Optimal Allocation of Multi-Type Vaccines in a Two-Dose Vaccination Campaign for Epidemic Control: A Case Study of COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Compartmental Epidemic Model
- (1)
- Those who are unvaccinated;
- (2)
- Those who received the first dose but are yet to be protected;
- (3)
- Those who are protected by the single dose after they received the first dose;
- (4)
- Those who received the second dose but do not yet have enhanced vaccine-generated immunity;
- (5)
- Those who are protected by the two doses after they received the second dose. In addition, the vaccinated groups are further divided based on the type of vaccine that they received.
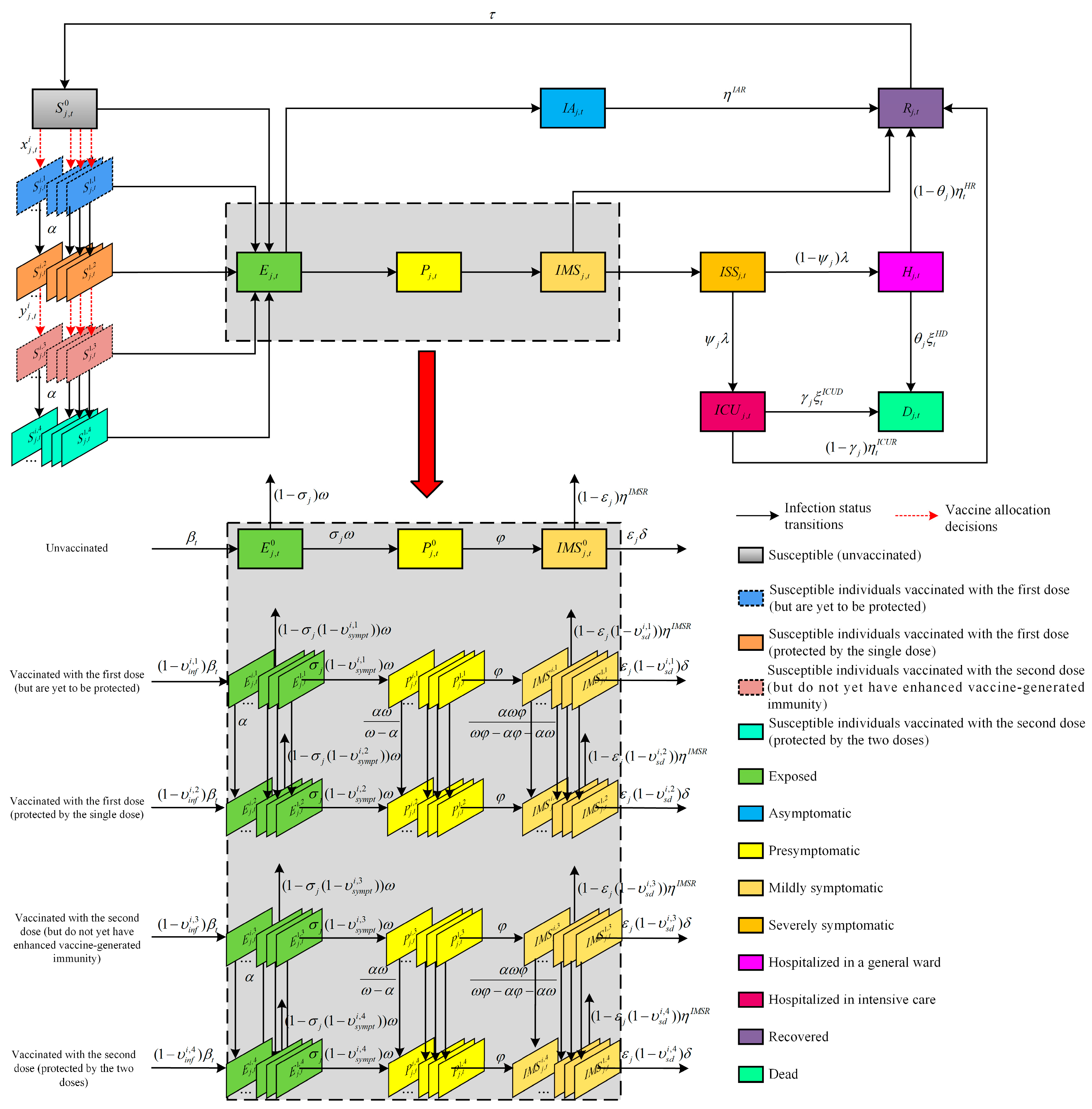
2.2. Model Notations
2.3. Model Assumptions
- Firstly, we assume that the population is homogeneously mixed. We do not consider natural births and deaths since the duration of the outbreak is much shorter than the human life expectancy [17]. Additionally, we also do not consider the population flow from region to region, i.e., the population size is constant within each public health region, which is judged to be a reasonable assumption over a short-term time horizon [45].
- Secondly, we assume that severely symptomatic individuals requiring hospitalization are either admitted to a general ward or an ICU, and patients cannot be transferred between the two. As in previous work (Moghadas et al. [46] and Zhu et al. [40]), we assume that all severely symptomatic individuals who are not hospitalized voluntarily undertake self-quarantine and, thus, do not transmit the virus to others. Moreover, once they are admitted, individuals are assumed to no longer be infectious because personnel and visitors who have contact with them are strictly required to adopt personal protective measures. Similar to the work of Hogan et al. [47], we assume that all infection-related deaths occur during hospitalization.
- Thirdly, we make the simplifying assumption that only susceptible individuals are eligible for vaccination (a similar assumption can be found in Yang et al. [15] and Han et al. [19]). In addition, vaccinated and unvaccinated individuals with SARS-CoV-2 infection are assumed to be equally infectious. We also assume that everyone who receives the first dose also receives the second dose of the vaccine.
- Lastly, the proposed model considers a continuous relaxation of the state and decision variables to make the problem computationally tractable [48]. This is a common assumption in studies aiming at optimizing infectious disease interventions, and this relaxation of the integer variables has been shown to guarantee a high-quality result [45].
2.4. Mathematical Formulation
3. Results
3.1. Data Sources
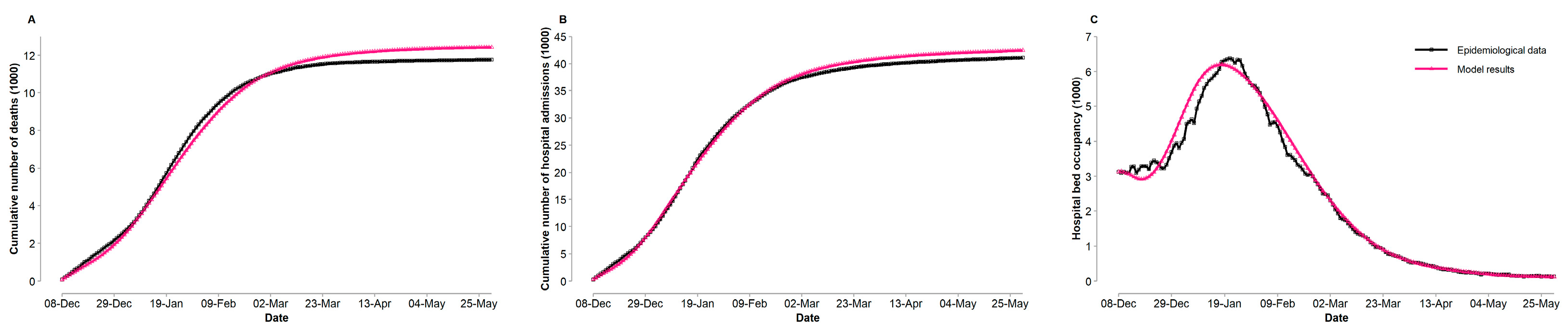
3.2. Model Validation
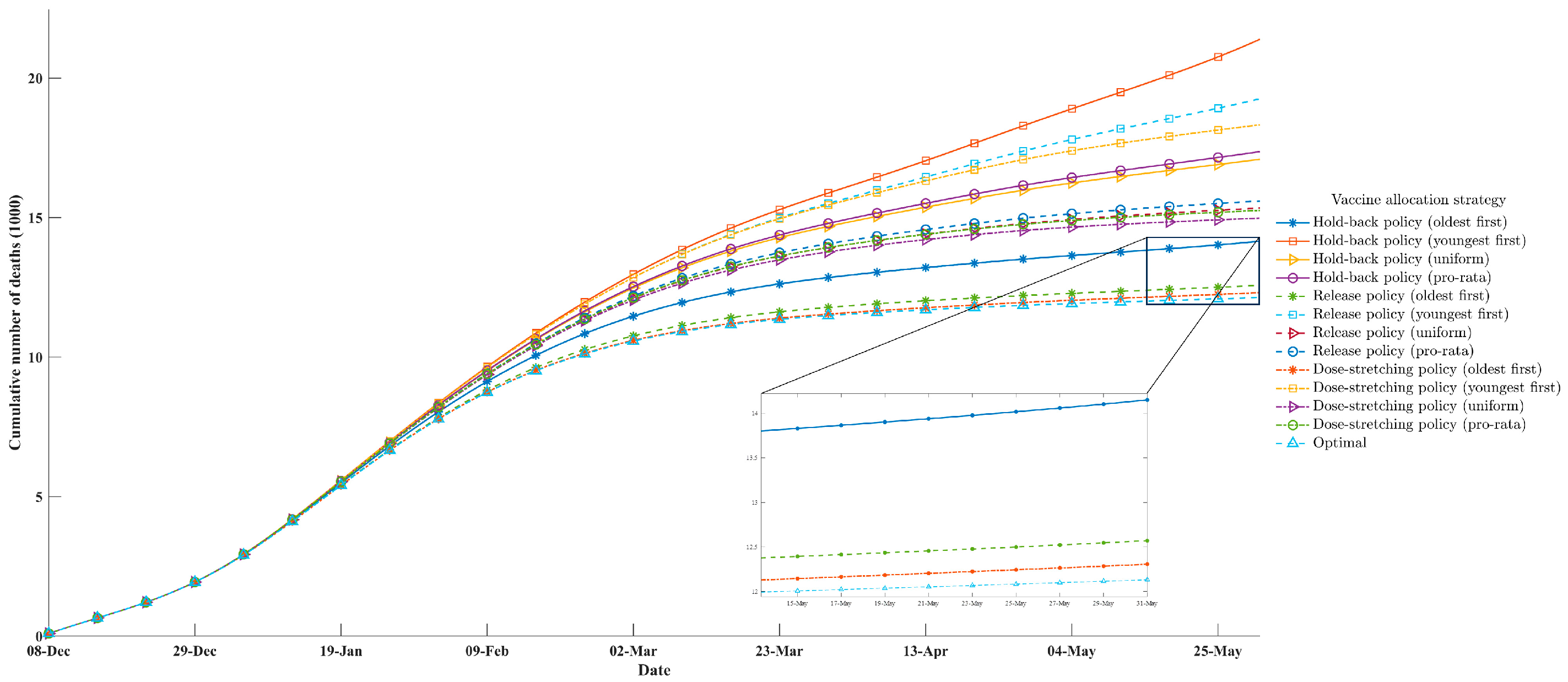
3.3. Comparative Studies
- Oldest first: Prioritization of the allocation of vaccines to the oldest group and then to younger groups in decreasing order of age.
- Youngest first: Prioritization of the allocation of vaccines to the youngest groups and then to older groups in increasing order of age.
- Pro-rata: The vaccines were allocated according to the population proportion within each age group.
- Uniform: The vaccines were uniformly allocated to each age group.
- Hold-back policy: The United States initially implemented a two-dose vaccination rollout policy under the Trump administration [59]. One additional vaccine dose was immediately put into storage when an individual received the first dose, and it was given to them once they returned to receive the second dose.
- Release policy: In the other two-dose vaccination rollout policy that then-President Joe Biden declared, the United States increased the release speeds of the available vaccine resources starting on 8 January 2021, which replaced the original hold-back policy [60]. In brief, all available vaccine resources in each period were used as either first doses for individuals with primary vaccinations or second doses for revaccinated individuals. Furthermore, the release policy stated that the available vaccine resources were to be first given to returning individuals who were eligible for their second dose. After that, all the remaining unused doses were given to individuals with a primary vaccination.
- Dose-stretching policy: The UK was the first country in the world to pursue this two-dose vaccination rollout policy [18]. The dose-stretching policy was similar to the release policy but extended the interval between the two doses of COVID-19 vaccines. Specifically, this vaccination rollout policy did not immediately provide a dose of vaccine for a recipient eligible to receive their second dose but delayed the administration of the second dose to provide the first dose to more individuals on the premise of guaranteeing the efficacy of the vaccines.
4. Discussion
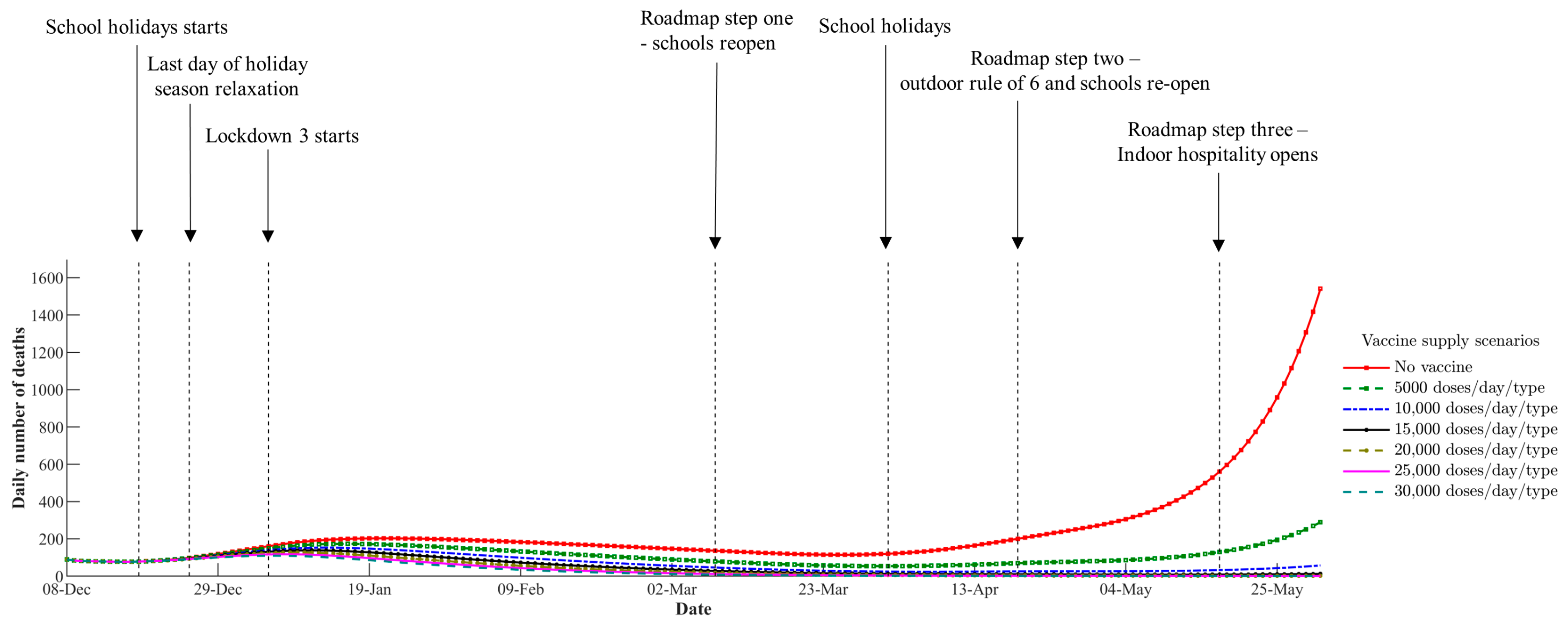
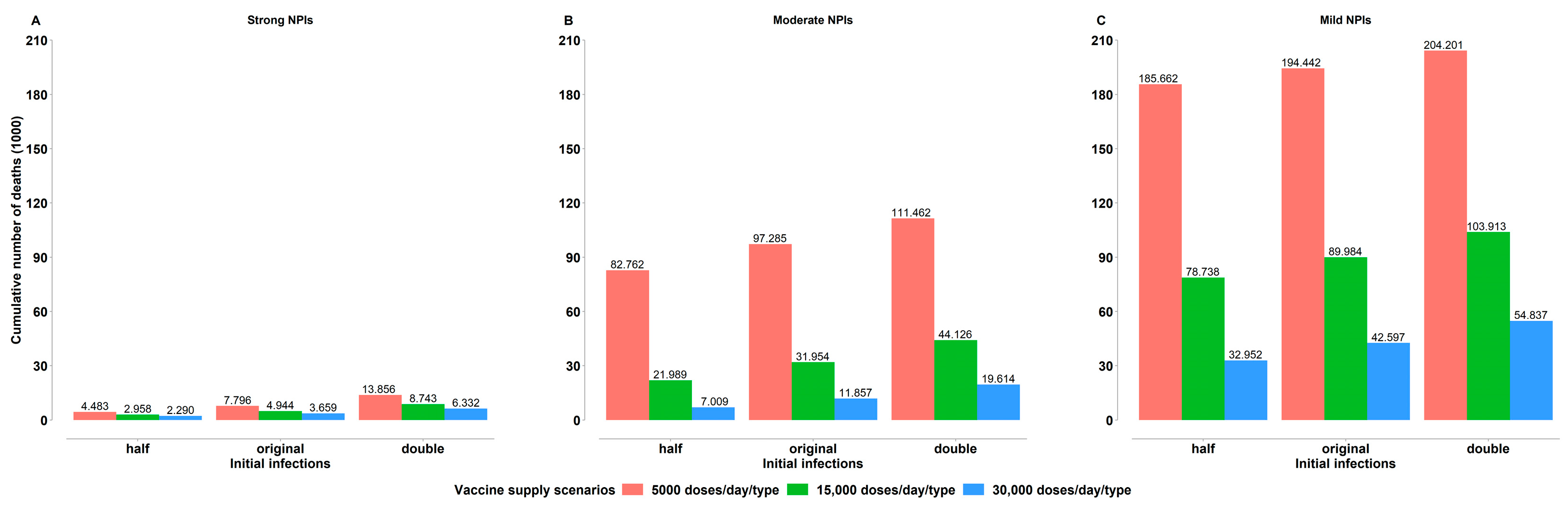
4.1. Impact of Vaccine Supply Levels
4.2. Impact of Non-Pharmaceutical Interventions
4.3. Impact of the Initial Infections
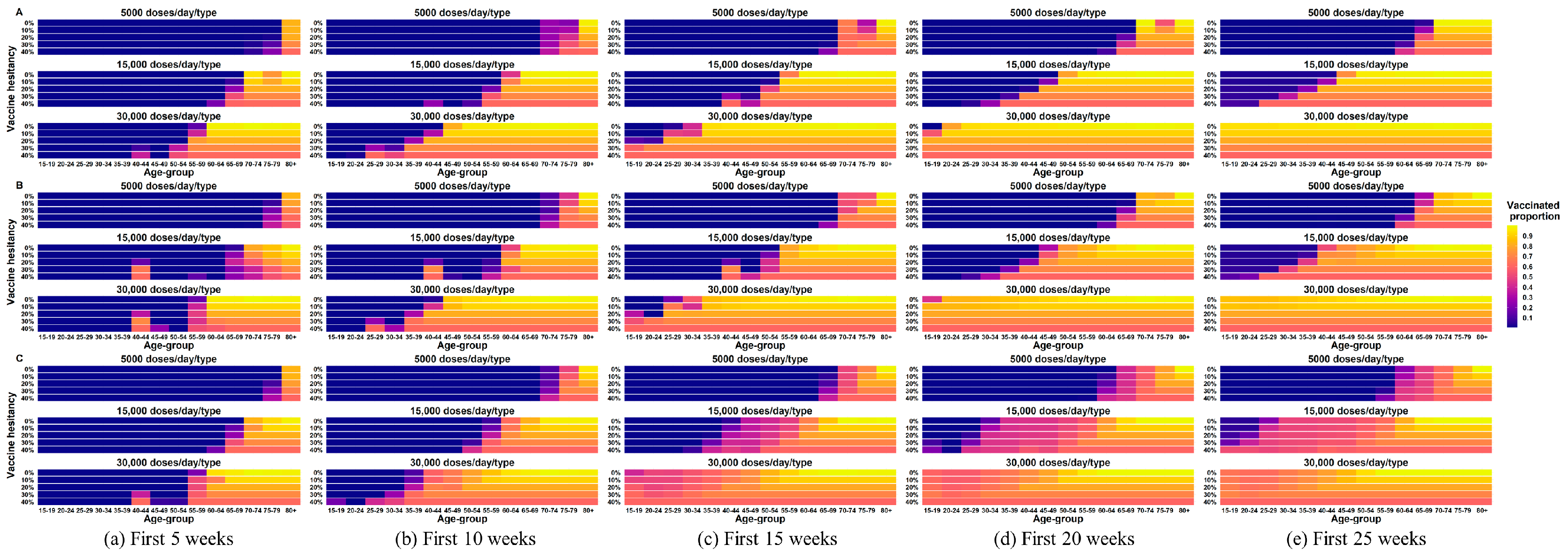
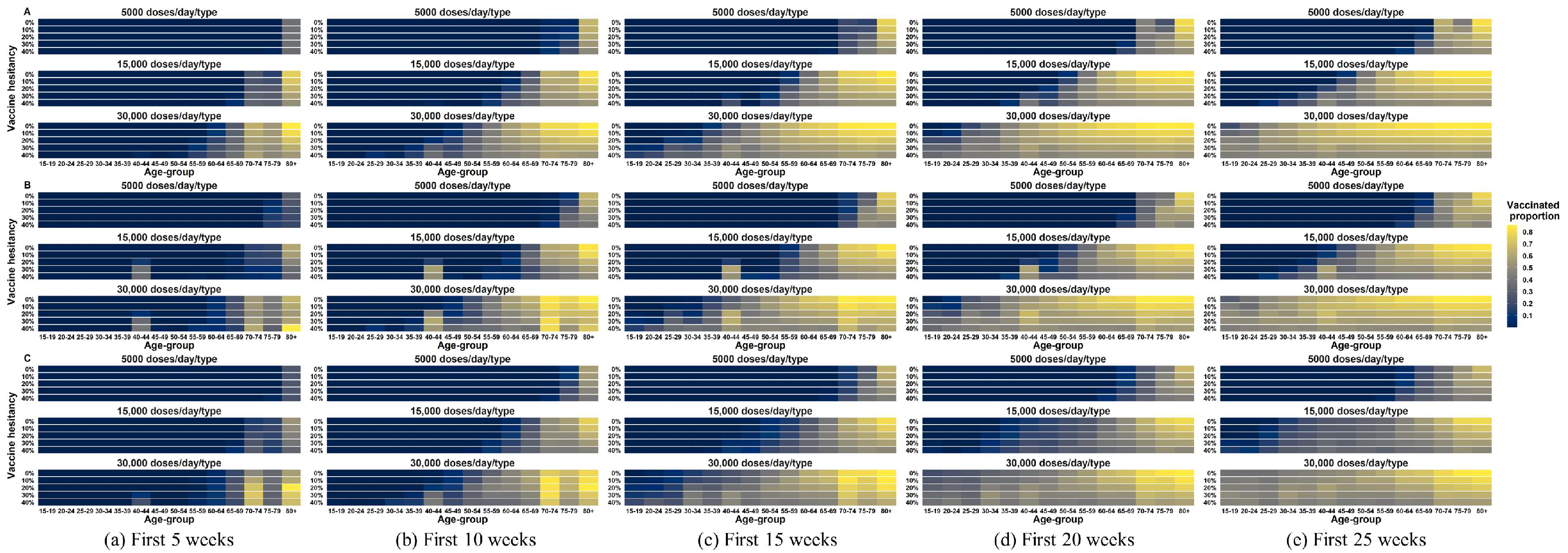
4.4. Impact of Vaccine Hesitancy
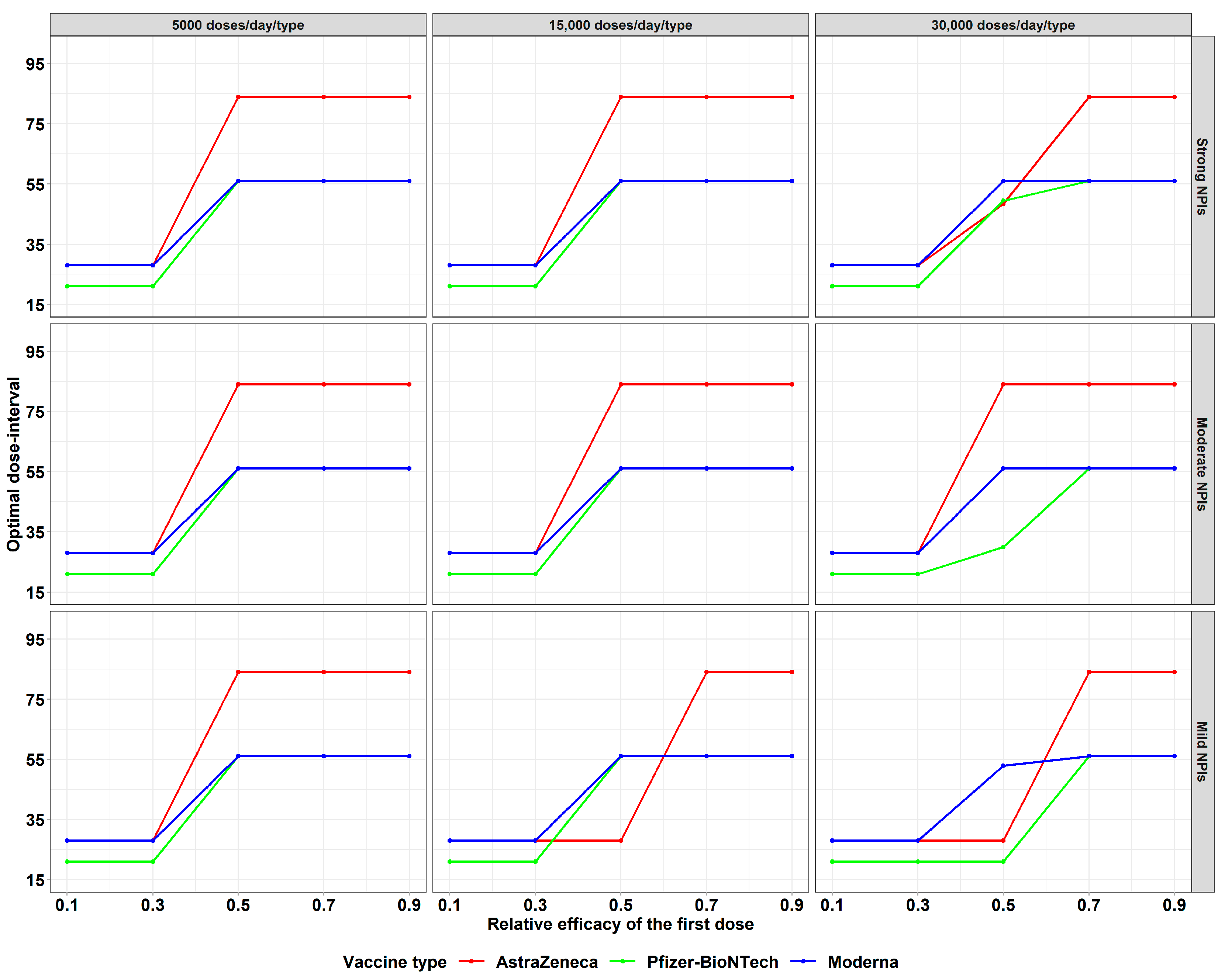
4.5. Impact of the Relative Efficacy of the First Dose
5. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choudhury, N.A.; Ramkumar, M.; Schoenherr, T.; Singh, S. The role of operations and supply chain management during epidemics and pandemics: Potential and future research opportunities. Transp. Res. E Logist. Transp. Rev 2023, 175, 103139. [Google Scholar] [CrossRef]
- Biswas, D.; Alfandari, L. Designing an optimal sequence of non-pharmaceutical interventions for controlling COVID-19. Eur. J. Oper. Res 2022, 303, 1372–1391. [Google Scholar] [CrossRef]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017, 389, 505–518. [Google Scholar] [CrossRef]
- Giuntella, O.; Hyde, K.; Saccardo, S.; Sadoff, S. Lifestyle and mental health disruptions during COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2016632118. [Google Scholar] [CrossRef]
- WHO. WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/deaths?n=o (accessed on 6 July 2024).
- Vermeulen-Miltz, E.; Clifford-Holmes, J.K.; Snow, B.; Lombard, A.T. Exploring the Impacts of COVID-19 on Coastal Tourism to Inform Recovery Strategies in Nelson Mandela Bay, South Africa. Systems 2022, 10, 120. [Google Scholar] [CrossRef]
- Huberts, N.F.D.; Thijssen, J.J.J. Optimal timing of non-pharmaceutical interventions during an epidemic. Eur. J. Oper. Res. 2023, 305, 1366–1389. [Google Scholar] [CrossRef]
- IMF. The Great Lockdown: Worst Economic Downturn Since the Great Depression. Available online: https://www.imf.org/zh/Blogs/Articles/2020/04/14/blog-weo-the-great-lockdown-worst-economic-downturn-since-the-great-depression (accessed on 8 June 2023).
- WHO. COVID-19 Pandemic Triggers 25% Increase in Prevalence of Anxiety and Depression Worldwide. Available online: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide (accessed on 8 September 2023).
- Silva, P.J.S.; Sagastizábal, C.; Nonato, L.G.; Struchiner, C.J.; Pereira, T. Optimized delay of the second COVID-19 vaccine dose reduces ICU admissions. Proc. Natl. Acad. Sci. USA 2021, 118, e2104640118. [Google Scholar] [CrossRef]
- Bertsimas, D.; Li, M.L.; Liu, X.; Xu, J.; Khan, N. Data-Driven COVID-19 Vaccine Development for Janssen. INFORMS J. Appl. Anal. 2023, 53, 70–84. [Google Scholar] [CrossRef]
- Joshi, K.; Rumpler, E.; Kennedy-Shaffer, L.; Bosan, R.; Lipsitch, M. Comparative performance of between-population vaccine allocation strategies with applications for emerging pandemics. Vaccine 2023, 41, 1864–1874. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzo, E.; Mari, L.; Pasetto, D.; Miccoli, S.; Casagrandi, R.; Gatto, M.; Rinaldo, A. The geography of COVID-19 spread in Italy and implications for the relaxation of confinement measures. Nat. Commun. 2020, 11, 4264. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Blanchini, F.; Bruno, R.; Colaneri, P.; Di Filippo, A.; Di Matteo, A.; Colaneri, M. Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy. Nat. Med. 2020, 26, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Marziano, V.; Deng, X.; Guzzetta, G.; Zhang, J.; Trentini, F.; Cai, J.; Poletti, P.; Zheng, W.; Wang, W.; et al. Despite vaccination, China needs non-pharmaceutical interventions to prevent widespread outbreaks of COVID-19 in 2021. Nat. Hum. Behav. 2021, 5, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Buckner, J.H.; Chowell, G.; Springborn, M.R. Dynamic prioritization of COVID-19 vaccines when social distancing is limited for essential workers. Proc. Natl. Acad. Sci. USA 2021, 118, e2025786118. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.F.; Becker, A.D.; Grenfell, B.T.; Metcalf, C.J.E. Disease and healthcare burden of COVID-19 in the United States. Nat. Med. 2020, 26, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Sonabend, R.; Whittles, L.K.; Imai, N.; Perez-Guzman, P.N.; Knock, E.S.; Rawson, T.; Gaythorpe, K.A.M.; Djaafara, B.A.; Hinsley, W.; FitzJohn, R.G.; et al. Non-pharmaceutical interventions, vaccination, and the SARS-CoV-2 delta variant in England: A mathematical modelling study. Lancet 2021, 398, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Cai, J.; Yang, J.; Zhang, J.; Wu, Q.; Zheng, W.; Shi, H.; Ajelli, M.; Zhou, X.H.; Yu, H. Time-varying optimization of COVID-19 vaccine prioritization in the context of limited vaccination capacity. Nat. Commun. 2021, 12, 4673. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S.; Callaway, E. What scientists do and don’t know about the Oxford-AstraZeneca COVID vaccine. Nature 2021, 592, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Nahofti Kohneh, J.; Amirdadi, M.; Teimoury, E. An optimization framework for COVID-19 vaccine allocation and inventory management: A case study. Appl. Soft. Comput. 2023, 132, 109801. [Google Scholar] [CrossRef] [PubMed]
- CDC. Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html (accessed on 20 June 2023).
- Hill, E.M.; Keeling, M.J. Comparison between one and two dose SARS-CoV-2 vaccine prioritization for a fixed number of vaccine doses. J. R. Soc. Interface 2021, 18, 20210214. [Google Scholar] [CrossRef]
- Abbas, H.W.; Sajid, Z.; Dao, U. Assessing the Impact of Risk Factors on Vaccination Uptake Policy Decisions Using a Bayesian Network (BN) Approach. Systems 2024, 12, 167. [Google Scholar] [CrossRef]
- Duijzer, L.E.; van Jaarsveld, W.; Dekker, R. Literature review: The vaccine supply chain. Eur. J. Oper. Res. 2018, 268, 174–192. [Google Scholar] [CrossRef]
- Medlock, J.; Galvani, A.P. Optimizing Influenza Vaccine Distribution. Science 2009, 325, 1705–1708. [Google Scholar] [CrossRef]
- Enayati, S.; Özaltın, O.Y. Optimal influenza vaccine distribution with equity. Eur. J. Oper. Res. 2020, 283, 714–725. [Google Scholar] [CrossRef]
- Venkatramanan, S.; Chen, J.; Fadikar, A.; Gupta, S.; Higdon, D.; Lewis, B.; Marathe, M.; Mortveit, H.; Vullikanti, A. Optimizing spatial allocation of seasonal influenza vaccine under temporal constraints. PLoS Comput. Biol. 2019, 15, e1007111. [Google Scholar] [CrossRef]
- Ferguson, N.; Matrajt, L.; Halloran, M.E.; Longini, I.M. Optimal Vaccine Allocation for the Early Mitigation of Pandemic Influenza. PLoS Comput. Biol. 2013, 9, e1002964. [Google Scholar] [CrossRef]
- Koks-Leensen, M.C.J.; Schalk, B.W.M.; Bakker-van Gijssel, E.J.; Timen, A.; Nagele, M.E.; van den Bemd, M.; Leusink, G.L.; Cuypers, M.; Naaldenberg, J. Risk for Severe COVID-19 Outcomes among Persons with Intellectual Disabilities, the Netherlands. Emerg. Infect. Dis. 2023, 29, 118–126. [Google Scholar] [CrossRef]
- Ibrahim, D.; Kis, Z.; Papathanasiou, M.M.; Kontoravdi, C.; Chachuat, B.; Shah, N. Strategic Planning of a Joint SARS-CoV-2 and Influenza Vaccination Campaign in the UK. Vaccines 2024, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Guttieres, D.; Sinskey, A.J.; Springs, S.L. Modeling Framework to Evaluate Vaccine Strategies against the COVID-19 Pandemic. Systems 2021, 9, 4. [Google Scholar] [CrossRef]
- Bolcato, M.; Rodriguez, D.; Feola, A.; Di Mizio, G.; Bonsignore, A.; Ciliberti, R.; Tettamanti, C.; Trabucco Aurilio, M.; Aprile, A. COVID-19 Pandemic and Equal Access to Vaccines. Vaccines 2021, 9, 538. [Google Scholar] [CrossRef]
- Babus, A.; Das, S.; Lee, S. The optimal allocation of COVID-19 vaccines. Econ. Lett. 2023, 224, 111008. [Google Scholar] [CrossRef]
- Bubar, K.M.; Reinholt, K.; Kissler, S.M.; Lipsitch, M.; Cobey, S.; Grad, Y.H.; Larremore, D.B. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science 2021, 371, 916–921. [Google Scholar] [CrossRef]
- Shim, E. Optimal Allocation of the Limited COVID-19 Vaccine Supply in South Korea. J. Clin. Med. 2021, 10, 591. [Google Scholar] [CrossRef]
- Foy, B.H.; Wahl, B.; Mehta, K.; Shet, A.; Menon, G.I.; Britto, C. Comparing COVID-19 vaccine allocation strategies in India: A mathematical modelling study. Int. J. Infect. Dis. 2021, 103, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Hill, E.M.; Dyson, L.; Tildesley, M.J.; Keeling, M.J. Modelling optimal vaccination strategy for SARS-CoV-2 in the UK. PLoS Comput. Biol. 2021, 17, e1008849. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, L.; Eaton, J.; Leung, T.; Dimitrov, D.; Schiffer, J.T.; Swan, D.A.; Janes, H. Optimizing vaccine allocation for COVID-19 vaccines shows the potential role of single-dose vaccination. Nat. Commun. 2021, 12, 3449. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Q.; Huang, M. Optimizing two-dose vaccine resource allocation to combat a pandemic in the context of limited supply: The case of COVID-19. Front. Public Health 2023, 11, 1129183. [Google Scholar] [CrossRef] [PubMed]
- Mak, H.Y.; Dai, T.; Tang, C.S. Managing two-dose COVID-19 vaccine rollouts with limited supply: Operations strategies for distributing time-sensitive resources. Prod. Oper. Manag. 2022, 31, 4424–4442. [Google Scholar] [CrossRef] [PubMed]
- Shumsky, R.A.; Smith, J.; Hoen, A.; Gilbert, M. Allocating COVID-19 vaccines: Save one for the second dose? Tuck School of Business Working Paper 2021. [Google Scholar] [CrossRef]
- Miura, F.; Leung, K.Y.; Klinkenberg, D.; Ainslie, K.E.C.; Wallinga, J. Optimal vaccine allocation for COVID-19 in the Netherlands: A data-driven prioritization. PLoS Comput. Biol. 2021, 17, e1009697. [Google Scholar] [CrossRef]
- Schwartz, I.B.; Smith, H.L. Infinite subharmonic bifurcation in an SEIR epidemic model. J. Math. Biol. 1983, 18, 233–253. [Google Scholar] [CrossRef]
- Abdin, A.F.; Fang, Y.P.; Caunhye, A.; Alem, D.; Barros, A.; Zio, E. An optimization model for planning testing and control strategies to limit the spread of a pandemic—The case of COVID-19. Eur. J. Oper. Res. 2023, 304, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Nourbakhsh, S.; Sah, P.; Fitzpatrick, M.C.; Galvani, A.P. Evaluation of COVID-19 vaccination strategies with a delayed second dose. PLoS Biol. 2021, 19, e3001211. [Google Scholar] [CrossRef] [PubMed]
- Hogan, A.B.; Winskill, P.; Watson, O.J.; Walker, P.G.T.; Whittaker, C.; Baguelin, M.; Brazeau, N.F.; Charles, G.D.; Gaythorpe, K.A.M.; Hamlet, A.; et al. Within-country age-based prioritisation, global allocation, and public health impact of a vaccine against SARS-CoV-2: A mathematical modelling analysis. Vaccine 2021, 39, 2995–3006. [Google Scholar] [CrossRef] [PubMed]
- Parino, F.; Zino, L.; Calafiore, G.C.; Rizzo, A. A model predictive control approach to optimally devise a two-dose vaccination rollout: A case study on COVID-19 in Italy. Int. J. Robust Nonlinear Control 2023, 33, 4808–4823. [Google Scholar] [CrossRef] [PubMed]
- Zibadi, S.; Samieefar, N.; Sodeifian, F.; Mosavari, N.; Rezaei, N. COVID-19 Vaccination: What Challenges Are We Going to Face. Acta Biomed. 2021, 92, e2021379. [Google Scholar] [CrossRef]
- Dunning, I.; Huchette, J.; Lubin, M. JuMP: A Modeling Language for Mathematical Optimization. SIAM Rev. 2017, 59, 295–320. [Google Scholar] [CrossRef]
- Wächter, A.; Biegler, L.T. On the implementation of an interior-point filter line-search algorithm for large-scale nonlinear programming. Math. Program. 2006, 106, 25–57. [Google Scholar] [CrossRef]
- HSL. The HSL Mathematical Software Library. Available online: https://www.hsl.rl.ac.uk/ (accessed on 20 April 2023).
- Prem, K.; Cook, A.R.; Jit, M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput. Biol. 2017, 13, e1005697. [Google Scholar] [CrossRef]
- ONS. Coronavirus (COVID-19) Infection Survey, UK: 18 December 2020. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/18december2020 (accessed on 9 June 2022).
- UK, GOV. Coronavirus (COVID-19) in the UK. Available online: https://coronavirus.data.gov.uk (accessed on 9 June 2022).
- ONS. Coronavirus (COVID-19) Infection Survey: Characteristics of People Testing Positive for COVID-19 in England and Antibody Data for the UK: December 2020. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsinthecommunityinengland/december2020 (accessed on 12 June 2022).
- Molla, J.; Ponce de Leon Chavez, A.; Hiraoka, T.; Ala-Nissila, T.; Kivela, M.; Leskela, L. Adaptive and optimized COVID-19 vaccination strategies across geographical regions and age groups. PLoS Comput. Biol. 2022, 18, e1009974. [Google Scholar] [CrossRef]
- ONS. Estimates of the Population for the UK, England and Wales, Scotland and Northern Ireland. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland (accessed on 6 June 2022).
- Gottlieb, S. Pharmacies Can Get Shots in Arms. Available online: https://www.wsj.com/articles/pharmacies-can-get-shots-in-arms-11609710279 (accessed on 25 June 2023).
- Staff, A. A Timeline of COVID-19 Vaccine Developments in 2021. Available online: https://www.ajmc.com/view/a-timeline-of-covid-19-vaccine-developments-in-2021 (accessed on 15 June 2023).




| Data | Metric | ||
|---|---|---|---|
| Mean Absolute Percent Error Weighted | Normalized Root Mean Squared Error | Explained Variance (%) | |
| Cumulative number of deaths | 0.0425 | 0.0483 | 98.95 |
| Cumulative number of hospital admissions | 0.0256 | 0.0295 | 99.72 |
| Hospital bed occupancy | 0.0601 | 0.1008 | 98.78 |
| Policy | Cumulative Number of Deaths | Proportion of Deaths Averted Compared with the Non-Vaccination Policy (%) |
|---|---|---|
| Non-vaccination policy | 42,579 | - |
| Actual policy | 12,433 | 70.8 |
| Optimal policy | 12,133 | 71.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Wang, Q.; Huang, M. Optimal Allocation of Multi-Type Vaccines in a Two-Dose Vaccination Campaign for Epidemic Control: A Case Study of COVID-19. Systems 2024, 12, 286. https://doi.org/10.3390/systems12080286
Zhu J, Wang Q, Huang M. Optimal Allocation of Multi-Type Vaccines in a Two-Dose Vaccination Campaign for Epidemic Control: A Case Study of COVID-19. Systems. 2024; 12(8):286. https://doi.org/10.3390/systems12080286
Chicago/Turabian StyleZhu, Jin, Qing Wang, and Min Huang. 2024. "Optimal Allocation of Multi-Type Vaccines in a Two-Dose Vaccination Campaign for Epidemic Control: A Case Study of COVID-19" Systems 12, no. 8: 286. https://doi.org/10.3390/systems12080286
APA StyleZhu, J., Wang, Q., & Huang, M. (2024). Optimal Allocation of Multi-Type Vaccines in a Two-Dose Vaccination Campaign for Epidemic Control: A Case Study of COVID-19. Systems, 12(8), 286. https://doi.org/10.3390/systems12080286






