Salmonid Antibacterial Immunity: An Aquaculture Perspective
Simple Summary
Abstract
1. The Impact of Global Aquaculture
2. Common Bacterial Diseases in Salmonid Culture
3. Teleostean Immunity: Our Current Understanding of the Antibacterial Response
3.1. Innate Immunity of Fish
3.1.1. Cells of Innate Immunity
3.1.2. Pattern Recognition Receptors (PRRs)
3.1.3. Antimicrobial Peptides
3.1.4. Respiratory Burst Activity
3.2. Adaptive Immunity of Fish
3.2.1. Cells of Adaptive Immunity
3.2.2. Major Histocompatibility (MH) Genes
3.2.3. Antibody Development
3.3. Cytokines
3.3.1. Chemokines
3.3.2. Proinflammatory Cytokines
3.3.3. Anti-Inflammatory Cytokines
4. The Benefit of Understanding Bony Fish Immunology
5. Current Methods of Bacterial Disease Prevention in Aquaculture
5.1. Heritable Differences in Selectively Bred Fish
5.2. Vaccinations and Their Efficacy
5.3. Understanding Bacterial Pathogens and the Resulting Disease State
5.4. Adjuvants/Immunostimulants for Aquaculture
5.5. Alternative Study Models—The Value of Fish Cell Lines for Immune Analyses
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taşbozan, O.; Gökçe, M.A. Fatty Acids in Fish. In Fatty Acids; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- WHO. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Kvamsdal, S.F.; Eide, A.; Ekerhovd, N.-A.; Enberg, K.; Gudmundsdottir, A.; Hoel, A.H.; Mills, K.E.; Mueter, F.J.; Ravn-Jonsen, L.; Sandal, L.K.; et al. Harvest Control Rules in Modern Fisheries Management. Elem. Sci. Anthr. 2016, 4, 000114. [Google Scholar] [CrossRef]
- Van Anrooy, R. Review of the Current State of World Aquaculture Insurance; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Brummett, R.E.; Alvial, A.; Kibenge, F.; Forster, J.; Burgos, J.M.; Ibarra, R.; St-Hilaire, S.; Chamberlain, G.C.; Lightner, D.V.; van Khoa, L.; et al. Reducing Disease Risk in Aquaculture; Agriculture and Environmental Services Discussion Paper; No. 9; World Bank Group: Washington, DC, USA, 2014. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Lucas, J.S.; Southgate, P.C. Aquaculture: Farming Aquatic Animals and Plants, 2nd ed.; John Wiley & Sons: West Sussex, UK, 2012. [Google Scholar]
- Meyer, F.P. Aquaculture Disease and Health Management. J. Anim. Sci. 1991, 69, 4201–4208. [Google Scholar] [CrossRef]
- Wedemeyer, G.A.; Nelson, N.C. Survival of Two Bacterial Fish Pathogens (Aeromonas salmonicida and the Enteric Redmouth Bacterium) in Ozonated, Chlorinated, and Untreated Waters. J. Fish. Res. Board Can. 1977, 34, 429–432. [Google Scholar] [CrossRef]
- Hoff, K.A. Survival of Vibrio anguillarum and Vibrio salmonicida at different salinities. Appl. Environ. Microbiol. 1989, 55, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Madetoja, J.; Nystedt, S.; Wiklund, T. Survival and Virulence of Flavobacterium psychrophilum in Water Microcosms. FEMS Microbiol. Ecol. 2003, 43, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, E. Henderson’s Dictionary of Biology; Pearson Benjamin Cummings Prentice Hall: London, UK, 2008. [Google Scholar]
- Ringø, E.; Myklebust, R.; Mayhew, T.M.; Olsen, R.E. Bacterial Translocation and Pathogenesis in the Digestive Tract of Larvae and Fry. Aquaculture 2007, 268, 251–264. [Google Scholar] [CrossRef]
- Defoirdt, T. Virulence Mechanisms of Bacterial Aquaculture Pathogens and Antivirulence Therapy for Aquaculture. Rev. Aquac. 2014, 6, 100–114. [Google Scholar] [CrossRef]
- Glasauer, S.M.K.; Neuhauss, S.C.F. Whole-Genome Duplication in Teleost Fishes and Its Evolutionary Consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Ravi, V.; Venkatesh, B. The Divergent Genomes of Teleosts. Annu. Rev. Anim. Biosci. 2018, 6, 47–68. [Google Scholar] [CrossRef]
- Helfman, G.; Collette, B.B.; Facey, D.E.; Bowen, B.W. The Diversity of Fishes: Biology, Evolution, and Ecology, 2nd ed.; Helfman, G., Collette, B.B., Facey, D.E., Bowen, B.W., Eds.; Wiley & Sons Ltd.: West Sussex, UK, 2009. [Google Scholar]
- Hodgkinson, J.W.; Grayfer, L.; Belosevic, M. Biology of Bony Fish Macrophages. Biology 2015, 4, 881–906. [Google Scholar] [CrossRef]
- Grayfer, L.; Kerimoglu, B.; Yaparla, A.; Hodgkinson, J.W.; Xie, J.; Belosevic, M. Mechanisms of Fish Macrophage Antimicrobial Immunity. Front. Immunol. 2018, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qin, Z.; Liu, H.; Lin, L.; Ye, J.; Li, J. Recent Advances on Phagocytic B Cells in Teleost Fish. Front. Immunol. 2020, 824. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Brown, M.L. A Review of Immune System Components, Cytokines, and Immunostimulants in Cultured Finfish Species. Open J. Anim. Sci. 2017, 7, 267–288. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. The Function of Fish Cytokines. Biology 2016, 5, 23. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Maisey, K.; Reyes-Lpez, F.; Toro-Ascuy, D.; Mara, A.; Imarai, M. Fish Cytokines and Immune Response. In New Advances and Contributions to Fish Biology; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Shamri, R.; Xenakis, J.J.; Spencer, L.A. Eosinophils in Innate Immunity: An Evolving Story. Cell Tissue Res. 2011, 343, 57–83. [Google Scholar] [CrossRef]
- Varricchi, G.; Raap, U.; Rivellese, F.; Marone, G.; Gibbs, B.F. Human Mast Cells and Basophils—How Are They Similar How Are They Different? Immunol. Rev. 2018, 282, 8–34. [Google Scholar] [CrossRef]
- Voehringer, D. Recent Advances in Understanding Basophil Functions in vivo. F1000Research 2017, 6, 1464. [Google Scholar] [CrossRef]
- Sheshachalam, A.; Srivastava, N.; Mitchell, T.; Lacy, P.; Eitzen, G. Granule Protein Processing and Regulated Secretion in Neutrophils. Front. Immunol. 2014, 5, 448. [Google Scholar] [CrossRef]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte Differentiation and Antigen-Presenting Functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Mathur, A.; Tripathi, A.; Kuse, M. Scalable System for Classification of White Blood Cells from Leishman Stained Blood Stain Images. J. Pathol. Inform. 2013, 4, 15. [Google Scholar] [CrossRef]
- Houwen, B. The Differential Cell Count. Lab Hematol. 2001, 7, 89–100. [Google Scholar]
- Niimi, A.J.; Lowe-Jinde, L. Differential Blood Cell Ratios of Rainbow Trout (Salmo gairdneri) Exposed to Methylmercury and Chlorobenzenes. Arch. Environ. Contam. Toxicol. 1984, 13, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Blaxhall, P.C.; Daisley, K.W. Routine Haematological Methods for Use with Fish Blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-Resident Macrophages Self-Maintain Locally throughout Adult Life with Minimal Contribution from Circulating Monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Plüddemann, A. Tissue Macrophages: Heterogeneity and Functions. BMC Biol. 2017, 15, 53. [Google Scholar] [CrossRef]
- Soza-Ried, C.; Hess, I.; Netuschil, N.; Schorpp, M.; Boehm, T. Essential Role of C-myb in Definitive Hematopoiesis Is Evolutionarily Conserved. Proc. Natl. Acad. Sci. USA 2010, 107, 17304–17308. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Zhou, D.; Huang, C.; Lin, Z.; Zhan, S.; Kong, L.; Fang, C.; Li, J. Macrophage Polarization and Function with Emphasis on the Evolving Roles of Coordinated Regulation of Cellular Signaling Pathways. Cell Signal. 2014, 26, 192–197. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Punt, J.; Stranford, S.; Jones, P.; Owen, J. Kuby Immunology, 8th ed.; W.H. Freeman: New York, NY, USA, 2019. [Google Scholar]
- Taguchi, T.; Mukai, K. Innate Immunity Signalling and Membrane Trafficking. Curr. Opin. Cell Biol. 2019, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.J.; Lu, X.J.; Chen, Q.; Chen, J. Molecular Characterization and Functional Analysis of a Novel C-Type Lectin Receptor-like Gene from a Teleost Fish, Plecoglossus altivelis. Fish Shellfish Immunol. 2015, 44, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, Z.; Yu, L.; Zhang, B.; Wang, J.; Zhou, J. Nucleotide-Binding and Oligomerization Domain (NOD)-like Receptors in Teleost Fish: Current Knowledge and Future Perspectives. J. Fish Dis. 2018, 41, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Zou, P.F.; Nie, P. Retinoic Acid-Inducible Gene I (RIG-I)-like Receptors (RLRs) in fish: Current Knowledge and Future Perspectives. Immunology 2017, 151, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Poynter, S.; Lisser, G.; Monjo, A.; DeWitte-Orr, S. Sensors of Infection: Viral Nucleic Acid PRRs in Fish. Biology 2015, 4, 460–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ji, J.; Jiang, X.; Shao, T.; Fan, D.; Jiang, X.; Lin, A.; Xiang, L.; Shao, J. Characterization of CGAS Homologs in Innate and Adaptive Mucosal Immunities in Zebrafish Gives Evolutionary Insights into CGAS-STING Pathway. FASEB J. 2020, 34, 7786–7809. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Zhou, C.; Li, L.; Nie, G.; Li, X. Toll-like Receptor Recognition of Bacteria in Fish: Ligand Specificity and Signal. Pathways. Fish Shellfish Immunol. 2014, 41, 380–388. [Google Scholar] [CrossRef]
- Nie, L.; Cai, S.-Y.; Shao, J.-Z.; Chen, J. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Front. Immunol. 2018, 9, 1523. [Google Scholar] [CrossRef]
- Samanta, M.; Swain, B.; Basu, M.; Panda, P.; Mohapatra, G.B.; Sahoo, B.R.; Maiti, N.K. Molecular Characterization of Toll-like Receptor 2 (TLR2), Analysis of Its Inductive Expression and Associated down-Stream Signaling Molecules Following Ligands Exposure and Bacterial Infection in the Indian Major Carp, Rohu (Labeo rohita). Fish Shellfish Immunol. 2012, 32, 411–425. [Google Scholar] [CrossRef]
- Basu, M.; Swain, B.; Sahoo, B.R.; Maiti, N.K.; Samanta, M. Induction of Toll-like Receptor (TLR) 2, and MyD88-Dependent TLR-Signaling in Response to Ligand Stimulation and Bacterial Infections in the Indian Major Carp, Mrigal (Cirrhinus mrigala). Mol. Biol. Rep. 2012, 39, 6015–6028. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.M.S.; Hermsen, T.; Taverne-Thiele, A.J.; Savelkoul, H.F.J.; Wiegertjes, G.F. Evolution of Recognition of Ligands from Gram-Positive Bacteria: Similarities and Differences in the TLR2-Mediated Response between Mammalian Vertebrates and Teleost Fish. J. Immunol. 2010, 184, 2355–2368. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.D.; Asahi, T.; Kondo, H.; Hirono, I.; Aoki, T. Molecular Cloning and Expression Study on Toll-like Receptor 5 Paralogs in Japanese Flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2010, 29, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.; Sepulcre, M.P.; Meseguer, J.; Mulero, V. Molecular Cloning, Phylogenetic Analysis and Functional Characterization of Soluble Toll-like Receptor 5 in Gilthead Seabream, Sparus aurata. Fish Shellfish Immunol. 2013, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, T.; Tsukada, H.; Nakao, M.; Oshiumi, H.; Matsumoto, M.; Seya, T. Sensing Bacterial Flagellin by Membrane and Soluble Orthologs of Toll-like Receptor 5 in Rainbow Trout (Onchorhynchus mykiss). J. Biol. Chem. 2004, 279, 48588–48597. [Google Scholar] [CrossRef] [PubMed]
- Iliev, D.B.; Skjæveland, I.; Jørgensen, J.B. CpG Oligonucleotides Bind TLR9 and RRM-Containing Proteins in Atlantic Salmon (Salmo salar). BMC Immunol. 2013, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Franch, R.; Cardazzo, B.; Antonello, J.; Castagnaro, M.; Patarnello, T.; Bargelloni, L. Full-Length Sequence and Expression Analysis of Toll-like Receptor 9 in the Gilthead Seabream (Sparus aurata L.). Gene 2006, 378, 42–51. [Google Scholar] [CrossRef]
- Gao, H.; Wu, L.; Sun, J.S.; Geng, X.Y.; Pan, B.P. Molecular Characterization and Expression Analysis of Toll-like Receptor 21 CDNA from Paralichthys olivaceus. Fish Shellfish Immunol. 2013, 35, 1138–1145. [Google Scholar] [CrossRef]
- Wang, W.; Shen, Y.; Pandit, N.P.; Li, J. Molecular Cloning, Characterization and Immunological Response Analysis of Toll-like Receptor 21 (TLR21) Gene in Grass Carp, Ctenopharyngodon idella. Dev. Comp. Immunol. 2013, 40, 227–231. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Li, D.; Huang, A.; Bu, G.; Meng, F.; Kong, F.; Cao, X.; Han, X.; Pan, X.; et al. Teleost-Specific TLR25 Identified from Schizothorax prenanti May Recognize Bacterial/Viral Components and Activate NF-ΚB and Type I IFNs Signaling Pathways. Fish Shellfish Immunol. 2018, 82, 361–370. [Google Scholar] [CrossRef]
- Shan, S.; Liu, D.; Liu, R.; Zhu, Y.; Li, T.; Zhang, F.; An, L.; Yang, G.; Li, H. Non-Mammalian Toll-like Receptor 18 (Tlr18) Recognizes Bacterial Pathogens in Common Carp (Cyprinus carpio L.): Indications for a Role of Participation in the NF-ΚB Signaling Pathway. Fish Shellfish Immunol. 2018, 72, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.D.; Kondo, H.; Hirono, I.; Aoki, T. Molecular Cloning and Characterization of Toll-like Receptor 14 in Japanese Flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2011, 30, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chu, Q.; Xu, T. MiR-122 Involved in the Regulation of Toll-like Receptor Signaling Pathway after Vibrio anguillarum Infection by Targeting TLR14 in Miiuy Croaker. Fish Shellfish Immunol. 2016, 58, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Gallo, R.L. Antimicrobial Peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up Immunity: How Antimicrobial Peptides Have Multiple Roles in Immune Defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Lee, J.K.; Luchian, T.; Park, Y. New Antimicrobial Peptide Kills Drug-Resistant Pathogens without Detectable Resistance. Oncotarget 2018, 9, 15616–15634. [Google Scholar] [CrossRef]
- O’Neill, H.C.; Griffiths, K.L.; Periasamy, P.; Hinton, R.A.; Hey, Y.-Y.; Petvises, S.; Tan, J.K.H. Spleen as a Site for Hematopoiesis of a Distinct Antigen Presenting Cell Type. Stem Cells Int. 2011, 2011, 954275. [Google Scholar] [CrossRef]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A Novel Human Antibiotic Peptide Secreted by Sweat Glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Bahar, A.A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Kang, H.-K.; Kim, C.; Seo, C.H.; Park, Y. The Therapeutic Applications of Antimicrobial Peptides (AMPs): A Patent Review. J. Microbiol. 2017, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L.; Cudic, M. Broth Microdilution Antibacterial Assay of Peptides. Methods Mol. Biol. 2007, 386, 309–320. [Google Scholar] [CrossRef]
- Elssner, A.; Duncan, M.; Gavrilin, M.; Wewers, M.D. A Novel P2X 7 Receptor Activator, the Human Cathelicidin-Derived Peptide LL37, Induces IL-1β Processing and Release. J. Immunol. 2004, 172, 4987–4994. [Google Scholar] [CrossRef]
- Yu, J.; Mookherjee, N.; Wee, K.; Bowdish, D.M.E.; Pistolic, J.; Li, Y.; Rehaume, L.; Hancock, R.E.W. Host Defense Peptide LL-37, in Synergy with Inflammatory Mediator IL-1beta, Augments Immune Responses by Multiple Pathways. J. Immunol. 2007, 179, 7684–7691. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Brown, K.L.; Bowdish, D.M.E.; Doria, S.; Falsafi, R.; Hokamp, K.; Roche, F.M.; Mu, R.; Doho, G.H.; Pistolic, J.; et al. Modulation of the TLR-Mediated Inflammatory Response by the Endogenous Human Host Defense Peptide LL-37. J. Immunol. 2006, 176, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- di Nardo, A.; Braff, M.H.; Taylor, K.R.; Na, C.; Granstein, R.D.; McInturff, J.E.; Krutzik, S.; Modlin, R.L.; Gallo, R.L. Cathelicidin Antimicrobial Peptides Block Dendritic Cell TLR4 Activation and Allergic Contact Sensitization. J. Immunol. 2007, 178, 1829–1834. [Google Scholar] [CrossRef]
- Falco, A.; Martinez-Lopez, A.; Coll, J.P.; Estepa, A. The Potential for Antimicrobial Peptides to Improve Fish Health in Aquaculture. In Infectious Disease in Aquaculture, Brian Austin; Woodhead Publishing Limited: Philadelphia, PA, USA, 2012; pp. 457–479. [Google Scholar] [CrossRef]
- Rajanbabu, V.; Chen, J.Y. Applications of Antimicrobial Peptides from Fish and Perspectives for the Future. Peptides 2011, 32, 415–420. [Google Scholar] [CrossRef]
- Valero, Y.; Saraiva-Fraga, M.; Costas, B.; Guardiola, F.A. Antimicrobial Peptides from Fish: Beyond the Fight against Pathogens. Rev. Aquac. 2020, 12, 224–253. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH Oxidases: An Overview from Structure to Innate Immunity-Associated Pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Quinn, M.T. Assembly of the Phagocyte NADPH Oxidase: Molecular Interaction of Oxidase Proteins. J. Leukoc. Biol. 1996, 60, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Nauseef, W.M. The Phagocyte NOX2 NADPH Oxidase in Microbial Killing and Cell Signaling. Curr. Opin. Immunol. 2019, 60, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Stafford, J.L.; Galvez, F.; Goss, G.G.; Belosevic, M. Induction of Nitric Oxide and Respiratory Burst Response in Activated Goldfish Macrophages Requires Potassium Channel Activity. Dev. Comp. Immunol. 2002, 26, 445–459. [Google Scholar] [CrossRef]
- Sepulcre, M.P.; López-Castejón, G.; Meseguer, J.; Mulero, V. The Activation of Gilthead Seabream Professional Phagocytes by Different PAMPs Underlines the Behavioural Diversity of the Main Innate Immune Cells of Bony Fish. Mol. Immunol. 2007, 44, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Boltaña, S.; Doñate, C.; Goetz, F.W.; MacKenzie, S.; Balasch, J.C. Characterization and Expression of NADPH Oxidase in LPS-, Poly(I:C)- and Zymosan-Stimulated Trout (Oncorhynchus mykiss W.) Macrophages. Fish Shellfish Immunol. 2009, 26, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Sharp, G.J.E.; Secombes, C.J. The Role of Reactive Oxygen Species in the Killing of the Bacterial Fish Pathogen Aeromonas salmonicida by Rainbow Trout Macrophages. Fish Shellfish Immunol. 1993, 3, 119–129. [Google Scholar] [CrossRef]
- Ardó, L.; Jeney, Z.; Adams, A.; Jeney, G. Immune Responses of Resistant and Sensitive Common Carp Families Following Experimental Challenge with Aeromonas hydrophila. Fish Shellfish Immunol. 2010, 29, 111–116. [Google Scholar] [CrossRef]
- Hodgkinson, J.W.; Ge, J.Q.; Grayfer, L.; Stafford, J.; Belosevic, M. Analysis of the Immune Response in Infections of the Goldfish (Carassius auratus L.) with Mycobacterium marinum. Dev. Comp. Immunol. 2012, 38, 456–465. [Google Scholar] [CrossRef]
- Havixbeck, J.J.; Rieger, A.M.; Churchill, L.J.; Barreda, D.R. Neutrophils Exert Protection in Early Aeromonas veronii Infections through the Clearance of Both Bacteria and Dying Macrophages. Fish Shellfish Immunol. 2017, 63, 18–30. [Google Scholar] [CrossRef]
- Collazos, M.E.; Ortega, E.; Barriga, C. Effect of Temperature on the Immune System of a Cyprinid Fish (Tinca tinca, L). Blood Phagocyte Function at Low Temperature. Fish Shellfish Immunol. 1994, 4, 231–238. [Google Scholar] [CrossRef]
- Collazos, M.E.; Barriga, C.; Ortega, E. Enhanced Granulocyte Phagocytosis at Low Winter Temperature and High Summer Temperature in the Tench (Tinca tinca L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1994, 109, 643–648. [Google Scholar] [CrossRef]
- Nikoskelainen, S.; Bylund, G.; Lilius, E.M. Effect of Environmental Temperature on Rainbow Trout (Oncorhynchus mykiss) Innate Immunity. Dev. Comp. Immunol. 2004, 28, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Le Morvan, C.; Troutaud, D.; Deschaux, P. Differential Effects of Temperature on Specific and Nonspecific Immune Defences in Fish. J. Exp. Biol. 1998, 201, 165–168. [Google Scholar] [PubMed]
- Cooper, M.D.; Alder, M.N. The Evolution of Adaptive Immune Systems. Cell 2006, 124, 815–822. [Google Scholar] [CrossRef]
- Flajnik, M.F.; Kasahara, M. Origin and Evolution of the Adaptive Immune System: Genetic Events and Selective Pressures. Nat. Rev. Genet. 2010, 11, 47–59. [Google Scholar] [CrossRef]
- Nemazee, D. Role of B Cell Antigen Receptor in Regulation of V(D)J Recombination and Cell Survival. Immunol. Res. 2000, 21, 259–263. [Google Scholar] [CrossRef]
- Abbas, A.K.; Murphy, K.M.; Sher, A. Functional Diversity of Helper T Lymphocytes. Nature 1996, 383, 787–793. [Google Scholar] [CrossRef]
- Barry, M.; Bleackley, R.C. Cytotoxic T Lymphocytes: All Roads Lead to Death. Nat. Rev. Immunol. 2002, 2, 401–409. [Google Scholar] [CrossRef]
- Lebien, T.W.; Tedder, T.F. B Lymphocytes: How They Develop and Function. Blood 2008, 112, 1570–1580. [Google Scholar] [CrossRef]
- Da Silva Pitombeira, M.; Martins, J.M.; Martins, J.M. Hematology of the Spanish Mackerel, Scomberomorus maculatus. Copeia 1970, 1970, 182. [Google Scholar] [CrossRef]
- Zhang, Y.-A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; Lapatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a Primitive Immunoglobulin Class Specialized in Mucosal Immunity. Nat. Immunol. 2012, 11, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Maisey, K.; Toro-Ascuy, D.; Montero, R.; Reyes-López, F.E.; Imarai, M. Identification of CD3ε, CD4, CD8β Splice Variants of Atlantic Salmon. Fish Shellfish Immunol. 2011, 31, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, F.; Dijkstra, J.M.; Kotterba, P.; Korytář, T.; Kock, H.; Köllner, B.; Jaureguiberry, B.; Nakanishi, T.; Fischer, U. The Expression of CD8α Discriminates Distinct T Cell Subsets in Teleost Fish. Dev. Comp. Immunol. 2011, 35, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.; Stet, R.J.M. The Relationship between Major Histocompatibility Receptors and Innate Immunity in Teleost Fish. Dev. Comp. Immunol. 2001, 25, 683–699. [Google Scholar] [CrossRef]
- Neefjes, J.; Jongsma, M.L.M.; Paul, P.; Bakke, O. Towards a Systems Understanding of MHC Class I and MHC Class II Antigen Presentation. Nat. Rev. Immunol. 2011, 11, 823–836. [Google Scholar] [CrossRef]
- Landsverk, O.J.B.; Bakke, O.; Gregers, T.F. MHC II and the Endocytic Pathway: Regulation by Invariant Chain. Scand. J. Immunol. 2009, 70, 184–193. [Google Scholar] [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Choo, S.Y. The HLA System: Genetics, Immunology, Clinical Testing, and Clinical Implications. Yonsei Med. J. 2007, 48, 11–23. [Google Scholar] [CrossRef]
- Garcia, M.A.A.; Yebra, B.G.; Flores, A.L.L.; Guerra, E.G. The Major Histocompatibility Complex in Transplantation. J. Transplant. 2012, 2012, 842141. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Dijkstra, J.M. Major Histocompatibility Complex (MHC) Genes and Disease Resistance in Fish. Cells 2019, 8, 378. [Google Scholar] [CrossRef]
- Lenz, T.L. Computational Prediction of MHC II-Antigen Binding Supports Divergent Allele Advantage and Explains Trans-Species Polymorphism. Evolution 2011, 65, 2380–2390. [Google Scholar] [CrossRef]
- Pierini, F.; Lenz, T.L. Divergent Allele Advantage at Human MHC Genes: Signatures of Past and Ongoing Selection. Mol. Biol. Evol. 2018, 35, 2145–2158. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.L.; Neff, B.D. Major Histocompatibility Complex Heterozygote Advantage and Widespread Bacterial Infections in Populations of Chinook Salmon (Oncorhynchus tshawytscha). Mol. Ecol. 2009, 18, 4716–4729. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.A.; Kirkland, M.; Heath, J.W.; Heath, D.D.; Dixon, B. Breeding Strategy and Rearing Environment Effects on the Disease Resistance of Cultured Chinook Salmon (Oncorhynchus tshawytscha). Aquaculture 2014, 422–423, 160–166. [Google Scholar] [CrossRef]
- Pazderka, F.; Longenecker, B.M.; Law, G.R.J.; Stone, H.A.; Ruth, R.F. Histocompatibility of Chicken Populations Selected for Resistance to Marek’s Disease. Immunogenetics 1975, 2, 93–100. [Google Scholar] [CrossRef]
- Hill, A.V.S.; Elvin, J.; Willis, A.C.; Aidoo, M.; Allsopp, C.E.M.; Gotch, F.M.; Ming Gao, X.; Takiguchis, M.; Greenwood, B.M.; Townsend, A.R.M.; et al. Molecular Analysis of the Association of HLA-B53 and Resistance to Severe Malaria. Nature 1992, 360, 434–439. [Google Scholar] [CrossRef]
- Shen, H.; Han, G.; Jia, B.; Jiang, S.; Du, Y. MHC-DRB1/DQB1 Gene Polymorphism and Its Association with Resistance/Susceptibility to Cystic echinococcosis in Chinese Merino Sheep. J. Parasitol. Res. 2014, 2014, 272601. [Google Scholar] [CrossRef]
- Forthal, D.N. Functions of Antibodies. Microbiol. Spectr. 2014, 2, 23–48. [Google Scholar]
- Sunyer, J.O. Fishing for Mammalian Paradigms in the Teleost Immune System. Nat. Immunol. 2013, 14, 320–326. [Google Scholar] [CrossRef]
- Mashoof, S.; Criscitiello, M.F. Fish Immunoglobulins. Biology 2016, 5, 45. [Google Scholar] [CrossRef]
- Parra, D.; Reyes-Lopez, F.E.; Tort, L. Mucosal Immunity and B Cells in Teleosts: Effect of Vaccination and Stress. Front. Immunol. 2015, 6, 354. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, C.; Chen, K.; Cerutti, A. The Enigmatic Function of IgD: Some Answers at Last. Eur. J. Immunol. 2018, 48, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.D.; Landis, E.D.; Phillips, R.B. Discovery of a Unique Ig Heavy-Chain (IgT) in Rainbow Trout: Implications for a Distinctive B Cell Developmental Pathway in Teleost Fish. Proc. Natl. Acad. Sci. USA 2005, 102, 6919–6924. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Parra, D.; Gómez, D.; Salinas, I.; Zhang, Y.A.; von Gersdorff Jørgensen, L.; Heinecke, R.D.; Buchmann, K.; LaPatra, S.; Oriol Sunyer, J. Teleost Skin, an Ancient Mucosal Surface That Elicits Gut-like Immune Responses. Proc. Natl. Acad. Sci. USA 2013, 110, 13097–13102. [Google Scholar] [CrossRef]
- Piazzon, M.C.; Galindo-Villegas, J.; Pereiro, P.; Estensoro, I.; Calduch-Giner, J.A.; Gómez-Casado, E.; Novoa, B.; Mulero, V.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Differential Modulation of IgT and IgM upon Parasitic, Bacterial, Viral, and Dietary Challenges in a Perciform Fish. Front. Immunol. 2016, 7, 637. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Kong, W.; Yin, Y.X.; Dong, F.; Huang, Z.Y.; Yin, G.M.; Dong, S.; Salinas, I.; Zhang, Y.A.; Xu, Z. Mucosal Immunoglobulins Protect the Olfactory Organ of Teleost Fish against Parasitic Infection. PLoS Pathog. 2018, 14, e1007251. [Google Scholar] [CrossRef]
- Solem, S.T.; Stenvik, J. Antibody Repertoire Development in—A Review with Emphasis on Salmonids and Gadus morhua L. Dev. Comp. Immunol. 2006, 30, 57–76. [Google Scholar] [CrossRef]
- Kaattari, S.L.; Zhang, H.L.; Khor, I.W.; Kaattari, I.M.; Shapiro, D.A. Affinity Maturation in Trout: Clonal Dominance of High Affinity Antibodies Late in the Immune Response. Dev. Comp. Immunol. 2002, 26, 191–200. [Google Scholar] [CrossRef]
- Dickerson, H.W.; Findly, R.C. Vertebrate Adaptive Immunity-Comparative Insights from a Teleost Model. Front. Immunol. 2017, 8, 1379. [Google Scholar] [CrossRef]
- Barreto, V.M.; Pan-Hammarstrom, Q.; Zhao, Y.; Hammarstrom, L.; Misulovin, Z.; Nussenzweig, M.C. AID from Bony Fish Catalyzes Class Switch Recombination. J. Exp. 2005, 202, 733–738. [Google Scholar] [CrossRef]
- Wakae, K.; Magor, B.G.; Saunders, H.; Nagaoka, H.; Kawamura, A.; Kinoshita, K.; Honjo, T.; Muramatsu, M. Evolution of Class Switch Recombination Function in Fish Activation-Induced Cytidine Deaminase, AID. Int. Immunol. 2006, 18, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Craig Findly, R.; Zhao, X.; Noe, J.; Camus, A.C.; Dickerson, H.W. B Cell Memory Following Infection and Challenge of Channel Catfish with Ichthyophthirius multifiliis. Dev. Comp. Immunol. 2013, 39, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Bromage, E.S.; Kaattari, I.M.; Zwollo, P.; Kaattari, S.L. Plasmablast and Plasma Cell Production and Distribution in Trout Immune Tissues. J. Immunol. 2004, 173, 7317–7323. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Kaattari, I.; Kaattari, S. Plasmablasts and Plasma Cells: Reconsidering Teleost Immune System Organization. Dev. Comp. Immunol. 2011, 35, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, V.; Brennan, S.; Chambers, G.; George, P. The Albumins of Chinook Salmon (Oncorhynchus tshawytscha) and Brown Trout (Salmo trutta) Appear to Lack a Propeptide. Arch. Biochem. Biophys. 1998, 350, 239–244. [Google Scholar] [CrossRef]
- Grayfer, L.; Belosevic, M. Cytokine Regulation of Teleost Inflammatory Responses. In New Advances and Contributions to Fish Biology; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. CSH Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and Chemokine Receptors: Positioning Cells for Host Defense and Immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef]
- Dixon, B.; Shum, B.; Adams, E.J.; Magor, K.; Hedrick, R.P.; Muir, D.G.; Parham, P. CK-1, a Putative Chemokine of Rainbow Trout (Oncorhynchus mykiss). Immunol. Rev. 1998, 166, 341–348. [Google Scholar] [CrossRef]
- Alejo, A.; Tafalla, C. Chemokines in Teleost Fish Species. Dev. Comp. Immunol. 2011, 35, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Tafalla, C. Teleost Chemokines and Their Receptors. Biology 2015, 4, 756–784. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, H.; Hieshima, K.; Osada, N.; Kato-Unoki, Y.; Otsuka-Ono, K.; Takegawa, S.; Izawa, T.; Yoshizawa, A.; Kikuchi, Y.; Tanase, S.; et al. Extensive Expansion and Diversification of the Chemokine Gene Family in Zebrafish: Identification of a Novel Chemokine Subfamily CX. BMC Genom. 2008, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Peatman, E.; Liu, Z. Evolution of CC Chemokines in Teleost Fish: A Case Study in Gene Duplication and Implications for Immune Diversity. Immunogenetics 2007, 59, 613–623. [Google Scholar] [CrossRef]
- Kobayashi, Y. Neutrophil Infiltration and Chemokines. Crit. Rev. Immunol. 2006, 26, 307–315. [Google Scholar] [CrossRef]
- Martin, P.; Leibovich, S.J. Inflammatory Cells during Wound Repair: The Good, the Bad and the Ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef]
- Feghali, C.A.; Wright, T.M. Cytokines in Acute and Chronic Inflammation. Front. Biosci. 1997, 2, d12–d26. [Google Scholar] [CrossRef]
- Wulff, B.C.; Wilgus, T.A. Mast Cell Activity in the Healing Wound: More than Meets the Eye? Exp. Dermatol. 2013, 22, 507–510. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast Cells and Inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of Inflammation: The Beginning Programs the End. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Sfacteria, A.; Brines, M.; Blank, U. The Mast Cell Plays a Central Role in the Immune System of Teleost Fish. Mol. Immunol. 2015, 63, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-Deficient Mice Develop Chronic Enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Reyes-López, F.E.; Toro-Ascuy, D.; Ibañez, J.; Maisey, K.; Sandino, A.M.; Imarai, M. IPNV Modulation of pro and anti-Inflammatory Cytokine Expression in Atlantic Salmon Might Help the Establishment of Infection and Persistence. Fish Shellfish Immunol. 2012, 32, 291–300. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Reyes-López, F.; Toro-Ascuy, D.; Montero, R.; Maisey, K.; Acuña-Castillo, C.; Sunyer, J.O.; Parra, D.; Sandino, A.M.; Imarai, M. Induction of Anti-Inflammatory Cytokine Expression by IPNV in Persistent Infection. Fish Shellfish Immunol. 2014, 41, 172–182. [Google Scholar] [CrossRef]
- Wei, H.; Yin, L.; Feng, S.; Wang, X.; Yang, K.; Zhang, A.; Zhou, H. Dual-Parallel Inhibition of IL-10 and TGF-Β1 Controls LPS-Induced Inflammatory Response via NF-ΚB Signaling in Grass Carp Monocytes/Macrophages. Fish Shellfish Immunol. 2015, 44, 445–452. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F. Fishing down Marine Food Webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Wipfli, M.S.; Baxter, C.V. Linking Ecosystems, Food Webs, and Fish Production: Subsidies in Salmonid Watersheds. Fisheries 2010, 35, 373–387. [Google Scholar] [CrossRef]
- Mercier, F.E.; Ragu, C.; Scadden, D.T. The Bone Marrow at the Crossroads of Blood and Immunity. Nat. Rev. Immunol. 2012, 12, 49–60. [Google Scholar] [CrossRef]
- Secombes, C.J.; Wang, T. The Innate and Adaptive Immune System of Fish. In Infectious Disease in Aquaculture, Brian Austin; Woodhead Publishing Limited: Philadelphia, PA, USA, 2012; pp. 3–68. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and Adaptive Immunity in Teleost Fish: A Review. Vet. Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
- Palti, Y. Toll-like Receptors in Bony Fish: From Genomics to Function. Dev. Comp. Immunol. 2011, 35, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Phelan, P.E.; Mellon, M.T.; Kim, C.H. Functional Characterization of Full-Length TLR3, IRAK-4, and TRAF6 in Zebrafish (Danio rerio). Mol. Immunol. 2005, 42, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, A.; Oshiumi, H.; Tsujita, T.; Mitani, H.; Kasai, H.; Yoshimizu, M.; Matsumoto, M.; Seya, T. Teleost TLR22 Recognizes RNA Duplex to Induce IFN and Protect Cells from Birnaviruses. J. Immunol. 2008, 181, 3474–3485. [Google Scholar] [CrossRef]
- Volff, J.N. Genome Evolution and Biodiversity in Teleost Fish. Heredity 2005, 94, 280–294. [Google Scholar] [CrossRef]
- Zschaler, J.; Schlorke, D.; Arnhold, J. Differences in Innate Immune Response between Man and Mouse. Crit. Rev. Immunol. 2014, 34, 433–454. [Google Scholar] [CrossRef]
- Tao, L.; Reese, T.A. Making Mouse Models That Reflect Human Immune Responses. Trends Immunol. 2017, 38, 181–193. [Google Scholar] [CrossRef]
- Brazeau, M.D.; Friedman, M. The Origin and Early Phylogenetic History of Jawed Vertebrates. Nature 2015, 520, 490–498. [Google Scholar] [CrossRef]
- Star, B.; Nederbragt, A.J.; Jentoft, S.; Grimholt, U.; Malmstrøm, M.; Gregers, T.F.; Rounge, T.B.; Paulsen, J.; Solbakken, M.H.; Sharma, A.; et al. The Genome Sequence of Atlantic Cod Reveals a Unique Immune System. Nature 2011, 477, 207–210. [Google Scholar] [CrossRef]
- Haase, D.; Roth, O.; Kalbe, M.; Schmiedeskamp, G.; Scharsack, J.P.; Rosenstiel, P.; Reusch, T.B.H. Absence of Major Histocompatibility Complex Class II Mediated Immunity in Pipefish, Syngnathus typhle: Evidence from Deep Transcriptome Sequencing. Biol. Lett. 2013, 9, 20130044. [Google Scholar] [CrossRef]
- Solbakken, M.H.; Tørresen, O.K.; Nederbragt, A.J.; Seppola, M.; Gregers, T.F.; Jakobsen, K.S.; Jentoft, S. Evolutionary Redesign of the Atlantic Cod (Gadus morhua L.) Toll-like Receptor Repertoire by Gene Losses and Expansions. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gjedrem, T.; Robinson, N. Advances by Selective Breeding for Aquatic Species: A Review. Agric. Sci. 2014, 5, 1152–1158. [Google Scholar] [CrossRef]
- Thodesen, J.; Grisdale-Helland, B.; Helland, S.J.; Gjerde, B. Feed Intake, Growth and Feed Utilization of Offspring from Wild and Selected Atlantic Salmon (Salmo salar). Aquaculture 1999, 180, 237–246. [Google Scholar] [CrossRef]
- Perry, G.M.L.; Martyniuk, C.M.; Ferguson, M.M.; Danzmann, R.G. Genetic Parameters for Upper Thermal Tolerance and Growth-Related Traits in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2005, 250, 120–128. [Google Scholar] [CrossRef]
- Bentsen, H.B.; Gjerde, B.; Eknath, A.E.; de Vera, M.S.P.; Velasco, R.R.; Danting, J.C.; Dionisio, E.E.; Longalong, F.M.; Reyes, R.A.; Abella, T.A.; et al. Genetic Improvement of Farmed Tilapias: Response to Five Generations of Selection for Increased Body Weight at Harvest in Oreochromis niloticus and the Further Impact of the Project. Aquaculture 2017, 468, 206–217. [Google Scholar] [CrossRef]
- Ye, B.; Wan, Z.; Wang, L.; Pang, H.; Wen, Y.; Liu, H.; Liang, B.; Lim, H.S.; Jiang, J.; Yue, G. Heritability of Growth Traits in the Asian Seabass (Lates calcarifer). Aquac. Fish. 2017, 2, 112–118. [Google Scholar] [CrossRef]
- Moav, R.; Wohlfarth, G. Two-Way Selection for Growth Rate in the Common Carp (Cyprinus carpio L.). Genetics 1976, 82, 83–101. [Google Scholar]
- Hulata, G.; Wohlfarth, G.W.; Halevy, A. Mass Selection for Growth Rate in the Nile Tilapia (Oreochromis niloticus). Aquaculture 1986, 57, 177–184. [Google Scholar] [CrossRef]
- Bentsen, H.B.; Eknath, A.E.; Palada-de Vera, M.S.; Danting, J.C.; Bolivar, H.L.; Reyes, R.A.; Dionisio, E.E.; Longalong, F.M.; Circa, A.V.; Tayamen, M.M.; et al. Genetic Improvement of Farmed Tilapias: Growth Performance in a Complete Diallel Cross Experiment with Eight Strains of Oreochromis niloticus. Aquaculture 1998, 160, 145–173. [Google Scholar] [CrossRef]
- Bentsen, H.B.; Olesen, I. Designing Aquaculture Mass Selection Programs to Avoid High Inbreeding Rates. Aquaculture 2002, 204, 349–359. [Google Scholar] [CrossRef]
- Rodgveller, C.J.; Smoker, W.W.; Gray, A.K.; Joyce, J.E.; Gharrett, A.J. Effects of Inbreeding and Family Origin on Variation of Size of Chinook Salmon Oncorhynchus tshawytscha. Alsk. Fish. Res. Bull. 2005, 11, 73–81. [Google Scholar]
- D’Ambrosio, J.; Phocas, F.; Haffray, P.; Bestin, A.; Brard-Fudulea, S.; Poncet, C.; Quillet, E.; Dechamp, N.; Fraslin, C.; Charles, M.; et al. Genome-Wide Estimates of Genetic Diversity, Inbreeding and Effective Size of Experimental and Commercial Rainbow Trout Lines Undergoing Selective Breeding. Genet. Sel. Evol. 2019, 51, 26. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.M.; Houston, R.D.; Newman, S. Genetics and Genomics of Disease Resistance in Salmonid Species. Front. Genet. 2014, 5, 415. [Google Scholar] [CrossRef] [PubMed]
- Imsland, A.K.; Jonassen, T.M.; Langston, A.; Hoare, R.; Wergeland, H.; FitzGerald, R.; Mulcahy, M.; Stefansson, S.O. The Interrelation of Growth and Disease Resistance of Different Populations of Juvenile Atlantic Halibut (Hippoglossus hippoglossus L.). Aquaculture 2002, 204, 167–177. [Google Scholar] [CrossRef]
- Silverstein, J.T.; Vallejo, R.L.; Palti, Y.; Leeds, T.D.; Rexroad, C.E.; Welch, T.J.; Wiens, G.D.; Ducrocq, V. Rainbow Trout Resistance to Bacterial Cold-Water Disease Is Moderately Heritable and Is Not Adversely Correlated with Growth. J. Anim. Sci. 2009, 87, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Evenhuis, J.P.; Leeds, T.D.; Marancik, D.P.; Lapatra, S.E.; Wiens, G.D. Rainbow Trout (Oncorhynchus mykiss) Resistance to Columnaris Disease Is Heritable and Favorably Correlated with Bacterial Cold Water Disease Resistance. J. Anim. Sci. 2015, 93, 1546–1554. [Google Scholar] [CrossRef]
- Fevolden, S.E.; Refstie, T.; Røed, K.H. Disease Resistance in Rainbow Trout (Oncorhynchus mykiss) Selected for Stress Response. Aquaculture 1992, 104, 19–29. [Google Scholar] [CrossRef]
- Gjøen, H.M.; Refstie, T.; Ulla, O.; Gjerde, B. Genetic Correlations between Survival of Atlantic Salmon in Challenge and Field Tests. Aquaculture 1997, 158, 277–288. [Google Scholar] [CrossRef]
- Grimholt, U.; Larsen, S.; Nordmo, R.; Midtlyng, P.; Kjoeglum, S.; Storset, A.; Saebø, S.; Stet, R.J.M. MHC Polymorphism and Disease Resistance in Atlantic Salmon (Salmo salar); Facing Pathogens with Single Expressed Major Histocompatibility Class I and Class II Loci. Immunogenetics 2003, 55, 210–219. [Google Scholar] [CrossRef]
- Yáñez, J.M.; Bangera, R.; Lhorente, J.P.; Barría, A.; Oyarzún, M.; Neira, R.; Newman, S. Negative Genetic Correlation between Resistance against Piscirickettsia salmonis and Harvest Weight in Coho Salmon (Oncorhynchus kisutch). Aquaculture 2016, 459, 8–13. [Google Scholar] [CrossRef]
- Trang, T.T.; Hung, N.H.; Ninh, N.H.; Knibb, W.; Nguyen, N.H. Genetic Variation in Disease Resistance against White Spot Syndrome Virus (WSSV) in Liptopenaeus vannamei. Front. Genet. 2019, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Ødegård, J.; Baranski, M.; Gjerde, B.; Gjedrem, T. Methodology for Genetic Evaluation of Disease Resistance in Aquaculture Species: Challenges and Future Prospects. Aquac. Res. 2011, 42, 103–114. [Google Scholar] [CrossRef]
- Murray, C.B.; Evelyn, T.P.T.; Beacham, T.D.; Barner, L.W.; Ketcheson, J.E.; Prosperi-Porta, L. Experimental Induction of Bacterial Kidney Disease in Chinook Salmon by Immersion and Cohabitation Challenges. Dis. Aquat. Org. 1992, 12, 91–96. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Santi, N.; Fredriksen, B.N.; Løkling, K.E.; Evensen, Ø. A Systematic Approach towards Optimizing a Cohabitation Challenge Model for Infectious Pancreatic Necrosis Virus in Atlantic Salmon (Salmo salar L). PLoS ONE 2016, 11, e0148467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, D.H.; Shoemaker, C. Experimental Induction of Motile Aeromonas Septicemia in Channel Catfish (Ictalurus punctatus) by Waterborne Challenge with Virulent Aeromonas hydrophila. Aquac. Rep. 2016, 3, 18–23. [Google Scholar] [CrossRef]
- Long, A.; Fehringer, T.R.; Lafrentz, B.R.; Call, D.R.; Cain, K.D. Development of a Waterborne Challenge Model for Flavobacterium psychrophilum. FEMS Microbiol. Lett. 2014, 359, 154–160. [Google Scholar] [CrossRef]
- Frisch, K.; Småge, S.B.; Vallestad, C.; Duesund, H.; Brevik, Ø.J.; Klevan, A.; Olsen, R.H.; Sjaatil, S.T.; Gauthier, D.; Brudeseth, B.; et al. Experimental Induction of Mouthrot in Atlantic Salmon Smolts Using Tenacibaculum maritimum from Western Canada. J. Fish Dis. 2018, 41, 1247–1258. [Google Scholar] [CrossRef]
- Taksdal, T.; Ramstad, A.; Stangeland, K.; Dannevig, B.H. Induction of Infectious Pancreatic Necrosis (IPN) in Covertly Infected Atlantic Salmon, Salmo salar L., Post-Smolts by Stress Exposure, by Injection of IPN Virus (IPNV) and by Cohabitation. J. Fish Dis. 1998, 21, 193–204. [Google Scholar] [CrossRef]
- Henriksen, M.M.M.; Madsen, L.; Dalsgaard, I. Effect of Hydrogen Peroxide on Immersion Challenge of Rainbow Trout Fry with Flavobacterium psychrophilum. PLoS ONE 2013, 8, 62590. [Google Scholar] [CrossRef]
- Semple, S.L.; Kellendonk, C.J.; Al-Hussinee, L.; Macinnes, J.I.; Lumsden, J.S.; Dixon, B. Serum IgM, MH Class IIβ Genotype and Respiratory Burst Activity Do Not Differ between Rainbow Trout Families Displaying Resistance or Susceptibility to the Coldwater Pathogen, Flavobacterium psychrophilum. Aquaculture 2018, 483, 131–140. [Google Scholar] [CrossRef]
- Good, C.M.; Thorburn, M.A.; Stevenson, R.M.W. Factors Associated with the Incidence of Bacterial Gill Disease in Salmonid Lots Reared in Ontario, Canada Government Hatcheries. Prev. Vet. Med. 2008, 83, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.J.; Pearson, M.D. Infectious Pancreatic Necrosis in Atlantic Salmon, Salmo salar L. J. Fish Dis. 2005, 28, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Wiens, G.D.; Palti, Y.; Leeds, T.D. Three Generations of Selective Breeding Improved Rainbow Trout (Oncorhynchus mykiss) Disease Resistance against Natural Challenge with Flavobacterium psychrophilum during Early Life-Stage Rearing. Aquaculture 2018, 497, 414–421. [Google Scholar] [CrossRef]
- Marancik, D.; Gao, G.; Paneru, B.; Ma, H.; Hernandez, A.G.; Salem, M.; Yao, J.; Palti, Y.; Wiens, G.D. Whole-Body Transcriptome of Selectively Bred, Resistant-, Control-, and Susceptible-Line Rainbow Trout Following Experimental Challenge with Flavobacterium psychrophilum. Front. Genet. 2014, 5, 453. [Google Scholar] [CrossRef]
- Wiens, G.D.; Vallejo, R.L.; Leeds, T.D.; Palti, Y.; Hadidi, S.; Liu, S.; Evenhuis, J.P.; Welch, T.J.; Rexroad, C.E. Assessment of Genetic Correlation between Bacterial Cold Water Disease Resistance and Spleen Index in a Domesticated Population of Rainbow Trout: Identification of QTL on Chromosome Omy19. PLoS ONE 2013, 8, e75749. [Google Scholar] [CrossRef]
- Johnson, N.A.; Vallejo, R.L.; Silverstein, J.T.; Welch, T.J.; Wiens, G.D.; Hallerman, E.M.; Palti, Y. Suggestive Association of Major Histocompatibility IB Genetic Markers with Resistance to Bacterial Cold Water Disease in Rainbow Trout (Oncorhynchus mykiss). Mar. Biotechnol. 2008, 10, 429–437. [Google Scholar] [CrossRef]
- Hadidi, S.; Glenney, G.W.; Welch, T.J.; Silverstein, J.T.; Wiens, G.D. Spleen Size Predicts Resistance of Rainbow Trout to Flavobacterium psychrophilum Challenge. J. Immunol. 2008, 180, 4156–4165. [Google Scholar] [CrossRef]
- Zwollo, P.; Hennessey, E.; Moore, C.; Marancik, D.P.; Wiens, G.D.; Epp, L. A BCWD-Resistant Line of Rainbow Trout Exhibits Higher Abundance of IgT+ B Cells and Heavy Chain Tau Transcripts Compared to a Susceptible Line Following Challenge with Flavobacterium psychrophilum. Dev. Comp. Immunol. 2017, 74, 190–199. [Google Scholar] [CrossRef]
- Brown, R.M.; Wiens, G.D.; Salinas, I. Analysis of the Gut and Gill Microbiome of Resistant and Susceptible Lines of Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 86, 497–506. [Google Scholar] [CrossRef]
- Wiens, G.D.; Marancik, D.P.; Zwollo, P.; Kaattari, S.L. Reduction of Rainbow Trout Spleen Size by Splenectomy Does Not Alter Resistance against Bacterial Cold Water Disease. Dev. Comp. Immunol. 2015, 49, 31–37. [Google Scholar] [CrossRef]
- Assefa, A.; Abunna, F. Maintenance of Fish Health in Aquaculture: Review of Epidemiological Approaches for Prevention and Control of Infectious Disease of Fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef] [PubMed]
- Dejaegere, A.; Choulier, L.; Lafont, V.; de Genst, E.; Altschuh, D. Variations in Antigen-Antibody Association Kinetics as a Function of PH and Salt Concentration: A QSAR and Molecular Modeling Study. Biochemistry 2005, 44, 14409–14418. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W.; Andrew, S.M.; Hogarth, M.P.; Pietersz, G.A.; McKenzie, I.F.C. The Effect of Temperature on the Binding Kinetics and Equilibrium Constants of Monoclonal Antibodies to Cell Surface Antigens. Mol. Immunol. 1990, 27, 327–333. [Google Scholar] [CrossRef]
- Encarnação, J.C.; Barta, P.; Fornstedt, T.; Andersson, K. Impact of Assay Temperature on Antibody Binding Characteristics in Living Cells: A Case Study. Biomed. Rep. 2017, 7, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Le Morvan, C.; Deschaux, P.; Troutaud, D. Effects and Mechanisms of Environmental Temperature on Carp (Cyprinus carpio) Anti-DNP Antibody Response and Non-Specific Cytotoxic Cell Activity: A Kinetic Study. Dev. Comp. Immunol. 1996, 20, 331–340. [Google Scholar] [CrossRef]
- Avtalion, R.R. Temperature Effect on Antibody Production and Immunological Memory, in Carp (Cyprinus carpio) Immunized against Bovine Serum Albumin (BSA). Immunology 1969, 17, 927–931. [Google Scholar]
- Grabowski, L.D.; LaPatra, S.E.; Cain, K.D. Systemic and Mucosal Antibody Response in Tilapia, Oreochromis niloticus (L.), Following Immunization with Flavobacterium columnare. J. Fish Dis. 2004, 27, 573–581. [Google Scholar] [CrossRef]
- Rijkers, G.T.; Frederix-Wolters, E.M.; van Muiswinkel, W.B. The Immune System of Cyprinid Fish. Kinetics and Temperature Dependence of Antibody-Producing Cells in Carp (Cyprinus carpio). Immunology 1980, 41, 91–97. [Google Scholar]
- Plumb, J.A.; Wise, M.L.; Rogers, W.A. Modulary Effects of Temperature on Antibody Response and Specific Resistance to Challenge of Channel Catfish, Ictalurus punctatus, Immunized against Edwardsiella ictaluri. Vet. Immunol. Immunopathol. 1986, 12, 297–304. [Google Scholar] [CrossRef]
- Lange, M.D.; Webster, C.D. The Effect of Temperature on the Mucosal IgM Antibody Response to DNP-KLH in Channel Catfish (Ictalurus punctatus). Fish Shellfish Immunol. 2017, 70, 493–497. [Google Scholar] [CrossRef]
- Mikkelsen, H.; Lindenstrøm, T.; Nielsen, M.E. Effects of Temperature on Production and Specificity of Antibodies in Rainbow Trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2006, 37, 518–522. [Google Scholar] [CrossRef]
- Alcorn, S.W.; Murray, A.L.; Pascho, R.J. Effects of Rearing Temperature on Immune Functions in Sockeye Salmon (Oncorhynchus nerka). Fish Shellfish Immunol. 2002, 12, 303–334. [Google Scholar] [CrossRef] [PubMed]
- Paterson, W.D.; Fryer, J.L. Effect of Temperature and Antigen Dose on the Antibody Response of Juvenile Coho Salmon (Oncorhynchus kisutch) to Aeromonas salmonicida Endotoxin. J. Fish Res. Board Can. 1974, 31, 1743–1749. [Google Scholar] [CrossRef]
- Lillehaug, A.; Ramstad, A.; Bækken, K.; Reitan, L.J. Protective Immunity in Atlantic Salmon (Salmo salar L.) Vaccinated at Different Water Temperatures. Fish Shellfish Immunol. 1993, 3, 143–156. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, Ø. An Overview of Challenges Limiting the Design of Protective Mucosal Vaccines for Finfish. Front. Immunol. 2015, 6, 542. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, H.; Chang, X.; Tang, Y.; Liu, Q.; Zhang, Y. Notable Mucosal Immune Responses Induced in the Intestine of Zebrafish (Danio rerio) Bath-Vaccinated with a Live Attenuated Vibrio anguillarum Vaccine. Fish Shellfish Immunol. 2014, 40, 99–108. [Google Scholar] [CrossRef]
- Hoare, R.; Ngo, T.P.H.; Bartie, K.L.; Adams, A. Efficacy of a Polyvalent Immersion Vaccine against Flavobacterium psychrophilum and Evaluation of Immune Response to Vaccination in Rainbow Trout Fry (Onchorynchus mykiss L.). Vet. Res. 2017, 48. [Google Scholar] [CrossRef]
- Sudheesh, P.S.; Cain, K.D. Prospects and Challenges of Developing and Commercializing Immersion Vaccines for Aquaculture. Int. Biol. Rev. 2017, 1, 1–20. [Google Scholar] [CrossRef]
- Semple, S.L. Improving Aquaculture: The Impact of Bacterial Disease Treatments on Salmonid Immune Performance. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2019. [Google Scholar]
- Figueroa, C.; Veloso, P.; Espin, L.; Dixon, B.; Afonso, J.M.; Soto, C.; Conejeros, P.; Gallardo, J.A. Host Genetic Variation Explains Reduced Protection of Commercial Vaccines against Piscirickettsia salmonis in Atlantic Salmon. Sci. Rep. 2020, in press. [Google Scholar]
- Ye, J.; Kaattari, I.M.; Kaattari, S.L. The Differential Dynamics of Antibody Subpopulation Expression during Affinity Maturation in a Teleost. Fish Shellfish Immunol. 2011, 30, 372–377. [Google Scholar] [CrossRef]
- LaFrentz, B.R.; LaPatra, S.E.; Jones, G.R.; Congleton, J.L.; Sun, B.; Cain, K.D. Characterization of Serum and Mucosal Antibody Responses and Relative per Cent Survival in Rainbow Trout, Oncorhynchus mykiss (Walbaum), Following Immunization and Challenge with Flavobacterium psychrophilum. J. Fish Dis. 2002, 25, 703–713. [Google Scholar] [CrossRef]
- Liang, J.L.; Tiwari, T.; Moro, P.; Messonnier, N.E.; Reingold, A.; Sawyer, M.; Clark, T.A. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2018, 67. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.L.; Bols, N.C.; Lumsden, J.S.; Dixon, B. Understanding the Pathogenesis of Flavobacterium psychrophilum Using the Rainbow Trout Monocyte/Macrophage-like Cell Line, RTS11, as an Infection Model. Microb. Pathog. 2020, 139, 103910. [Google Scholar] [CrossRef] [PubMed]
- Magarinos, B.; Santos, Y.; Romalde, J.L.; Rivas, C.; Barja, J.L.; Toranzo, A.E. Pathogenic Activities of Live Cells and Extracellular Products of the Fish Pathogen Pasteurella piscicida. J. Gen. Microbiol. 1992, 138, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, E.; Oshima, S.; Nomura, S. Toxicity and Immunogenicity of Aeromonas salmonicida Extracellular Products to Salmonids. J. Fish Dis. 1990, 13, 495–503. [Google Scholar] [CrossRef]
- Vanya Ewart, K.; Williams, J.; Richards, R.C.; Gallant, J.W.; Melville, K.; Douglas, S.E. The Early Response of Atlantic Salmon (Salmo salar) Macrophages Exposed in vitro to Aeromonas salmonicida Cultured in Broth and in Fish. Dev. Comp. Immunol. 2008, 32, 380–390. [Google Scholar] [CrossRef]
- Munro, A.L.S.; Hastings, T.S.; Ellis, A.E.; Liversidge, J. Studies on an Ichthyotoxic Material Produced Extracellularly by the Furunculosis Bacterium Aeromonas salmonicida. In Fish Diseases; Ahne, W., Ed.; Springer: Berlin/Heidelberg, Germany. [CrossRef]
- Bjornsdottir, B.; Gudmundsdottir, T.; Gudmundsdottir, B.K. Virulence Properties of Moritella viscosa Extracellular Products. J. Fish Dis. 2011, 34, 333–343. [Google Scholar] [CrossRef]
- Bjornsdottir, B.; Fast, M.D.; Sperker, S.A.; Brown, L.L.; Gudmundsdottir, B.K. Effects of Moritella viscosa Antigens on Pro-Inflammatory Gene Expression in an Atlantic Salmon (Salmo salar Linnaeus) Cell Line (SHK-1). Fish Shellfish Immunol. 2009, 26, 858–863. [Google Scholar] [CrossRef]
- MacKinnon, B.; Groman, D.; Fast, M.D.; Manning, A.J.; Jones, P.; St-Hilaire, S. Atlantic Salmon Challenged with Extracellular Products from Moritella viscosa. Dis. Aquat. Org. 2019, 133, 119–125. [Google Scholar] [CrossRef]
- Powell, M.D.; Briand, H.A.; Wright, G.M.; Burka, J.F. Rainbow Trout (Oncorhynchus mykiss Walbaum) Intestinal Eosinophilic Granule Cell (Egc) Response to Aeromonas salmonicida and Vibrio anguillarum Extracellular Products. Fish Shellfish Immunol. 1993, 3, 279–289. [Google Scholar] [CrossRef]
- Ostland, V.E.; Byrne, P.J.; Hoover, G.; Ferguson, H.W. Necrotic Myositis of Rainbow Trout, Oncorhynchus mykiss (Walbaum): Proteolytic Characteristics of a Crude Extracellular Preparation from Flavobacterium psychrophilum. J. Fish Dis. 2000, 23, 329–336. [Google Scholar] [CrossRef]
- Pérez-Pascual, D.; Rochat, T.; Kerouault, B.; Gómez, E.; Neulat-Ripoll, F.; Henry, C.; Quillet, E.; Guijarro, J.A.; Bernardet, J.F.; Duchaud, E. More Than Gliding: Involvement of GldD and GldG in the Virulence of Flavobacterium psychrophilum. Front. Microbiol. 2017, 8, 2168. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pascual, D.; Gómez, E.; Álvarez, B.; Méndez, J.; Reimundo, P.; Navais, R.; Duchaud, E.; Guijarro, J.A. Comparative Analysis and Mutation Effects of Fpp2-Fpp1 Tandem Genes Encoding Proteolytic Extracellular Enzymes of Flavobacterium psychrophilum. Microbiology 2011, 157, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Secades, P.; Alvarez, B.; Guijarro, J.A. Purification and Properties of a New Psychrophilic Metalloprotease (Fpp2) in the Fish Pathogen Flavobacterium psychrophilum. FEMS Microbiol. Lett. 2003, 226, 273–279. [Google Scholar] [CrossRef]
- Secades, P.; Alvarez, B.; Guijarro, J.A. Purification and Characterization of a Psychrophilic, Calcium-Induced, Growth-Phase-Dependent Metalloprotease from the Fish Pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 2001, 67, 2436–2444. [Google Scholar] [CrossRef]
- Pérez-Pascual, D.; Gómez, E.; Guijarro, J.A. Lack of a Type-2 Glycosyltransferase in the Fish Pathogen Flavobacterium psychrophilum Determines Pleiotropic Changes and Loss of Virulence. Vet. Res. 2015, 46, 1. [Google Scholar] [CrossRef]
- Lages, M.A.; Balado, M.; Lemos, M.L. The Expression of Virulence Factors in Vibrio anguillarum Is Dually Regulated by Iron Levels and Temperature. Front. Microbiol. 2019, 10, 2335. [Google Scholar] [CrossRef]
- Milton, D.L.; O’Toole, R.; Hörstedt, P.; Wolf-Watz, H. Flagellin A Is Essential for the Virulence of Vibrio anguillarum. J. Bacteriol. 1996, 178, 1310–1319. [Google Scholar] [CrossRef]
- Burr, S.; Wahli, T.; Segner, H.; Pugovkin, D.; Frey, J. Association of Type III Secretion Genes with Virulence of Aeromonas salmonicida Subsp. Salmonicida. Dis. Aquat. Org. 2003, 57, 167–171. [Google Scholar] [CrossRef]
- Bandin, I.; Santos, Y.; Bruno, D.W.; Raynard, R.S.; Toranzo, A.E.; Barja, J.L. Lack of Biological Activities in the Extracellular Products of Renibacterium salmoninarum. Can. J. Fish Aquat. Sci. 1991, 48, 421–425. [Google Scholar] [CrossRef]
- Ellis, A.E.; Burrows, A.S.; Stapleton, K.J. Lack of Relationship between Virulence of Aeromonas salmonicida and the Putative Virulence Factors: A-Layer, Extracellular Proteases and Extracellular Haemolysins. J. Fish Dis. 1988, 11, 309–323. [Google Scholar] [CrossRef]
- Ekman, E.; Norrgren, L. Pathology and Immunohistochemistry in Three Species of Salmonids after Experimental Infection with Flavobacterium psychrophilum. J. Fish Dis. 2003, 26, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.; Bliska, J.B. Turning Yersinia Pathogenesis Outside in: Subversion of Macrophage Function by Intracellular Yersiniae. Clin. Immunol. 2005, 114, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Gutenberger, S.; Duimstra, J.; Rohovec, J.; Fryer, J. Intracellular Survival of Renibacterium salmoninarum in Trout Mononuclear Phagocytes. Dis. Aquat. Org. 1997, 28, 93–106. [Google Scholar] [CrossRef]
- Grayson, T.H.; Cooper, L.F.; Wrathmell, A.B.; Roper, J.; Evenden, A.J.; Gilpin, M.L. Host Responses to Renibacterium salmoninarum and Specific Components of the Pathogen Reveal the Mechanisms of Immune Suppression and Activation. Immunology 2002, 106, 273–283. [Google Scholar] [CrossRef]
- Ramírez, R.; Ǵomez, F.A.; Marshall, S.H. The Infection Process of Piscirickettsia salmonis in Fish Macrophages Is Dependent upon Interaction with Host-Cell Clathrin and Actin. FEMS Microbiol. Lett. 2015, 362, 1–8. [Google Scholar] [CrossRef]
- Álvarez, C.A.; Gomez, F.A.; Mercado, L.; Ramírez, R.; Marshall, S.H. Piscirickettsia salmonis Imbalances the Innate Immune Response to Succeed in a Productive Infection in a Salmonid Cell Line Model. PLoS ONE 2016, 11, e0163943. [Google Scholar] [CrossRef]
- Nematollahi, A.; Pasmans, F.; Haesebrouck, F.; Decostere, A. Early Interactions of Flavobacterium psychrophilum with Macrophages of Rainbow Trout Oncorhynchus mykiss. Dis. Aquat. Org 2005, 64, 23–28. [Google Scholar] [CrossRef]
- Nilsen, H.; Olsen, A.B.; Vaagnes, Ø.; Hellberg, H.; Bottolfsen, K.; Skjelstad, H.; Colquhoun, D.J. Systemic Flavobacterium psychrophilum Infection in Rainbow Trout, Oncorhynchus mykiss (Walbaum), Farmed in Fresh and Brackish Water in Norway. J. Fish Dis. 2011, 34, 403–408. [Google Scholar] [CrossRef]
- de la Maza, L.M.; Zhong, G.; Brunham, R.C. Update on Chlamydia Trachomatis Vaccinology. Am. Soc. Microbiol. 2017, 24, e00543-16. [Google Scholar] [CrossRef]
- Henao-Tamayo, M.; Ordway, D.J.; Orme, I.M. Memory T Cell Subsets in Tuberculosis: What Should We Be Targeting? Tuberculosis 2014, 94, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Titball, R.W. Vaccines against Intracellular Bacterial Pathogens. Drug Discov. Today 2008, 13, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.L.; Khader, S.A. Novel Vaccine Approaches for Protection against Intracellular Pathogens. Curr. Opin. Immunol. 2014, 28, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.G.; Wiens, G.D.; Hammell, K.L.; Rhodes, L.D. Vaccination against Bacterial Kidney Disease. In Fish Vaccination; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 255–272. ISBN 9780470674550. [Google Scholar] [CrossRef]
- Burnley, T.A.; Stryhn, H.; Burnley, H.J.; Hammell, K.L. Randomized Clinical Field Trial of a Bacterial Kidney Disease Vaccine in Atlantic Salmon, Salmo salar L. J. Fish Dis. 2010, 33, 545–557. [Google Scholar] [CrossRef]
- Decostere, A.; D’Haese, E.; Lammens, M.; Nelis, H.; Haesebrouck, F. In vivo Study of Phagocytosis, Intracellular Survival and Multiplication of Flavobacterium psychrophilum in Rainbow Trout, Oncorhynchus mykiss (Walbaum), Spleen Phagocytes. J. Fish Dis. 2001, 24, 481–487. [Google Scholar] [CrossRef]
- Hoare, R.; Jung, S.J.; Ngo, T.P.H.; Bartie, K.L.; Thompson, K.D.; Adams, A. Efficacy of a Polyvalent Injectable Vaccine against Flavobacterium psychrophilum Administered to Rainbow Trout (Oncorhynchus mykiss L.). J. Fish Dis. 2019, 42, 229–236. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Sudheesh, P.S.; Knupp, C.; Loch, T.P.; Faisal, M.; Cain, K.D. Assessment of Cross-Protection to Heterologous Strains of Flavobacterium psychrophilum Following Vaccination with a Live-Attenuated Coldwater Disease Immersion Vaccine. J. Fish Dis. 2019, 42, 75–84. [Google Scholar] [CrossRef]
- Fredriksen, B.N.; Olsen, R.H.; Furevik, A.; Souhoka, R.A.; Gauthier, D.; Brudeseth, B. Efficacy of a Divalent and a Multivalent Water-in-Oil Formulated Vaccine against a Highly Virulent Strain of Flavobacterium psychrophilum after Intramuscular Challenge of Rainbow Trout (Oncorhynchus mykiss). Vaccine 2013, 31, 1994–1998. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key Roles of Adjuvants in Modern Vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Preiss, S.; da Silva, F.T.; Garçon, N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and Immunostimulants in Fish Vaccines: Current Knowledge and Future Perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, V.; Miquel, A.; Burzio, L.O.; Rosemblatt, M.; Engel, E.; Valenzuela, S.; Parada, G.; Valenzuela, P.D.T. A Vaccine against the Salmonid Pathogen Piscirickettsia salmonis Based on Recombinant Proteins. Vaccine 2006, 24, 5083–5091. [Google Scholar] [CrossRef] [PubMed]
- Tyler, J.W.; Klesius, P.H. Protection against Enteric Septicemia of Catfish (Ictalurus punctatus) by Immunization with the R-Mutant, Escherichia coli (J5). Am. J. Vet. Res. 1994, 55, 1256–1260. [Google Scholar] [PubMed]
- Wang, E.; Wang, J.; Long, B.; Wang, K.; He, Y.; Yang, Q.; Chen, D.; Geng, Y.; Huang, X.; Ouyang, P.; et al. Molecular Cloning, Expression and the Adjuvant Effects of Interleukin-8 of Channel Catfish (Ictalurus punctatus) against Streptococcus iniae. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Araki, K.; Hayashi, K.; Takeuchi, Y.; Shiozaki, K.; Suetake, H.; Yamamoto, A. Adjuvant Effect of Recombinant Interleukin-12 in the Nocardiosis Formalin-Killed Vaccine of the Amberjack Seriola dumerili. Fish Shellfish Immunol. 2017, 67, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Peddie, S.; Campos-Pérez, J.J.; Zou, J.; Secombes, C.J. The Effect of Intraperitoneally Administered Recombinant IL-1β on Immune Parameters and Resistance to Aeromonas salmonicida in the Rainbow Trout (Oncorhynchus mykiss). Dev. Comp. Immunol. 2003, 27, 801–812. [Google Scholar] [CrossRef]
- Buonocore, F.; Mazzini, M.; Forlenza, M.; Randelli, E.; Secombes, C.J.; Zou, J.; Scapigliati, G. Expression in Escherchia coli and Purification of Sea Bass (Dicentrarchus labrax) Interleukin 1β, a Possible Immunoadjuvant in Aquaculture. Mar. Biotechnol. 2004, 6, 53–59. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Q.; Xu, L.; Li, S.; Wang, D.; Zhao, J.; Liu, H.; Feng, J.; Lu, T. Effects of Different Cytokines on Immune Responses of Rainbow Trout in a Virus DNA Vaccination Model. Oncotarget 2017, 8, 112222–112235. [Google Scholar] [CrossRef]
- Guo, M.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. The Effects of IL-1β, IL-8, G-CSF and TNF-α as Molecular Adjuvant on the Immune Response to an E. Tarda Subunit Vaccine in Flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2018, 77, 374–384. [Google Scholar] [CrossRef]
- Wang, E.; Long, B.; Wang, K.; Wang, J.; He, Y.; Wang, X.; Yang, Q.; Liu, T.; Chen, D.; Geng, Y.; et al. Interleukin-8 Holds Promise to Serve as a Molecular Adjuvant in DNA Vaccination Model against Streptococcus iniae Infection in Fish. Oncotarget 2016, 7, 83938–83950. [Google Scholar] [CrossRef]
- Dixon, B.; Barreda, D.R.; Sunyer, J.O. Perspective on the Development and Validation of Ab Reagents to Fish Immune Proteins for the Correct Assessment of Immune Function. Front. Immunol. 2018, 9, 2957. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Bhat, R.A.H.; Pande, A. Antimicrobial Peptides of Fish: Innocuous Alternatives to Antibiotics. Rev. Aquac. 2020, 12, 85–106. [Google Scholar] [CrossRef]
- Brunner, S.R.; Varga, J.F.A.; Dixon, B. Antimicrobial Peptides of Salmonid Fish: From Form to Function. Biology 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.; Carpio, Y.; Valdés, I.; Velázquez, J.; Zamora, Y.; Morales, R.; Morales, A.; Rodríguez, E.; Estrada, M.P. Co-Administration of Tilapia Alpha-Helical Antimicrobial Peptides with Subunit Antigens Boost Immunogenicity in Mice and Tilapia (Oreochromis niloticus). Vaccine 2014, 32, 223–229. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wang, P.; Zhang, N.; Chen, D.D.; Nie, P.; Li, J.-L.; Zhang, Y.A. B Cell Functions Can Be Modulated by Antimicrobial Peptides in Rainbow Trout Oncorhynchus mykiss: Novel Insights into the Innate Nature of B Cells in Fish. Front. Immunol. 2017, 8, 388. [Google Scholar] [CrossRef]
- Bridle, A.; Nosworthy, E.; Polinski, M.; Nowak, B. Evidence of an Antimicrobial-Immunomodulatory Role of Atlantic Salmon Cathelicidins during Infection with Yersinia ruckeri. PLoS ONE 2011, 6, e23417. [Google Scholar] [CrossRef]
- Peter Chiou, P.; Khoo, J.; Bols, N.C.; Douglas, S.; Chen, T.T. Effects of Linear Cationic α-Helical Antimicrobial Peptides on Immune-Relevant Genes in Trout Macrophages. Dev. Comp. Immunol. 2006, 30, 797–806. [Google Scholar] [CrossRef]
- Jia, X.; Patrzykat, A.; Devlin, R.H.; Ackerman, P.A.; Iwama, G.K.; Hancock, R.E.W. Antimicrobial Peptides Protect Coho Salmon from Vibrio anguillarum Infections. Appl. Environ. Microbiol. 2000, 66, 1928–1932. [Google Scholar] [CrossRef]
- Furlan, M.; Rosani, U.; Gambato, S.; Irato, P.; Manfrin, A.; Mardirossian, M.; Venier, P.; Pallavicini, A.; Scocchi, M. Induced Expression of Cathelicidins in Trout (Oncorhynchus mykiss) Challenged with Four Different Bacterial Pathogens. J. Pept. Sci. 2018, 24, e3089. [Google Scholar] [CrossRef]
- Kitani, Y.; Fernandes, J.M.O.; Kiron, V. Identification of the Atlantic Cod L-Amino Acid Oxidase and Its Alterations Following Bacterial Exposure. Dev. Comp. Immunol. 2015, 50, 116–120. [Google Scholar] [CrossRef]
- Kitani, Y.; Hieu, D.Q.; Kiron, V. Cloning of Selected Body Surface Antimicrobial Peptide/Protein Genes of Atlantic Salmon and Their Responses to Aeromonas salmonicida. Fish Sci. 2019, 85, 847–858. [Google Scholar] [CrossRef]
- Ruangsri, J.; Kitani, Y.; Kiron, V.; Lokesh, J.; Brinchmann, M.F.; Karlsen, B.O.; Fernandes, J.M.O. A Novel Beta-Defensin Antimicrobial Peptide in Atlantic Cod with Stimulatory Effect on Phagocytic Activity. PLoS ONE 2013, 8, e62302. [Google Scholar] [CrossRef] [PubMed]
- Lauth, X.; Babon, J.J.; Stannard, J.A.; Singh, S.; Nizet, V.; Carlberg, J.M.; Ostland, V.E.; Pennington, M.W.; Norton, R.S.; Westerman, M.E. Bass Hepcidin Synthesis, Solution Structure, Antimicrobial Activities and Synergism, and in vivo Hepatic Response to Bacterial Infections. J. Biol. Chem. 2005, 280, 9272–9282. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.; Noga, E.J. Evidence for Synergism of the Antimicrobial Peptide Piscidin 2 with Antiparasitic and Antioomycete Drugs. J. Fish Dis. 2010, 33, 995–1003. [Google Scholar] [CrossRef]
- Semple, S.L.; Rodríguez-Ramos, T.; Carpio, Y.; Lumsden, J.S.; Estrada, M.P.; Dixon, B. PACAP Is Lethal to Flavobacterium psychrophilum Through Either Direct Membrane Permeabilization or Indirectly, by Priming the Immune Response in Rainbow Trout Macrophages. Front. Immunol. 2019, 10, 926. [Google Scholar] [CrossRef]
- Niyonsaba, F.; Ushio, H.; Nakano, N.; Ng, W.; Sayama, K.; Hashimoto, K.; Nagaoka, I.; Okumura, K.; Ogawa, H. Antimicrobial Peptides Human β-Defensins Stimulate Epidermal Keratinocyte Migration, Proliferation and Production of Proinflammatory Cytokines and Chemokines. J. Investig. Dermatol. 2007, 127, 594–604. [Google Scholar] [CrossRef]
- Zhang, H.; Porro, G.; Orzech, N.; Müllen, B.; Liu, M.; Slutsky, A.S. Neutrophil Defensins Mediate Acute Inflammatory Response and Lung Dysfunction in Dose-Related Fashion. Am. J. Physiol. Cell. Mol. Physiol. 2001, 280, L947–L954. [Google Scholar] [CrossRef]
- Chaly, Y.V.; Paleolog, E.M.; Kolesnikova, T.S.; Tikhonov, I.I.; Petratchenko, E.V.; Voitenok, N.N. Neutrophil Alpha-Defensin Human Neutrophil Peptide Modulates Cytokine Production in Human Monocytes and Adhesion Molecule Expression in Endothelial Cells. Eur. Cytokine Netw. 2000, 11, 257–266. [Google Scholar]
- Martinez, C.; Abad, C.; Delgado, M.; Arranz, A.; Juarranz, M.G.; Rodriguez-Henche, N.; Brabet, P.; Leceta, J.; Gomariz, R.P. Anti-Inflammatory Role in Septic Shock of Pituitary Adenylate Cyclase-Activating Polypeptide Receptor. Proc. Natl. Acad. Sci. USA 2002, 99, 1053–1058. [Google Scholar] [CrossRef]
- Delgado, M.; Miller Jonakait, G.; Ganea, D. Vasoactive Intestinal Peptide and Pituitary Adenylate Cyclase-Activating Polypeptide Inhibit Chemokine Production in Activated Microglia. Glia 2002, 39, 148–161. [Google Scholar] [CrossRef]
- Delgado, M.; Munoz-Elias, E.J.; Gomariz, R.P.; Ganea, D. VIP and PACAP Inhibit IL-12 Production in LPS-Stimulated Macrophages. Subsequent Effect on IFNγ Synthesis by T Cells. J. Neuroimmunol. 1999, 96, 167–181. [Google Scholar] [CrossRef]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a Novel 38 Residue-Hypothalamic Polypeptide Which Stimulates Adenylate Cyclase in Pituitary Cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef]
- Lugo, J.M.; Rodriguez, A.; Helguera, Y.; Morales, R.; Gonzalez, O.; Acosta, J.; Besada, V.; Sanchez, A.; Estrada, M.P. Recombinant Novel Pituitary Adenylate Cyclase-Activating Polypeptide from African Catfish (Clarias gariepinus) Authenticates Its Biological Function as a Growth-Promoting Factor in Low Vertebrates. J. Endocrinol. 2008, 197, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.J.; Boulding, E.G. Associations between Single Nucleotide Polymorphisms in Candidate Genes and Growth Rate in Arctic Charr (Salvelinus alpinus L.). Heredity 2003, 91, 60–69. [Google Scholar] [CrossRef]
- Lugo, J.M.; Oliva, A.; Morales, A.; Reyes, O.; Garay, H.E.; Herrera, F.; Cabrales, A.; Pérez, E.; Estrada, M.P. The Biological Role of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in Growth and Feeding Behavior in Juvenile Fish. J. Pept. Sci. 2010, 16, 633–643. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.Z.; Wang, A.; Zhou, Z. Probiotics as Means of Diseases Control in Aquaculture, a Review of Current Knowledge and Future Perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef]
- Newaj-Fyzul, A.; Al-Harbi, A.H.; Austin, B. Review: Developments in the Use of Probiotics for Disease Control in Aquaculture. Aquaculture 2014, 431, 1–11. [Google Scholar] [CrossRef]
- Newaj-Fyzul, A.; Adesiyun, A.A.; Mutani, A.; Ramsubhag, A.; Brunt, J.; Austin, B. Bacillus Subtilis AB1 Controls Aeromonas Infection in Rainbow Trout (Oncorhynchus mykiss, Walbaum). J. Appl. Microbiol. 2007, 103, 1699–1706. [Google Scholar] [CrossRef]
- Sharifuzzaman, S.M.; Austin, B. Influence of Probiotic Feeding Duration on Disease Resistance and Immune Parameters in Rainbow Trout. Fish Shellfish Immunol. 2009, 27, 440–445. [Google Scholar] [CrossRef]
- Balcázar, J.L.; de Blas, I.; Ruiz-Zarzuela, I.; Vendrell, D.; Gironés, O.; Muzquiz, J.L. Enhancement of the Immune Response and Protection Induced by Probiotic Lactic Acid Bacteria against Furunculosis in Rainbow Trout (Oncorhynchus mykiss). FEMS Immunol. Med. Microbiol. 2007, 51, 185–193. [Google Scholar] [CrossRef]
- Brunt, J.; Newaj-Fyzul, A.; Austin, B. The Development of Probiotics for the Control of Multiple Bacterial Diseases of Rainbow Trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2007, 30, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Sharifuzzaman, S.M.; Austin, B. Development of Protection in Rainbow Trout (Oncorhynchus mykiss, Walbaum) to Vibrio anguillarum Following Use of the Probiotic Kocuria SM1. Fish Shellfish Immunol. 2010, 29, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Sharifuzzaman, S.M.; Abbass, A.; Tinsley, J.W.; Austin, B. Subcellular Components of Probiotics Kocuria SM1 and Rhodococcus SM2 Induce Protective Immunity in Rainbow Trout (Oncorhynchus mykiss, Walbaum) against Vibrio anguillarum. Fish Shellfish Immunol. 2011, 30, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Jöborn, A.; Olsson, J.C.; Westerdahl, A.; Conway, P.L.; Kjelleberg, S. Colonization in the Fish Intestinal Tract and Production of Inhibitory Substances in Intestinal Mucus and Faecal Extracts by Carnobacterium Sp. Strain K1. J. Fish Dis. 1997, 20, 383–392. [Google Scholar] [CrossRef]
- Tukmechi, A.; Rahmati Andani, H.R.; Manaffar, R.; Sheikhzadeh, N. Dietary Administration of Beta-Mercapto-Ethanol Treated Saccharomyces cerevisiae Enhanced the Growth, Innate Immune Response and Disease Resistance of the Rainbow Trout, Oncorhynchus mykiss. Fish Shellfish Immunol. 2011, 30, 923–928. [Google Scholar] [CrossRef]
- Burbank, D.R.; Shah, D.H.; LaPatra, S.E.; Fornshell, G.; Cain, K.D. Enhanced Resistance to Coldwater Disease Following Feeding of Probiotic Bacterial Strains to Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2011, 321, 185–190. [Google Scholar] [CrossRef]
- Abbass, A.; Sharifuzzaman, S.M.; Austin, B. Cellular Components of Probiotics Control Yersinia ruckeri Infection in Rainbow Trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2010, 33, 31–37. [Google Scholar] [CrossRef]
- Park, Y.; Lee, S.; Hong, J.; Kim, D.; Moniruzzaman, M.; Bai, S.C. Use of Probiotics to Enhance Growth, Stimulate Immunity and Confer Disease Resistance to Aeromonas salmonicida in Rainbow Trout (Oncorhynchus mykiss). Aquac. Res. 2017, 48, 2672–2682. [Google Scholar] [CrossRef]
- Balcázar, J.L.; de Blas, I.; Ruiz-Zarzuela, I.; Vendrell, D.; Calvo, A.C.; Márquez, I.; Gironés, O.; Muzquiz, J.L. Changes in Intestinal Microbiota and Humoral Immune Response Following Probiotic Administration in Brown Trout (Salmo trutta). Br. J. Nutr. 2007, 97, 522–527. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Sun, G.; Li, X.; Liu, Z. Growth, Immune Response, Antioxidant Capability, and Disease Resistance of Juvenile Atlantic Salmon (Salmo salar L.) Fed Bacillus velezensis V4 and Rhodotorula mucilaginosa Compound. Aquaculture 2019, 500, 65–74. [Google Scholar] [CrossRef]
- Robertson, P.A.W.; O’Dowd, C.; Burrells, C.; Williams, P.; Austin, B. Use of Carnobacterium Sp. as a Probiotic for Atlantic Salmon (Salmo salar L.) and Rainbow Trout (Oncorhynchus mykiss, Walbaum). Aquaculture 2000, 185, 235–243. [Google Scholar] [CrossRef]
- Soltani, M. Effect of the Probiotic, Lactobacillus plantarum on Growth Performance and Haematological Indices of Rainbow Trout (Oncorhynchus mykiss) Immunized with Bivalent Streptococcosis/Lactococcosis Vaccine. Iran. J. Fish Sci. 2019, 18, 283–295. [Google Scholar] [CrossRef]
- Soltani, M.; Pakzad, K.; Taheri-Mirghaed, A.; Mirzargar, S.; Shekarabi, S.P.H.; Yosefi, P.; Soleymani, N. Dietary Application of the Probiotic Lactobacillus plantarum 426951 Enhances Immune Status and Growth of Rainbow Trout (Oncorhynchus mykiss) Vaccinated Against Yersinia ruckeri. Probiotics Antimicrob. Proteins 2019, 11, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.M.; Soltani, M.; Ali Ebrahimzahe-Mousavi, H.; Pakzad, K. Influence of Probiotic, Lactobacillus plantarum on Serum Biochemical and Immune Parameters in Vaccinated Rainbow Trout (Oncorhynchus mykiss) against Streptococcosis/Lactococosis. Int. J. Aquat. Biol. 2016, 4, 285–294. [Google Scholar]
- Kim, D.H.; Austin, B. Innate Immune Responses in Rainbow Trout (Oncorhynchus mykiss, W.) Induced by Probiotics. Fish Shellfish Immunol. 2006, 21, 513–524. [Google Scholar] [CrossRef]
- Pérez-Sánchez, T.; Balcázar, J.L.; Merrifield, D.L.; Carnevali, O.; Gioacchini, G.; de Blas, I.; Ruiz-Zarzuela, I. Expression of Immune-Related Genes in Rainbow Trout (Oncorhynchus mykiss) Induced by Probiotic Bacteria during Lactococcus garvieae Infection. Fish Shellfish Immunol. 2011, 31, 196–201. [Google Scholar] [CrossRef]
- Al-Hisnawi, A.; Rodiles, A.; Rawling, M.D.; Castex, M.; Waines, P.; Gioacchini, G.; Carnevali, O.; Merrifield, D.L. Dietary Probiotic Pediococcus Acidilactici MA18/5M Modulates the Intestinal Microbiota and Stimulates Intestinal Immunity in Rainbow Trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2019, 50, 1133–1151. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Roosta, H.; Masoumi, H.; Farhadi, A.; Jeffs, A. Long-Term Effects of Three Probiotics, Singular or Combined, on Serum Innate Immune Parameters and Expressions of Cytokine Genes in Rainbow Trout during Grow-Out. Fish Shellfish Immunol. 2020, 98, 748–757. [Google Scholar] [CrossRef]
- Panigrahi, A.; Viswanath, K.; Satoh, S. Real-Time Quantification of the Immune Gene Expression in Rainbow Trout Fed Different Forms of Probiotic Bacteria Lactobacillus rhamnosus. Aquac. Res. 2011, 42, 906–917. [Google Scholar] [CrossRef]
- Vasanth, G.K.; Kiron, V.; Kulkarni, A.; Dahle, D.; Lokesh, J.; Kitani, Y. A Microbial Feed Additive Abates Intestinal Inflammation in Atlantic Salmon. Front. Immunol. 2015, 6, 409. [Google Scholar] [CrossRef]
- Ringø, E.; Salinas, I.; Olsen, R.E.; Nyhaug, A.; Myklebust, R.; Mayhew, T.M. Histological Changes in Intestine of Atlantic Salmon (Salmo salar L.) Following in vitro Exposure to Pathogenic and Probiotic Bacterial Strains. Cell Tissue Res. 2007, 328, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Salinas, I.; Myklebust, R.; Esteban, M.A.; Olsen, R.E.; Meseguer, J.; Ringø, E. In vitro Studies of Lactobacillus delbrueckii Subsp. Lactis in Atlantic Salmon (Salmo salar L.) Foregut: Tissue Responses and Evidence of Protection against Aeromonas salmonicida Subsp. Salmonicida Epithelial Damage. Vet. Microbiol. 2008, 128, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Klakegg, Ø.; Salonius, K.; Nilsen, A.; Fülberth, M.; Sørum, H. Enhanced Growth and Decreased Mortality in Atlantic Salmon (Salmo salar) after Probiotic Bath. J. Appl. Microbiol. 2020, 129, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Quimby, M.C. Established Eurythermic Line of Fish Cells in vitro. Science 1962, 135, 1065–1066. [Google Scholar] [CrossRef] [PubMed]
- Fryer, J.L.; Lannan, C.N. Three Decades of Fish Cell Culture: A Current Listing of Cell Lines Derived from Fishes. J. Tissue Cult. Methods 1994, 16, 87–94. [Google Scholar] [CrossRef]
- Lakra, W.S.; Swaminathan, T.R.; Joy, K.P. Development, Characterization, Conservation and Storage of Fish Cell Lines: A Review. Fish Physiol. Biochem. 2011, 37, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Menanteau-Ledouble, S.; Lawrence, M.L.; El-Matbouli, M. Invasion and Replication of Yersinia ruckeri in Fish Cell Cultures. BMC Vet. Res. 2018, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Soto, E.; Yun, S.; Lewis, J.; Kearney, M.T.; Hansen, J. Interaction of Francisella noatunensis Subsp. Orientalis with Oreochromis mossambicus Bulbus Arteriosus Cell Line. Microb. Pathog. 2017, 105, 326–333. [Google Scholar] [CrossRef]
- Ormonde, P.; Hörstedt, P.; O’Toole, R.; Milton, D.L. Role of Motility in Adherence to and Invasion of a Fish Cell Line by Vibrio anguillarum. J. Bacteriol. 2000, 182, 2326–2328. [Google Scholar] [CrossRef]
- López-Dóriga, M.V.; Barnes, A.C.; dos Santos, N.M.S.; Ellis, A.E. Invasion of Fish Epithelial Cells by Photobacterium damselae Subsp. Piscicida: Evidence for Receptor Specificity, and Effect of Capsule and Serum. Microbiology 2000, 146, 21–30. [Google Scholar] [CrossRef]
- Castro, R.; Jouneau, L.; Tacchi, L.; Macqueen, D.J.; Alzaid, A.; Secombes, C.J.; Martin, S.A.M.; Boudinot, P. Disparate Developmental Patterns of Immune Responses to Bacterial and Viral Infections in Fish. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Balebona, M.C.; Krovacek, K.; Moriñigo, M.A.; Mansson, I.; Faris, A.; Borrego, J.J. Neurotoxic Effect on Two Fish Species and a PC12 Cell Line of the Supernate of Vibrio alginolyticus and Vibrio anguillarum. Vet. Microbiol. 1998, 63, 61–69. [Google Scholar] [CrossRef]
- Toranzo, A.E.; Santos, Y.; Lemos, M.L.; Ledo, A.; Bolinches, J. Homology of Vibrio anguillarum Strains Causing Epizootics in Turbot, Salmon and Trout Reared on the Atlantic Coast of Spain. Aquaculture 1987, 67, 41–52. [Google Scholar] [CrossRef]
- Krovacek, K.; Faris, A.; Ahne, W.; MÃ¥nsson, I. Adhesion of Aeromonas hydrophila and Vibrio anguillarum to Fish Cells and to Mucus-Coated Glass Slides. FEMS Microbiol. Lett. 1987, 42, 85–89. [Google Scholar] [CrossRef]
- Chaves-Pozo, E.; Pelegrín, P.; García-Castillo, J.; García-Ayala, A.; Mulero, V.; Meseguer, J. Acidophilic Granulocytes of the Marine Fish Gilthead Seabream (Sparus aurata L.) Produce Interleukin-1β Following Infection with Vibrio anguillarum. Cell Tissue Res. 2004, 316, 189–195. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.; Yang, G.; Zhang, X.-H.; Li, Y. Mutational Analysis of the Zinc Metalloprotease EmpA of Vibrio anguillarum. FEMS Microbiol. Lett. 2007, 267, 56–63. [Google Scholar] [CrossRef]
- Sarmasik, A.; Chen, T.T. Bactericidal Activity of Cecropin B and Cecropin P1 Expressed in Fish Cells (CHSE-214): Application in Controlling Fish Bacterial Pathogens. Aquaculture 2003, 220, 183–194. [Google Scholar] [CrossRef]
- Semple, S.L.; Vo, N.T.K.; Poynter, S.J.; Li, M.; Heath, D.D.; DeWitte-Orr, S.J.; Dixon, B. Extracellular dsRNA Induces a Type I Interferon Response Mediated via Class A Scavenger Receptors in a Novel Chinook Salmon Derived Spleen Cell Line. Dev. Comp. Immunol. 2018. [Google Scholar] [CrossRef]
- Monjo, A.L.; Poynter, S.J.; DeWitte-Orr, S.J. CHSE-214: A Model for Studying Extracellular dsRNA Sensing in vitro. Fish Shellfish Immunol. 2017, 68, 266–271. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of Phenotypic and Functional Stability of RAW 264.7 Cell Line through Serial Passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Chung, C.Y.; Iida-Klein, A.; Wyatt, L.E.; Rudkin, G.H.; Ishida, K.; Yamaguchi, D.T.; Miller, T.A. Serial Passage of MC3T3-E1 Cells Alters Osteoblastic Function and Responsiveness to Transforming Growth Factor-Β1 and Bone Morphogenetic Protein-2. Biochem. Biophys. Res. Commun. 1999, 265, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Kwist, K.; Bridges, W.C.; Burg, K.J.L. The Effect of Cell Passage Number on Osteogenic and Adipogenic Characteristics of D1 Cells. Cytotechnology 2016, 68, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Jia, Q.; Di, K.Q.; Gao, S.M.; Wen, X.H.; Zhou, R.Y.; Wei, W.; Wang, L.Z. Passage Number Affects the Pluripotency of Mouse Embryonic Stem Cells as Judged by Tetraploid Embryo Aggregation. Cell Tissue Res. 2007, 327, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, R.; Malick, L.; Kennedy, S. Ganglioside and Morphological Changes in Mouse Embryo Cells with Time. Cancer Lett. 1978, 4, 13–19. [Google Scholar] [CrossRef]
- Frattini, A.; Fabbri, M.; Valli, R.; de Paoli, E.; Montalbano, G.; Gribaldo, L.; Pasquali, F.; Maserati, E. High Variability of Genomic Instability and Gene Expression Profiling in Different HeLa Clones. Sci. Rep. 2015, 5, 15377. [Google Scholar] [CrossRef]
- Mohammadi Farsani, T.; Motevaseli, E.; Neyazi, N.; Khorramizadeh, M.R.; Zafarvahedian, E.; Ghahremani, M.H. Effect of Passage Number and Culture Time on the Expression and Activity of Insulin-Degrading Enzyme in Caco-2 Cells. Iran. Biomed. J. 2018, 22, 70–75. [Google Scholar] [CrossRef]
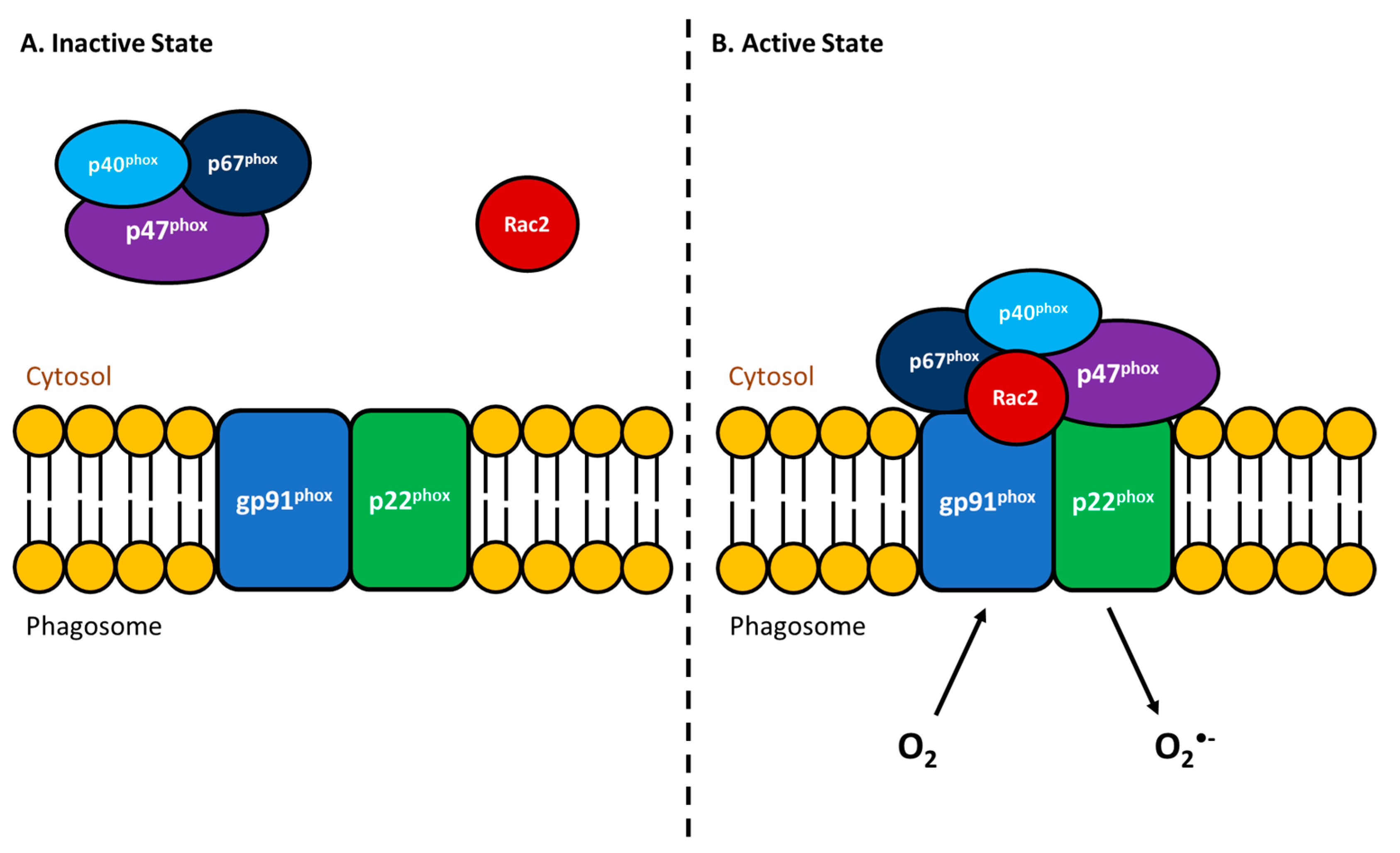
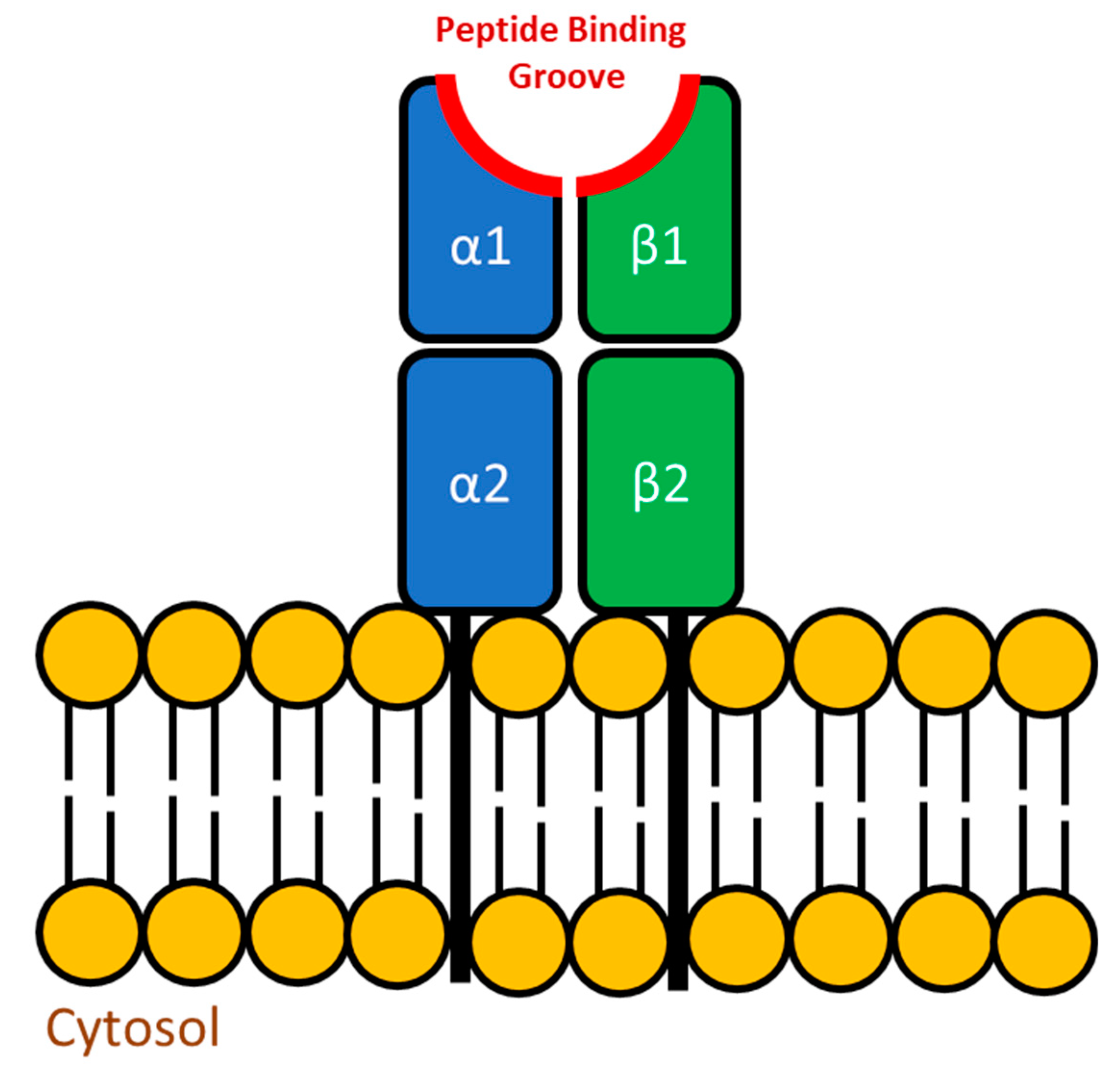
| Bacterial Species | Gram Stain Reaction | Disease |
|---|---|---|
| Yersinia ruckeri | − | Enteric Redmouth Disease |
| Flavobacterium columnare | − | Columnaris Disease |
| Flavobacterium psychrophilum | − | Bacterial Coldwater Disease (BCWD) |
| Flavobacterium branchiophilum | − | Bacterial Gill Disease (BGD) |
| Moritella viscosa | − | Winter Ulcer |
| Edwardsiella tarda | − | Edwardsiellosis |
| Piscirickettsia salmonis | − | Piscirickettsiosis |
| Aeromonas salmonicida | − | Furunculosis |
| Aeromonas hydrophila | − | Motile Aeromonas Septicemia |
| Tenacibaculum maritimum | − | Mouthrot |
| Vibrio salmonicida | − | Hitra Disease, Coldwater Vibriosis |
| Vibrio veronii | − | Epizootic Ulcerative Syndrome |
| Vibrio anguillarum | − | Vibriosis |
| Renibacterium salmoninarum | + | Bacterial Kidney Disease (BKD) |
| Mycobacterium marinum | + | Mycobacteriosis |
| Streptococcus phocae | + | Streptococcosis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semple, S.L.; Dixon, B. Salmonid Antibacterial Immunity: An Aquaculture Perspective. Biology 2020, 9, 331. https://doi.org/10.3390/biology9100331
Semple SL, Dixon B. Salmonid Antibacterial Immunity: An Aquaculture Perspective. Biology. 2020; 9(10):331. https://doi.org/10.3390/biology9100331
Chicago/Turabian StyleSemple, Shawna L., and Brian Dixon. 2020. "Salmonid Antibacterial Immunity: An Aquaculture Perspective" Biology 9, no. 10: 331. https://doi.org/10.3390/biology9100331
APA StyleSemple, S. L., & Dixon, B. (2020). Salmonid Antibacterial Immunity: An Aquaculture Perspective. Biology, 9(10), 331. https://doi.org/10.3390/biology9100331






