The Role of Offspring Genotype-by-Sex Interactions, Independently of Environmental Cues, on the Phenotype Traits of an Obese Swine Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Animals and Experimental Design
2.3. Assessment of Morphological and Homeostatic Features of Piglets during Early Postnatal Development
2.4. Assessment of Morphological and Homeostatic Features of Piglets during Juvenile Development
2.5. Evaluation of Body Composition and Organs Weight of Piglets at 60 and 210 Days-Old
2.6. Evaluation of the Oxidant/Antioxidant Status of the Piglets
2.7. Evaluation of the Metabolic Status of Piglets
2.8. Evaluation of the Fat Content and Fatty Acid Composition of Tissue Samples
2.9. Statistical Analysis
3. Results
3.1. Effects of Genotype and Sexon Litter Characteristics and Prenatal Development of the Piglets
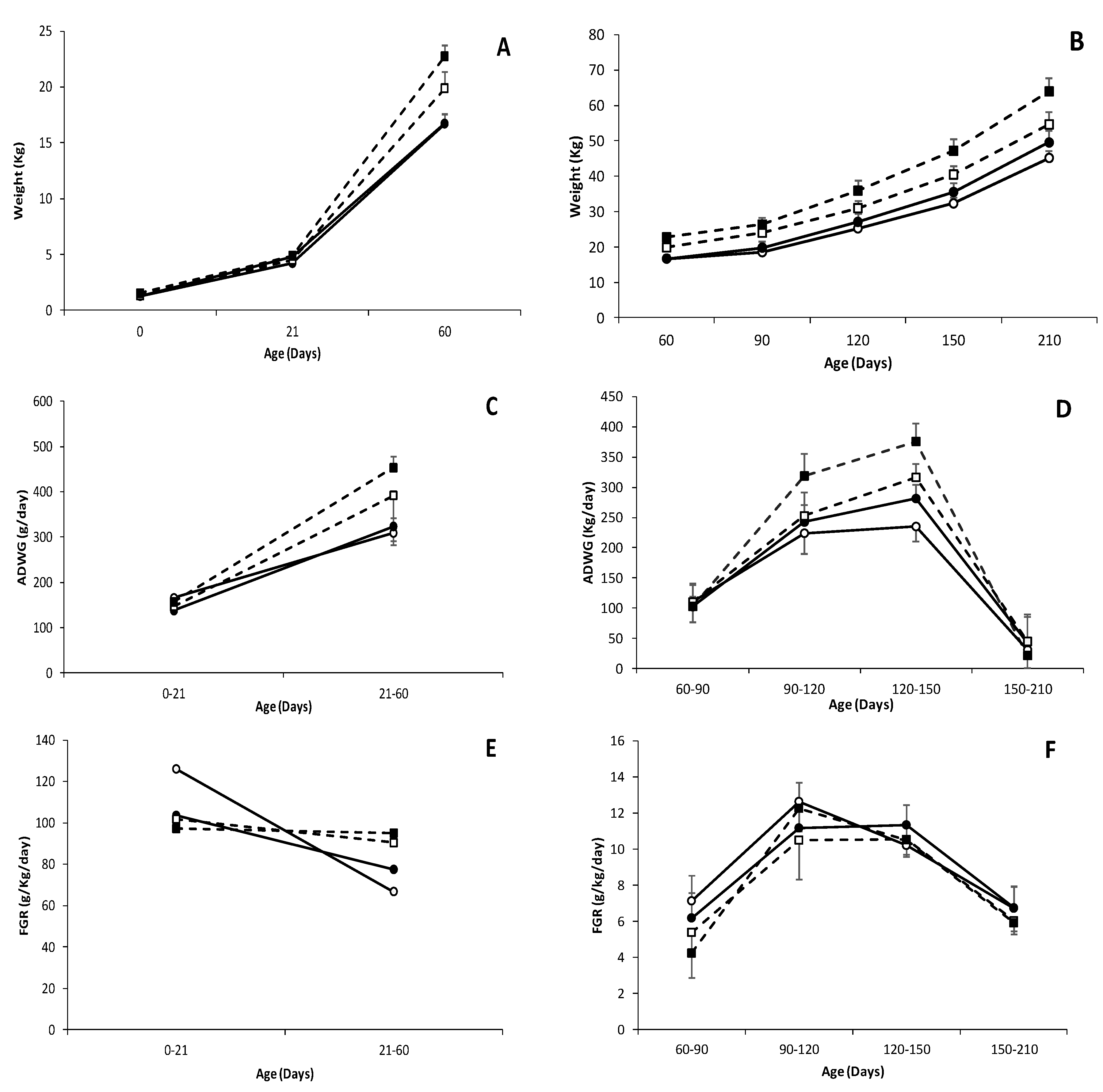
3.2. Effects of Genotype and Sex on Postnatal Patterns of Growth and Development of Piglets
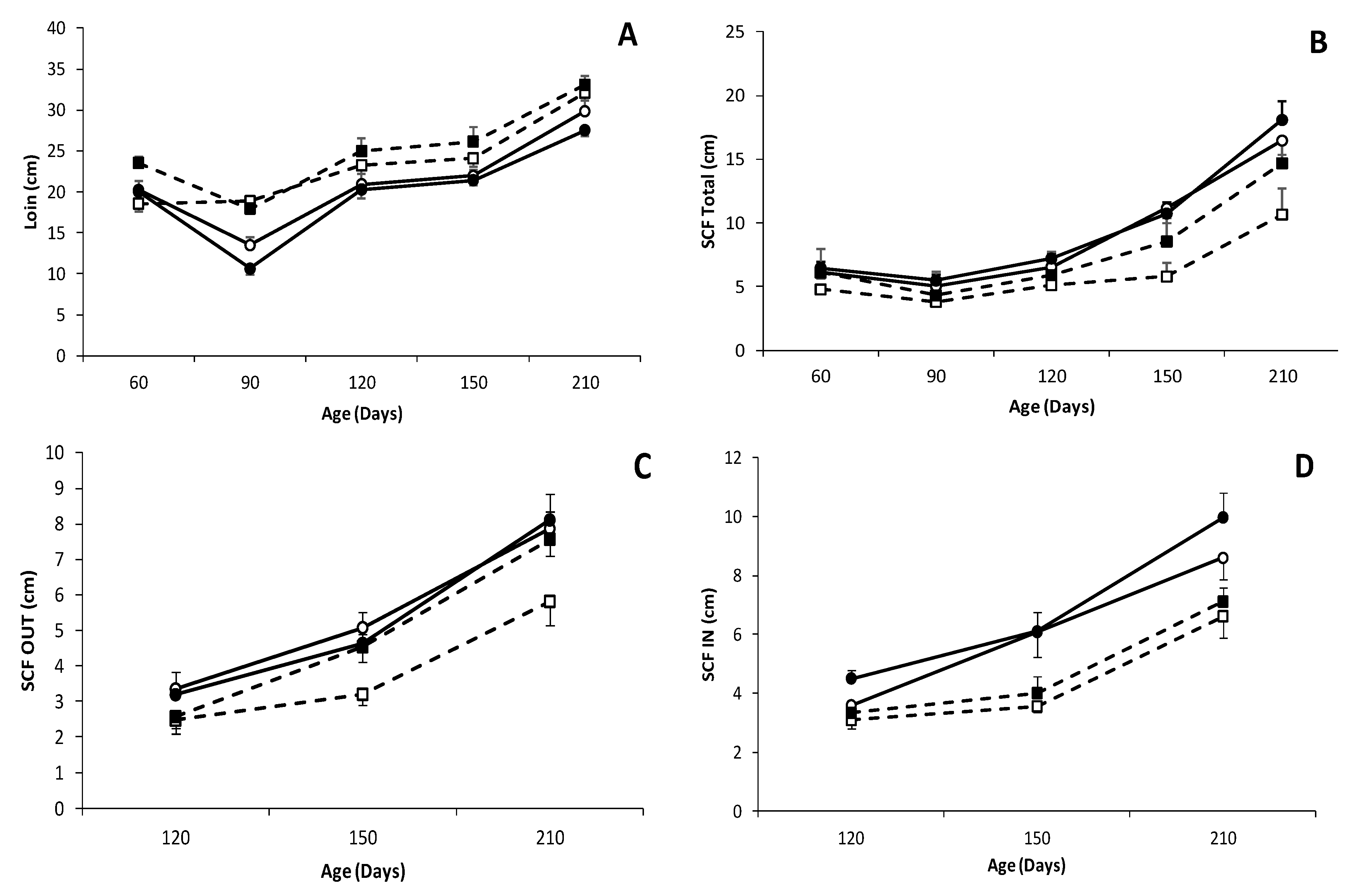
3.3. Effects of Genotype and Sex on Body Composition, Muscle Accretion and Adiposity
3.4. Effects of Genotype and Sex on the Antioxidant Capacity and Oxidative Stress of Piglets
3.5. Effects of Genotype and Sex on Metabolic Status of Piglets
3.6. Effects of Genotype and Sex on Fatty Acid Composition of the Piglets
3.7. Overview of Interactions between Genotype and Sex on Fatty Acid Composition of the Piglets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J.E. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Song, Y.; Caballero, B.; Cheskin, L. Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int. 2008, 73, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Finer, N.; Garnett, S.P.; Bruun, J.M. COVID-19 and obesity. Clin. Obes. 2020, 10, 12365. [Google Scholar] [CrossRef]
- Scheen, A.J. Obesity and risk of severe COVID-19. Rev. Med. Suisse 2020, 16, 1115–1119. [Google Scholar] [PubMed]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2012, 8, 228–236. [Google Scholar] [CrossRef]
- Scully, T. Diabetes in numbers. Nat. Cell Biol. 2012, 485, S2–S3. [Google Scholar] [CrossRef]
- Gluckman, P.D. Living with the Past: Evolution, Development, and Patterns of Disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef]
- Shetty, P. Public health: India’s diabetes time bomb. Nat. Cell Biol. 2012, 485, S14–S16. [Google Scholar] [CrossRef]
- Kong, A.P.; Xu, G.; Brown, N.; So, W.-Y.; Ma, R.C.W.; Chan, J.C.N. Diabetes and its comorbidities—Where East meets West. Nat. Rev. Endocrinol. 2013, 9, 537–547. [Google Scholar] [CrossRef]
- Prentice, A.M. Obesity in Emerging Nations: Evolutionary Origins and the Impact of a Rapid Nutrition Transition. In Meeting Micronutrient Requirements for Health and Development; Karger, A.G., Ed.; Karger Publishers: Berlin, Germany, 2009; Volume 63, pp. 47–57. [Google Scholar]
- Wells, J.C.K. Ethnic variability in adiposity and cardiovascular risk: The variable disease selection hypothesis. Int. J. Epidemiol. 2009, 38, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N. Beyond the Barker hypothesis and the thrifty genotype—The womb, ethnicity, genes and the environment—Recent perspectives on the evolution of diabetes and the metabolic syndrome in India. Indian J. Endocrinol. Metab. 2012, 16, S142–S146. [Google Scholar] [PubMed]
- Prentice, A.M. Nutrition and Chronic Disease: Lessons from the Developing and Developed World. Nestlé Nutr. Inst. Workshop Series 2014, 78, 155–160. [Google Scholar]
- Pandey, A.; Chawla, S.; Guchhait, P. Type-2 diabetes: Current understanding and future perspectives. IUBMB Life 2015, 67, 506–513. [Google Scholar] [CrossRef]
- Arner, P. Resistin: Yet another adipokine tells us that men are not mice. Diabetologia 2005, 48, 2203–2205. [Google Scholar] [CrossRef]
- Bähr, A.; Wolf, E. Domestic Animal Models for Biomedical Research. Reprod. Domest. Anim. 2012, 47, 59–71. [Google Scholar] [CrossRef]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J.; Frazier, K.S. Swine as Models in Biomedical Research and Toxicology Testing. Veter. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Russell, J.C.; Proctor, S.D. Small animal models of cardiovascular disease: Tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc. Pathol. 2006, 15, 318–330. [Google Scholar] [CrossRef]
- Hamernik, D.L. Farm animals are important biomedical models. Anim. Front. 2019, 9, 3–5. [Google Scholar] [CrossRef]
- Barbaux, S.; Erwich, J.; Favaron, P.; Gil, S.; Gallot, D.; Golos, T.; Gonzalez-Bulnes, A.; Guibourdenche, J.; Heazell, A.E.P.; Jansson, T.; et al. IFPA meeting 2014 workshop report: Animal models to study pregnancy pathologies; new approaches to study human placental exposure to xenobiotics; biomarkers of pregnancy pathologies; placental genetics and epigenetics; the placenta and stillbirth and fetal growth restriction. Placenta 2015, 36, S5–S10. [Google Scholar] [CrossRef]
- Bee, G. Effect of early gestation feeding, birth weight, and gender of progeny on muscle fiber characteristics of pigs at slaughter1. J. Anim. Sci. 2004, 82, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Quiniou, N.; Dagorn, J.; Gaudré, D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 2002, 78, 63–70. [Google Scholar] [CrossRef]
- Rehfeldt, C.; Kuhn, G. Consequences of birth weight for postnatal growth performance and carcass quality in pigs as related to myogenesis1. J. Anim. Sci. 2006, 84, E113–E123. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.P.; Caton, J.S. Role of the pre- and post-natal environment in developmental programming of health and productivity. Mol. Cell. Endocrinol. 2012, 354, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K. Advances in Swine Biomedical Model Genomics. Int. J. Biol. Sci. 2007, 3, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Weisgraber, K.H.; Innerarity, T.; Brewer, H.B.; Assmann, G. Swine lipoproteins and atherosclerosis. Changes in the plasma lipoproteins and apoproteins induced by cholesterol feeding. Biochemistry 1975, 14, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Dyson, M.C.; Alloosh, M.; Vuchetich, J.P.; Mokelke, E.A.; Sturek, M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp. Med. 2006, 56, 35–45. [Google Scholar]
- Spurlock, M.E.; Gabler, N.K. The Development of Porcine Models of Obesity and the Metabolic Syndrome. J. Nutr. 2008, 138, 397–402. [Google Scholar] [CrossRef]
- Guilloteau, P.; Zabielski, R.; Hammon, H.M.; Metges, C.C. Nutritional program-ming of gastrointestinal tract development. Is the pig a good model for man? Nutr. Res. Rev. 2010, 23, 4–22. [Google Scholar] [CrossRef]
- Walsh-Hentges, L.S.; Martin, R.J. Influence of genetic obesity on maternal andfetal serum and lipoprotein lipids in swine. Int. J. Obes. 1988, 12, 49–57. [Google Scholar]
- Torres-Rovira, L.; Astiz, S.; Caro, A.; Lopez-Bote, C.; Ovilo, C.; Pallares, P.; Perez-Solana, M.L.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Diet-Induced Swine Model with Obesity/Leptin Resistance for the Study of Metabolic Syndrome and Type 2 Diabetes. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rovira, L.; Gonzalez-Anover, P.; Astiz, S.; Caro, A.; Lopez-Bote, C.; Ovilo, C.; Pallares, P.; Perez-Solana, M.L.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Effect of an Obesogenic Diet During the Juvenile Period on Growth Pattern, Fatness and Metabolic, Cardiovascular and Reproductive Features of Swine with Obesity/Leptin Resistance. Endocr. Metab. Immune Disord. Drug Targets 2013, 13, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Augustine, K.A.; Rossi, R.M. Rodent mutant models of obesity and their correlations to human obesity. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1999, 257, 64–72. [Google Scholar] [CrossRef]
- Óvilo, C.; I Fernández, A.; Noguera, J.L.; Barragán, C.; Letón, R.; Rodríguez, C.; Mercadé, A.; Alves, E.; Folch, J.M.; Varona, L.; et al. Fine mapping of porcine chromosome 6 QTL and LEPR effects on body composition in multiple generations of an Iberian by Landrace intercross. Genet. Res. 2005, 85, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.; Óvilo, C.; Silió, L.; Tomás, A.; Noguera, J.L.; Rodríguez, M.C. Single- and joint-population analyses of two experimental pig crosses to confirm quantitative trait loci on Sus scrofa chromosome 6 and leptin receptor effects on fatness and growth traits1. J. Anim. Sci. 2009, 87, 459–468. [Google Scholar] [CrossRef]
- Fernández-Fígares, I.; Lachica, M.; Nieto, R.; Rivera-Ferre, M.G.; Aguilera, J. Serum profile of metabolites and hormones in obese (Iberian) and lean (Landrace) growing gilts fed balanced or lysine deficient diets. Livest. Sci. 2007, 110, 73–81. [Google Scholar] [CrossRef]
- Óvilo, C.; Fernández, A.; I Fernández, A.; Folch, J.M.; Varona, L.; Benítez, R.; Nüñez, Y.; Rodriguez, C.; Silió, L. Hypothalamic expression of porcine leptin receptor (LEPR), neuropeptide Y (NPY), and cocaine- and amphetamine-regulated transcript (CART) genes is influenced by LEPR genotype. Mamm. Genome 2010, 21, 583–591. [Google Scholar] [CrossRef]
- Óvilo, C.; Gonzalez-Bulnes, A.; Benítez, R.; Ayuso, M.; Barbero, A.; Pérez-Solana, M.L.; Barragán, C.; Astiz, S.; Fernández, A.; López-Bote, C. Prenatal programming in an obese swine model: Sex-related effects of maternal energy restriction on morphology, metabolism and hypothalamic gene expression. Br. J. Nutr. 2014, 111, 735–746. [Google Scholar] [CrossRef]
- Ayuso, M.; Fernández, A.; Núñez, Y.; Benítez, R.; Isabel, B.; Barragán, C.; Fernández, A.I.; Rey, A.I.; Medrano, J.F.; Cánovas, Á.; et al. Comparative Analysis of Muscle Transcriptome between Pig Genotypes Identifies Genes and Regulatory Mechanisms Associated to Growth, Fatness and Metabolism. PLoS ONE 2015, 10, e0145162. [Google Scholar] [CrossRef]
- Ayuso, M.; Fernández, A.; Núñez, Y.; Benítez, R.; Isabel, B.; Fernández, A.I.; Rey, A.I.; González-Bulnes, A.; Medrano, J.F.; Cánovas, Á.; et al. Developmental Stage, Muscle and Genetic Type Modify Muscle Transcriptome in Pigs: Effects on Gene Expression and Regulatory Factors Involved in Growth and Metabolism. PLoS ONE 2016, 11, e0167858. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.; Torres-Rovira, L.; Barbero, A.; Ayuso, M.; Garcia-Contreras, C.; Vazquez-Gomez, M. Developmental Origins of Health and Disease in swine: Implications for animal production and biomedical research. Theriogenology 2016, 86, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bulnes, A.; Óvilo, C.; López-Bote, C.; Astiz, S.; Ayuso, M.; Perez-Solana, M.; Sanchez-Sanchez, R.; Torres-Rovira, L. Fetal and Early-Postnatal Developmental Patterns of Obese-Genotype Piglets Exposed to Prenatal Programming by Maternal Over- and Undernutrition. Endocr. Metab. Immune Disord. Drug Targets 2013, 13, 240–249. [Google Scholar] [CrossRef]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Barbero, A.; Pesantez-Pacheco, J.L.; Zinellu, A.; Berlinguer, F.; Gonzalez-Añover, P.; Gonzalez, J.; Encinas, T.; Torres-Rovira, L.; et al. Polyphenols and IUGR Pregnancies: Effects of Maternal Hydroxytyrosol Supplementation on Placental Gene Expression and Fetal Antioxidant Status, DNA-Methylation and Phenotype. Int. J. Mol. Sci. 2019, 20, 1187. [Google Scholar] [CrossRef]
- Vazquez-Gomez, M.; Garcia-Contreras, C.; Torres-Rovira, L.; Pesantez, J.L.; Gonzalez-Añover, P.; Gómez-Fidalgo, E.; Sánchez-Sánchez, R.; Ovilo, C.; Isabel, B.; Astiz, S.; et al. Polyphenols and IUGR pregnancies: Maternal hydroxytyrosol supplementation improves prenatal and early-postnatal growth and metabolism of the offspring. PLoS ONE 2017, 12, e0177593. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Gomez, M.; Heras-Molina, A.; Garcia-Contreras, C.; Pesantez-Pacheco, J.L.; Torres-Rovira, L.; Martinez-Fernandez, B.; Gonzalez, J.; Encinas, T.; Astiz, S.; Óvilo, C.; et al. Polyphenols and IUGR Pregnancies: Effects of Maternal Hydroxytyrosol Supplementation on Postnatal Growth, Metabolism and Body Composition of the Offspring. Antioxidants 2019, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bulnes, A.; Astiz, S.; Encinas, T.; Gonzalez-Añover, P.; Perez-Solana, M.; Sanchez-Sanchez, R.; Torres-Rovira, L.; Tresguerres, J.A. Characterization of a distinctive pattern of periovulatory leptin secretion and its relationship with ovulation rate and luteal function in swine with obesity/leptin resistance. Peptides 2012, 37, 290–293. [Google Scholar] [CrossRef] [PubMed]
- González-Valero, L.; Rodríguez-López, J.M.; Lachica, M.; Fernández-Fígares, I. Metabolic differences in hepatocytes of obese and lean pigs. Animal 2014, 8, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Cogollos, L.; Garcia-Contreras, C.; Vazquez-Gomez, M.; Astiz, S.; Sanchez-Sanchez, R.; Gomez-Fidalgo, E.; Ovilo, C.; Isabel, B.; Gonzalez-Bulnes, A. Effects of fetal genotype and sex on developmental response to maternal malnutrition. Reprod. Fertil. Dev. 2017, 29, 1155–1168. [Google Scholar] [CrossRef]
- García-Contreras, C.; Madsen, O.; Groenen, M.A.M.; López-García, A.; Vázquez-Gómez, M.; Astiz, S.; Núñez, Y.; Benítez, R.; Fernández, A.; Isabel, B.; et al. Impact of genotype, body weight and sex on the prenatal muscle transcriptome of Iberian pigs. PLoS ONE 2020, 15, e0227861. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012; p. 420. [Google Scholar] [CrossRef]
- Daza, A.; Latorre, M.A.; Olivares, A.; Bote, C.L. The effects of male and female immunocastration on growth performances and carcass and meat quality of pigs intended for dry-cured ham production: A preliminary study. Livest. Sci. 2016, 190, 20–26. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Segura, J.; López-Bote, C. A laboratory efficient method for intramuscular fat analysis. Food Chem. 2014, 145, 821–825. [Google Scholar] [CrossRef]
- Ruiz-Carrascal, J.; Antequera, T.; Andres, A.; Petron, M.; Muriel, E. Improvement of a solid phase extraction method for analysis of lipid fractions in muscle foods. Anal. Chim. Acta 2004, 520, 201–205. [Google Scholar] [CrossRef]
- Lopez-Bote, C.; Rey, A.I.; Ruiz, J.R.; Isabel, B.; Arias, R.S. Effect of feeding diets high in monounsaturated fatty acids and α-tocopheryl acetate to rabbits on resulting carcass fatty acid profile and lipid oxidation. Anim. Sci. 1997, 64, 177–186. [Google Scholar] [CrossRef]
- Segura, J.; Escudero, R.; de Ávila, M.R.; Cambero, M.; López-Bote, C. Effect of fatty acid composition and positional distribution within the triglyceride on selected physical properties of dry-cured ham subcutaneous fat. Meat Sci. 2015, 103, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Pamplona, R.; Buffenstein, R.; Buttemer, W.A. Life and Death: Metabolic Rate, Membrane Composition, and Life Span of Animals. Physiol. Rev. 2007, 87, 1175–1213. [Google Scholar] [CrossRef] [PubMed]
- Hulver, M.W.; Berggren, J.R.; Carper, M.J.; Miyazaki, M.; Ntambi, J.M.; Hoffman, E.P.; Thyfault, J.P.; Stevens, R.; Dohm, G.L.; Houmard, J.A.; et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005, 2, 251–261. [Google Scholar] [CrossRef]
- Torres-Rovira, L.; Tarrade, A.; Astiz, S.; Mourier, E.; Perez-Solana, M.; de la Cruz, P.; Gómez-Fidalgo, E.; Sánchez-Sánchez, R.; Chavatte-Palmer, P.; Gonzalez-Bulnes, A. Sex and Breed-Dependent Organ Development and Metabolic Responses in Foetuses from Lean and Obese/Leptin Resistant Swine. PLoS ONE 2013, 8, e66728. [Google Scholar] [CrossRef][Green Version]
- Charneca, R.N.; le Dividich, J. Body composition and blood parameters of newborn piglets from Alentejano and conventional (Large White x Landrace) genotype. Spanish J. Agric. Res. 2010, 2, 317–325. [Google Scholar] [CrossRef]
- Palma-Granados, P.; Haro, A.; Seiquer, I.; Lara, L.; Aguilera, J.; Nieto, R. Similar effects of lysine deficiency in muscle biochemical characteristics of fatty and lean piglets1. J. Anim. Sci. 2017, 95, 3025–3036. [Google Scholar] [CrossRef] [PubMed]
- Barea, R.; Nieto, R.; Vitari, F.; Domeneghini, C.; Aguilera, J.F. Effects of pig genotype (Iberian v. Landrace × Large White) on nutrient digestibility, relative organ weight and small intestine structure at two stages of growth. Animal 2011, 5, 547–557. [Google Scholar] [CrossRef]
- O’Hea, E.K.; Leveille, G.A. Significance of Adipose Tissue and Liver as Sites of Fatty Acid Synthesis in the Pig and the Efficiency of Utilization of Various Substrates for Lipogenesis. J. Nutr. 1969, 99, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Wronska, A.; Kmieć, Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol. 2012, 205, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, J.; Ma, J.; Jiang, A.; Tang, G.; Mai, M.; Zhu, L.; Bai, L.; Li, M.; Li, X. Gene expression profiling reveals distinct features of various porcine adipose tissues. Lipids Heal. Dis. 2013, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H. Stearoyl-CoA Desaturase: A Vital Checkpoint in the Development and Progression of Obesity. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 217–231. [Google Scholar] [CrossRef]
- Roden, M.; Price, T.B.; Perseghin, G.; Petersen, K.F.; Rothman, D.L.; Cline, G.W.; I Shulman, G. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Investig. 1996, 97, 2859–2865. [Google Scholar] [CrossRef]
- Barbero, A.; Astiz, S.; Lopez-Bote, C.J.; Perez-Solana, M.L.; Ayuso, M.; Garcia-Real, I.; Gonzalez-Bulnes, A. Maternal Malnutrition and Offspring Sex Determine Juvenile Obesity and Metabolic Disorders in a Swine Model of Leptin Resistance. PLoS ONE 2013, 8, e78424. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.J.; Sanchez-Sanchez, R.; Perez-Solana, M.L.; Torres-Rovira, L.; Ayuso, M.; Gonzalez, J. Early-postnatal changes in adiposity and lipids profile by transgenerational developmental programming in swine with obesity/leptin resistance. J. Endocrinol. 2014, 223, M17–M29. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Omega-3 polyunsaturated fatty acids and human health outcomes. BioFactors 2009, 35, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Świątkiewicz, S.; Arczewska-Wlosek, A.; Józefiak, D. The relationship between dietary fat sources and immune response in poultry and pigs: An updated review. Livest. Sci. 2015, 180, 237–246. [Google Scholar] [CrossRef]
- Rutting, S.; Papanicolaou, M.; Xenaki, D.; Wood, L.G.; Mullin, A.M.; Hansbro, P.M.; Oliver, B.G. Dietary ω-6 polyunsaturated fatty acid arachidonic acid increases inflammation but inhibits ECM protein expression in COPD. Respir. Res. 2018, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Izaola-Jauregui, O.; Dd Luis, D.; Sajoux, I.; Domingo, J.C.; Vidal, M. Inflammation and obesity (lipoinflammation)]. Nutr. Hospital. 2015, 31, 2352–2358. [Google Scholar]
- Kuroda, M.; Sakaue, H. Adipocyte Death and Chronic Inflammation in Obesity. J. Med Investig. 2017, 64, 193–196. [Google Scholar] [CrossRef]
- Arango, J.; Misztal, I.; Tsuruta, S.; Culbertson, M.; Holl, J.; Herring, W. Genetic study of individual preweaning mortality and birth weight in Large White piglets using threshold-linear models. Livest. Sci. 2006, 101, 208–218. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Ovilo, C.; Lopez-Bote, C.J.; Astiz, S.; Ayuso, M.; Perez-Solana, M.L.; Sanchez-Sanchez, R.; Torres-Rovira, L. Gender-specific early postnatal catch-up growth after intrauterine growth retardation by food restriction in swine with obesity/leptin resistance. Reproduction 2012, 144, 269–278. [Google Scholar] [CrossRef]
- Aiken, C.E.; Ozanne, S.E. Sex differences in developmental programming models. Reproduction 2013, 145, R1–R13. [Google Scholar] [CrossRef]
- Fowden, A.L.; Moore, T. Maternal-fetal resource allocation: Co-operation and conflict. Placenta 2012, 33, e11–e15. [Google Scholar] [CrossRef]
- Lopez-Tello, J.; Arias-Alvarez, M.; Martinez, M.-A.J.; García-García, R.M.; Rodriguez, M.; Gonzalez, P.L.L.; Bermejo-Poza, R.; Gonzalez-Bulnes, A.; Rebollar, P.G. Competition for Materno-Fetal Resource Partitioning in a Rabbit Model of Undernourished Pregnancy. PLoS ONE 2017, 12, e0169194. [Google Scholar] [CrossRef]
- Rickard, I.J.; Russell, A.F.; Lummaa, V. Producing sons reduces lifetime reproductive success of subsequent offspring in pre-industrial Finns. Proc. R. Soc. B Boil. Sci. 2007, 274, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Mathews, F.; Johnson, P.J.; Neil, A. You are what your mother eats: Evidence for maternal preconception diet influencing foetal sex in humans. Proc. R. Soc. B Boil. Sci. 2008, 275, 1661–1668. [Google Scholar] [CrossRef] [PubMed]


| Parameter | IB × IB | IB × LW | Total | F vs M | IB × IB vs IB × LW | Gen × Sex | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Days) | Total | Females | Males | Total | Females | Males | IB × IB | IB × LW | F | M | |||
| FRAP (µmol/mL) | 60 | 17.4 ± 1.76 | 18.0 ± 3.35 | 16.9 ± 1.97 | 26.9 ± 6.10 | 10.3 ± 1.88 | 31.7 ± 5.01 | NS | NS | *** | + | + | *** |
| 120 | 24.8 ± 1.88 | 25.0 ± 2.38 | 24.6 ± 3.05 | 22.9 ± 1.64 | 18.9 ± 1.85 | 26.8 ± 0.98 | NS | NS | * | NS | NS | NS | |
| MDA (µmol/L) | 60 | 64.1 ± 12.2 | 62.6 ± 10.2 | 65.2 ± 13.6 | 73.1 ± 12.3 | 77.4 ± 12.3 | 69.5 ± 12.0 | * | NS | NS | * | NS | NS |
| 120 | 54.4 ± 6.41 | 54.3 ± 5.04 | 54.6 ± 7.87 | 52.8 ± 4.39 | 52.8 ± 5.55 | 52.9 ± 3.58 | NS | NS | NS | NS | NS | NS | |
| mg/dL | 60 Days | 120 Days | 150 Days | 210 Days | ||||
|---|---|---|---|---|---|---|---|---|
| IB × IB | IB × LW | IB × IB | IB × LW | IB × IB | IB × LW | IB × IB | IB × LW | |
| GLU | 99.8 ± 4.79 | 101 ± 4.17 | 85.1 ± 2.32 | 89.17 ± 3.80 | 85.1 a ± 2.23 | 95.3 b ± 5.76 | 82.6 ± 4.26 | 84.2 ± 4.02 |
| FRU | 222 ± 6.76 | 226 ± 9.4 | 222 ± 6.76 | 227 ± 4.83 | 227 ± 8.19 | 241 ± 5.36 | 252 ± 5.74 | 254 ± 7.47 |
| CHO | 76.6 ± 3.35 | 82.9 ± 3.29 | 122 c ± 3.53 | 100 d ± 4.04 | 124 c ± 3.75 | 103 d ± 3.86 | 109 a ± 3.54 | 95.2 b ± 5.45 |
| HDL-c | 25.6 ± 1.49 | 29.2 ± 1.59 | 64.7 e ± 1.78 | 53.1 f ± 2.67 | 67.0 c ± 1.62 | 60.2 d ± 2.08 | 55.0 ± 2.46 | 52.3 ± 6.31 |
| LDL-c | 39.5 ± 2.26 | 45.4 ± 3.27 | 56.8 a ± 3.57 | 41.6 b ± 1.89 | 58.2 a ± 3.70 | 44.4 b ± 2.67 | 48.8 ± 2.67 | 43.2 ± 3.78 |
| TG | 34.6 ± 2.50 | 34.9 ± 2.18 | 65.3 ± 3.83 | 55.0 ± 6.92 | 64.6 c ± 4.05 | 46.8 d ± 2.50 | 49.4 c ± 2.72 | 34.2 d ± 2.46 |
| UREA | 17.9 ± 1.45 | 14.7 ± 0.85 | 27.4 ± 1.16 | 22.6 ± 2.54 | 23.8 ± 1.21 | 22.8 ± 1.52 | 18.3 ± 1.07 | 21.6 ± 1.94 |
| LAC | 61.1 a ± 4.41 | 112 b ± 5.90 | 61.1 ± 5.87 | 54.6 ± 7.83 | 65.6 ± 5.07 | 69.6 ± 10.5 | 64.8 ± 8.44 | 56.0 ± 7.71 |
| Tissue | Fraction | Variable | IB × IB | IB × LW |
|---|---|---|---|---|
| SCF | Out | MUFA (g/100 g) | 50.9 c ± 0.49 | 48.3 d ± 0.49 |
| PUFA (g/100 g) | 13.0 e ± 0.26 | 15.8 f ± 0.37 | ||
| UI | 0.80 c ± 0.01 | 0.84 d ± 0.01 | ||
| N3 (g/100 g) | 1.13 c ± 0.03 | 1.35 d ± 0.05 | ||
| N6 (g/100 g) | 11.8 e ± 0.25 | 14.5 f ± 0.34 | ||
| In | MUFA (g/100 g) | 49.7 c ± 0.43 | 47.1 d ± 0.54 | |
| PUFA (g/100 g) | 13.0 e ± 0.31 | 15.3 f ± 0.37 | ||
| UI | 0.78 a ± 0.01 | 0.81 b ± 0.01 | ||
| MUFA/SFA | 1.34 a ± 0.03 | 1.26 b ± 0.03 | ||
| N3 (g/100 g) | 1.06 c ± 0.03 | 1.23 d ± 0.05 | ||
| N6 (g/100 g) | 11.9 e ± 0.29 | 14.1 f ± 0.34 | ||
| D9 | 0.55 a ± 0.01 | 0.53 b ± 0.01 | ||
| DI | 3.78 a ± 0.10 | 3.38 b ± 0.12 | ||
| LD | Polar | PUFA (g/100 g) | 43.4 a ± 0.68 | 40.2 b ± 1.60 |
| N6 (g/100 g) | 38.9 a ± 0.65 | 36.0 b ± 1.45 | ||
| N6/N3 | 8.90 a ± 0.22 | 8.01 b ± 0.24 | ||
| BF | Neutral | N3 (g/100 g) | 2.19 a ± 0.12 | 3.04 b ± 0.39 |
| DN3 | 0.26 a ± 0.02 | 0.35 b ± 0.05 | ||
| Polar | N6/N3 | 9.99 a ± 0.21 | 9.20 b ± 0.27 | |
| Liver | Neutral | DI | 0.39 a ± 0.02 | 0.46 b ± 0.03 |
| Tissue | Fraction | Variable | IB × IB | IB × LW |
|---|---|---|---|---|
| SCF | Out | MUFA (g/100 g) | 48.4 c ± 0.30 | 47.0 d ± 0.36 |
| PUFA (g/100 g) | 13.6 e ± 0.30 | 15.7 f ± 0.46 | ||
| UI | 0.78 c ± 0.01 | 0.81 d ± 0.01 | ||
| N3 (g/100 g) | 1.36 c ± 0.04 | 1.55 d ± 0.05 | ||
| N6 (g/100 g) | 12.3 e ± 0.26 | 14.2 f ± 0.41 | ||
| DN6 | 0.00195 a ± 0.00 | 0.00201 b ± 0.00 | ||
| In | SFA (g/100 g) | 41.2 a ± 0.55 | 39.7 b ± 0.61 | |
| PUFA (g/100 g) | 12.3 e ± 0.36 | 14.5 f ± 0.52 | ||
| UI | 0.73 c ± 0.01 | 0.77 d ± 0.01 | ||
| N3 (g/100 g) | 1.33 a ± 0.05 | 1.51 b ± 0.05 | ||
| N6 (g/100 g) | 10.9 e ± 0.31 | 13.0 f ± 0.48 | ||
| DN6 | 0.0025 e ± 0.00 | 0.0018 f ± 0.00 | ||
| VCF | SFA (g/100 g) | 48.3 a ± 0.38 | 46.4 b ± 0.81 | |
| PUFA (g/100 g) | 9.87 e ± 0.22 | 12.2 f ± 0.52 | ||
| UI | 0.63 c ± 0.01 | 0.68 d ± 0.01 | ||
| N3 (g/100 g) | 0.98 e ± 0.02 | 1.19 f ± 0.05 | ||
| N6 (g/100 g) | 8.89 e ± 0.20 | 11.0 f ± 0.47 | ||
| DN6 | 0.003 c ± 0.00 | 0.002 d ± 0.00 | ||
| LD | Polar | DN3 | 1.09 a ± 0.13 | 0.81 b ± 0.05 |
| BF | Neutral | DN6 | 0.08 e ± 0.02 | 0.06 f ± 0.00 |
| Polar | MUFA (g/100 g) | 20.5 c ± 0.26 | 19.8 d ± 0.14 | |
| UI | 1.43 c ± 0.01 | 1.46 d ± 0.01 | ||
| MUFA/SFA | 0.57 c ± 0.01 | 0.55 d ± 0.00 | ||
| N3 (g/100 g) | 2.60 c ± 0.04 | 2.82 d ± 0.04 | ||
| N6/N3 | 15.9 a ± 0.24 | 14.8 b ± 0.28 | ||
| D9 | 0.32 a ± 0.00 | 0.31 b ± 0.00 | ||
| DN3 | 0.33 a ± 0.02 | 0.38 b ± 0.01 |
| Age (Days) | Parameter | IB × IB | IB × LW | Gen × Sex | |||
|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | ||||
| 0 | Body measures | Body weight (kg) | 1.27 ± 0.04 | 1.27 ± 0.05 | 1.32 ± 0.09 | 1.52 ± 0.10 | + |
| 21 | Body measures | Body weight (kg) | 4.81 ± 0.15 | 4.22 ± 0.18 | 4.51 ± 0.38 | 4.88 ± 0.34 | * |
| BPD (cm) | 6.03 ± 0.04 | 5.88 ± 0.06 | 5.94 ± 0.14 | 6.19 ± 0.09 | ** | ||
| ONL (cm) | 14.9 ± 0.10 | 14.8 ± 0.14 | 13.7 ± 0.34 | 15.3 ± 1.17 | * | ||
| TC (cm) | 37.3 ± 0.49 | 35.7 ± 0.63 | 35.5 ± 1.17 | 36.8 ± 1.16 | * | ||
| AC (cm) | 33.3 ± 0.51 | 31.1 ± 0.67 | 30.9 ± 1.11 | 32.5 ± 0.99 | * | ||
| 60 | Body measures | BPD (cm) | 7.49 ± 0.09 | 7.34 ± 0.07 | 7.81 ± 0.15 | 8.17 ± 0.13 | * |
| Viscera weight | Brain (g) | 71.7 ± 1.51 | 71.2 ± 1.39 | 63.0 ± 2.88 | 71.7 ± 1.38 | + | |
| Lungs (g) | 229 ± 15.4 | 196 ± 8.87 | 215 ± 22.3 | 241 ± 9.32 | + | ||
| Pancreas (g) | 37.6 ± 3.61 | 34.3 ± 2.56 | 36.2 ± 3.87 | 46.9 ± 2.97 | + | ||
| Spleen (g) | 54.0 ± 4.39 | 46.2 ± 3.31 | 46.6 ± 7.02 | 58.3 ± 3.35 | + | ||
| Kidneys (g) | 84.7 ± 5.55 | 78.5 ± 4.38 | 74.0 ± 7.86 | 93.3 ± 3.34 | + | ||
| Metabolites | Fruc (mg/dL) | 212 ± 14.5 | 229 ± 9.18 | 248 ± 14.4 | 203 ± 3.80 | * | |
| Fatty acids composition | UI BFN | 1.09 ± 0.04 | 1.09 ± 0.03 | 1.28 ± 0.05 | 1.05 ± 0.06 | + | |
| N6 BFN (g/100 g) | 24.9 ± 1.85 | 24.8 ± 1.46 | 31.5 ± 1.26 | 22.3 ± 2.24 | + | ||
| SFA BFN (g/100 g) | 39.9 ± 0.19 | 38.6 ± 0.52 | 38.8 ± 0.16 | 39.7 ± 0.38 | ** | ||
| N6/N3 BFP | 9.60 ± 0.27 | 10.3 ± 0.28 | 9.28 ± 0.37 | 9.11 ± 0.42 | + | ||
| DN6 LVP (g/100 g) | 0.87 ± 0.04 | 0.75 ± 0.03 | 0.76 ± 0.04 | 0.81 ± 0.05 | + | ||
| 210 | Metabolites | Fruc (mg/dL) | 239 ± 6.87 | 267 ± 6.12 | 258 ± 8.69 | 250 ± 12.2 | + |
| Urea (mg/dL) | 18.5 ± 1.19 | 18.1 ± 1.92 | 16.7 ± 2.11 | 25.9 ± 2.09 | ** | ||
| Fatty acids composition | MUFA SCFO (g/100 g) | 48.9 ± 0.38 | 47.8 ± 0.41 | 46.1 ± 0.33 | 47.7 ± 0.44 | ** | |
| PUFA SCFO (g/100 g) | 14.1 ± 0.34 | 13.1 ± 0.44 | 17.2 ± 0.52 | 14.7 ± 0.29 | + | ||
| MUFA/SFA SCFO | 1.32 ± 0.02 | 1.23 ± 0.02 | 1.26 ± 0.02 | 1.27 ± 0.03 | + | ||
| N6 SCFO (g/100 g) | 12.7 ± 0.31 | 11.8 ± 0.40 | 15.5 ± 0.45 | 13.2 ± 0.27 | + | ||
| D9 SCFO | 0.56 ± 0.00 | 0.54 ± 0.00 | 0.55 ± 0.00 | 0.55 ± 0.01 | + | ||
| DI SCFO | 3.61 ± 0.09 | 3.18 ± 0.10 | 3.39 ± 0.11 | 3.53 ± 0.14 | * | ||
| MUFA SCFI (g/100 g) | 47.2 ± 0.59 | 45.8 ± 0.43 | 45.1 ± 0.64 | 46.2 ± 0.56 | * | ||
| MUFA/SFA SCFI | 1.20 ± 0.03 | 1.07 ± 0.02 | 1.15 ± 0.03 | 1.16 ± 0.04 | + | ||
| N6/N3 SCFI | 8.15 ± 0.23 | 8.48 ± 0.12 | 9.16 ± 0.12 | 8.26 ± 0.08 | ** | ||
| D9 SCFI | 0.53 ± 0.01 | 0.51 ± 0.01 | 0.53 ± 0.01 | 0.52 ± 0.01 | + | ||
| DI SCFI | 2.95 ± 0.09 | 2.54 ± 0.06 | 2.90 ± 0.13 | 2.91 ± 0.14 | + | ||
| PUFA VF (g/100 g) | 10.3 ± 0.30 | 9.46 ± 0.26 | 13.5 ± 0.74 | 11.1 ± 0.42 | + | ||
| N3 VF (g/100 g) | 1.01 ± 0.03 | 0.95 ± 0.02 | 1.28 ± 0.08 | 1.10 ± 0.05 | + | ||
| N6 VF (g/100 g) | 9.27 ± 0.28 | 8.51 ± 0.25 | 12.2 ± 0.68 | 10.0 ± 0.38 | + | ||
| PUFA BFN (g/100 g) | 7.73 ± 1.77 | 9.94 ± 1.10 | 9.47 ± 0.34 | 8.45 ± 0.41 | *** | ||
| UI BFN | 0.77 ± 0.03 | 0.82 ± 0.02 | 0.80 ± 0.01 | 0.79 ± 0.01 | *** | ||
| N6 BFN (g/100 g) | 6.90 ± 1.69 | 8.91 ± 1.08 | 8.50 ± 0.30 | 7.45 ± 0.41 | *** | ||
| N6/N3 BFN | 8.33 ± 0.75 | 8.86 ± 0.95 | 8.84 ± 0.23 | 7.63 ± 0.61 | * | ||
| DN6 BFN | 0.06 ± 0.03 | 0.11 ± 0.02 | 0.05 ± 0.00 | 0.06 ± 0.00 | *** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heras-Molina, A.; Pesantez, J.L.; Astiz, S.; Garcia-Contreras, C.; Vazquez-Gomez, M.; Isabel, B.; Ovilo, C.; Gonzalez-Bulnes, A. The Role of Offspring Genotype-by-Sex Interactions, Independently of Environmental Cues, on the Phenotype Traits of an Obese Swine Model. Biology 2020, 9, 445. https://doi.org/10.3390/biology9120445
Heras-Molina A, Pesantez JL, Astiz S, Garcia-Contreras C, Vazquez-Gomez M, Isabel B, Ovilo C, Gonzalez-Bulnes A. The Role of Offspring Genotype-by-Sex Interactions, Independently of Environmental Cues, on the Phenotype Traits of an Obese Swine Model. Biology. 2020; 9(12):445. https://doi.org/10.3390/biology9120445
Chicago/Turabian StyleHeras-Molina, Ana, José Luis Pesantez, Susana Astiz, Consolación Garcia-Contreras, Marta Vazquez-Gomez, Beatriz Isabel, Cristina Ovilo, and Antonio Gonzalez-Bulnes. 2020. "The Role of Offspring Genotype-by-Sex Interactions, Independently of Environmental Cues, on the Phenotype Traits of an Obese Swine Model" Biology 9, no. 12: 445. https://doi.org/10.3390/biology9120445
APA StyleHeras-Molina, A., Pesantez, J. L., Astiz, S., Garcia-Contreras, C., Vazquez-Gomez, M., Isabel, B., Ovilo, C., & Gonzalez-Bulnes, A. (2020). The Role of Offspring Genotype-by-Sex Interactions, Independently of Environmental Cues, on the Phenotype Traits of an Obese Swine Model. Biology, 9(12), 445. https://doi.org/10.3390/biology9120445






