Dawn to Dusk: Diurnal Rhythm of the Immune Response in Rainbow Trout (Oncorhynchus Mykiss)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish
2.2. Sampling and Leukocyte Preparation
2.3. Flow Cytometry
2.4. Phagocytosis of Latex Beads
2.5. Aeromonas salmonicida for Stimulation Experiments
2.6. In Vivo Stimulation Experiment
2.7. In Vitro Stimulation Experiment
2.8. Bacterial Growth in Serum
2.9. Statistical Analysis
3. Results
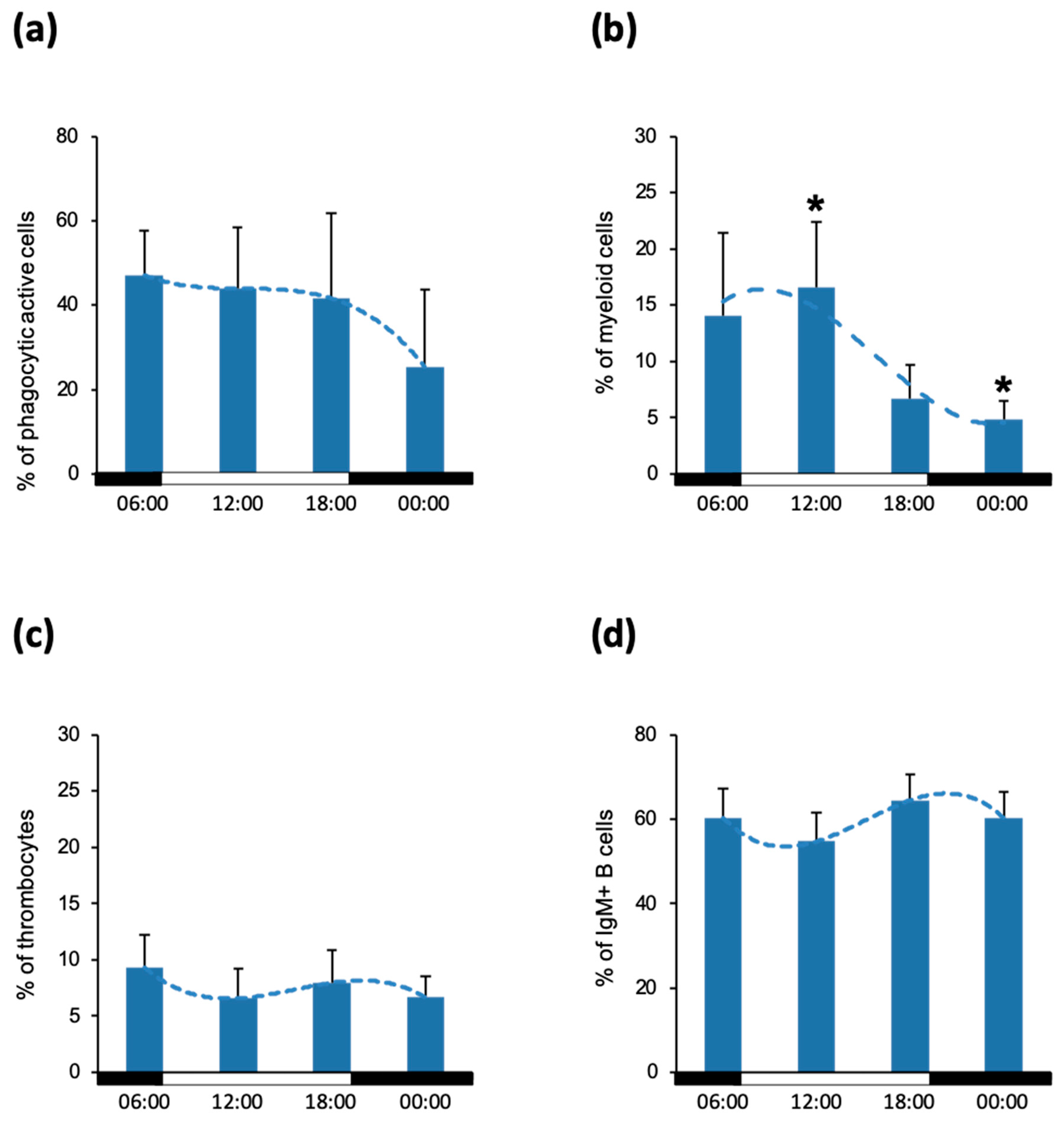
3.1. Cell Composition of Blood and Head Kidney Leukocytes
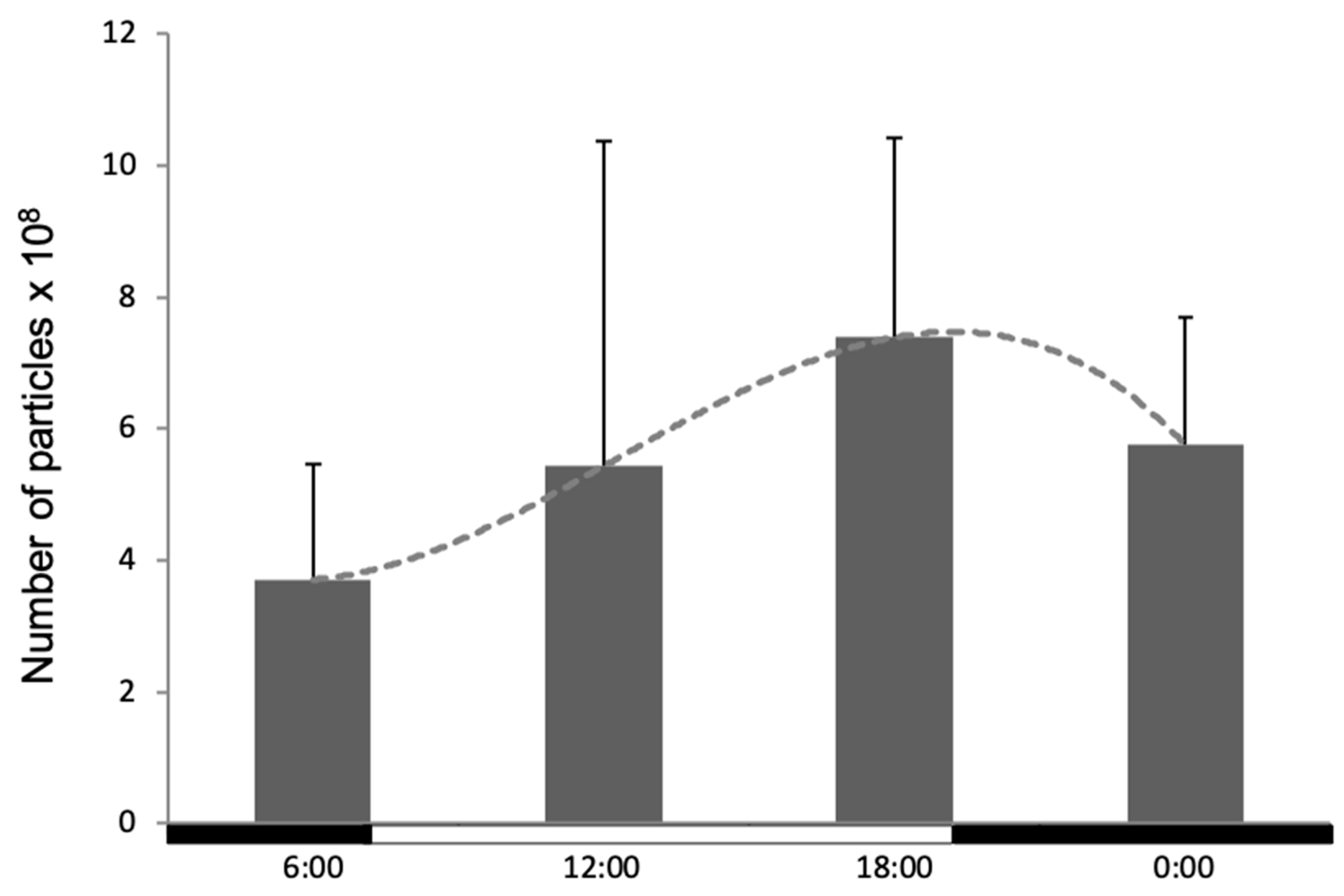
3.2. Phagocytic Activity of Blood Leukocytes
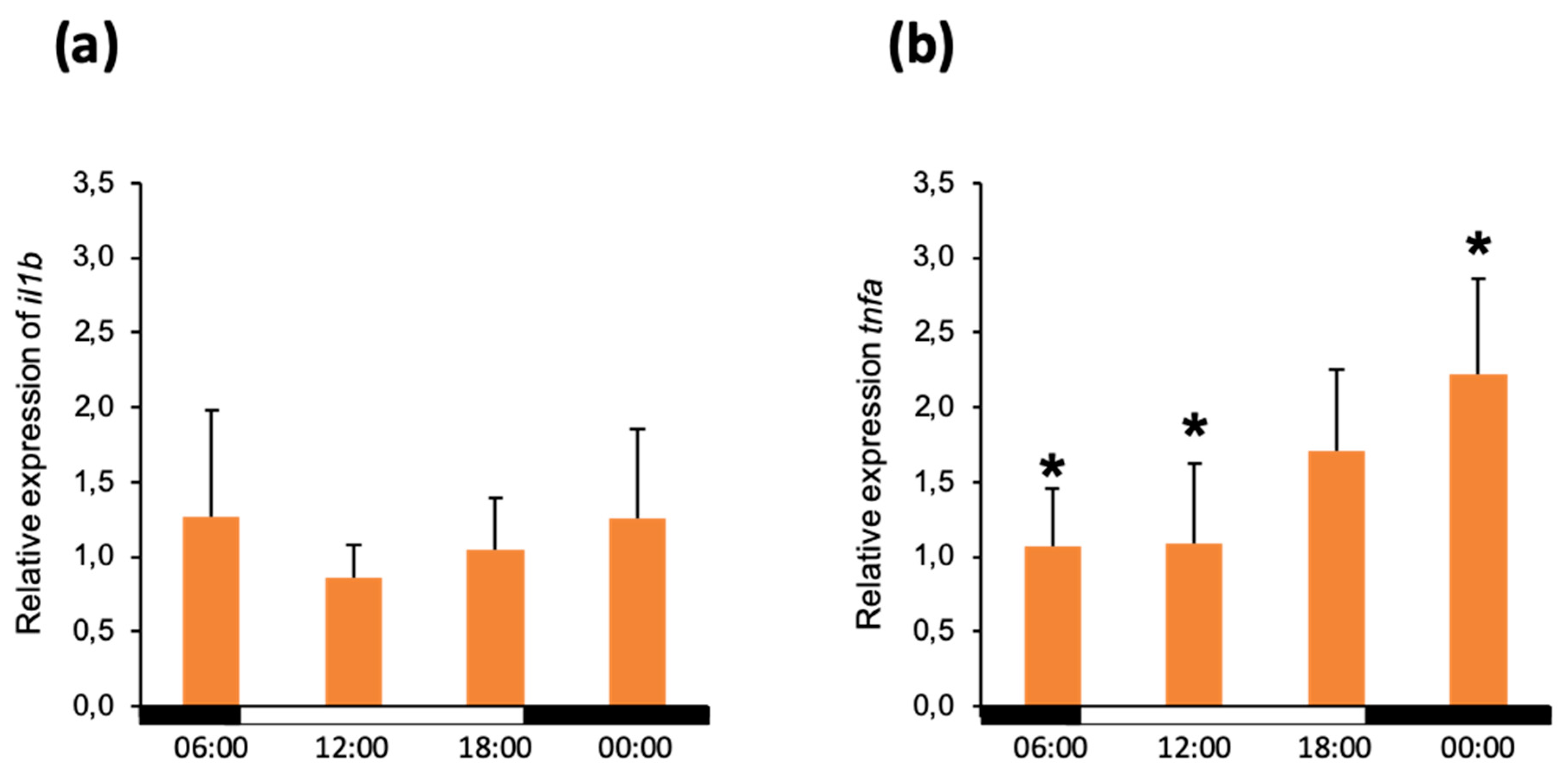
3.3. In Vitro Stimulation of Head Kidney Leukocytes
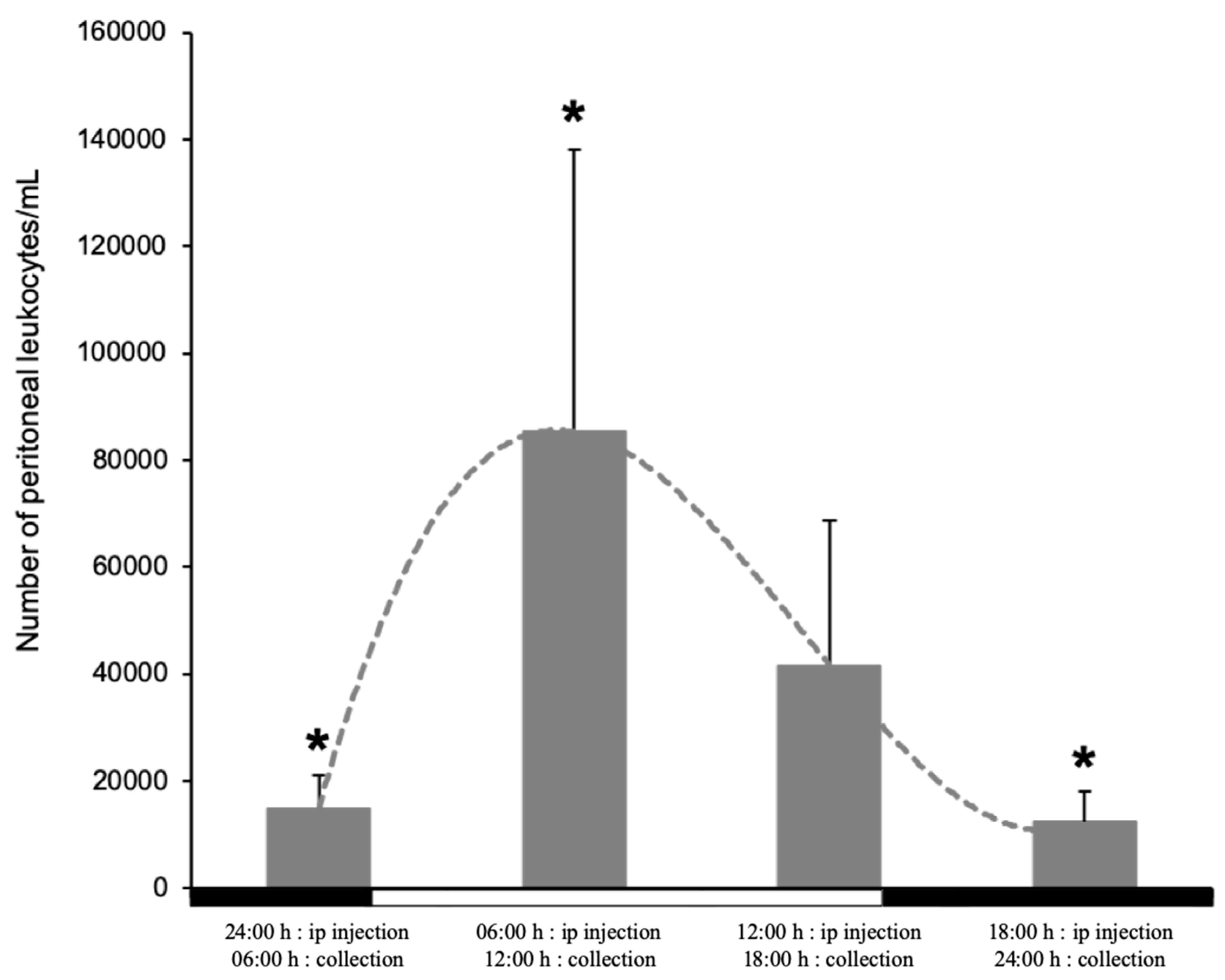
3.4. Recruitment of Leukocytes into the Peritoneal Cavity
3.5. Bacterial Proliferation in Serum
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edgar, R.S.; Green, E.W.; Zhao, Y.; Van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.C.; Lewis, W.G.; Kay, S.A. Mammalian circadian signaling networks and therapeutic targets. Nat. Chem. Biol. 2007, 3, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, G.A.; Johnston, J.D.; Andersson, H.; Wagner, G.; Hazlerigg, D.G. Photorefractoriness in Mammals: Dissociating a Seasonal Timer from the Circadian-Based Photoperiod Response. Endocrinology 2005, 146, 3782–3790. [Google Scholar] [CrossRef]
- Reebs, S.G. Plasticity of diel and circadian activity rhythms in fishes. Rev. Fish Biol. Fish. 2002, 12, 349–371. [Google Scholar] [CrossRef]
- Hurd, M.W.; Debruyne, J.; Straume, M.; Cahill, G.M. Circadian rhythms of locomotor activity in zebrafish. Physiol. Behav. 1998, 65, 465–472. [Google Scholar] [CrossRef]
- Gerkema, M.P.; Videler, J.J.; De Wiljes, J.; Van Lavieren, H.; Gerritsen, H.; Karel, M. Photic entrainment of circadian activity patterns in the tropical labrid fish Halichoeres chrysus. Chronobiol. Int. 2000, 17, 613–622. [Google Scholar] [CrossRef][Green Version]
- Vera, L.M.; Cairns, L.; Sánchez-Vázquez, F.J.; Migaud, H. Circadian Rhythms of Locomotor Activity in the Nile Tilapia Oreochromis niloticus. Chronobiol. Int. 2009, 26, 666–681. [Google Scholar] [CrossRef]
- Haus, E.; Smolensky, M.H. Biologic rhythms in the immune system. Chronobiol. Int. 1999, 16, 581–622. [Google Scholar] [CrossRef]
- Abo, T.; Kawate, T.; Itoh, K.; Kumagai, K. Studies on the bioperiodicity of the immune response. I. Circadian rhythms of human T, B, and K cell traffic in the peripheral blood. J. Immunol. 1981, 126, 1360–1363. [Google Scholar]
- Haus, E.; Lakatua, D.J.; Swoyer, J.; Sackett-Lundeen, L. Chronobiology in hematology and immunology. Am. J. Anat. 1983, 168, 467–517. [Google Scholar] [CrossRef] [PubMed]
- Hriscu, M.L. Modulatory Factors of Circadian Phagocytic Activity. Ann. N. Y. Acad. Sci. 2005, 1057, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Arjona, A.; Sarkar, D.K. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J. Immunol. 2005, 174, 7618–7624. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Canon, C.; Di Palma, M.; Florentin, I.; Misset, J.L. When should the immune clock be reset? From circadian pharmacodynamics to temporally optimized drug delivery. Ann. N. Y. Acad. Sci. 1991, 618, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Young, M.R.; Matthews, J.P.; Kanabrocki, E.L.; Sothern, R.B.; Roitman-Johnson, B.; Scheving, L.E. Circadian rhythmometry of serum interleukin-2, interleukin-10, tumor necrosis factor-alpha, and granulocyte-macrophage colony-stimulating factor in men. Chronobiol. Int. 1995, 12, 19–27. [Google Scholar] [CrossRef]
- Esquifino, A.I.; Selgas, L.; Arce, A.; Della Maggiore, V.; Cardinali, D.P. Twenty-Four-Hour Rhythms in Immune Responses in Rat Submaxillary Lymph Nodes and Spleen: Effect of Cyclosporine. Brain Behav. Immun. 1996, 10, 92–102. [Google Scholar] [CrossRef]
- Fernandes, G.; Halberg, F.; Yunis, E.J.; Good, R.A. Circadian rhythmic plaque-forming cell response of spleens from mice immunized with SRBC. J. Immunol. 1976, 117, 962–966. [Google Scholar]
- Shackelford, P.G.; Feigin, R.D. Periodicity of Susceptibility to Pneumococcal Infection: Influence of Light and Adrenocortical Secretions. Science 1973, 182, 285–287. [Google Scholar] [CrossRef]
- Castanon-Cervantes, O.; Wu, M.; Ehlen, J.C.; Paul, K.; Gamble, K.L.; Johnson, R.L.; Besing, R.C.; Menaker, M.; Gewirtz, A.T.; Davidson, A.J. Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 2010, 185, 5796–5805. [Google Scholar] [CrossRef]
- Tort, L.; Balasch, J.C.; Mackenzie, S. Fish immune system. A crossroads between innate and adaptive responses. Immunología 2003, 22, 277–286. [Google Scholar]
- Li, J.; Barreda, D.R.; Zhang, Y.A.; Boshra, H.; Gelman, A.E.; LaPatra, S.; Tort, L.; Sunyer, J.O. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat. Immunol. 2006, 7, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Parra, D.; Rieger, A.M.; Li, J.; Zhang, Y.A.; Randall, L.M.; Hunter, C.A.; Barreda, D.R.; Sunyer, J.O. Pivotal advance: Peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J. Leukoc. Biol. 2012, 91, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, W.T.; Wong, W.M.; Ward, E.; Thrasher, A.J.; Goldblatt, D.; Osman, M.; Digard, P.; Canaday, D.H.; Gustafsson, K. Human gamma delta T cells: A lymphoid lineage cell capable of professional phagocytosis. J. Immunol. 2009, 183, 5622–5629. [Google Scholar] [CrossRef] [PubMed]
- Köllner, B.; Fischer, U.; Rombout, J.; Taverne-Thiele, J.; Hansen, J. Potential involvement of rainbow trout thrombocytes in immune functions: A study using a panel of monoclonal antibodies and RT-PCR. Dev. Comp. Immunol. 2004, 28, 1049–1062. [Google Scholar] [CrossRef]
- DeLuca, D.; Wilson, M.; Warr, G.W. Lymphocyte heterogeneity in the trout, Salmo gairdneri, defined with monoclonal antibodies to IgM. Eur. J. Immunol. 1983, 13, 546–551. [Google Scholar] [CrossRef]
- Korytář, T.; Thi, H.D.; Takizawa, F.; Köllner, B. A multicolour flow cytometry identifying defined leukocyte subsets of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2013, 35, 2017–2019. [Google Scholar] [CrossRef]
- Korytář, T.; Nipkow, M.; Altmann, S.; Goldammer, T.; Köllner, B.; Rebl, A. Adverse Husbandry of Maraena Whitefish Directs the Immune System to Increase Mobilization of Myeloid Cells and Proinflammatory Responses. Front. Immunol. 2016, 7, 631. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.D.; Kramer, A.; Maier, B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412. [Google Scholar] [CrossRef]
- Ackermann, K.; Revell, V.L.; Lao, O.; Rombouts, E.J.; Skene, D.J.; Kayser, M. Diurnal Rhythms in Blood Cell Populations and the Effect of Acute Sleep Deprivation in Healthy Young Men. Sleep 2012, 35, 933–940. [Google Scholar] [CrossRef]
- Cermakian, N.; Lange, T.; Golombek, D.; Sarkar, D.; Nakao, A.; Shibata, S.; Mazzoccoli, G. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol. Int. 2013, 30, 870–888. [Google Scholar] [CrossRef] [PubMed]
- Kawate, T.; Abo, T.; Hinuma, S.; Kumagai, K. Studies of the bioperiodicity of the immune response. II. Co-variations of murine T and B cells and a role of corticosteroid. J. Immunol. 1981, 126, 1364–1367. [Google Scholar] [PubMed]
- Méndez-Ferrer, S.; Lucas, D.; Battista, M.; Frenette, P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008, 452, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.; Battista, M.; Shi, P.A.; Isola, L.; Frenette, P.S. Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell 2008, 3, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, J.; Esteban, M. Ángeles; Rodríguez, A. Are thrombocytes and platelets true phagocytes? Microsc. Res. Tech. 2002, 57, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Øverland, H.S.; Pettersen, E.F.; Rønneseth, A.; Wergeland, H.I. Phagocytosis by B-cells and neutrophils in Atlantic salmon (Salmo salar L.) and Atlantic cod (Gadus morhua L.). Fish Shellfish Immunol. 2010, 28, 193–204. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Lucas, D.; Chow, A.; Jang, J.E.; Zhang, D.; Hashimoto, D.; Merad, M.; Frenette, P.S. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 2012, 37, 290–301. [Google Scholar] [CrossRef]
- Korytář, T.; Jaros, J.; Verleih, M.; Rebl, A.; Kotterba, G.; Kühn, C.; Goldammer, T.; Köllner, B. Novel insights into the peritoneal inflammation of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2013, 35, 1192–1199. [Google Scholar] [CrossRef]
- Loza-Correa, M.; Gomez-Valero, L.; Buchrieser, C. Circadian Clock Proteins in Prokaryotes: Hidden Rhythms? Front. Microbiol. 2010, 1, 130. [Google Scholar] [CrossRef]
- Johnson, C.H.; Zhao, C.; Xu, Y.; Mori, T. Timing the day: What makes bacterial clocks tick? Nat. Rev. Genet. 2017, 15, 232–242. [Google Scholar] [CrossRef]
- Rijo-Ferreira, F.; Takahashi, J.S.; Figueiredo, L.M. Circadian rhythms in parasites. PLoS Pathog. 2017, 13, e1006590. [Google Scholar] [CrossRef] [PubMed]
- Prior, K.F.; O’Donnell, A.J.; Rund, S.S.C.; Savill, N.J.; Van Der Veen, D.R.; Reece, S.E. Host circadian rhythms are disrupted during malaria infection in parasite genotype-specific manners. Sci. Rep. 2019, 9, 10905. [Google Scholar] [CrossRef] [PubMed]
- Westwood, M.L.; O’Donnell, A.J.; De Bekker, C.; Lively, C.M.; Zuk, M.; Reece, S.E. The evolutionary ecology of circadian rhythms in infection. Nat. Ecol. Evol. 2019, 3, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Rambhatla, S.B.; Lai, A.G.; McKeating, J.A. Interplay between circadian clock and viral infection. J. Mol. Med. 2017, 95, 1283–1289. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Berg, I.; Brinchmann, M.F.; Kiron, V. Short-term crowding stress in Atlantic cod, Gadus morhua L. modulates the humoral immune response. Aquaculture 2009, 295, 110–115. [Google Scholar] [CrossRef]
- Lazado, C.C.; Gesto, M.; Madsen, L.; Jokumsen, A. Interplay between daily rhythmic serum-mediated bacterial killing activity and immune defence factors in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 72, 418–425. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montero, R.; Strzelczyk, J.E.; Tze Ho Chan, J.; Verleih, M.; Rebl, A.; Goldammer, T.; Köllner, B.; Korytář, T. Dawn to Dusk: Diurnal Rhythm of the Immune Response in Rainbow Trout (Oncorhynchus Mykiss). Biology 2020, 9, 8. https://doi.org/10.3390/biology9010008
Montero R, Strzelczyk JE, Tze Ho Chan J, Verleih M, Rebl A, Goldammer T, Köllner B, Korytář T. Dawn to Dusk: Diurnal Rhythm of the Immune Response in Rainbow Trout (Oncorhynchus Mykiss). Biology. 2020; 9(1):8. https://doi.org/10.3390/biology9010008
Chicago/Turabian StyleMontero, Ruth, Joanna Ewa Strzelczyk, Justin Tze Ho Chan, Marieke Verleih, Alexander Rebl, Tom Goldammer, Bernd Köllner, and Tomáš Korytář. 2020. "Dawn to Dusk: Diurnal Rhythm of the Immune Response in Rainbow Trout (Oncorhynchus Mykiss)" Biology 9, no. 1: 8. https://doi.org/10.3390/biology9010008
APA StyleMontero, R., Strzelczyk, J. E., Tze Ho Chan, J., Verleih, M., Rebl, A., Goldammer, T., Köllner, B., & Korytář, T. (2020). Dawn to Dusk: Diurnal Rhythm of the Immune Response in Rainbow Trout (Oncorhynchus Mykiss). Biology, 9(1), 8. https://doi.org/10.3390/biology9010008






