Data-Driven Management of Vaccination and Its Consequences
Abstract
:1. Introduction
- Monitoring the progress of the vaccination campaign. This task involves providing a clear understanding of the size and structure of the population that has received a dose of vaccine. These actions make it possible to assess the progress of the campaign and the extent to which the vaccination plan has been fulfilled.
- Obtaining data for planning the next stages of the vaccination campaign. Understanding the current progress of the campaign and the structure of the vaccinated population allows planning the next stages of the campaign in terms of timing, vaccine volumes, required medical personnel, orders to vaccination manufacturers, vaccine logistics by region and vaccination sites, and other parameters for future stages of the campaign.
- Monitoring the effects of vaccination. Monitoring the possible effects and side effects of vaccines is essential, both in terms of predicting their occurrence in certain groups of patients and in terms of refining vaccines for the next cycle of administration. Despite the proven benefits of vaccination, vaccines can cause complications in certain groups of patients with certain combinations of health factors. Despite this background, there is still a certain amount of hesitancy and skepticism about vaccination in some communities—such patients are not in favor of vaccination because of the lack of knowledge of all the consequences. However, it is important for developers to be aware of the actual side effects and possible complications of their product in order to create the safest vaccine possible.
2. Methodology
3. Literature Review
3.1. Analyzing Research on the Effects and Side Effects of Vaccination
3.2. Decision Analysis of Data Collection and Analysis of Vaccination Outcome Data
4. Results
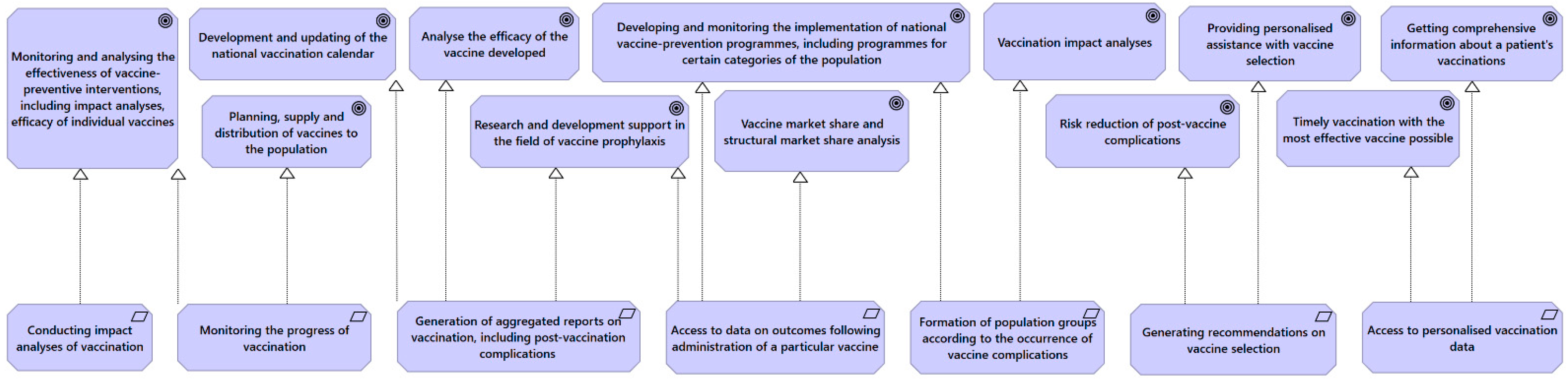
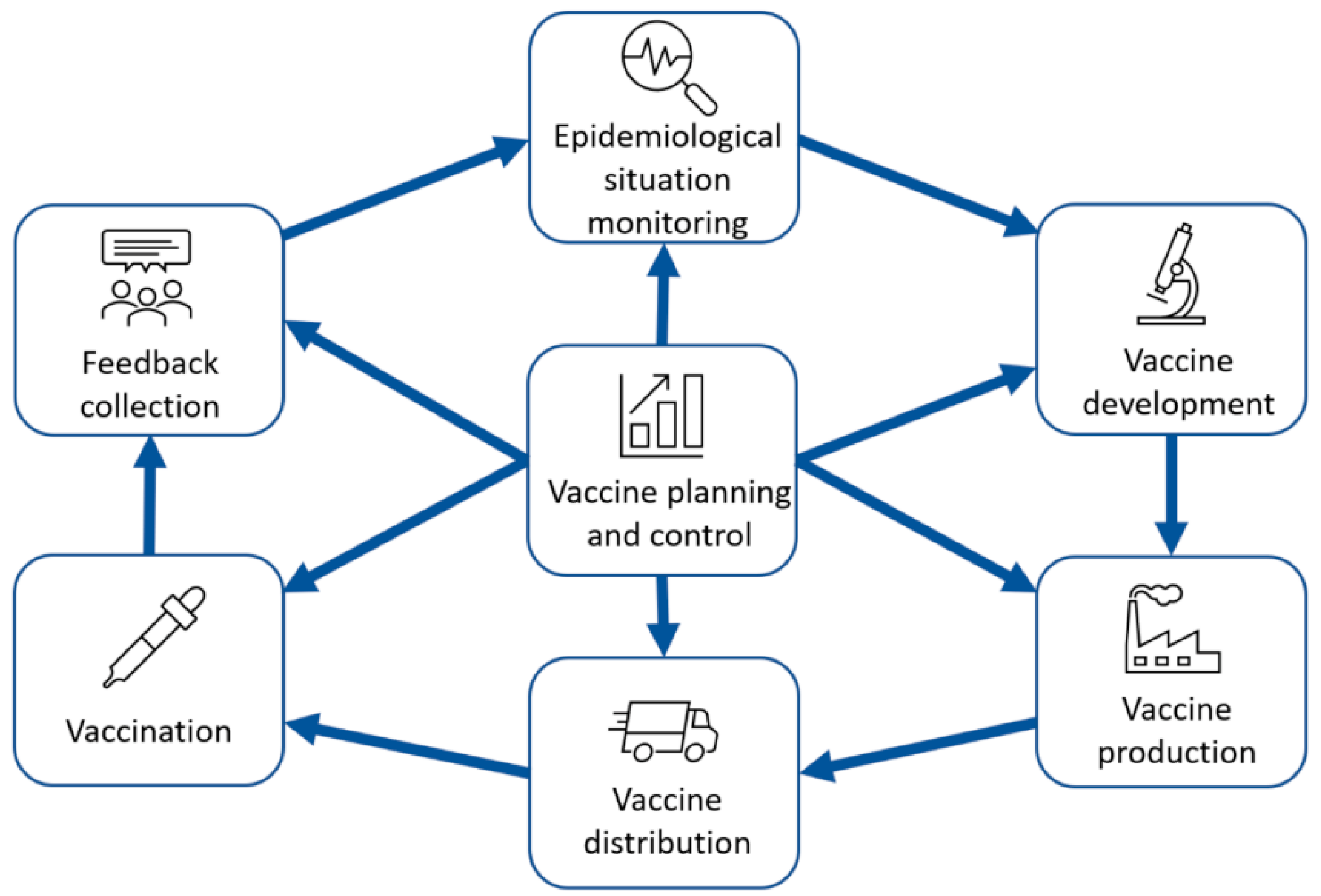
4.1. Requirement to the Vaccination Data Collection and Analysis System
- Monitoring of the epidemiological situation;
- Vaccine development;
- Vaccination;
- Vaccine planning and monitoring.
- Monitoring and analysis of the effectiveness of vaccine-preventive measures, including impact analysis and effectiveness of individual vaccines;
- Planning, provision and distribution of vaccines to the population;
- Development and updating of the national vaccination calendar;
- Support for research and development in the field of vaccine prophylaxis;
- Developing and monitoring the implementation of national vaccine prevention programs, including programs for certain categories of the population.
- –
- Analyzing the efficacy of the developed vaccine;
- –
- Vaccine market share and structural market share analysis;
- –
- Analyzing the effects of vaccination.
- –
- Reducing the risks of postvaccine complications;
- –
- Providing personalized help with vaccine selection;
- –
- Getting comprehensive information about a patient’s immunizations.
- successfully completed vaccination,
- completed vaccination with disease acquisition,
- incomplete vaccination,
- fatalities.
- allow for analysis of the effects of vaccination;
- allow monitoring of vaccination progress;
- be able to generate aggregated reports on vaccination, including on post-vaccine complications;
- allow access to data on outcomes following the administration of a particular vaccine;
- allow the formation of population groups according to postvaccine complications that have occurred.
- allow generating aggregated reports on vaccination, including on post-vaccination complications;
- provide access to data on outcomes following administration of a particular vaccine;
- allow the formation of population groups according to postvaccine complications that have occurred.
- generate data for the Physician Decision Support System (PDSS) to make recommendations for vaccine selection;
- provide access to personalized vaccination data.
- allow for analysis of the effects of vaccination;
- allow monitoring of vaccination progress;
- be able to generate aggregated reports on vaccination, including on post-vaccine complications;
- allow access to data on outcomes following the administration of a particular vaccine;
- allow the formation of population groups according to postvaccine complications that have occurred.
- provide recommendations for vaccine selection;
- provide access to personalized vaccination data.
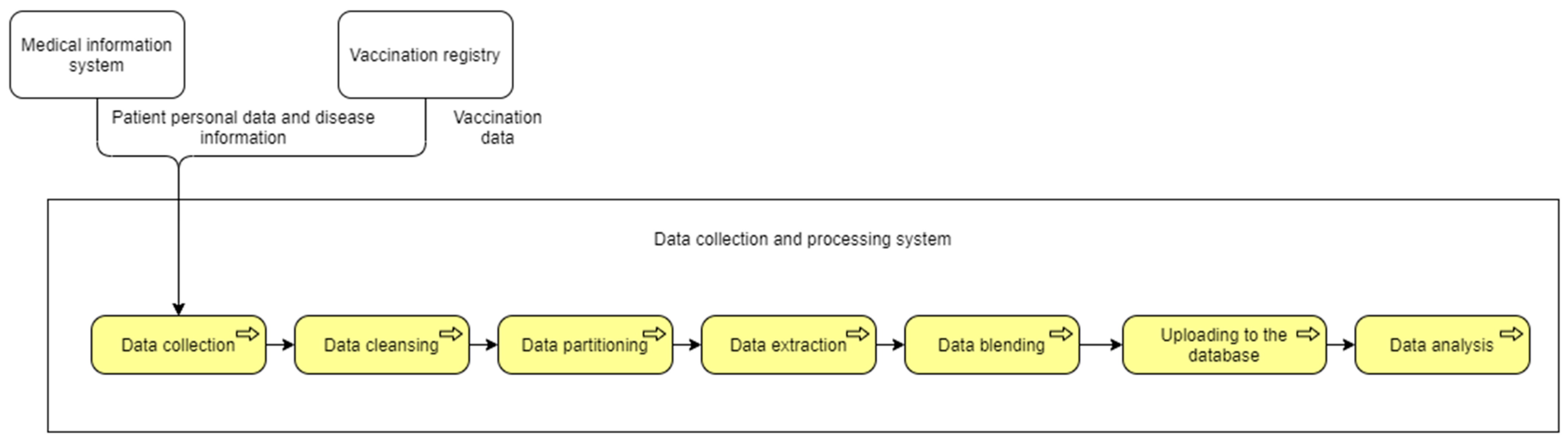
4.2. Architecture of the Vaccination Data Collection and Analysis System
5. Discussion
- –
- Patients: maintenance of vaccination history and the possibility to take into account the specifics of the medical history when choosing a vaccine;
- –
- Medical organizations/physicians: awareness of the patient’s vaccination history and the ability to predict complications and side effects based on the patient’s medical history;
- –
- Vaccine developers: information on complications and side effects of the vaccine (including for certain populations) for further refinement of the vaccine and development of new vaccines;
- –
- State health authorities: understanding the results of vaccine campaigns (including by population groups), bottlenecks and areas of development of vaccination systems.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riedel, S. Edward Jenner and the History of Smallpox and Vaccination. Bayl. Univ. Med. Cent. Proc. 2005, 18, 21–25. [Google Scholar] [CrossRef] [PubMed]
- WHO. This Page Allows You to Request a Table with AFP/Polio Data 2023. Available online: https://extranet.who.int/polis/public/CaseCount.aspx (accessed on 10 February 2023).
- Ilin, I.; Levina, A.; Frolov, K.; Borremans, A.; Ershova, A.; Tick, A.; Averina, M. Life-Cycle Contract as an Innovative Business Model for High-Tech Medical Organizations. J. Open Innov. Technol. Mark. Complex. 2022, 8, 207. [Google Scholar] [CrossRef]
- Ilin, I.; Levina, A.; Frolov, K. Innovative Ecosystem Model of Vaccine Lifecycle Management. J. Open Innov. Technol. Mark. Complex. 2022, 8, 5. [Google Scholar] [CrossRef]
- Canadian Cardiovascular Society. Canadian Cardiovascular Society Receives $1.6 Million to Study Myocarditis and/or Pericarditis after mRNA COVID-19 Vaccination. Can. J. Cardiol. 2022, 38, A9–A12. [Google Scholar] [CrossRef]
- CDC. Health Topics. Available online: https://www.cdc.gov/health-topics.html (accessed on 9 February 2023).
- Drew, D.A.; Nguyen, L.H.; Steves, C.J.; Menni, C.; Freydin, M.; Varsavsky, T.; Sudre, C.H.; Cardoso, M.J.; Ourselin, S.; Wolf, J.; et al. Rapid Implementation of Mobile Technology for Real-Time Epidemiology of COVID-19. Science 2020, 368, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Finnish Institute for Health and Welfare Finnish National Vaccination Register and Monitoring of the Vaccination Programme. Available online: thl.fi/en/web/infectious-diseases-and-vaccinations/surveillance-and-registers/finnish-national-vaccination-register-and-monitoring-of-the-vaccination-programme (accessed on 9 February 2023).
- Gogov Federal Register of Those Vaccinated against COVID-19. Available online: gogov.ru/login/covid-v-registr (accessed on 9 February 2023).
- Greyson, D.; Carpiano, R.M.; Bettinger, J.A. Support for a Vaccination Documentation Mandate in British Columbia, Canada. Vaccine 2022, 40, 7415–7425. [Google Scholar] [CrossRef] [PubMed]
- Mehilainen COVID-19 Vaccination. Available online: https://www.mehilainen.fi/en/coronavirus/covid-19-vaccination (accessed on 31 March 2023).
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; El-Sayed Moustafa, J.S.; et al. Real-Time Tracking of Self-Reported Symptoms to Predict Potential COVID-19. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef]
- S2 Smart Technology How IBM Paralysed the Canadian Medical Industry. Available online: https://vc.ru/services/486869-kak-ibm-paralizovala-rabotu-kanadskoy-mediciny (accessed on 9 February 2023).
- Writing team for the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network Vaccine Coverage Theme Group Why Collect Individual-Level Vaccination Data? Can. Med. Assoc. J. 2010, 182, 273–275. [CrossRef]
- Ilin, I.V.; Levina, A.I.; Dubgorn, A.S.; Abran, A. Investment Models for Enterprise Architecture (EA) and IT Architecture Projects within the Open Innovation Concept. J. Open Innov. Technol. Mark. Complex. 2021, 7, 69. [Google Scholar] [CrossRef]
- Lankhorst, M. Enterprise Architecture at Work; The Enterprise Engineering Series; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-29650-5. [Google Scholar]
- Hornford, D.; Hornford, T.; Sabesan, S.; Scotch, S.; Street, K.; Toder, S. The TOGAF® Standard, 10th ed.; Van Haren Publishing: Hertogenbosch, The Netherlands, 2022. [Google Scholar]
- The Open Group. The ArchiMate 3.0 Enterprise Architecture Modeling Language 2016. Available online: https://www.opengroup.org/archimate-forum/archimate-overview (accessed on 15 February 2023).
- Nguyen, X.-H.; Saoudi, A.; Liblau, R.S. Vaccine-Associated Inflammatory Diseases of the Central Nervous System: From Signals to Causation. Curr. Opin. Neurol. 2016, 29, 362–371. [Google Scholar] [CrossRef]
- Beyer, W.E.P.; Palache, A.M.; Kerstens, R.; Masurel, N. Gender Differences in Local and Systemic Reactions to Inactivated Influenza Vaccine, Established by a Meta-Analysis of Fourteen Independent Studies. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of Immune Responses to Viral Vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Nichol, K.L.; Margolis, K.L.; Lind, A.; Murdoch, M.; McFadden, R.; Hauge, M.; Magnan, S.; Drake, M. Side Effects Associated with Influenza Vaccination in Healthy Working Adults. A Randomized, Placebo-Controlled Trial. Arch. Intern. Med. 1996, 156, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T. Causal Relationship between Immunological Responses and Adverse Reactions Following Vaccination. Vaccine 2019, 37, 366–371. [Google Scholar] [CrossRef]
- Martins, R.M.; Maia, M.d.L.S.; Farias, R.H.G.; Camacho, L.A.B.; Freire, M.S.; Galler, R.; Yamamura, A.M.Y.; Almeida, L.F.C.; Lima, S.M.B.; Nogueira, R.M.R.; et al. 17DD Yellow Fever Vaccine: A Double Blind, Randomized Clinical Trial of Immunogenicity and Safety on a Dose-Response Study. Hum. Vaccines Immunother. 2013, 9, 879–888. [Google Scholar] [CrossRef]
- CDC. Possible Side Effects from Vaccines. Available online: https://www.cdc.gov/vaccines/vac-gen/side-effects.htm (accessed on 2 May 2022).
- Palmer, M.; Bhakdi, S. Vascular and Organ Damage Induced by mRNA Vaccines: Irrefutable Proof of Causality; 2022; Doctors for COVID Ethics; Available online: https://doctors4covidethics.org/vascular-and-organ-damage-induced-by-mrna-vaccines-irrefutable-proof-of-causality/ (accessed on 28 March 2023).
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.; Shuai, Z.; Ye, D.; Pan, H. New-onset Autoimmune Phenomena post-COVID-19 Vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk Factors and Disease Profile of Post-Vaccination SARS-CoV-2 Infection in UK Users of the COVID Symptom Study App: A Prospective, Community-Based, Nested, Case-Control Study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Liozon, E.; Filloux, M.; Parreau, S.; Gondran, G.; Bezanahary, H.; Ly, K.-H.; Fauchais, A.-L. Immune-Mediated Diseases Following COVID-19 Vaccination: Report of a Teaching Hospital-Based Case-Series. J. Clin. Med. 2022, 11, 7484. [Google Scholar] [CrossRef]
- Possible Side Effects after Getting a COVID-19 Vaccine. In National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases; CDC: Atlanta, GA, USA, 2022.
- SeyedAlinaghi, S.; Afsahi, A.M.; MohsseniPour, M.; Behnezhad, F.; Salehi, M.A.; Barzegary, A.; Mirzapour, P.; Mehraeen, E.; Dadras, O. Late Complications of COVID-19; a Systematic Review of Current Evidence. Arch. Acad. Emerg. Med. 2021, 9, e14. [Google Scholar] [CrossRef]
- Rink, D.R.; Swan, J.E. Product Life Cycle Research: A Literature Review. J. Bus. Res. 1979, 7, 219–242. [Google Scholar] [CrossRef]
- Sonnemann, G.; Margni, M. (Eds.) LCA Compendium—The Complete World of Life Cycle Assessment; Springer Netherlands: Dordrecht, The Netherlands, 2015; ISBN 978-94-017-7220-4. [Google Scholar]
- Jabłoński, A.; Jabłoński, M. Research on Business Models in Their Life Cycle. Sustainability 2016, 8, 430. [Google Scholar] [CrossRef]
- Denysenko, Y.; Dynnyk, O.; Yashyna, T.; Malovana, N.; Zaloga, V. Implementation of CALS-Technologies in Quality Management of Product Life Cycle Processes. In Advances in Design, Simulation and Manufacturing; Ivanov, V., Rong, Y., Trojanowska, J., Venus, J., Liaposhchenko, O., Zajac, J., Pavlenko, I., Edl, M., Perakovic, D., Eds.; Lecture Notes in Mechanical Engineering; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–12. ISBN 978-3-319-93586-7. [Google Scholar]
- Koiesar, P.J. What Deming Told the Japanese in 1950. Qual. Manag. J. 1994, 2, 9–24. [Google Scholar] [CrossRef]
- Ilin, I.; Voronova, O.; Pavlov, D.; Kochkarov, A.; Tick, A.; Khusainov, B. System of Project Management at a Medical Hub as an Instrument for Implementation of Open Innovation. Systems 2023, 11, 182. [Google Scholar] [CrossRef]
- Zakharov, V.; Balykina, Y.; Ilin, I.; Tick, A. Forecasting a New Type of Virus Spread: A Case Study of COVID-19 with Stochastic Parameters. Mathematics 2022, 10, 3725. [Google Scholar] [CrossRef]
- Babaee, E.; Amirkafi, A.; Tehrani-Banihashemi, A.; SoleimanvandiAzar, N.; Eshrati, B.; Rampisheh, Z.; Asadi-Aliabadi, M.; Nojomi, M. Adverse Effects Following COVID-19 Vaccination in Iran. BMC Infect. Dis. 2022, 22, 476. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Al Ketbi, L.M.B.; Al Kaabi, N.; Al Mansoori, M.; Al Maskari, N.N.; Al Shamsi, M.S.; Alderei, A.S.; El Eissaee, H.N.; Al Ketbi, R.M.; Al Shamsi, N.S.; et al. Vaccine Side Effects Following COVID-19 Vaccination Among the Residents of the UAE—An Observational Study. Front. Public Health 2022, 10, 876336. [Google Scholar] [CrossRef]
- Hatmal, M.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Mohamud, R.; Fawaz, M.; Kateeb, E.T.; Alkhairy, O.K.; Tayyem, R.; Lounis, M.; Al-Raeei, M.; et al. Reported Adverse Effects and Attitudes among Arab Populations Following COVID-19 Vaccination: A Large-Scale Multinational Study Implementing Machine Learning Tools in Predicting Post-Vaccination Adverse Effects Based on Predisposing Factors. Vaccines 2022, 10, 366. [Google Scholar] [CrossRef]
- Sriwastava, S.; Sharma, K.; Khalid, S.; Bhansali, S.; Shrestha, A.; Elkhooly, M.; Srivastava, S.; Khan, E.; Jaiswal, S.; Wen, S. COVID-19 Vaccination and Neurological Manifestations: A Review of Case Reports and Case Series. Brain Sci. 2022, 12, 407. [Google Scholar] [CrossRef]
- Abu Ali, K.; Alyounis, S. CyberSecurity in Healthcare Industry. In Proceedings of the 2021 International Conference on Information Technology (ICIT), Amman, Jordan, 14 July 2021; pp. 695–701. [Google Scholar]
- Burrell, D.N. Cybersecurity in Healthcare Through the 7-S Model Strategy. Sci. Bull. 2023, 28, 26–35. [Google Scholar] [CrossRef]
- Piricz, N. A review of prosumers’ behaviours in smart grids and importance of smart grid management. Ekonomski Vjesnik/Econviews 2022, 35, 483–496. [Google Scholar] [CrossRef]
- Piricz, N.; Révész, B. Lessons Learned from an Operational Smart Grid Through the Example of a Local Government in Hungary. Public Financ. Q. 2022, 67, 396–412. [Google Scholar] [CrossRef]
- Cartwright, A.J. The Elephant in the Room: Cybersecurity in Healthcare. J. Clin. Monit. Comput. 2023, 37, 1123–1132. [Google Scholar] [CrossRef]
- Coventry, L.; Branley, D. Cybersecurity in Healthcare: A Narrative Review of Trends, Threats and Ways Forward. Maturitas 2018, 113, 48–52. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, N.; Martin, G.; Grass, E.; Durkin, M.; Darzi, A.; Ghafur, S. Cybersecurity in Healthcare: Comparing Cybersecurity Maturity and Experiences Across Global Healthcare Organizations. SSRN J. 2020. [Google Scholar] [CrossRef]


| Children and Teenagers | Adults 18 Years of Age and Older | ||
|---|---|---|---|
| From 6 Months to 3 Years Old | From 4 Years Old to 17 Years Old | ||
| At the injection site | Pain in the leg or arm where the injection was given | Pain, swelling and redness in the arm where the injection was given | |
| All over the body | Swollen lymph nodes Irritability or tearfulness Drowsiness Loss of appetite | Swollen lymph nodes Fatigue Headache Muscle pain Chills | Fatigue Headache Muscle pain Chills Fever Nausea |
| Groups of Side Effects | Patient Category | Number of Studies |
|---|---|---|
| Neurological diseases | Hospitalized patients | 1 |
| Patients with an established diagnosis of COVID-19 | 15 | |
| Lung disease | COVID-19 patients with existing diseases | 1 |
| Hospitalized patients | 6 | |
| Patients with an established diagnosis of COVID-19 | 14 | |
| Patients recently recovered from COVID-19 | 1 | |
| Liver disease | Patients with an established diagnosis of COVID-19 | 5 |
| Heart disease | COVID-19 patients with existing diseases | 1 |
| Hospitalized patients | 3 | |
| Patients with an established diagnosis of COVID-19 | 14 | |
| Patients recently recovered from COVID-19 | 1 | |
| Thrombosis | Hospitalized patients | 4 |
| Patients with an established diagnosis of COVID-19 | 13 | |
| Patients recently recovered from COVID-19 | 1 | |
| Kidney disease | Patients with an established diagnosis of COVID-19 | 8 |
| Hospitalized patients | 1 | |
| Stroke | Patients with an established diagnosis of COVID-19 | 23 |
| Hospitalized patients | 14 | |
| Other | All population groups | 37 |
| Input Data | Analytical Data |
|---|---|
|
|
| EHR | Vaccination Registry |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levina, A.; Ilin, I.; Trifonova, N.; Tick, A. Data-Driven Management of Vaccination and Its Consequences. Systems 2023, 11, 553. https://doi.org/10.3390/systems11110553
Levina A, Ilin I, Trifonova N, Tick A. Data-Driven Management of Vaccination and Its Consequences. Systems. 2023; 11(11):553. https://doi.org/10.3390/systems11110553
Chicago/Turabian StyleLevina, Anastasia, Igor Ilin, Nina Trifonova, and Andrea Tick. 2023. "Data-Driven Management of Vaccination and Its Consequences" Systems 11, no. 11: 553. https://doi.org/10.3390/systems11110553
APA StyleLevina, A., Ilin, I., Trifonova, N., & Tick, A. (2023). Data-Driven Management of Vaccination and Its Consequences. Systems, 11(11), 553. https://doi.org/10.3390/systems11110553








