Effects of Propranolol on Growth, Lipids and Energy Metabolism and Oxidative Stress Response of Phaeodactylum tricornutum
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Diatom Cell Density Measurements and Pellet Collection
2.3. Chlorophyll a Pulse Amplitude Modulated Fluorometry
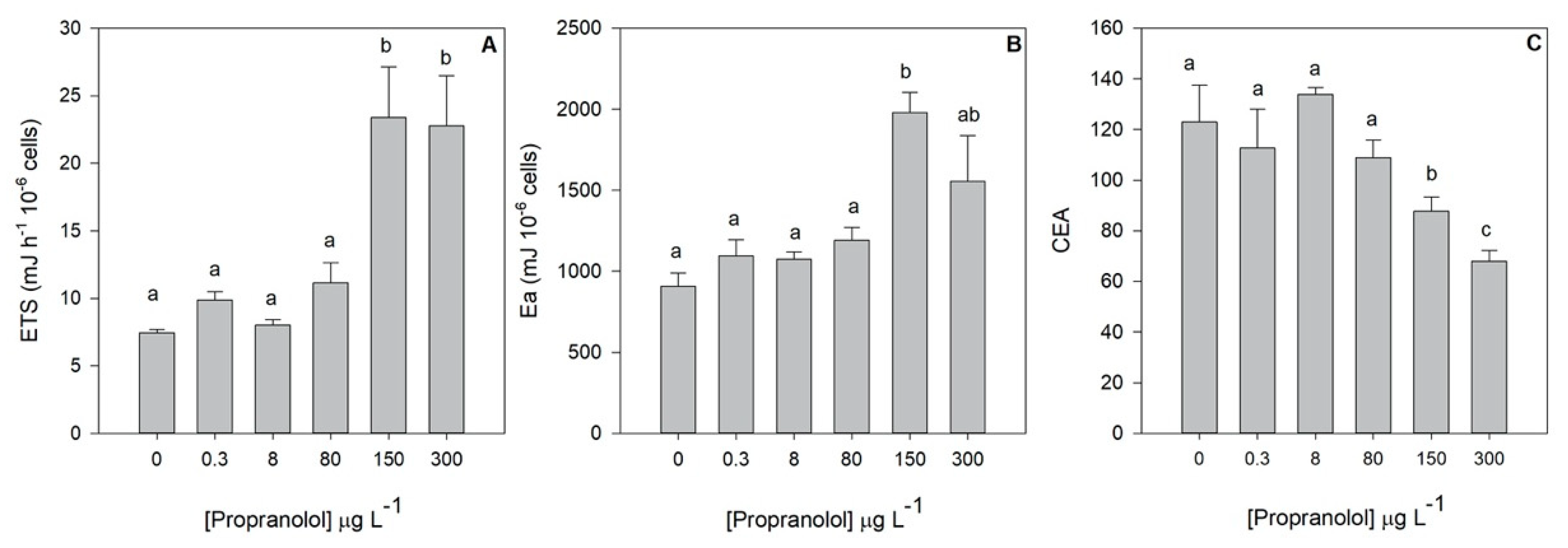
2.4. Cell Energy Allocation and Mitochondrial Metabolism
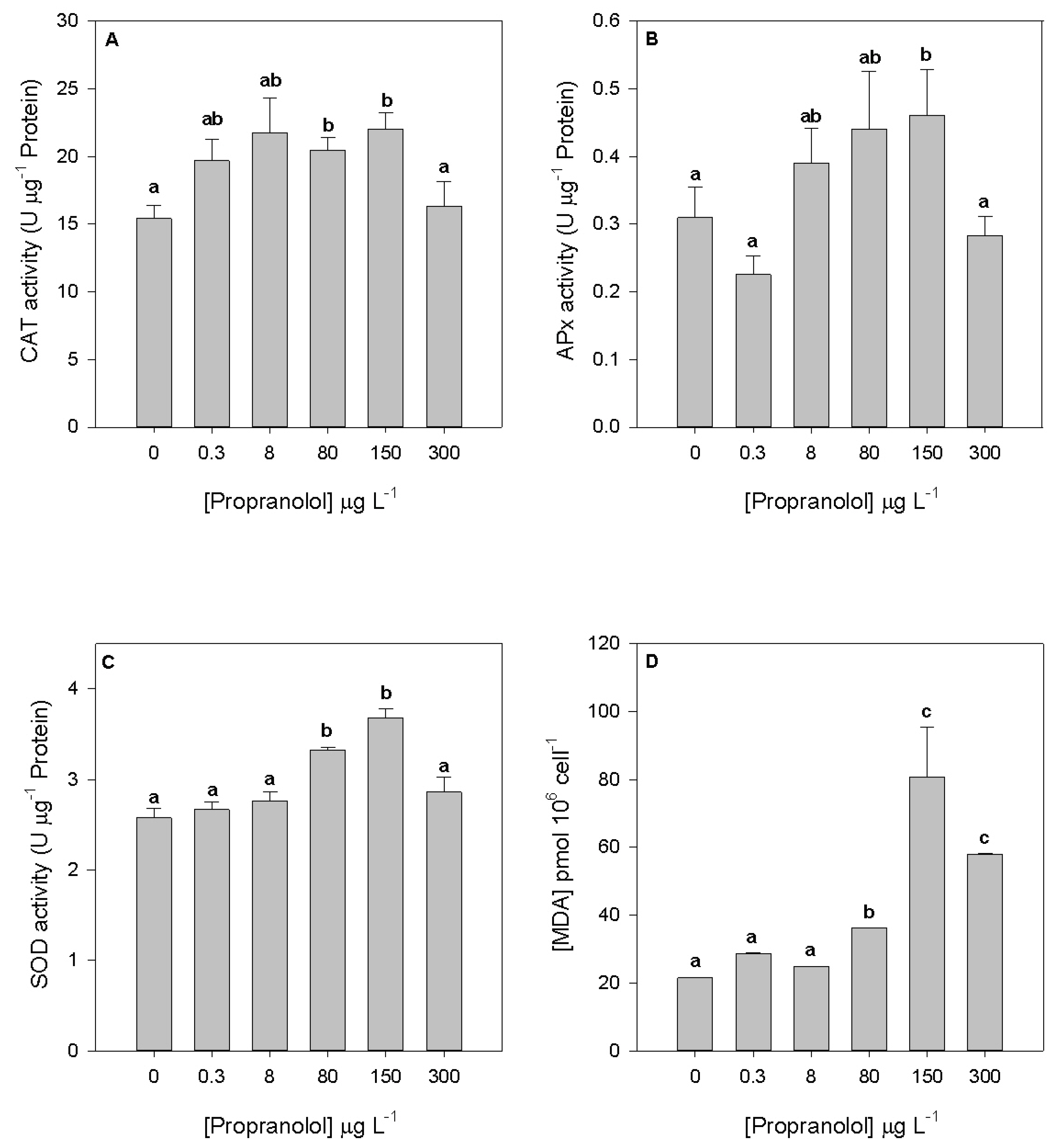
2.5. Oxidative Stress
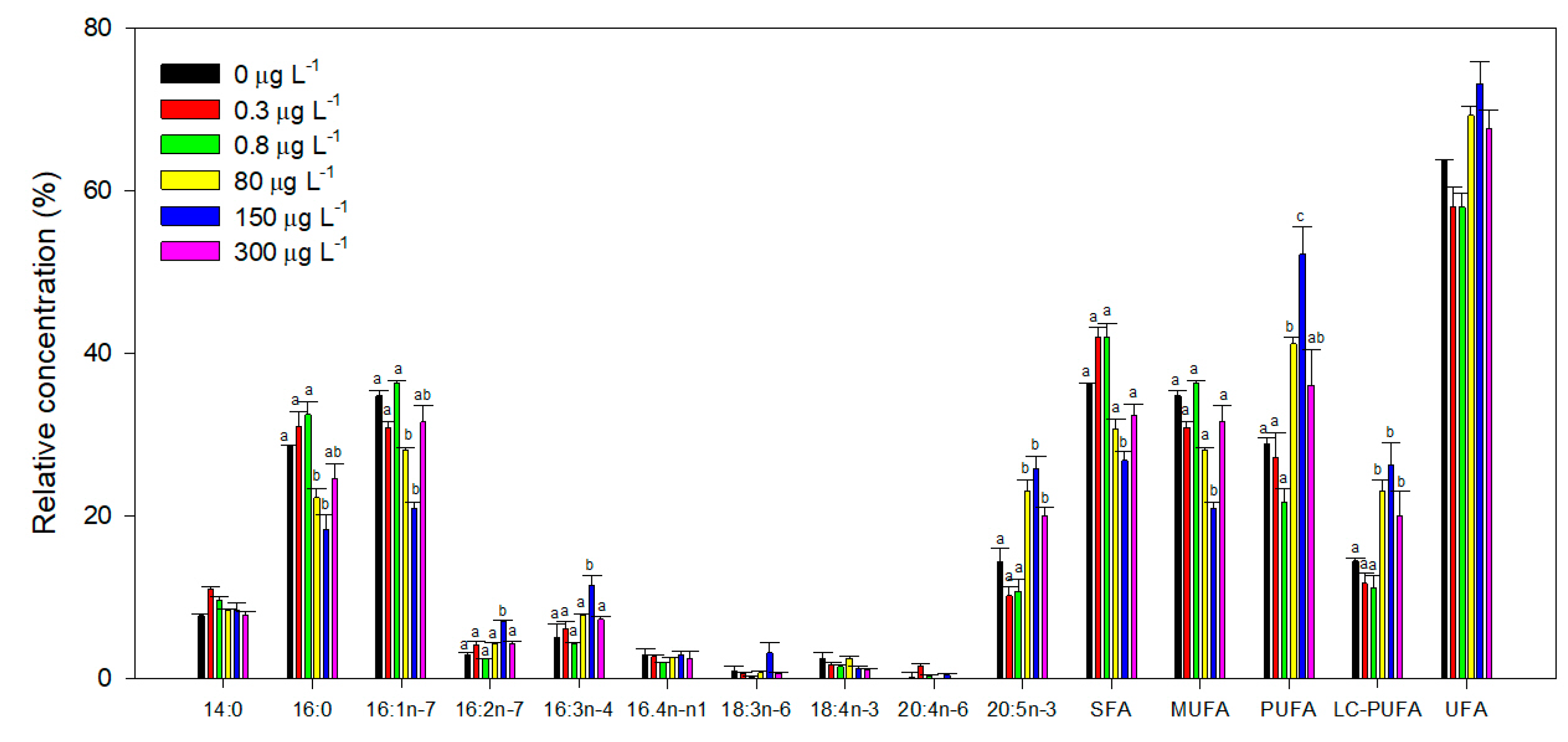
2.6. Fatty Acids Profile
2.7. Statistical Analysis
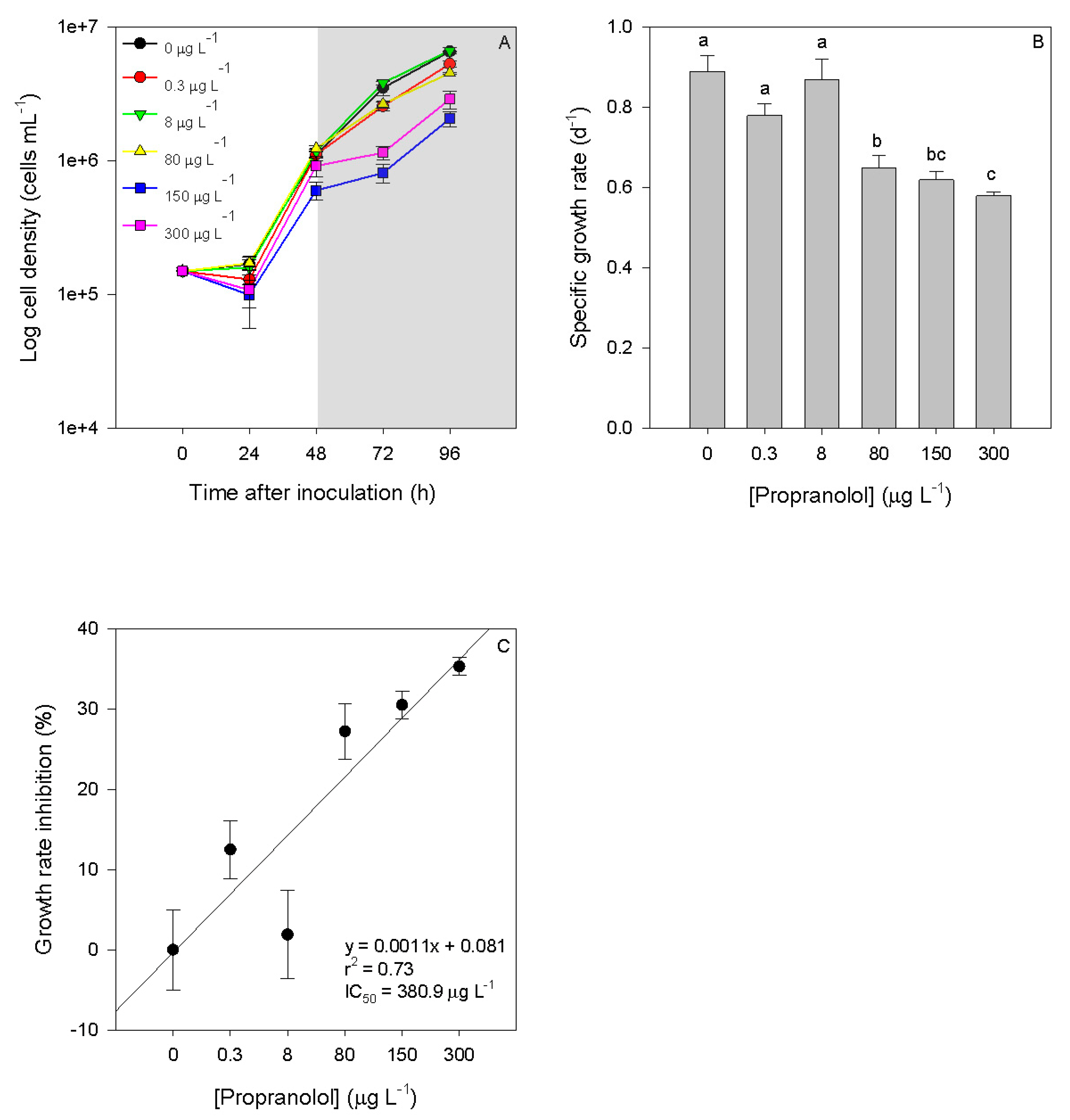
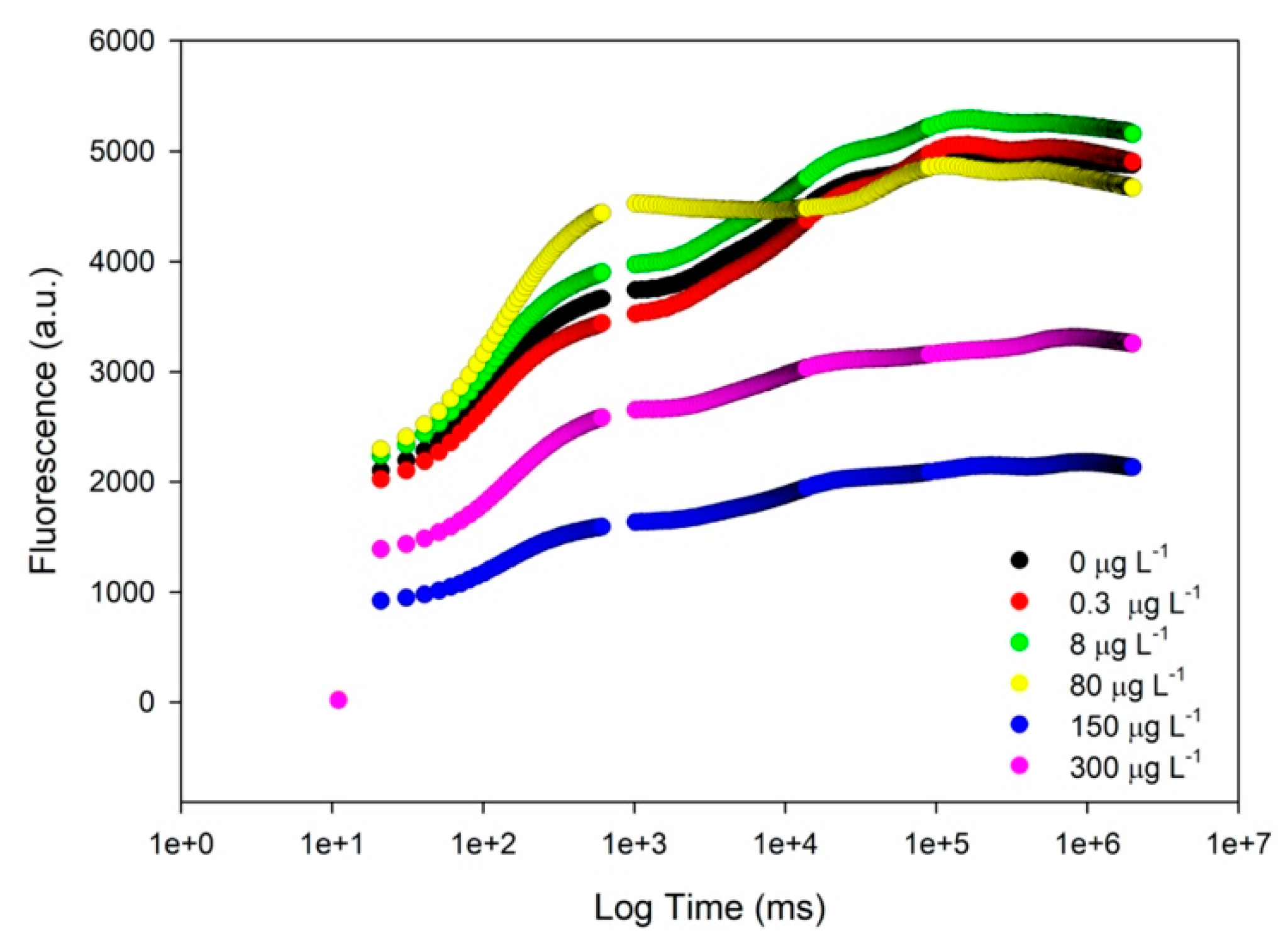
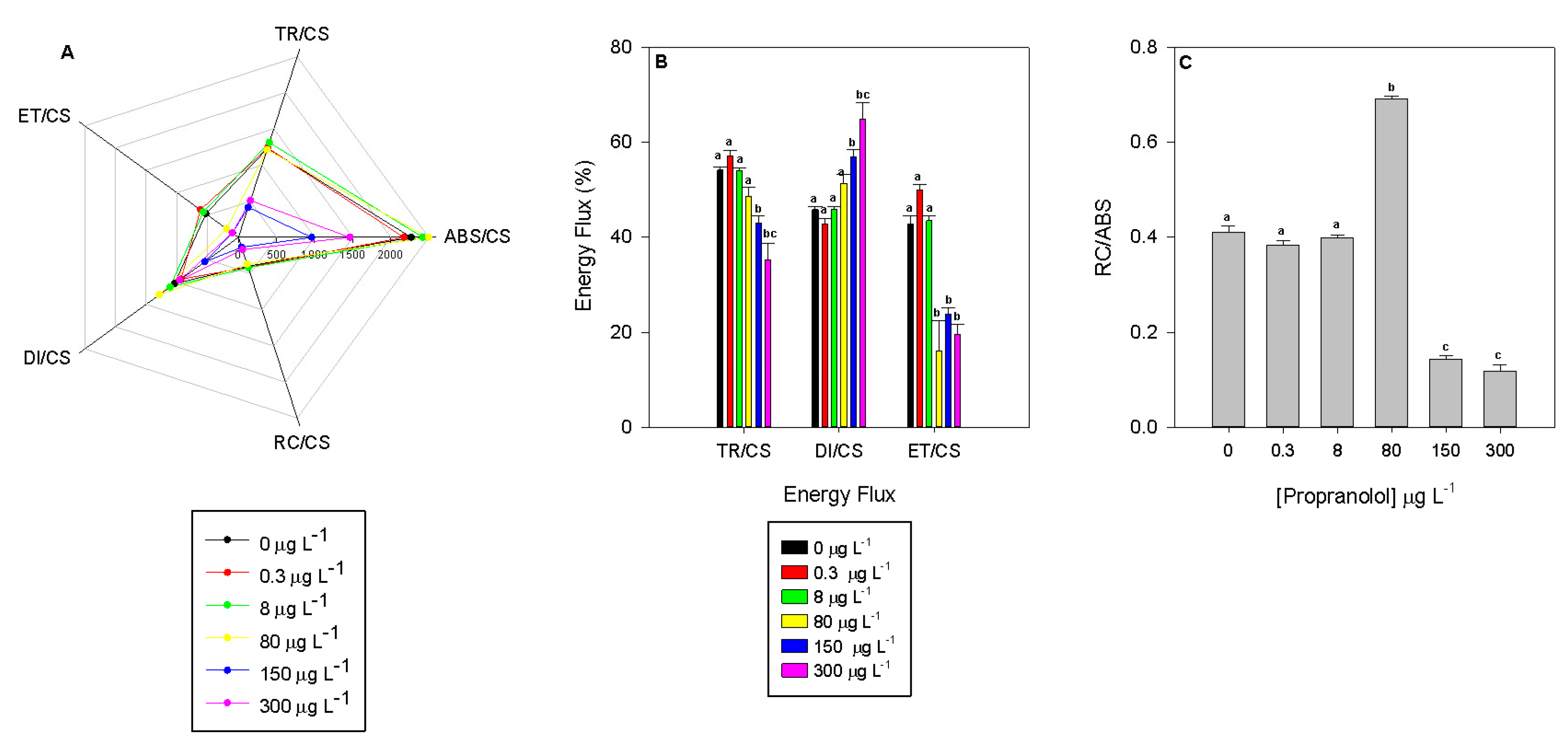
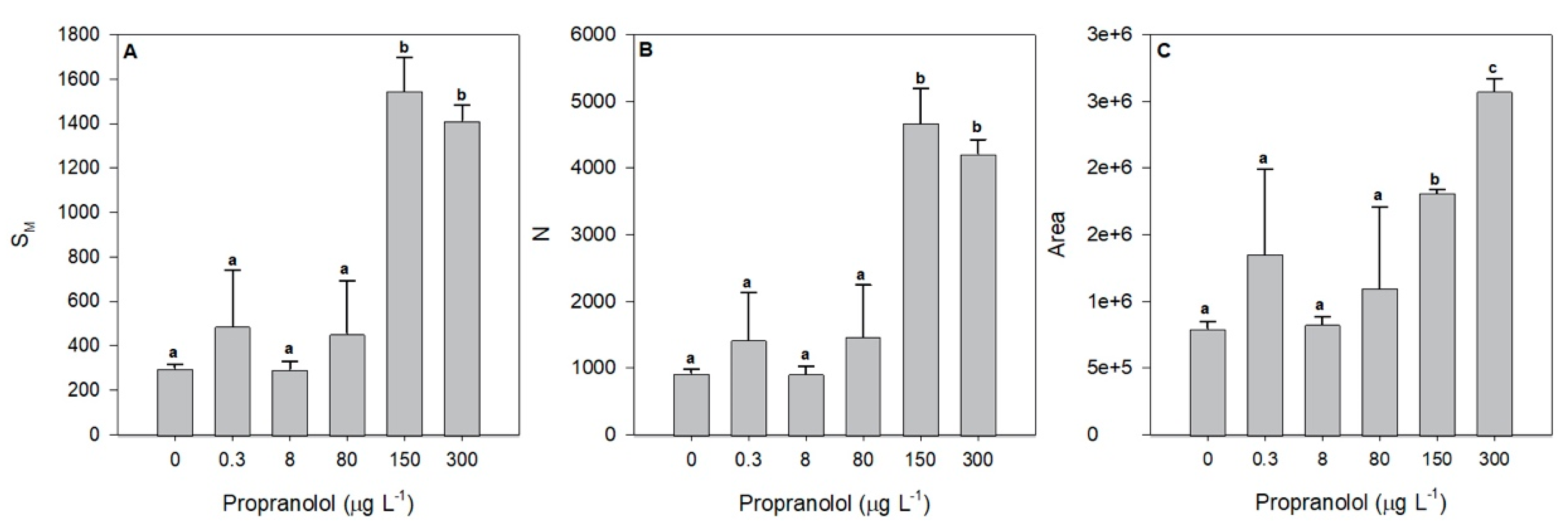
3. Results
3.1. Growth-Related Features
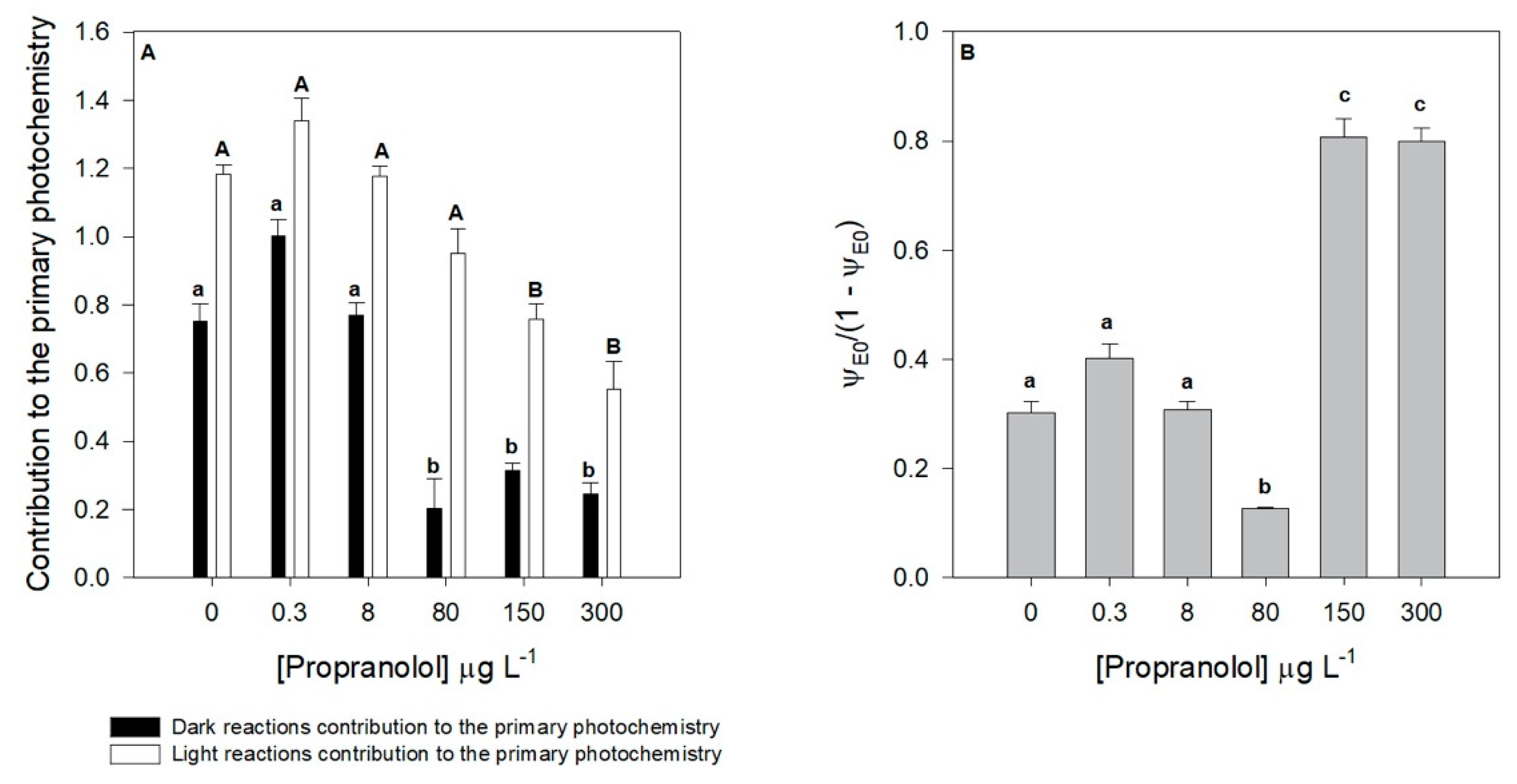
3.2. Photobiological Traits
3.3. Energy Allocation and Consumption
3.4. Oxidative Stress
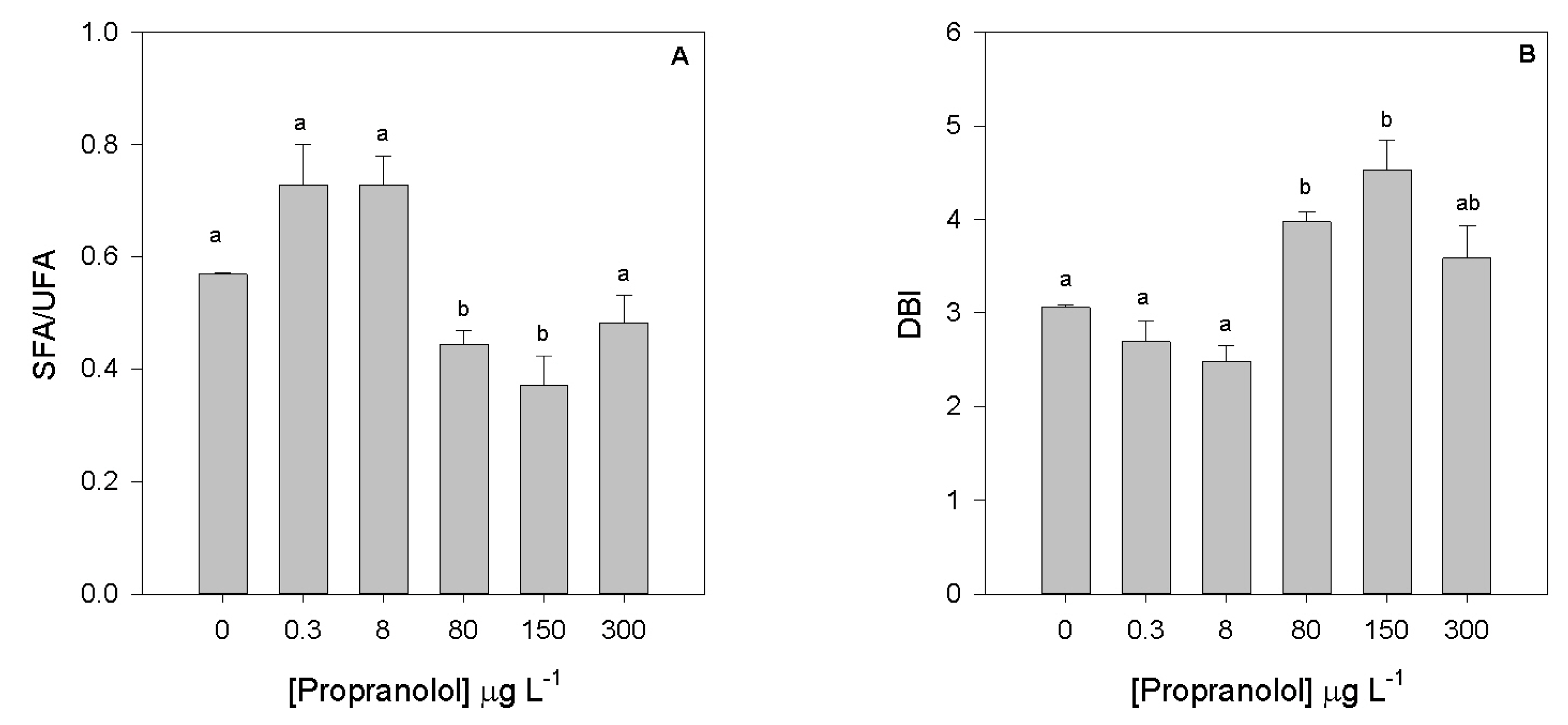
3.5. Fatty Acids Profile
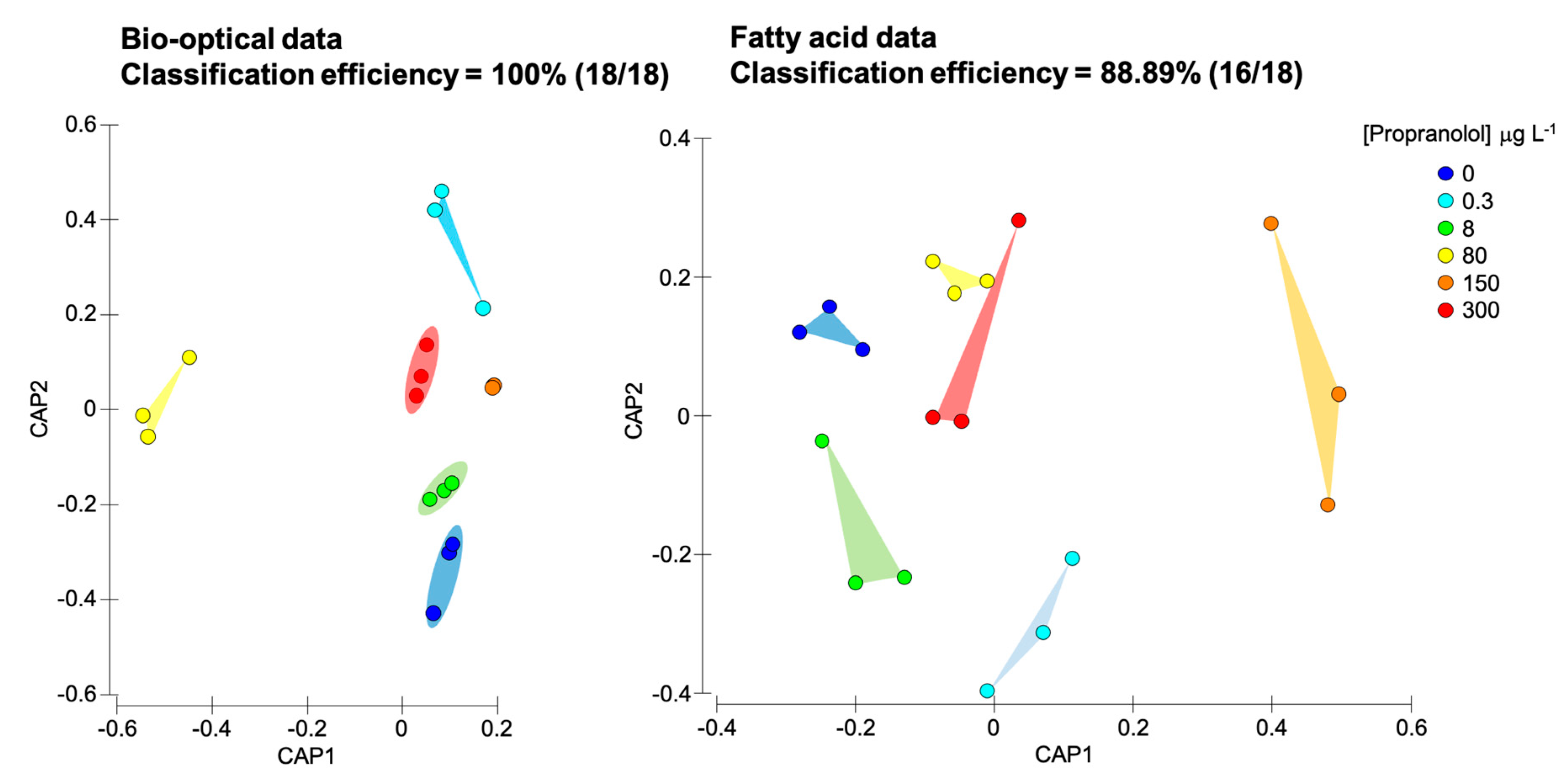
3.6. Overall Metabolic Impacts of Propranolol Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Martínez, M.L.; Intralawan, A.; Vázquez, G.; Pérez-Maqueo, O.; Sutton, P.; Landgrave, R. The coasts of our world: Ecological, economic and social importance. Ecol. Econ. 2007, 63, 254–272. [Google Scholar] [CrossRef]
- Li, H. Management of coastal mega-cities—A new challenge in the 21st century. Mar. Policy 2003, 27, 333–337. [Google Scholar] [CrossRef]
- Von Glasow, R.; Jickells, T.D.; Baklanov, A.; Carmichael, G.R.; Church, T.M.; Gallardo, L.; Hughes, C.; Kanakidou, M.; Liss, P.S.; Mee, L.; et al. Megacities and Large Urban Agglomerations in the Coastal Zone: Interactions between Atmosphere, Land, and Marine Ecosystems. Ambio 2013, 42, 13–28. [Google Scholar] [CrossRef]
- Gaw, S.; Thomas, K.V.; Hutchinson, T.H. Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130572. [Google Scholar] [CrossRef]
- Reis-Santos, P.; Pais, M.; Duarte, B.; Caçador, I.; Freitas, A.; Vila Pouca, A.S.; Barbosa, J.; Leston, S.; Rosa, J.; Ramos, F.; et al. Screening of human and veterinary pharmaceuticals in estuarine waters: A baseline assessment for the Tejo estuary. Mar. Pollut. Bull. 2018, 135, 1079–1084. [Google Scholar] [CrossRef]
- Anette, K.; Nicole, A. Pharmaceuticals in the environment: Scientific evidence of risks and its regulation. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130587. [Google Scholar]
- Crain, C.M.; Halpern, B.S.; Beck, M.W.; Kappel, C. V Understanding and managing human threats to the coastal marine environment. Ann. N. Y. Acad. Sci. 2009, 1162, 39–62. [Google Scholar] [CrossRef]
- Franzellitti, S.; Buratti, S.; Valbonesi, P.; Capuzzo, A.; Fabbri, E. The β-blocker propranolol affects cAMP-dependent signaling and induces the stress response in Mediterranean mussels, Mytilus galloprovincialis. Aquat. Toxicol. 2011, 101, 299–308. [Google Scholar] [CrossRef]
- Bendz, D.; Paxéus, N.A.; Ginn, T.R.; Loge, F.J. Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Höje River in Sweden. J. Hazard. Mater. 2005, 122, 195–204. [Google Scholar] [CrossRef]
- Woldegiorgis, A.; Andersson, J.; Remberger, M.; Kaj, L.; Ekheden, Y.; Blom, L.; Brorström-Lundén, E.; Anders Borgen, C.D.; Schlabach, M. Results from the Swedish National Screening Programme 2005 Subreport 3: Perfluorinated Alkylated Substances (PFAS); Tehnical Report, IVL: Stockholm, Sweden, 2006. [Google Scholar]
- Solé, M.; Shaw, J.P.; Frickers, P.E.; Readman, J.W.; Hutchinson, T.H. Effects on feeding rate and biomarker responses of marine mussels experimentally exposed to propranolol and acetaminophen. Anal. Bioanal. Chem. 2010, 396, 649–656. [Google Scholar] [CrossRef]
- Maurer, M.; Escher, B.I.; Richle, P.; Schaffner, C.; Alder, A.C. Elimination of β-blockers in sewage treatment plants. Water Res. 2007, 41, 1614–1622. [Google Scholar] [CrossRef]
- Breton, R.; Boxall, A. Pharmaceuticals and personal care products in the environment: Regulatory drivers and research needs. Proc. QSAR Comb. Sci. 2003, 22, 399–409. [Google Scholar] [CrossRef]
- Johnson, A.C.; Keller, V.; Williams, R.J.; Young, A. A practical demonstration in modelling diclofenac and propranolol river water concentrations using a GIS hydrology model in a rural UK catchment. Environ. Pollut. 2007, 146, 155–165. [Google Scholar] [CrossRef]
- Ericson, H.; Thorsén, G.; Kumblad, L. Physiological effects of diclofenac, ibuprofen and propranolol on Baltic Sea blue mussels. Aquat. Toxicol. 2010, 99, 223–231. [Google Scholar] [CrossRef]
- Thomas, K.V.; Hilton, M.J. The occurrence of selected human pharmaceutical compounds in UK estuaries. Mar. Pollut. Bull. 2004, 49, 436–444. [Google Scholar] [CrossRef]
- Ashton, D.; Hilton, M.; Thomas, K.V. Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom. Sci. Total Environ. 2004, 333, 167–184. [Google Scholar] [CrossRef]
- Huggett, D.B.; Brooks, B.W.; Peterson, B.; Foran, C.M.; Schlenk, D. Toxicity of select beta adrenergic receptor-blocking pharmaceuticals (B-blockers) on aquatic organisms. Arch. Environ. Contam. Toxicol. 2002, 43, 229–235. [Google Scholar] [CrossRef]
- Santos, L.H.M.L.M.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef]
- Franzellitti, S.; Buratti, S.; Du, B.; Haddad, S.P.; Chambliss, C.K.; Brooks, B.W.; Fabbri, E. A multibiomarker approach to explore interactive effects of propranolol and fluoxetine in marine mussels. Environ. Pollut. 2015, 205, 60–69. [Google Scholar] [CrossRef]
- Owen, S.F.; Giltrow, E.; Huggett, D.B.; Hutchinson, T.H.; Saye, J.A.; Winter, M.J.; Sumpter, J.P. Comparative physiology, pharmacology and toxicology of β-blockers: Mammals versus fish. Aquat. Toxicol. 2007, 82, 145–162. [Google Scholar] [CrossRef]
- Galindo-Trigo, S.; Gray, J.E.; Smith, L.M. Conserved roles of CrRLK1L receptor-like kinases in cell expansion and reproduction from algae to angiosperms. Front. Plant Sci. 2016, 7, 1269. [Google Scholar] [CrossRef] [PubMed]
- Schulze, B.; Buhmann, M.T.; Río Bártulos, C.; Kroth, P.G.; Bártulos, C.R.; Kroth, P.G. Comprehensive computational analysis of leucine-rich repeat (LRR) proteins encoded in the genome of the diatom Phaeodactylum tricornutum. Mar. Genom. 2015, 21, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Scalco, E.; Audic, S.; Vincent, F.; Veluchamy, A.; Poulain, J.; Wincker, P.; Iudicone, D.; de Vargas, C.; Bittner, L.; et al. Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl. Acad. Sci. USA 2016, 113, E1516–E1525. [Google Scholar] [CrossRef] [PubMed]
- Domingues, N.; Matos, A.R.; da Silva, J.M.; Cartaxana, P. Response of the Diatom Phaeodactylum tricornutum to photooxidative stress resulting from high light exposure. PLoS ONE 2012, 7, e38162. [Google Scholar] [CrossRef] [PubMed]
- Benoiston, A.-S.; Ibarbalz, F.M.; Bittner, L.; Guidi, L.; Jahn, O.; Dutkiewicz, S.; Bowler, C. The evolution of diatoms and their biogeochemical functions. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160397. [Google Scholar] [CrossRef]
- Wiktorowska-Owczarek, A.; Berezińska, M.; Nowak, J. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef]
- Arts, M.T.; Ackman, R.G.; Holub, B.J. “Essential fatty acids” in aquatic ecosystems: A crucial link between diet and human health and evolution. Can. J. Fish. Aquat. Sci. 2001, 58, 122–137. [Google Scholar] [CrossRef]
- Parrish, C.C. Lipids in Marine Ecosystems. ISRN Oceanogr. 2013, 2013, 604045. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Duarte, B.; Gameiro, C.; Godinho, R.M.; Caçador, I. Photochemical features and trace element substituted chlorophylls as early detection biomarkers of metal exposure in the model diatom Phaeodactylum tricornutum. Ecol. Indic. 2018, 95, 1038–1052. [Google Scholar] [CrossRef]
- Bowler, C.; Allen, A.E.; Badger, J.H.; Grimwood, J.; Jabbari, K.; Kuo, A.; Maheswari, U.; Martens, C.; Maumus, F.; Otillar, R.P.; et al. The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 2008, 456, 239–244. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Raimundo, J.; Pereira, P.; Vale, C. Immobilised Phaeodactylum tricornutum as biomonitor of trace element availability in the water column during dredging. Environ. Sci. Pollut. Res. 2014, 21, 3572–3581. [Google Scholar] [CrossRef] [PubMed]
- Feijão, E.; Gameiro, C.; Franzitta, M.; Duarte, B.; Caçador, I.; Cabrita, M.T.; Matos, A.R. Heat wave impacts on the model diatom Phaeodactylum tricornutum: Searching for photochemical and fatty acid biomarkers of thermal stress. Ecol. Indic. 2018, 95, 1026–1037. [Google Scholar] [CrossRef]
- Dodson, V.J.; Mouget, J.L.; Dahmen, J.L.; Leblond, J.D. The long and short of it: Temperature-dependent modifications of fatty acid chain length and unsaturation in the galactolipid profiles of the diatoms Haslea ostrearia and Phaeodactylum tricornutum. Hydrobiologia 2014, 727, 95–107. [Google Scholar] [CrossRef]
- Matos, A.R.; Gameiro, C.L.; Duarte, B.; Caçador, I.; Cabrita, M.T. Effects of nickel on the fatty acid composition of the diatom Phaeodactylum tricornutum. In Proceedings of the IMMR|International Meeting on Marine Research 2016, Peniche, Portugal, 14–15 July 2016. [Google Scholar]
- Abida, H.; Dolch, L.-J.J.; Meï, C.; Villanova, V.; Conte, M.; Block, M.A.; Finazzi, G.; Bastien, O.; Tirichine, L.; Bowler, C.; et al. Membrane glycerolipid remodeling triggered by nitrogen and phosphorus starvation in Phaeodactylum tricornutum. Plant Physiol. 2015, 167, 118–136. [Google Scholar] [CrossRef]
- Duarte, B.; Prata, D.; Matos, A.R.; Cabrita, M.T.; Caçador, I.; Marques, J.C.; Cabral, H.N.; Reis-Santos, P.; Fonseca, V.F. Ecotoxicity of the lipid-lowering drug bezafibrate on the bioenergetics and lipid metabolism of the diatom Phaeodactylum tricornutum. Sci. Total Environ. 2019, 650, 2085–2094. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, X.; Wang, Y.; Yang, Y.; Zhang, W.; Zhao, Y.; Zhang, X. ROS changes are responsible for tributyl phosphate (TBP)-induced toxicity in the alga Phaeodactylum tricornutum. Aquat. Toxicol. 2019, 208, 168–178. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of Marine Planktonic Diatoms: I. Cyclotella Nana Hustedt, and Detonula Confervacea (cleve) gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals. Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD: Paris, France, 2011; pp. 1–25. [Google Scholar]
- Claessens, M.; Vanhaecke, L.; Wille, K.; Janssen, C.R. Emerging contaminants in Belgian marine waters: Single toxicant and mixture risks of pharmaceuticals. Mar. Pollut. Bull. 2013, 71, 41–50. [Google Scholar] [CrossRef]
- Santos-Ballardo, D.U.; Rossi, S.; Hernández, V.; Gómez, R.V.; del Carmen Rendón-Unceta, M.; Caro-Corrales, J.; Valdez-Ortiz, A. A simple spectrophotometric method for biomass measurement of important microalgae species in aquaculture. Aquaculture 2015, 448, 87–92. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. Crop Improvement for Food Security; Behl, R.K., Punia, M.S., Lather, B.P.S., Eds.; SSARM: Hisar, India, 1999; pp. 72–115. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration Series; Govindjee, G., Papageorgiou, G., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- De Coen, W.M.; Janssen, C.R. The use of biomarkers in Daphnia magna toxicity testing. IV. Cellular Energy Allocation: A new methodology to assess the energy budget of toxicant-stressed Daphnia populations. J. Aquat. Ecosyst. Stress Recover. 1997, 6, 43–55. [Google Scholar] [CrossRef]
- Gnaiger, E. Calculation of Energetic and Biochemical Equivalents of Respiratory Oxygen Consumption. In Proceedings of the Polarographic Oxygen Sensors; Gnaiger, E., Forstner, H., Eds.; Springer: Berlin, Germany, 1983; pp. 337–345. [Google Scholar]
- King, F.D.; Packard, T.T. Respiration and the activity of the respiratory electron transport system in marine zooplankton. Limnol. Oceanogr. 1975, 20, 849–854. [Google Scholar] [CrossRef]
- Aderemi, A.O.; Novais, S.C.; Lemos, M.F.; Alves, L.M.; Hunter, C.; Pahl, O. Oxidative stress responses and cellular energy allocation changes in microalgae following exposure to widely used human antibiotics. Aquat. Toxicol. 2018, 203, 130–139. [Google Scholar] [CrossRef]
- Verslycke, T.; Ghekiere, A.; Janssen, C.R. Seasonal and spatial patterns in cellular energy allocation in the estuarine mysid Neomysis integer (Crustacea: Mysidacea) of the Scheldt estuary (The Netherlands). J. Exp. Mar. Bio Ecol. 2004, 306, 245–267. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Teranishi, Y.; Tanaka, A.; Osumi, M.; Fukui, S. Catalase activities of hydrocarbon-utilizing candida yeasts. Agric. Biol. Chem. 1974, 38, 1213–1220. [Google Scholar] [CrossRef]
- Tiryakioglu, M.; Eker, S.; Ozkutlu, F.; Husted, S.; Cakmak, I. Antioxidant defense system and cadmium uptake in barley genotypes differing in cadmium tolerance. J. Trace Elem. Med. Biol. 2006, 20, 181–189. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Matos, A.R.; Hourton-Cabassa, C.; Ciçek, D.; Rezé, N.; Arrabaça, J.D.; Zachowski, A.; Moreau, F. Alternative oxidase involvement in cold stress response of Arabidopsis thaliana fad2 and FAD3+ cell suspensions altered in membrane lipid composition. Plant Cell Physiol. 2007, 48, 856–865. [Google Scholar] [CrossRef]
- Duarte, B.; Pedro, S.; Marques, J.C.; Adão, H.; Caçador, I. Zostera noltii development probing using chlorophyll a transient analysis (JIP-test) under field conditions: Integrating physiological insights into a photochemical stress index. Ecol. Indic. 2017, 76, 219–229. [Google Scholar] [CrossRef]
- Feijão, E.; Cruz de Carvalho, R.; Duarte, I.A.; Matos, A.R.; Cabrita, M.T.; Novais, S.C.; Lemos, M.F.L.; Caçador, I.; Marques, J.C.; Reis-Santos, P.; et al. Fluoxetine Arrests Growth of the Model Diatom Phaeodactylum tricornutum by Increasing Oxidative Stress and Altering Energetic and Lipid Metabolism. Front. Microbiol. 2020, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.; Cabrita, M.T.; Vidal, T.; Pereira, J.L.; Pacheco, M.; Pereira, P.; Canário, J.; Gonçalves, F.J.M.; Matos, A.R.; Rosa, R.; et al. Phytoplankton community-level bio-optical assessment in a naturally mercury contaminated Antarctic ecosystem (Deception Island). Mar. Environ. Res. 2018, 140, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2006; 192p. [Google Scholar]
- Weir, M.R. β-Blockers in the Treatment of Hypertension: Are There Clinically Relevant Differences? Postgrad. Med. 2009, 121, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Burdiak, P.; Rusaczonek, A.; Witoń, D.; Głów, D.; Karpiński, S. Cysteine-rich receptor-like kinase CRK5 as a regulator of growth, development, and ultraviolet radiation responses in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 3325–3337. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Sharkey, T.D. Stomatal Conductance and Photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.-Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Havurinne, V.; Tyystjärvi, E. Action spectrum of photoinhibition in the diatom Phaeodactylum tricornutum. Plant Cell Physiol. 2017, 58, 2217–2225. [Google Scholar] [CrossRef]
- Lavaud, J.; van Gorkom, H.; Etienne, A.-L. Photosystem II electron transfer cycle and chlorespiration in planktonic diatoms. Photosynth. Res. 2002, 74, 51–59. [Google Scholar] [CrossRef]
- Sakurai, I.; Mizusawa, N.; Wada, H.; Sato, N. Digalactosyldiacylglycerol is required for stabilization of the oxygen-evolving complex in photosystem II. Plant Physiol. 2007, 145, 1361–1370. [Google Scholar] [CrossRef]
- Kern, J.; Guskov, A. Lipids in photosystem II: Multifunctional cofactors. J. Photochem. Photobiol. B Biol. 2011, 104, 19–34. [Google Scholar] [CrossRef]
- Mizusawa, N.; Wada, H. The role of lipids in photosystem II. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 194–208. [Google Scholar] [CrossRef]
- Li, Y.-F.; Gao, Y.; Chai, Z.; Chen, C. Nanometallomics: An emerging field studying the biological effects of metal-related nanomaterials. Metallomics 2014, 6, 220. [Google Scholar] [CrossRef]
- Wong, D.M.; Franz, A.K. A comparison of lipid storage in phaeodactylum tricornutum and tetraselmis suecica using laser scanning confocal microscopy. J. Microbiol. Methods 2013, 95, 122–128. [Google Scholar] [CrossRef]
- Chai, M.Q.; Chen, J.S.; Zhao, S.; Song, J.G. Propranolol increases phosphatidic acid level via activation of phospholipase D. Acta Pharmacol. Sin. 2001, 22, 777–784. [Google Scholar]
- Cerón García, M.C.; Sánchez Mirón, A.; Fernández Sevilla, J.M.; Molina Grima, E.; García Camacho, F. Mixotrophic growth of the microalga Phaeodactylum tricornutum: Influence of different nitrogen and organic carbon sources on productivity and biomass composition. Process Biochem. 2005, 40, 297–305. [Google Scholar] [CrossRef]
- Maeng, S.K.; You, S.H.; Nam, J.Y.; Ryu, H.; Timmes, T.C.; Kim, H.C. The growth of Scenedesmus quadricauda in RO concentrate and the impacts on refractory organic matter, Escherichia coli, and trace organic compounds. Water Res. 2018, 134, 292–300. [Google Scholar] [CrossRef]
- Sample, K.T.; Cain, R.B.; Schmidt, S.; Semple, K.T.; Cain, R.B.; Schmidt, S. Biodegradation of aromatic compounds by microalgae. FEMS Microbiol. Lett. 1999, 170, 291–300. [Google Scholar] [CrossRef]
- Kloner, R.A.; Fishbein, M.C.; Braunwald, E.; Maroko, P.R. Effect of propranolol on mitochondrial morphology during acute myocardial ischemia. Am. J. Cardiol. 1978, 41, 880–886. [Google Scholar] [CrossRef]
- Nayler, W.G.; Ferrari, R.; Williams, A. Protective effect of pretreatment with verapamil, nifedipine and propranolol on mitochondrial function in the ischemic and reperfused myocardium. Am. J. Cardiol. 1980, 46, 242–248. [Google Scholar] [CrossRef]
- Sakurada, A.; Voss, D.O.; Brandão, D.; Campello, A.P. Effects of propranolol on heart muscle mitochondria. Biochem. Pharmacol. 1972, 21, 535–540. [Google Scholar] [CrossRef]
- Huggett, D.B.; Cook, J.C.; Ericson, J.F.; Williams, R.T. A theoretical model for utilizing mammalian pharmacology and safety data to prioritize potential impacts of human pharmaceuticals to fish. Hum. Ecol. Risk Assess. 2003, 9, 1789–1799. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Brausch, J.M.; Connors, K.A.; Brooks, B.W.; Rand, G.M. Human Pharmaceuticals in the Aquatic Environment: A Review of Recent Toxicological Studies and Considerations for Toxicity Testing. In Reviews of Environmental Contamination and Toxicology Volume 218; Whitacre, D.M., Ed.; Springer: Boston, MA, USA, 2012; pp. 1–99. [Google Scholar]
| OJIP-Test | Parameter Description |
|---|---|
| Area | Corresponds to the oxidized quinone pool size available for reduction and is a function of the area above the Kautsky plot |
| N | Reaction center turnover rate |
| SM | Corresponds to the energy needed to close all reaction centers |
| PG | Grouping probability between the two PSII units |
| ABS/CS | Absorbed energy flux per cross-section |
| TR/CS | Trapped energy flux per cross-section |
| ET/CS | Electron transport energy flux per cross-section |
| DI/CS | Dissipated energy flux per cross-section |
| RC/CS | Number of available reaction centers per cross-section |
| %TR | Relative trapped energy flux per cross-section (%TR = TR/CS/ABS/CS) |
| %ET | Relative electron transport energy flux per cross-section (%ET = ET/CS/TR/CS) |
| %DI | Relative dissipated energy flux per cross-section (%DI = DI/CS/ABS/CS) |
| TR0/DI0 | Contribution or partial performance due to the light reactions for primary photochemistry |
| φo/(1 − φo) | Contribution or partial performance due to the dark reactions for primary photochemistry |
| ψE0/(1 − ψE0) | Equilibrium constant for the redox reactions between PS II and PS I |
| RC/ABS | Reaction center II density within the antenna chlorophyll bed of PS II |
| [Propranolol] μg L−1 | Proteins (mJ 10−6 Cells) | Carbohydrates (mJ 10−6 Cells) | Lipids (mJ 10−6 Cells) |
|---|---|---|---|
| 0 | 183.4 ± 22.7 a | 106.7 ± 15.5 a | 618.0 ± 43.8 a |
| 0.3 | 277.1 ± 47.3 a | 62.4 ± 19.7 ab | 756.4 ± 41.3 a |
| 8 | 239.6 ± 25.0 a | 111.3 ± 5.0 a | 723.1 ± 28.8 a |
| 80 | 422.9 ± 33.7 b | 33.3 ± 7.4 b | 734.6 ± 59.5 a |
| 150 | 515.4 ± 49.1 b | 6.5 ± 1.8 c | 1458.6 ± 73.5 b |
| 300 | 571.2 ± 139.46 b | 51.2 ± 14.3 ab | 933.3 ± 154.9 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, B.; Feijão, E.; Cruz de Carvalho, R.; Duarte, I.A.; Silva, M.; Matos, A.R.; Cabrita, M.T.; Novais, S.C.; Lemos, M.F.L.; Marques, J.C.; et al. Effects of Propranolol on Growth, Lipids and Energy Metabolism and Oxidative Stress Response of Phaeodactylum tricornutum. Biology 2020, 9, 478. https://doi.org/10.3390/biology9120478
Duarte B, Feijão E, Cruz de Carvalho R, Duarte IA, Silva M, Matos AR, Cabrita MT, Novais SC, Lemos MFL, Marques JC, et al. Effects of Propranolol on Growth, Lipids and Energy Metabolism and Oxidative Stress Response of Phaeodactylum tricornutum. Biology. 2020; 9(12):478. https://doi.org/10.3390/biology9120478
Chicago/Turabian StyleDuarte, Bernardo, Eduardo Feijão, Ricardo Cruz de Carvalho, Irina A. Duarte, Marisa Silva, Ana Rita Matos, Maria Teresa Cabrita, Sara C. Novais, Marco F. L. Lemos, João Carlos Marques, and et al. 2020. "Effects of Propranolol on Growth, Lipids and Energy Metabolism and Oxidative Stress Response of Phaeodactylum tricornutum" Biology 9, no. 12: 478. https://doi.org/10.3390/biology9120478
APA StyleDuarte, B., Feijão, E., Cruz de Carvalho, R., Duarte, I. A., Silva, M., Matos, A. R., Cabrita, M. T., Novais, S. C., Lemos, M. F. L., Marques, J. C., Caçador, I., Reis-Santos, P., & Fonseca, V. F. (2020). Effects of Propranolol on Growth, Lipids and Energy Metabolism and Oxidative Stress Response of Phaeodactylum tricornutum. Biology, 9(12), 478. https://doi.org/10.3390/biology9120478










