Development of Open-Field Behaviour in the Medaka, Oryzias latipes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
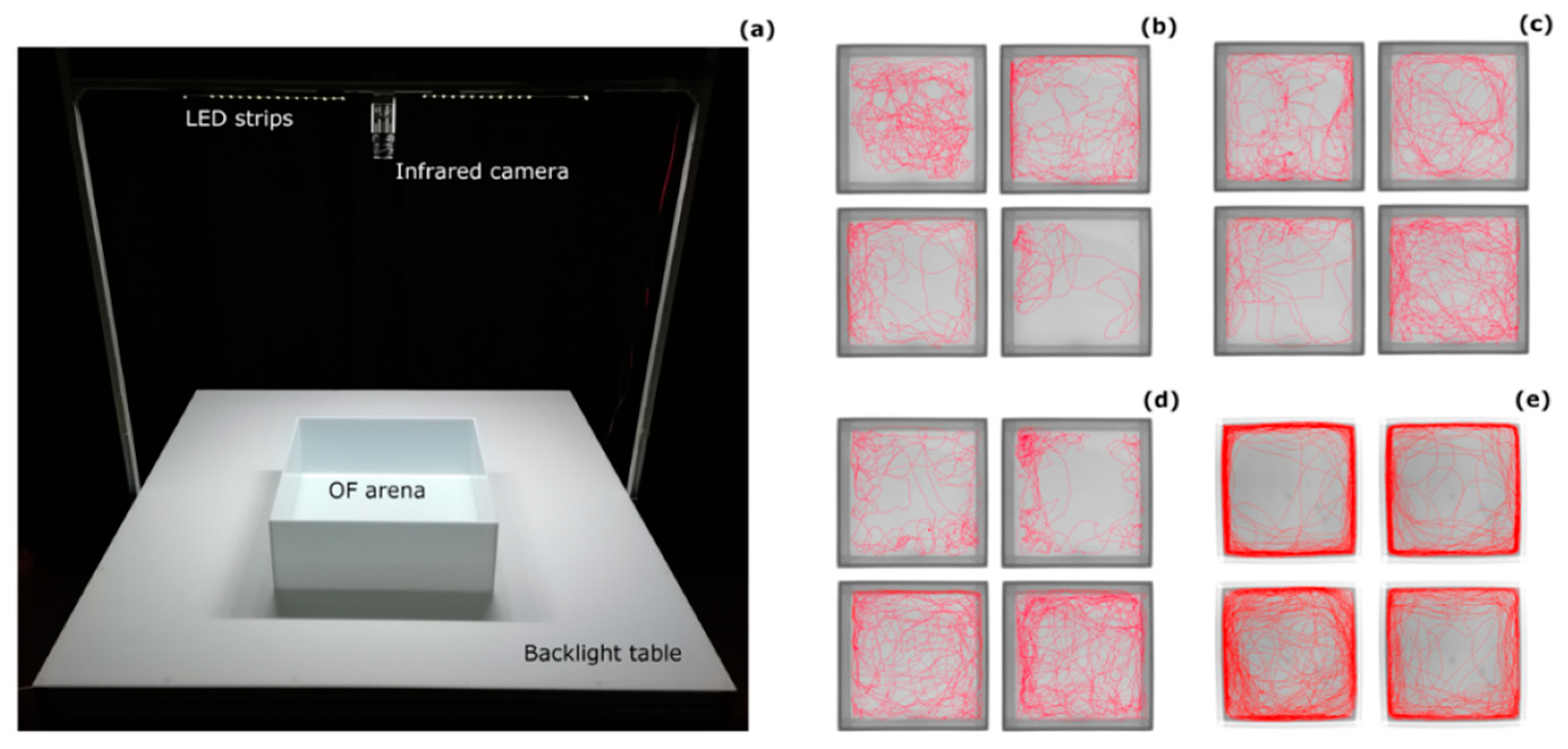
2.2. Open-Field Test
- 1 dph: 8 cm × 8 cm, filled with 2.5 cm of ERM;
- 10 dph: 8 cm × 8 cm, filled with 2.5 cm of ERM;
- 30 dph: 12 cm × 12 cm, filled with 4 cm of FW;
- 120 dph: 40 cm × 40 cm, filled with 12 cm of water.
2.3. Statistical Analysis
2.4. Ethical Approval
3. Results
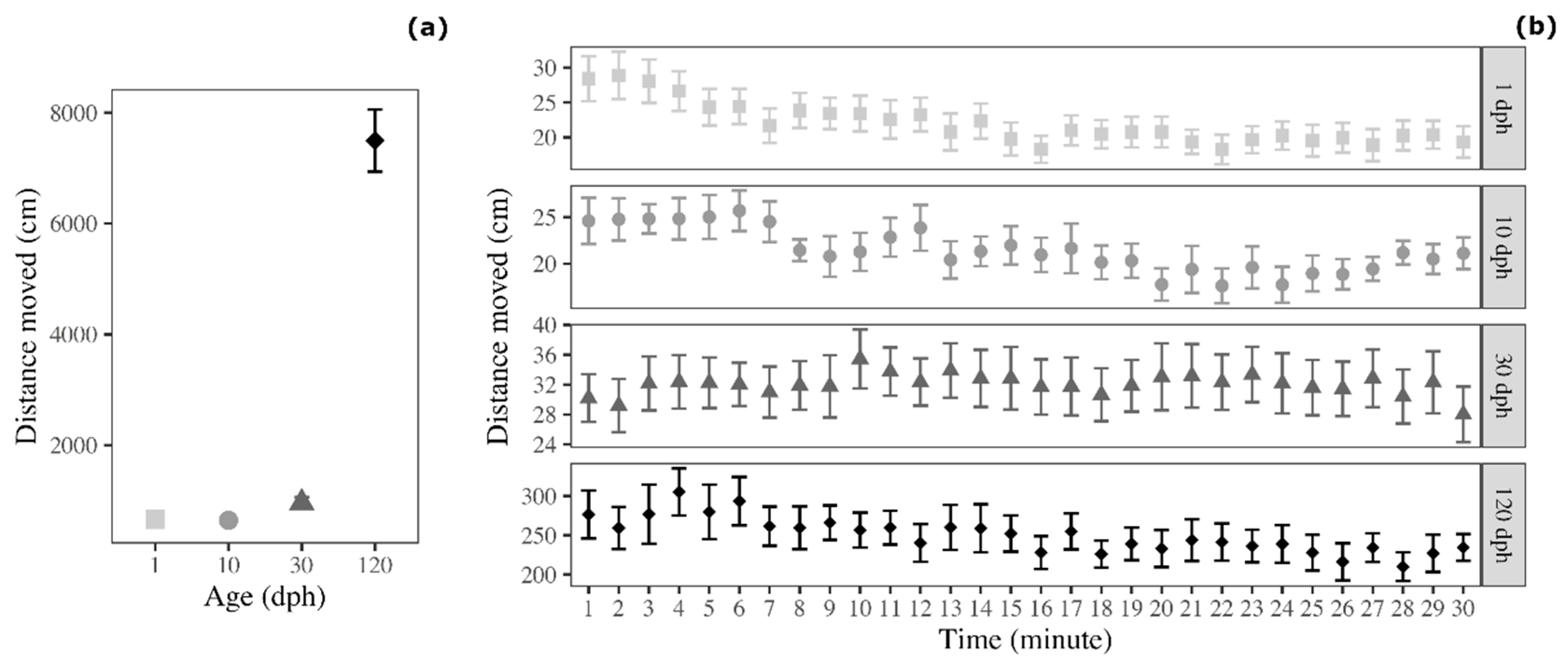
3.1. Distance Moved
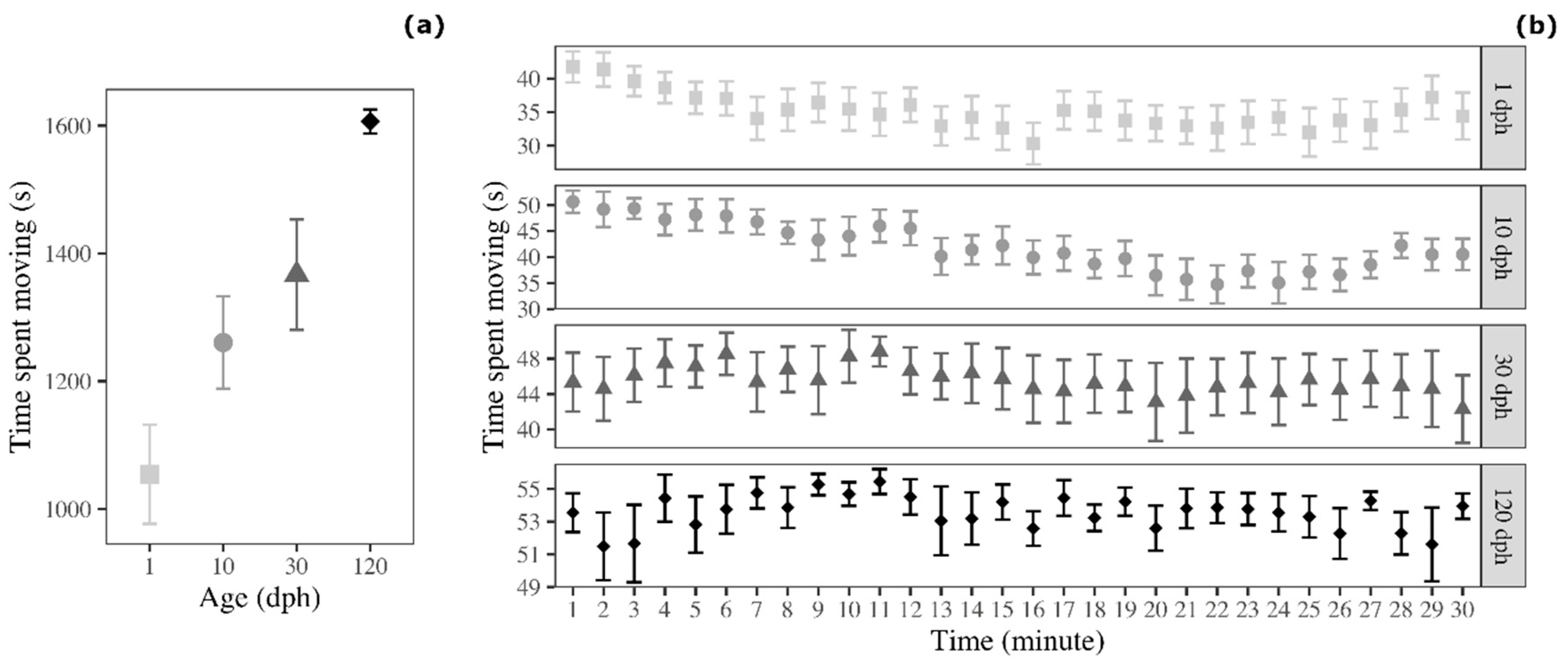
3.2. Time Spent Moving
3.3. Time Spent in the Centre of the Arena
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hong, X.; Zha, J. Fish behavior: A promising model for aquatic toxicology research. Sci. Total Environ. 2019, 686, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Braubach, O.; Spitsbergen, J.; Gerlai, R.; Kalueff, A.V. Zebrafish models for translational neuroscience research: From tank to bedside. Trends Neurosci. 2014, 37, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The open field test. In Mood and Anxiety Related Phenotypes in Mice; Humana Press: Totowa, NJ, USA, 2009; pp. 1–20. [Google Scholar]
- Kulikov, A.V.; Tikhonova, M.A.; Kulikov, V.A. Automated measurement of spatial preference in the open field test with transmitted lighting. J. Neurosci. Methods 2008, 170, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.G. The validity of three tests of temperament in guppies (Poecilia reticulata). J. Comp. Psychol. 2008, 122, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.; Sawyer, S.; Perrin, F.; Oxendine, S.E.; Kezios, Z.D. Adapting the open field test to assess anxiety-related behavior in zebrafish. In Zebrafish Protocols for Neurobehavioral Research; Humana Press: Totowa, NJ, USA, 2012; pp. 181–189. [Google Scholar]
- Liu, C.-X.; Li, C.-Y.; Hu, C.-C.; Wang, Y.; Lin, J.; Jiang, Y.-H.; Li, Q.; Xu, X. CRISPR/Cas9-induced shank3b mutant zebrafish display autism-like behaviors. Mol. Autism 2018, 9, 23. [Google Scholar] [CrossRef]
- Grossman, L.; Utterback, E.; Stewart, A.; Gaikwad, S.; Chung, K.M.; Suciu, C.; Wong, K.; Elegante, M.; Elkhayat, S.; Tan, J.; et al. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav. Brain Res. 2010, 214, 277–284. [Google Scholar] [CrossRef]
- Bruzzone, M.; Gatto, E.; Xiccato, T.L.; Valle, L.D.; Fontana, C.M.; Meneghetti, G.; Bisazza, A. Measuring recognition memory in zebrafish larvae: Issues and limitations. PeerJ 2020, 8, e8890. [Google Scholar] [CrossRef]
- Colwill, R.M.; Creton, R. Locomotor behaviors in zebrafish (Danio rerio) larvae. Behav. Process. 2011, 86, 222–229. [Google Scholar] [CrossRef]
- Fero, K.; Yokogawa, T.; Burgess, H.A. The behavioral repertoire of larval zebrafish. In Zebrafish Models in Neurobehavioral Research; Humana Press: Totowa, NJ, USA, 2011; pp. 249–291. [Google Scholar]
- Stewart, W.J.; Cardenas, G.S.; McHenry, M.J. Zebrafish larvae evade predators by sensing water flow. J. Exp. Biol. 2013, 216, 388–398. [Google Scholar] [CrossRef]
- Norton, W.H.J. Toward developmental models of psychiatric disorders in zebrafish. Front. Neural Circuits 2013, 7, 79. [Google Scholar] [CrossRef]
- Armant, O.; März, M.; Schmidt, R.; Ferg, M.; Diotel, N.; Ertzer, R.; Bryne, J.C.; Yang, L.; Baader, I.; Reischl, M.; et al. Genome-wide, whole mount in situ analysis of transcriptional regulators in zebrafish embryos. Dev. Biol. 2013, 380, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Wang, Z.; Chai, Y.; Hang, W.; Shang, C.; Yang, W.; Bai, L.; Du, J.; Wang, K.; Wen, Q. Rapid whole brain imaging of neural activity in freely behaving larval zebrafish (Danio rerio). eLife 2017, 6, e28158. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Richardson, M.K. Exploratory behaviour in the open field test adapted for larval zebrafish: Impact of environmental complexity. Behav. Process. 2013, 92, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Polverino, G.; Cigliano, C.; Nakayama, S.; Mehner, T. Emergence and development of personality over the ontogeny of fish in absence of environmental stress factors. Behav. Ecol. Sociobiol. 2016, 70, 2027–2037. [Google Scholar] [CrossRef]
- Kasahara, M.; Naruse, K.; Sasaki, S.; Nakatani, Y.; Qu, W.; Ahsan, B.; Yamada, T.; Nagayasu, Y.; Doi, K.; Kasai, Y.; et al. The medaka draft genome and insights into vertebrate genome evolution. Nat. Cell Biol. 2007, 447, 714–719. [Google Scholar] [CrossRef]
- Wittbrodt, J.; Shima, A.; Schartl, M. Medaka—A model organism from the far east. Nat. Rev. Genet. 2002, 3, 53–64. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chiang, C.-Y.; Tsai, H.-J. Zebrafish and Medaka: New model organisms for modern biomedical research. J. Biomed. Sci. 2016, 23, 1–11. [Google Scholar] [CrossRef]
- Myklatun, A.; Lauri, A.; Eder, S.H.; Cappetta, M.; Shcherbakov, D.; Wurst, W.; Winklhofer, M.; Westmeyer, G.G. Zebrafish and medaka offer insights into the neurobehavioral correlates of vertebrate magnetoreception. Nat. Commun. 2018, 9, 1–10. [Google Scholar]
- Otsuka, A.; Shimomura, K.; Niwa, H.; Kagawa, N. The presence of a conspecific induces risk-taking behaviour and enlargement of somata size of dopaminergic neurons in the brain of male medaka fish. J. Fish Biol. 2020, 96, 1014–1023. [Google Scholar] [CrossRef]
- Signore, I.A.; Guerrero, N.; Loosli, F.; Colombo, A.; Villalon, A.; Wittbrodt, J.; Concha, M.L. Zebrafish and medaka: Model organisms for a comparative developmental approach of brain asymmetry. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 991–1003. [Google Scholar] [CrossRef]
- Chiffre, A.; Clérandeau, C.; Dwoinikoff, C.; Le Bihanic, F.; Budzinski, H.; Geret, F.; Cachot, J. Psychotropic drugs in mixture alter swimming behaviour of Japanese medaka (Oryzias latipes) larvae above environmental concentrations. Environ. Sci. Pollut. Res. 2016, 23, 4964–4977. [Google Scholar] [CrossRef] [PubMed]
- Ansai, S.; Hosokawa, H.; Maegawa, S.; Kinoshita, M. Chronic fluoxetine treatment induces anxiolytic responses and altered social behaviors in medaka, Oryzias latipes. Behav. Brain Res. 2016, 303, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Nomichi, S.; Chen, K.; Honda, M.; Kang, I.J.; Shimasaki, Y.; Oshima, Y. Short-term and persistent impacts on behaviors related to locomotion, anxiety, and startle responses of Japanese medaka (Oryzias latipes) induced by acute, sublethal exposure to chlorpyrifos. Aquat. Toxicol. 2017, 192, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, W.; Watanabe, E. Habituation of medaka (Oryzias latipes) demonstrated by open-field testing. Behav. Process. 2010, 85, 142–150. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Montalbano, G.; Bertolucci, C. Personality traits covary with individual differences in inhibitory abilities in 2 species of fish. Curr. Zoöl. 2020, 66, 187–195. [Google Scholar] [CrossRef]
- Kotrschal, A.; Lievens, E.J.; Dahlbom, J.; Bundsen, A.; Semenova, S.; Sundvik, M.; Maklakov, M.M.; Winberg, S.; Panula, P.; Kolm, N. Artificial selection on relative brain size reveals a positive genetic correlation between brain size and proactive personality in the guppy. Evolution 2014, 68, 1139–1149. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Mazzoldi, C.; Griggio, M. Sex composition modulates the effects of familiarity in new environment. Behav. Process. 2017, 140, 133–138. [Google Scholar] [CrossRef]
- Øverli, Ø.; Winberg, S.; Pottinger, T.G. Behavioral and neuroendocrine correlates of selection for stress responsiveness in rainbow trout—A review. Integr. Comp. Biol. 2005, 45, 463–474. [Google Scholar] [CrossRef]
- Domenici, P. The scaling of locomotor performance in predator–prey encounters: From fish to killer whales. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 131, 169–182. [Google Scholar] [CrossRef]
- Fuiman, L.A.; Webb, P.W. Ontogeny of routine swimming activity and performance in zebra danios (Teleostei: Cyprinidae). Anim. Behav. 1988, 36, 250–261. [Google Scholar] [CrossRef]
- Jeon, J.K.; Kim, P.K.; Park, Y.J.; Myoung, J.G.; Kim, J.M. Changes of serum cortisol concentration and stress responses in cohe salmon (Oncorhynchus kisutch) to netting. Korean J. Fish. Aquat. Sci. 2000, 33, 115–118. [Google Scholar]
- Blaser, R.E.; Chadwick, L.; McGinnis, G. Behavioral measures of anxiety in zebrafish (Danio rerio). Behav. Brain Res. 2010, 208, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.M.; Feist, G.W.; Varga, Z.M.; Westerfield, M.; Kent, M.L.; Schreck, C.B. Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture 2006, 258, 565–574. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; De Russi, G.; Bertolucci, C. A novel-odour exploration test for measuring anxiety in adult and larval zebrafish. J. Neurosci. Methods 2020, 335, 108619. [Google Scholar] [CrossRef] [PubMed]
- Petrazzini, M.E.M.; Agrillo, C.; Piffer, L.; Dadda, M.; Bisazza, A. Development and application of a new method to investigate cognition in newborn guppies. Behav. Brain Res. 2012, 233, 443–449. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Griggio, M. Shoal sex composition affects exploration in the Mediterranean killifish. Ethology 2017, 123, 818–824. [Google Scholar] [CrossRef]
- Norton, W.H. Measuring larval zebrafish behavior: Locomotion, thigmotaxis, and startle. In Zebrafish Protocols for Neurobehavioral Research; Humana Press: Totowa, NJ, USA, 2012; pp. 3–20. [Google Scholar]
- Schnörr, S.; Steenbergen, P.; Richardson, M.; Champagne, D. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef]
- Doria, M.D.; Morand-Ferron, J.; Bertram, S.M. Spatial cognitive performance is linked to thigmotaxis in field crickets. Anim. Behav. 2019, 150, 15–25. [Google Scholar] [CrossRef]
- Liu, Y.C.; Bailey, I.; Hale, M.E. Alternative startle motor patterns and behaviors in the larval zebrafish (Danio rerio). J. Comp. Physiol. A 2012, 198, 11–24. [Google Scholar] [CrossRef]
- Lucon-Xiccato, T.; Di Mauro, G.; Bisazza, A.; Bertolucci, C. Alarm cue-mediated response and learning in zebrafish larvae. Behav. Brain Res. 2019, 380, 112446. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucon-Xiccato, T.; Conti, F.; Loosli, F.; Foulkes, N.S.; Bertolucci, C. Development of Open-Field Behaviour in the Medaka, Oryzias latipes. Biology 2020, 9, 389. https://doi.org/10.3390/biology9110389
Lucon-Xiccato T, Conti F, Loosli F, Foulkes NS, Bertolucci C. Development of Open-Field Behaviour in the Medaka, Oryzias latipes. Biology. 2020; 9(11):389. https://doi.org/10.3390/biology9110389
Chicago/Turabian StyleLucon-Xiccato, Tyrone, Francesca Conti, Felix Loosli, Nicholas S. Foulkes, and Cristiano Bertolucci. 2020. "Development of Open-Field Behaviour in the Medaka, Oryzias latipes" Biology 9, no. 11: 389. https://doi.org/10.3390/biology9110389
APA StyleLucon-Xiccato, T., Conti, F., Loosli, F., Foulkes, N. S., & Bertolucci, C. (2020). Development of Open-Field Behaviour in the Medaka, Oryzias latipes. Biology, 9(11), 389. https://doi.org/10.3390/biology9110389






