Simulation-Based Assessment of Cholera Epidemic Response: A Case Study of Al-Hudaydah, Yemen
Abstract
1. Introduction
2. Materials and Methods
2.1. Cholera Susceptible-Infected-Recovered/Susceptible
2.1.1. Indirect Infection
2.1.2. Asymptomatic Reinforcing Feedback Loop (R)
2.1.3. Bacteria Shedding
- bacteria shedding from symptomatic is 10^4, hence, normalized to 104/106 = 0.01
- bacteria shedding from a mildly infected individual is 108, hence, normalized to 108/106 = 100
- bacteria shedding from a severely infected individual is 10^12, hence, normalized to 1012/106 = 1,000,000
2.1.4. Symptomatic Reinforcing Feedback Loops (R)
2.1.5. Recovered Balancing Feedback Loops (B)
2.1.6. Immunity Waning
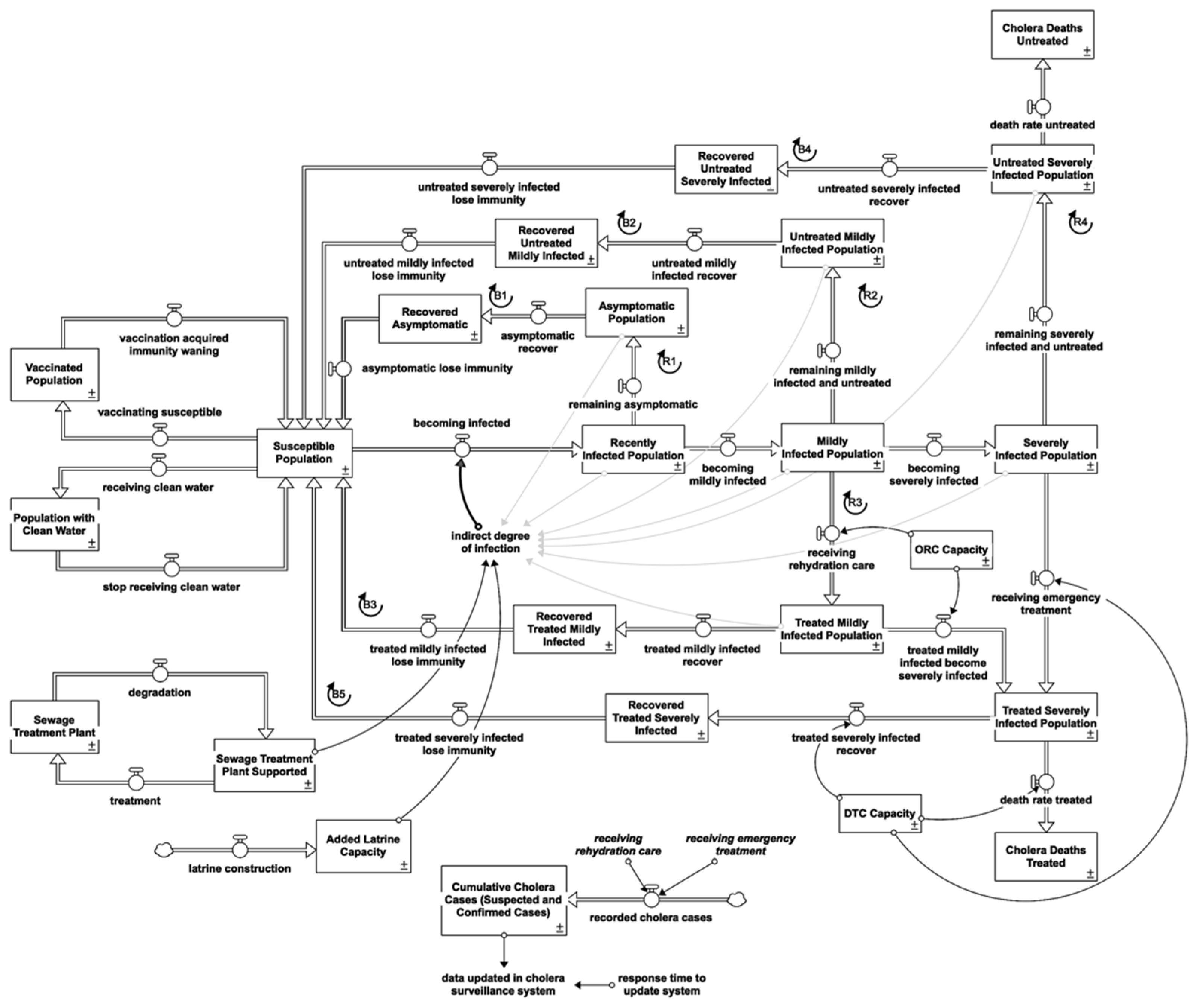
2.2. Cholera Response-Intervention Structure
2.2.1. Water, Sanitation and Hygiene Interventions (WASH)
Clean Water Provision
Sewage Treatment Plant
Latrine Construction
2.2.2. Healthcare Interventions
Diarrhea Treatment Centre (DTC)
Oral Rehydration Corner (ORC)
Vaccination
2.2.3. Surveillance System
2.3. Other Model Settings
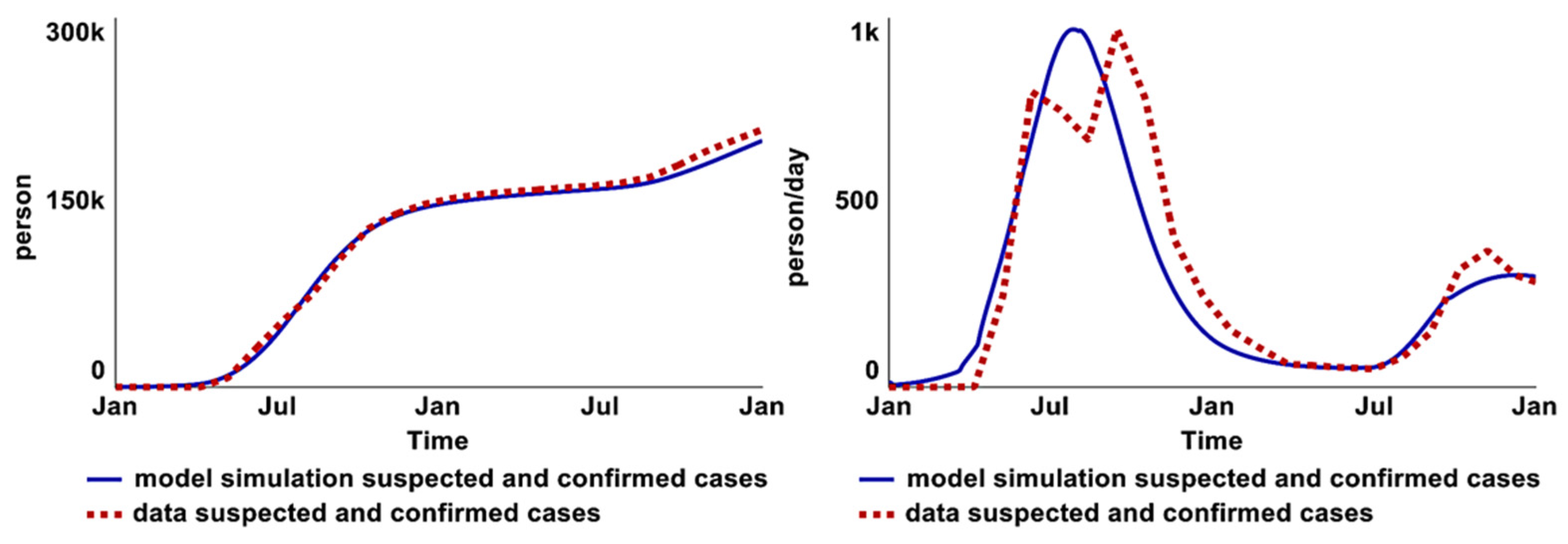
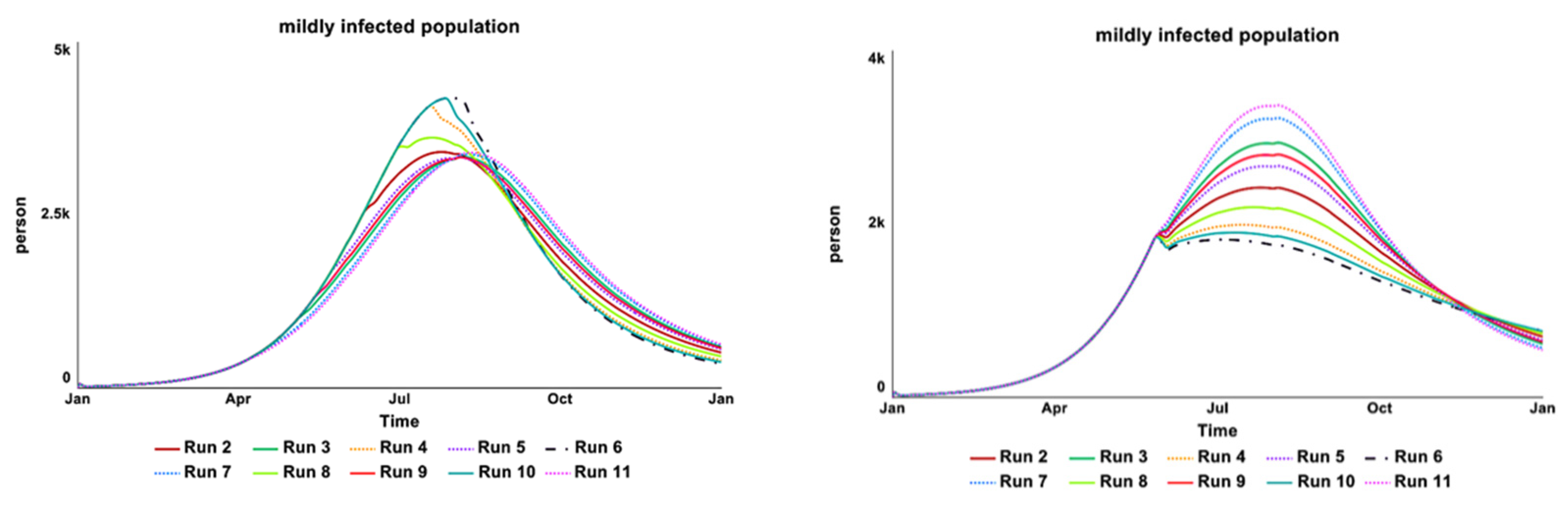
2.4. Model Validation
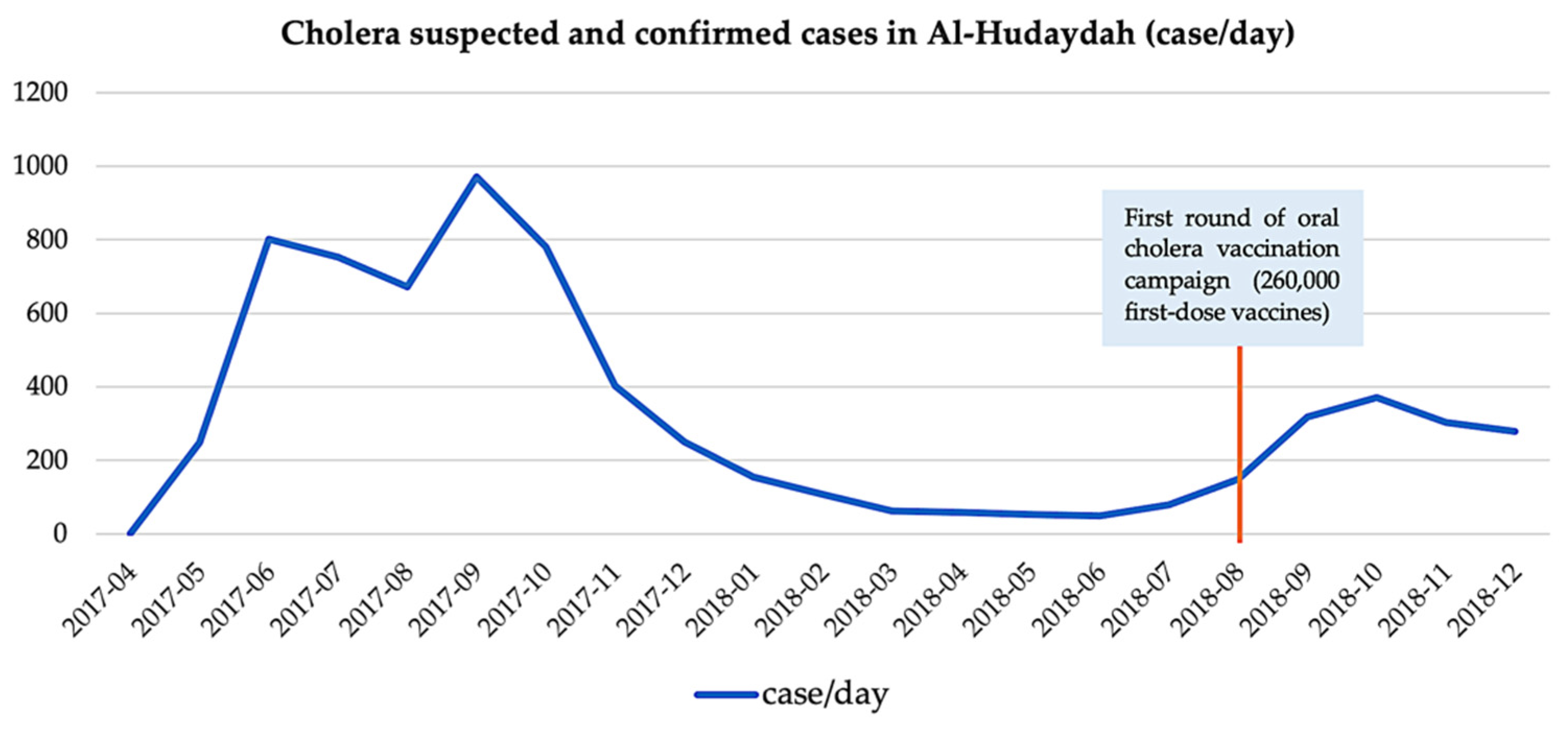
2.4.1. Comparison to Historical Data
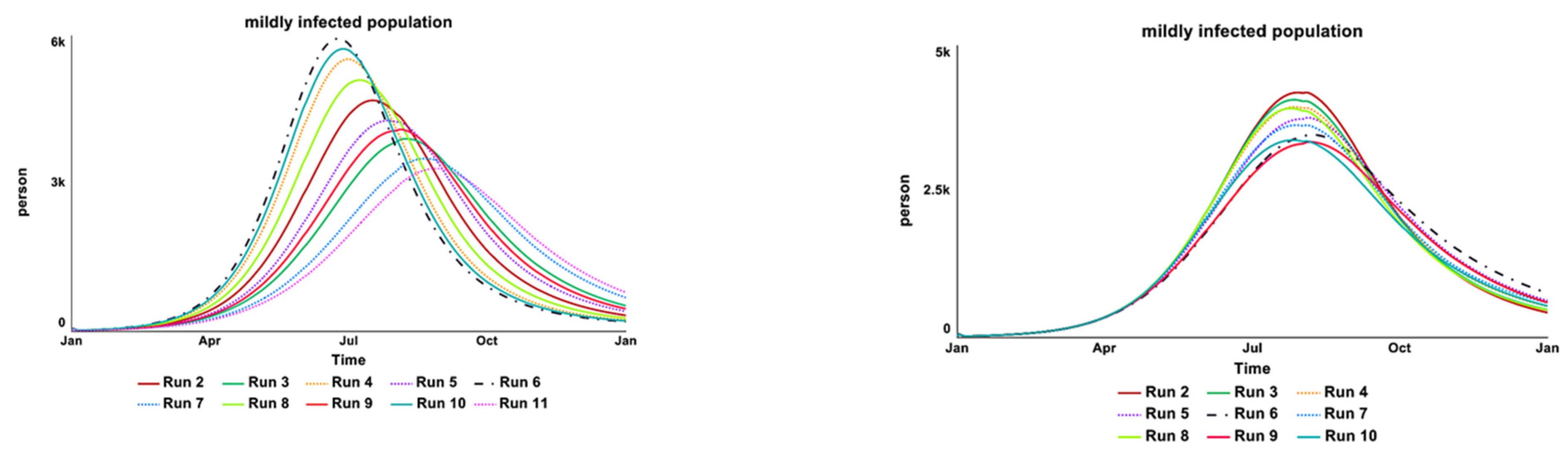
2.4.2. Sensitivity Test
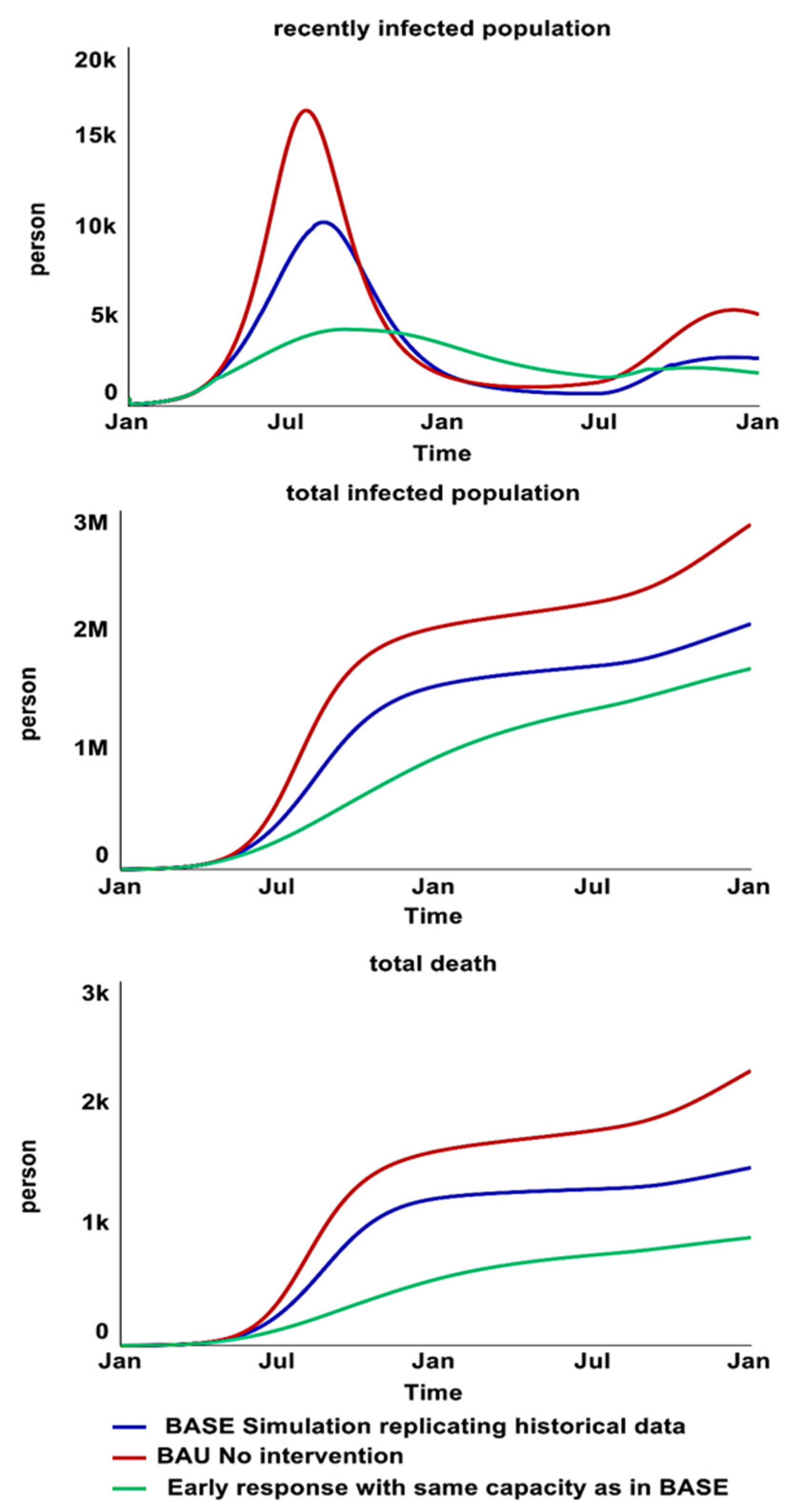
3. Scenario Analysis and Discussion
BAU-BASE-Early Response
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Médecins Sans Frontières. Management of a Cholera Epidemic. 2018th ed. 2018. Available online: https://medicalguidelines.msf.org/viewport/CHOL/english/management-of-a-cholera-epidemic-23444438.html (accessed on 20 September 2022).
- World Health Organization. Guidelines for Cholera Control; World Health Organization: Geneva, Switzerland, 1993; Available online: https://apps.who.int/iris/handle/10665/36837 (accessed on 20 September 2022).
- Federspiel, F.; Ali, M. The Cholera Outbreak in Yemen: Lessons Learned and Way Forward. BMC Public Health 2018, 18, 1338. [Google Scholar] [CrossRef] [PubMed]
- Harpring, R.; Maghsoudi, A.; Fikar, C.; Piotrowicz, W.D.; Heaslip, G. An Analysis of Compounding Factors of Epidemics in Complex Emergencies: A System Dynamics Approach. J. Humanit. Logist. Supply Chain Manag. 2021, 11, 198–226. [Google Scholar]
- Spiegel, P.; Ratnayake, R.; Hellman, N.; Lantagne, D.S.; Ververs, M.; Ngwa, M.; Wise, P.H. Cholera in Yemen: A Case Study of Epidemic Preparedness and Response; Johns Hopkins Center for Humanitarian Health: Baltimore, MD, USA, 2018. [Google Scholar]
- Davis, W.; Narra, R.; Mintz, E.D. Cholera. Curr. Epidemiol. Rep. 2018, 5, 303–315. [Google Scholar] [CrossRef]
- World Health Organization. Cholera Situation in Yemen December 2020 [Infographic]. Available online: https://applications.emro.who.int/docs/WHOEMCSR314E-eng.pdf?ua=1 (accessed on 20 September 2022).
- Burki, T. Yemen’s Neglected Health and Humanitarian Crisis. Lancet 2016, 387, 734–735. [Google Scholar] [CrossRef]
- Qadri, F.; Islam, T.; Clemens, J.D. Cholera in Yemen—An Old Foe Rearing Its Ugly Head. N. Engl. J. Med. 2017, 377, 2005–2007. [Google Scholar] [CrossRef]
- Emergency Operation Center. Cholera Response Health Actors and Partner Activities. 2021. Available online: https://app.powerbi.com/view?r=eyJrIjoiNTY3YmU0NTItMmFjYy00OTUxLWI2NzEtOTU5N2Q0MDBjMjE5IiwidCI6ImI3ZTNlYmJjLTE2ZTctNGVmMi05NmE5LTVkODc4ZDg3MDM5ZCIsImMiOjl9 (accessed on 20 September 2022).
- United Nations Office for the Coordination of Humanitarian Affairs. Yemen: Cholera Outbreak Tracker Governorate Profiles. Available online: https://public.tableau.com/views/001CholeraYemenTracker/FacilityBaseline?%3Aembed=y&%3AshowVizHome=no&%3Adisplay_count=y&%3Adisplay_static_image=y#!%2Fpublish-confirm (accessed on 20 September 2022).
- UNICEF. UNICEF Yemen Humanitarian Situation Report. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/UNICEFYemenHumanitarianSituationReport-August2018.pdf (accessed on 20 September 2022).
- Al-Mekhlafi, H.M. Yemen in a Time of Cholera: Current Situation and Challenges. Am. J. Trop. Med. Hyg. 2018, 98, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, S.; Pichierri, G.; Cegolon, L.; Panu Napodano, C.M.; Ali Maher, O. Coordination during Cholera Outbreak Response: Critical Insights from Yemen. Am. J. Trop. Med. Hyg. 2021, 105, 1155–1156. [Google Scholar] [CrossRef]
- Barciela, R.; Bilge, T.; Brown, K.; Champion Christophe, A.S.; Shields, M.; Ticehurst, H.; Jutla, A.; Usmani, M.; Colwell, R. Early Action for Cholera Project. Yemen Case Study; Met Office: Exeter, UK, 2021. [Google Scholar]
- Pruyt, E. Small System Dynamics Models for Big Issues: Triple Jump towards Real-World Complexity; TU Delft Library: Delft, The Netherlands, 2013. [Google Scholar]
- Fung, I.C.-H. Cholera Transmission Dynamic Models for Public Health Practitioners. Emerg. Themes Epidemiol. 2014, 11, 1. [Google Scholar] [CrossRef]
- Richardson, G.P. System Dynamics: Simulation for Policy Analysis from a Feedback Perspective. In Qualitative Simulation Modeling and Analysis; Springer: Berlin/Heidelberg, Germany, 1991; pp. 144–169. [Google Scholar]
- Rocca, R. Complex Systems Modeling for Humanitarian Action: Methods and Opportunities; United Nations Office for the Coordination of Humanitarian Affairs: Geneva, Switzerland, 2021. [Google Scholar]
- Diphtheria & Cholera Response. Available online: https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/documents/files/eoc_sitrep_25_yemen.pdf (accessed on 20 September 2022).
- Camacho, A.; Bouhenia, M.; Alyusfi, R.; Alkohlani, A.; Naji, M.A.M.; de Radiguès, X.; Abubakar, A.M.; Almoalmi, A.; Seguin, C.; Sagrado, M.J.; et al. Cholera Epidemic in Yemen, 2016–2018: An Analysis of Surveillance Data. Lancet Glob. Health 2018, 6, e680–e690. [Google Scholar] [CrossRef]
- Sterman, J. Business Dynamics. Systems Thinking and Modeling for a Complex World; McGraw Hill Higher Education: Boston, MA, USA, 2000. [Google Scholar]
- Pruyt, E. Making System Dynamics Cool? Using Hot Testing & Teaching Cases. In Proceedings of the 27th International Conference of the System Dynamics Society. System Dynamics Society, Albuquerque, New Mexico, 26 July 2009. [Google Scholar]
- Mwasa, A.; Tchuenche, J.M. Mathematical Analysis of a Cholera Model with Public Health Interventions. Biosystems 2011, 105, 190–200. [Google Scholar] [CrossRef]
- Wolfe, M.; Kaur, M.; Yates, T.; Woodin, M.; Lantagne, D. A Systematic Review and Meta-Analysis of the Association between Water, Sanitation, and Hygiene Exposures and Cholera in Case-Control Studies. Am. J. Trop. Med. Hyg. 2018, 99, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Okoh, A.I.; Sibanda, T.; Nongogo, V.; Adefisoye, M.; Olayemi, O.O.; Nontongana, N. Prevalence and Characterisation of Non-Cholerae Vibrio Spp. in Final Effluents of Wastewater Treatment Facilities in Two Districts of the Eastern Cape Province of South Africa: Implications for Public Health. Environ. Sci. Pollut. Res. 2015, 22, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; De Deyn, M.L.Z.Q.; Loke, W.; Yeo, W.S. Yemen’s Cholera Epidemic Is a One Health Issue. J. Prev. Med. Public Health 2020, 53, 289. [Google Scholar] [CrossRef] [PubMed]
- Grad, Y.H.; Miller, J.C.; Lipsitch, M. Cholera Modeling: Challenges to Quantitative Analysis and Predicting the Impact of Interventions. Epidemiology 2012, 23, 523–530. [Google Scholar] [CrossRef]
- Chao, D.L.; Longini, I.M., Jr.; Morris, J.G., Jr. Modeling Cholera Outbreaks. Curr. Top. Microbiol. Immunol. 2014, 379, 195–209. [Google Scholar] [CrossRef]
- Kaper, J.B.; Morris, J.G., Jr.; Levine, M.M. Cholera. Clin. Microbiol. Rev. 1995, 8, 48–86. [Google Scholar] [CrossRef]
- Leung, T.; Matrajt, L. Protection Afforded by Previous Vibrio Cholerae Infection against Subsequent Disease and Infection: A Review. PLoS Negl. Trop. Dis. 2021, 15, e0009383. [Google Scholar] [CrossRef]
- Nelson, E.J.; Harris, J.B.; Morris, J.G., Jr.; Calderwood, S.B.; Camilli, A. Cholera Transmission: The Host, Pathogen and Bacteriophage Dynamic. Nat. Rev. Microbiol. 2009, 7, 693–702. [Google Scholar] [CrossRef]
- Chao, D.L.; Halloran, M.E.; Longini, I.M. Vaccination Strategies for Epidemic Cholera in Haiti with Implications for the Developing World. Proc. Natl. Acad. Sci. USA 2011, 108, 7081–7085. [Google Scholar] [CrossRef]
- Global Task Force on Cholera Control. Roadmap 2030. Available online: https://www.gtfcc.org/about-gtfcc/roadmap-2030/ (accessed on 20 September 2022).
- Al-Gheethi, A.A.S.; Abdul-Monem, M.O.; Al-Zubeiry, A.H.S.; Efaq, A.N.; Shamar, A.M.; Al-Amery, R.M.A. Effectiveness of Selected Wastewater Treatment Plants in Yemen for Reduction of Faecal Indicators and Pathogenic Bacteria in Secondary Effluents and Sludge. Water Pract. Technol. 2014, 9, 293–306. [Google Scholar] [CrossRef]
- Miller Neilan, R.L.; Schaefer, E.; Gaff, H.; Fister, K.R.; Lenhart, S. Modeling Optimal Intervention Strategies for Cholera. Bull. Math. Biol. 2010, 72, 2004–2018. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.A.; Rumunu, J.; Jamet, C.; Kenyi, Y.; Lino, R.L.; Wamala, J.F.; Mpairwe, A.M.; Ciglenecki, I.; Luquero, F.J.; Azman, A.S.; et al. Adapting to the Global Shortage of Cholera Vaccines: Targeted Single Dose Cholera Vaccine in Response to an Outbreak in South Sudan. Lancet Infect. Dis. 2017, 17, e123–e127. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ending Cholera a Global Roadmap to 2030. In Ending Cholera a Global Roadmap to 2030; UNICEF: Yemen, Yemen, 2017; p. 32. [Google Scholar]
- LaRocque, R.; Harris, J.B. Cholera: Clinical Features, Diagnosis, Treatment, and Prevention. This Top. Available online: https://www.uptodate.com/contents/cholera-clinical-features-diagnosis-treatment-and-prevention (accessed on 20 September 2022).
- Barlas, Y. Formal Aspects of Model Validity and Validation in System Dynamics. Syst. Dyn. Rev. 1996, 12, 183–210. [Google Scholar] [CrossRef]
- Turner, B.L. Model Laboratories: A Quick-Start Guide for Design of Simulation Experiments for Dynamic Systems Models. Ecol. Modell. 2020, 434, 109246. [Google Scholar] [CrossRef]
- Tuite, A.R.; Tien, J.; Eisenberg, M.; Earn, D.J.; Ma, J.; Fisman, D.N. Cholera Epidemic in Haiti, 2010: Using a Transmission Model to Explain Spatial Spread of Disease and Identify Optimal Control Interventions. Ann. Intern. Med. 2011, 154, 593–601. [Google Scholar] [CrossRef]
- Abu-Lohom, N.; Muzenda, D.; Mumssen, Y.U. A WASH Response to Yemen’s Cholera Outbreak. World Bank Blogs 2018. Available online: https://blogs.worldbank.org/water/wash-response-yemen-s-cholera-outbreak (accessed on 20 September 2022).
- Al-Gheethi, A.; Noman, E.; Jeremiah David, B.; Mohamed, R.; Abdullah, A.; Nagapan, S.; Hashim Mohd, A. A Review of Potential Factors Contributing to Epidemic Cholera in Yemen. J. Water Health 2018, 16, 667–680. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Treatment. Available online: https://www.cdc.gov/cholera/treatment/antibiotic-treatment.html (accessed on 20 September 2022).
- International Organization for Migration. Task Force for Population Movement Yemen August 2018. 2021. Available online: https://displacement.iom.int/yemen (accessed on 20 September 2022).
- Ali, A. IDPs in Hudaydah: Where Aid, Protection Don’t Always Reach; 2021. Available online: https://sanaacenter.org/ypf/idps-in-hudaydah/ (accessed on 20 September 2022).
- McCrickard, L.; Massay, A.E.; Narra, R.; Mghamba, J.; Mohamed, A.A.; Kishimba, R.S.; Urio, L.J.; Rusibayamila, N.; Magembe, G.; Bakari, M.; et al. Cholera Mortality during Urban Epidemic, Dar Es Salaam, Tanzania, August 16, 2015–January 16, 2016. Emerg. Infect. Dis. J. 2017, 23, 13. [Google Scholar] [CrossRef]
- UNICEF. UNCEF Cholera Tookit 2013. Available online: https://sites.unicef.org/cholera/Cholera-Toolkit-2013.pdf (accessed on 20 September 2022).
- Ochoa, B.; Surawicz, C.M. Diarrheal Diseases–Acute and Chronic. Available online: https://gi.org/topics/diarrhea-acute-and-chronic/ (accessed on 20 September 2022).
- Michas, F. Number of Patients That Physicians in the U.S. Saw per Day from 2012 to 2018. 2020. Available online: https://www.statista.com/statistics/613959/us-physicans-patients-seen-per-day/ (accessed on 20 September 2022).
- Günther, I.; Niwagaba, C.B.; Lüthi, C.; Horst, A.; Mosler, H.-J.; Tumwebaze, I.K. When Is Shared Sanitation Improved Sanitation?-The Correlation between Number of Users and Toilet Hygiene. 2012. Available online: https://www.ircwash.org/resources/when-shared-sanitation-improved-sanitation-correlation-between-number-users-and-toilet (accessed on 22 September 2022).
- Worldbank. People Practicing Open Defecation, Urban (% of urban population)-Yemen, Rep. Available online: https://data.worldbank.org/indicator/SH.STA.ODFC.UR.ZS?locations=YE (accessed on 20 September 2022).
- Ministry of Electricity and Water. Environmental Impact Assessment. Available online: Chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://documents1.worldbank.org/curated/en/279131468335060537/pdf/E4940V60P0576020Box353756B01PUBLIC1.pdf (accessed on 20 September 2022).
- Pezzoli, L. Global Oral Cholera Vaccine Use, 2013–2018. Vaccine 2020, 38, A132–A140. [Google Scholar] [CrossRef]
- Durham, L.K.; Longini, I.M., Jr.; Halloran, M.E.; Clemens, J.D.; Azhar, N.; Rao, M. Estimation of Vaccine Efficacy in the Presence of Waning: Application to Cholera Vaccines. Am. J. Epidemiol. 1998, 147, 948–959. [Google Scholar] [CrossRef]
- Shim, E.; Galvani, A.P. Distinguishing Vaccine Efficacy and Effectiveness. Vaccine 2012, 30, 6700–6705. [Google Scholar] [CrossRef]
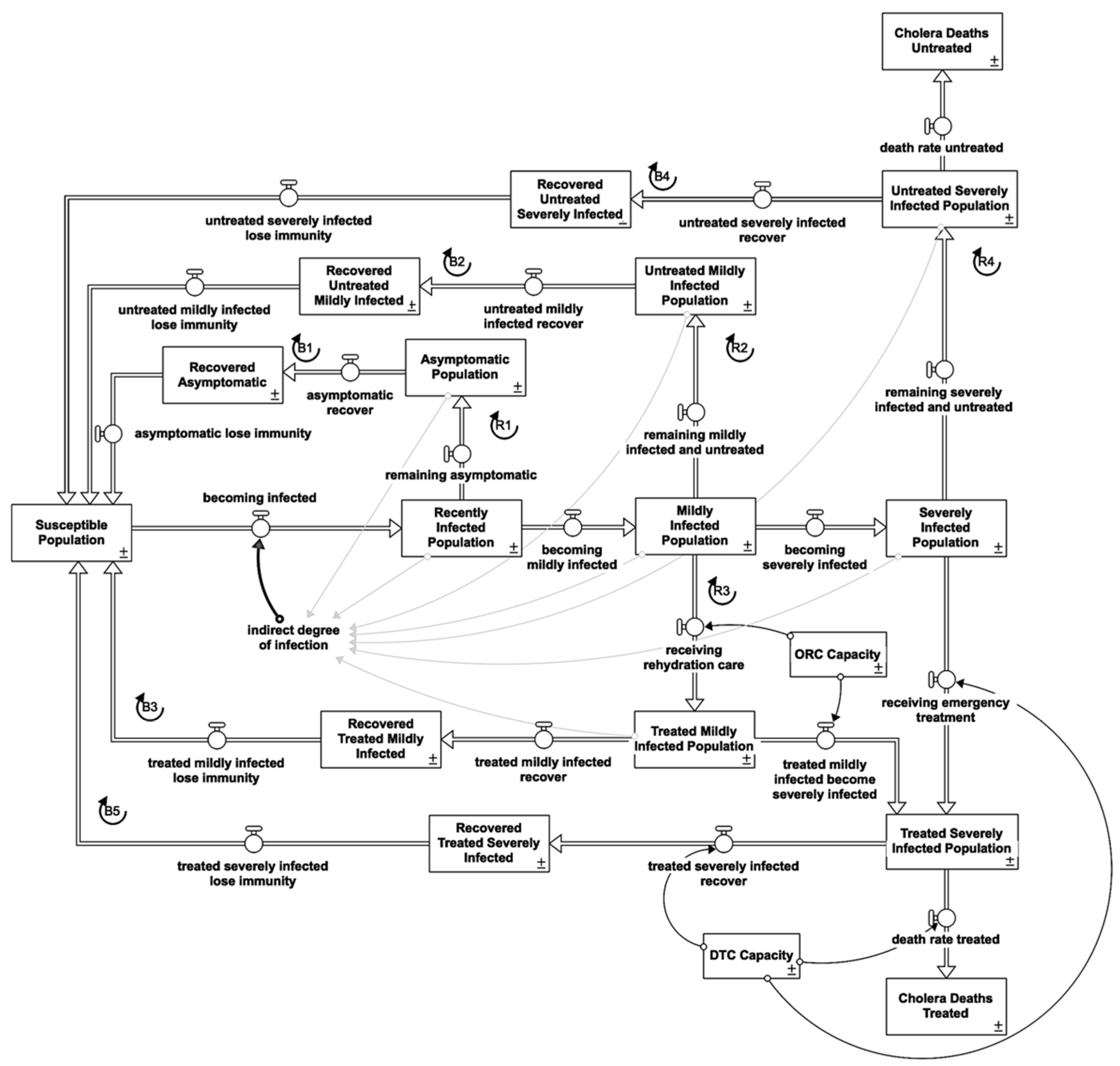


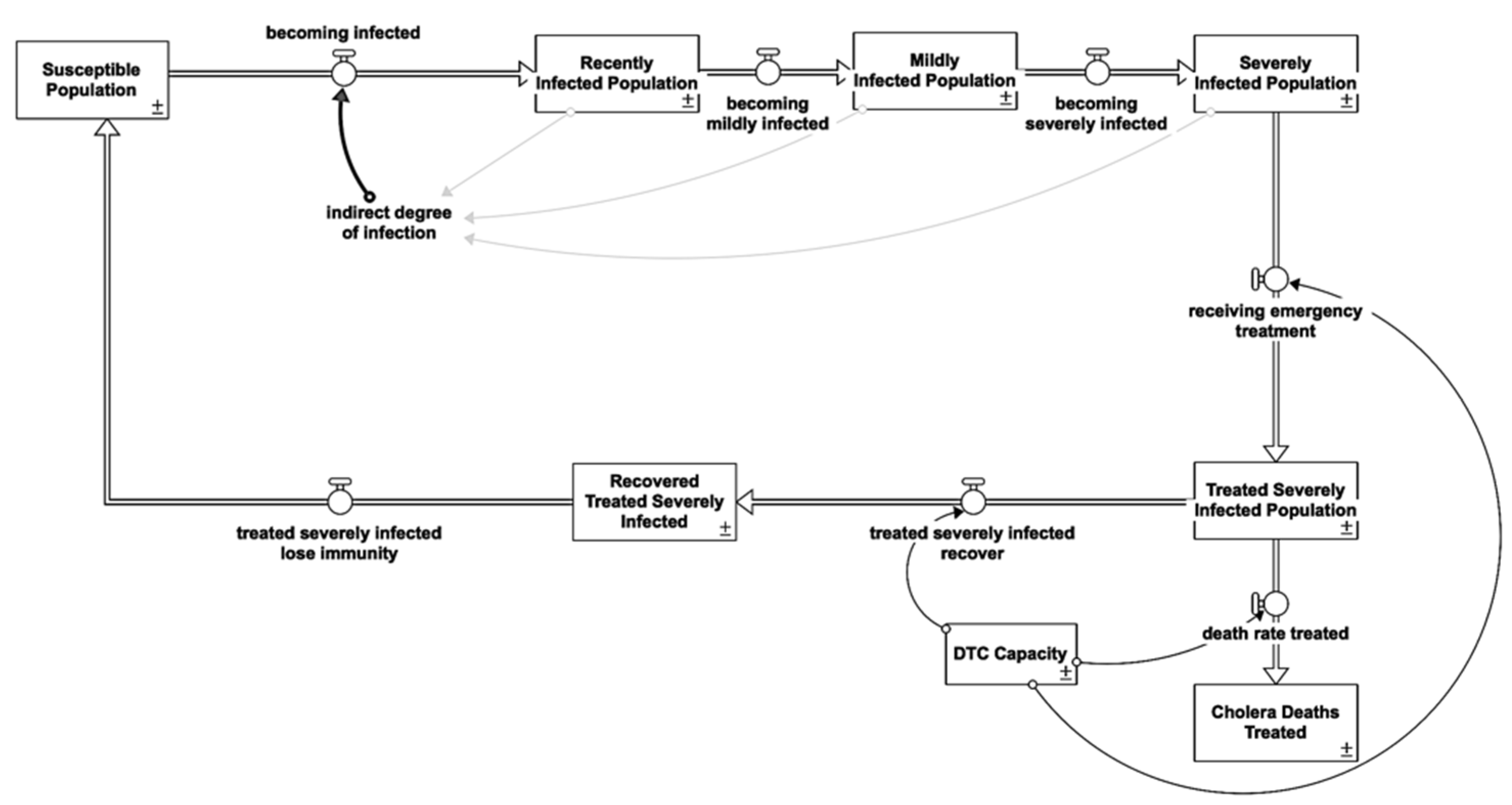
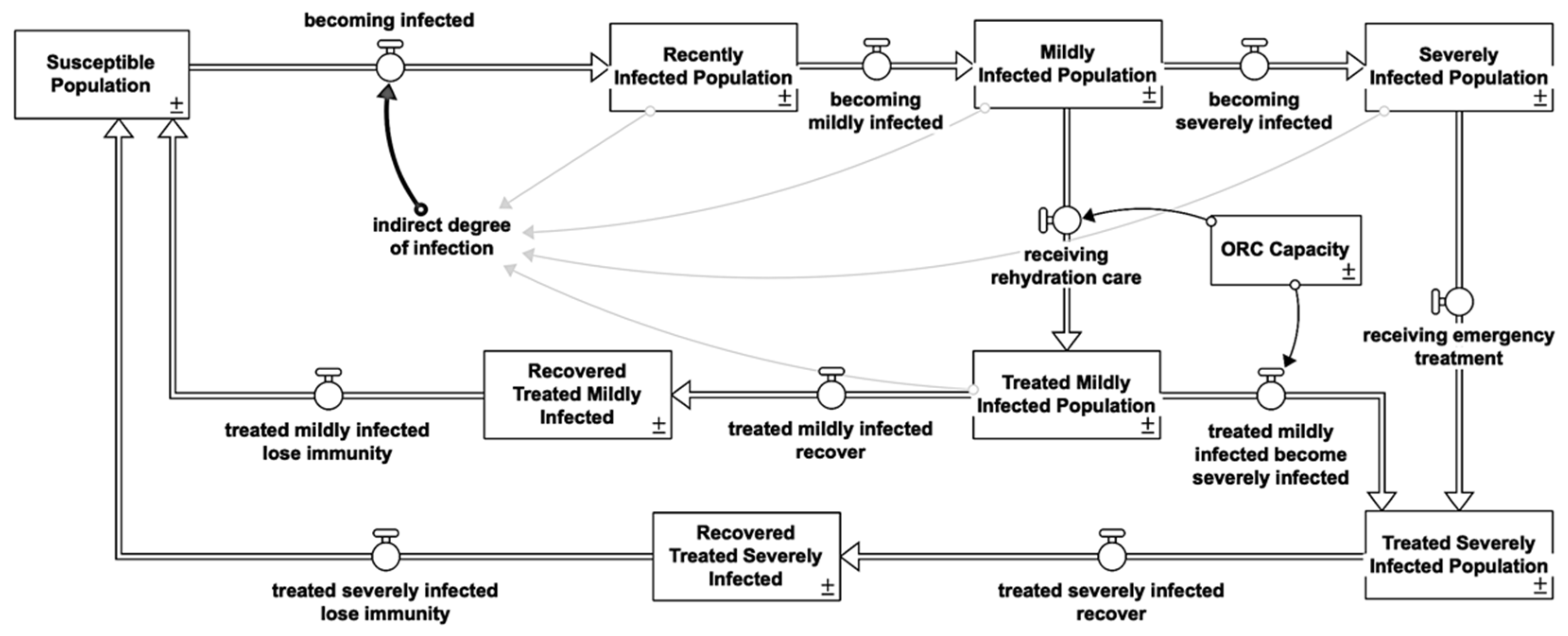
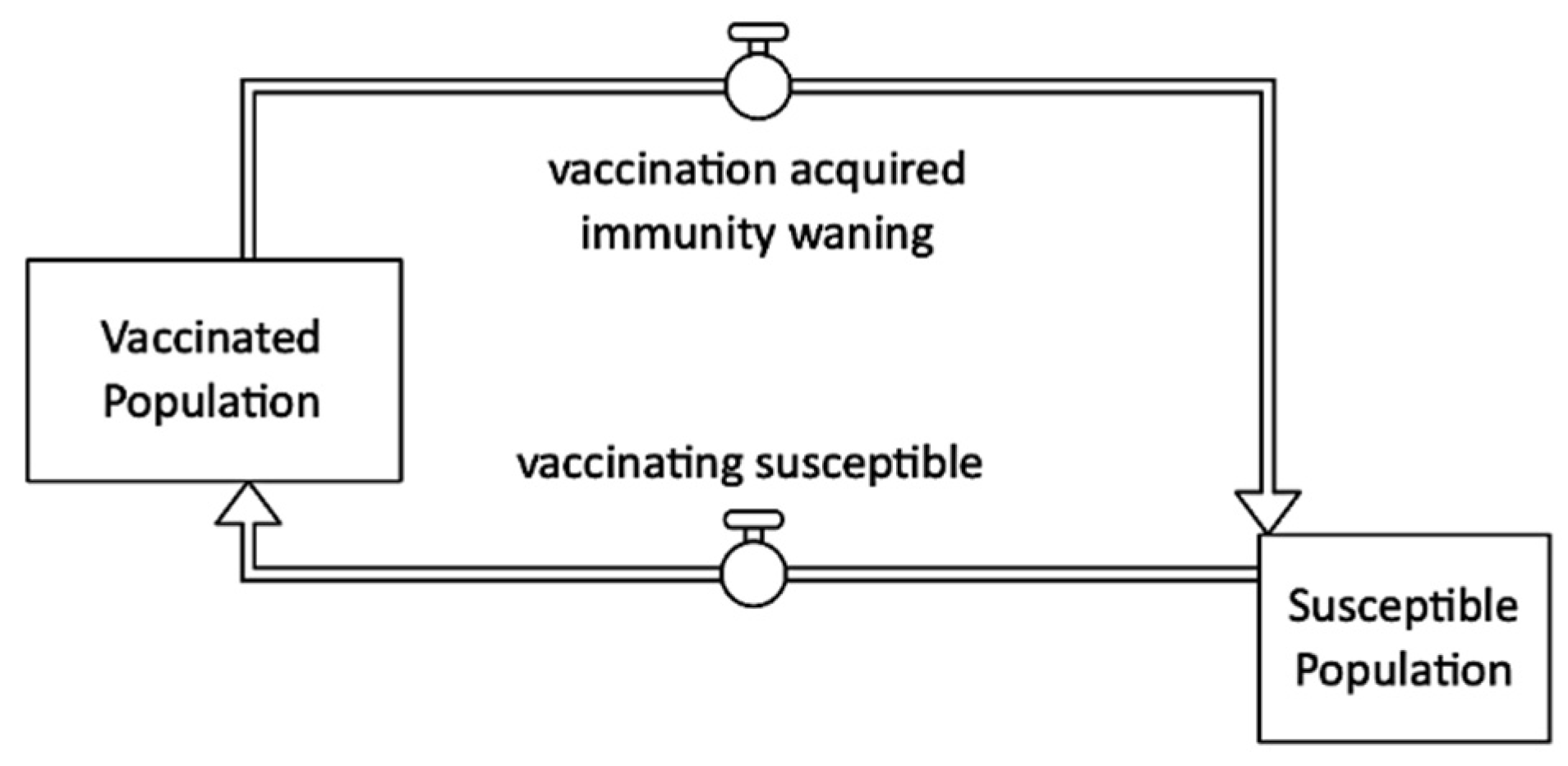


| Infectious State | Treatment | Loop | Shown |
|---|---|---|---|
| Asymptomatic (75%) | No | Asymptomatic infected loop | R1 |
| Asymptomatic recovered loop | B1 | ||
| Mild symptoms (15%) | No | Untreated mildly infected loop | R2 |
| No | Untreated mildly recovered loop | B2 | |
| Yes | Treated mildly infected loop | R3 | |
| Yes | Treated mildly recovered loop | B3 | |
| Severe symptoms (10%) | No | Untreated severely infected loop | R4 |
| No | Untreated severely recovered loop | B4 | |
| Yes | Treated severely recovered loop | B5 |
| No | Parameters | Sensitivity Test (Numerical) | Values | Unit | Sources |
|---|---|---|---|---|---|
| 1 | connectedness of aquifers | 0.02 | 1/day | Calibrated; [14,38] | |
| 2 | time to affect water in aquifers | 3.5 | day | Calibrated; [14] | |
| 3 | ratio of asymptomatic | 0.75 | dmnl | [1,30] | |
| 4 | average incubation time | 1 | day | [1,30,32] | |
| 5 | average duration of illness asymptomatic | 5 | day | [1,29,30] | |
| 6 | susceptible population | 3,238,199 | person | [18] | |
| 7 | recently infected population | 500 | person | [18] | |
| 8 | normal ratio of severe disease | 0.3 | dmnl | [1,30] | |
| 9 | average duration of illness symptomatic | 9 | day | [32,39] | |
| 10 | average asymptomatic infection acquired immunity period | 180 | day | [31] | |
| 11 | average symptomatic infection acquired immunity period | 1095 | day | [30,31] | |
| 12 | fraction mildly infected seeking care | 0.3 | dmnl | Estimation from Camacho et al. [21] | |
| 13 | fraction severely infected seeking care | 0.4 | dmnl | [1,21] | |
| 14 | treated fatality fraction | 0.0021 | dmnl | [18] | |
| 15 | bacteria shedding from asymptomatic | 0.67 | dmnl | [30] (normalized value) | |
| 16 | bacteria shedding from mildly infected | 1.33 | dmnl | [32] (normalized value) | |
| 17 | bacteria shedding from severely infected | 2 | dmnl | [30] (normalized value) |
 Sensitive
Sensitive  Highly sensitive.
Highly sensitive.| Scenario | Total Infected Population | Total Death |
|---|---|---|
| BASE | 2,055,712 | 1,468 |
| BAU | 2,888,484 | 2,268 |
| +41% | +55% | |
| Early Response | 1,681,105 | 891 |
| −18% | −39% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loo, P.S.; Aguiar, A.; Kopainsky, B. Simulation-Based Assessment of Cholera Epidemic Response: A Case Study of Al-Hudaydah, Yemen. Systems 2023, 11, 3. https://doi.org/10.3390/systems11010003
Loo PS, Aguiar A, Kopainsky B. Simulation-Based Assessment of Cholera Epidemic Response: A Case Study of Al-Hudaydah, Yemen. Systems. 2023; 11(1):3. https://doi.org/10.3390/systems11010003
Chicago/Turabian StyleLoo, Pei Shan, Anaely Aguiar, and Birgit Kopainsky. 2023. "Simulation-Based Assessment of Cholera Epidemic Response: A Case Study of Al-Hudaydah, Yemen" Systems 11, no. 1: 3. https://doi.org/10.3390/systems11010003
APA StyleLoo, P. S., Aguiar, A., & Kopainsky, B. (2023). Simulation-Based Assessment of Cholera Epidemic Response: A Case Study of Al-Hudaydah, Yemen. Systems, 11(1), 3. https://doi.org/10.3390/systems11010003






