IL-6, NLR, and SII Markers and Their Relation with Alterations in CD8+ T-Lymphocyte Subpopulations in Patients Treated for Lung Adenocarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Studied
2.2. Expression of IL-6 in Lung Adenocarcinoma Biopsies
2.3. Blood Sample Collection
2.4. Quantification of Th1/Th2/Th17 Cytokines Using Cytometric Bead Array
2.5. Quantification of TGF-β and HMGB-1 by Enzyme-Linked Immunosorbent Assays (ELISA)
2.6. Data Collection from Cancer Patients
2.7. Panel of Antibodies for Phenotyping of T-Lymphocyte Subpopulation
2.8. Quantification of CD4+ and CD8+ T-Lymphocyte and Their Subpopulations
Staining Procedure and Cytometric Analysis
2.9. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
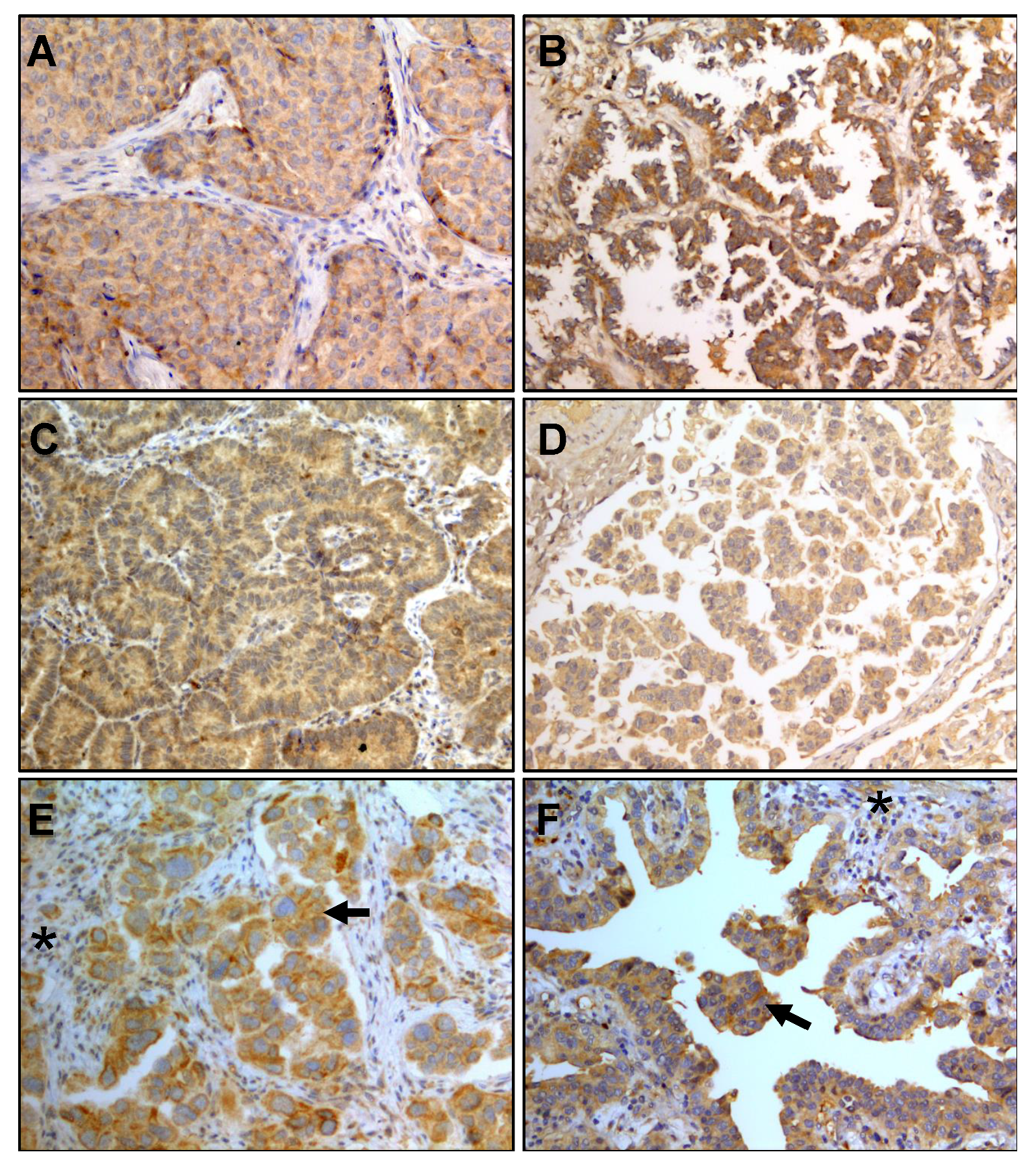
3.2. IL-6 Staining in Lung Adenocarcinoma Biopsies
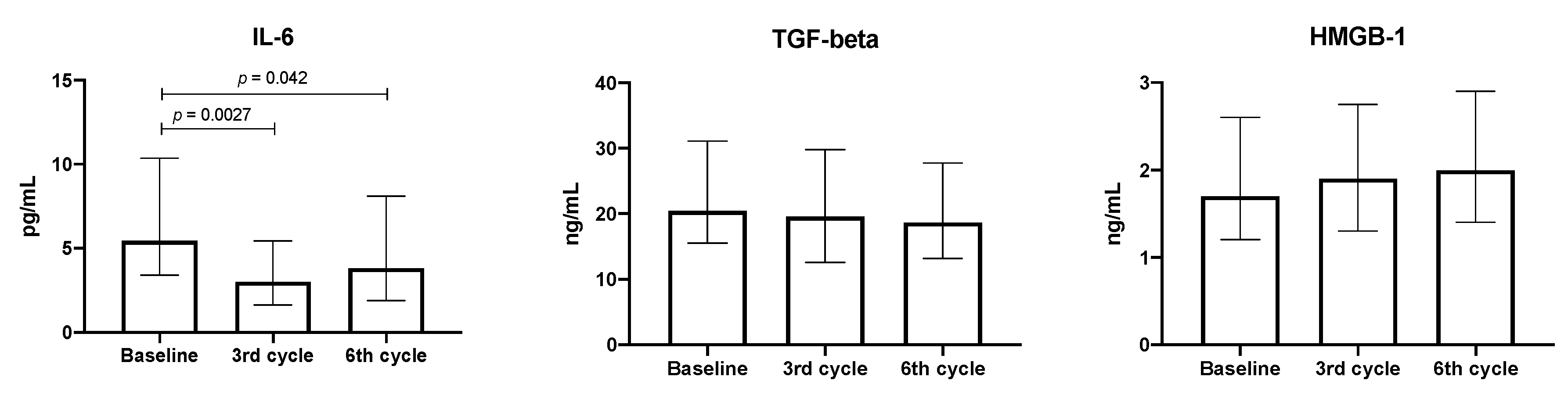
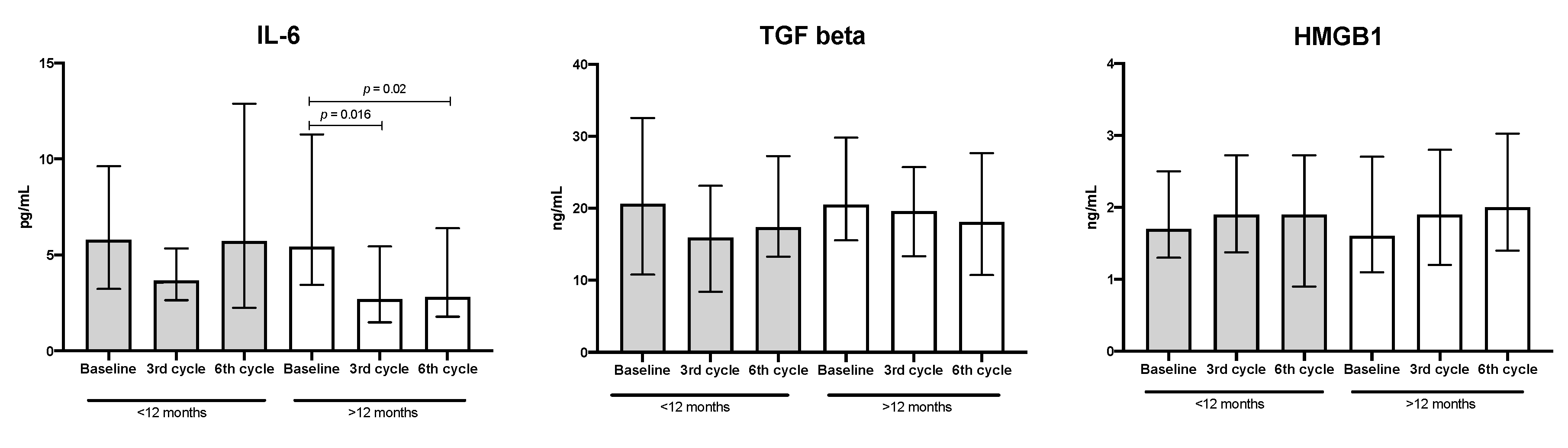
3.3. Quantification of Cytokines, TGF-β, and HMGB-1 in the Plasma from Patients with Lung Adenocarcinoma
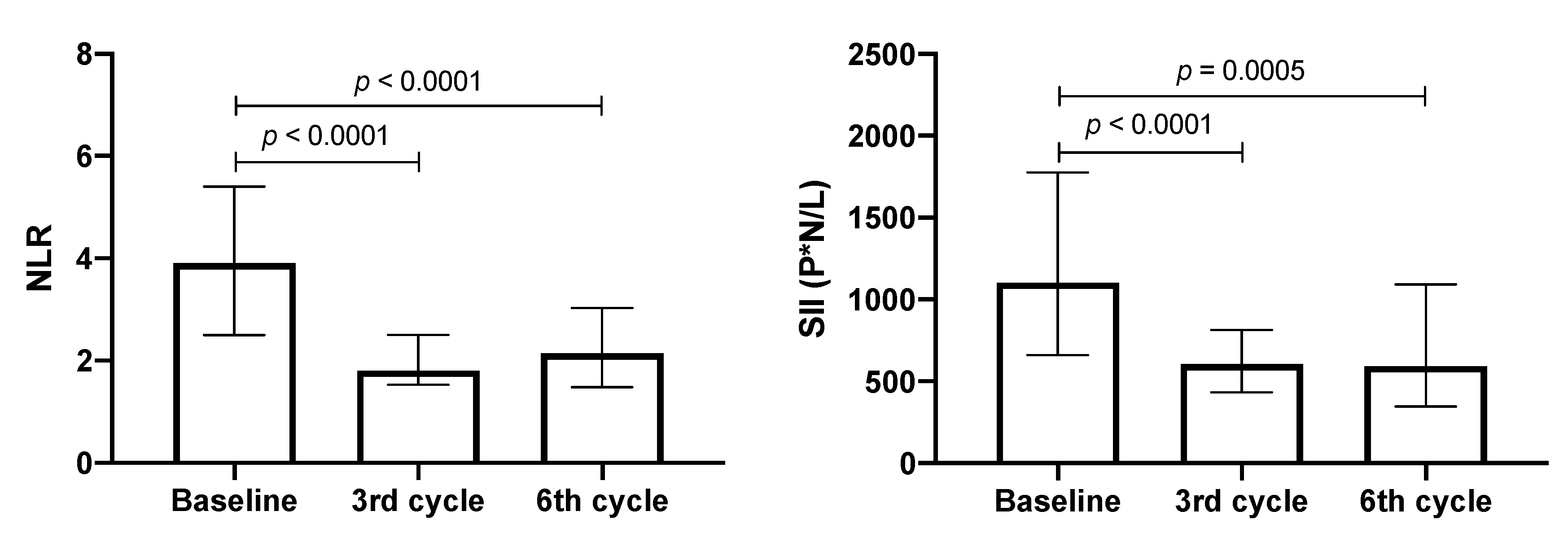
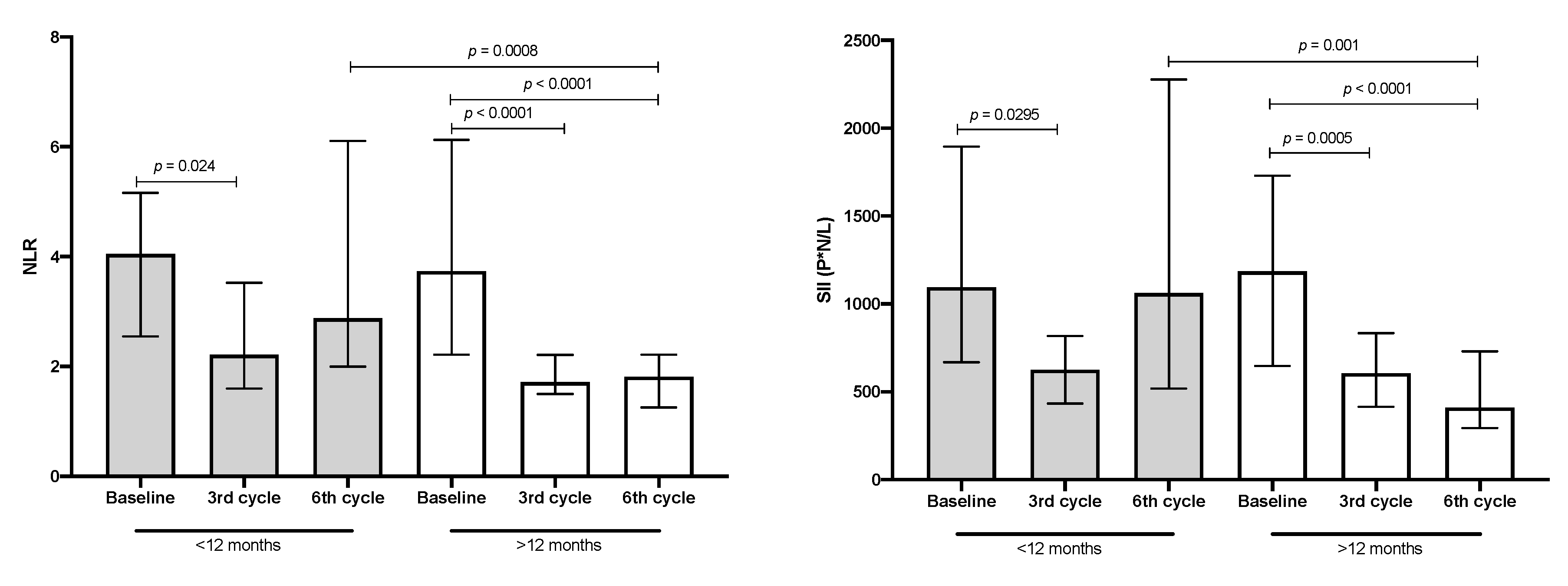
3.4. NLR and SII Values in Patients with Lung Adenocarcinoma
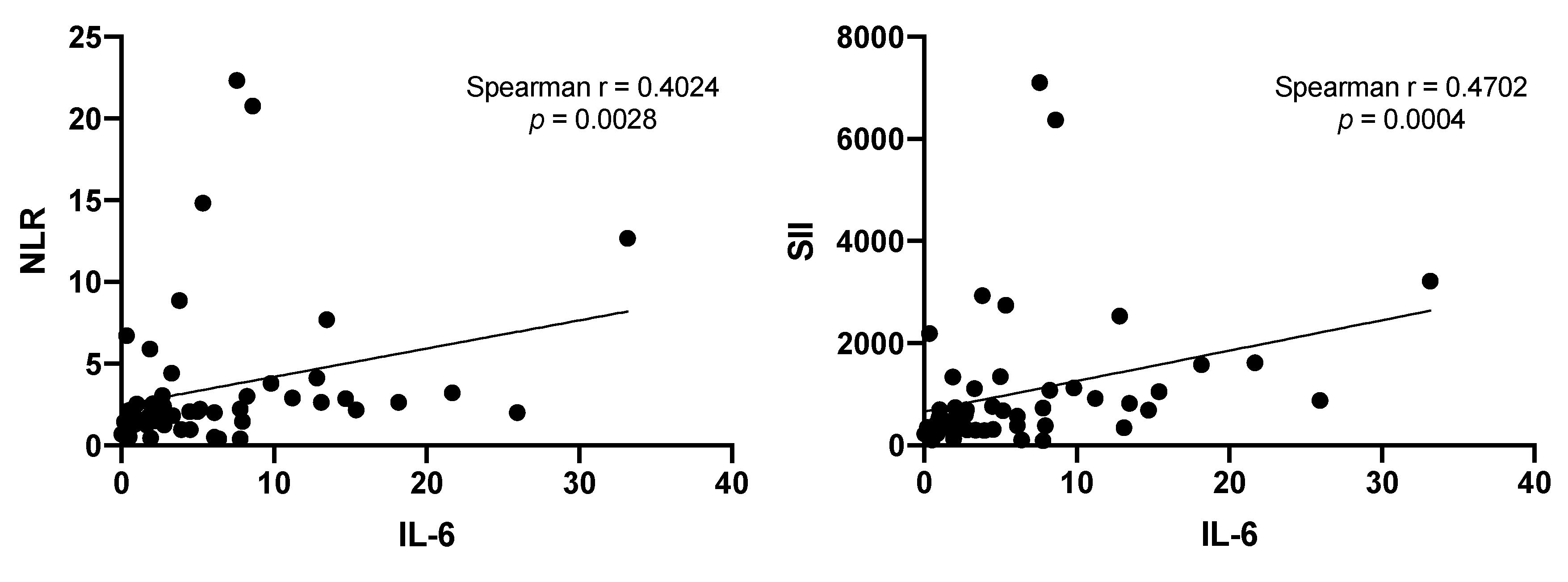
3.5. Correlation of NLR and SII Markers with IL-6
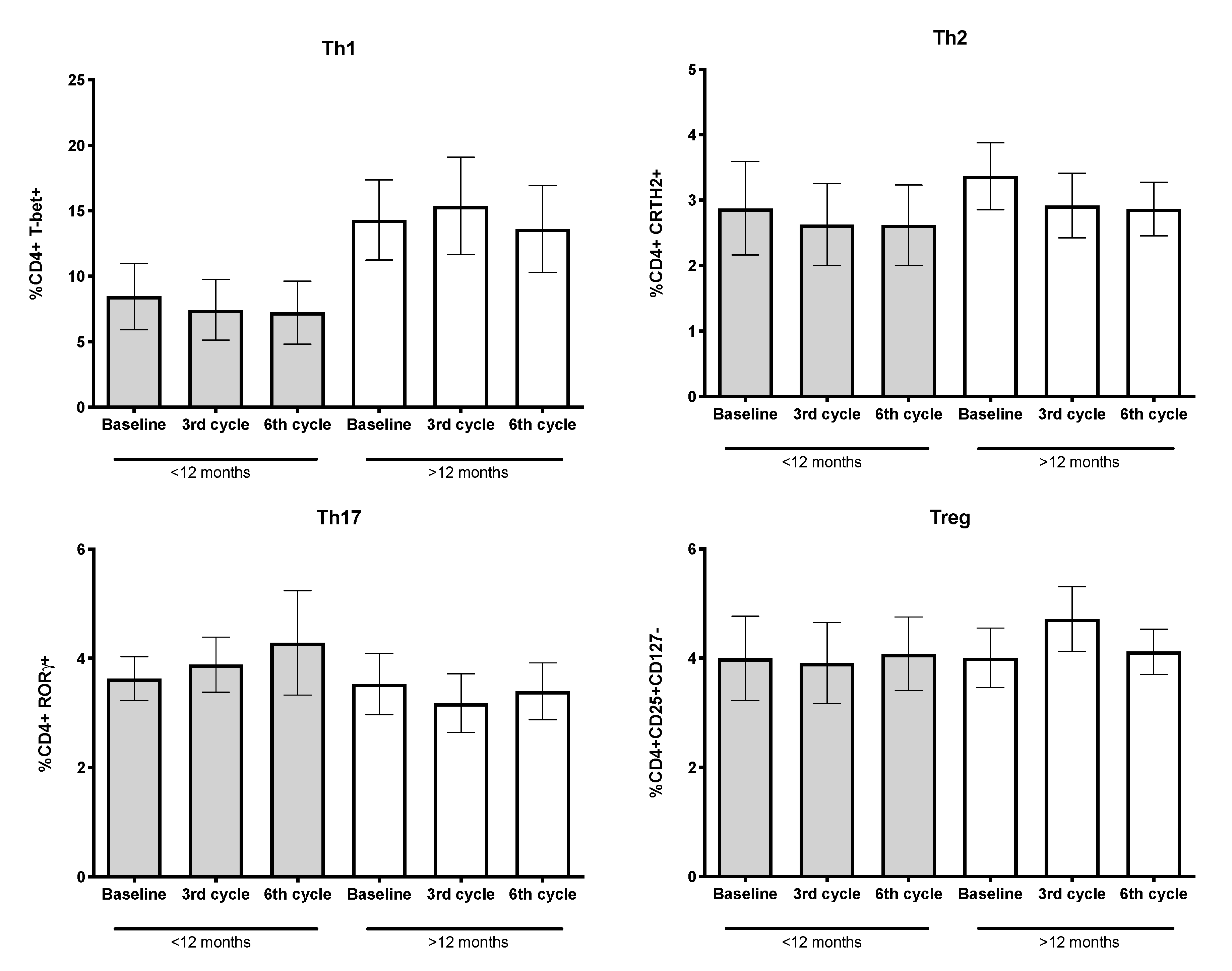
3.6. Percentages of CD4+ T-Lymphocytes and Their Subpopulations
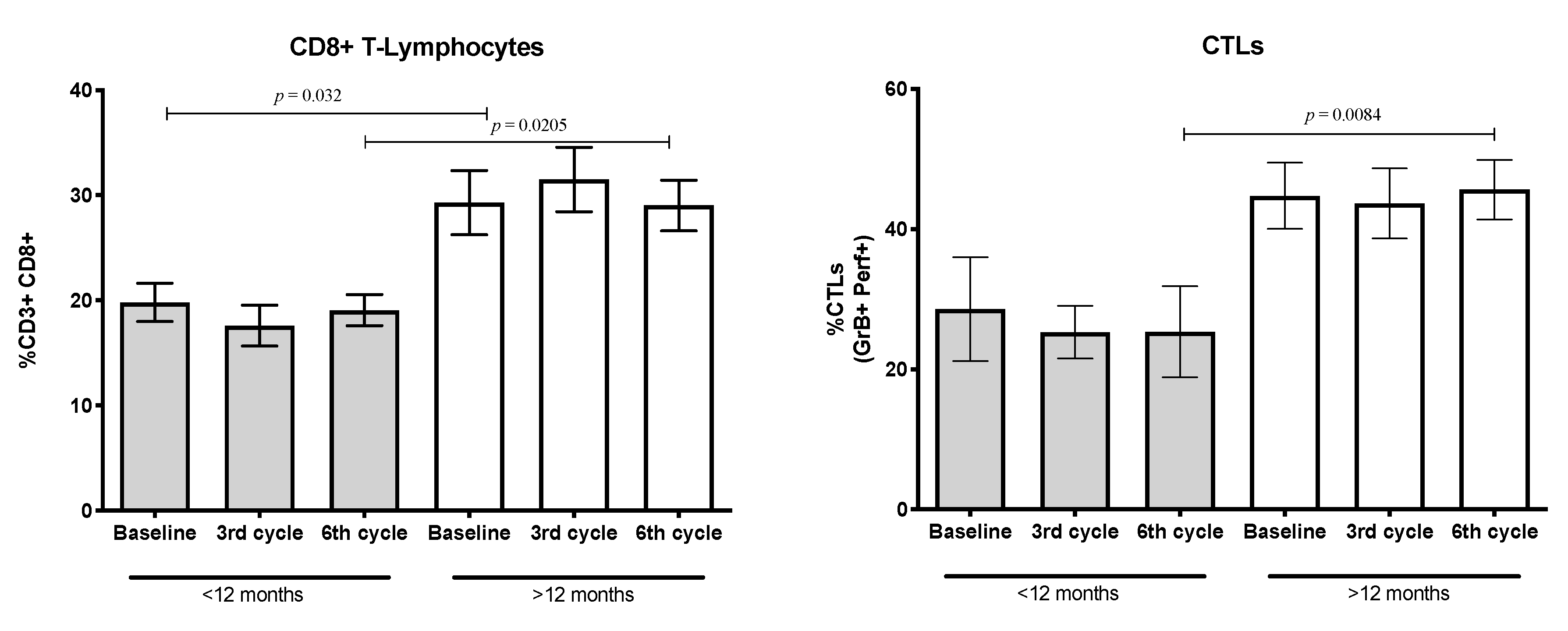
3.7. Percentages of CD8+ T-Lymphocytes and Cytotoxic T-Lymphocytes (CTLs) Expressing the Cytolytic Machinery
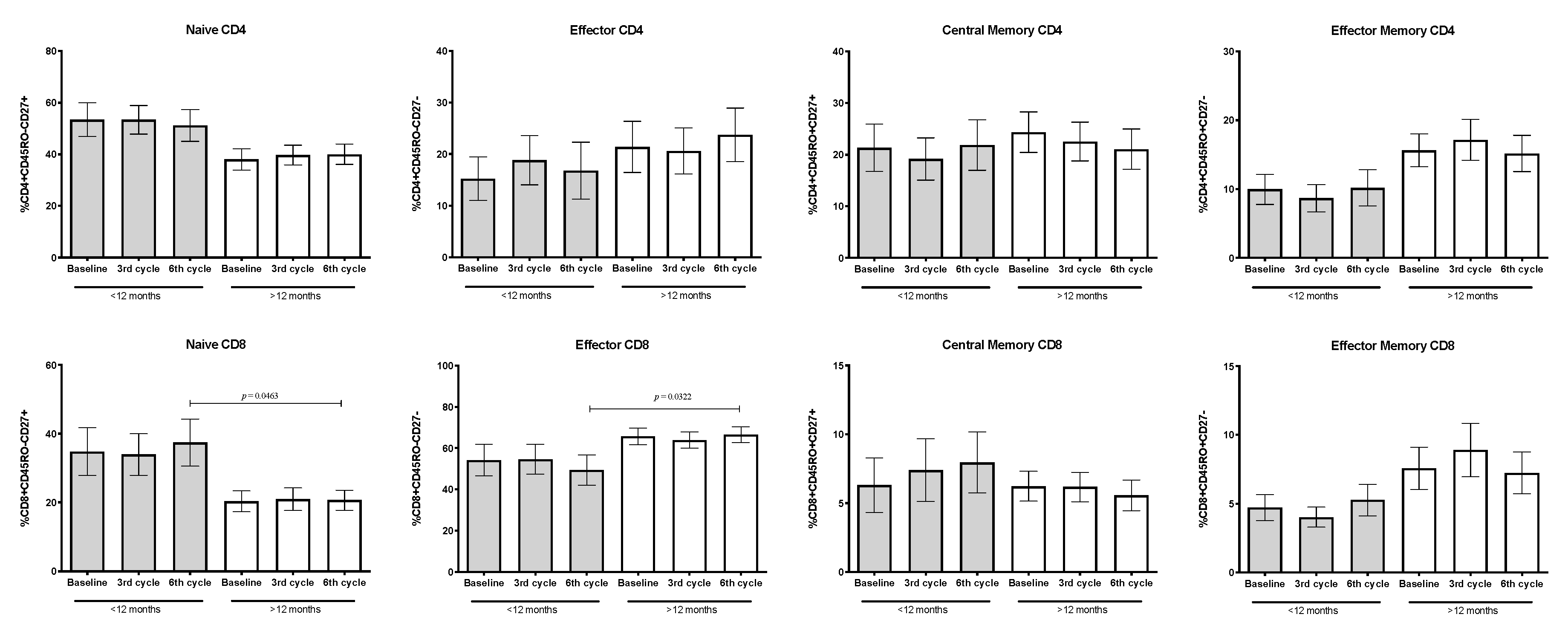
3.8. Percentages of Naïve, Memory, and Effector Subpopulations from CD4+ and CD8+ T-Lymphocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yang, X.; Huang, Y.; Zhao, M.; Li, M.; Ma, K.; Yin, J.; Zhan, C.; Wang, Q. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res. 2019, 11, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef]
- Bromberg, J.; Wang, T.C. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell 2009, 15, 79–80. [Google Scholar] [CrossRef]
- Chang, C.H.; Hsiao, C.F.; Yeh, Y.M.; Chang, G.C.; Tsai, Y.H.; Chen, Y.M.; Huang, M.S.; Chen, H.L.; Li, Y.J.; Yang, P.C.; et al. Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int. J. Cancer 2013, 132, 1977–1985. [Google Scholar] [CrossRef]
- Unver, N.; McAllister, F. IL-6 family cytokines: Key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev. 2018, 41, 10–17. [Google Scholar] [CrossRef]
- Kang, M.H.; Go, S.I.; Song, H.N.; Lee, A.; Kim, S.H.; Kang, J.H.; Jeong, B.K.; Kang, K.M.; Ling, H.; Lee, G.W. The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br. J. Cancer 2014, 111, 452–460. [Google Scholar] [CrossRef]
- Li, C.; Tian, W.; Zhao, F.; Li, M.; Ye, Q.; Wei, Y.; Li, T.; Xie, K. Systemic immune-inflammation index, SII, for prognosis of elderly patients with newly diagnosed tumors. Oncotarget 2018, 9, 35293–35299. [Google Scholar] [CrossRef]
- Tong, Y.S.; Tan, J.; Zhou, X.L.; Song, Y.Q.; Song, Y.J. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J. Transl. Med. 2017, 15, 221. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chang, Q.; Meng, X.; Gao, N.; Wang, W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J. Cancer 2018, 9, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, B.; Wang, L.; Wang, R.; Yang, X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: A meta-analysis. Medicine (Baltimore) 2019, 98, e13788. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Available online: www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 20 October 2020).
- Da Cunha Santos, G.; Shepherd, F.A.; Tsao, M.S. EGFR mutations and lung cancer. Annu. Rev. Pathol. 2011, 6, 49–69. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Venereau, E.; Ceriotti, C.; Bianchi, M.E. DAMPs from Cell Death to New Life. Front Immunol. 2015, 6, 422. [Google Scholar] [CrossRef]
- Hato, S.V.; Khong, A.; de Vries, I.J.; Lesterhuis, W.J. Molecular pathways: The immunogenic effects of platinum-based chemotherapeutics. Clin. Cancer Res. 2014, 20, 2831–2837. [Google Scholar] [CrossRef]
- Sedletska, Y.; Giraud-Panis, M.J.; Malinge, J.M. Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: Importance of apoptotic pathways. Curr. Med. Chem. Anticancer Agents 2005, 5, 251–265. [Google Scholar] [CrossRef]
- Venereau, E.; De Leo, F.; Mezzapelle, R.; Careccia, G.; Musco, G.; Bianchi, M.E. HMGB1 as biomarker and drug target. Pharmacol. Res. 2016, 111, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front Immunol. 2020, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, B.L.; Steel, H.C.; Theron, A.J.; Heyman, L.; Smit, T.; Ramdas, Y.; Anderson, R. High Mobility Group Box 1 in Human Cancer. Cells 2020, 9, 1664. [Google Scholar] [CrossRef]
- Wang, J.L.; Wu, D.W.; Cheng, Z.Z.; Han, W.Z.; Xu, S.W.; Sun, N.N. Expression of high mobility group box - B1 (HMGB-1) and matrix metalloproteinase-9 (MMP-9) in non-small cell lung cancer (NSCLC). Asian Pac. J. Cancer Prev. 2014, 15, 4865–4869. [Google Scholar] [CrossRef]
- Islas-Vazquez, L.; Prado-Garcia, H.; Aguilar-Cazares, D.; Meneses-Flores, M.; Galicia-Velasco, M.; Romero-Garcia, S.; Camacho-Mendoza, C.; Lopez-Gonzalez, J.S. LAP TGF-Beta Subset of CD4(+)CD25(+)CD127(-) Treg Cells is Increased and Overexpresses LAP TGF-Beta in Lung Adenocarcinoma Patients. Biomed. Res. Int. 2015, 2015, 430943. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J. Immunol. Res. 2015, 2015, 983698. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, B.; Huang, R.Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.Q.; Gao, C.Y.; Wang, B.L.; Zhang, Y.M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta 2014, 1845, 182–201. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Lippitz, B.E. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013, 14, e218–e228. [Google Scholar] [CrossRef]
- Li, J.; Lan, T.; Zhang, C.; Zeng, C.; Hou, J.; Yang, Z.; Zhang, M.; Liu, J.; Liu, B. Reciprocal activation between IL-6/STAT3 and NOX4/Akt signalings promotes proliferation and survival of non-small cell lung cancer cells. Oncotarget 2015, 6, 1031–1048. [Google Scholar] [CrossRef]
- Haura, E.B.; Livingston, S.; Coppola, D. Autocrine interleukin-6/interleukin-6 receptor stimulation in non-small-cell lung cancer. Clin. Lung Cancer 2006, 7, 273–275. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Chen, P.; Xu, W.; Wu, Y.; Che, G. Prognostic value of the pretreatment systemic immune-inflammation index (SII) in patients with non-small cell lung cancer: A meta-analysis. Ann. Transl. Med. 2019, 7, 433. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, L. The function and mechanism of HMGB1 in lung cancer and its potential therapeutic implications. Oncol. Lett. 2018, 15, 6799–6805. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, K.; Naumnik, W.; Niklinska, W.; Chyczewska, E. Clinical Significance of HMGB-1 and TGF-beta Level in Serum and BALF of Advanced Non-Small Cell Lung Cancer. Adv. Exp. Med. Biol. 2015, 852, 49–58. [Google Scholar] [CrossRef]
- Sims, G.P.; Rowe, D.C.; Rietdijk, S.T.; Herbst, R.; Coyle, A.J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar] [CrossRef]
- Lee, S.H. Chemotherapy for Lung Cancer in the Era of Personalized Medicine. Tuberc. Respir. Dis. (Seoul) 2019, 82, 179–189. [Google Scholar] [CrossRef]
| Characteristic | Total Group | Patients With: | p | |
|---|---|---|---|---|
| Shorter Survival 1 | Longer Survival 1 | |||
| n | 53 | 22 | 31 | - |
| Age (years) 2 | 60 (31–89) | 52 (31–75) | 61 (37–89) | 0.04 |
| Female | 22 | 9 | 13 | - |
| Male | 31 | 13 | 18 | |
| Stage | ||||
| IIIb-c | 13 | 6 | 7 | - |
| IV | 40 | 16 | 24 | |
| ECOG | ||||
| 1 | 36 | 16 | 23 | - |
| 2 | 17 | 6 | 8 | |
| Treatment | ||||
| CDDP/Paclitaxel | 28 | 10 | 18 | - |
| CDDP/Pemetrexed | 14 | 8 | 6 | |
| CBDCA/Paclitaxel | 7 | 3 | 4 | |
| CBDCA/Pemetrexed | 4 | 1 | 3 | |
| Median OS months 2 | 12 | 9 (5–12) | 21 (13–35) | 0.0001 |
| Clinical parameters | ||||
| Leukocytes (103/mm3) 3 | 8.6 (7–11.5) | 10.2 (6.9–12.4) | 8 (6.9–9.8) | 0.0925 |
| Lymphocytes (103/mm3) 3 | 1.6 (1.2–2.1) | 1.8 (1.3–2.3) | 1.6 (1.1–2.1) | 0.4379 |
| TGF-β (ng/mL) 3 | 20.5 (15.5–31.1) | 20.6 (10.8–32.5) | 20.5 (15.5–29.8) | 0.9524 |
| HMGB-1 (ng/mL) 3 | 1.7 (1.2–2.6) | 1.7 (1.3–2.5) | 1.6 (1.1–2.7) | 0.7588 |
| IL-6 (pg/mL) 3 | 5.4 (3.4–21.4) | 5.8 (3.2–9.6) | 5.4 (3.4–11.3) | 0.9679 |
| NLR 3 | 3.9 (2.5–5.4) | 4.1 (2.5–5.2) | 3.7 (2.2–6.1) | 0.9608 |
| SII 3 | 1104 (661.7–1777) | 1094 (666.9–1894) | 1186 (647.5–1730) | >0.999 |
| T-lymphocytes CD4+ (%) 4 | 38.37 ± 3.29 | 43.40 ± 5.34 | 35.58 ± 4.13 | 0.1867 |
| Th1 (%) 4 | 12.21 ± 2.20 | 8.46 ± 2.53 | 14.30 ± 3.06 | 0.1749 |
| Th2 (%) 4 | 3.19 ± 0.41 | 2.88 ± 0.71 | 3.37 ± 0.51 | 0.4289 |
| Th17 (%) 4 | 3.56 ± 0.38 | 3.63 ± 0.40 | 3.53 ± 0.56 | 0.3879 |
| Treg (%) 4 | 4.00 ± 0.44 | 3.99 ± 0.77 | 4.01 ± 0.54 | 0.9063 |
| CD8+ (%) 4 | 25.91 ± 2.22 | 19.81 ± 1.82 | 29.30 ± 3.05 | 0.032 |
| CTLs (GrB+Perf+) (%) 4 | 38.98 ± 4.23 | 28.59 ± 7.43 | 44.75 ± 4.73 | 0.0987 |
| Adenocarcinoma Subtype | Total | Intensity | |||
|---|---|---|---|---|---|
| Strong | Moderate | Low | None | ||
| Solid | 18 | 12 | 4 | 0 | 2 |
| Acinar | 12 | 10 | 2 | 0 | 0 |
| Lepidic | 6 | 5 | 0 | 0 | 1 |
| Papillary | 5 | 3 | 1 | 0 | 1 |
| Micropapillary | 2 | 1 | 1 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islas-Vazquez, L.; Aguilar-Cazares, D.; Galicia-Velasco, M.; Rumbo-Nava, U.; Meneses-Flores, M.; Luna-Rivero, C.; Lopez-Gonzalez, J.S. IL-6, NLR, and SII Markers and Their Relation with Alterations in CD8+ T-Lymphocyte Subpopulations in Patients Treated for Lung Adenocarcinoma. Biology 2020, 9, 376. https://doi.org/10.3390/biology9110376
Islas-Vazquez L, Aguilar-Cazares D, Galicia-Velasco M, Rumbo-Nava U, Meneses-Flores M, Luna-Rivero C, Lopez-Gonzalez JS. IL-6, NLR, and SII Markers and Their Relation with Alterations in CD8+ T-Lymphocyte Subpopulations in Patients Treated for Lung Adenocarcinoma. Biology. 2020; 9(11):376. https://doi.org/10.3390/biology9110376
Chicago/Turabian StyleIslas-Vazquez, Lorenzo, Dolores Aguilar-Cazares, Miriam Galicia-Velasco, Uriel Rumbo-Nava, Manuel Meneses-Flores, Cesar Luna-Rivero, and Jose Sullivan Lopez-Gonzalez. 2020. "IL-6, NLR, and SII Markers and Their Relation with Alterations in CD8+ T-Lymphocyte Subpopulations in Patients Treated for Lung Adenocarcinoma" Biology 9, no. 11: 376. https://doi.org/10.3390/biology9110376
APA StyleIslas-Vazquez, L., Aguilar-Cazares, D., Galicia-Velasco, M., Rumbo-Nava, U., Meneses-Flores, M., Luna-Rivero, C., & Lopez-Gonzalez, J. S. (2020). IL-6, NLR, and SII Markers and Their Relation with Alterations in CD8+ T-Lymphocyte Subpopulations in Patients Treated for Lung Adenocarcinoma. Biology, 9(11), 376. https://doi.org/10.3390/biology9110376






