P.Y.; Hashim, H.; Ho, W.S. Opportunities and challenges: Landfill gas to biomethane injection into natural gas distribution grid through pipeline. J. Clean. Prod. 2018, 175, 409–419. [Google Scholar] [CrossRef]
- Horschig, T.; Adams, P.W.R.; Gawel, E.; Thrän, D. How to decarbonize the natural gas sector: A dynamic simulation approach for the market development estimation of renewable gas in Germany. Appl. Energy 2018, 213, 555–572. [Google Scholar] [CrossRef]
- Peters, D.; Van der Leun, K.; Terlouw, W.; van Tilburg, J.; Berg, T.; Schimmel, M.; Van der Hoorn, I.; Buseman, M.; Staats, M.; Schenkel, M.; et al. Gas Decarbonisation Pathways 2020–2050: Gas for Climate; Guidehouse Stadsplateau 15: Utrecht, The Netherlands, 2020. [Google Scholar]
- European Parliament. Directive. (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the promotion of the use of energy from renewable sources. Off. J. Eur. Union 2018, L328, 82–209. [Google Scholar]
- Bedoić, R.; Jurić, F.; Ćosić, B.; Pukšec, T.; Čuček, L.; Duić, N. Beyond energy crops and subsidised electricity—A study on sustainable biogas production and utilisation in advanced energy markets. Energy 2020, 201, 117651. [Google Scholar] [CrossRef]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.C.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Impact of hydrothermal pretreatment on anaerobic digestion efficiency for lignocellulosic biomass: Influence of pretreatment temperature on the formation of biomass-degrading byproducts. Chemosphere 2020, 256, 127116. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B.I. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef] [PubMed]
- Sabeeh, M.; Zeshan; Liaquat, R.; Maryam, A. Effect of alkaline and alkaline-photocatalytic pretreatment on characteristics and biogas production of rice straw. Bioresour. Technol. 2020, 309, 123449. [Google Scholar] [CrossRef]
- Fjørtoft, K.; Morken, J.; Hanssen, J.F.; Briseid, T. Pre-treatment methods for straw for farm-scale biogas plants. Biomass Bioenerg. 2019, 124, 88–94. [Google Scholar] [CrossRef]
- Govumoni, S.P.; Koti, S.; Kothagouni, S.Y.; Venkateshwar, S.; Linga, V.R. Evaluation of pretreatment methods for enzymatic saccharification of wheat straw for bioethanol production. Carbohydr. Polym. 2013, 91, 646–650. [Google Scholar] [CrossRef]
- Rajput, A.A.; Zeshan; Hassan, M. Enhancing biogas production through co-digestion and thermal pretreatment of wheat straw and sunflower meal. Renew. Energy 2021, 168, 1–10. [Google Scholar] [CrossRef]
- Gunes, B.; Stokes, J.; Davis, P.; Connolly, C.; Lawler, J. Modelling and optimisation of the biogas yield after hybrid alkaline-ultrasonic pre-treatment in the early stages of anaerobic digestion of pot ale to shorten the processing time. Process. Saf. Environ. Prot. 2021, 146, 43–53. [Google Scholar] [CrossRef]
- Zieliński, M.; Kisielewska, M.; Dudek, M.; Rusanowska, P.; Nowicka, A.; Krzemieniewski, M.; Kazimierowicz, J.; Dębowski, M. Comparison of microwave thermohydrolysis and liquid hot water pretreatment of energy crop Sida hermaphrodita for enhanced methane production. Biomass Bioenerg. 2019, 128, 105324. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef] [PubMed]
- Millati, R.; Wikandari, R.; Ariyanto, T.; Putri, R.U.; Taherzadeh, M.J. Pretreatment technologies for anaerobic digestion of lignocelluloses and toxic feedstocks. Bioresour. Technol. 2020, 304, 122998. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Yusup, S.; Akil, H.M. Pretreatment of oil palm biomass with ionic liquids: A new approach for fabrication of green composite board. J. Clean. Prod. 2016, 126, 677–685. [Google Scholar] [CrossRef]
- Gao, J.; Chen, L.; Yuan, K.; Huang, H.; Yan, Z. Ionic liquid pretreatment to enhance the anaerobic digestion of lignocellulosic biomass. Bioresour. Technol. 2013, 150, 352–358. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Lee, J.M. Recovery of lignin and ionic liquid by using organic solvents. J. Ind. Eng. Chem. 2017, 49, 122–132. [Google Scholar] [CrossRef]
- Financie, R.; Moniruzzaman, M.; Uemura, Y. Enhanced enzymatic delignification of oil palm biomass with ionic liquid pretreatment. Biochem. Eng. J. 2016, 110, 1–7. [Google Scholar] [CrossRef]
- Allison, B.J.; Cádiz, J.C.; Karuna, N.; Jeoh, T.; Simmons, C.W. The effect of ionic liquid pretreatment on the bioconversion of tomato processing waste to fermentable sugars and biogas. Appl. Biochem. Biotechnol. 2016, 179, 1227–1247. [Google Scholar] [CrossRef]
- Vitz, J.; Erdmenger, T.; Haensch, C.; Schubert, U.S. Extended dissolution studies of cellulose in imidazolium based ionic liquids. Green Chem. 2009, 11, 417–424. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Chen, H. Enzymatic hydrolysis of cellulose materials treated with ionic liquid [BMIM] Cl. Chin. Sci. Bull. 2006, 51, 2432–2436. [Google Scholar] [CrossRef]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2009, 102, 1368–1376. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, Y.; Kwak, S.Y. Comparing the influence of acetate and chloride anions on the structure of ionic liquid pretreated lignocellulosic biomass. Biomass Bioenerg. 2016, 93, 243–253. [Google Scholar] [CrossRef]
- Poornejad, N.; Karimi, K.; Behzad, T. Improvement of saccharification and ethanol production from rice straw by NMMO and [BMIM][OAc] pretreatments. Ind. Crops Prod. 2013, 41, 408–413. [Google Scholar] [CrossRef]
- Mahjoub, N.; Sahebi, H.; Mazdeh, M.; Teymouri, A. Optimal design of the second and third generation biofuel supply network by a multi-objective model. J. Clean. Prod. 2020, 256, 120355. [Google Scholar] [CrossRef]
- Liu, C.Z.; Wang, F.; Stiles, A.R.; Guo, C. Ionic liquids for biofuel production: Opportunities and challenges. Appl. Energy 2012, 92, 406–414. [Google Scholar] [CrossRef]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M. Pretreatment of agricultural biomass for anaerobic digestion: Current state and challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech. 2015, 5, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; Qiu, L.; Luo, S.; Li, S.; Yu, X.; Guo, X. Effect of ionic liquids pretreatment on anaerobic digestion of potato stem leaves. Nongye Jixie Xuebao/Trans. Chin. Soc. Agric. Mac. 2017, 48, 266–271. [Google Scholar]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Solvent pretreatments of lignocellulosic materials to enhance biogas production: A review. Energy Fuels 2016, 30, 1892–1903. [Google Scholar] [CrossRef]
- Papa, G.; Rodriguez, S.; George, A.; Schievano, A.; Orzi, V.; Sale, K.L.; Singh, S.; Adani, F.; Simmons, B.A. Comparison of different pretreatments for the production of bioethanol and biomethane from corn stover and switchgrass. Bioresour. Technol. 2015, 183, 101–110. [Google Scholar] [CrossRef]
- Allison, B.J.; Simmons, C.W. Valorization of tomato pomace by sequential lycopene extraction and anaerobic digestion. Biomass Bioenerg. 2017, 105, 331–341. [Google Scholar] [CrossRef]
- Villamil, J.A.; Mohedano, A.F.; Rodriguez, J.J.; de la Rubia, M.A. Valorisation of the liquid fraction from hydrothermal carbonisation of sewage sludge by anaerobic digestion. J. Chem. Technol. Biotechnol. 2018, 93, 450–456. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; Part 1000–3000; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Raposo, F.; de la Rubia, M.A.; Borja, R.; Alaiz, M. Assessment of a modified and optimised method for determining chemical oxygen demand of solid substrates and solutions with high suspended solid content. Talanta 2008, 76, 448–453. [Google Scholar] [CrossRef]
- Rozzi, A.; Remigi, E. Methods of assessing microbial activity and inhibition under anaerobic conditions: A literature review. Rev. Environ. Sci. Biotechnol. 2004, 3, 93–115. [Google Scholar] [CrossRef]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef] [PubMed]
- De la Rubia, M.A.; Villamil, J.A.; Rodriguez, J.J.; Borja, R.; Mohedano, A.F. Mesophilic anaerobic co-digestion of the organic fraction of municipal solid waste with the liquid fraction from hydrothermal carbonization of sewage sludge. Waste Manag. 2018, 76, 315–322. [Google Scholar] [CrossRef]
- ASTM International. ASTM International. ASTM D7582-15. In Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Padrino, B.; Lara-Serrano, M.; Morales-de la Rosa, S.; Campos-Martín, J.M.; García Fierro, J.L.; Martínez, F.; Melero, J.A.; Puyol, D. Resource recovery potential from lignocellulosic feedstock upon lysis with ionic liquids. Front. Bioeng. Biotechnol. 2018, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef] [Green Version]
- De Diego-Díaz, B.; Duran, A.; Álvarez-García, M.R.; Fernández-Rodríguez, J. New trends in physicochemical characterization of solid lignocellulosic waste in anaerobic digestion. Fuel 2019, 245, 240–246. [Google Scholar] [CrossRef]
- Clough, M.T.; Geyer, K.; Hunt, P.A.; Mertes, J.; Welton, T. Thermal decomposition of carboxylate ionic liquids: Trends and mechanisms. Phys. Chem. Chem. Phys. 2013, 15, 20480–20495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.N.; Khan, M.N.; Rehman, N.; Sharif, A.; Ahmed, E.; Farooqi, Z.H.; Din, M.I. Environmentally benign extraction of cellulose from dunchi fiber for nanocellulose fabrication. Int. J. Biol. Macromol. 2020, 153, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.; Moniruzzaman, M.; Iqbal, T.; Yusup, S.; Rashid, M.; Raza, A. Comparative effect of ionic liquids pretreatment on thermogravimetric kinetics of crude oil palm biomass for possible sustainable exploitation. J. Mol. Liq. 2019, 282, 88–96. [Google Scholar] [CrossRef]
- Sidi-Yacoub, B.; Oudghiri, F.; Belkadi, M.; Rodríguez-Barroso, R. Characterization of lignocellulosic components in exhausted sugar beet pulp waste by TG/FTIR analysis. J. Therm. Anal. Calorim. 2019, 138, 1801–1809. [Google Scholar] [CrossRef]
- De la Rubia, M.A.; Fernández-Cegrí, V.; Raposo, F.; Borja, R. Influence of particle size and chemical composition on the performance and kinetics of anaerobic digestion process of sunflower oil cake in batch mode. Biochem. Eng. J. 2011, 58, 162–167. [Google Scholar] [CrossRef]
| Wheat Straw | Barley Straw | Grape Stem | |
|---|---|---|---|
| TS (g kg–1) | 963 ± 0.65 | 963 ± 0.65 | 963 ± 0.65 |
| VS (g kg–1) | 897 ± 1.25 | 896 ± 1.25 | 897 ± 0.50 |
| TCOD (g kg–1) | 334 ± 1.40 | 297 ± 1.25 | 344 ± 1.80 |
| C (%) | 43.32 ± 0.02 | 43.89 ± 0.03 | 47.25 ± 0.09 |
| H (%) | 5.62 ± 0.03 | 5.75 ± 0.05 | 5.60 ± 0.07 |
| N (%) | 0.63 ± 0.01 | 0.56 ± 0.01 | 1.48 ± 0.01 |
| S (%) | 0.17 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.01 |
| O * (%) | 40.58 ± 0.02 | 42.39 ± 0.01 | 11.35 ± 0.02 |
| VM (%) | 80.30 ± 0.03 | 82.70 ± 0.02 | 65.80 ± 0.01 |
| Ash (%) | 9.7 ± 0.03 | 7.3 ± 0.02 | 24.2 ± 0.01 |
| Biomass | Raw | HT | IL1 | IL3 | IL5 |
|---|---|---|---|---|---|
| Wheat straw | 54.8 | 55.7 | 53.9 | 51.4 | 50 |
| Barley straw | 53.8 | 53.6 | 52.4 | 51.2 | 50.9 |
| Grape stem | 32.5 | 32.1 | 28.2 | 27.8 | 27.5 |
| Pretreatment | Sugar Yield (mg Glucose g−1 Biomass) | ||
|---|---|---|---|
| WS | BS | GS | |
| HT | 123.4 ± 1.1 | 41.2 ± 1.3 | 47.8 ± 0.6 |
| IL1 | 127.6 ± 3.2 | 42.4 ± 0.8 | 50.0 ± 0.1 |
| IL3 | 143.0 ± 4.8 | 45.5 ± 0.9 | 94.5 ± 0.7 |
| IL5 | 158.2 ± 5.1 | 47.9 ± 1.6 | 188.1 ± 6.8 |
| Assay | Total Alkalinity (mg CaCO3 L–1) | IA/TA | TAN (mg L–1) | |||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | |
| WS Raw | 592 ± 4 | 2778 ± 1 | 0.35 | 0.14 | 56 ± 1 | 826 ± 20 |
| WS HT | 524 ± 16 | 2473 ± 11 | 0.26 | 0.11 | 70 ± 7 | 868 ± 30 |
| WS IL5 | 530 ± 12 | 2495 ± 1 | 0.27 | 0.12 | 140 ± 3 | 1036 ± 40 |
| GS Raw | 484 ± 5 | 2926 ± 2 | 0.24 | 0.11 | 28 ± 1 | 518 ± 20 |
| GS HT | 409 ± 22 | 2502 ± 1 | 0.29 | 0.05 | 70 ± 5 | 658 ± 59 |
| GS IL5 | 680 ± 15 | 2761 ± 3 | 0.23 | 0.14 | 70 ± 8 | 672 ± 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin-Batista, J.D.; Mohedano, A.F.; de la Rubia, A. Pretreatment of Lignocellulosic Biomass with 1-Ethyl-3-methylimidazolium Acetate for Its Eventual Valorization by Anaerobic Digestion. Resources 2021, 10, 118. https://doi.org/10.3390/resources10120118
Marin-Batista JD, Mohedano AF, de la Rubia A. Pretreatment of Lignocellulosic Biomass with 1-Ethyl-3-methylimidazolium Acetate for Its Eventual Valorization by Anaerobic Digestion. Resources. 2021; 10(12):118. https://doi.org/10.3390/resources10120118
Chicago/Turabian StyleMarin-Batista, Jose D., Angel F. Mohedano, and Angeles de la Rubia. 2021. "Pretreatment of Lignocellulosic Biomass with 1-Ethyl-3-methylimidazolium Acetate for Its Eventual Valorization by Anaerobic Digestion" Resources 10, no. 12: 118. https://doi.org/10.3390/resources10120118
APA StyleMarin-Batista, J. D., Mohedano, A. F., & de la Rubia, A. (2021). Pretreatment of Lignocellulosic Biomass with 1-Ethyl-3-methylimidazolium Acetate for Its Eventual Valorization by Anaerobic Digestion. Resources, 10(12), 118. https://doi.org/10.3390/resources10120118








