AI in Thyroid Cancer Diagnosis: Techniques, Trends, and Future Directions
Abstract
1. Introduction
1.1. Background
1.2. Our Contribution
- An overview of existing frameworks and specifics of various AI techniques, including supervised learning (DL, artificial neural networks, traditional classification, and PMs) and USL (clustering and dimensionality reduction) methods, as well as ensemble methods (bagging and boosting).
- An examination of multiple TCDs, exploring the characteristics of these datasets, as well as the methods employed for selecting and extracting features in different research studies.
- An outline of standard assessment criteria used to evaluate the performance of AI-based thyroid cancer detection methods, encompassing classification and regression metrics, statistical metrics, computer vision metrics, and ranking metrics.
- A critical analysis and discussion highlighting limitations, hurdles, current trends, and open challenges in the field.
- A discussion of future research directions, emphasizing areas requiring more attention to overcome existing barriers and improve thyroid cancer detection solutions.
- An emphasis on the potential of AI in advancing thyroid cancer detection while advocating a continuous critical evaluation for responsible and effective use.
1.3. Road Map
2. Overview of Existing Frameworks
2.1. Objective of AI-Based Analysis (O)
- O1. Classification: Thyroid carcinoma classification refers to the categorization of thyroid cancers based on their histopathological features, clinical behavior, and prognosis. There are several types of thyroid carcinomas, each of which has distinct characteristics. The primary categories include: (i) PTC: The most common type, accounting for about 80% of all thyroid cancers. PTC tends to grow very slowly, but it often spreads to lymph nodes in the neck. Despite this, it is usually curable with treatment; (ii) FTC: the second most common type, FTC can invade blood vessels and spread to distant parts of the body, but it is less likely to spread to lymph nodes; (iii) MTC: This type of thyroid cancer starts in the thyroid’s parafollicular cells, also called C cells, which produce the hormone calcitonin. Elevated levels of calcitonin in the blood can indicate MTC; (iv) ATC: A very aggressive and rare form of thyroid cancer, ATC often spreads quickly to other parts of the neck and body. It is difficult to treat.
- O2. Segmentation: The segmentation of thyroid carcinoma refers to the process of identifying and delineating the region of an image that corresponds to a thyroid tumor. The goal of segmentation is to separate the areas of interest, in this case, the thyroid tumor, from the surrounding tissues in medical images. This can be done manually by an expert radiologist, or it can be automated using machine learning algorithms [64,65]. Segmentation is a crucial step in medical image analysis because it helps to accurately determine the location, size, and shape of the tumor, which are vital parameters for diagnosis, treatment planning, and prognosis prediction. A variety of methods can be used to perform image segmentation, including thresholding, edge detection, region-growing methods, and more complex machine learning and DL techniques.
- O3. Prediction: The prediction of thyroid carcinoma involves the use of various diagnostic tools, tests, and techniques—often employing machine learning models—to anticipate the probability of a patient developing thyroid cancer. This predictive analysis can be based on several factors, including but not limited to (i) genetic predisposition: individuals with a family history of thyroid cancer are at a higher risk; (ii) gender and age: thyroid cancer is more common in women and people aged between 25 and 65; (iii) radiation exposure: exposure to high levels of radiation, especially during childhood, increases the risk of developing thyroid cancer; (iv) diet and lifestyle: a lack of iodine in the diet and certain lifestyle factors may contribute to an increased risk. In a medical context, prediction does not necessarily mean a certain future outcome, but rather it points to an increased risk or likelihood based on current data and predictive models. For thyroid carcinoma, predictive tools and tests are typically used in conjunction with each other to achieve more accurate results. For instance, machine learning algorithms can be trained on historical medical data to predict the likelihood of a nodule being benign or malignant, aiding in early detection and more effective treatment planning. Various studies have been proposed to predict thyroid cancer. For instance, in [66], the authors employed the use of an artificial neural network (ANN) and a logistic regression (LR) to make predictions. Another study [67] detailed the creation of a predictive machine using a CNN to analyze 10,068 microscopic thyroid cancer images from South Asian populations. The thyroid cancer images were a part of pharmacogenomic datasets, encompassing genomics and a variation analysis of individual differences associated with the predisposition to the disease.
2.2. Preprocessing
2.3. Supervised Learning (SL)
Traditional Classification (TCL)
- T1. K-nearest neighbors (KNN): The KNN algorithm is a type of nonparametric supervised machine learning method used for regression and classification. The method relies on the utilization of K training samples for predictions. In a study conducted by Chandel et al. in [73], the KNN method was applied to classify thyroid disease based on TSH, T4, and goiter parameters. Liu et al. [74] also employed the fuzzy KNN approach to differentiate between hyperthyroidism, hypothyroidism, and normal cases. There is a growing interest in larger datasets for future research, as noted in [75].
- T2. Support vector machine (SVM): An SVM is a machine learning method used for classification and regression tasks. In a study published in [76], an SVM approach was proposed for differentiating benign from malignant thyroid nodules by utilizing 98 thyroid nodule (TN) samples (82 benign and 16 malignant). Another study in [77] employed six SVMs to classify nodular thyroid lesions by selecting the most important textural characteristics. The authors reported that the proposed method achieved the correct classification. In [78], a generalized discriminant analysis and wavelet carrier vector machine system (GDA-WSVM) was introduced for diagnosing TN, consisting of feature extraction, classification, and testing phases.
- T3. Decision trees (DT): DT learning is a method for data mining that uses a predictive model for decision-making, where the output values are represented by the leaves and the input variables are represented by branches. This approach has been applied to uncover underlying thyroid diseases as demonstrated in various studies such as [79,80,81,82].
- T4. Logistic regression (LR): In [83], the LR model was used to determine the specific characteristics of thyroid microcarcinoma in 63 patients, based on the combination of contrast-enhanced ultrasound (CEUS) and conventional US values. Another study, conducted in northern Iran and reported in [84], applied LR to analyze 33,530 cases of thyroid cancer. LR is a widely used binomial regression model in machine learning.
2.4. Unsupervised Learning (USL)
Clustering (C)
- C1. K-means (KM): The K-means (KM) method is a technique for data partitioning and a combinatorial optimization challenge. It is commonly utilized in USL, in which observations are separated into K groups. In [96], the authors explore the utilization of an ANN and improvised K-means method for normalizing raw data. The study used thyroid data from the UCI dataset containing 215 instances.
- C2. Entropy-based (EB): In [97], a parameter-free calculation framework named DeMine was developed to predict microRNA regulatory modules (MRMs). DeMine is a three-step method based on information entropy. Firstly, the miRNA regulation network is transformed into a synergistic miRNA–miRNA network. Then, miRNA clusters are detected by maximizing the entropy density of the target cluster. Finally, the coregulated miRNAs are integrated into the corresponding clusters to form the final MRMs. The proposed method not only provides improved accuracy but also identifies more miRNAs as potential tumor markers for tumor diagnosis.
2.5. Deep Learning (DL)
2.5.1. Extreme Learning Machine (ELM)
2.5.2. Multilayer Perceptron (MLP)
2.5.3. Radial Basis Function (RBF)
2.5.4. Denoising Autoencoder (DAE)
2.5.5. Convolutional Neural Network (CNN)
2.5.6. Recurrent Neural Network (RNN)
2.5.7. Restricted Boltzmann Machine (RBM)
2.5.8. Generative Adversarial Network (GAN)
2.5.9. Probabilistic Models (PM)
2.6. Ensemble Methods (EMs)
2.6.1. Bagging (B)
- B1. Bootstrap aggregation (BA): The bootstrap aggregating technique is a widely utilized ensemble method aimed at improving the accuracy of machine learning algorithms, particularly for the purposes of classification, regression, and variance reduction. In [143], this approach was employed for diagnosing thyroid abnormalities.
- B2. Feature bagging (FB): In [144], FB is introduced as a method of ensemble learning with the goal of minimizing the correlation between the individual models in the ensemble. FB achieves this by training the models on a randomly selected subset of features, instead of all features in the dataset. The method was applied to distinguish between benign and malignant thyroid cancer cases [145].
2.6.2. Boosting (O)
- O1. AdaBoost In the study by Pan et al. [146], a new method called AdaBoost was utilized to diagnose thyroid nodules using the standard UCI dataset. The RF and PCA techniques were employed for classification purposes and to maintain data variability, respectively.
- O2. Gradient tree boosting (XGBoost): In [147], the XGBoost algorithm was introduced as a fast and efficient implementation of gradient-boosted decision trees. Since its introduction, the XGBoost algorithm has been applied to a range of research topics, including civil engineering [148], time-series classification [149], sport and health monitoring [150], and ischemic stroke readmission [151].
3. Thyroid Cancer Datasets
- ThyroidOmics: This is a dataset developed by the Thyroid Working Group of the CHARGE Consortium that aims to examine the underlying factors and consequences of TD using various omics techniques such as genomics, epigenomics, transcriptomics, proteomics, and metabolomics. The dataset consists of the results of the discovery stage of the genomewide association analysis (GWAS) meta-analysis for thyrotropin (TSH), free thyroxine (FT4), increased TSH (hypothyroidism), and decreased TSH (hyperthyroidism) as reported in [179,180].
- Thyroid Disease Data Set (TDDS): The dataset utilized for classifying using ANN is referred to as the Thyroid database and features 3772 training instances and 3428 testing instances, with a combination of 15 categorical and 6 real attributes. The three defined classes in this dataset include normal (not hypothyroid), hyperfunctioning, and subnormal functioning [181].
- KEEL Thyroid Dataset: The KEEL dataset provides a set of benchmarks to evaluate the effectiveness of various learning methods. This dataset includes several types of classification, such as standard, multi-instance, imbalanced data, semi-supervised classification, regression, time series, and USL, which can be used as reference points for a performance analysis [182].
- TNM8 Dataset: A dataset was created for the purpose of reporting pathologies of thyroid resection specimens associated with carcinoma. The data do not include core needle biopsy specimens or metastasis to the thyroid gland. The dataset also does not encompass noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), tumors of uncertain malignancy, thyroid carcinomas originating from struma ovarii, carcinomas originating in thyroglossal duct cysts, sarcomas, or lymphomas.
- Gene Expression Omnibus (GEO): The GEO database is a genomics repository that follows the guidelines of the minimum information about a microarray experiment. This database is designed to store gene expression datasets, arrays, and sequences and provides researchers with access to a vast collection of experiment results, gene profiles, and platform records in GEO [183].
- Surveillance, Epidemiology, and End Results (SEER): The creators of this dataset aim to supply a collection of clinical characteristics from thyroid carcinoma patients, which includes 34 details such as age, gender, lymph nodes, and others.
- Digital Database Thyroid Image (DDTI): The DDTI dataset serves as a valuable resource for researchers and new radiologists looking to develop algorithm-based CAD systems for thyroid nodule analysis. The dataset comprises 99 cases and 134 images, with each patient’s data stored in an XML file format [184]. Figure 4 provides an illustration of six samples from each of the thyroid carcinoma tissue types in the DDTI dataset.
- Cancer Genome Atlas (TCGA): The TCGA is a comprehensive collection of data gathered from 11,000 patients diagnosed with various types of cancer over a period of 12 years. The data consist of detailed genomic, epigenomic, transcriptomic, and proteomic information, amounting to a total of 2.5 petabytes. This extensive dataset has been instrumental in advancing the research, diagnosis, and treatment of cancer.
- National Cancer Data Repository (NCDR): The NCDR serves as a resource for healthcare and research with the goal of capturing all recorded cases of cancer in England. These data are sourced from the office for national statistics [185].
- Prostate, Lung, Colorectal, and Ovarian (PLCO) dataset The National Cancer Institute supports the PLCO cancer screening trial, aimed at examining the direct factors that contribute to cancer in both men and women. The trial has records of 155,000 participants, and all studies regarding thyroid cancer incidence and mortality can be found within it [186].
4. Features
4.1. Feature Selection Methods (FS)
- FS1. Information gain (IG): Information gain (IG) is a straightforward method for classifying thyroid cancer features. This method evaluates the likelihood of having cancer by comparing the entropy before and after the examination. Typically, a higher gain value corresponds to a lower entropy. IG has been used extensively in several applications for the diagnosis of cancerous diseases, such as in filtering informative genes for precise cancer classification [201], selecting breast cancer treatment factors based on the entropy formula [202], analyzing and classifying medical data of breast cancer [203], reducing the dimensionality of genes in multiclass cancer microarray gene expression datasets [204], and filtering irrelevant and redundant genes of cancer [201]. In [205], IG is utilized as a feature selection technique to eliminate redundant and irrelevant symptoms in datasets related to diabetes, breast cancer, and heart disease. Additionally, the IG-SVM approach, combining IG and SVM, has been employed and its results served as input for the LIBSVM classifier [201].
- FS2. Correlation-based feature selection (CFS): CFS is a technique frequently used for evaluating the correlation between different cancer features. In various studies, the CFS algorithm has been integrated into attribute selection methods for improved classification, such as in [206], where it was applied to thyroid, hepatitis, and breast cancer data from the UCI ML repository. In [141], the authors proposed a hybrid method that combined learning algorithm tools and feature selection techniques for disease diagnosis. The CFS was utilized in [207] for feature selection in microarray datasets to minimize the data’s dimensionality and identify discriminatory genes. A hybrid model incorporating the CFS and a binary particle swarm optimization (BPSO) was proposed in [208] to classify cancer types and was applied to 11 benchmark microarray datasets. The CSVM-RFE, which involves a CFS, was used in [209] to reduce the number of cancer features and eliminate irrelevant ones. In [176], the authors utilized CFS techniques to identify key RNA expression features.
- FS3. Relief (R): The relief algorithm, commonly known as RA, is an effective method used in selecting important features by assessing their differentiation quality by assigning scores. This technique calculates the weight of various features based on the correlation between cancer attributes. In a study published in [210], a feature selection method based on the relief algorithm was proposed as a means of improving efficiency.
- FS4. Consistency-based subset evaluation (CSE): The study in [211] presents a hybrid classification model for breast cancer, which is based on dividing cancer data into single-class subsets. The effectiveness of the model is evaluated using the Wisconsin Breast Cancer Dataset (WBCD).
4.2. Feature Extraction Methods (FE)
- FE1. Principal components analysis (PCA): The use of PCA has been highlighted in several studies as a method to reduce the dimensionality of data and decorrelate the attributes of cancer features. For instance, in [69], PCA was applied to the dual-tree complex wavelet (DTCW) transform to select the optimum features of thyroid cancer. In [70], PCA was proposed as a tool for classifying different thyroid cancer subtypes such as papillary, follicular, and undifferentiated. The implementation of PCA and linear discriminant analysis was also explored in [212] for classifying Raman spectra of different thyroid cancer subtypes. Finally, in [213], the authors utilized PCA on cDNA microarray data to uncover the biological basis of breast cancers.
- FE2. Texture description: Texture analysis is a commonly used method for extracting relevant information in the classification, segmentation, and prediction of thyroid cancer. There are numerous texture analysis techniques in the literature, including wavelet transform, binary descriptors, and statistical descriptors. The discrete wavelet transform (DWT), in particular, has received significant attention for its ability to perfectly decorrelate data. Many studies have utilized wavelets for thyroid cancer detection, such as in [214], where wavelet techniques were employed to identify cancer regions in thyroid, breast, ovarian, and prostate tumors. In [215], texture information was used to diagnose TN malignancy through a two-level 2D wavelet transform. Other works exploring this area can be found in [216,217].
- FE3. Active contour (AC): The active contour (AC), first introduced by Kass and Witkin in 1987, is a dynamic structure primarily used in image processing. There are several approaches for solving the problem of contour segmentation using a deformable curve model, which has seen numerous applications in the field of detection of thyroid cancer, as demonstrated in [218,219,220].
- FE4. Local binary patterns (LBP): The LBP are features employed in computer vision to recognize textures or objects in digital images. LBP have been utilized to detect thyroid cancer in [216]. The combination of LBP and DL has also been proposed to classify benign and malignant thyroid nodules in [221,222].
- FE5. Gray-level co-occurrence matrix (GLCM): The GLCM is a matrix that represents the distribution of values of pixels that occur together at a specified offset in an image. In [223], GLCM was used to extract features to differentiate between different types of thyroid cancer. In [224], the differences between an individual with Hashimoto’s thyroiditis-associated PTC and one with Hashimoto’s thyroiditis alone were investigated based on GLCM comparison.
- FE6. Independent component analysis (ICA): In an ICA, information is gathered into a set of contributing features for the purpose of feature extraction. ICA is utilized to separate multivariate signals into their individual components. In [225], ICA is used to extract 29 attributes as independent and useful features for classifying data into either hypothyroid or hyperthyroid using an SVM.
5. Standard Assessment Criteria
- Classification and Regression MetricsTable 7. Summary of classification and regression metrics used in evaluating AI-based thyroid cancer detection schemes.Table 7. Summary of classification and regression metrics used in evaluating AI-based thyroid cancer detection schemes.
Metric Mathematical Formula Description Accuracy (ACC) Gives the correct percent of the total number of positive and negative predictions. Specificity It is the ratio of correctly predicted negative samples to the total negative samples. Sensitivity It is a quantifiable measure metric of real positive cases that were predicted as true positive cases. Precision (P) Measures the proportion of true positive predictions made by the model, out of all the positive predictions made by the model. F1 score (F1) It is the harmonic mean of precision and sensitivity of the classification. Error rate (ER) It is equivalent to one minus accuracy. Root-mean-square error (RMSE) It is the standard deviation of the predicted error between the training and testing dataset, its lower value means that the classifier is an excellent one. The negative predictive value (NPV) It is the proportion of negative results in diagnostic tests; a higher value means the accuracy of the diagnosis. Jaccard similarity index (JSI) It has been proposed by Paul Jaccard to gauge the similarity and variety in samples. Fallout or false positive rate (FPR) Measures the proportion of negative samples that are incorrectly classified as positive by the model. Volumetric overlap error (VOE) Evaluates the similarity between the segmented region and the ground-truth region. VOE measures the amount of overlap between the two regions and is defined as the ratio of the volume of the union of the segmented region and the ground-truth region to the volume of their intersection. Mean absolute error (MAE) It is the average of the difference between the original values and the predicted values. Mean squared error (MSE) It is the average of the square of the difference between the original values and the predicted values. - Statistical MetricsTable 8. Summary of statistical metrics used in assessing AI-based thyroid cancer detection schemes.Table 8. Summary of statistical metrics used in assessing AI-based thyroid cancer detection schemes.
Metric Mathematical Formula Description Standard deviation (SD) It is a measure of the amount of variation or dispersion in a set of data. Correlation (Corr) It describes the degree of association or relationship between two or more variables. Kappa de Cohen It measures the degree of concordance between two evaluators, relative to chance. - Computer Vision MetricsTable 9. Summary of computer vision metrics used in assessing AI-based thyroid cancer detection schemes.Table 9. Summary of computer vision metrics used in assessing AI-based thyroid cancer detection schemes.
Metric Mathematical Formula Description Peak signal-to-noise ratio (PSNR) It measures the ratio of the maximum possible power of a signal to the power of the noise that affects the fidelity of its representation. Structural similarity index (SSIM) It evaluates the similarity between two images or videos by comparing their luminance, contrast, and structural information. Visual information fidelity (VIF) It evaluates the quality of a reconstructed or compressed image or video compared to the original signal. It measures the amount of visual information preserved in the processed image or video, taking into account the spatial and frequency characteristics of the image. Normalized cross-correlation (NCC) Measures the similarity between two images (or videos) by subtracting the mean value of each signal from the signal itself. Then, the signals are normalized by dividing them by their standard deviation. Finally, the cross-correlation between the two normalized signals is calculated. Structural content (SC) A higher value of structural content shows that the image is of poor quality. Weight PSNR It takes into account the image texture [232]. Noise visibility function (NVF) It estimates the texture content in the image. is the luminance variance. Visual signal-to-noise ratio (VSNR) It is based on the specified thresholds of distortions in the image based on the computing of contrast thresholds and a wavelet transform. If the distortions are lower than the threshold, the VSNR is perfect. is the RMS contrast of the original image I, and is the visual distortion [233]. Weighted signal-to-noise ratio (WSNR) It is based on the contrast sensitivity function (CSF). , , and represent discrete Fourier transforms (2D TFD) [234]. Normalized absolute error (NAE): It evaluates the accuracy of an ML model’s predictions. It measures the difference between the predicted values and the actual values, as a proportion of the range of the actual values. Laplacian mean squared error (LMSE) It is a variant of the mean squared error () that uses the Laplacian distribution instead of the Gaussian distribution. is the Laplacian operator. - Ranking Metrics
- M1. Mean reciprocal rank (MRR): The MRR is a statistic measure for evaluating the mean reciprocal rank of results for a sample of queries [235].where ranki refers to the rank position of the first relevant document for the ith query.
- M2. The discounted cumulative gain (DCG): the DCG is used to measure the ranking quality [236].
6. Example of Thyroid Cancer Detection Using AI
7. Critical Analysis and Discussion
Limitations and Open Challenges
- Insufficient clean data and accuracy: The lack of comprehensive and annotated datasets regarding the incidence and spread of cancer, specifically thyroid cancer, is a major hindrance to accurate cancer diagnoses and efficient treatment. Medical statistics often do not properly record the number of deaths caused by thyroid cancer, making data collection and validation challenging [265]. This results in a limited quantity of data typically collected from one center, due to the absence of a dedicated thyroid cancer clinical database shared among institutions. The accuracy of AI algorithms in diagnosing thyroid cancer is also limited by the scarcity of available labeled cases for clinical outcomes [266]. Researchers acknowledge that a large quantity of data is necessary for a neural network to yield accurate results, but caution must be taken in regard to the data added during the learning phase, as it can introduce noise.
- Thyroid gland imaging: In the diagnostic evaluation of thyroid cancer, computed tomography (CT) and MRI are available options, but they are not considered the preferred methods due to their high cost and unavailability in certain cases [55]. Instead, ultrasound is commonly used as an alternative to physical exams, radioisotope scans, or fine-needle aspiration biopsies. During an ultrasound examination, the doctor is able to assess the activity of the gland by observing the echo of the node and determining its echogenicity, size, limits, and the presence of calcifications. However, the results obtained from ultrasound tests are not always accurate enough to differentiate between benign and malignant nodes and the images obtained can be more prone to noise [267].
- DL models’ hyperparameters: Choosing the right DL algorithm is crucial in addressing various issues, particularly those related to thyroid cancer diagnosis. Due to the close similarities between benign and malignant tumors, as well as between tumors and other types of lymphocytes, it is challenging to differentiate between them accurately [268]. To achieve this, a significant increase in the number of layers for feature extraction may be required. However, this results in a longer processing time, especially when dealing with large quantities of data, which can impact the timeliness of the diagnosis for cancer patients [54].
- Computation cost and storage space: In the field of algorithms, time computing is a metric that assesses the computational complexity of an algorithm, which predicts the time it takes to run the algorithm by calculating the number of basic operations it performs, as well as its dependence on the size of the input. Typically, time computing is expressed as , where n represents the size of the input, measured in terms of the number of bits required to represent it [269]. Researchers in the AI field, especially those working on thyroid cancer or other types of cancer diagnosis, face the challenge of finding algorithms that are both highly accurate and efficient in terms of processing time. They aim to develop algorithms that can analyze vast quantities of data quickly while still providing accurate results. Moreover, the volume of data used in these algorithms can sometimes exceed the available storage space [54].
- Imbalanced dataset: The distribution of cancer elements within categories related to thyroid tissue cells is often uneven, as these cells often make up a minority of the total tissue cell dataset. As a result, the dataset is highly imbalanced, consisting of both cancer cells and normal cells. This unbalanced distribution of features in cancer cell detection datasets often results in the suboptimal performance of AI algorithms used for the detection [270].
- Sparse labels: Labeling is a crucial aspect of computed tomography (CT) detection, specifically for distinguishing between normal and abnormal cancer cells. However, the process can be time-consuming and costly due to the limited number of available labels. This scarcity results in inconsistent decisions and can negatively impact the accuracy of AI algorithms, which heavily rely on labeled data. This can eventually undermine the trust and credibility of this type of application [270].
- The volume of data: At present, with the advancement in technology, especially in the field of thyroid cancer diagnosis and the growing volume of medical and patient data, researchers are facing challenges in suggesting algorithms that can effectively handle a limited number of samples, noisy samples, unannotated samples, sparse samples, incomplete samples, and high-dimensional samples. This requires AI algorithms that are highly efficient and capable of processing vast quantities of data exchanged between healthcare providers and patients or among specialist physicians [271].
- Error susceptibility: Despite AI being self-sufficient, it is still susceptible to errors. For instance, when training an algorithm with TCDs to diagnose cancerous regions, it can result in biased predictions if the training sets are biased. This can lead to a series of incorrect results that may go unnoticed for an extended period. If detected, identifying and correcting the source of the problem can be a time-consuming process [272].
- Data form: Despite the numerous advancements in the use of AI for thyroid cancer detection, several limitations persist and pose a challenge to its progress. With the growing demand for various medical imaging technologies that result in vast quantities of data needed for AI algorithms, coordinating and organizing this information has become a daunting task. This can largely be attributed to the absence of proper labeling, annotation, or segmentation of the data, making it difficult to manage effectively [273].
- Unexplainable AI: The utilization of AI in the medical field can sometimes yield results that are unclear and lack proper justification, known as a “black box”. This leaves doctors unsure about the accuracy of the results and may lead to erroneous decisions and treatments for patients with thyroid cancer. Essentially, AI can behave like a black box and fail to provide understandable explanations for its outputs [274].
- Lack of cancer detection platform: One of the major barriers to detecting various cancers, particularly thyroid cancer, is the limited availability of platforms for reproducing and examining previous results. This shortage represents a significant weakness and hinders the comparison of AI algorithm performance, making it challenging to improve their efficacy [159]. The presence of online platforms with comprehensive datasets, cutting-edge algorithms, and expert recommendations is vital in aiding doctors, researchers, developers, and specialists to make informed decisions with a low margin of error. Such platforms also provide a crucial supplement to clinical diagnoses by allowing for a more comprehensive experimentation and comparison [275].
- The digitization and loss data: The digitization of medical records has become a necessity, particularly in the realm of cancer diagnosis, due to the widespread adoption of various technologies such as whole-slide images. These latter serve as digital versions of glass slides, facilitating the application of AI techniques for pathological analysis [276]. Despite its benefits, digitization in the medical field is confronted with certain limitations, such as the risk of significant information loss during the quantification and inaccuracies that may arise from data compression utilized in autoencoder algorithms. Hence, it is crucial to be mindful in selecting the right digitization technology to preserve the information and maintain the originality of the data [277,278].
- Contrast: The absence of sufficient contrast in the tissues neighboring the TG complicates the process of accurately analyzing and diagnosing thyroid cancer.
8. Future Research Directions
8.1. Explainable Artificial Intelligence (XAI)
8.2. Edge, Fog, and Cloud Computing for Implementation
8.3. Reinforcement Learning (RL)
8.4. Transfer Learning (TL)
8.5. Panoptic Segmentation (PS)
8.6. Internet of Medical Imaging Things (IoMIT)
8.7. Three-Dimensional Thyroid Cancer Detection (3D-TCD)
8.8. AI in Thyroid Surgery (AI-TS)
8.9. Wavelet-Based AI
8.10. Learning with Reduced Data
8.11. Recommender Systems (RSs)
8.12. Federated Learning (FL):
8.13. Generative Chatbots
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Active contour |
| AI | Artificial intelligence |
| ANN | Artificial neural network |
| ATC | Anaplastic thyroid carcinoma |
| BA | Bootstrap aggregation |
| Bi-LSTM | Bidirectional LSTM |
| BN | Bayesian network |
| CAD | Computer-aided diagnosis |
| CFS | Correlation-based feature selection |
| CNN | Convolutional neural network |
| CT | Computed tomography |
| DAE | Denoising autoencoder |
| DCG | Discounted cumulative gain |
| DCNN | Deep convolutional neural network |
| DDTI | Digital Database Thyroid Image |
| DL | Deep learning |
| DNN | Deep neural network |
| DR | Dimensionality reduction |
| DT | Decision trees |
| DTCW | Double-tree complex wavelet transform |
| DWT | Discrete wavelet transfer |
| ELM | Extreme learning machine |
| ER | Error rate |
| FB | Feature bagging |
| FL | Federated learning |
| FNAB | Fine-needle aspiration biopsy |
| FTC | Follicular thyroid carcinoma |
| GAN | Generative adversarial network |
| GEO | Gene expression omnibus |
| GLCM | Gray-level co-occurrence matrix |
| HOG | Histogram of oriented gradient |
| ICA | Independent component analysis |
| IG | Information gain |
| IoMIT | Internet of medical imaging things |
| KM | K-means |
| KNN | K-nearest neighbors |
| LBP | Local binary patterns |
| LR | Logistic regression |
| LSTM | lLong short-term memory |
| ML | Machine learning |
| MLP | Multilayer perceptron |
| MRI | Magnetic resonance imaging |
| MRM | MicroRNA regulatory module |
| MRR | Mean reciprocal rank |
| MSE | Mean squared error |
| MTC | Medullary thyroid carcinoma |
| NCDR | National Cancer Data Repository |
| PCA | Principal component analysis |
| PLCO | Prostate, Lung, Colorectal, and Ovarian |
| PM | Probabilistic models |
| PS | Panoptic segmentation |
| PSNR | Peak signal to noise ratio |
| PTC | Papillary carcinoma |
| RBF | Radial basis function |
| RBM | Restricted Boltzmann machine |
| RF | Random forest |
| RL | Reinforcement learning |
| RMSE | Root-mean-square error |
| RNN | Recurrent neural network |
| RS | Recommender systems |
| SEER | Surveillance, Epidemiology, and End Results |
| SL | Supervised learning |
| SVM | Support vector machine |
| TCD | Thyroid cancer dataset |
| TCGA | The Cancer Genome Atlas |
| TCL | Traditional classification |
| TD | Thyroid disease |
| TDDS | Thyroid Disease Data Set |
| TG | Thyroid gland |
| TIRADS | Thyroid Imaging, Reporting, and Data System |
| TI-RADS | Thyroid Imaging, Reporting, and Data System |
| TL | Transfer learning |
| TN | Thyroid nodules |
| USL | Unsupervised learning |
| XAI | Explainable AI |
| XAI | Explainable artificial intelligence |
| XGBoost | Gradient tree boosting |
References
- Himeur, Y.; Al-Maadeed, S.; Varlamis, I.; Al-Maadeed, N.; Abualsaud, K.; Mohamed, A. Face mask detection in smart cities using deep and transfer learning: Lessons learned from the COVID-19 pandemic. Systems 2023, 11, 107. [Google Scholar] [CrossRef]
- Himeur, Y.; Al-Maadeed, S.; Almaadeed, N.; Abualsaud, K.; Mohamed, A.; Khattab, T.; Elharrouss, O. Deep visual social distancing monitoring to combat COVID-19: A comprehensive survey. Sustain. Cities Soc. 2022, 85, 104064. [Google Scholar] [CrossRef]
- Sohail, S.S.; Farhat, F.; Himeur, Y.; Nadeem, M.; Madsen, D.Ø.; Singh, Y.; Atalla, S.; Mansoor, W. Decoding ChatGPT: A Taxonomy of Existing Research, Current Challenges, and Possible Future Directions. J. King Saud Univ. Comput. Inf. Sci. 2023, 35, 101675. [Google Scholar]
- Himeur, Y.; Elnour, M.; Fadli, F.; Meskin, N.; Petri, I.; Rezgui, Y.; Bensaali, F.; Amira, A. AI-big data analytics for building automation and management systems: A survey, actual challenges and future perspectives. Artif. Intell. Rev. 2023, 56, 4929–5021. [Google Scholar] [CrossRef]
- Calisto, F.M.; Nunes, N.; Nascimento, J.C. Modeling adoption of intelligent agents in medical imaging. Int. J. Hum. Comput. Stud. 2022, 168, 102922. [Google Scholar] [CrossRef]
- Deng, Y.; Li, H.; Wang, M.; Li, N.; Tian, T.; Wu, Y.; Xu, P.; Yang, S.; Zhai, Z.; Zhou, L.; et al. Global burden of thyroid cancer from 1990 to 2017. JAMA Netw. Open 2020, 3, e208759. [Google Scholar] [CrossRef]
- Hammouda, D.; Aoun, M.; Bouzerar, K.; Namaoui, M.; Rezzik, L.; Meguerba, O.; Belaidi, A.; Kherroubi, S. Registre des Tumeurs d’Alger; Ministere de la Sante et de la Population Institut National de Sante Publique: Paris, France, 2006.
- Abid, L. Le cancer de la thyroide en Algérie. In Guide de la Santé en Algérie: Actualité Pathologie; Santedz: Alger, Algeria, 2008. [Google Scholar]
- NIPH. Available online: http://www.insp.dz/index.php/Non-categorise/registre-des-tumeurs-d-alger.html (accessed on 17 January 2021).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Hitu, L.; Gabora, K.; Bonci, E.A.; Piciu, A.; Hitu, A.C.; Ștefan, P.A.; Piciu, D. MicroRNA in Papillary Thyroid Carcinoma: A Systematic Review from 2018 to June 2020. Cancers 2020, 12, 3118. [Google Scholar] [CrossRef]
- Castellana, M.; Piccardo, A.; Virili, C.; Scappaticcio, L.; Grani, G.; Durante, C.; Giovanella, L.; Trimboli, P. Can ultrasound systems for risk stratification of thyroid nodules identify follicular carcinoma? Cancer Cytopathol. 2020, 128, 250–259. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Elia, G.; Ragusa, F.; Ruffilli, I.; La Motta, C.; Paparo, S.R.; Patrizio, A.; Vita, R.; Benvenga, S.; Materazzi, G.; et al. Novel treatments for anaplastic thyroid carcinoma. Gland. Surg. 2020, 9, S28. [Google Scholar] [CrossRef]
- Giovanella, L.; Treglia, G.; Iakovou, I.; Mihailovic, J.; Verburg, F.A.; Luster, M. EANM practice guideline for PET/CT imaging in medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 61–77. [Google Scholar] [CrossRef]
- Carling, T.; Udelsman, R. Thyroid cancer. Annu. Rev. Med. 2014, 65, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zou, X.; Zeng, H.; Zhao, Y.; Ma, X. Comparison of Diagnostic Performance of Five Different Ultrasound TI-RADS Classification Guidelines for Thyroid Nodules. Front. Oncol. 2020, 10, 598225. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Fujimoto, T.; Ota, H.; Hirokawa, M.; Yabuta, T.; Masuoka, H.; Fukushima, M.; Higashiyama, T.; Kihara, M.; Ito, Y.; et al. Calcifications in thyroid tumors on ultrasonography: Calcification types and relationship with histopathological type. Ultrasound Int. Open 2018, 4, E45. [Google Scholar] [CrossRef] [PubMed]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G. Thyroid imaging reporting and data system (TI-RADS): A user’s guide. Radiology 2018, 287, 29–36. [Google Scholar] [CrossRef]
- Genomic Data Commons Data Portal. Available online: https://portal.gdc.cancer.gov/ (accessed on 10 January 2021).
- TI-RADS Calculator. Available online: http://tiradscalculator.com/ (accessed on 10 January 2021).
- AI TI-RADS Calculator. Available online: https://deckard.duhs.duke.edu/~ai-ti-rads/ (accessed on 10 January 2021).
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Wettasinghe, M.C.; Rosairo, S.; Ratnatunga, N.; Wickramasinghe, N.D. Diagnostic accuracy of ultrasound characteristics in the identification of malignant thyroid nodules. BMC Res. Notes 2019, 12, 193. [Google Scholar] [CrossRef]
- Nayak, R.; Nawar, N.; Webb, J.; Fatemi, M.; Alizad, A. Impact of imaging cross-section on visualization of thyroid microvessels using ultrasound: Pilot study. Sci. Rep. 2020, 10, 415. [Google Scholar] [CrossRef]
- Singh Ospina, N.; Maraka, S.; Espinosa DeYcaza, A.; O’Keeffe, D.; Brito, J.P.; Gionfriddo, M.R.; Castro, M.R.; Morris, J.C.; Erwin, P.; Montori, V.M. Diagnostic accuracy of thyroid nodule growth to predict malignancy in thyroid nodules with benign cytology: Systematic review and meta-analysis. Clin. Endocrinol. 2016, 85, 122–131. [Google Scholar] [CrossRef]
- Kumar, V.; Webb, J.; Gregory, A.; Meixner, D.D.; Knudsen, J.M.; Callstrom, M.; Fatemi, M.; Alizad, A. Automated segmentation of thyroid nodule, gland, and cystic components from ultrasound images using deep learning. IEEE Access 2020, 8, 63482–63496. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, F.; Jiang, T.; Zhao, Q.; Kong, D. Ultrasound image-based thyroid nodule automatic segmentation using convolutional neural networks. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 1895–1910. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.A.; Hart, R.D. Fine-needle aspiration biopsy of thyroid nodules: Determining when it is necessary. Can. Fam. Physician 2018, 64, 127. [Google Scholar]
- Hahn, S.Y.; Shin, J.H.; Oh, Y.L.; Park, K.W.; Lim, Y. Comparison between fine needle Aspiration and core needle Biopsy for the Diagnosis of thyroid Nodules: Effective Indications according to US Findings. Sci. Rep. 2020, 10, 4969. [Google Scholar] [CrossRef]
- Ullah, H.; Saba, T.; Islam, N.; Abbas, N.; Rehman, A.; Mehmood, Z.; Anjum, A. An ensemble classification of exudates in color fundus images using an evolutionary algorithm based optimal features selection. Microsc. Res. Tech. 2019, 82, 361–372. [Google Scholar] [CrossRef]
- Saba, T.; Bokhari, S.T.F.; Sharif, M.; Yasmin, M.; Raza, M. Fundus image classification methods for the detection of glaucoma: A review. Microsc. Res. Tech. 2018, 81, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Mughal, B.; Muhammad, N.; Sharif, M.; Rehman, A.; Saba, T. Removal of pectoral muscle based on topographic map and shape-shifting silhouette. BMC Cancer 2018, 18, 778. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Calisto, F.M.; Santiago, C.; Aleluia, C.; Nascimento, J.C. Classification of Breast Cancer in Mri with Multimodal Fusion. In Proceedings of the 2023 IEEE 20th International Symposium on Biomedical Imaging (ISBI), Cartagena de Indias, Colombia, 18–21 April 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 1–4. [Google Scholar]
- Diogo, P.; Morais, M.; Calisto, F.M.; Santiago, C.; Aleluia, C.; Nascimento, J.C. Weakly-Supervised Diagnosis and Detection of Breast Cancer Using Deep Multiple Instance Learning. In Proceedings of the 2023 IEEE 20th International Symposium on Biomedical Imaging (ISBI), Cartagena de Indias, Colombia, 18–21 April 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 1–4. [Google Scholar]
- Wang, X.; Zhang, J.; Yang, S.; Xiang, J.; Luo, F.; Wang, M.; Zhang, J.; Yang, W.; Huang, J.; Han, X. A generalizable and robust deep learning algorithm for mitosis detection in multicenter breast histopathological images. Med. Image Anal. 2023, 84, 102703. [Google Scholar] [CrossRef] [PubMed]
- Mughal, B.; Sharif, M.; Muhammad, N.; Saba, T. A novel classification scheme to decline the mortality rate among women due to breast tumor. Microsc. Res. Tech. 2018, 81, 171–180. [Google Scholar] [CrossRef]
- Abbas, N.; Saba, T.; Mehmood, Z.; Rehman, A.; Islam, N.; Ahmed, K.T. An automated nuclei segmentation of leukocytes from microscopic digital images. Pak. J. Pharm. Sci. 2019, 32, 2123–2138. [Google Scholar]
- Abbas, N.; Saba, T.; Rehman, A.; Mehmood, Z.; Javaid, N.; Tahir, M.; Khan, N.U.; Ahmed, K.T.; Shah, R. Plasmodium species aware based quantification of malaria parasitemia in light microscopy thin blood smear. Microsc. Res. Tech. 2019, 82, 1198–1214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yue, W.; Li, X.; Liu, S.; Guo, L.; Xu, H.; Zhang, H.; Yang, G. Comparison study of radiomics and deep learning-based methods for thyroid nodules classification using ultrasound images. IEEE Access 2020, 8, 52010–52017. [Google Scholar] [CrossRef]
- Qin, P.; Wu, K.; Hu, Y.; Zeng, J.; Chai, X. Diagnosis of benign and malignant thyroid nodules using combined conventional ultrasound and ultrasound elasticity imaging. IEEE J. Biomed. Health Informatics 2019, 24, 1028–1036. [Google Scholar] [CrossRef]
- Wu, H.; Deng, Z.; Zhang, B.; Liu, Q.; Chen, J. Classifier model based on machine learning algorithms: Application to differential diagnosis of suspicious thyroid nodules via sonography. Am. J. Roentgenol. 2016, 207, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, J.; Pei, S.; Chen, Y.; He, X.; Dong, Y.; Zhang, L.; Mo, X.; Huang, W.; Cong, S.; et al. Machine learning–assisted system for thyroid nodule diagnosis. Thyroid 2019, 29, 858–867. [Google Scholar] [CrossRef]
- Sollini, M.; Cozzi, L.; Chiti, A.; Kirienko, M. Texture analysis and machine learning to characterize suspected thyroid nodules and differentiated thyroid cancer: Where do we stand? Eur. J. Radiol. 2018, 99, 1–8. [Google Scholar] [CrossRef]
- Yang, C.Q.; Gardiner, L.; Wang, H.; Hueman, M.T.; Chen, D. Creating prognostic systems for well-differentiated thyroid cancer using machine learning. Front. Endocrinol. 2019, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Abbad Ur Rehman, H.; Lin, C.Y.; Mushtaq, Z. Effective K-Nearest Neighbor Algorithms Performance Analysis of Thyroid Disease. J. Chin. Inst. Eng. 2021, 44, 77–87. [Google Scholar] [CrossRef]
- Taylor, J.N.; Mochizuki, K.; Hashimoto, K.; Kumamoto, Y.; Harada, Y.; Fujita, K.; Komatsuzaki, T. High-resolution Raman microscopic detection of follicular thyroid cancer cells with unsupervised machine learning. J. Phys. Chem. B 2019, 123, 4358–4372. [Google Scholar] [CrossRef]
- Chandio, J.A.; Mallah, G.A.; Shaikh, N.A. Decision Support System for Classification Medullary Thyroid Cancer. IEEE Access 2020, 8, 145216–145226. [Google Scholar] [CrossRef]
- Lee, J.H.; Chai, Y.J. A deep-learning model to assist thyroid nodule diagnosis and management. Lancet Digit. Health 2021, 3, e410. [Google Scholar] [CrossRef] [PubMed]
- Buda, M.; Wildman-Tobriner, B.; Hoang, J.K.; Thayer, D.; Tessler, F.N.; Middleton, W.D.; Mazurowski, M.A. Management of thyroid nodules seen on US images: Deep learning may match performance of radiologists. Radiology 2019, 292, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, J.; Peng, S.; Wang, W.; Xiao, H. A deep-learning model to assist thyroid nodule diagnosis and management–Authors’ reply. Lancet Digit. Health 2021, 3, e411–e412. [Google Scholar] [CrossRef]
- Iesato, A.; Nucera, C. Role of regulatory non-coding RNAs in aggressive thyroid cancer: Prospective applications of neural network analysis. Molecules 2021, 26, 3022. [Google Scholar] [CrossRef]
- Sharifi, Y.; Bakhshali, M.A.; Dehghani, T.; DanaiAshgzari, M.; Sargolzaei, M.; Eslami, S. Deep learning on ultrasound images of thyroid nodules. Biocybern. Biomed. Eng. 2021, 41, 636–655. [Google Scholar] [CrossRef]
- Lin, Y.J.; Chao, T.K.; Khalil, M.A.; Lee, Y.C.; Hong, D.Z.; Wu, J.J.; Wang, C.W. Deep learning fast screening approach on cytological whole slides for thyroid cancer diagnosis. Cancers 2021, 13, 3891. [Google Scholar] [CrossRef]
- Ha, E.J.; Baek, J.H. Applications of machine learning and deep learning to thyroid imaging: Where do we stand? Ultrasonography 2021, 40, 23. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Li, M.; Cui, X.W.; Xu, G. Deep multimodal learning for lymph node metastasis prediction of primary thyroid cancer. Phys. Med. Biol. 2022, 67, 035008. [Google Scholar] [CrossRef]
- Pavithra, S.; Yamuna, G.; Arunkumar, R. Deep Learning Method for Classifying Thyroid Nodules Using Ultrasound Images. In Proceedings of the 2022 International Conference on Smart Technologies and Systems for Next Generation Computing (ICSTSN), Villupuram, India, 25–26 March 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–6. [Google Scholar]
- Paul, R.; Juliano, A.; Faquin, W.; Chan, A.W. An Artificial Intelligence Ultrasound Platform for Screening and Staging of Thyroid Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, e8. [Google Scholar] [CrossRef]
- Ilyas, M.; Malik, H.; Adnan, M.; Bashir, U.; Bukhari, W.A.; Khan, M.I.A.; Ahmad, A. Deep Learning based Classification of Thyroid Cancer using Different Medical Imaging Modalities: A Systematic Review. Vfast Trans. Softw. Eng. 2022, 9, 1–17. [Google Scholar]
- Liu, C.; Huang, Y.; Ozolek, J.A.; Hanna, M.G.; Singh, R.; Rohde, G.K. SetSVM: An approach to set classification in nuclei-based cancer detection. IEEE J. Biomed. Health Informatics 2018, 23, 351–361. [Google Scholar] [CrossRef]
- Zhang, S.; Du, H.; Jin, Z.; Zhu, Y.; Zhang, Y.; Xie, F.; Zhang, M.; Jiao, Z.; Tian, X.; Zhang, J.; et al. Integrating Clinical Knowledge in a Thyroid Nodule Classification Model Based on. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 2334–2336. [Google Scholar]
- Zhang, H.; Zhao, C.; Guo, L.; Li, X.; Luo, Y.; Lu, J.; Xu, H. Diagnosis of Thyroid Nodules in Ultrasound Images Using Two Combined Classification Modules. In Proceedings of the 2019 12th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Suzhou, China, 19–21 October 2019; IEEE: San Francisco, CA, USA, 2019; pp. 1–5. [Google Scholar]
- Chen, D.; Zhang, J.; Li, W. Thyroid Nodule Classification Using Two Levels Attention-Based Bi-Directional LSTM with Ultrasound Reports. In Proceedings of the 2018 9th International Conference on Information Technology in Medicine and Education (ITME), Hangzhou, China, 19–21 October 2018; IEEE: San Francisco, CA, USA, 2018; pp. 309–312. [Google Scholar]
- Ma, X.; Sun, B.; Liu, W.; Sui, D.; Chen, J.; Tian, Z. AMSeg: A Novel Adversarial Architecture based Multi-scale Fusion Framework for Thyroid Nodule Segmentation. IEEE Access 2023, 11, 72911–72924. [Google Scholar] [CrossRef]
- Yadav, N.; Dass, R.; Virmani, J. Assessment of encoder-decoder-based segmentation models for thyroid ultrasound images. Med. Biol. Eng. Comput. 2023, 61, 2159–2195. [Google Scholar] [CrossRef]
- Jajroudi, M.; Baniasadi, T.; Kamkar, L.; Arbabi, F.; Sanei, M.; Ahmadzade, M. Prediction of survival in thyroid cancer using data mining technique. Technol. Cancer Res. Treat. 2014, 13, 353–359. [Google Scholar] [CrossRef]
- Sajeev, V.; Vyshnavi, A.H.; Namboori, P.K. Thyroid Cancer Prediction Using Gene Expression Profile, Pharmacogenomic Variants And Quantum Image Processing in Deep Learning Platform—A Theranostic Approach. In Proceedings of the 2020 International Conference for Emerging Technology (INCET), Belgaum, India, 5–7 June 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1–5. [Google Scholar]
- Tarkov, M.; Chiglintsev, E. Data space dimensionality reduction in the problem of diagnosing a thyroid disease. Bull. Novosib. Comput. Cent. Ser. Comput. Sci. 2012, 33, 79–84. [Google Scholar]
- Shankarlal, B.; Sathya, P. Performance Analysis of Thyroid Tumor Detection and Segmentation Using PCA-Based Random Classification Method. In Innovations in Electrical and Electronics Engineering; Springer: New York, NY, USA, 2020; pp. 833–841. [Google Scholar]
- Soulaymani, A.; Aschawa, H. Epidemiological Study of Thyroid Carcinoma Using Principal Component Analysis. J. Clin. Epigenetics 2018, 4, 9. [Google Scholar]
- Liu, N.; Fenster, A.; Tessier, D.; Gou, S.; Chong, J. Self-supervised learning enhanced ultrasound video thyroid nodule tracking. In Medical Imaging 2023: Image Processing; SPIE: Stockholm, Sweden, 2023; Volume 12464, pp. 683–687. [Google Scholar]
- Hou, Y.; Sang, Q. Boosting Ultrasonic Image Classification via Self-Supervised Representation Learning. In Proceedings of the 2023 3rd International Conference on Computer, Control and Robotics (ICCCR), Shanghai, China, 24–26 March 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 116–120. [Google Scholar]
- Chandel, K.; Kunwar, V.; Sabitha, S.; Choudhury, T.; Mukherjee, S. A comparative study on thyroid disease detection using K-nearest neighbor and Naive Bayes classification techniques. CSI Trans. ICT 2016, 4, 313–319. [Google Scholar] [CrossRef]
- Liu, D.Y.; Chen, H.L.; Yang, B.; Lv, X.E.; Li, L.N.; Liu, J. Design of an enhanced fuzzy k-nearest neighbor classifier based computer aided diagnostic system for thyroid disease. J. Med. Syst. 2012, 36, 3243–3254. [Google Scholar] [CrossRef]
- Geetha, K.; Baboo, S.S. An empirical model for thyroid disease classification using evolutionary multivariate Bayseian prediction method. Glob. J. Comput. Sci. Technol. 2016, 16, 1–10. [Google Scholar]
- Ma, J.; Luo, S.; Dighe, M.; Lim, D.J.; Kim, Y. Differential diagnosis of thyroid nodules with ultrasound elastography based on support vector machines. In Proceedings of the 2010 IEEE International Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 1372–1375. [Google Scholar]
- Chang, C.Y.; Chen, S.J.; Tsai, M.F. Application of support-vector-machine-based method for feature selection and classification of thyroid nodules in ultrasound images. Pattern Recognit. 2010, 43, 3494–3506. [Google Scholar] [CrossRef]
- Dogantekin, E.; Dogantekin, A.; Avci, D. An expert system based on Generalized Discriminant Analysis and Wavelet Support Vector Machine for diagnosis of thyroid diseases. Expert Syst. Appl. 2011, 38, 146–150. [Google Scholar] [CrossRef]
- Yadav, D.C.; Pal, S. Prediction of thyroid disease using decision tree ensemble method. Hum.-Intell. Syst. Integr. 2020, 2, 89–95. [Google Scholar] [CrossRef]
- Hao, Y.; Zuo, W.; Shi, Z.; Yue, L.; Xue, S.; He, F. Prognosis of thyroid disease using MS-apriori improved decision tree. In Proceedings of the International Conference on Knowledge Science, Engineering and Management, Changchun, China, 17–19 August 2018; Springer: New York, NY, USA, 2018; pp. 452–460. [Google Scholar]
- Dharmarajan, K.; Balasree, K.; Arunachalam, A.; Abirmai, K. Thyroid Disease Classification Using Decision Tree and SVM. Exec. Ed. 2020, 11, 3234. [Google Scholar]
- Yadav, D.C.; Pal, S. Decision tree ensemble techniques to predict thyroid disease. Int. J. Recent Technol. Eng. 2019, 8, 8242–8246. [Google Scholar] [CrossRef]
- Zhao, R.N.; Zhang, B.; Yang, X.; Jiang, Y.X.; Lai, X.J.; Zhang, X.Y. Logistic regression analysis of contrast-enhanced ultrasound and conventional ultrasound characteristics of sub-centimeter thyroid nodules. Ultrasound Med. Biol. 2015, 41, 3102–3108. [Google Scholar] [CrossRef] [PubMed]
- Yazdani-Charati, J.; Akha, O.; Khosravi, F. Factors Affecting Thyroid Cancer in Patients with Thyroid Nodules Using Logistic Regression in Interval Censored Data. Int. J. Cancer Manag. 2018, 11, e9111. [Google Scholar] [CrossRef]
- Kate, M.D.; Kale, V. Check for updates The Role of Machine Learning in Thyroid Cancer Diagnosis. In Proceedings of the International Conference on Applications of Machine Intelligence and Data Analytics (ICAMIDA 2022), Aurangabad, India, 22–24 December 2022; Springer Nature: London, UK, 2023; Volume 105, p. 276. [Google Scholar]
- Nobile, M.S.; Capitoli, G.; Sowirono, V.; Clerici, F.; Piga, I.; van Abeelen, K.; Magni, F.; Pagni, F.; Galimberti, S.; Cazzaniga, P.; et al. Unsupervised neural networks as a support tool for pathology diagnosis in MALDI-MSI experiments: A case study on thyroid biopsies. Expert Syst. Appl. 2023, 215, 119296. [Google Scholar] [CrossRef]
- Manogaran, G.; Vijayakumar, V.; Varatharajan, R.; Kumar, P.M.; Sundarasekar, R.; Hsu, C.H. Machine learning based big data processing framework for cancer diagnosis using hidden Markov model and GM clustering. Wirel. Pers. Commun. 2018, 102, 2099–2116. [Google Scholar] [CrossRef]
- Agrawal, U.; Soria, D.; Wagner, C.; Garibaldi, J.; Ellis, I.O.; Bartlett, J.M.; Cameron, D.; Rakha, E.A.; Green, A.R. Combining clustering and classification ensembles: A novel pipeline to identify breast cancer profiles. Artif. Intell. Med. 2019, 97, 27–37. [Google Scholar] [CrossRef]
- de Souto, M.C.; Costa, I.G.; de Araujo, D.S.; Ludermir, T.B.; Schliep, A. Clustering cancer gene expression data: A comparative study. BMC Bioinform. 2008, 9, 497. [Google Scholar] [CrossRef]
- Anas, M.; Gupta, K.; Ahmad, S. Skin cancer classification using K-means clustering. Int. J. Tech. Res. Appl. 2017, 5, 62–65. [Google Scholar]
- Khan, A.R.; Khan, S.; Harouni, M.; Abbasi, R.; Iqbal, S.; Mehmood, Z. Brain tumor segmentation using K-means clustering and deep learning with synthetic data augmentation for classification. Microsc. Res. Tech. 2021, 84, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yu, G.; Wang, J. Clustering cancer gene expression data by projective clustering ensemble. PLoS ONE 2017, 12, e0171429. [Google Scholar] [CrossRef] [PubMed]
- Chandel, K.; Kunwar, V.; Sabitha, A.S.; Bansal, A.; Choudhury, T. Analysing thyroid disease using density-based clustering technique. Int. J. Bus. Intell. Data Min. 2020, 17, 273–297. [Google Scholar] [CrossRef]
- Katikireddy Srinivas, D.K. Performa analysis of clustering of thyroid drug data using fuzzy and m-clust. J. Crit. Rev. 2020, 7, 2128–2141. [Google Scholar]
- Venkataramana, B.; Padmasree, L.; Rao, M.S.; Latha, D.; Ganesan, G. Comparative Study on performance of Fuzzy clustering algorithms on Liver and Thyroid Data. J. Fuzzy Set Valued Anal. 2018, 2018, 9240389. [Google Scholar] [CrossRef][Green Version]
- Mahurkar, K.K.; Gaikwad, D. Normalization using Improvised K-Means applied in diagnosing thyroid disease with ANN. In Proceedings of the 2017 International Conference on Trends in Electronics and Informatics (ICEI), Tirunelveli, India, 11–12 May 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 579–583. [Google Scholar]
- Yang, Y.; Song, Y.; Cao, B. An Information Entropy-based Method to Detect microRNA Regulatory Module. IPSJ Trans. Bioinform. 2019, 12, 1–8. [Google Scholar] [CrossRef]
- Canton, S.P.; Dadashzadeh, E.; Yip, L.; Forsythe, R.; Handzel, R. Automatic Detection of Thyroid and Adrenal Incidentals Using Radiology Reports and Deep Learning. J. Surg. Res. 2021, 266, 192–200. [Google Scholar] [CrossRef]
- Peng, S.; Liu, Y.; Lv, W.; Liu, L.; Zhou, Q.; Yang, H.; Ren, J.; Liu, G.; Wang, X.; Zhang, X.; et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: A multicentre diagnostic study. Lancet Digit. Health 2021, 3, e250–e259. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, Y.; Du, J.; Qin, Y.; Lu, H.; Xiang, J.; Wang, F. Deep learning based classification of ultrasound images for thyroid nodules: A large scale of pilot study. Ann. Transl. Med. 2019, 7, 137. [Google Scholar] [CrossRef]
- Li, L.N.; Ouyang, J.H.; Chen, H.L.; Liu, D.Y. A computer aided diagnosis system for thyroid disease using extreme learning machine. J. Med. Syst. 2012, 36, 3327–3337. [Google Scholar] [CrossRef]
- Ma, C.; Guan, J.; Zhao, W.; Wang, C. An efficient diagnosis system for Thyroid disease based on enhanced Kernelized Extreme Learning Machine Approach. In Proceedings of the International Conference on Cognitive Computing, San Francisco, CA, USA, 2–7 July 2018; Springer: New York, NY, USA, 2018; pp. 86–101. [Google Scholar]
- Xia, J.; Chen, H.; Li, Q.; Zhou, M.; Chen, L.; Cai, Z.; Fang, Y.; Zhou, H. Ultrasound-based differentiation of malignant and benign thyroid Nodules: An extreme learning machine approach. Comput. Methods Programs Biomed. 2017, 147, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, R.; Parthiban, L. Optimal Deep Learning with Kernel Extreme Learning Machine Based Thyroid Disease Diagnosis and Classification Model. J. Comput. Theor. Nanosci. 2021, 18, 639–649. [Google Scholar]
- Rao, B.N.; Reddy, D.L.S.; Bhaskar, G. Thyroid Diagnosis Using Multilayer Perceptron. In Proceedings of the International Conference on E-Business and Telecommunications, Prague, Czech Republic, 26–28 July 2019; Springer: New York, NY, USA, 2019; pp. 452–459. [Google Scholar]
- Hosseinzadeh, M.; Ahmed, O.H.; Ghafour, M.Y.; Safara, F.; Ali, S.; Vo, B.; Chiang, H.S. A multiple multilayer perceptron neural network with an adaptive learning algorithm for thyroid disease diagnosis in the internet of medical things. J. Supercomput. 2020, 77, 1–22. [Google Scholar] [CrossRef]
- Isa, I.; Saad, Z.; Omar, S.; Osman, M.; Ahmad, K.; Sakim, H.M. Suitable MLP network activation functions for breast cancer and thyroid disease detection. In Proceedings of the 2010 Second International Conference on Computational Intelligence, Modelling and Simulation, Bali, Indonesia, 28–30 September 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 39–44. [Google Scholar]
- Mourad, M.; Moubayed, S.; Dezube, A.; Mourad, Y.; Park, K.; Torreblanca-Zanca, A.; Torrecilla, J.S.; Cancilla, J.C.; Wang, J. Machine Learning and feature Selection Applied to SeeR Data to Reliably Assess thyroid cancer prognosis. Sci. Rep. 2020, 10, 5176. [Google Scholar] [CrossRef]
- Erol, R.; Oğulata, S.N.; Şahin, C.; Alparslan, Z.N. A radial basis function neural network (RBFNN) approach for structural classification of thyroid diseases. J. Med. Syst. 2008, 32, 215–220. [Google Scholar] [CrossRef]
- Ferreira, M.F.; Camacho, R.; Teixeira, L.F. Autoencoders as weight initialization of deep classification networks applied to papillary thyroid carcinoma. In Proceedings of the 2018 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Madrid, Spain, 3–6 December 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 629–632. [Google Scholar]
- Teixeira, V.; Camacho, R.; Ferreira, P.G. Learning influential genes on cancer gene expression data with stacked denoising autoencoders. In Proceedings of the 2017 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Kansas City, MO, USA, 13–16 November 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1201–1205. [Google Scholar]
- Liu, T.; Guo, Q.; Lian, C.; Ren, X.; Liang, S.; Yu, J.; Niu, L.; Sun, W.; Shen, D. Automated detection and classification of thyroid nodules in ultrasound images using clinical-knowledge-guided convolutional neural networks. Med. Image Anal. 2019, 58, 101555. [Google Scholar] [CrossRef]
- Ha, E.J.; Baek, J.H.; Na, D.G. Deep convolutional neural network models for the diagnosis of thyroid cancer. Lancet Oncol. 2019, 20, e130. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Zhou, S. The Detection of Hyperthyroidism by the Modified LeNet-5 Network. Indian J. Pharm. Sci. 2020, 82, 108–114. [Google Scholar] [CrossRef]
- Qiao, T.; Liu, S.; Cui, Z.; Yu, X.; Cai, H.; Zhang, H.; Sun, M.; Lv, Z.; Li, D. Deep learning for intelligent diagnosis in thyroid scintigraphy. J. Int. Med. Res. 2021, 49, 0300060520982842. [Google Scholar] [CrossRef]
- Cox, J.; Rubin, S.; Adams, J.; Pereira, C.; Dighe, M.; Alessio, A. Hyperparameter selection for ResNet classification of malignancy from thyroid ultrasound images. In Medical Imaging 2020: Computer-Aided Diagnosis. International Society for Optics and Photonics; SPIE: New York, NY, USA, 2020; Volume 11314, p. 1131447. [Google Scholar]
- Chi, J.; Walia, E.; Babyn, P.; Wang, J.; Groot, G.; Eramian, M. Thyroid nodule classification in ultrasound images by fine-tuning deep convolutional neural network. J. Digit. Imaging 2017, 30, 477–486. [Google Scholar] [CrossRef]
- Tekchandani, H.; Verma, S.; Londhe, N.D.; Jain, R.R.; Tiwari, A. Severity Assessment of Cervical Lymph Nodes using Modified VGG-Net, and Squeeze and Excitation Concept. In Proceedings of the 2021 IEEE 11th Annual Computing and Communication Workshop and Conference (CCWC), Virtual, 27–30 January 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 709–714. [Google Scholar]
- Ke, L.; Deng, Y.; Xia, W.; Qiang, M.; Chen, X.; Liu, K.; Jing, B.; He, C.; Xie, C.; Guo, X.; et al. Development of a self-constrained 3D DenseNet model in automatic detection and segmentation of nasopharyngeal carcinoma using magnetic resonance images. Oral Oncol. 2020, 110, 104862. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, S.; Zhang, Q.; Wei, X.; Pan, Y.; Zhao, J.; Xin, X.; Qin, C.; Wang, X.; Li, J.; et al. Diagnosis of thyroid cancer using deep convolutional neural network models applied to sonographic images: A retrospective, multicohort, diagnostic study. Lancet Oncol. 2019, 20, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yu, J.; Liu, T.; Chang, Q.; Niu, L.; Sun, W. Thyroid Nodule Detection in Ultrasound Images with Convolutional Neural Networks. In Proceedings of the 2019 14th IEEE Conference on Industrial Electronics and Applications (ICIEA), Xi’an, China, 19–21 June 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1442–1446. [Google Scholar]
- Koh, J.; Lee, E.; Han, K.; Kim, E.K.; Son, E.J.; Sohn, Y.M.; Seo, M.; Kwon, M.R.; Yoon, J.H.; Lee, J.H.; et al. Diagnosis of thyroid nodules on ultrasonography by a deep convolutional neural network. Sci. Rep. 2020, 10, 15245. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yu, J.; Liao, J.; Chen, Z. Convolutional Neural Network for Breast and Thyroid Nodules Diagnosis in Ultrasound Imaging. BioMed Res. Int. 2020, 2020, 1763803. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, S.; Yu, R.; Liu, Z.; Gao, H.; Yue, B.; Liu, X.; Zheng, X.; Gao, M.; Wei, X. An efficient deep convolutional neural network model for visual localization and automatic diagnosis of thyroid nodules on ultrasound images. Quant. Imaging Med. Surg. 2021, 11, 1368. [Google Scholar] [CrossRef]
- Zhao, C.K.; Ren, T.T.; Yin, Y.F.; Shi, H.; Wang, H.X.; Zhou, B.Y.; Wang, X.R.; Li, X.; Zhang, Y.F.; Liu, C.; et al. A comparative analysis of two machine learning-based diagnostic patterns with thyroid imaging reporting and data system for thyroid nodules: Diagnostic performance and unnecessary biopsy rate. Thyroid 2021, 31, 470–481. [Google Scholar] [CrossRef]
- Han, M.; Ha, E.; Park, J. Computer-aided diagnostic system for thyroid nodules on ultrasonography: Diagnostic performance based on the thyroid imaging reporting and data system classification and dichotomous outcomes. Am. J. Neuroradiol. 2021, 42, 559–565. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, S.; Liu, Q.; Xie, C.; Dai, Y.; Peng, C.; Chen, X.; Zou, R. Thyroid nodule recognition using a joint convolutional neural network with information fusion of ultrasound images and radiofrequency data. Eur. Radiol. 2021, 31, 5001–5011. [Google Scholar] [CrossRef]
- Wei, Q.; Zeng, S.E.; Wang, L.P.; Yan, Y.J.; Wang, T.; Xu, J.W.; Zhang, M.Y.; Lv, W.Z.; Cui, X.W.; Dietrich, C.F. The value of S-Detect in improving the diagnostic performance of radiologists for the differential diagnosis of thyroid nodules. Med. Ultrason. 2020, 22, 415–423. [Google Scholar] [CrossRef]
- Stib, M.T.; Pan, I.; Merck, D.; Middleton, W.D.; Beland, M.D. Thyroid Nodule Malignancy Risk Stratification Using a Convolutional Neural Network. Ultrasound Q. 2020, 36, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Hang, J.; Chen, X.; Xu, D.; Chen, J.; Ye, X.; Zhang, D. An intelligent platform for ultrasound diagnosis of thyroid nodules. Sci. Rep. 2020, 10, 13223. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.; Kim, Y.J.; Han, K.; Lee, E.; Kim, H.J.; Shin, J.H.; Moon, H.J.; Youk, J.H.; Kim, K.G.; Kwak, J.Y. Application of machine learning to ultrasound images to differentiate follicular neoplasms of the thyroid gland. Ultrasonography 2020, 39, 257. [Google Scholar] [CrossRef] [PubMed]
- Park, V.Y.; Han, K.; Seong, Y.K.; Park, M.H.; Kim, E.K.; Moon, H.J.; Yoon, J.H.; Kwak, J.Y. Diagnosis of thyroid nodules: Performance of a deep learning convolutional neural network model vs. radiologists. Sci. Rep. 2019, 9, 110335. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Yao, J.; Zhou, W.; Dong, Y.; Xu, S.; Zhou, J.; Zhan, W. A computer-aided diagnosing system in the evaluation of thyroid nodules–experience in a specialized thyroid center. World J. Surg. Oncol. 2019, 17, 210. [Google Scholar] [CrossRef]
- Chen, D.; Shi, C.; Wang, M.; Pan, Q. Thyroid Nodule Classification Using Hierarchical Recurrent Neural Network with Multiple Ultrasound Reports. In Proceedings of the International Conference on Neural Information Processing, Guangzhou, China, 14–18 November 2017; Springer: New York, NY, USA, 2017; pp. 765–773. [Google Scholar]
- Smolensky, P.; McClelland, J.L. Information Processing in Dynamical Systems: Foundations of Harmony Theory; MIT Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Vairale, V.S.; Shukla, S. Physical Fitness Recommender Framework for Thyroid Patients using Restricted Boltzmann Machines. Int. J. Intell. Eng. Syst. 2020, 13, 247–256. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, J.; Qiang, Y.; Yang, X.; Dong, Y.; Du, Q.; Shi, G.; Zia, M.B. DScGANS: Integrate domain knowledge in training dual-path semi-supervised conditional generative adversarial networks and s3vm for ultrasonography thyroid nodules classification. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Shenzhen, China, 13–17 October 2019; Springer: New York, NY, USA, 2019; pp. 558–566. [Google Scholar]
- Yoo, T.K.; Choi, J.Y.; Kim, H.K. A generative adversarial network approach to predicting postoperative appearance after orbital decompression surgery for thyroid eye disease. Comput. Biol. Med. 2020, 118, 103628. [Google Scholar] [CrossRef]
- Liu, Y.I.; Kamaya, A.; Desser, T.S.; Rubin, D.L. A bayesian network for differentiating benign from malignant thyroid nodules using sonographic and demographic features. Am. J. Roentgenol. 2011, 196, W598–W605. [Google Scholar] [CrossRef]
- Liu, Y.I.; Kamaya, A.; Desser, T.S.; Rubin, D.L. A controlled vocabulary to represent sonographic features of the thyroid and its application in a Bayesian network to predict thyroid nodule malignancy. Summit Transl. Bioinform. 2009, 2009, 68. [Google Scholar]
- Ashraf, M.; Chetty, G.; Tran, D.; Sharma, D. Hybrid approach for diagnosing thyroid, hepatitis, and breast cancer based on correlation based feature selection and Naïve bayes. In Proceedings of the International Conference on Neural Information Processing, Doha, Qatar, 12–15 November 2012; Springer: New York, NY, USA, 2012; pp. 272–280. [Google Scholar]
- Chandran, V.; Sumithra, M.; Karthick, A.; George, T.; Deivakani, M.; Elakkiya, B.; Subramaniam, U.; Manoharan, S. Diagnosis of Cervical Cancer based on Ensemble Deep Learning Network using Colposcopy Images. BioMed Res. Int. 2021, 2021, 5584004. [Google Scholar] [CrossRef]
- Awujoola Olalekan, J.; Ogwueleka, F.; Odion, P. Effective and Accurate Bootstrap Aggregating (Bagging) Ensemble Algorithm Model for Prediction and Classification of Hypothyroid Disease. Int. J. Comput. Appl. 2020, 975, 8887. [Google Scholar]
- Chen, D.; Hu, J.; Zhu, M.; Tang, N.; Yang, Y.; Feng, Y. Diagnosis of thyroid nodules for ultrasonographic characteristics indicative of malignancy using random forest. BioData Min. 2020, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Himeur, Y.; Ghanem, K.; Alsalemi, A.; Bensaali, F.; Amira, A. Artificial intelligence based anomaly detection of energy consumption in buildings: A review, current trends and new perspectives. Appl. Energy 2021, 287, 116601. [Google Scholar] [CrossRef]
- Pan, Q.; Zhang, Y.; Zuo, M.; Xiang, L.; Chen, D. Improved ensemble classification method of thyroid disease based on random forest. In Proceedings of the 2016 8th International Conference on Information Technology in Medicine and Education (ITME), Fuzhou, China, 23–25 December 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 567–571. [Google Scholar]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd ACM Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Lim, S.; Chi, S. Xgboost application on bridge management systems for proactive damage estimation. Adv. Eng. Inform. 2019, 41, 100922. [Google Scholar] [CrossRef]
- Ji, C.; Zou, X.; Hu, Y.; Liu, S.; Lyu, L.; Zheng, X. XG-SF: An XGBoost classifier based on shapelet features for time series classification. Procedia Comput. Sci. 2019, 147, 24–28. [Google Scholar] [CrossRef]
- Guo, J.; Yang, L.; Bie, R.; Yu, J.; Gao, Y.; Shen, Y.; Kos, A. An XGBoost-based physical fitness evaluation model using advanced feature selection and Bayesian hyper-parameter optimization for wearable running monitoring. Comput. Netw. 2019, 151, 166–180. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Huang, H.; Peng, C.; Ge, Y.; Wu, H.; Wang, J.; Xiong, G.; Yi, Y. Extreme gradient boosting model has a better performance in predicting the risk of 90-day readmissions in patients with ischaemic stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 104441. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Zhang, X.; Jin, J.; Shen, Y. Computer aided diagnosis of thyroid nodules based on the devised small-datasets multi-view ensemble learning. Med. Image Anal. 2020, 67, 101819. [Google Scholar] [CrossRef]
- Thomas, J.; Haertling, T. AIBx, artificial intelligence model to risk stratify thyroid nodules. Thyroid 2020, 30, 878–884. [Google Scholar] [CrossRef]
- Kezlarian, B.; Lin, O. Artificial Intelligence in Thyroid Fine Needle Aspiration Biopsies. Acta Cytol. 2020, 65, 324–329. [Google Scholar] [CrossRef]
- Sanyal, P.; Mukherjee, T.; Barui, S.; Das, A.; Gangopadhyay, P. Artificial intelligence in cytopathology: A neural network to identify papillary carcinoma on thyroid fine-needle aspiration cytology smears. J. Pathol. Inform. 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lee, E.; Koo, J.S.; Yoon, J.H.; Nam, K.H.; Lee, J.; Jo, Y.S.; Moon, H.J.; Park, V.Y.; Kwak, J.Y. Artificial intelligence to predict the BRAFV600E mutation in patients with thyroid cancer. PLoS ONE 2020, 15, e0242806. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kang, J.K.; Pham, T.D.; Batchuluun, G.; Park, K.R. Ultrasound image-based diagnosis of malignant thyroid nodule using artificial intelligence. Sensors 2020, 20, 1822. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xie, S.; Yu, J.; Niu, L.; Sun, W. Classification of thyroid nodules in ultrasound images using deep model based transfer learning and hybrid features. In Proceedings of the 2017 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), New Orleans, LA, USA, 5–9 March 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 919–923. [Google Scholar]
- Abdolali, F.; Kapur, J.; Jaremko, J.L.; Noga, M.; Hareendranathan, A.R.; Punithakumar, K. Automated thyroid nodule detection from ultrasound imaging using deep convolutional neural networks. Comput. Biol. Med. 2020, 122, 103871. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Wei, X.; Zhu, J.; Yu, R.; Zhao, M.; Yu, M.; Liu, Z.; Liu, S. Fully convolutional networks for ultrasound image segmentation of thyroid nodules. In Proceedings of the 2018 IEEE 20th International Conference on High Performance Computing and Communications; IEEE 16th International Conference on Smart City; IEEE 4th International Conference on Data Science and Systems (HPCC/SmartCity/DSS), Exeter, UK, 28–30 June 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 886–890. [Google Scholar]
- Kim, E.; Corte-Real, M.; Baloch, Z. A deep semantic mobile application for thyroid cytopathology. In Medical Imaging 2016: PACS and Imaging Informatics: Next Generation and Innovations. International Society for Optics and Photonics; SPIE: Bellingham, WA, USA, 2016; Volume 9789, p. 97890A. [Google Scholar]
- Ma, L.; Ma, C.; Liu, Y.; Wang, X. Thyroid diagnosis from SPECT images using convolutional neural network with optimization. Comput. Intell. Neurosci. 2019, 2019, 6212759. [Google Scholar] [CrossRef]
- Chai, Y.; Song, J.; Shear, M. Artificial Intelligence for thyroid nodule ultrasound image analysis. Ann. Thyroid. 2020, 5, 8. [Google Scholar] [CrossRef]
- Song, J.; Chai, Y.J.; Masuoka, H.; Park, S.W.; Kim, S.J.; Choi, J.Y.; Kong, H.J.; Lee, K.E.; Lee, J.; Kwak, N.; et al. Ultrasound image analysis using deep learning algorithm for the diagnosis of thyroid nodules. Medicine 2019, 98, e15133. [Google Scholar] [CrossRef]
- Barczyński, M.; Stopa-Barczyńska, M.; Wojtczak, B.; Czarniecka, A.; Konturek, A. Clinical validation of S-DetectTM mode in semi-automated ultrasound classification of thyroid lesions in surgical office. Gland. Surg. 2020, 9, S77. [Google Scholar] [CrossRef]
- Choi, Y.J.; Baek, J.H.; Park, H.S.; Shim, W.H.; Kim, T.Y.; Shong, Y.K.; Lee, J.H. A computer-aided diagnosis system using artificial intelligence for the diagnosis and characterization of thyroid nodules on ultrasound: Initial clinical assessment. Thyroid 2017, 27, 546–552. [Google Scholar] [CrossRef]
- Fragopoulos, C.; Pouliakis, A.; Meristoudis, C.; Mastorakis, E.; Margari, N.; Chroniaris, N.; Koufopoulos, N.; Delides, A.G.; Machairas, N.; Ntomi, V.; et al. Radial Basis Function Artificial Neural Network for the Investigation of Thyroid Cytological Lesions. J. Thyroid. Res. 2020, 2020, 5464787. [Google Scholar] [CrossRef]
- Savala, R.; Dey, P.; Gupta, N. Artificial neural network model to distinguish follicular adenoma from follicular carcinoma on fine needle aspiration of thyroid. Diagn. Cytopathol. 2018, 46, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Li, L.R.; Du, B.; Liu, H.Q.; Chen, C. Artificial Intelligence for Personalized Medicine in Thyroid Cancer: Current Status and Future Perspectives. Front. Oncol. 2021, 10, 3360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, L.; Mao, T.; Zhong, L. Assessment of risk based on variant pathways and establishment of an artificial neural network model of thyroid cancer. BMC Med. Genet. 2019, 20, 92. [Google Scholar] [CrossRef]
- Wildman-Tobriner, B.; Buda, M.; Hoang, J.K.; Middleton, W.D.; Thayer, D.; Short, R.G.; Tessler, F.N.; Mazurowski, M.A. Using artificial intelligence to revise ACR TI-RADS risk stratification of thyroid nodules: Diagnostic accuracy and utility. Radiology 2019, 292, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, S.; Yang, S.; Zhao, C.; Tian, G.; Gao, Y.; Chen, Y.; Lu, Y. Automatic thyroid nodule recognition and diagnosis in ultrasound imaging with the YOLOv2 neural network. World J. Surg. Oncol. 2019, 17, 12. [Google Scholar] [CrossRef]
- Ozolek, J.A.; Tosun, A.B.; Wang, W.; Chen, C.; Kolouri, S.; Basu, S.; Huang, H.; Rohde, G.K. Accurate diagnosis of thyroid follicular lesions from nuclear morphology using supervised learning. Med. Image Anal. 2014, 18, 772–780. [Google Scholar] [CrossRef]
- Wang, W.; Ozolek, J.A.; Rohde, G.K. Detection and classification of thyroid follicular lesions based on nuclear structure from histopathology images. Cytom. Part A J. Int. Soc. Adv. Cytom. 2010, 77, 485–494. [Google Scholar] [CrossRef]
- Zhu, Y.; Sang, Q.; Jia, S.; Wang, Y.; Deyer, T. Deep neural networks could differentiate Bethesda class III versus class IV/V/VI. Ann. Transl. Med. 2019, 7, 231. [Google Scholar] [CrossRef]
- Bhalla, S.; Kaur, H.; Kaur, R.; Sharma, S.; Raghava, G.P. Expression based biomarkers and models to classify early and late-stage samples of Papillary Thyroid Carcinoma. PLoS ONE 2020, 15, e0231629. [Google Scholar] [CrossRef]
- Dolezal, J.M.; Trzcinska, A.; Liao, C.Y.; Kochanny, S.; Blair, E.; Agrawal, N.; Keutgen, X.M.; Angelos, P.; Cipriani, N.A.; Pearson, A.T. Deep learning prediction of BRAF-RAS gene expression signature identifies noninvasive follicular thyroid neoplasms with papillary-like nuclear features. Mod. Pathol. 2020, 34, 862–874. [Google Scholar] [CrossRef]
- Daniels, K.; Gummadi, S.; Zhu, Z.; Wang, S.; Patel, J.; Swendseid, B.; Lyshchik, A.; Curry, J.; Cottrill, E.; Eisenbrey, J. Machine learning by ultrasonography for genetic risk stratification of thyroid nodules. JAMA Otolaryngol.–Head Neck Surg. 2020, 146, 36–41. [Google Scholar] [CrossRef]
- Teumer, A.; Chaker, L.; Groeneweg, S.; Li, Y.; Di Munno, C.; Barbieri, C.; Schultheiss, U.T.; Traglia, M.; Ahluwalia, T.S.; Akiyama, M.; et al. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat. Commun. 2018, 9, 4455. [Google Scholar] [CrossRef] [PubMed]
- The ThyroidOmics Consortium. Available online: https://transfer.sysepi.medizin.uni-greifswald.de/thyroidomics/ (accessed on 1 March 2021).
- Thyroid Disease Data Set. Available online: https://archive.ics.uci.edu/ml/datasets/thyroid+disease (accessed on 1 March 2021).
- Knowledge Extraction Based on Evolutionary Learning. Available online: https://sci2s.ugr.es/keel/dataset.php?cod=67 (accessed on 1 March 2021).
- Gene Expression Omnibus. Available online: https://www.ncbi.nlm.nih.gov/geo/ (accessed on 1 March 2021).
- The Digital Database of Thyroid Ultrasound Images. Available online: http://cimalab.intec.co/?lang=en&mod=project&id=31 (accessed on 1 March 2021).
- The National Cancer Registration and Analysis Service. Available online: http://www.ncin.org.uk/about_ncin/ (accessed on 1 March 2021).
- The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Available online: https://prevention.cancer.gov/major-programs/prostate-lung-colorectal-and-ovarian-cancer-screening-trial (accessed on 1 March 2021).
- Ouyang, F.S.; Guo, B.L.; Ouyang, L.Z.; Liu, Z.W.; Lin, S.J.; Meng, W.; Huang, X.Y.; Chen, H.X.; Qiu-Gen, H.; Yang, S.M. Comparison between linear and nonlinear machine-learning algorithms for the classification of thyroid nodules. Eur. J. Radiol. 2019, 113, 251–257. [Google Scholar] [CrossRef]
- Moon, J.H.; Steinhubl, S.R. Digital medicine in thyroidology: A new era of managing thyroid disease. Endocrinol. Metab. 2019, 34, 124–131. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Chang, Q.; Liu, T.; Zhang, S.; Wang, X.; Guo, Q.; Yao, J.; Sun, W.; Niu, L. Evaluation of a deep learning-based computer-aided diagnosis system for distinguishing benign from malignant thyroid nodules in ultrasound images. Med. Phys. 2020, 47, 3952–3960. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, K.; Wan, P. A method of ultrasonic image recognition for thyroid papillary carcinoma based on deep convolution neural network. NeuroQuantology 2018, 16, 757–768. [Google Scholar] [CrossRef]
- Xu, L.; Gao, J.; Wang, Q.; Yin, J.; Yu, P.; Bai, B.; Pei, R.; Chen, D.; Yang, G.; Wang, S.; et al. Computer-aided diagnosis systems in diagnosing malignant thyroid nodules on ultrasonography: A systematic review and meta-analysis. Eur. Thyroid. J. 2020, 9, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, K.; Zhang, L.; Wei, C.; Yang, P.; Xu, W. Classification of Thyroid Nodules with Stacked Denoising Sparse Autoencoder. Int. J. Endocrinol. 2020, 2020, 9015713. [Google Scholar] [CrossRef]
- Ko, S.Y.; Lee, J.H.; Yoon, J.H.; Na, H.; Hong, E.; Han, K.; Jung, I.; Kim, E.K.; Moon, H.J.; Park, V.Y.; et al. Deep convolutional neural network for the diagnosis of thyroid nodules on ultrasound. Head Neck 2019, 41, 885–891. [Google Scholar] [CrossRef]
- Lee, C.; Kim, Y.; Kim, Y.S.; Jang, J. Automatic disease annotation from radiology reports using artificial intelligence implemented by a recurrent neural network. Am. J. Roentgenol. 2019, 212, 734–740. [Google Scholar] [CrossRef]
- Sharifi, A.; Alizadeh, K. Comparison of the Particle Swarm Optimization with the Genetic Algorithms as a Training for Multilayer Perceptron Technique to Diagnose Thyroid Functional Disease. Shiraz E-Med. J. 2021, 22, 100351. [Google Scholar] [CrossRef]
- Vairale, V.S.; Shukla, S. Recommendation of Food Items for Thyroid Patients Using Content-Based KNN Method. In Data Science and Security; Springer: New York, NY, USA, 2021; pp. 71–77. [Google Scholar]
- Shen, Y.; Lai, Y.; Xu, D.; Xu, L.; Song, L.; Zhou, J.; Song, C.; Wang, J. Diagnosis of thyroid neoplasm using support vector machine algorithms based on platelet RNA-seq. Endocrine 2020, 72, 758–783. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rao, K.; Liu, J.; Han, C.; Gong, L.; Chong, Y.; Liu, Z.; Xu, X. Machine Learning Algorithms for the Prediction of Central Lymph Node Metastasis in Patients with Papillary Thyroid Cancer. Front. Endocrinol. 2020, 11, 816. [Google Scholar] [CrossRef]
- McDow, A.D.; Roman, B.R.; Saucke, M.C.; Jensen, C.B.; Zaborek, N.; Jennings, J.L.; Davies, L.; Brito, J.P.; Pitt, S.C. Factors associated with physicians’ recommendations for managing low-risk papillary thyroid cancer. Am. J. Surg. 2020, 222, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, D.; Zhang, J. ARB: Knowledge Discovery and Disease Diagnosis on Thyroid Disease Diagnosis integrating Association Rule with Bagging Algorithm. Eng. Lett. 2020, 28, 390–399. [Google Scholar]
- Gao, L.; Ye, M.; Lu, X.; Huang, D. Hybrid method based on information gain and support vector machine for gene selection in cancer classification. Genom. Proteom. Bioinform. 2017, 15, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Fahrudin, T.M.; Syarif, I.; Barakbah, A.R. Data mining approach for breast cancer patient recovery. EMITTER Int. J. Eng. Technol. 2017, 5, 36–71. [Google Scholar] [CrossRef][Green Version]
- Hamsagayathri, P.; Sampath, P. Performance analysis of breast cancer classification using decision tree classifiers. Int. J. Curr. Pharm. Res. 2017, 9, 19–25. [Google Scholar] [CrossRef]
- Chinnaswamy, A.; Srinivasan, R. Hybrid information gain based fuzzy roughset feature selection in cancer microarray data. In Proceedings of the 2017 Innovations in Power and Advanced Computing Technologies (i-PACT), Vellore, India, 21–22 April 2017; IEEE: San Francisco, CA, USA, 2017; pp. 1–6. [Google Scholar]
- Mishra, S.; Mallick, P.K.; Tripathy, H.K.; Bhoi, A.K.; González-Briones, A. Performance Evaluation of a Proposed Machine Learning Model for Chronic Disease Datasets Using an Integrated Attribute Evaluator and an Improved Decision Tree Classifier. Appl. Sci. 2020, 10, 8137. [Google Scholar] [CrossRef]
- Ashraf, M.; Chetty, G.; Tran, D. Feature selection techniques on thyroid, hepatitis, and breast cancer datasets. Int. J. Data Min. Intell. Inf. Technol. Appl. 2013, 3, 1. [Google Scholar]
- Al-Batah, M.; Zaqaibeh, B.; Alomari, S.A.; Alzboon, M.S. Gene Microarray Cancer Classification using Correlation Based Feature Selection Algorithm and Rules Classifiers. Int. J. Online Biomed. Eng. 2019, 15, 62–73. [Google Scholar] [CrossRef]
- Jain, I.; Jain, V.K.; Jain, R. Correlation feature selection based improved-binary particle swarm optimization for gene selection and cancer classification. Appl. Soft Comput. 2018, 62, 203–215. [Google Scholar] [CrossRef]
- Rustam, Z.; Maghfirah, N. Correlated based SVM-RFE as feature selection for cancer classification using microarray databases. In Proceedings of the AIP Conference Proceedings, 3rd International Symposium on Current Progress in Mathematics and Sciences 2017, ISCPMS 2017, Bali, Indonesia, 26–27 July 2017; AIP Publishing LLC: Melville, NY, USA, 2018; Volume 2023, p. 020235. [Google Scholar]
- Cui, L.; Ge, L.; Gan, H.; Liu, X.; Zhang, Y. Ovarian Cancer Identification Based on Feature Weighting for High-throughput Mass Spectrometry Data. J. Syst. Biol. 2018, 1, 1. [Google Scholar]
- Onan, A. A fuzzy-rough nearest neighbor classifier combined with consistency-based subset evaluation and instance selection for automated diagnosis of breast cancer. Expert Syst. Appl. 2015, 42, 6844–6852. [Google Scholar] [CrossRef]
- O’Dea, D.; Bongiovanni, M.; Sykiotis, G.P.; Ziros, P.G.; Meade, A.D.; Lyng, F.M.; Malkin, A. Raman spectroscopy for the preoperative diagnosis of thyroid cancer and its subtypes: An in vitro proof-of-concept study. Cytopathology 2019, 30, 51–60. [Google Scholar] [CrossRef]
- Selaru, F.M.; Yin, J.; Olaru, A.; Mori, Y.; Xu, Y.; Epstein, S.H.; Sato, F.; Deacu, E.; Wang, S.; Sterian, A.; et al. An unsupervised approach to identify molecular phenotypic components influencing breast cancer features. Cancer Res. 2004, 64, 1584–1588. [Google Scholar] [CrossRef]
- Sudarshan, V.K.; Mookiah, M.R.K.; Acharya, U.R.; Chandran, V.; Molinari, F.; Fujita, H.; Ng, K.H. Application of wavelet techniques for cancer diagnosis using ultrasound images: A review. Comput. Biol. Med. 2016, 69, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Haji, S.O.; Yousif, R.Z. A Novel Run-length based wavelet features for Screening Thyroid Nodule Malignancy. Braz. Arch. Biol. Technol. 2019, 62, e19170821. [Google Scholar] [CrossRef]
- Yu, B.; Wang, Z.; Zhu, R.; Feng, X.; Qi, M.; Li, J.; Zhao, R.; Huang, L.; Xin, R.; Li, F.; et al. The transverse ultrasonogram of thyroid papillary carcinoma has a better prediction accuracy than the longitudinal one. IEEE Access 2019, 7, 100763–100770. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Pham, T.D.; Batchuluun, G.; Yoon, H.S.; Park, K.R. Artificial intelligence-based thyroid nodule classification using information from spatial and frequency domains. J. Clin. Med. 2019, 8, 1976. [Google Scholar] [CrossRef]
- Poudel, P.; Illanes, A.; Arens, C.; Hansen, C.; Friebe, M. Active contours extension and similarity indicators for improved 3D segmentation of thyroid ultrasound images. In Proceedings of the Medical Imaging 2017: Imaging Informatics for Healthcare, Research, and Applications. International Society for Optics and Photonics, Orlando, FL, USA, 11–16 February 2017; Volume 10138, p. 1013803. [Google Scholar]
- Poudel, P.; Hansen, C.; Sprung, J.; Friebe, M. 3D segmentation of thyroid ultrasound images using active contours. Curr. Dir. Biomed. Eng. 2016, 2, 467–470. [Google Scholar] [CrossRef]
- Nugroho, H.A.; Nugroho, A.; Choridah, L. Thyroid nodule segmentation using active contour bilateral filtering on ultrasound images. In Proceedings of the 2015 International Conference on Quality in Research (QiR), Lombok, Indonesia, 10–13 August 2015; IEEE: Washington, DC, USA, 2015; pp. 43–46. [Google Scholar]
- Xie, J.; Guo, L.; Zhao, C.; Li, X.; Luo, Y.; Jianwei, L. A Hybrid Deep Learning and Handcrafted Features based Approach for Thyroid Nodule Classification in Ultrasound Images. In Journal of Physics: Conference Series, Proceedings of the 2020 3rd International Conference on Computer Information Science and Artificial Intelligence (CISAI), Inner Mongolia, China, 25–27 September 2020; IOP Publishing: Bristol, UK, 2020; Volume 1693, p. 012160. [Google Scholar]
- Mei, X.; Dong, X.; Deyer, T.; Zeng, J.; Trafalis, T.; Fang, Y. Thyroid nodule benignty prediction by deep feature extraction. In Proceedings of the 2017 IEEE 17th International Conference on Bioinformatics and Bioengineering (BIBE), Washington, DC, USA, 23–25 October 2017; IEEE: Washington, DC, USA, 2017; pp. 241–245. [Google Scholar]
- Song, G.; Xue, F.; Zhang, C. A model using texture features to differentiate the nature of thyroid nodules on sonography. J. Ultrasound Med. 2015, 34, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Dinčić, M.; Todorović, J.; Ostojić, J.N.; Kovačević, S.; Dunđerović, D.; Lopičić, S.; Spasić, S.; Radojević-Škodrić, S.; Stanisavljević, D.; Ilić, A.Ž. The fractal and GLCM textural parameters of chromatin may be potential biomarkers of papillary thyroid carcinoma in Hashimoto’s thyroiditis specimens. Microsc. Microanal. 2020, 26, 717–730. [Google Scholar] [CrossRef]
- Kalaimani, I. Analysis for the Prediction of Thyroid Disease by Using ICA and Optimal Kernel SVM Approach. Int. J. Emerg. Technol. Innov. Eng. 2019, 5, 39–55. [Google Scholar]
- Ahmad, W.; Huang, L.; Ahmad, A.; Shah, F.; Iqbal, A.; Saeed, A. Thyroid diseases forecasting using a hybrid decision support system based on ANFIS, k-NN and information gain method. J. Appl. Environ. Biol. Sci. 2017, 7, 78–85. [Google Scholar]
- Zulfanahri; Nugroho, H.A.; Nugroho, A.; Frannita, E.L.; Ardiyanto, I. Classification of thyroid ultrasound images based on shape features analysis. In Proceedings of the 2017 10th Biomedical Engineering International Conference (BMEiCON), Hokkaido, Japan, 31 August–2 September 2017; IEEE: Washington, DC, USA, 2017; pp. 1–5. [Google Scholar]
- Song, H.; Dong, C.; Zhang, X.; Wu, W.; Chen, C.; Ma, B.; Chen, F.; Chen, C.; Lv, X. Rapid identification of papillary thyroid carcinoma and papillary microcarcinoma based on serum Raman spectroscopy combined with machine learning models. Photodiagn. Photodyn. Ther. 2022, 37, 102647. [Google Scholar] [CrossRef]
- Acharya, U.R.; Faust, O.; Sree, S.V.; Molinari, F.; Suri, J.S. ThyroScreen system: High resolution ultrasound thyroid image characterization into benign and malignant classes using novel combination of texture and discrete wavelet transform. Comput. Methods Programs Biomed. 2012, 107, 233–241. [Google Scholar] [CrossRef]
- Nugroho, H.A.; Zulfanahri; Frannita, E.L.; Ardiyanto, I.; Choridah, L. Computer aided diagnosis for thyroid cancer system based on internal and external characteristics. J. King Saud Univ.-Comput. Inf. Sci. 2021, 33, 329–339. [Google Scholar]
- Liu, C.; Chen, S.; Yang, Y.; Shao, D.; Peng, W.; Wang, Y.; Chen, Y.; Wang, Y. The value of the computer-aided diagnosis system for thyroid lesions based on computed tomography images. Quant. Imaging Med. Surg. 2019, 9, 642. [Google Scholar] [CrossRef]
- Erfurt, J.; Helmrich, C.R.; Bosse, S.; Schwarz, H.; Marpe, D.; Wiegand, T. A study of the perceptually weighted peak signal-to-noise ratio (WPSNR) for image compression. In Proceedings of the 2019 IEEE International Conference on Image Processing (ICIP), Taipei, Taiwan, 22–29 September 2019; IEEE: Washington, DC, USA, 2019; pp. 2339–2343. [Google Scholar]
- Chandler, D.M.; Hemami, S.S. VSNR: A wavelet-based visual signal-to-noise ratio for natural images. IEEE Trans. Image Process. 2007, 16, 2284–2298. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Xie, W. Weighted signal-to-noise ratio robust design for a new double sampling npx chart. Comput. Ind. Eng. 2020, 139, 106124. [Google Scholar] [CrossRef]
- Lasseck, M. Audio-based Bird Species Identification with Deep Convolutional Neural Networks. In Proceedings of the CLEF (Working Notes), Avignon, France, 10–14 September 2018. [Google Scholar]
- Agarwal, A.; Zaitsev, I.; Joachims, T. Counterfactual learning-to-rank for additive metrics and deep models. arXiv 2018, arXiv:1805.00065. [Google Scholar]
- Murphy, P.M. UCI Repository of Machine Learning Databases. 1994. Available online: https://archive.ics.uci.edu/ (accessed on 1 March 2021).
- Bai, Z.; Chang, L.; Yu, R.; Li, X.; Wei, X.; Yu, M.; Liu, Z.; Gao, J.; Zhu, J.; Zhang, Y.; et al. Thyroid nodules risk stratification through deep learning based on ultrasound images. Med. Phys. 2020, 47, 6355–6365. [Google Scholar] [CrossRef] [PubMed]
- Colakoglu, B.; Alis, D.; Yergin, M. Diagnostic value of machine learning-based quantitative texture analysis in differentiating benign and malignant thyroid nodules. J. Oncol. 2019, 2019, 6328329. [Google Scholar] [CrossRef] [PubMed]
- Park, V.Y.; Lee, E.; Lee, H.S.; Kim, H.J.; Yoon, J.; Son, J.; Song, K.; Moon, H.J.; Yoon, J.H.; Kim, G.R.; et al. Combining radiomics with ultrasound-based risk stratification systems for thyroid nodules: An approach for improving performance. Eur. Radiol. 2021, 31, 2405–2413. [Google Scholar] [CrossRef]
- Duc, N.T.; Lee, Y.M.; Park, J.H.; Lee, B. An ensemble deep learning for automatic prediction of papillary thyroid carcinoma using fine needle aspiration cytology. Expert Syst. Appl. 2022, 188, 115927. [Google Scholar] [CrossRef]
- Vadhiraj, V.V.; Simpkin, A.; O’Connell, J.; Singh Ospina, N.; Maraka, S.; O’Keeffe, D.T. Ultrasound image classification of thyroid nodules using machine learning techniques. Medicina 2021, 57, 527. [Google Scholar] [CrossRef]
- Gild, M.L.; Chan, M.; Gajera, J.; Lurie, B.; Gandomkar, Z.; Clifton-Bligh, R.J. Risk stratification of indeterminate thyroid nodules using ultrasound and machine learning algorithms. Clin. Endocrinol. 2022, 96, 646–652. [Google Scholar] [CrossRef]
- Ma, J.; Wu, F.; Zhu, J.; Xu, D.; Kong, D. A pre-trained convolutional neural network based method for thyroid nodule diagnosis. Ultrasonics 2017, 73, 221–230. [Google Scholar] [CrossRef]
- Zhu, Y.; Fu, Z.; Fei, J. An image augmentation method using convolutional network for thyroid nodule classification by transfer learning. In Proceedings of the 2017 3rd IEEE international conference on computer and communications (ICCC), Chengdu, China, 13–16 December 2017; IEEE: Washington, DC, USA, 2017; pp. 1819–1823. [Google Scholar]
- Gao, L.; Liu, R.; Jiang, Y.; Song, W.; Wang, Y.; Liu, J.; Wang, J.; Wu, D.; Li, S.; Hao, A.; et al. Computer-aided system for diagnosing thyroid nodules on ultrasound: A comparison with radiologist-based clinical assessments. Head Neck 2018, 40, 778–783. [Google Scholar] [CrossRef]
- Zuo, D.; Han, L.; Chen, K.; Li, C.; Hua, Z.; Lin, J. Extraction of calcification in ultrasonic images based on convolution neural network. Sheng Xue Gong Cheng Xue Zhi/J. Biomed. Eng./Shengwu Yixue Gongchengxue Zazhi 2018, 35, 679–687. [Google Scholar]
- Kim, Y.J.; Choi, Y.; Hur, S.J.; Park, K.S.; Kim, H.J.; Seo, M.; Lee, M.K.; Jung, S.L.; Jung, C.K. Deep convolutional neural network for classification of thyroid nodules on ultrasound: Comparison of the diagnostic performance with that of radiologists. Eur. J. Radiol. 2022, 152, 110335. [Google Scholar] [CrossRef]
- Lee, J.H.; Ha, E.J.; Kim, J.H. Application of deep learning to the diagnosis of cervical lymph node metastasis from thyroid cancer with CT. Eur. Radiol. 2019, 29, 5452–5457. [Google Scholar] [CrossRef] [PubMed]
- Tsou, P.; Wu, C.J. Mapping driver mutations to histopathological subtypes in papillary thyroid carcinoma: Applying a deep convolutional neural network. J. Clin. Med. 2019, 8, 1675. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, Y.; Chen, W. Application of deep learning in the prediction of benign and malignant thyroid nodules on ultrasound images. IEEE Access 2020, 8, 221468–221480. [Google Scholar] [CrossRef]
- Kwon, S.W.; Choi, I.J.; Kang, J.Y.; Jang, W.I.; Lee, G.H.; Lee, M.C. Ultrasonographic thyroid nodule classification using a deep convolutional neural network with surgical pathology. J. Digit. Imaging 2020, 33, 1202–1208. [Google Scholar] [CrossRef]
- Chan, W.K.; Sun, J.H.; Liou, M.J.; Li, Y.R.; Chou, W.Y.; Liu, F.H.; Chen, S.T.; Peng, S.J. Using deep convolutional neural networks for enhanced ultrasonographic image diagnosis of differentiated thyroid cancer. Biomedicines 2021, 9, 1771. [Google Scholar] [CrossRef]
- Kim, G.; Lee, E.; Kim, H.; Yoon, J.; Park, V.; Kwak, J. Convolutional neural network to stratify the malignancy risk of thyroid nodules: Diagnostic performance compared with the American college of radiology thyroid imaging reporting and data system implemented by experienced radiologists. Am. J. Neuroradiol. 2021, 42, 1513–1519. [Google Scholar] [CrossRef]
- Wu, G.G.; Lv, W.Z.; Yin, R.; Xu, J.W.; Yan, Y.J.; Chen, R.X.; Wang, J.Y.; Zhang, B.; Cui, X.W.; Dietrich, C.F. Deep learning based on ACR TI-RADS can improve the differential diagnosis of thyroid nodules. Front. Oncol. 2021, 11, 575166. [Google Scholar] [CrossRef]
- Jin, Z.; Zhu, Y.; Zhang, S.; Xie, F.; Zhang, M.; Zhang, Y.; Tian, X.; Zhang, J.; Luo, Y.; Cao, J. Ultrasound computer-aided diagnosis (CAD) based on the thyroid imaging reporting and data system (TI-RADS) to distinguish benign from malignant thyroid nodules and the diagnostic performance of radiologists with different diagnostic experience. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e918452-1. [Google Scholar] [CrossRef]
- Wang, X.; Agyekum, E.A.; Ren, Y.; Zhang, J.; Zhang, Q.; Sun, H.; Zhang, G.; Xu, F.; Bo, X.; Lv, W.; et al. A radiomic nomogram for the ultrasound-based evaluation of extrathyroidal extension in papillary thyroid carcinoma. Front. Oncol. 2021, 11, 625646. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wang, H.; Wang, L.; Hu, W.; Sun, X.; Dai, Z.; Zhu, J.; Li, H.; Ge, Y.; Song, B. Radiomics based on multiparametric MRI for extrathyroidal extension feature prediction in papillary thyroid cancer. BMC Med. Imaging 2021, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jin, Y.; Dai, L.; Zhang, M.; Qiu, Y.; Wang, K.; Tian, J.; Zheng, J. Differential diagnosis of benign and malignant thyroid nodules using deep learning radiomics of thyroid ultrasound images. Eur. J. Radiol. 2020, 127, 108992. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhu, J.; Qiu, Q.; Wang, Y.; Bai, T.; Yin, Y. Prediction of immunohistochemistry of suspected thyroid nodules by use of machine learning–based radiomics. Am. J. Roentgenol. 2019, 213, 1348–1357. [Google Scholar] [CrossRef]
- Park, V.Y.; Han, K.; Lee, E.; Kim, E.K.; Moon, H.J.; Yoon, J.H.; Kwak, J.Y. Association between radiomics signature and disease-free survival in conventional papillary thyroid carcinoma. Sci. Rep. 2019, 9, 4501. [Google Scholar] [CrossRef] [PubMed]
- Himeur, Y.; Varlamis, I.; Kheddar, H.; Amira, A.; Atalla, S.; Singh, Y.; Bensaali, F.; Mansoor, W. Federated Learning for Computer Vision. arXiv 2023, arXiv:2308.13558. [Google Scholar]
- Shah, A.A.; Malik, H.A.M.; Muhammad, A.; Alourani, A.; Butt, Z.A. Deep learning ensemble 2D CNN approach towards the detection of lung cancer. Sci. Rep. 2023, 13, 2987. [Google Scholar] [CrossRef]
- Salazar-Vega, J.; Ortiz-Prado, E.; Solis-Pazmino, P.; Gómez-Barreno, L.; Simbaña-Rivera, K.; Henriquez-Trujillo, A.R.; Brito, J.P.; Toulkeridis, T.; Coral-Almeida, M. Thyroid Cancer in Ecuador, a 16 years population-based analysis (2001–2016). BMC Cancer 2019, 19, 294. [Google Scholar] [CrossRef]
- Elmore, L.W.; Greer, S.F.; Daniels, E.C.; Saxe, C.C.; Melner, M.H.; Krawiec, G.M.; Cance, W.G.; Phelps, W.C. Blueprint for cancer research: Critical gaps and opportunities. CA Cancer J. Clin. 2021, 71, 107–139. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, J.; Byeon, J.S. Key principles of clinical validation, device approval, and insurance coverage decisions of artificial intelligence. Korean J. Radiol. 2021, 22, 442. [Google Scholar] [CrossRef]
- Zhu, Y.C.; AlZoubi, A.; Jassim, S.; Jiang, Q.; Zhang, Y.; Wang, Y.B.; Ye, X.D.; Hongbo, D. A generic deep learning framework to classify thyroid and breast lesions in ultrasound images. Ultrasonics 2021, 110, 106300. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Lin, K.Y.; Lin, Y.J.; Khalil, M.A.; Chu, K.L.; Chao, T.K. A soft label deep learning to assist breast cancer target therapy and thyroid cancer diagnosis. Cancers 2022, 14, 5312. [Google Scholar] [CrossRef]
- Al-Qurayshi, Z.; Randolph, G.W.; Kandil, E. Cost-effectiveness of computed tomography nodal scan in patients with papillary thyroid carcinoma. Oral Oncol. 2021, 118, 105326. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Lei, Z.; Yue, W.; Feng, B.; Li, W.; Ou, D.; Feng, N.; Lu, Y.; Xu, J.; Chen, W.; et al. DeepThy-Net: A Multimodal Deep Learning Method for Predicting Cervical Lymph Node Metastasis in Papillary Thyroid Cancer. Adv. Intell. Syst. 2022, 4, 2200100. [Google Scholar] [CrossRef]
- Sayed, A.N.; Himeur, Y.; Bensaali, F. From time-series to 2d images for building occupancy prediction using deep transfer learning. Eng. Appl. Artif. Intell. 2023, 119, 105786. [Google Scholar] [CrossRef]
- Karsa, A.; Punwani, S.; Shmueli, K. An optimized and highly repeatable MRI acquisition and processing pipeline for quantitative susceptibility mapping in the head-and-neck region. Magn. Reson. Med. 2020, 84, 3206–3222. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.I.; Boo, D.; Kim, B.; Choi, I.Y.; Ko, S.; Yoo, I.R.; Kim, K.; Kim, J.; Joo, Y.; et al. Second primary malignancy risk in thyroid cancer and matched patients with and without radioiodine therapy analysis from the observational health data sciences and informatics. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3547–3556. [Google Scholar] [CrossRef]
- Sardianos, C.; Varlamis, I.; Chronis, C.; Dimitrakopoulos, G.; Alsalemi, A.; Himeur, Y.; Bensaali, F.; Amira, A. The emergence of explainability of intelligent systems: Delivering explainable and personalized recommendations for energy efficiency. Int. J. Intell. Syst. 2021, 36, 656–680. [Google Scholar] [CrossRef]
- Masuda, T.; Nakaura, T.; Funama, Y.; Sugino, K.; Sato, T.; Yoshiura, T.; Baba, Y.; Awai, K. Machine learning to identify lymph node metastasis from thyroid cancer in patients undergoing contrast-enhanced CT studies. Radiography 2021, 27, 920–926. [Google Scholar] [CrossRef]
- Dov, D.; Kovalsky, S.; Cohen, J.; Range, D.; Henao, R.; Carin, L. Thyroid cancer malignancy prediction from whole slide cytopathology images. arXiv 2019, arXiv:1904.00839. [Google Scholar]
- Dov, D.; Kovalsky, S.Z.; Cohen, J.; Range, D.E.; Henao, R.; Carin, L. AI-Assisted Thyroid Malignancy Prediction from Whole-Slide Images; Standford University: Palo Alto, CA, USA, 2019. [Google Scholar]
- Halicek, M.; Shahedi, M.; Little, J.V.; Chen, A.Y.; Myers, L.L.; Sumer, B.D.; Fei, B. Head and neck cancer detection in digitized whole-slide histology using convolutional neural networks. Sci. Rep. 2019, 9, 14043. [Google Scholar] [CrossRef]
- Lamy, J.B.; Sekar, B.; Guezennec, G.; Bouaud, J.; Séroussi, B. Explainable artificial intelligence for breast cancer: A visual case-based reasoning approach. Artif. Intell. Med. 2019, 94, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Kobylińska, K.; Mikołajczyk, T.; Adamek, M.; Orłowski, T.; Biecek, P. Explainable machine learning for modeling of early postoperative mortality in lung cancer. In Artificial Intelligence in Medicine: Knowledge Representation and Transparent and Explainable Systems; Springer: New York, NY, USA, 2019; pp. 161–174. [Google Scholar]
- Pintelas, E.; Liaskos, M.; Livieris, I.E.; Kotsiantis, S.; Pintelas, P. Explainable Machine Learning Framework for Image Classification Problems: Case Study on Glioma Cancer Prediction. J. Imaging 2020, 6, 37. [Google Scholar] [CrossRef]
- Lamy, J.B.; Sekar, B.D.; Guezennec, G.; Bouaud, J.; Séroussi, B. Intelligence Artificielle Explicable Pour le Cancer du Sein: Une Approche Visuelle de Raisonnement à Partir de cas; EGC: Bruxelles, Belgique, 2020; pp. 457–466. [Google Scholar]
- Poceviciute, M.; Eilertsen, G.; Lundström, C. Survey of XAI in Digital Pathology. Artif. Intell. Mach. Learn. Digit. Pathol. State-Art Future Challenges 2020, 12090, 56. [Google Scholar]
- Sayed, A.N.; Bensaali, F.; Himeur, Y.; Houchati, M. Edge-Based Real-Time Occupancy Detection System through a Non-Intrusive Sensing System. Energies 2023, 16, 2388. [Google Scholar] [CrossRef]
- Alsalemi, A.; Himeur, Y.; Bensaali, F.; Amira, A. An innovative edge-based internet of energy solution for promoting energy saving in buildings. Sustain. Cities Soc. 2022, 78, 103571. [Google Scholar] [CrossRef]
- Sayed, A.; Himeur, Y.; Alsalemi, A.; Bensaali, F.; Amira, A. Intelligent edge-based recommender system for internet of energy applications. IEEE Syst. J. 2021, 16, 5001–5010. [Google Scholar] [CrossRef]
- Charteros, E.; Koutsopoulos, I. Edge Computing for Having an Edge on Cancer Treatment: A Mobile App for Breast Image Analysis. In Proceedings of the 2020 IEEE International Conference on Communications Workshops (ICC Workshops), Dublin, Ireland, 7–11 June 2020; IEEE: Washington, DC, USA, 2020; pp. 1–6. [Google Scholar]
- Sufian, A.; Ghosh, A.; Sadiq, A.S.; Smarandache, F. A survey on deep transfer learning to edge computing for mitigating the COVID-19 pandemic. J. Syst. Archit. 2020, 108, 101830. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Rong, H.; Bilal, K.; Yang, N.; Li, K. A disease diagnosis and treatment recommendation system based on big data mining and cloud computing. Inf. Sci. 2018, 435, 124–149. [Google Scholar] [CrossRef]
- Chai, X. Diagnosis Method of Thyroid Disease Combining Knowledge Graph and Deep Learning. IEEE Access 2020, 8, 149787–149795. [Google Scholar] [CrossRef]
- Jagtap, P.; Jagdale, P.; Gawade, S.; Javalkar, P. Online Healthcare System Using the Concept of Cloud Computing. Int. J. Sci. Res. Sci. Eng. Technol. IJSRSET 2016, 2, 943–946. [Google Scholar]
- Anuradha, M.; Jayasankar, T.; Prakash, N.; Sikkandar, M.Y.; Hemalakshmi, G.; Bharatiraja, C.; Britto, A.S.F. IoT enabled cancer prediction system to enhance the authentication and security using cloud computing. Microprocess. Microsyst. 2021, 80, 103301. [Google Scholar] [CrossRef]
- Kečo, D.; Subasi, A.; Kevric, J. Cloud computing-based parallel genetic algorithm for gene selection in cancer classification. Neural Comput. Appl. 2018, 30, 1601–1610. [Google Scholar] [CrossRef]
- Rajan, J.P.; Rajan, S.E.; Martis, R.J.; Panigrahi, B.K. Fog computing employed computer aided cancer classification system using deep neural network in internet of things based healthcare system. J. Med. Syst. 2020, 44, 34. [Google Scholar] [CrossRef] [PubMed]
- Mutlag, A.A.; Abd Ghani, M.K.; Arunkumar, N.A.; Mohammed, M.A.; Mohd, O. Enabling technologies for fog computing in healthcare IoT systems. Future Gener. Comput. Syst. 2019, 90, 62–78. [Google Scholar] [CrossRef]
- Hartmann, M.; Hashmi, U.S.; Imran, A. Edge computing in smart health care systems: Review, challenges, and research directions. Trans. Emerg. Telecommun. Technol. 2019, 33, e3710. [Google Scholar] [CrossRef]
- Corchado Rodríguez, J.M. AI, Blockchain and Edge Computing for Industrial Predictive Maintenance. In Proceedings of the 9th Workshop on Service Oriented, Holonic and Multi-Agent Manufacturing Systems for Industry of the Future, SOHOMA 2019, Valencia, Spain, 3–4 October 2019. [Google Scholar]
- Balaprakash, P.; Egele, R.; Salim, M.; Wild, S.; Vishwanath, V.; Xia, F.; Brettin, T.; Stevens, R. Scalable reinforcement-learning-based neural architecture search for cancer deep learning research. In Proceedings of the International Conference for High Performance Computing, Networking, Storage and Analysis, Denver, CO, USA, 17–22 November 2019; pp. 1–33. [Google Scholar]
- Li, Z.; Xia, Y. Deep reinforcement learning for weakly-supervised lymph node segmentation in ct images. IEEE J. Biomed. Health Informatics 2020, 25, 774–783. [Google Scholar] [CrossRef]
- Kerdjidj, O.; Himeur, Y.; Sohail, S.S.; Amira, A.; Fadli, F.; Atalla, S.; Mansoor, W.; Copiaco, A.; Daradkeh, M.; Gawanmeh, A.; et al. Uncovering the Potential of Indoor Localization: Role of Deep and Transfer Learning. Preprints 2023, 2023062249. [Google Scholar] [CrossRef]
- Kheddar, H.; Himeur, Y.; Awad, A.I. Deep Transfer Learning Applications in Intrusion Detection Systems: A Comprehensive Review. arXiv 2023, arXiv:2304.10550. [Google Scholar]
- Himeur, Y.; Al-Maadeed, S.; Kheddar, H.; Al-Maadeed, N.; Abualsaud, K.; Mohamed, A.; Khattab, T. Video surveillance using deep transfer learning and deep domain adaptation: Towards better generalization. Eng. Appl. Artif. Intell. 2023, 119, 105698. [Google Scholar] [CrossRef]
- Kheddar, H.; Himeur, Y.; Al-Maadeed, S.; Amira, A.; Bensaali, F. Deep transfer learning for automatic speech recognition: Towards better generalization. Knowl. Based Syst. 2023, 277, 110851. [Google Scholar] [CrossRef]
- Narayan, V.; Mall, P.K.; Alkhayyat, A.; Abhishek, K.; Kumar, S.; Pandey, P. Enhance-Net: An Approach to Boost the Performance of Deep Learning Model Based on Real-Time Medical Images. J. Sensors 2023, 2023, 8276738. [Google Scholar] [CrossRef]
- Yu, J.; Deng, Y.; Liu, T.; Zhou, J.; Jia, X.; Xiao, T.; Zhou, S.; Li, J.; Guo, Y.; Wang, Y.; et al. Lymph node metastasis prediction of papillary thyroid carcinoma based on transfer learning radiomics. Nat. Commun. 2020, 11, 4807. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, Q.; Lao, I.; Wang, L.; Wu, Y.; Li, D.; Ji, Q.; Wang, Y.; Zhu, Y.; Lu, H.; et al. Using deep convolutional neural networks for multi-classification of thyroid tumor by histopathology: A large-scale pilot study. Ann. Transl. Med. 2019, 7, 468. [Google Scholar] [CrossRef] [PubMed]
- Sundar, K.S.; Sai, S.S.S. Exploring Transfer Learning, Fine-tuning of Thyroid Ultrasound Images. In Proceedings of the 1st Conference on Medical Imaging with Deep Learning (MIDL 2018), Amsterdam, The Netherlands, 4–6 July 2018. [Google Scholar]
- Liu, D.; Zhang, D.; Song, Y.; Zhang, C.; Zhang, F.; O’Donnell, L.; Cai, W. Nuclei Segmentation via a Deep Panoptic Model with Semantic Feature Fusion. In Proceedings of the IJCAI, 2019 International Joint Conference on Artificial Intelligence, Macao, China, 10–16 August 2019; pp. 861–868. [Google Scholar]
- Elharrouss, O.; Al-Maadeed, S.; Subramanian, N.; Ottakath, N.; Almaadeed, N.; Himeur, Y. Panoptic segmentation: A review. arXiv 2021, arXiv:2111.10250. [Google Scholar]
- Yu, X.; Lou, B.; Zhang, D.; Winkel, D.; Arrahmane, N.; Diallo, M.; Meng, T.; von Busch, H.; Grimm, R.; Kiefer, B.; et al. Deep Attentive Panoptic Model for Prostate Cancer Detection Using Biparametric MRI Scans. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Lima, Peru, 4–8 October 2020; Springer: New York, NY, USA, 2020; pp. 594–604. [Google Scholar]
- Cai, W.; Xiong, Z.; Sun, X.; Rosin, P.L.; Jin, L.; Peng, X. Panoptic Segmentation-Based Attention for Image Captioning. Appl. Sci. 2020, 10, 391. [Google Scholar] [CrossRef]
- Ivanova, D. Artificial Intelligence in Internet of Medical Imaging Things: The Power of Thyroid Cancer Detection. In Proceedings of the 2018 International Conference on Information Technologies (InfoTech), Varna, Bulgaria, 20–21 September 2018; IEEE: Washington, DC, USA, 2018; pp. 1–4. [Google Scholar]
- Borovska, P.; Ivanova, D.; Draganov, I. Internet of Medical Imaging Things and Analytics in Support of Precision Medicine for the Case Study of Thyroid Cancer Early Diagnostics. Serdica J. Comput. Bulg. Acad. Sci. Inst. Math. Inform. 2018, 12, 47–64. [Google Scholar]
- Ivanova, D. Internet of Medical Imaging Things and the Application of Information Technologies for Early Detection of Thyroid Cancer. Silico Intellect 2017, 1, 20–25. [Google Scholar]
- Seifert, P.; Ullrich, S.L.; Kühnel, C.; Gühne, F.; Drescher, R.; Winkens, T.; Freesmeyer, M. Optimization of Thyroid Volume Determination by Stitched 3D-Ultrasound Data Sets in Patients with Structural Thyroid Disease. Biomedicines 2023, 11, 381. [Google Scholar] [CrossRef]
- Li, W.B.; Zhang, B.; Zhu, Q.L.; Jiang, Y.X.; Sun, J.; Yang, M.; Li, J.C. Comparison between thin-slice 3-D volumetric ultrasound and conventional ultrasound in the differentiation of benign and malignant thyroid lesions. Ultrasound Med. Biol. 2015, 41, 3096–3101. [Google Scholar] [CrossRef]
- Lyshchik, A.; Drozd, V.; Reiners, C. Accuracy of three-dimensional ultrasound for thyroid volume measurement in children and adolescents. Thyroid 2004, 14, 113–120. [Google Scholar] [CrossRef]
- Ying, M.; Yung, D.M.; Ho, K.K. Two-dimensional ultrasound measurement of thyroid gland volume: A new equation with higher correlation with 3-D ultrasound measurement. Ultrasound Med. Biol. 2008, 34, 56–63. [Google Scholar] [CrossRef]
- Pakkasjärvi, N.; Luthra, T.; Anand, S. Artificial Intelligence in Surgical Learning. Surgeries 2023, 4, 86–97. [Google Scholar] [CrossRef]
- Bodenstedt, S.; Wagner, M.; Müller-Stich, B.P.; Weitz, J.; Speidel, S. Artificial intelligence-assisted surgery: Potential and challenges. Visc. Med. 2020, 36, 450–455. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Guo, Y.; Shen, M.; Yang, G.Z. Artificial intelligence in surgery. arXiv 2019, arXiv:2001.00627. [Google Scholar]
- Zhou, X.Y.; Guo, Y.; Shen, M.; Yang, G.Z. Application of artificial intelligence in surgery. Front. Med. 2020, 14, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Berikol, G.B.; Berikol, G.; Bozdereli, D. Artificial intelligence in neuro, head, and neck surgery. In Artificial Intelligence in Precision Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 393–404. [Google Scholar]
- Tan, L.; Tivey, D.; Kopunic, H.; Babidge, W.; Langley, S.; Maddern, G. Part 1: Artificial intelligence technology in surgery. ANZ J. Surg. 2020, 90, 2409–2414. [Google Scholar] [CrossRef]
- Habal, M.B. Brave New Surgical Innovations: The Impact of Bioprinting, Machine Learning, and Artificial Intelligence in Craniofacial Surgery. J. Craniofac. Surg. 2020, 31, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yu, H.W.; Kwon, H.; Kong, H.J.; Lee, K.E.; Kim, H.C. Evaluation of Surgical Skills during Robotic Surgery by Deep Learning-Based Multiple Surgical Instrument Tracking in Training and Actual Operations. J. Clin. Med. 2020, 9, 1964. [Google Scholar] [CrossRef] [PubMed]
- Voglis, S.; van Niftrik, C.H.; Staartjes, V.E.; Brandi, G.; Tschopp, O.; Regli, L.; Serra, C. Feasibility of machine learning based predictive modelling of postoperative hyponatremia after pituitary surgery. Pituitary 2020, 23, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Welton, A.J.; Hashimoto, D.A. Current applications of artificial intelligence for intraoperative decision support in surgery. Front. Med. 2020, 14, 369381. [Google Scholar] [CrossRef] [PubMed]
- Melarkode, N.; Srinivasan, K.; Qaisar, S.M.; Plawiak, P. AI-Powered Diagnosis of Skin Cancer: A Contemporary Review, Open Challenges and Future Research Directions. Cancers 2023, 15, 1183. [Google Scholar] [CrossRef]
- Kumar, Y.; Koul, A.; Singla, R.; Ijaz, M.F. Artificial intelligence in disease diagnosis: A systematic literature review, synthesizing framework and future research agenda. J. Ambient. Intell. Humaniz. Comput. 2022, 14, 8459–8486. [Google Scholar] [CrossRef]
- Abdolali, F.; Shahroudnejad, A.; Hareendranathan, A.R.; Jaremko, J.L.; Noga, M.; Punithakumar, K. A systematic review on the role of artificial intelligence in sonographic diagnosis of thyroid cancer: Past, present and future. Front. Biomed. Technol. 2020, 7, 266–280. [Google Scholar] [CrossRef]
- Petersson, L.; Larsson, I.; Nygren, J.M.; Nilsen, P.; Neher, M.; Reed, J.E.; Tyskbo, D.; Svedberg, P. Challenges to implementing artificial intelligence in healthcare: A qualitative interview study with healthcare leaders in Sweden. BMC Health Serv. Res. 2022, 22, 850. [Google Scholar] [CrossRef]
- Sarker, I.H. Ai-based modeling: Techniques, applications and research issues towards automation, intelligent and smart systems. SN Comput. Sci. 2022, 3, 158. [Google Scholar] [CrossRef]
- Himeur, Y.; Alsalemi, A.; Al-Kababji, A.; Bensaali, F.; Amira, A.; Sardianos, C.; Dimitrakopoulos, G.; Varlamis, I. A survey of recommender systems for energy efficiency in buildings: Principles, challenges and prospects. Inf. Fusion 2021, 72, 1–21. [Google Scholar] [CrossRef]
- Areeb, Q.M.; Nadeem, M.; Sohail, S.S.; Imam, R.; Doctor, F.; Himeur, Y.; Hussain, A.; Amira, A. Filter bubbles in recommender systems: Fact or fallacy—A systematic review. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2023, e1512. [Google Scholar] [CrossRef]
- Varlamis, I.; Sardianos, C.; Chronis, C.; Dimitrakopoulos, G.; Himeur, Y.; Alsalemi, A.; Bensaali, F.; Amira, A. Smart fusion of sensor data and human feedback for personalized energy-saving recommendations. Appl. Energy 2022, 305, 117775. [Google Scholar] [CrossRef]
- Atalla, S.; Daradkeh, M.; Gawanmeh, A.; Khalil, H.; Mansoor, W.; Miniaoui, S.; Himeur, Y. An Intelligent Recommendation System for Automating Academic Advising Based on Curriculum Analysis and Performance Modeling. Mathematics 2023, 11, 1098. [Google Scholar] [CrossRef]
- Antunes, R.S.; André da Costa, C.; Küderle, A.; Yari, I.A.; Eskofier, B. Federated learning for healthcare: Systematic review and architecture proposal. ACM Trans. Intell. Syst. Technol. (TIST) 2022, 13, 1–23. [Google Scholar] [CrossRef]
- Lee, H.; Chai, Y.J.; Joo, H.; Lee, K.; Hwang, J.Y.; Kim, S.M.; Kim, K.; Nam, I.C.; Choi, J.Y.; Yu, H.W.; et al. Federated learning for thyroid ultrasound image analysis to protect personal information: Validation study in a real health care environment. JMIR Med. Informatics 2021, 9, e25869. [Google Scholar] [CrossRef] [PubMed]
- Sohail, S.S.; Madsen, D.Ø.; Himeur, Y.; Ashraf, M. Using ChatGPT to Navigate Ambivalent and Contradictory Research Findings on Artificial Intelligence. Front. Artif. Intell. 2023, 6, 1195797. [Google Scholar] [CrossRef] [PubMed]
- Farhat, F.; Silva, E.S.; Hassani, H.; Madsen, D.Ø.; Sohail, S.S.; Himeur, Y.; Alam, M.A.; Zafar, A. Analyzing the scholarly footprint of ChatGPT: Mapping the progress and identifying future trends. Preprints 2023, 2023062100. [Google Scholar] [CrossRef]
- Sohail, S.S.; Farhat, F.; Himeur, Y.; Nadeem, M.; Madsen, D.Ø.; Singh, Y.; Atalla, S.; Mansoor, W. The future of gpt: A taxonomy of existing chatgpt research, current challenges, and possible future directions. Curr. Challenges, Possible Future Dir. 2023. [Google Scholar] [CrossRef]
- Haver, H.L.; Ambinder, E.B.; Bahl, M.; Oluyemi, E.T.; Jeudy, J.; Yi, P.H. Appropriateness of Breast Cancer Prevention and Screening Recommendations Provided by ChatGPT. Radiology 2023, 307, e230424. [Google Scholar] [CrossRef]
- Cao, J.J.; Kwon, D.H.; Ghaziani, T.T.; Kwo, P.; Tse, G.; Kesselman, A.; Kamaya, A.; Tse, J.R. Accuracy of Information Provided by ChatGPT Regarding Liver Cancer Surveillance and Diagnosis. Am. J. Roentgenol. 2023, 221, 556–559. [Google Scholar] [CrossRef]
- Sorin, V.; Klang, E.; Sklair-Levy, M.; Cohen, I.; Zippel, D.B.; Balint Lahat, N.; Konen, E.; Barash, Y. Large language model (ChatGPT) as a support tool for breast tumor board. NPJ Breast Cancer 2023, 9, 44. [Google Scholar] [CrossRef]
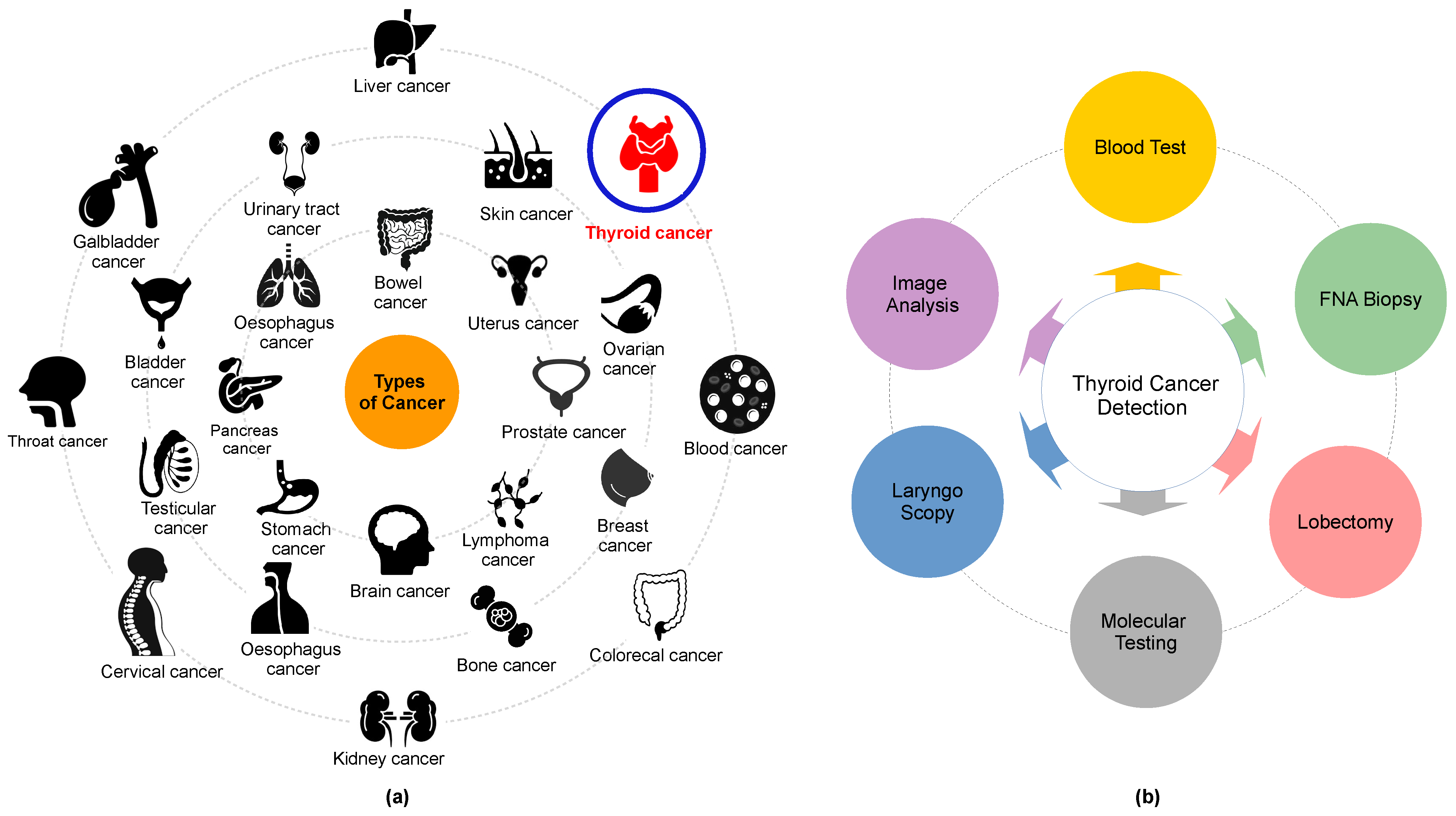
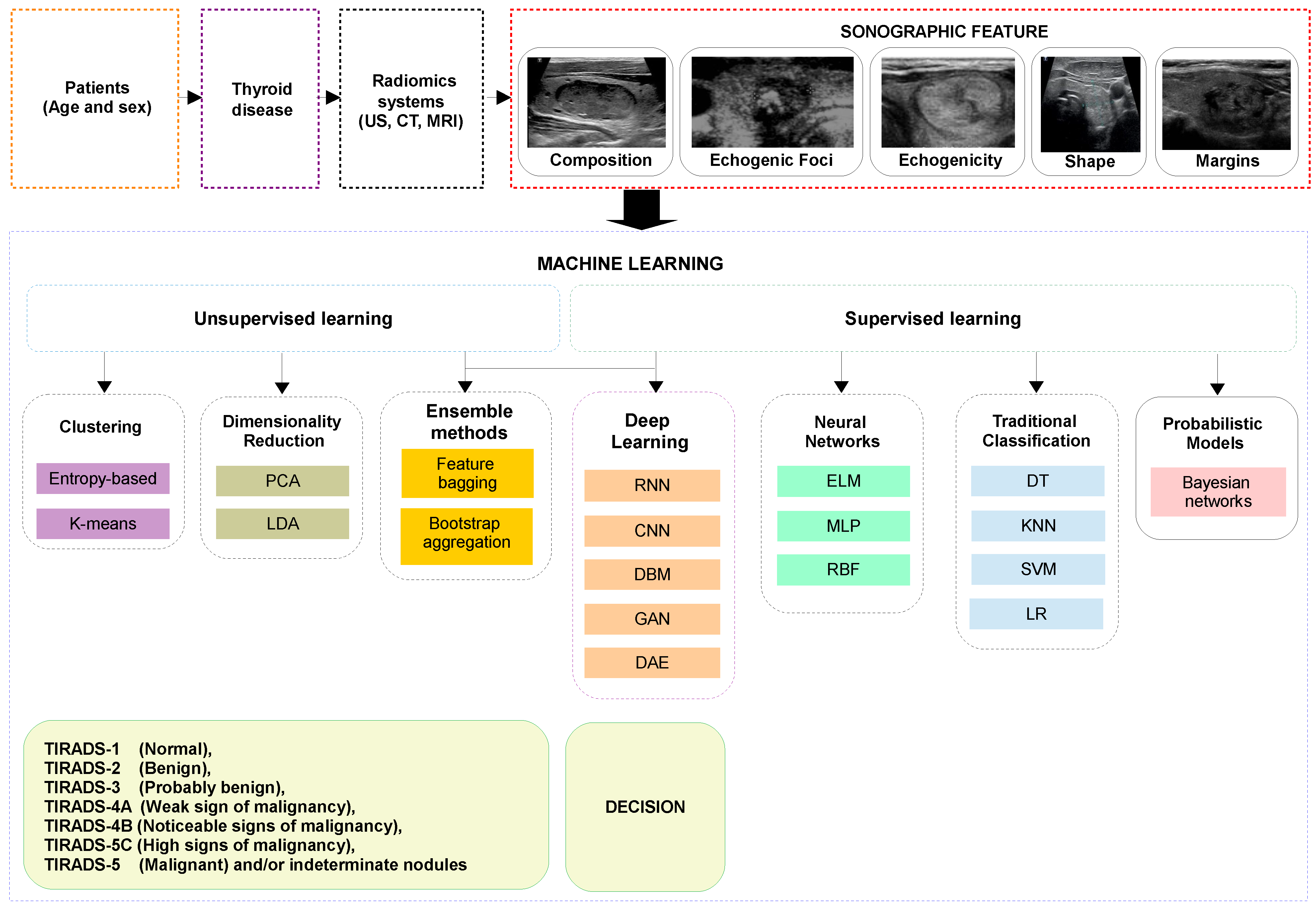
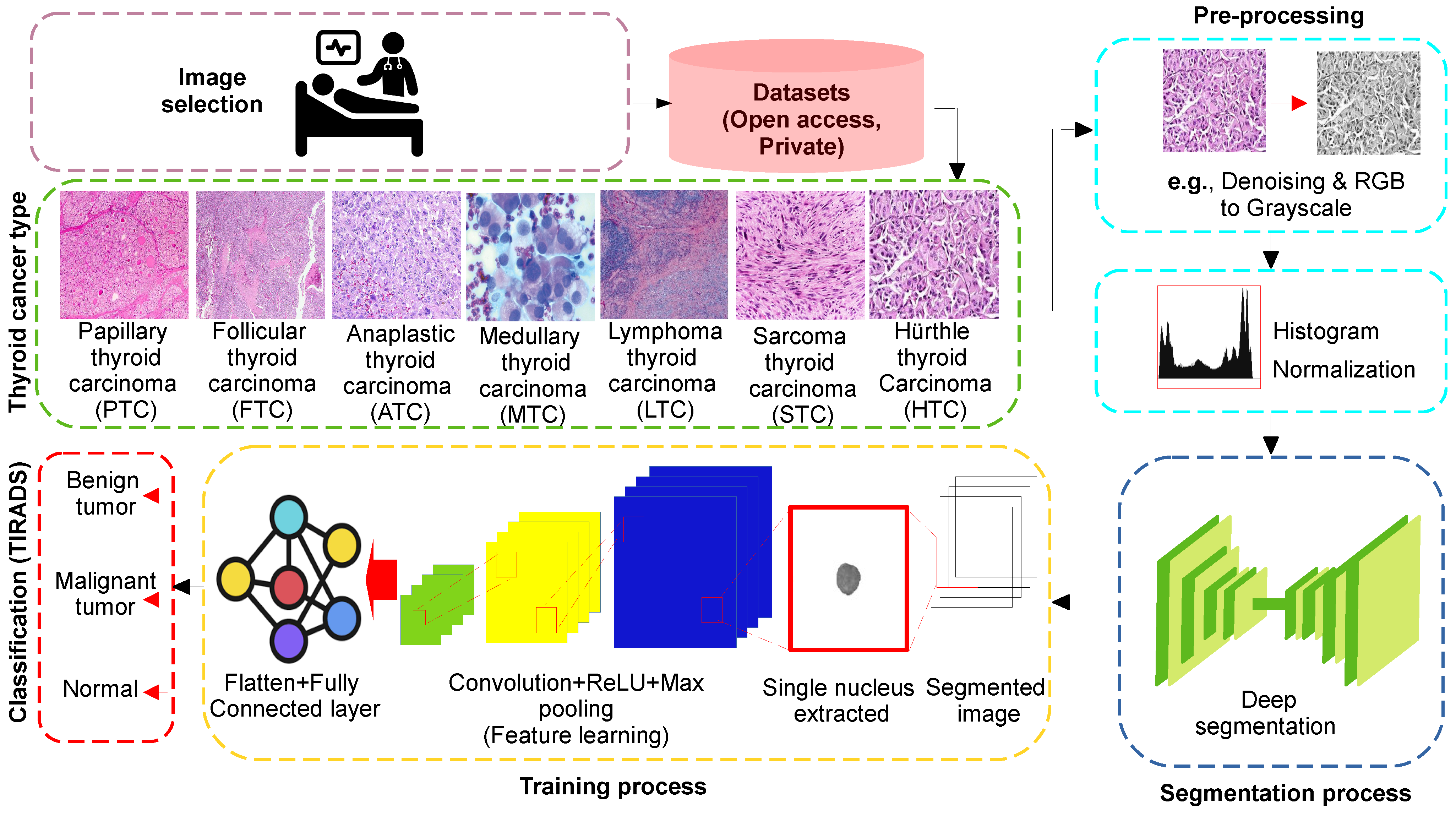


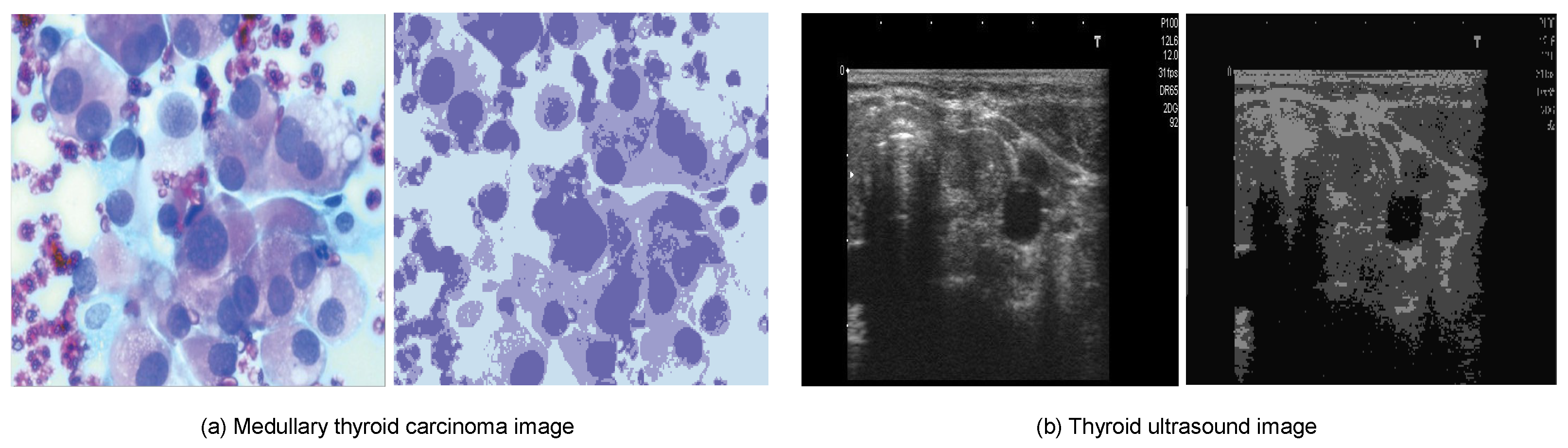
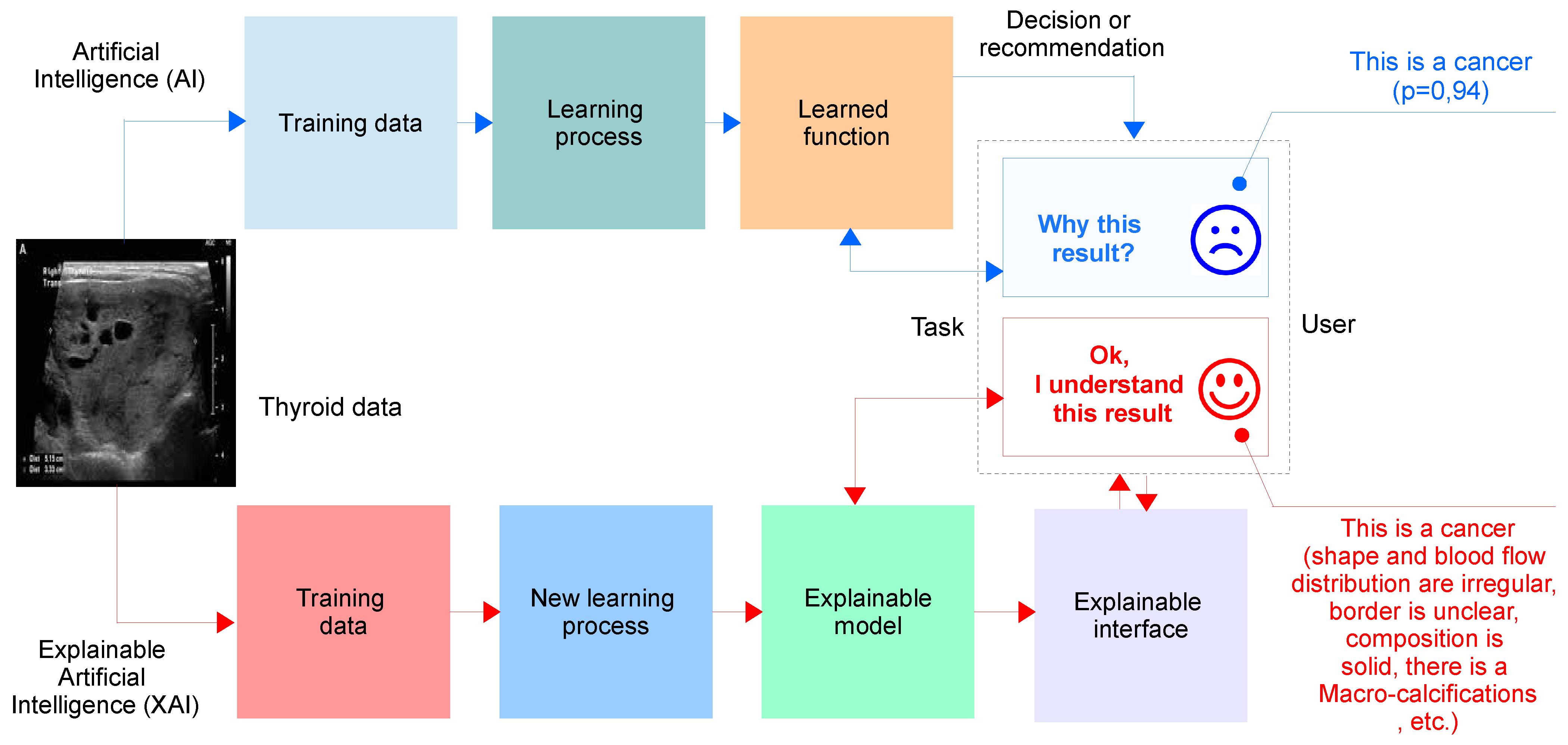


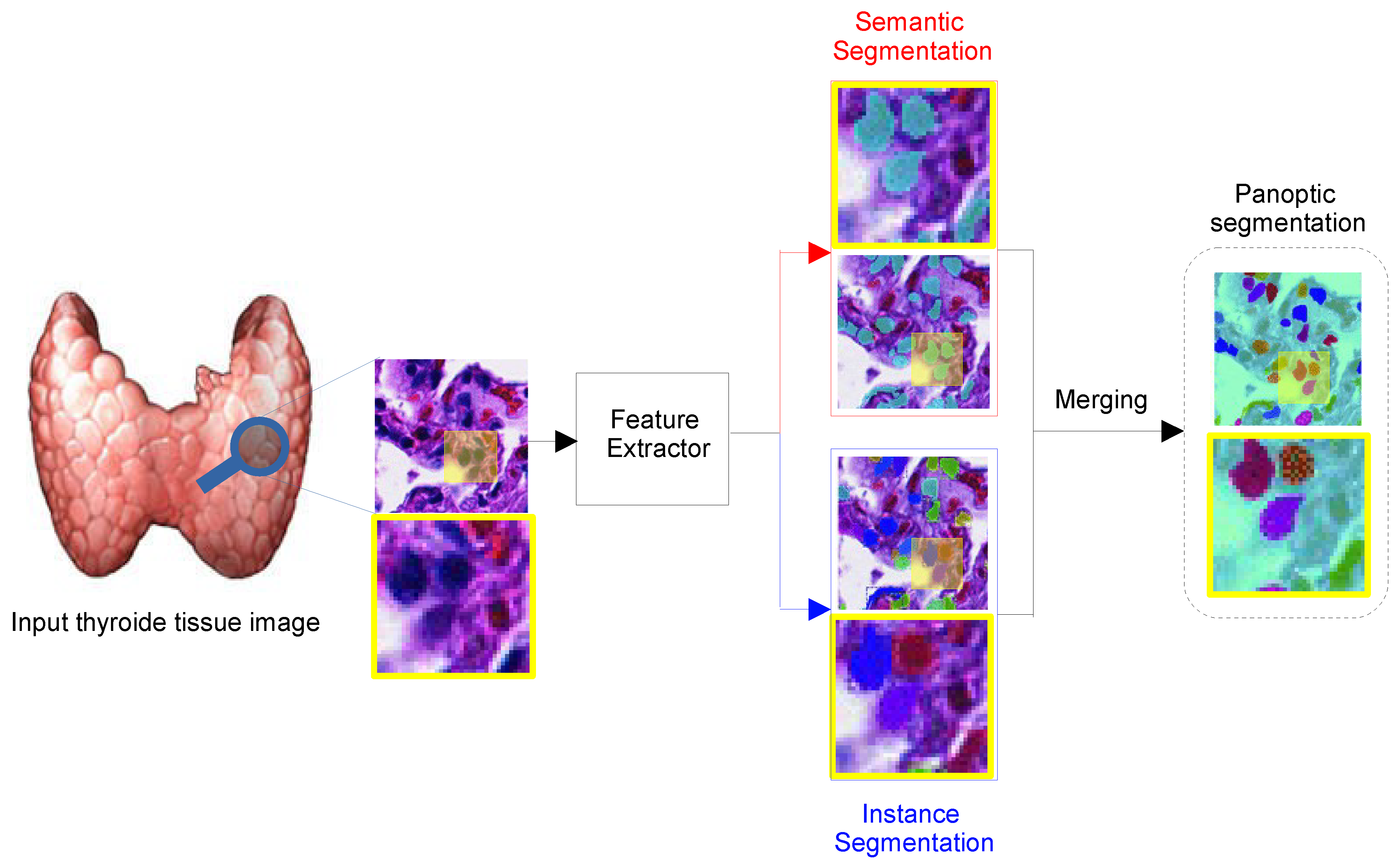
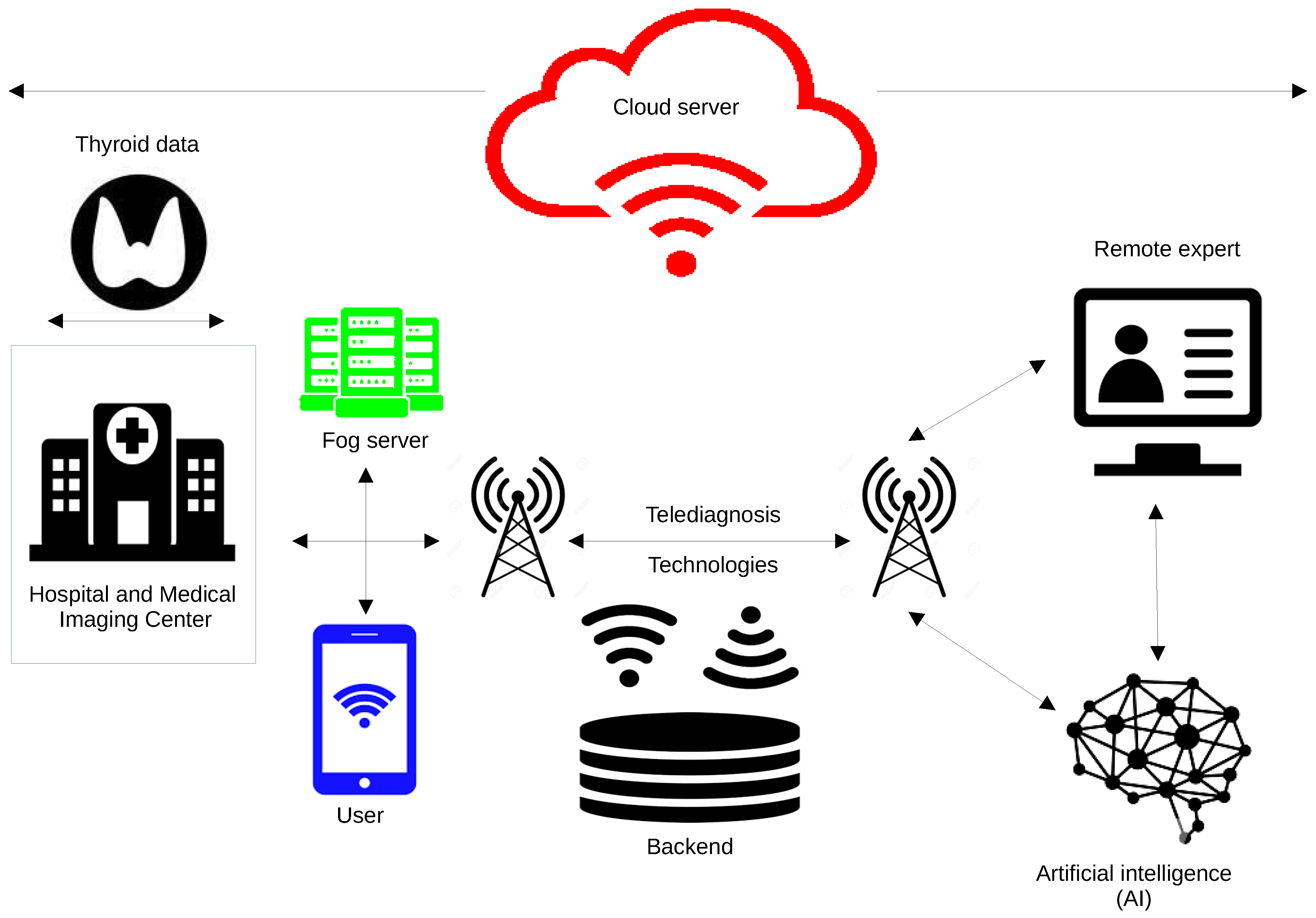
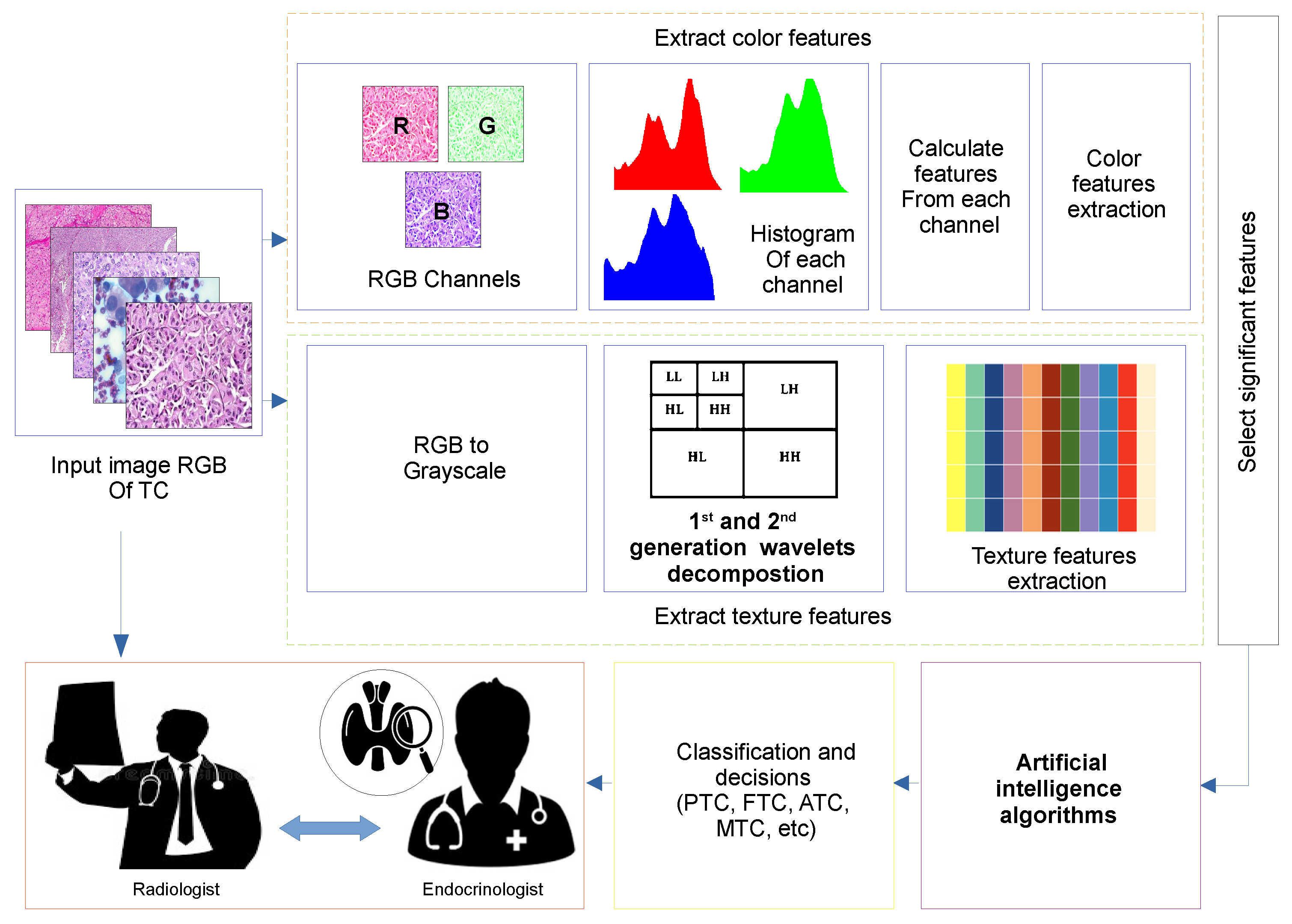
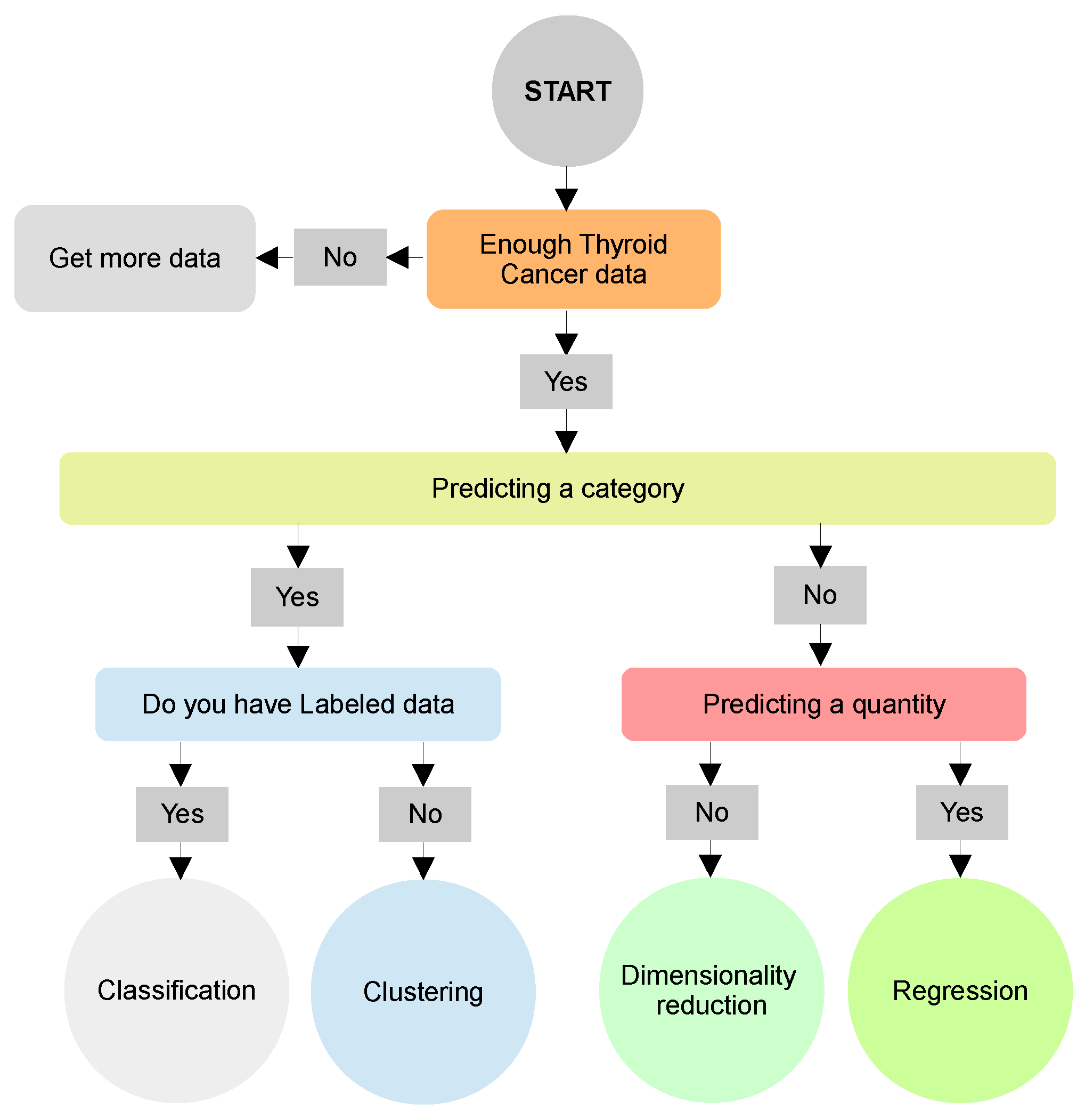

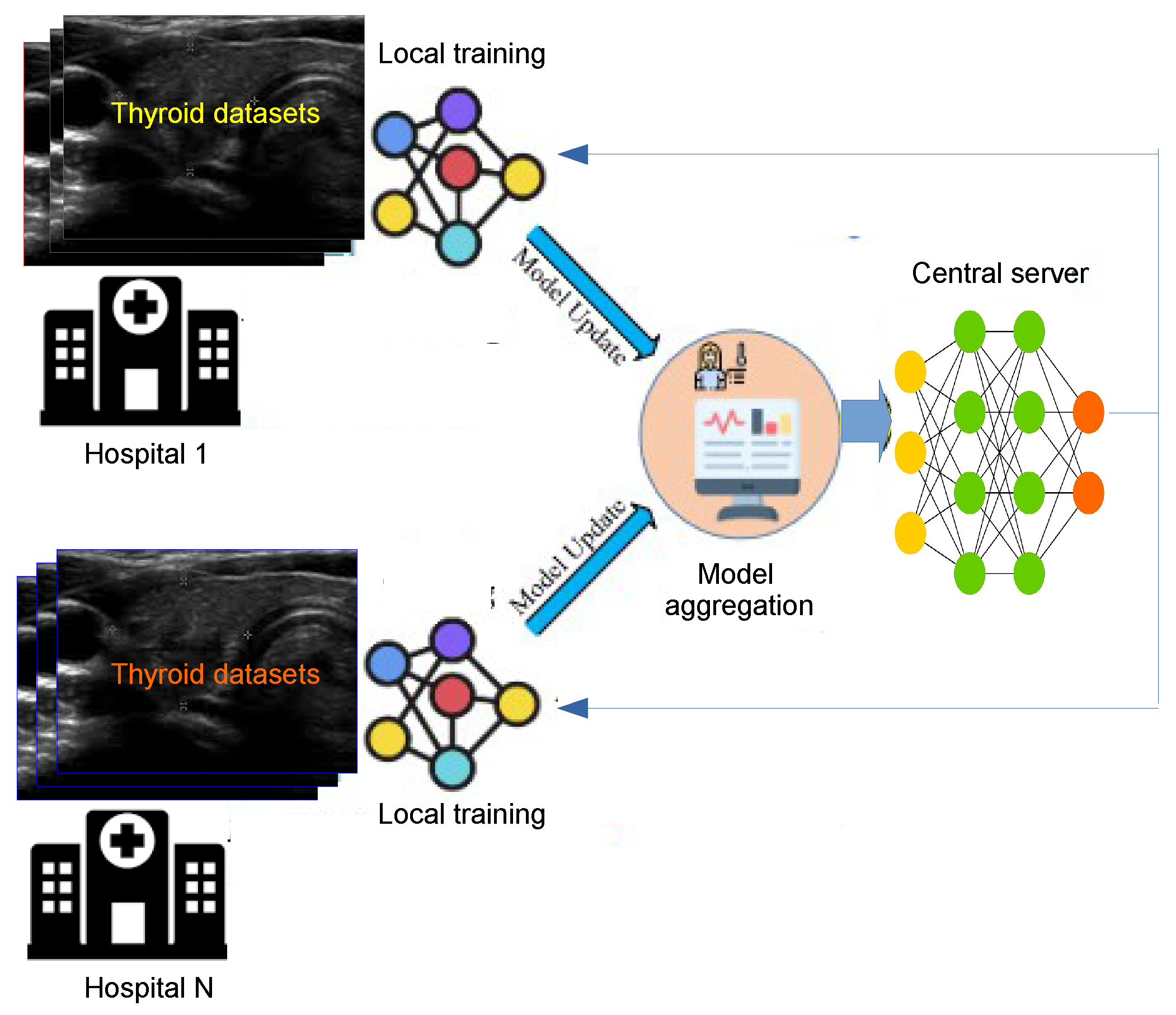
| Ref | Year | PPY | TCDS | AIA | Open Challenges | Future Directions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCDA | RDLA | PP | XAI | EFC-AI | RL | PS | IoMIT | RS | |||||
| [51] | 2021 | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| [52] | 2021 | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| [53] | 2021 | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| [54] | 2021 | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| [55] | 2021 | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| [56] | 2022 | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| [57] | 2022 | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| [58] | 2022 | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| [59] | 2022 | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| Ours | - | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ref. | Year | Country | NP | NM | NF | NN | NBN | NMN | TP | TN | FP | FN | ACC | Sens. | Spec. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [124] | 2021 | China | 102 | 00 | 102 | 104 | 57 | 47 | 38 | 07 | 07 | 50 | 44.12 | 43.18 | 50.00 |
| [125] | 2021 | China | 102 | 25 | 77 | 103 | 73 | 33 | 27 | 12 | 06 | 61 | 36.79 | 30.68 | 66.67 |
| [126] | 2021 | Koria | 325 | 61 | 264 | 325 | 257 | 68 | 48 | 52 | 20 | 205 | 30.77 | 18.97 | 72.22 |
| [127] | 2021 | China | 2775 | 726 | 2049 | 2775 | 2472 | 303 | 271 | 363 | 32 | 2109 | 22.85 | 11.39 | 91.90 |
| [127] | 2021 | China | 163 | 48 | 115 | 175 | 67 | 108 | 86 | 09 | 22 | 58 | 54.29 | 59.72 | 29.03 |
| [128] | 2020 | China | 2489 | 614 | 1875 | 2489 | 1021 | 1468 | 1280 | 258 | 188 | 763 | 61.79 | 62.65 | 57.85 |
| [129] | 2020 | USA | 571 | 234 | 337 | 651 | 500 | 151 | 133 | 214 | 18 | 287 | 53.22 | 31.67 | 92.24 |
| [130] | 2020 | China | 166 | 46 | 100 | 209 | 109 | 100 | 87 | 16 | 13 | 93 | 49.28 | 48.33 | 55.17 |
| [122] | 2020 | Korea | 200 | 49 | 151 | 200 | 102 | 98 | 90 | 41 | 08 | 61 | 65.50 | 59.60 | 83.67 |
| [131] | 2020 | Korea | 340 | 79 | 261 | 348 | 252 | 96 | 31 | 25 | 65 | 227 | 16.09 | 12.02 | 27.78 |
| [132] | 2019 | Korea | 106 | 29 | 77 | 2018 | 132 | 86 | 69 | 23 | 17 | 109 | 42.20 | 38.76 | 57.50 |
| [133] | 2019 | China | 171 | 32 | 139 | 180 | 85 | 95 | 86 | 50 | 09 | 35 | 75.56 | 71.07 | 84.75 |
| Ref. | Category | Classifier | DD | Dataset | O | SV | APP |
|---|---|---|---|---|---|---|---|
| [110] | DL | DAE | PTC | TCGA | O1 | 18,985 features | US |
| [111] | DL | DAE | PTC | TCGA | O1 | 510 samples | Omics |
| [67] | DL | CNN | TC | PD | O1 | 10,068 images | Omics |
| [153] | DL | CNN | TC | PD | O1 | 482 images | Omics |
| [154] | DL | CNN | PTC, FTC | NA | NA | NA | FNAB |
| [155] | DL | CNN | PTC | PD | O1 | 370 microphotographs | FNAB |
| [156] | DL | CNN | PTC | PD | O3 | 469 patients | FNAB |
| [157] | DL | CNN | TC | DDTI | O1 | 298 patients | US |
| [158] | DL | CNN | TC | PD | O1 | 1037 images | US |
| [159] | DL | CNN | TN | PD | O2 | 80 patients | US |
| [160] | DL | CNN | TN | PD | O2 | 300 images | US |
| [161] | DL | CNN | TC | PD | O1 | 459 labeled | US |
| [162] | DL | CNN | TD | ImageNet | O1 | 2888 samples | US |
| [120] | DL | CNN | TC | PD | O1 | 17,627 patients | US |
| [121] | DL | CNN | TC | PD | O1 | 1110 images | US |
| [123] | DL | CNN | TN | PD | O1, S1 | 537 images | US |
| [134] | DL | RNN | TN | PD | O1 | 13,592 patients | US |
| [136] | DL | DBM | TD | PD | O1 | 94 users | Fitness |
| [138] | DL | GAN | TC | PD | O3 | 109 images | Surgery |
| [163] | DL | NA | TC | NA | NA | NA | US |
| [164] | DL | NA | TC | PD | O1 | 1358 images | US |
| [165] | AI | NA | TC | PD | O1 | 50 patients | Surgery |
| [166] | AI | NA | TC | PD | O1 | 89 patients | US |
| [101] | ANN | ELM | TD | UCI | O1 | 215 patients | US |
| [102] | ANN | ELM | TD | UCI | O1 | 215 patients | US |
| [103] | ANN | ELM | TD | PD | O1 | 187 patients | US |
| [105] | ANN | MLP | TD | PD | O1 | 7200 samples | US |
| [106] | ANN | MLP | TD | UCI | O1 | 7200 patients | US |
| [109] | ANN | RBF | TD | PD | O1 | 487 patients | US |
| [167] | ANN | RBF | TD | PD | O1 | 447 patients | Cytopathological |
| [168] | ANN | NA | FTC | PD | O1 | 57 smears | FNAB |
| [169] | ANN | NA | FTC | NA | NA | NA | FNAB |
| [170] | ANN | NA | TC | TCGA | O3 | 482 samples | Histopathological |
| [171] | ANN | NA | TC | PD | O1 | 1264 patients | FNAB |
| [172] | ANN | NA | TN | PD | O1 | 276 patients | US |
| [73] | TCL | KNN | TD | PD | O1 | 7200 instances | US |
| [173] | TCL | KNN | FTC | PD | O1, O2 | 94 patients | Histopathological |
| [174] | TCL | SVM | FTC | PD | O1 | 43 nuclei | Histopathological |
| [175] | TCL | SVM | TN | PD | O1 | 467 TN | US |
| [76] | TCL | SVM | TC | PD | O1 | 92 subjects | US |
| [176] | TCL | SVM | PTC | TCGA | O1 | 500 patients | Omics |
| [177] | DL | DL | PTC | TCGA | O3 | 115 slides | Omics |
| [178] | ML | ML | TN | PD | O1 | 121 patients | Omics |
| [79] | TCL | DT | TC | UCI | O1 | 3739 patients | US |
| [81] | TCL | DT | TC | NA | O1 | NA | US |
| [82] | TCL | DT | TC | UCI | O1 | 499 patients | US |
| [83] | TCL | LR | TC | PD | O1 | 63 patients | US |
| [84] | TCL | LR | TN | PD | O1 | 33,530 patients | US |
| [139] | PM | BN | TD | UCI | O1 | 93 adult patients | US |
| [140] | PM | BN | TC | NA | O1 | 37 patients | US |
| [96] | C | KM | TC | UCI | O1 | 215 instances | US |
| [97] | C | EB | TC | Private data | O1 | 734 cases | US |
| [70] | DR | PCA | TC | PD | O1 | NA | NA |
| [144] | B | FB | TN | PD | O1 | 1480 patients | US |
| Ref | Year | TCD | IT | IF | Instance | M/F | DA |
|---|---|---|---|---|---|---|---|
| [44] | 2018 | BMU | Sonographic | PNG | 1077 | 4309 | Public |
| [172] | 2019 | TCCC | US | PNG | 370 | 370 | Public |
| [187] | 2019 | Clinical | US | JPEG | 117 | 2108 | Public |
| [188] | 2019 | Hospital | US | JPEG | 62 | 12/60 | Public |
| [189] | 2020 | TIRADS | US | JPEG | 5278 | NA | Public |
| [190] | 2018 | Peking Union | US | JPEG | 4309 | 1179 | Private |
| [120] | 2019 | Medical Center | US | PNG | 1425 | 2064 | Private |
| [191] | 2020 | PubMed | CT scans | JPEG | 2108 | 54/253 | Private |
| [156] | 2021 | ACR | DICOM | DICOM | 1629 | 83/289 | Private |
| [126] | 2021 | Clinical | US | PNG | 40 | 407 | Private |
| Ref. | AI Meth. | Achieve. (%) | Advantages | Drawbacks |
|---|---|---|---|---|
| [192] | DAE | ACC = 92.9 | No need for labels for thyroid cancer | Insufficient training data and need relevant data |
| [193] | CNN | AUC = 85.0 | High thyroid cancer detection | Insufficient labels for thyroid cancer and weak in interpretability |
| [194] | RNN | ACC = 98.2 | No need for labels for thyroid cancer | Slow computation and difficulty in training |
| [195] | MLP | ACC = 95.0 | Adaptive learning for thyroid cancer | Limited in its results |
| [196] | KNN | P = 93.0 | High sensitivity to thyroid cancer detection | Insufficient labels for thyroid cancer |
| [197] | SVM | ACC = 97.0 | High sensitivity to thyroid cancer detection | Weak in interpretability and long training time |
| [198] | DT | AUC = 73.10 | Does not require scaling and normalization of data | Unstable |
| [199] | LR | – | Low-cost training and easier implementation | Difficulty to label data |
| [200] | B | ACC = 94.88 | High detection of thyroid cancer | Loss of interpretability and high computational cost |
| Ref. | Year | Classifier | Features | Contributions |
|---|---|---|---|---|
| [226] | 2017 | KNN | FC/IG | - Avoids data redundancy and reduces computation time. The KNN algorithm deals with the missing data, and the ANFIS algorithm is provided with the resultant data as input. |
| [227] | 2017 | SVM | FC/CFS | - Extracts the geometric and moment features while some kernels of the SVM classifier classify the extracted features. |
| [108] | 2020 | CNN | FC/R | - Combines ML and feature selection algorithms (namely, Fisher’s discriminant ratio, Kruskal–Wallis’ analysis, and Relief-F) to analyze the SEER database. |
| [228] | 2022 | CNN | FE/PCA | - The influence of unbalanced serum Raman data on the prediction results was minimized by using an oversampling algorithm in this study. PCA was used to reduce the data dimension before classifying the data using RF and adaptive boosting. |
| [229] | 2012 | O | FE/TD | - Combines CAD and DWT and texture feature extraction. The AdaBoost classifier uses the extracted features to classify images into benign or malignant thyroid images. |
| [230] | 2021 | CNN | FE/AC | - Image enhancement, segmentation, and multifeature extraction, encompassing both geometric and texture features. Each characteristic is then classified using an MLP and SVM, resulting in a determination of either benign or malignant. |
| [189] | 2020 | SVM | FE/LBP | - Deep features are extracted by a CNN and are combined with handcrafted features, including a histogram of oriented gradient (HOG), and scale-invariant feature transform to create fused features. These fused features are then used for classification by an SVM. |
| [231] | 2019 | SVM | FE/GLCM | - Uses a median filter to reduce noise and delineates the contours before extracting features from thyroid regions, including GLCM texture features. SVM, RF, and bootstrap aggregating (bagging) are then used to identify the benign and malignant nodules. |
| [225] | 2019 | SVM | FE/ICA | - A multikernel-based SVM is used as a classifier to distinguish the thyroid disease. |
| Ref. | AI Model | Dataset | ACC | SPE | SEN | PPV | F1 | NPV | AUC |
|---|---|---|---|---|---|---|---|---|---|
| [238] | CNN | PD | 88.00 | 79.10 | 98.10 | - | - | - | - |
| [103] | ELM | PD | 87.72 | 94.55 | 78.89 | - | - | - | - |
| [108] | MLP | PD | 87.16 | 87.05 | 91.18 | 16.20 | 27.50 | 99.70 | - |
| [142] | SVM | PD | 63.27 | 71.85 | 38.46 | 32.43 | - | 76.87 | - |
| [239] | RF | PD | 86.80 | 87.90 | 85.20 | - | - | - | 92.00 |
| [240] | LR | PD | 77.80 | 79.80 | 70.60 | - | - | - | 75.00 |
| [231] | B | PD | 84.69 | 86.96 | 82.69 | 87.76 | - | 81.63 | 88.52 |
| [241] | Ensemble DL | Cytological images | 99.71 | - | - | - | - | - | - |
| [100] | VGG-16 | Cytological images | 97.66 | - | - | - | - | - | - |
| [54] | VGG-16 | 99.00 | 86.00 | 94.00 | - | 88.00 | - | - | |
| [43] | RF | Ultrasound | - | - | - | - | - | - | 94.00 |
| [187] | k-SVM | Ultrasound | - | - | - | - | - | - | 95.00 |
| [131] | ANN | Ultrasound | - | - | - | - | - | - | 69.00 |
| [125] | SVM RF | Ultrasound | - | - | - | - | - | - | 95.10 |
| [242] | ANN SVM | Ultrasound | 96.00 | - | - | - | - | - | - |
| [243] | RF | Ultrasound | - | - | - | - | - | - | 75.00 |
| [244] | CNN | DICOM | 83.00 | 85.00 | 82.40 | - | - | - | - |
| [28] | CNN | DICOM | - | 91.50 | - | - | - | - | - |
| [117] | Fine-tuned DCNN | PD | 99.10 | - | - | - | - | - | - |
| [245] | ResNet18-based network | PD | 93.80 | - | - | - | - | - | - |
| [246] | Multiple-scale CNN | PD | 82.20 | - | - | - | - | - | - |
| [99] | ThyNet | PD | - | - | - | - | - | - | 92.10 |
| [247] | Alexnet CNN | PD | 86.00 | - | - | - | - | - | - |
| [175] | DNN | ACR TIRADS | 87.20 | - | - | - | - | - | - |
| [124] | CNN (BETNET) | Ultrasound | 98.30 | - | - | - | - | - | - |
| [248] | ResNet | TIRADS | 75.00 | - | - | - | - | - | - |
| [249] | Xception | CT images | 89.00 | 92.00 | 86.00 | - | - | - | - |
| [120] | DCNN | Sonographic images | 89.00 | 86.00 | 93.00 | - | - | - | - |
| [250] | Google inception v3 | Histopathology images | 95.00 | - | - | - | - | - | - |
| [251] | Cascade MaskR-CNN | Ultrasound | 94.00 | 95.00 | 93.00 | - | - | - | - |
| [252] | VGG16 | Ultrasound | - | 92.00 | 70.00 | - | - | - | - |
| [253] | VGG19 | Ultrasound | 77.60 | 81.40 | 72.50 | - | - | - | - |
| [40] | VGG16 | Ultrasound | 74.00 | 80.00 | 63.00 | - | - | - | - |
| [189] | SVM CNN | Ultrasound | 92.50 | 83.10 | 96.40 | - | - | - | - |
| [254] | CNN | TIRADS | 85.10 | 86.10 | 81.80 | - | - | - | - |
| [255] | CNN | TIRADS | 82.10 | 85.00 | 78.00 | - | - | - | - |
| [256] | CNN | TIRADS | 80.30 | 80.10 | 80.60 | - | - | - | - |
| [257] | CNN | US | 83.00 | 47.00 | 65.00 | - | - | - | - |
| [258] | CNN | MRI | 79.00 | 80.00 | 65.00 | - | - | - | - |
| [259] | CNN | US | 97.00 | 84.10 | 89.50 | - | - | - | - |
| [260] | CNN | CT image | 84.00 | 73.00 | 93.00 | - | - | - | - |
| [261] | CNN | US | 77.00 | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habchi, Y.; Himeur, Y.; Kheddar, H.; Boukabou, A.; Atalla, S.; Chouchane, A.; Ouamane, A.; Mansoor, W. AI in Thyroid Cancer Diagnosis: Techniques, Trends, and Future Directions. Systems 2023, 11, 519. https://doi.org/10.3390/systems11100519
Habchi Y, Himeur Y, Kheddar H, Boukabou A, Atalla S, Chouchane A, Ouamane A, Mansoor W. AI in Thyroid Cancer Diagnosis: Techniques, Trends, and Future Directions. Systems. 2023; 11(10):519. https://doi.org/10.3390/systems11100519
Chicago/Turabian StyleHabchi, Yassine, Yassine Himeur, Hamza Kheddar, Abdelkrim Boukabou, Shadi Atalla, Ammar Chouchane, Abdelmalik Ouamane, and Wathiq Mansoor. 2023. "AI in Thyroid Cancer Diagnosis: Techniques, Trends, and Future Directions" Systems 11, no. 10: 519. https://doi.org/10.3390/systems11100519
APA StyleHabchi, Y., Himeur, Y., Kheddar, H., Boukabou, A., Atalla, S., Chouchane, A., Ouamane, A., & Mansoor, W. (2023). AI in Thyroid Cancer Diagnosis: Techniques, Trends, and Future Directions. Systems, 11(10), 519. https://doi.org/10.3390/systems11100519







