Mechanotransduction in Development: A Focus on Angiogenesis
Simple Summary
Abstract
1. Introduction
1.1. Mechanosensors and Their Classification
1.2. The ECM–Membrane–Cytoskeleton–Nucleus Axis in Mechanosensing
2. Mechanosensitive Ion Channels
2.1. Transient Receptor Potential (TRP) Family Members
2.2. Piezo Channels
2.3. Intracellular Mechanotransduction: Receptors for Soluble Ligands as Mechanosensors
3. Mechanosensing in Embryo Development
4. Mechanotransduction in Vasculature
4.1. Mechanical Regulation of Endothelial Tip Cell Sprouting
4.2. Biomechanical Control of Angiogenesis
4.3. Mechanosensitive Ion Channels in Vascular Development
4.4. Mechanotransduction in Lymphangiogenesis
5. Mechanosensing of the Blood-Brain Barrier
6. Hemodynamics in Pathological Vascular Phenotypes
7. Discussion
8. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AJ | Adherens Junction |
| AVM | ArterioVenous Malformation |
| BBB | Blood-Brain Barrier |
| BK | Big Potassium |
| BMP | Bone Morphogenetic Protein |
| CNS | Central Nervous System |
| EC | Endothelial Cell |
| ECM | Extracellular Matrix |
| ENaC | Epithelial Sodium Channel |
| EndMT | Endothelial-to-Mesenchymal Transition |
| ENG | Endoglin |
| ETC | Endothelial Tip Cell |
| FAK | Focal Adhesion Kinase |
| GPCR | G-Protein Coupled Receptor |
| HHT | Hereditary Hemorrhagic Telangiectasia |
| ICH | Intracerebral Haemorrhage |
| KLF | Krüppel-Like Factor |
| LINC | Linker of Nucleoskeleton and Cytoskeleton |
| LTP | Long Term Potentiation |
| MC | Mechanosensitive Channel |
| MSC | Mesenchymal Stem Cell |
| NO | Nitric Oxide |
| NVU | NeuroVascular Unit |
| SOCE | Store Operated Ca2+ Entry |
| TAZ | Transcriptional coactivator with PDZ-binding motif |
| TJ | Tight junction |
| TREK | TWIK-Related K+ |
| TRP | Transient Receptor Potential |
| VEC | Vascular Endothelial Cadherin |
| VEGF | Vascular Endothelial Growth Factor |
| VSMC | Vascular Smooth Muscle Cell |
| YAP | Yes-Associated Protein |
References
- Cai, J.; Deng, Y.; Min, Z.; Li, C.; Zhao, Z.; Jing, D. Deciphering the dynamics: Exploring the impact of mechanical forces on histone acetylation. FASEB J. 2024, 38, e23849. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Schmid, H.; Münzberg, C.; Maass, U.; Krndija, D.; Adler, G.; Seufferlein, T.; Liedert, A.; Ignatius, A.; Oswald, F.; et al. Phosphorylation and turnover of paxillin in focal contacts is controlled by force and defines the dynamic state of the adhesion site. Cytoskeleton 2015, 72, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Panagaki, F.; Tapia-Rojo, R.; Zhu, T.; Milmoe, N.; Paracuellos, P.; Board, S.; Mora, M.; Walker, J.; Rostkova, E.; Stannard, A.; et al. Structural anisotropy results in mechano-directional transport of proteins across nuclear pores. Nat. Phys. 2024, 20, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Bae, Y.; Balakrishnan, S.; Kim, D.H. Editorial: Mechanical coupling between extracellular and intracellular microenvironment. Front. Cell Dev. Biol. 2024, 12, 1427439. [Google Scholar] [CrossRef]
- Do, T.D.; Katsuyoshi, J.; Cai, H.; Ohashi, T. Mechanical Properties of Isolated Primary Cilia Measured by Micro-tensile Test and Atomic Force Microscopy. Front. Bioeng. Biotechnol. 2021, 9, 753805. [Google Scholar] [CrossRef]
- Nishizawa, K.; Lin, S.Z.; Chardès, C.; Rupprecht, J.F.; Lenne, P.F. Two-point optical manipulation reveals mechanosensitive remodeling of cell-cell contacts in vivo. Proc. Natl. Acad. Sci. USA 2023, 120, e2212389120. [Google Scholar] [CrossRef]
- McCormick, L.E.; Gupton, S.L. Mechanistic advances in axon pathfinding. Curr. Opin. Cell Biol. 2020, 63, 11–19. [Google Scholar] [CrossRef]
- Morales-Camilo, N.; Liu, J.; Ramírez, M.J.; Canales-Salgado, P.; Alegría, J.J.; Liu, X.; Ong, H.T.; Barrera, N.P.; Fierro, A.; Toyama, Y.; et al. Alternative molecular mechanisms for force transmission at adherens junctions via β-catenin-vinculin interaction. Nat. Commun. 2024, 15, 5608. [Google Scholar] [CrossRef]
- Chen, K.; Kwon, S.H.; Henn, D.; Kuehlmann, B.A.; Tevlin, R.; Bonham, C.A.; Griffin, M.; Trotsyuk, A.A.; Borrelli, M.R.; Noishiki, C.; et al. Disrupting biological sensors of force promotes tissue regeneration in large organisms. Nat. Commun. 2021, 12, 5256. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, W.; Wei, Y.; Li, Q.; Chen, N.; Xia, Q.; Wang, L.; Hu, J.; Zhou, X.; Sun, Y.; et al. Mechanical stress-induced autophagy is cytoskeleton dependent. Cell Prolif. 2024, 57, e13728. [Google Scholar] [CrossRef]
- Bays, J.L.; Campbell, H.K.; Heidema, C.; Sebbagh, M.; DeMali, K.A. Linking E-cadherin mechanotransduction to cell metabolism through force-mediated activation of AMPK. Nat. Cell Biol. 2017, 19, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Welhaven, H.D.; McCutchen, C.N.; June, R.K. Effects of mechanical stimulation on metabolomic profiles of SW1353 chondrocytes: Shear and compression. Biol. Open 2022, 11, bio058895. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M.; Liu, W.; Zabirnyk, O. Proline metabolism and microenvironmental stress. Annu. Rev. Nutr. 2010, 30, 441–463. [Google Scholar] [CrossRef]
- Arnadóttir, J.; Chalfie, M. Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 2010, 39, 111–137. [Google Scholar] [CrossRef] [PubMed]
- Hamill, O.P.; Martinac, B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 2001, 81, 685–740. [Google Scholar] [CrossRef]
- Anishkin, A.; Loukin, S.H.; Teng, J.; Kung, C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl. Acad. Sci. USA 2014, 111, 7898–7905. [Google Scholar] [CrossRef]
- Hu, X.; Margadant, F.M.; Yao, M.; Sheetz, M.P. Molecular stretching modulates mechanosensing pathways. Protein Sci. 2017, 26, 1337–1351. [Google Scholar] [CrossRef]
- Fu, Y.; Jing, Z.; Chen, T.; Xu, X.; Wang, X.; Ren, M.; Wu, Y.; Wu, T.; Li, Y.; Zhang, H.; et al. Nanotube patterning reduces macrophage inflammatory response via nuclear mechanotransduction. J. Nanobiotechnol. 2023, 21, 229. [Google Scholar] [CrossRef]
- Vivo, M.; Rosti, V.; Cervone, S.; Lanzuolo, C. Chromatin plasticity in mechanotransduction. Curr. Opin. Cell Biol. 2024, 88, 102376. [Google Scholar] [CrossRef]
- Grudtsyna, V.; Packirisamy, S.; Bidone, T.C.; Swaminathan, V. Extracellular matrix sensing via modulation of orientational order of integrins and F-actin in focal adhesions. Life Sci. Alliance 2023, 6, e202301898. [Google Scholar] [CrossRef]
- Benke, K.; Ágg, B.; Szilveszter, B.; Tarr, F.; Nagy, Z.B.; Pólos, M.; Daróczi, L.; Merkely, B.; Szabolcs, Z. The role of transforming growth factor-beta in Marfan syndrome. Cardiol. J. 2013, 20, 227–234. [Google Scholar] [CrossRef]
- Pei, F.; Guo, T.; Zhang, M.; Ma, L.; Jing, J.; Feng, J.; Ho, T.V.; Wen, Q.; Chai, Y. FGF signaling modulates mechanotransduction/WNT signaling in progenitors during tooth root development. Bone Res. 2024, 12, 37. [Google Scholar] [CrossRef]
- Steinberg, T.; Dieterle, M.P.; Ramminger, I.; Klein, C.; Brossette, J.; Husari, A.; Tomakidi, P. On the Value of In Vitro Cell Systems for Mechanobiology from the Perspective of Yes-Associated Protein/Transcriptional Co-Activator with a PDZ-Binding Motif and Focal Adhesion Kinase and Their Involvement in Wound Healing, Cancer, Aging, and Senescence. Int. J. Mol. Sci. 2023, 24, 12677. [Google Scholar] [CrossRef]
- Gautel, M. Cytoskeletal protein kinases: Titin and its relations in mechanosensing. Pflugers Arch. 2011, 462, 119–134. [Google Scholar] [CrossRef]
- Yao, M.; Goult, B.T.; Klapholz, B.; Hu, X.; Toseland, C.P.; Guo, Y.; Cong, P.; Sheetz, M.P.; Yan, J. The mechanical response of talin. Nat. Commun. 2016, 7, 11966. [Google Scholar] [CrossRef]
- Takata, T.; Matsumura, M. The LINC Complex Assists the Nuclear Import of Mechanosensitive Transcriptional Regulators. Results Probl. Cell Differ. 2022, 70, 315–337. [Google Scholar] [CrossRef]
- García-González, A.; Jacchetti, E.; Marotta, R.; Tunesi, M.; Rodríguez Matas, J.F.; Raimondi, M.T. The Effect of Cell Morphology on the Permeability of the Nuclear Envelope to Diffusive Factors. Front. Physiol. 2018, 9, 925. [Google Scholar] [CrossRef]
- Galbraith, C.G.; Yamada, K.M.; Sheetz, M.P. The relationship between force and focal complex development. J. Cell Biol. 2002, 159, 695–705. [Google Scholar] [CrossRef]
- Harrison, O.J.; Jin, X.; Hong, S.; Bahna, F.; Ahlsen, G.; Brasch, J.; Wu, Y.; Vendome, J.; Felsovalyi, K.; Hampton, C.M.; et al. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure 2011, 19, 244–256. [Google Scholar] [CrossRef]
- Meili, R.; Alonso-Latorre, B.; del Alamo, J.C.; Firtel, R.A.; Lasheras, J.C. Myosin II is essential for the spatiotemporal organization of traction forces during cell motility. Mol. Biol. Cell 2010, 21, 405–417. [Google Scholar] [CrossRef]
- Jacquemet, G.; Stubb, A.; Saup, R.; Miihkinen, M.; Kremneva, E.; Hamidi, H.; Ivaska, J. Filopodome Mapping Identifies p130Cas as a Mechanosensitive Regulator of Filopodia Stability. Curr. Biol. 2019, 29, 202–216.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Wang, Y.; Lu, C.; Hu, X.; Kawazoe, N.; Yang, Y.; Chen, G. Focal adhesion and actin orientation regulated by cellular geometry determine stem cell differentiation via mechanotransduction. Acta Biomater. 2024, 182, 81–92. [Google Scholar] [CrossRef] [PubMed]
- McGillivary, R.M.; Starr, D.A.; Luxton, G.W.G. Building and breaking mechanical bridges between the nucleus and cytoskeleton: Regulation of LINC complex assembly and disassembly. Curr. Opin. Cell Biol. 2023, 85, 102260. [Google Scholar] [CrossRef]
- Miralles, F.; Posern, G.; Zaromytidou, A.I.; Treisman, R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 2003, 113, 329–342. [Google Scholar] [CrossRef]
- Maciejowski, J.; Hatch, E.M. Nuclear Membrane Rupture and Its Consequences. Annu. Rev. Cell Dev. Biol. 2020, 36, 85–114. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Ikram, S.; Bibi, N.; Mir, A. Hutchinson-Gilford Progeria Syndrome: A Premature Aging Disease. Mol. Neurobiol. 2018, 55, 4417–4427. [Google Scholar] [CrossRef]
- Greenberg, C.R.; Rimoin, D.L.; Gruber, H.E.; DeSa, D.J.; Reed, M.; Lachman, R.S. A new autosomal recessive lethal chondrodystrophy with congenital hydrops. Am. J. Med. Genet. 1988, 29, 623–632. [Google Scholar] [CrossRef]
- Eriksson, M.; Brown, W.T.; Gordon, L.B.; Glynn, M.W.; Singer, J.; Scott, L.; Erdos, M.R.; Robbins, C.M.; Moses, T.Y.; Berglund, P.; et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 2003, 423, 293–298. [Google Scholar] [CrossRef]
- Li, L.; Du, Y.; Kong, X.; Li, Z.; Jia, Z.; Cui, J.; Gao, J.; Wang, G.; Xie, K. Lamin B1 is a novel therapeutic target of betulinic acid in pancreatic cancer. Clin. Cancer Res. 2013, 19, 4651–4661. [Google Scholar] [CrossRef]
- Moss, S.F.; Krivosheyev, V.; de Souza, A.; Chin, K.; Gaetz, H.P.; Chaudhary, N.; Worman, H.J.; Holt, P.R. Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms. Gut 1999, 45, 723–729. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Chen, C.C. Force from Filaments: The Role of the Cytoskeleton and Extracellular Matrix in the Gating of Mechanosensitive Channels. Front. Cell Dev. Biol. 2022, 10, 886048. [Google Scholar] [CrossRef]
- Buncha, V.; Cherezova, A.; Alexander, S.; Baranovskaya, I.; Coleman, K.A.; Cherian-Shaw, M.; Brands, M.W.; Sullivan, J.C.; O’Connor, P.M.; Mamenko, M. Aldosterone Antagonism Is More Effective at Reducing Blood Pressure and Excessive Renal ENaC Activity in AngII-Infused Female Rats Than in Males. Hypertension 2023, 80, 2196–2208. [Google Scholar] [CrossRef]
- Nickerson, A.J.; Sheng, S.; Cox, N.A.; Szekely, K.G.; Marciszyn, A.L.; Lam, T.; Chen, J.; Gingras, S.; Kashlan, O.B.; Kirabo, A.; et al. Loss of the alpha subunit distal furin cleavage site blunts ENaC activation following Na+ restriction. J. Physiol. 2024, 602, 4309–4326. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Jiang, B.Y.; Chen, C.C. Acid-sensing ion channels: Dual function proteins for chemo-sensing and mechano-sensing. J. Biomed. Sci. 2018, 25, 46. [Google Scholar] [CrossRef]
- Sorum, B.; Docter, T.; Panico, V.; Rietmeijer, R.A.; Brohawn, S.G. Tension activation of mechanosensitive two-pore domain K+ channels TRAAK, TREK-1, and TREK-2. Nat. Commun. 2024, 15, 3142. [Google Scholar] [CrossRef]
- Dupont, C.; Blake, B.; Voss, A.A.; Rich, M.M. BK channels promote action potential repolarization in skeletal muscle but contribute little to myotonia. Pflugers Arch. 2024, 476, 1693–1702. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Ning, F.; Ren, D.; Tao, J.; Xie, W.; Eaton, D.C.; Jiang, G.; Farris, A.B.; Xin, H.; et al. A novel role of BK potassium channel activity in preventing the development of kidney fibrosis. Kidney Int. 2022, 101, 945–962. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, M.; Cao, N.; Zhang, L.; He, Q.; Wang, J.; Wang, R.; Wang, L.; Zhao, L.; Si, J. Emodin activates BK channel in vascular smooth muscle cells and relaxes the interlobar renal artery of rat. Biomed. Pharmacother. 2022, 153, 113452. [Google Scholar] [CrossRef]
- Contet, C.; Goulding, S.P.; Kuljis, D.A.; Barth, A.L. BK Channels in the Central Nervous System. Int. Rev. Neurobiol. 2016, 128, 281–342. [Google Scholar] [CrossRef]
- Echeverría, F.; Gonzalez-Sanabria, N.; Alvarado-Sanchez, R.; Fernández, M.; Castillo, K.; Latorre, R. Large conductance voltage-and calcium-activated K+ (BK) channel in health and disease. Front. Pharmacol. 2024, 15, 1373507. [Google Scholar] [CrossRef]
- Lin, H.H.; Ng, K.F.; Chen, T.C.; Tseng, W.Y. Ligands and Beyond: Mechanosensitive Adhesion GPCRs. Pharmaceuticals 2022, 15, 219. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 2016, 343, 42–53. [Google Scholar] [CrossRef]
- Pang, V.Y.; Yang, Z.; Wu, S.M.; Pang, J.J. The co-expression of the depolarizing and hyperpolarizing mechanosensitive ion channels in mammalian retinal neurons. Front. Med. 2024, 11, 1463898. [Google Scholar] [CrossRef]
- Edosuyi, O.; Adesuyi, A.; Choi, M.; Igbe, I.; Oyekan, A. Malate reduced blood pressure and exerted differential effects on renal hemodynamics; role of the nitric oxide system and renal epithelial sodium channels (ENaC). Eur. J. Pharmacol. 2023, 938, 175441. [Google Scholar] [CrossRef]
- Sandoz, G.; Lesage, F. Protein complex analysis of native brain potassium channels by proteomics. Methods Mol. Biol. 2008, 491, 113–123. [Google Scholar] [CrossRef]
- Sandoz, G.; Tardy, M.P.; Thümmler, S.; Feliciangeli, S.; Lazdunski, M.; Lesage, F. Mtap2 is a constituent of the protein network that regulates twik-related K+ channel expression and trafficking. J. Neurosci. 2008, 28, 8545–8552. [Google Scholar] [CrossRef]
- Li, Z.; Yu, Y.; Cao, X.; Wang, Y.; Lu, J.; Feng, Y.; Jiang, Y.; Lu, Y. Mechanism of Ca2+ overload caused by STIM1/ORAI1 activation of store-operated Ca2+ entry (SOCE) in hydrogen peroxide-induced mitochondrial damage and apoptosis in human primary melanocytes. Mol. Biol. Rep. 2025, 52, 223. [Google Scholar] [CrossRef]
- Storch, U.; Mederos y Schnitzler, M.; Gudermann, T. G protein-mediated stretch reception. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1241–H1249. [Google Scholar] [CrossRef]
- Gualdani, R.; Gailly, P. How TRPC Channels Modulate Hippocampal Function. Int. J. Mol. Sci. 2020, 21, 3915. [Google Scholar] [CrossRef]
- Rosenberg, P.; Hawkins, A.; Stiber, J.; Shelton, J.M.; Hutcheson, K.; Bassel-Duby, R.; Shin, D.M.; Yan, Z.; Williams, R.S. TRPC3 channels confer cellular memory of recent neuromuscular activity. Proc. Natl. Acad. Sci. USA 2004, 101, 9387–9392. [Google Scholar] [CrossRef]
- Higashida, H.; Yuhi, T.; Akther, S.; Amina, S.; Zhong, J.; Liang, M.; Nishimura, T.; Liu, H.X.; Lopatina, O. Oxytocin release via activation of TRPM2 and CD38 in the hypothalamus during hyperthermia in mice: Implication for autism spectrum disorder. Neurochem. Int. 2018, 119, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Tong, Y.; Zheng, J.; Shi, R.; Liang, H.; Li, M.; Meng, Y.; Shi, J.; Zhao, D.; Seehus, C.R.; et al. TRPM7 contributes to pyroptosis and its involvement in status epilepticus. J. Neuroinflammation 2024, 21, 315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Y.; Zuo, M.; Yu, Y.; Huang, X. Advances in the Study for Modulators of Transient Receptor Potential Vanilloid (TRPV) Channel Family. Curr. Top Med. Chem. 2025, 25, 1–48. [Google Scholar] [CrossRef]
- Wang, X.D.; Lin, Z.K.; Ji, S.X.; Bi, S.Y.; Liu, W.X.; Zhang, G.F.; Wan, F.H.; Lü, Z.C. Molecular Characterization of TRPA Subfamily Genes and Function in Temperature Preference in Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Int. J. Mol. Sci. 2021, 22, 7157. [Google Scholar] [CrossRef]
- Huang, P.; Dong, R.Y.; Wang, P.; Xu, M.; Sun, X.; Dong, X.P. MCOLN/TRPML channels in the regulation of MTORC1 and autophagy. Autophagy 2024, 20, 1203–1204. [Google Scholar] [CrossRef]
- Lakhia, R.; Ramalingam, H.; Chang, C.M.; Cobo-Stark, P.; Biggers, L.; Flaten, A.; Alvarez, J.; Valencia, T.; Wallace, D.P.; Lee, E.C.; et al. PKD1 and PKD2 mRNA cis-inhibition drives polycystic kidney disease progression. Nat. Commun. 2022, 13, 4765. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, W.; Dou, W.; Luo, H.; Ouyang, X. Pleiotropic physiological functions of Piezo1 in human body and its effect on malignant behavior of tumors. Front. Physiol. 2024, 15, 1377329. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Leung, V.; Seradj, S.H.; Sonmez, U.; Servin-Vences, R.; Lipomi, D.; Ye, L.; Patapoutian, A. A key role of PIEZO2 mechanosensitive ion channel in adipose sensory innervation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Cox, C.D.; Poole, K.; Martinac, B. Re-evaluating TRP channel mechanosensitivity. Trends Biochem. Sci. 2024, 49, 693–702. [Google Scholar] [CrossRef]
- Huang, J.; Korsunsky, A.; Yazdani, M.; Chen, J. Targeting TRP channels: Recent advances in structure, ligand binding, and molecular mechanisms. Front. Mol. Neurosci. 2024, 16, 1334370. [Google Scholar] [CrossRef]
- Wang, Y.; Bu, J.; Shen, H.; Li, H.; Wang, Z.; Chen, G. Targeting Transient Receptor Potential Canonical Channels for Diseases of the Nervous System. Curr. Drug Targets 2017, 18, 1460–1465. [Google Scholar] [CrossRef]
- Wes, P.D.; Chevesich, J.; Jeromin, A.; Rosenberg, C.; Stetten, G.; Montell, C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. USA 1995, 92, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Obukhov, A.G.; Harteneck, C.; Zobel, A.; Harhammer, R.; Kalkbrenner, F.; Leopoldt, D.; Lückhoff, A.; Nürnberg, B.; Schultz, G. Direct activation of trpl cation channels by G alpha11 subunits. EMBO J. 1996, 15, 5833–5838. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.J.; Jardin, I.; Sanchez-Collado, J.; Salido, G.M.; Smani, T.; Rosado, J.A. TRPC Channels in the SOCE Scenario. Cells 2020, 9, 126. [Google Scholar] [CrossRef]
- Wang, M.; Wen, W.; Chen, Y.; Yishajiang, S.; Li, Y.; Li, Z.; Zhang, X. TRPC5 channel participates in myocardial injury in chronic intermittent hypoxia. Clinics 2024, 79, 100368. [Google Scholar] [CrossRef]
- Yang, L.; Peng, Z.; Gong, F.; Yan, W.; Shi, Y.; Li, H.; Zhou, C.; Yao, H.; Yuan, M.; Yu, F.; et al. TRPC4 aggravates hypoxic pulmonary hypertension by promoting pulmonary endothelial cell apoptosis. Free Radic. Biol. Med. 2024, 219, 141–152. [Google Scholar] [CrossRef]
- Zong, P.; Li, C.X.; Feng, J.; Cicchetti, M.; Yue, L. TRP Channels in Stroke. Neurosci. Bull. 2024, 40, 1141–1159. [Google Scholar] [CrossRef]
- Bröker-Lai, J.; Rego Terol, J.; Richter, C.; Mathar, I.; Wirth, A.; Kopf, S.; Moreno-Pérez, A.; Büttner, M.; Tan, L.L.; Makke, M.; et al. TRPC5 controls the adrenaline-mediated counter regulation of hypoglycemia. EMBO J. 2024, 43, 5813–5836. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Hu, Y.; Wang, L.; Lv, G.; Feng, Y.; Sun, Z.; Cao, Z.; Liu, Y.; Wang, H. Discovery of N-alkyl-N-benzyl thiazoles as novel TRPC antagonists for the treatment of glioblastoma multiforme. Eur. J. Med. Chem. 2024, 265, 116066. [Google Scholar] [CrossRef]
- Qi, H.; Wu, F.; Wang, H. Function of TRPC1 in modulating hepatocellular carcinoma progression. Med. Oncol. 2023, 40, 97. [Google Scholar] [CrossRef]
- Becker, A.; Mannebach, S.; Mathar, I.; Weissgerber, P.; Freichel, M.; Loodin, A.P.; Fecher-Trost, C.; Belkacemi, A.; Beck, A.; Philipp, S.E. Control of Insulin Release by Transient Receptor Potential Melastatin 3 (TRPM3) Ion Channels. Cell Physiol. Biochem. 2020, 54, 1115–1131. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Götz, C.; Montenarh, M.; Philipp, S.E. Control of TRPM3 Ion Channels by Protein Kinase CK2-Mediated Phosphorylation in Pancreatic β-Cells of the Line INS-1. Int. J. Mol. Sci. 2021, 22, 13133. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, T.H.O.; Pereira-Figueiredo, D.; Veroneze, L.; Nascimento, A.A.; De Logu, F.; Nassini, R.; Campello-Costa, P.; Faria-Melibeu, A.D.C.; Souza Monteiro de Araújo, D.; Calaza, K.C. Functions of TRPs in retinal tissue in physiological and pathological conditions. Front. Mol. Neurosci. 2024, 17, 1459083. [Google Scholar] [CrossRef]
- Liu, D.Q.; Mei, W.; Zhou, Y.Q.; Xi, H. Targeting TRPM channels for cerebral ischemia-reperfusion injury. Trends Pharmacol. Sci. 2024, 45, 862–867. [Google Scholar] [CrossRef]
- Liman, E.R. The Ca2+-Activated TRP Channels: TRPM4 and TRPM5. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Chapter 15; Liedtke, W.B., Heller, S., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Holderby, K.G.; Kozak, J.A. Use of tetraethylammonium (TEA) and Tris loading for blocking TRPM7 channels in intact cells. Front. Pharmacol. 2024, 15, 1341799. [Google Scholar] [CrossRef]
- Huang, P.; Qu, C.; Rao, Z.; Wu, D.; Zhao, J. Bidirectional regulation mechanism of TRPM2 channel: Role in oxidative stress, inflammation and ischemia-reperfusion injury. Front. Immunol. 2024, 15, 1391355. [Google Scholar] [CrossRef]
- Landen, J.G.; Vandendoren, M.; Killmer, S.; Bedford, N.L.; Nelson, A.C. Huddling substates in mice facilitate dynamic changes in body temperature and are modulated by Shank3b and Trpm8 mutation. Commun. Biol. 2024, 7, 1186. [Google Scholar] [CrossRef]
- King, J.W.; Bennett, A.S.W.; Wood, H.M.; Baker, C.C.; Alsaadi, H.; Topley, M.; Vanner, S.A.; Reed, D.E.; Lomax, A.E. Expression and function of transient receptor potential melastatin 3 in the spinal afferent innervation of the mouse colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 326, G176–G186. [Google Scholar] [CrossRef]
- Sinica, V.; Vlachová, V. Transient receptor potential ankyrin 1 channel: An evolutionarily tuned thermosensor. Physiol. Res. 2021, 70, 363–381. [Google Scholar] [CrossRef]
- Stueber, T.; Eberhardt, M.J.; Caspi, Y.; Lev, S.; Binshtok, A.; Leffler, A. Differential cytotoxicity and intracellular calcium-signalling following activation of the calcium-permeable ion channels TRPV1 and TRPA1. Cell Calcium 2017, 68, 34–44. [Google Scholar] [CrossRef]
- Fila, M.; Przyslo, L.; Derwich, M.; Sobczuk, P.; Pawlowska, E.; Blasiak, J. The TRPA1 Ion Channel Mediates Oxidative Stress-Related Migraine Pathogenesis. Molecules 2024, 29, 3385. [Google Scholar] [CrossRef]
- Pires, P.W.; Earley, S. Neuroprotective effects of TRPA1 channels in the cerebral endothelium following ischemic stroke. Elife 2018, 7, e35316. [Google Scholar] [CrossRef] [PubMed]
- Faris, P.; Rumolo, A.; Pellavio, G.; Tanzi, M.; Vismara, M.; Berra-Romani, R.; Gerbino, A.; Corallo, S.; Pedrazzoli, P.; Laforenza, U.; et al. Transient receptor potential ankyrin 1 (TRPA1) mediates reactive oxygen species-induced Ca2+ entry, mitochondrial dysfunction, and caspase-3/7 activation in primary cultures of metastatic colorectal carcinoma cells. Cell Death Discov. 2023, 9, 213. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Du, Q.; Gu, J.; Wu, J.; Liu, Q.; Li, Z.; Zhang, T.; Xu, J.; Xie, R. Research Progress on TRPA1 in Diseases. J. Membr. Biol. 2023, 256, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Kremeyer, B.; Lopera, F.; Cox, J.J.; Momin, A.; Rugiero, F.; Marsh, S.; Woods, C.G.; Jones, N.G.; Paterson, K.J.; Fricker, F.R.; et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 2010, 66, 671–680. [Google Scholar] [CrossRef]
- Rosato, A.S.; Tang, R.; Grimm, C. Two-pore and TRPML cation channels: Regulators of phagocytosis, autophagy and lysosomal exocytosis. Pharmacol. Ther. 2021, 220, 107713. [Google Scholar] [CrossRef]
- Chen, C.C.; Krogsaeter, E.; Grimm, C. Two-pore and TRP cation channels in endolysosomal osmo-/mechanosensation and volume regulation. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118921. [Google Scholar] [CrossRef]
- Bargal, R.; Avidan, N.; Ben-Asher, E.; Olender, Z.; Zeigler, M.; Frumkin, A.; Raas-Rothschild, A.; Glusman, G.; Lancet, D.; Bach, G. Identification of the gene causing mucolipidosis type IV. Nat. Genet. 2000, 26, 118–123. [Google Scholar] [CrossRef]
- Sun, M.; Goldin, E.; Stahl, S.; Falardeau, J.L.; Kennedy, J.C.; Acierno, J.S., Jr.; Bove, C.; Kaneski, C.R.; Nagle, J.; Bromley, M.C.; et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum. Mol. Genet. 2000, 9, 2471–2478. [Google Scholar] [CrossRef]
- Grimm, C.; Cuajungco, M.P.; van Aken, A.F.; Schnee, M.; Jörs, S.; Kros, C.J.; Ricci, A.J.; Heller, S. A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc. Natl. Acad. Sci. USA 2007, 104, 19583–19588. [Google Scholar] [CrossRef]
- Rinkenberger, N.; Schoggins, J.W. Mucolipin-2 Cation Channel Increases Trafficking Efficiency of Endocytosed Viruses. mBio 2018, 9, e02314-17. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-stimulated genes: Roles in viral pathogenesis. Curr. Opin. Virol. 2014, 6, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Sudarikova, A.V.; Vasileva, V.Y.; Sultanova, R.F.; Ilatovskaya, D.V. Recent advances in understanding ion transport mechanisms in polycystic kidney disease. Clin. Sci. 2021, 135, 2521–2540. [Google Scholar] [CrossRef]
- Kotsis, F.; Boehlke, C.; Kuehn, E.W. The ciliary flow sensor and polycystic kidney disease. Nephrol. Dial. Transplant. 2013, 28, 518–526. [Google Scholar] [CrossRef]
- Nigro, E.A.; Distefano, G.; Chiaravalli, M.; Matafora, V.; Castelli, M.; Pesenti Gritti, A.; Bachi, A.; Boletta, A. Polycystin-1 Regulates Actomyosin Contraction and the Cellular Response to Extracellular Stiffness. Sci. Rep. 2019, 9, 16640. [Google Scholar] [CrossRef]
- Peng, J.B.; Suzuki, Y.; Gyimesi, G.; Hediger, M.A. TRPV5 and TRPV6 Calcium-Selective Channels. In Calcium Entry Channels in Non-Excitable Cells; Chapter 13; Kozak, J.A., Putney, J.W., Jr., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Ma, L.; Lee, B.H.; Clifton, H.; Schaefer, S.; Zheng, J. Nicotinic acid is a common regulator of heat-sensing TRPV1-4 ion channels. Sci. Rep. 2015, 5, 8906. [Google Scholar] [CrossRef]
- Haustrate, A.; Prevarskaya, N.; Lehen’kyi, V. Role of the TRPV Channels in the Endoplasmic Reticulum Calcium Homeostasis. Cells 2020, 9, 317. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Petrova, R.S.; Sugiyama, Y.; Nagai, N.; Tamura, H.; Donaldson, P.J. Regulation of the Membrane Trafficking of the Mechanosensitive Ion Channels TRPV1 and TRPV4 by Zonular Tension, Osmotic Stress and Activators in the Mouse Lens. Int. J. Mol. Sci. 2021, 22, 12658. [Google Scholar] [CrossRef]
- Ji, C.; Wang, Y.; Wang, Q.; Wang, A.; Ali, A.; McCulloch, C.A. TRPV4 regulates β1 integrin-mediated cell-matrix adhesions and collagen remodeling. FASEB J. 2023, 37, e22946. [Google Scholar] [CrossRef]
- Berciano, J.; Baets, J.; Gallardo, E.; Zimoń, M.; García, A.; López-Laso, E.; Combarros, O.; Infante, J.; Timmerman, V.; Jordanova, A.; et al. Reduced penetrance in hereditary motor neuropathy caused by TRPV4 Arg269Cys mutation. J. Neurol. 2011, 258, 1413–1421. [Google Scholar] [CrossRef]
- Lorin, C.; Vögeli, I.; Niggli, E. Dystrophic cardiomyopathy: Role of TRPV2 channels in stretch-induced cell damage. Cardiovasc. Res. 2015, 106, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.; Zaguri, R.; Edvardson, S.; Maayan, C.; Elpeleg, O.; Lev, S.; Davidson, E.; Peters, M.; Kfir-Erenfeld, S.; Berger, E.; et al. Nociception and pain in humans lacking a functional TRPV1 channel. J. Clin. Investig. 2023, 133, e153558. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, Q.; Lee, M.; Cao, X.; Zhang, J.; Ma, D.; Chen, L.; Hu, X.; Wang, H.; Wang, X.; et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am. J. Hum. Genet. 2012, 90, 558–564. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Zeng, W.Z.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 2018, 362, 464–467. [Google Scholar] [CrossRef]
- Cox, C.D.; Bae, C.; Ziegler, L.; Hartley, S.; Nikolova-Krstevski, V.; Rohde, P.R.; Ng, C.A.; Sachs, F.; Gottlieb, P.A.; Martinac, B. Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat. Commun. 2016, 7, 10366. [Google Scholar] [CrossRef]
- Verkest, C.; Schaefer, I.; Nees, T.A.; Wang, N.; Jegelka, J.M.; Taberner, F.J.; Lechner, S.G. Intrinsically disordered intracellular domains control key features of the mechanically-gated ion channel PIEZO2. Nat. Commun. 2022, 13, 1365. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Yang, X.; Zhou, G.; Wang, L.; Xiao, B. Tethering Piezo channels to the actin cytoskeleton for mechanogating via the cadherin-β-catenin mechanotransduction complex. Cell Rep. 2022, 38, 110342. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Gong, H.; Kesteven, S.; Guo, Y.; Wu, J.; Li, J.V.; Cheng, D.; Zhou, Z.; Iismaa, S.E.; Kaidonis, X.; et al. Piezo1 is the cardiac mechanosensor that initiates the cardiomyocyte hypertrophic response to pressure overload in adult mice. Nat. Cardiovasc. Res. 2022, 1, 577–591. [Google Scholar] [CrossRef]
- Chuntharpursat-Bon, E.; Povstyan, O.V.; Ludlow, M.J.; Carrier, D.J.; Debant, M.; Shi, J.; Gaunt, H.J.; Bauer, C.C.; Curd, A.; Simon Futers, T.; et al. PIEZO1 and PECAM1 interact at cell-cell junctions and partner in endothelial force sensing. Commun. Biol. 2023, 6, 358. [Google Scholar] [CrossRef]
- Peyronnet, R.; Martins, J.R.; Duprat, F.; Demolombe, S.; Arhatte, M.; Jodar, M.; Tauc, M.; Duranton, C.; Paulais, M.; Teulon, J.; et al. Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO Rep. 2013, 14, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, P.; Hütte, M.; Kudryasheva, G.; Taberner, F.J.; Lechner, S.G.; Rehfeldt, F.; Gomez-Varela, D.; Schmidt, M. Myotubularin related protein-2 and its phospholipid substrate PIP2 control Piezo2-mediated mechanotransduction in peripheral sensory neurons. Elife 2018, 7, e32346. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.S.; Qiu, Z.; Woo, S.H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B.; et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA. 2014, 111, 10347–10352. [Google Scholar] [CrossRef]
- Retailleau, K.; Duprat, F.; Arhatte, M.; Ranade, S.S.; Peyronnet, R.; Martins, J.R.; Jodar, M.; Moro, C.; Offermanns, S.; Feng, Y.; et al. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep. 2015, 13, 1161–1171. [Google Scholar] [CrossRef]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Le, D.T.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16148–16153. [Google Scholar] [CrossRef]
- Jin, Y.; Li, J.; Wang, Y.; Ye, R.; Feng, X.; Jing, Z.; Zhao, Z. Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. Angle Orthod. 2015, 85, 87–94. [Google Scholar] [CrossRef]
- Michishita, M.; Yano, K.; Tomita, K.I.; Matsuzaki, O.; Kasahara, K.I. Piezo1 expression increases in rat bladder after partial bladder outlet obstruction. Life Sci. 2016, 166, 1–7. [Google Scholar] [CrossRef]
- Syeda, R.; Florendo, M.N.; Cox, C.D.; Kefauver, J.M.; Santos, J.S.; Martinac, B.; Patapoutian, A. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016, 17, 1739–1746. [Google Scholar] [CrossRef]
- Procès, A.; Luciano, M.; Kalukula, Y.; Ris, L.; Gabriele, S. Multiscale Mechanobiology in Brain Physiology and Diseases. Front. Cell Dev. Biol. 2022, 10, 823857. [Google Scholar] [CrossRef]
- Wang, B.; Ke, W.; Wang, K.; Li, G.; Ma, L.; Lu, S.; Xiang, Q.; Liao, Z.; Luo, R.; Song, Y.; et al. Mechanosensitive Ion Channel Piezo1 Activated by Matrix Stiffness Regulates Oxidative Stress-Induced Senescence and Apoptosis in Human Intervertebral Disc Degeneration. Oxid. Med. Cell Longev. 2021, 2021, 8884922. [Google Scholar] [CrossRef]
- Zarychanski, R.; Schulz, V.P.; Houston, B.L.; Maksimova, Y.; Houston, D.S.; Smith, B.; Rinehart, J.; Gallagher, P.G. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood 2012, 120, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Lukacs, V.; Mathur, J.; Mao, R.; Bayrak-Toydemir, P.; Procter, M.; Cahalan, S.M.; Kim, H.J.; Bandell, M.; Longo, N.; Day, R.W.; et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat. Commun. 2015, 6, 8329. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Guo, X.; Wang, X.; Lin, H.; Yu, Y.; Shu, J.; Dong, M.; Yang, L. A Novel Homozygous Missense Mutation of PIEZO1 Leading to Lymphatic Malformation-6 Identified in a Family with Three Adverse Pregnancy Outcomes due to Nonimmune Fetal Hydrops. Front. Genet. 2022, 13, 856046. [Google Scholar] [CrossRef]
- Scimone, C.; Donato, L.; Alibrandi, S.; D’Angelo, R.; Sidoti, A. Evidences of PIEZO1 involvement in cerebral cavernous malformation pathogenesis. Microvasc. Res. 2022, 141, 104342. [Google Scholar] [CrossRef]
- Beurg, M.; Fettiplace, R. PIEZO2 as the anomalous mechanotransducer channel in auditory hair cells. J. Physiol. 2017, 595, 7039–7048. [Google Scholar] [CrossRef]
- von Buchholtz, L.J.; Ghitani, N.; Lam, R.M.; Licholai, J.A.; Chesler, A.T.; Ryba, N.J.P. Decoding Cellular Mechanisms for Mechanosensory Discrimination. Neuron 2021, 109, 285–298.e5. [Google Scholar] [CrossRef]
- McMillin, M.J.; Beck, A.E.; Chong, J.X.; Shively, K.M.; Buckingham, K.J.; Gildersleeve, H.I.; Aracena, M.I.; Aylsworth, A.S.; Bitoun, P.; Carey, J.C.; et al. Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am. J. Hum. Genet. 2014, 94, 734–744. [Google Scholar] [CrossRef]
- Chesler, A.T.; Szczot, M.; Bharucha-Goebel, D.; Čeko, M.; Donkervoort, S.; Laubacher, C.; Hayes, L.H.; Alter, K.; Zampieri, C.; Stanley, C.; et al. The Role of PIEZO2 in Human Mechanosensation. N. Engl. J. Med. 2016, 375, 1355–1364. [Google Scholar] [CrossRef]
- Delle Vedove, A.; Storbeck, M.; Heller, R.; Hölker, I.; Hebbar, M.; Shukla, A.; Magnusson, O.; Cirak, S.; Girisha, K.M.; O’Driscoll, M.; et al. Biallelic Loss of Proprioception-Related PIEZO2 Causes Muscular Atrophy with Perinatal Respiratory Distress, Arthrogryposis, and Scoliosis. Am. J. Hum. Genet. 2016, 99, 1206–1216. [Google Scholar] [CrossRef]
- Miyazawa, K.; Itoh, Y.; Fu, H.; Miyazono, K. Receptor-activated transcription factors and beyond: Multiple modes of Smad2/3-dependent transmission of TGF-β signaling. J. Biol. Chem. 2024, 300, 107256. [Google Scholar] [CrossRef]
- Saha, S.; Ji, L.; de Pablo, J.J.; Palecek, S.P. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys. J. 2008, 94, 4123–4133. [Google Scholar] [CrossRef] [PubMed]
- Grabias, B.M.; Konstantopoulos, K. Notch4-dependent antagonism of canonical TGF-β1 signaling defines unique temporal fluctuations of SMAD3 activity in sheared proximal tubular epithelial cells. Am. J. Physiol. Renal Physiol. 2013, 305, F123–F133. [Google Scholar] [CrossRef]
- Kunnen, S.J.; Leonhard, W.N.; Semeins, C.; Hawinkels, L.J.A.C.; Poelma, C.; Ten Dijke, P.; Bakker, A.; Hierck, B.P.; Peters, D.J.M. Fluid shear stress-induced TGF-β/ALK5 signaling in renal epithelial cells is modulated by MEK1/2. Cell Mol. Life Sci. 2017, 74, 2283–2298. [Google Scholar] [CrossRef] [PubMed]
- Maruri, D.P.; Iyer, K.S.; Schmidtke, D.W.; Petroll, W.M.; Varner, V.D. Signaling Downstream of Focal Adhesions Regulates Stiffness-Dependent Differences in the TGF-β1-Mediated Myofibroblast Differentiation of Corneal Keratocytes. Front. Cell Dev. Biol. 2022, 10, 886759. [Google Scholar] [CrossRef]
- Stutchbury, B.; Atherton, P.; Tsang, R.; Wang, D.Y.; Ballestrem, C. Distinct focal adhesion protein modules control different aspects of mechanotransduction. J. Cell Sci. 2017, 130, 1612–1624. [Google Scholar] [CrossRef]
- Boogerd, C.J.; Perini, I.; Kyriakopoulou, E.; Han, S.J.; La, P.; van der Swaan, B.; Berkhout, J.B.; Versteeg, D.; Monshouwer-Kloots, J.; van Rooij, E. Cardiomyocyte proliferation is suppressed by ARID1A-mediated YAP inhibition during cardiac maturation. Nat. Commun. 2023, 14, 4716. [Google Scholar] [CrossRef]
- Marin, T.M.; Clemente, C.F.; Santos, A.M.; Picardi, P.K.; Pascoal, V.D.; Lopes-Cendes, I.; Saad, M.J.; Franchini, K.G. Shp2 negatively regulates growth in cardiomyocytes by controlling focal adhesion kinase/Src and mTOR pathways. Circ. Res. 2008, 103, 813–824. [Google Scholar] [CrossRef]
- Baba, H.A.; Stypmann, J.; Grabellus, F.; Kirchhof, P.; Sokoll, A.; Schäfers, M.; Takeda, A.; Wilhelm, M.J.; Scheld, H.H.; Takeda, N.; et al. Dynamic regulation of MEK/Erks and Akt/GSK-3beta in human end-stage heart failure after left ventricular mechanical support: Myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc. Res. 2003, 59, 390–399. [Google Scholar] [CrossRef]
- Kishimoto, H.; Iwasaki, M.; Wada, K.; Horitani, K.; Tsukamoto, O.; Kamikubo, K.; Nomura, S.; Matsumoto, S.; Harada, T.; Motooka, D.; et al. Wnt5a-YAP signaling axis mediates mechanotransduction in cardiac myocytes and contributes to contractile dysfunction induced by pressure overload. iScience 2023, 26, 107146. [Google Scholar] [CrossRef]
- Rickman, M.; Ghim, M.; Pang, K.; von Huelsen Rocha, A.C.; Drudi, E.M.; Sureda-Vives, M.; Ayoub, N.; Tajadura-Ortega, V.; George, S.J.; Weinberg, P.D.; et al. Disturbed flow increases endothelial inflammation and permeability via a Frizzled-4-β-catenin-dependent pathway. J. Cell Sci. 2023, 136, jcs260449. [Google Scholar] [CrossRef]
- Paolini, A.; Fontana, F.; Pham, V.C.; Rödel, C.J.; Abdelilah-Seyfried, S. Mechanosensitive Notch-Dll4 and Klf2-Wnt9 signaling pathways intersect in guiding valvulogenesis in zebrafish. Cell Rep. 2021, 37, 109782. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.O.; Alkhafaji, A.T.; Mohammed, J.S.; Bansal, P.; Kaur, H.; Ahmad, I.; Hjazi, A.; Mohammed, I.H.; Jawad, M.A.; Zwamel, A.H. LncRNA NEAT1 in the pathogenesis of liver-related diseases. Cell Biochem. Funct. 2024, 42, e4006. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Wang, X.; Li, S.; Tang, L. Substrate Stiffness Drives Epithelial to Mesenchymal Transition and Proliferation through the NEAT1-Wnt/β-Catenin Pathway in Liver Cancer. Int. J. Mol. Sci. 2021, 22, 12066. [Google Scholar] [CrossRef]
- Hino, N.; Rossetti, L.; Marín-Llauradó, A.; Aoki, K.; Trepat, X.; Matsuda, M.; Hirashima, T. ERK-Mediated Mechanochemical Waves Direct Collective Cell Polarization. Dev. Cell 2020, 53, 646–660.e8. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Gupta, M.; Vedula, S.R.; Lim, C.T.; Ladoux, B.; Sokabe, M. Actomyosin bundles serve as a tension sensor and a platform for ERK activation. EMBO Rep. 2015, 16, 250–257. [Google Scholar] [CrossRef]
- Shaya, O.; Binshtok, U.; Hersch, M.; Rivkin, D.; Weinreb, S.; Amir-Zilberstein, L.; Khamaisi, B.; Oppenheim, O.; Desai, R.A.; Goodyear, R.J.; et al. Cell-Cell Contact Area Affects Notch Signaling and Notch-Dependent Patterning. Dev. Cell 2017, 40, 505–511.e6. [Google Scholar] [CrossRef]
- He, X.; Liu, Y.; Dai, Z.; Chen, Y.; Liu, W.; Dai, H.; Hu, Y. Yoda1 pretreated BMSC derived exosomes accelerate osteogenesis by activating phospho-ErK signaling via Yoda1-mediated signal transmission. J. Nanobiotechnol. 2024, 22, 407. [Google Scholar] [CrossRef]
- Zheng, M.; Yao, Y.; Borkar, N.A.; Thompson, M.A.; Zhang, E.; Drake, L.Y.; Ye, X.; Vogel, E.R.; Pabelick, C.M.; Prakash, Y.S. Piezo channels modulate human lung fibroblast function. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 327, L547–L556. [Google Scholar] [CrossRef]
- Siddiqui, H.B.; Golcez, T.; Çelik, M.; Sevgin, B.; Çoban, M.; Süder, İ.; Kaya, Ö.; Özören, N.; Pekkan, K. Modulation of mechanosensitive genes during embryonic aortic arch development. Dev. Dyn. 2024, 254, 222–239. [Google Scholar] [CrossRef]
- Okamoto, S.; Chaya, T.; Omori, Y.; Kuwahara, R.; Kubo, S.; Sakaguchi, H.; Furukawa, T. Ick Ciliary Kinase Is Essential for Planar Cell Polarity Formation in Inner Ear Hair Cells and Hearing Function. J. Neurosci. 2017, 37, 2073–2085. [Google Scholar] [CrossRef]
- Takacs, Z.; Proikas-Cezanne, T. Primary cilia mechanosensing triggers autophagy-regulated cell volume control. Nat. Cell Biol. 2016, 18, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Krenn, P.W.; Montanez, E.; Costell, M.; Fässler, R. Integrins, anchors and signal transducers of hematopoietic stem cells during development and in adulthood. Curr. Top Dev. Biol. 2022, 149, 203–261. [Google Scholar] [CrossRef] [PubMed]
- Brunet, T.; Bouclet, A.; Ahmadi, P.; Mitrossilis, D.; Driquez, B.; Brunet, A.C.; Henry, L.; Serman, F.; Béalle, G.; Ménager, C.; et al. Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat. Commun. 2013, 4, 2821. [Google Scholar] [CrossRef] [PubMed]
- Röper, J.C.; Mitrossilis, D.; Stirnemann, G.; Waharte, F.; Brito, I.; Fernandez-Sanchez, M.E.; Baaden, M.; Salamero, J.; Farge, E. The major β-catenin/E-cadherin junctional binding site is a primary molecular mechano-transductor of differentiation in vivo. Elife 2018, 7, e33381. [Google Scholar] [CrossRef]
- Ayad, N.M.E.; Lakins, J.N.; Ghagre, A.; Ehrlicher, A.J.; Weaver, V.M. Tissue tension permits β-catenin phosphorylation to drive mesoderm specification in human embryonic stem cells. bioRxiv 2023. [Google Scholar] [CrossRef]
- Blum, M.; Andre, P.; Muders, K.; Schweickert, A.; Fischer, A.; Bitzer, E.; Bogusch, S.; Beyer, T.; van Straaten, H.W.; Viebahn, C. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation 2007, 75, 133–146. [Google Scholar] [CrossRef]
- Sampaio, P.; Ferreira, R.R.; Guerrero, A.; Pintado, P.; Tavares, B.; Amaro, J.; Smith, A.A.; Montenegro-Johnson, T.; Smith, D.J.; Lopes, S.S. Left-right organizer flow dynamics: How much cilia activity reliably yields laterality? Dev. Cell 2014, 29, 716–728. [Google Scholar] [CrossRef]
- Nonaka, S.; Tanaka, Y.; Okada, Y.; Takeda, S.; Harada, A.; Kanai, Y.; Kido, M.; Hirokawa, N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 1998, 95, 829–837. [Google Scholar] [CrossRef]
- Axelrod, J.D. Planar cell polarity signaling in the development of left-right asymmetry. Curr. Opin. Cell Biol. 2020, 62, 61–69. [Google Scholar] [CrossRef]
- Kamura, K.; Kobayashi, D.; Uehara, Y.; Koshida, S.; Iijima, N.; Kudo, A.; Yokoyama, T.; Takeda, H. Pkd1l1 complexes with Pkd2 on motile cilia and functions to establish the left-right axis. Development 2011, 138, 1121–1129. [Google Scholar] [CrossRef]
- Yoshiba, S.; Shiratori, H.; Kuo, I.Y.; Kawasumi, A.; Shinohara, K.; Nonaka, S.; Asai, Y.; Sasaki, G.; Belo, J.A.; Sasaki, H.; et al. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 2012, 338, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.T.; Keynton, J.L.; Buenavista, M.T.; Jin, X.; Patel, S.H.; Kyosuke, S.; Vibert, J.; Williams, D.J.; Hamada, H.; Hussain, R.; et al. Genetic Analysis Reveals a Hierarchy of Interactions between Polycystin-Encoding Genes and Genes Controlling Cilia Function during Left-Right Determination. PLoS Genet. 2016, 12, e1006070. [Google Scholar] [CrossRef] [PubMed]
- Logan, M.; Pagán-Westphal, S.M.; Smith, D.M.; Paganessi, L.; Tabin, C.J. The transcription factor Pitx2 mediates situs-specific morphogenesis in response to left-right asymmetric signals. Cell 1998, 94, 307–317. [Google Scholar] [CrossRef]
- Katoh, T.A.; Omori, T.; Mizuno, K.; Sai, X.; Minegishi, K.; Ikawa, Y.; Nishimura, H.; Itabashi, T.; Kajikawa, E.; Hiver, S.; et al. Immotile cilia mechanically sense the direction of fluid flow for left-right determination. Science 2023, 379, 66–71. [Google Scholar] [CrossRef]
- Katoh, T.A.; Lange, T.; Nakajima, Y.; Yashiro, K.; Okada, Y.; Hamada, H. BMP4 regulates asymmetric Pkd2 distribution in mouse nodal immotile cilia and ciliary mechanosensing required for left-right determination. Dev. Dyn. 2024, 10, 1–14. [Google Scholar] [CrossRef]
- Schwayer, C.; Shamipour, S.; Pranjic-Ferscha, K.; Schauer, A.; Balda, M.; Tada, M.; Matter, K.; Heisenberg, C.P. Mechanosensation of Tight Junctions Depends on ZO-1 Phase Separation and Flow. Cell 2019, 179, 937–952.e18. [Google Scholar] [CrossRef]
- Davidson, L.A.; Marsden, M.; Keller, R.; Desimone, D.W. Integrin alpha5beta1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr. Biol. 2006, 16, 833–844. [Google Scholar] [CrossRef]
- Marsden, M.; DeSimone, D.W. Integrin-ECM interactions regulate cadherin-dependent cell adhesion and are required for convergent extension in Xenopus. Curr. Biol. 2003, 13, 1182–1191. [Google Scholar] [CrossRef]
- Thompson, A.J.; Pillai, E.K.; Dimov, I.B.; Foster, S.K.; Holt, C.E.; Franze, K. Rapid changes in tissue mechanics regulate cell behaviour in the developing embryonic brain. Elife 2019, 8, e39356. [Google Scholar] [CrossRef]
- Barriga, E.H.; Franze, K.; Charras, G.; Mayor, R. Tissue stiffening coordinates morphogenesis by triggering collective cell migration in vivo. Nature 2018, 554, 523–527. [Google Scholar] [CrossRef]
- Shellard, A.; Mayor, R. Integrating chemical and mechanical signals in neural crest cell migration. Curr. Opin. Genet. Dev. 2019, 57, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Canales Coutiño, B.; Mayor, R. Neural crest mechanosensors: Seeing old proteins in a new light. Dev. Cell 2022, 57, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Q.; Xiao, Q.; Gong, P.; Kang, N. CHD7 Regulates Osteogenic Differentiation of Human Dental Follicle Cells via PTH1R Signaling. Stem Cells Int. 2020, 2020, 8882857. [Google Scholar] [CrossRef] [PubMed]
- Camargo-Sosa, K.; Colanesi, S.; Müller, J.; Schulte-Merker, S.; Stemple, D.; Patton, E.E.; Kelsh, R.N. Endothelin receptor Aa regulates proliferation and differentiation of Erb-dependent pigment progenitors in zebrafish. PLoS Genet. 2019, 15, e1007941. [Google Scholar] [CrossRef]
- Balczerski, B.; Matsutani, M.; Castillo, P.; Osborne, N.; Stainier, D.Y.; Crump, J.G. Analysis of sphingosine-1-phosphate signaling mutants reveals endodermal requirements for the growth but not dorsoventral patterning of jaw skeletal precursors. Dev. Biol. 2012, 362, 230–241. [Google Scholar] [CrossRef]
- Canales Coutiño, B.; Mayor, R. The mechanosensitive channel Piezo1 cooperates with semaphorins to control neural crest migration. Development 2021, 148, dev200001. [Google Scholar] [CrossRef]
- Cheng, C.; Cong, Q.; Liu, Y.; Hu, Y.; Liang, G.; Dioneda, K.M.M.; Yang, Y. Yap controls notochord formation and neural tube patterning by integrating mechanotransduction with FoxA2 and Shh expression. Sci. Adv. 2023, 9, eadf6927. [Google Scholar] [CrossRef]
- Voltes, A.; Hevia, C.F.; Engel-Pizcueta, C.; Dingare, C.; Calzolari, S.; Terriente, J.; Norden, C.; Lecaudey, V.; Pujades, C. Yap/Taz-TEAD activity links mechanical cues to progenitor cell behavior during zebrafish hindbrain segmentation. Development 2019, 146, dev176735. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Janairo, R.R.R.; Kwong, G.; Tsou, A.D.; Chu, J.S.; Wang, A.; Yu, J.; Wang, D.; Li, S. Matrix stiffness modulates the differentiation of neural crest stem cells in vivo. J. Cell Physiol. 2019, 234, 7569–7578. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Wilson, R.E.; Denisin, A.K.; Dunn, A.R.; Pruitt, B.L. 3D Microwell Platforms for Control of Single Cell 3D Geometry and Intracellular Organization. Cell Mol. Bioeng. 2020, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Saez, P.; Shirke, P.U.; Seth, J.R.; Alegre-Cebollada, J.; Majumder, A. Competing elastic and viscous gradients determine directional cell migration. Math Biosci. 2025, 380, 109362. [Google Scholar] [CrossRef]
- Rolfe, R.A.; Kenny, E.M.; Cormican, P.; Murphy, P. Transcriptome analysis of the mouse E14.5 (TS23) developing humerus and differential expression in muscle-less mutant embryos lacking mechanical stimulation. Genom. Data 2014, 2, 32–36. [Google Scholar] [CrossRef]
- Arvind, V.; Huang, A.H. Mechanobiology of limb musculoskeletal development. Ann. N. Y. Acad. Sci. 2017, 1409, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.H.; Carter, D.R. Mechanical induction in limb morphogenesis: The role of growth-generated strains and pressures. Bone 2002, 31, 645–653. [Google Scholar] [CrossRef]
- Foolen, J.; van Donkelaar, C.C.; Ito, K. Intracellular tension in periosteum/perichondrium cells regulates long bone growth. J. Orthop. Res. 2011, 29, 84–91. [Google Scholar] [CrossRef]
- Mikic, B.; Johnson, T.L.; Chhabra, A.B.; Schalet, B.J.; Wong, M.; Hunziker, E.B. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J. Rehabil. Res. Dev. 2000, 37, 127–133. [Google Scholar]
- Subramanian, A.; Kanzaki, L.F.; Schilling, T.F. Mechanical force regulates Sox9 expression at the developing enthesis. Development 2023, 150, dev201141. [Google Scholar] [CrossRef]
- Vermeulen, S.; Roumans, N.; Honig, F.; Carlier, A.; Hebels, D.G.A.J.; Eren, A.D.; Dijke, P.T.; Vasilevich, A.; de Boer, J. Mechanotransduction is a context-dependent activator of TGF-β signaling in mesenchymal stem cells. Biomaterials 2020, 259, 120331. [Google Scholar] [CrossRef]
- Langen, U.H.; Pitulescu, M.E.; Kim, J.M.; Enriquez-Gasca, R.; Sivaraj, K.K.; Kusumbe, A.P.; Singh, A.; Di Russo, J.; Bixel, M.G.; Zhou, B.; et al. Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. Nat. Cell Biol. 2017, 19, 189–201. [Google Scholar] [CrossRef]
- Wein, M.N. Blood and bones: Mechanical cues and Hippo signaling drive vascular invasion during limb formation. Dev. Cell 2024, 59, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Paivinen, P.; Xie, C.; Krup, A.L.; Makela, T.P.; Mostov, K.E.; Reiter, J.F. Ciliary Hedgehog signaling patterns the digestive system to generate mechanical forces driving elongation. Nat. Commun. 2021, 12, 7186. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Adamopoulos, C.; Vottis, C.T.; Papavassiliou, A.G.; Basdra, E.K. Runx2 and Polycystins in Bone Mechanotransduction: Challenges for Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 5291. [Google Scholar] [CrossRef] [PubMed]
- Shewale, B.; Ebrahim, T.; Samal, A.; Dubois, N. Molecular Regulation of Cardiomyocyte Maturation. Curr. Cardiol. Rep. 2025, 27, 32. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Z.; He, S.; Wang, C.; Li, R.; Zhang, R.; Li, J.; Yang, Z.; Li, H.; Liu, S.; et al. Developmental dynamics mimicking inversely engineered pericellular matrix for articular cartilage regeneration. Biomaterials 2025, 317, 123066. [Google Scholar] [CrossRef]
- Estévez, M.; Cicuéndez, M.; Colilla, M.; Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Magnetic colloidal nanoformulations to remotely trigger mechanotransduction for osteogenic differentiation. J. Colloid Interface Sci. 2024, 664, 454–468. [Google Scholar] [CrossRef]
- Ihalainen, T.O.; Aires, L.; Herzog, F.A.; Schwartlander, R.; Moeller, J.; Vogel, V. Differential basal-to-apical accessibility of lamin A/C epitopes in the nuclear lamina regulated by changes in cytoskeletal tension. Nat. Mater. 2015, 14, 1252–1261. [Google Scholar] [CrossRef]
- Sturm, G.; Cardenas, A.; Bind, M.A.; Horvath, S.; Wang, S.; Wang, Y.; Hägg, S.; Hirano, M.; Picard, M. Human aging DNA methylation signatures are conserved but accelerated in cultured fibroblasts. Epigenetics 2019, 14, 961–976. [Google Scholar] [CrossRef]
- Beedle, A.E.; Roca-Cusachs, P. The reversibility of cellular mechano-activation. Curr. Opin. Cell Biol. 2023, 84, 102229. [Google Scholar] [CrossRef]
- Dudaryeva, O.Y.; Bernhard, S.; Tibbitt, M.W.; Labouesse, C. Implications of Cellular Mechanical Memory in Bioengineering. ACS Biomater. Sci. Eng. 2023, 9, 5985–5998. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Sorboni, S.G.; Ranjbar, N.; Deyhimfar, R.; Abtahi, M.S.; Izady, M.; Kazemi, N.; Noori, A.; Pennisi, C.P. Mechanotransduction in tissue engineering: Insights into the interaction of stem cells with biomechanical cues. Exp. Cell Res. 2023, 431, 113766. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewicz, A.; Secomb, T.W.; Pries, A.R. Angioadaptation: Keeping the vascular system in shape. News Physiol. Sci. 2002, 17, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Angiogenesis and endothelial cell function. Arzneimittelforschung 1994, 44, 416–417. [Google Scholar]
- Bentley, K.; Gerhardt, H.; Bates, P.A. Agent-based simulation of notch-mediated tip cell selection in angiogenic sprout initialisation. J. Theor. Biol. 2008, 250, 25–36. [Google Scholar] [CrossRef]
- Hiepen, C.; Benamar, M.; Barrasa-Fano, J.; Condor, M.; Ilhan, M.; Münch, J.; Hastar, N.; Kerkhoff, Y.; Harms, G.S.; Mielke, T.; et al. Endothelial tip-cell position, filopodia formation and biomechanics require BMPR2 expression and signaling. Commun. Biol. 2025, 8, 21. [Google Scholar] [CrossRef]
- Ramirez, F.; Caescu, C.; Wondimu, E.; Galatioto, J. Marfan syndrome; A connective tissue disease at the crossroads of mechanotransduction, TGFβ signaling and cell stemness. Matrix Biol. 2018, 71, 82–89. [Google Scholar] [CrossRef]
- Alonso, F.; Dong, Y.; Li, L.; Jahjah, T.; Dupuy, J.W.; Fremaux, I.; Reinhardt, D.P.; Génot, E. Fibrillin-1 regulates endothelial sprouting during angiogenesis. Proc. Natl. Acad. Sci. USA 2023, 120, e2221742120. [Google Scholar] [CrossRef]
- Wang, W.; Zanotelli, M.R.; Sabo, L.N.; Fabiano, E.D.; Goldfield, N.M.; Le, C.; Techasiriwan, E.P.; Lopez, S.; Berestesky, E.D.; Reinhart-King, C.A. Collagen density regulates tip-stalk cell rearrangement during angiogenesis via cellular bioenergetics. APL Bioeng. 2024, 8, 026120. [Google Scholar] [CrossRef]
- Vaeyens, M.M.; Jorge-Peñas, A.; Barrasa-Fano, J.; Steuwe, C.; Heck, T.; Carmeliet, P.; Roeffaers, M.; Van Oosterwyck, H. Matrix deformations around angiogenic sprouts correlate to sprout dynamics and suggest pulling activity. Angiogenesis 2020, 23, 315–324. [Google Scholar] [CrossRef]
- Ahmed, T.; Ramonett, A.; Kwak, E.A.; Kumar, S.; Flores, P.C.; Ortiz, H.R.; Langlais, P.R.; Hund, T.J.; Mythreye, K.; Lee, N.Y. Endothelial tip/stalk cell selection requires BMP9-induced βIV-spectrin expression during sprouting angiogenesis. Mol. Biol. Cell 2023, 34, ar72. [Google Scholar] [CrossRef]
- Carvalho, J.R.; Fortunato, I.C.; Fonseca, C.G.; Pezzarossa, A.; Barbacena, P.; Dominguez-Cejudo, M.A.; Vasconcelos, F.F.; Santos, N.C.; Carvalho, F.A.; Franco, C.A. Non-canonical Wnt signaling regulates junctional mechanocoupling during angiogenic collective cell migration. Elife 2019, 8, e45853. [Google Scholar] [CrossRef] [PubMed]
- Burri, P.H.; Djonov, V. Intussusceptive angiogenesis--the alternative to capillary sprouting. Mol. Asp. Med. 2002, 23, S1–S27. [Google Scholar] [CrossRef] [PubMed]
- le Noble, F.; Moyon, D.; Pardanaud, L.; Yuan, L.; Djonov, V.; Matthijsen, R.; Bréant, C.; Fleury, V.; Eichmann, A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development 2004, 131, 361–375. [Google Scholar] [CrossRef]
- Perryn, E.D.; Czirók, A.; Little, C.D. Vascular sprout formation entails tissue deformations and VE-cadherin-dependent cell-autonomous motility. Dev. Biol. 2008, 313, 545–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gajdusek, C.M.; Luo, Z.; Mayberg, M.R. Basic fibroblast growth factor and transforming growth factor beta-1: Synergistic mediators of angiogenesis in vitro. J. Cell Physiol. 1993, 157, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Padget, R.L.; Mohite, S.S.; Hoog, T.G.; Justis, B.S.; Green, B.E.; Udan, R.S. Hemodynamic force is required for vascular smooth muscle cell recruitment to blood vessels during mouse embryonic development. Mech. Dev. 2019, 156, 8–19. [Google Scholar] [CrossRef]
- Lenard, A.; Daetwyler, S.; Betz, C.; Ellertsdottir, E.; Belting, H.G.; Huisken, J.; Affolter, M. Endothelial cell self-fusion during vascular pruning. PLoS Biol. 2015, 13, e1002126. [Google Scholar] [CrossRef]
- Sugden, W.W.; Meissner, R.; Aegerter-Wilmsen, T.; Tsaryk, R.; Leonard, E.V.; Bussmann, J.; Hamm, M.J.; Herzog, W.; Jin, Y.; Jakobsson, L.; et al. Endoglin controls blood vessel diameter through endothelial cell shape changes in response to haemodynamic cues. Nat. Cell Biol. 2017, 19, 653–665. [Google Scholar] [CrossRef]
- Meeson, A.P.; Argilla, M.; Ko, K.; Witte, L.; Lang, R.A. VEGF deprivation-induced apoptosis is a component of programmed capillary regression. Development 1999, 126, 1407–1415. [Google Scholar] [CrossRef]
- Nieminen, T.; Toivanen, P.I.; Rintanen, N.; Heikura, T.; Jauhiainen, S.; Airenne, K.J.; Alitalo, K.; Marjomäki, V.; Ylä-Herttuala, S. The impact of the receptor binding profiles of the vascular endothelial growth factors on their angiogenic features. Biochim. Biophys. Acta 2014, 1840, 454–463. [Google Scholar] [CrossRef]
- Echarri, A.; Del Pozo, M.A. Caveolae—Mechanosensitive membrane invaginations linked to actin filaments. J. Cell Sci. 2015, 128, 2747–2758. [Google Scholar] [CrossRef] [PubMed]
- Givens, C.; Tzima, E. Endothelial Mechanosignaling: Does One Sensor Fit All? Antioxid. Redox Signal 2016, 25, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Browning, E.A.; Hong, N.; DeBolt, K.; Sorokina, E.M.; Liu, W.; Birnbaum, M.J.; Fisher, A.B. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H105–H114. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Fisher, A.B. Mechanotransduction in the endothelium: Role of membrane proteins and reactive oxygen species in sensing, transduction, and transmission of the signal with altered blood flow. Antioxid. Redox Signal. 2014, 20, 899–913. [Google Scholar] [CrossRef]
- Takeda, H.; Komori, K.; Nishikimi, N.; Nimura, Y.; Sokabe, M.; Naruse, K. Bi-phasic activation of eNOS in response to uni-axial cyclic stretch is mediated by differential mechanisms in BAECs. Life Sci. 2006, 79, 233–239. [Google Scholar] [CrossRef]
- Gomes, A.M.; Pinto, T.S.; da Costa Fernandes, C.J.; da Silva, R.A.; Zambuzzi, W.F. Wortmannin targeting phosphatidylinositol 3-kinase suppresses angiogenic factors in shear-stressed endothelial cells. J. Cell Physiol. 2020, 235, 5256–5269. [Google Scholar] [CrossRef]
- Cronin, N.M.; Dawson, L.W.; DeMali, K.A. Mechanical activation of VE-cadherin stimulates AMPK to increase endothelial cell metabolism and vasodilation. bioRxiv 2024. [Google Scholar] [CrossRef]
- LaValley, D.J.; Zanotelli, M.R.; Bordeleau, F.; Wang, W.; Schwager, S.C.; Reinhart-King, C.A. Matrix Stiffness Enhances VEGFR-2 Internalization, Signaling, and Proliferation in Endothelial Cells. Converg. Sci. Phys. Oncol. 2017, 3, 044001. [Google Scholar] [CrossRef]
- Labrecque, L.; Royal, I.; Surprenant, D.S.; Patterson, C.; Gingras, D.; Béliveau, R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol. Biol. Cell. 2003, 14, 334–347. [Google Scholar] [CrossRef]
- Shin, H.; Haga, J.H.; Kosawada, T.; Kimura, K.; Li, Y.S.; Chien, S.; Schmid-Schönbein, G.W. Fine control of endothelial VEGFR-2 activation: Caveolae as fluid shear stress shelters for membrane receptors. Biomech. Model Mechanobiol. 2019, 18, 5–16. [Google Scholar] [CrossRef]
- Zhao, C.; Kam, H.T.; Chen, Y.; Gong, G.; Hoi, M.P.; Skalicka-Woźniak, K.; Dias, A.C.P.; Lee, S.M. Crocetin and Its Glycoside Crocin, Two Bioactive Constituents from Crocus sativus L. (Saffron), Differentially Inhibit Angiogenesis by Inhibiting Endothelial Cytoskeleton Organization and Cell Migration Through VEGFR2/SRC/FAK and VEGFR2/MEK/ERK Signaling Pathways. Front. Pharmacol. 2021, 12, 675359. [Google Scholar] [CrossRef]
- Matsuo, E.; Okamoto, T.; Ito, A.; Kawamoto, E.; Asanuma, K.; Wada, K.; Shimaoka, M.; Takao, M.; Shimamoto, A. Substrate stiffness modulates endothelial cell function via the YAP-Dll4-Notch1 pathway. Exp. Cell Res. 2021, 408, 112835. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, M.; Mamistvalov, R.; Sprinzak, D.; Vollmar, A.M.; Zahler, S. Matrix stiffness regulates Notch signaling activity in endothelial cells. J. Cell Sci. 2023, 136, jcs260442. [Google Scholar] [CrossRef] [PubMed]
- Mack, J.J.; Mosqueiro, T.S.; Archer, B.J.; Jones, W.M.; Sunshine, H.; Faas, G.C.; Briot, A.; Aragón, R.L.; Su, T.; Romay, M.C.; et al. NOTCH1 is a mechanosensor in adult arteries. Nat. Commun. 2017, 8, 1620. [Google Scholar] [CrossRef]
- Ruter, D.L.; Liu, Z.; Ngo, K.M.; Shaka, X.; Marvin, A.; Buglak, D.B.; Kidder, E.J.; Bautch, V.L. SMAD6 transduces endothelial cell flow responses required for blood vessel homeostasis. Angiogenesis 2021, 24, 387–398. [Google Scholar] [CrossRef]
- Hooglugt, A.; van der Stoel, M.M.; Shapeti, A.; Neep, B.F.; de Haan, A.; van Oosterwyck, H.; Boon, R.A.; Huveneers, S. DLC1 promotes mechanotransductive feedback for YAP via RhoGAP-mediated focal adhesion turnover. J. Cell Sci. 2024, 137, jcs261687. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef]
- Wang, K.C.; Yeh, Y.T.; Nguyen, P.; Limqueco, E.; Lopez, J.; Thorossian, S.; Guan, K.L.; Li, Y.J.; Chien, S. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc. Natl. Acad. Sci. USA 2016, 113, 11525–11530. [Google Scholar] [CrossRef]
- Rüdiger, D.; Kick, K.; Goychuk, A.; Vollmar, A.M.; Frey, E.; Zahler, S. Cell-Based Strain Remodeling of a Nonfibrous Matrix as an Organizing Principle for Vasculogenesis. Cell Rep. 2020, 32, 108015. [Google Scholar] [CrossRef]
- Kwon, H.B.; Wang, S.; Helker, C.S.; Rasouli, S.J.; Maischein, H.M.; Offermanns, S.; Herzog, W.; Stainier, D.Y. In vivo modulation of endothelial polarization by Apelin receptor signalling. Nat. Commun. 2016, 7, 11805. [Google Scholar] [CrossRef]
- Hoefer, I.E.; den Adel, B.; Daemen, M.J. Biomechanical factors as triggers of vascular growth. Cardiovasc. Res. 2013, 99, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.S.; Kim, K.; Hill, M.A. Regulation of blood flow in small arteries: Mechanosensory events underlying myogenic vasoconstriction. J. Exerc. Rehabil. 2020, 16, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Chen, M.; Prendergast, A.; Zhuang, Z.; Nasiri, A.; Joshi, D.; Hintzen, J.; Chung, M.; Kumar, A.; Mani, A.; et al. Latrophilin-2 mediates fluid shear stress mechanotransduction at endothelial junctions. EMBO J. 2024, 43, 3175–3191. [Google Scholar] [CrossRef]
- Yoshino, D.; Funamoto, K.; Sato, K.; Kenry Sato, M.; Lim, C.T. Hydrostatic pressure promotes endothelial tube formation through aquaporin 1 and Ras-ERK signaling. Commun. Biol. 2020, 3, 152. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ando, J. Endothelial cell and model membranes respond to shear stress by rapidly decreasing the order of their lipid phases. J. Cell Sci. 2013, 126, 1227–1234. [Google Scholar] [CrossRef]
- Lundbaek, J.A.; Collingwood, S.A.; Ingólfsson, H.I.; Kapoor, R.; Andersen, O.S. Lipid bilayer regulation of membrane protein function: Gramicidin channels as molecular force probes. J. R. Soc. Interface 2010, 7, 373–395. [Google Scholar] [CrossRef]
- Sun, X.; Fu, Y.; Gu, M.; Zhang, L.; Li, D.; Li, H.; Chien, S.; Shyy, J.Y.; Zhu, Y. Activation of integrin α5 mediated by flow requires its translocation to membrane lipid rafts in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 2016, 113, 769–774. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ando, J. Emerging Role of Plasma Membranes in Vascular Endothelial Mechanosensing. Circ. J. 2018, 82, 2691–2698. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Thang, B.Q.; Ramirez, K.; Shin, S.J.; Kohata, T.; Ohata, S.; Nguyen, T.A.V.; Ohtsuki, S.; Nagayama, K.; Yanagisawa, H. Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc. Natl. Acad. Sci. USA 2020, 117, 9896–9905. [Google Scholar] [CrossRef]
- Kotini, M.P.; van der Stoel, M.M.; Yin, J.; Han, M.K.; Kirchmaier, B.; de Rooij, J.; Affolter, M.; Huveneers, S.; Belting, H.G. Vinculin controls endothelial cell junction dynamics during vascular lumen formation. Cell Rep. 2022, 39, 110658. [Google Scholar] [CrossRef]
- van der Stoel, M.M.; Kotini, M.P.; Schoon, R.M.; Affolter, M.; Belting, H.G.; Huveneers, S. Vinculin strengthens the endothelial barrier during vascular development. Vasc. Biol. 2023, 5, e220012. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Tsai, H.Y.; Tsai, S.H.; Chu, P.H.; Huang, P.H.; Chen, J.W.; Lin, S.J. Deletion of the FHL2 gene attenuates intima-media thickening in a partially ligated carotid artery ligated mouse model. J. Cell Mol. Med. 2020, 24, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Devany, J.; Kim, H.R.; van Bodegraven, E.; Chmiel, T.; Tzu-Pin, S.; Chou, W.H.; Fang, Y.; Gardel, M.L. Mechanosensitive FHL2 tunes endothelial function. bioRxiv 2024. [Google Scholar] [CrossRef]
- Suresh Babu, S.; Wojtowicz, A.; Freichel, M.; Birnbaumer, L.; Hecker, M.; Cattaruzza, M. Mechanism of stretch-induced activation of the mechanotransducer zyxin in vascular cells. Sci. Signal 2012, 5, ra91. [Google Scholar] [CrossRef]
- Smyth, J.T.; Hwang, S.Y.; Tomita, T.; DeHaven, W.I.; Mercer, J.C.; Putney, J.W. Activation and regulation of store-operated calcium entry. J. Cell Mol. Med. 2010, 14, 2337–2349. [Google Scholar] [CrossRef]
- Thakore, P.; Earley, S. Transient Receptor Potential Channels and Endothelial Cell Calcium Signaling. Compr. Physiol. 2019, 9, 1249–1277. [Google Scholar] [CrossRef]
- Savage, A.M.; Kurusamy, S.; Chen, Y.; Jiang, Z.; Chhabria, K.; MacDonald, R.B.; Kim, H.R.; Wilson, H.L.; van Eeden, F.J.M.; Armesilla, A.L.; et al. tmem33 is essential for VEGF-mediated endothelial calcium oscillations and angiogenesis. Nat. Commun. 2019, 10, 732. [Google Scholar] [CrossRef]
- Antigny, F.; Girardin, N.; Frieden, M. Transient receptor potential canonical channels are required for in vitro endothelial tube formation. J. Biol. Chem. 2012, 287, 5917–5927. [Google Scholar] [CrossRef]
- Qin, W.; Xie, W.; Xia, N.; He, Q.; Sun, T. Silencing of Transient Receptor Potential Channel 4 Alleviates oxLDL-induced Angiogenesis in Human Coronary Artery Endothelial Cells by Inhibition of VEGF and NF-κB. Med. Sci. Monit. 2016, 22, 930–936. [Google Scholar] [CrossRef]
- Song, H.B.; Jun, H.O.; Kim, J.H.; Fruttiger, M.; Kim, J.H. Suppression of transient receptor potential canonical channel 4 inhibits vascular endothelial growth factor-induced retinal neovascularization. Cell Calcium 2015, 57, 101–108. [Google Scholar] [CrossRef]
- Maltaneri, R.E.; Schiappacasse, A.; Chamorro, M.E.; Nesse, A.B.; Vittori, D.C. Participation of membrane calcium channels in erythropoietin-induced endothelial cell migration. Eur. J. Cell Biol. 2018, 97, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.W.; Han, F.; Tauseef, M.; Birnbaumer, L.; Mehta, D.; Muller, W.A. TRPC6 is the endothelial calcium channel that regulates leukocyte transendothelial migration during the inflammatory response. J. Exp. Med. 2015, 212, 1883–1899. [Google Scholar] [CrossRef]
- Solano, A.S.; Lavanderos, B.; Metwally, E.; Earley, S. Transient Receptor Potential Channels in Vascular Mechanotransduction. Am. J. Hypertens. 2024, 23, hpae134. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Zheng, J. Proton block of proton-activated TRPV1 current. J. Gen. Physiol. 2015, 146, 147–159. [Google Scholar] [CrossRef]
- Parpaite, T.; Cardouat, G.; Mauroux, M.; Gillibert-Duplantier, J.; Robillard, P.; Quignard, J.F.; Marthan, R.; Savineau, J.P.; Ducret, T. Effect of hypoxia on TRPV1 and TRPV4 channels in rat pulmonary arterial smooth muscle cells. Pflugers Arch. 2016, 468, 111–130. [Google Scholar] [CrossRef]
- Su, L.; Zhang, Y.; He, K.; Wei, S.; Pei, H.; Wang, Q.; Yang, D.; Yang, Y. Activation of transient receptor potential vanilloid 1 accelerates re-endothelialization and inhibits neointimal formation after vascular injury. J. Vasc. Surg. 2017, 65, 197–205.e2. [Google Scholar] [CrossRef]
- Lu, Q.; Zemskov, E.A.; Sun, X.; Wang, H.; Yegambaram, M.; Wu, X.; Garcia-Flores, A.; Song, S.; Tang, H.; Kangath, A.; et al. Activation of the mechanosensitive Ca2+ channel TRPV4 induces endothelial barrier permeability via the disruption of mitochondrial bioenergetics. Redox Biol. 2021, 38, 101785. [Google Scholar] [CrossRef]
- Suresh, K.; Servinsky, L.; Jiang, H.; Bigham, Z.; Yun, X.; Kliment, C.; Huetsch, J.; Damarla, M.; Shimoda, L.A. Reactive oxygen species induced Ca2+ influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L893–L907. [Google Scholar] [CrossRef]
- O’Leary, C.; McGahon, M.K.; Ashraf, S.; McNaughten, J.; Friedel, T.; Cincolà, P.; Barabas, P.; Fernandez, J.A.; Stitt, A.W.; McGeown, J.G.; et al. Involvement of TRPV1 and TRPV4 Channels in Retinal Angiogenesis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3297–3309. [Google Scholar] [CrossRef]
- Cappelli, H.C.; Guarino, B.D.; Kanugula, A.K.; Adapala, R.K.; Perera, V.; Smith, M.A.; Paruchuri, S.; Thodeti, C.K. Transient receptor potential vanilloid 4 channel deletion regulates pathological but not developmental retinal angiogenesis. J. Cell Physiol. 2021, 236, 3770–3779. [Google Scholar] [CrossRef] [PubMed]
- Dragoni, S.; Guerra, G.; Fiorio Pla, A.; Bertoni, G.; Rappa, A.; Poletto, V.; Bottino, C.; Aronica, A.; Lodola, F.; Cinelli, M.P.; et al. A functional transient receptor potential vanilloid 4 (TRPV4) channel is expressed in human endothelial progenitor cells. J. Cell Physiol. 2015, 230, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, H.C.; Kanugula, A.K.; Adapala, R.K.; Amin, V.; Sharma, P.; Midha, P.; Paruchuri, S.; Thodeti, C.K. Mechanosensitive TRPV4 channels stabilize VE-cadherin junctions to regulate tumor vascular integrity and metastasis. Cancer Lett. 2019, 442, 15–20. [Google Scholar] [CrossRef]
- Lamalice, L.; Houle, F.; Huot, J. Phosphorylation of Tyr1214 within VEGFR-2 triggers the recruitment of Nck and activation of Fyn leading to SAPK2/p38 activation and endothelial cell migration in response to VEGF. J. Biol. Chem. 2006, 281, 34009–34020. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Chen, Y.; Wang, Y.; Li, X.; Hao, A.; Hu, Y.; Li, X. Correlation of vascular change with TRPV1, TRPV4, and TRPA1 in a rat model of inferior gluteal artery perforator flap. Wound Repair Regen. 2022, 30, 365–375. [Google Scholar] [CrossRef]
- Amoakon, J.P.; Lee, J.; Liyanage, P.; Arora, K.; Karlstaedt, A.; Mylavarapu, G.; Amin, R.; Naren, A.P. Defective CFTR modulates mechanosensitive channels TRPV4 and PIEZO1 and drives endothelial barrier failure. iScience 2024, 27, 110703. [Google Scholar] [CrossRef]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 integration of vascular architecture with physiological force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, G.; Jiang, S.; Ning, Y.; Deng, B.; Pan, X.; Liu, S.; He, Y.; Zhang, L.; Wan, R.; et al. The mechanosensitive Piezo1 orchestrating angiogenesis is essential in bone fracture repair. biorXiv 2019. [Google Scholar] [CrossRef]
- Abello, J.; Yin, Y.; Zhao, Y.; Maurer, J.; Lee, J.; Bodell, C.; Clevenger, A.J.; Burton, Z.; Goeckel, M.E.; Lin, M.; et al. Endothelial cell Piezo1 promotes vascular smooth muscle cell differentiation on large arteries. bioRxiv 2024. [Google Scholar] [CrossRef]
- Lai, A.; Zhou, Y.; Thurgood, P.; Chheang, C.; Chandra Sekar, N.; Nguyen, N.; Peter, K.; Khoshmanesh, K.; Baratchi, S. Endothelial Response to the Combined Biomechanics of Vessel Stiffness and Shear Stress Is Regulated via Piezo1. ACS Appl. Mater. Interfaces 2023, 15, 59103–59116. [Google Scholar] [CrossRef]
- Kang, H.; Hong, Z.; Zhong, M.; Klomp, J.; Bayless, K.J.; Mehta, D.; Karginov, A.V.; Hu, G.; Malik, A.B. Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am. J. Physiol. Cell Physiol. 2019, 316, C92–C103. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, Y.X.; Bu, W.J.; Li, P.; Chen, J.H.; Cao, M.; Dong, Y.C.; Sun, Z.J.; Dong, D.L. Piezo1 channel activation stimulates ATP production through enhancing mitochondrial respiration and glycolysis in vascular endothelial cells. Br. J. Pharmacol. 2023, 180, 1862–1877. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, M.; Jeon, N.L. Three-dimensional biomimetic model to reconstitute sprouting lymphangiogenesis in vitro. Biomaterials 2016, 78, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Park, E.; Yu, R.P.; Cooper, M.N.; Cho, I.T.; Choi, J.; Yu, J.; Zhao, L.; Yum, J.I.; Yu, J.S.; et al. Piezo1-Regulated Mechanotransduction Controls Flow-Activated Lymphatic Expansion. Circ. Res. 2022, 131, e2–e21. [Google Scholar] [CrossRef]
- Grimm, L.; Mason, E.; Yu, H.; Dudczig, S.; Panara, V.; Chen, T.; Bower, N.I.; Paterson, S.; Galeano, M.R.; Kobayashi, S.; et al. Single-cell analysis of lymphatic endothelial cell fate specification and differentiation during zebrafish development. EMBO J. 2023, 42, e112590. [Google Scholar] [CrossRef]
- Choi, D.; Park, E.; Jung, E.; Seong, Y.J.; Yoo, J.; Lee, E.; Hong, M.; Lee, S.; Ishida, H.; Burford, J.; et al. Laminar flow downregulates Notch activity to promote lymphatic sprouting. J. Clin. Investig. 2017, 127, 1225–1240. [Google Scholar] [CrossRef]
- Choi, D.; Park, E.; Jung, E.; Seong, Y.J.; Hong, M.; Lee, S.; Burford, J.; Gyarmati, G.; Peti-Peterdi, J.; Srikanth, S.; et al. ORAI1 Activates Proliferation of Lymphatic Endothelial Cells in Response to Laminar Flow Through Krüppel-Like Factors 2 and 4. Circ. Res. 2017, 120, 1426–1439. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, H.; Fang, Y.; Wu, G.; Chen, Q.; Xue, B.; Xu, R.; Zheng, K.; Jiang, H. Matrix Stiffness of GelMA Hydrogels Regulates Lymphatic Endothelial Cells toward Enhanced Lymphangiogenesis. ACS Appl. Mater. Interfaces 2024, 16, 55130–55141. [Google Scholar] [CrossRef]
- Ronaldson, P.T.; Davis, T.P. Transport Mechanisms at the Blood-Brain Barrier and in Cellular Compartments of the Neurovascular Unit: Focus on CNS Delivery of Small Molecule Drugs. Pharmaceutics 2022, 14, 1501. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Chen, Z.; Qin, H.; Zhang, Q.; Tian, B.; Li, X. Transcytosis: An effective mechanism to enhance nanoparticle extravasation and infiltration through biological barriers. Biomed. Mater. 2025, 20, 022003. [Google Scholar] [CrossRef]
- Gu, Y.; Cai, R.; Zhang, C.; Xue, Y.; Pan, Y.; Wang, J.; Zhang, Z. miR-132-3p boosts caveolae-mediated transcellular transport in glioma endothelial cells by targeting PTEN/PI3K/PKB/Src/Cav-1 signaling pathway. FASEB J. 2019, 33, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, I.; Nyúl-Tóth, Á.; Suciu, M.; Hermenean, A.; Krizbai, I.A. Heterogeneity of the blood-brain barrier. Tissue Barriers 2016, 4, e1143544. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Taddei, A.; Giampietro, C.; Conti, A.; Orsenigo, F.; Breviario, F.; Pirazzoli, V.; Potente, M.; Daly, C.; Dimmeler, S.; Dejana, E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat. Cell Biol. 2008, 10, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.A.; Zhang, X.; Predescu, D.; Huang, X.; Machado, R.F.; Göthert, J.R.; Malik, A.B.; Valyi-Nagy, T.; Zhao, Y.Y. Endothelial β-Catenin Signaling Is Required for Maintaining Adult Blood-Brain Barrier Integrity and Central Nervous System Homeostasis. Circulation 2016, 133, 177–186. [Google Scholar] [CrossRef]
- Stenman, J.M.; Rajagopal, J.; Carroll, T.J.; Ishibashi, M.; McMahon, J.; McMahon, A.P. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 2008, 322, 1247–1250. [Google Scholar] [CrossRef]
- Dormanns, K.; Brown, R.G.; David, T. The role of nitric oxide in neurovascular coupling. J. Theor. Biol. 2016, 394, 1–17. [Google Scholar] [CrossRef]
- Cheng, S.; Xia, I.F.; Wanner, R.; Abello, J.; Stratman, A.N.; Nicoli, S. Hemodynamics regulate spatiotemporal artery muscularization in the developing circle of Willis. Elife 2024, 13, RP94094. [Google Scholar] [CrossRef]
- Filosa, J.A.; Bonev, A.D.; Straub, S.V.; Meredith, A.L.; Wilkerson, M.K.; Aldrich, R.W.; Nelson, M.T. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 2006, 9, 1397–1403. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Kim, K.J.; Filosa, J.A. Advanced in vitro approach to study neurovascular coupling mechanisms in the brain microcirculation. J. Physiol. 2012, 590, 1757–1770. [Google Scholar] [CrossRef] [PubMed]
- Turovsky, E.A.; Braga, A.; Yu, Y.; Esteras, N.; Korsak, A.; Theparambil, S.M.; Hadjihambi, A.; Hosford, P.S.; Teschemacher, A.G.; Marina, N.; et al. Mechanosensory Signaling in Astrocytes. J. Neurosci. 2020, 40, 9364–9371. [Google Scholar] [CrossRef] [PubMed]
- Turovsky, E.A.; Plotnikov, E.Y.; Simakin, A.V.; Gudkov, S.V.; Varlamova, E.G. New magnetic iron nanoparticle doped with selenium nanoparticles and the mechanisms of their cytoprotective effect on cortical cells under ischemia-like conditions. Arch. Biochem. Biophys. 2025, 764, 110241. [Google Scholar] [CrossRef]
- Marina, N.; Turovsky, E.; Christie, I.N.; Hosford, P.S.; Hadjihambi, A.; Korsak, A.; Ang, R.; Mastitskaya, S.; Sheikhbahaei, S.; Theparambil, S.M.; et al. Brain metabolic sensing and metabolic signaling at the level of an astrocyte. Glia 2018, 66, 1185–1199. [Google Scholar] [CrossRef]
- Goetz, J.G.; Steed, E.; Ferreira, R.R.; Roth, S.; Ramspacher, C.; Boselli, F.; Charvin, G.; Liebling, M.; Wyart, C.; Schwab, Y.; et al. Endothelial cilia mediate low flow sensing during zebrafish vascular development. Cell Rep. 2014, 6, 799–808. [Google Scholar] [CrossRef]
- Eisa-Beygi, S.; Benslimane, F.M.; El-Rass, S.; Prabhudesai, S.; Abdelrasoul, M.K.A.; Simpson, P.M.; Yalcin, H.C.; Burrows, P.E.; Ramchandran, R. Characterization of Endothelial Cilia Distribution During Cerebral-Vascular Development in Zebrafish (Danio rerio). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2806–2818. [Google Scholar] [CrossRef]
- Pollock, L.M.; Perkins, B.; Anand-Apte, B. Primary cilia are present on endothelial cells of the hyaloid vasculature but are not required for the development of the blood-retinal barrier. PLoS ONE 2020, 15, e0225351. [Google Scholar] [CrossRef]
- Kallakuri, S.; Yu, J.A.; Li, J.; Li, Y.; Weinstein, B.M.; Nicoli, S.; Sun, Z. Endothelial cilia are essential for developmental vascular integrity in zebrafish. J. Am. Soc. Nephrol. 2015, 26, 864–875. [Google Scholar] [CrossRef]
- Chen, X.; Gays, D.; Milia, C.; Santoro, M.M. Cilia Control Vascular Mural Cell Recruitment in Vertebrates. Cell Rep. 2017, 18, 1033–1047. [Google Scholar] [CrossRef]
- Harraz, O.F.; Klug, N.R.; Senatore, A.J.; Hill-Eubanks, D.C.; Nelson, M.T. Piezo1 Is a Mechanosensor Channel in Central Nervous System Capillaries. Circ. Res. 2022, 130, 1531–1546. [Google Scholar] [CrossRef]
- Zi, H.; Peng, X.; Cao, J.; Xie, T.; Liu, T.; Li, H.; Bu, J.; Du, J.; Li, J. Piezo1-dependent regulation of pericyte proliferation by blood flow during brain vascular development. Cell Rep. 2024, 43, 113652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zlokovic, B.V. Endothelial Tip Cell Finds Its Way with Piezo1. Neuron 2020, 108, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Du, X.F.; Zhang, B.B.; Zi, H.X.; Yan, Y.; Yin, J.A.; Hou, H.; Gu, S.Y.; Chen, Q.; Du, J.L. Piezo1-Mediated Ca2+ Activities Regulate Brain Vascular Pathfinding during Development. Neuron 2020, 108, 180–192.e5. [Google Scholar] [CrossRef] [PubMed]
- Longden, T.A.; Mughal, A.; Hennig, G.W.; Harraz, O.F.; Shui, B.; Lee, F.K.; Lee, J.C.; Reining, S.; Kotlikoff, M.I.; König, G.M.; et al. Local IP3 receptor-mediated Ca2+ signals compound to direct blood flow in brain capillaries. Sci. Adv. 2021, 7, eabh0101. [Google Scholar] [CrossRef]
- Choi, D.; Park, E.; Choi, J.; Lu, R.; Yu, J.S.; Kim, C.; Zhao, L.; Yu, J.; Nakashima, B.; Lee, S.; et al. Piezo1 regulates meningeal lymphatic vessel drainage and alleviates excessive CSF accumulation. Nat. Neurosci. 2024, 27, 913–926. [Google Scholar] [CrossRef]
- Guo, T.; Chen, G.; Yang, L.; Deng, J.; Pan, Y. Piezo1 inhibitor isoquercitrin rescues neural impairment mediated by NLRP3 after intracerebral hemorrhage. Exp. Neurol. 2024, 379, 114852. [Google Scholar] [CrossRef]
- Qi, M.; Liu, R.; Zhang, F.; Yao, Z.; Zhou, M.L.; Jiang, X.; Ling, S. Roles of mechanosensitive ion channel PIEZO1 in the pathogenesis of brain injury after experimental intracerebral hemorrhage. Neuropharmacology 2024, 251, 109896. [Google Scholar] [CrossRef]
- Li, Y.; Baylie, R.L.; Tavares, M.J.; Brayden, J.E. TRPM4 channels couple purinergic receptor mechanoactivation and myogenic tone development in cerebral parenchymal arterioles. J. Cereb. Blood Flow Metab. 2014, 34, 1706–1714. [Google Scholar] [CrossRef]
- Toth, L.; Czigler, A.; Szarka, N.; Toth, P. The role of transient receptor potential channels in cerebral myogenic autoregulation in hypertension and aging. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H159–H161. [Google Scholar] [CrossRef]
- Alvarado, M.G.; Thakore, P.; Earley, S. Transient Receptor Potential Channel Ankyrin 1: A Unique Regulator of Vascular Function. Cells 2021, 10, 1167. [Google Scholar] [CrossRef]
- Luo, H.; Rossi, E.; Saubamea, B.; Chasseigneaux, S.; Cochois, V.; Choublier, N.; Smirnova, M.; Glacial, F.; Perrière, N.; Bourdoulous, S.; et al. Cannabidiol Increases Proliferation, Migration, Tubulogenesis, and Integrity of Human Brain Endothelial Cells through TRPV2 Activation. Mol. Pharm. 2019, 16, 1312–1326. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.; Augustine, J.; Karan, B.M.; Hernández, C.; Stitt, A.W.; Curtis, T.M.; Simó, R. Sitagliptin eye drops prevent the impairment of retinal neurovascular unit in the new Trpv2+/− rat model. J. Neuroinflammation 2024, 21, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Papadopoulos, P.; Hamel, E. Endothelial TRPV4 channels mediate dilation of cerebral arteries: Impairment and recovery in cerebrovascular pathologies related to Alzheimer’s disease. Br. J. Pharmacol. 2013, 170, 661–670. [Google Scholar] [CrossRef]
- Diaz-Otero, J.M.; Yen, T.C.; Ahmad, A.; Laimon-Thomson, E.; Abolibdeh, B.; Kelly, K.; Lewis, M.T.; Wiseman, R.W.; Jackson, W.F.; Dorrance, A.M. Transient receptor potential vanilloid 4 channels are important regulators of parenchymal arteriole dilation and cognitive function. Microcirculation 2019, 26, e12535. [Google Scholar] [CrossRef] [PubMed]
- Hatano, N.; Suzuki, H.; Itoh, Y.; Muraki, K. TRPV4 partially participates in proliferation of human brain capillary endothelial cells. Life Sci. 2013, 92, 317–324. [Google Scholar] [CrossRef]
- Chen, C.K.; Hsu, P.Y.; Wang, T.M.; Miao, Z.F.; Lin, R.T.; Juo, S.H. TRPV4 Activation Contributes Functional Recovery from Ischemic Stroke via Angiogenesis and Neurogenesis. Mol. Neurobiol. 2018, 55, 4127–4135. [Google Scholar] [CrossRef]
- Hansen, C.E.; Kamermans, A.; Mol, K.; Berve, K.; Rodriguez-Mogeda, C.; Fung, W.K.; van Het Hof, B.; Fontijn, R.D.; van der Pol, S.M.A.; Michalick, L.; et al. Inflammation-induced TRPV4 channels exacerbate blood-brain barrier dysfunction in multiple sclerosis. J. Neuroinflammation 2024, 21, 72. [Google Scholar] [CrossRef]
- Hakimizadeh, E.; Shamsizadeh, A.; Roohbakhsh, A.; Arababadi, M.K.; Hajizadeh, M.R.; Shariati, M.; Fatemi, I.; Moghadam-Ahmadi, A.; Bazmandegan, G.; Rezazadeh, H.; et al. TRPV1 receptor-mediated expression of Toll-like receptors 2 and 4 following permanent middle cerebral artery occlusion in rats. Iran J. Basic Med. Sci. 2017, 20, 863–869. [Google Scholar] [CrossRef]
- Miyanohara, J.; Shirakawa, H.; Sanpei, K.; Nakagawa, T.; Kaneko, S. A pathophysiological role of TRPV1 in ischemic injury after transient focal cerebral ischemia in mice. Biochem. Biophys. Res. Commun. 2015, 467, 478–483. [Google Scholar] [CrossRef]
- Al Tabosh, T.; Al Tarrass, M.; Tourvieilhe, L.; Guilhem, A.; Dupuis-Girod, S.; Bailly, S. Hereditary hemorrhagic telangiectasia: From signaling insights to therapeutic advances. J. Clin. Investig. 2024, 134, e176379. [Google Scholar] [CrossRef]
- Scimone, C.; Donato, L.; Alibrandi, S.; Conti, A.; Bortolotti, C.; Germanò, A.; Alafaci, C.; Vinci, S.L.; D’Angelo, R.; Sidoti, A. Methylome analysis of endothelial cells suggests new insights on sporadic brain arteriovenous malformation. Heliyon 2024, 10, e35126. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Granata, F.; Longo, M.; Mormina, E.; Turiaco, C.; Caragliano, A.A.; Donato, L.; Sidoti, A.; D’Angelo, R. Germline Mutation Enrichment in Pathways Controlling Endothelial Cell Homeostasis in Patients with Brain Arteriovenous Malformation: Implication for Molecular Diagnosis. Int. J. Mol. Sci. 2020, 21, 4321. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Donato, L.; Alafaci, C.; Granata, F.; Rinaldi, C.; Longo, M.; D’Angelo, R.; Sidoti, A. High-Throughput Sequencing to Detect Novel Likely Gene-Disrupting Variants in Pathogenesis of Sporadic Brain Arteriovenous Malformations. Front. Genet. 2020, 11, 46. [Google Scholar] [CrossRef]
- Park, H.; Furtado, J.; Poulet, M.; Chung, M.; Yun, S.; Lee, S.; Sessa, W.C.; Franco, C.A.; Schwartz, M.A.; Eichmann, A. Defective Flow-Migration Coupling Causes Arteriovenous Malformations in Hereditary Hemorrhagic Telangiectasia. Circulation 2021, 144, 805–822. [Google Scholar] [CrossRef]
- Baeyens, N.; Larrivée, B.; Ola, R.; Hayward-Piatkowskyi, B.; Dubrac, A.; Huang, B.; Ross, T.D.; Coon, B.G.; Min, E.; Tsarfati, M.; et al. Defective fluid shear stress mechanotransduction mediates hereditary hemorrhagic telangiectasia. J. Cell Biol. 2016, 214, 807–816. [Google Scholar] [CrossRef]
- Peacock, H.M.; Tabibian, A.; Criem, N.; Caolo, V.; Hamard, L.; Deryckere, A.; Haefliger, J.A.; Kwak, B.R.; Zwijsen, A.; Jones, E.A.V. Impaired SMAD1/5 Mechanotransduction and Cx37 (Connexin37) Expression Enable Pathological Vessel Enlargement and Shunting. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e87–e104. [Google Scholar] [CrossRef]
- Li, W.; Quigley, K. Bone morphogenetic protein signalling in pulmonary arterial hypertension: Revisiting the BMPRII connection. Biochem. Soc. Trans. 2024, 52, 1515–1528. [Google Scholar] [CrossRef]
- Hiepen, C.; Jatzlau, J.; Hildebrandt, S.; Kampfrath, B.; Goktas, M.; Murgai, A.; Cuellar Camacho, J.L.; Haag, R.; Ruppert, C.; Sengle, G.; et al. BMPR2 acts as a gatekeeper to protect endothelial cells from increased TGFβ responses and altered cell mechanics. PLoS Biol. 2019, 17, e3000557. [Google Scholar] [CrossRef]
- Zhong, A.; Mirzaei, Z.; Simmons, C.A. The Roles of Matrix Stiffness and ß-Catenin Signaling in Endothelial-to-Mesenchymal Transition of Aortic Valve Endothelial Cells. Cardiovasc. Eng. Technol. 2018, 9, 158–167. [Google Scholar] [CrossRef]
- Ragazzini, S.; Scocozza, F.; Bernava, G.; Auricchio, F.; Colombo, G.I.; Barbuto, M.; Conti, M.; Pesce, M.; Garoffolo, G. Mechanosensor YAP cooperates with TGF-β1 signaling to promote myofibroblast activation and matrix stiffening in a 3D model of human cardiac fibrosis. Acta Biomater. 2022, 152, 300–312. [Google Scholar] [CrossRef]
- Rolle, I.G.; Crivellari, I.; Zanello, A.; Mazzega, E.; Dalla, E.; Bulfoni, M.; Avolio, E.; Battistella, A.; Lazzarino, M.; Cellot, A.; et al. Heart failure impairs the mechanotransduction properties of human cardiac pericytes. J. Mol. Cell. Cardiol. 2021, 151, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Deng, X.W.; Xu, G.Q.; Lin, J.; Lu, H.Z.; Chen, J. Mechanical homeostasis imbalance in hepatic stellate cells activation and hepatic fibrosis. Front. Mol. Biosci. 2023, 10, 1183808. [Google Scholar] [CrossRef]

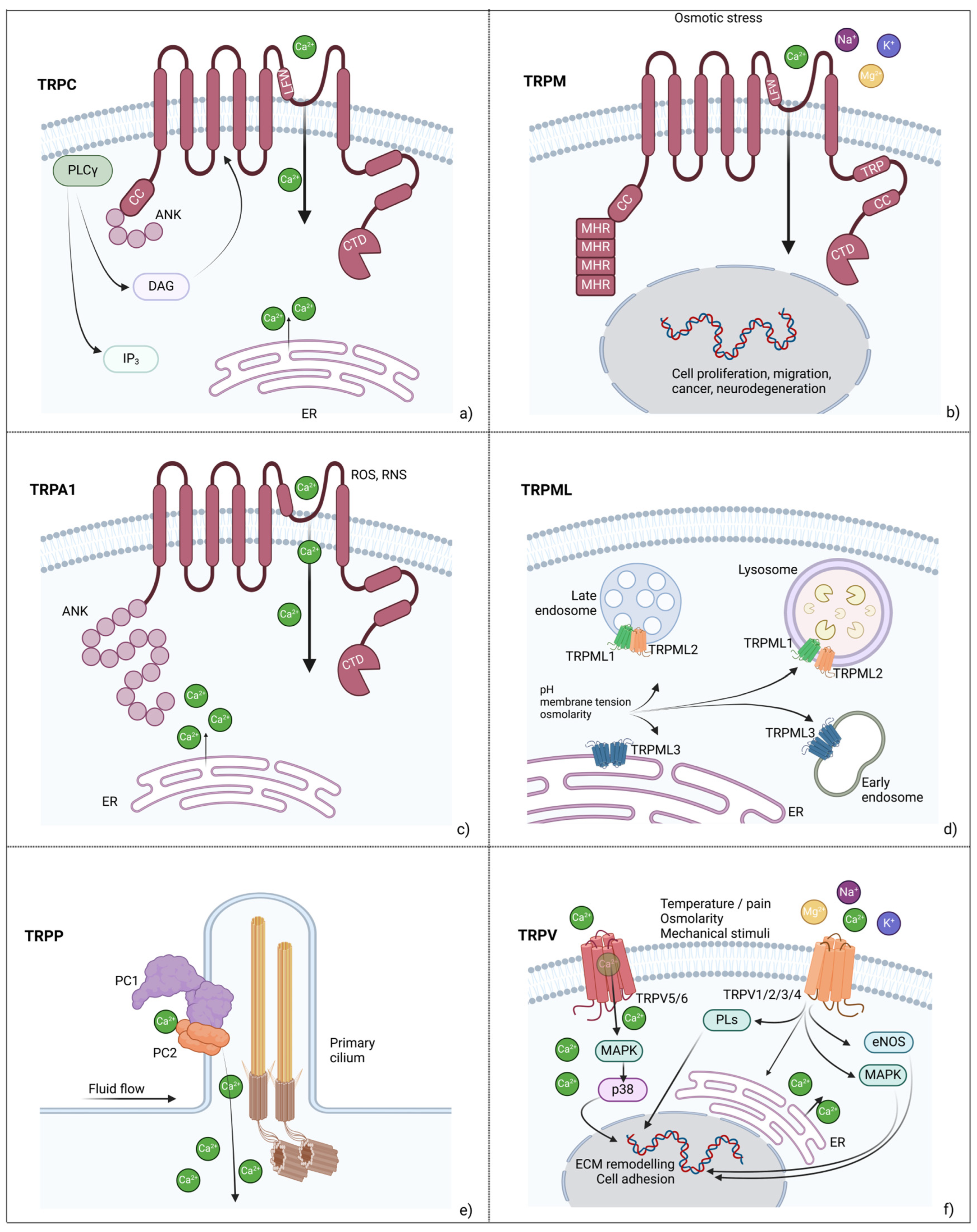
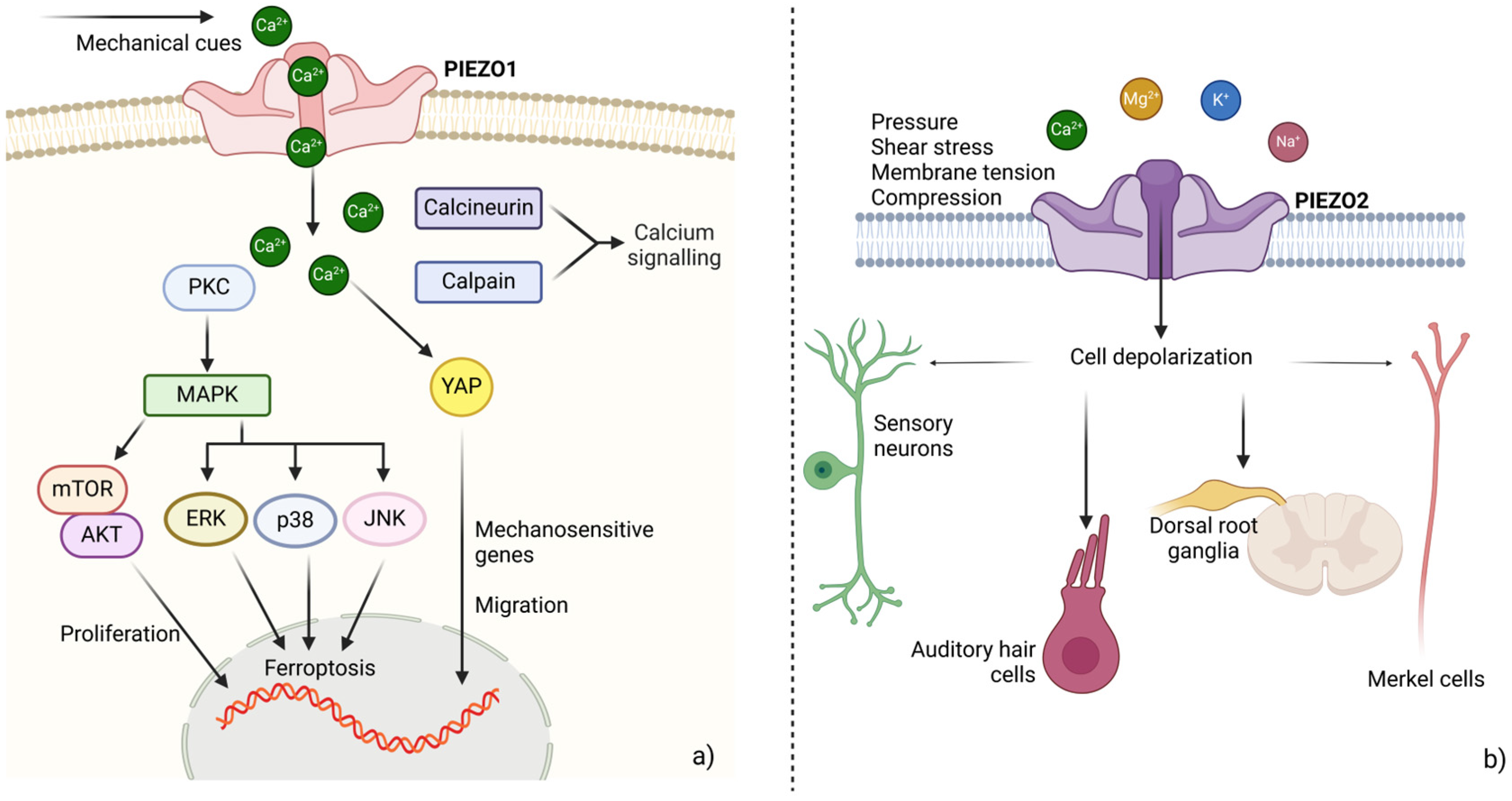
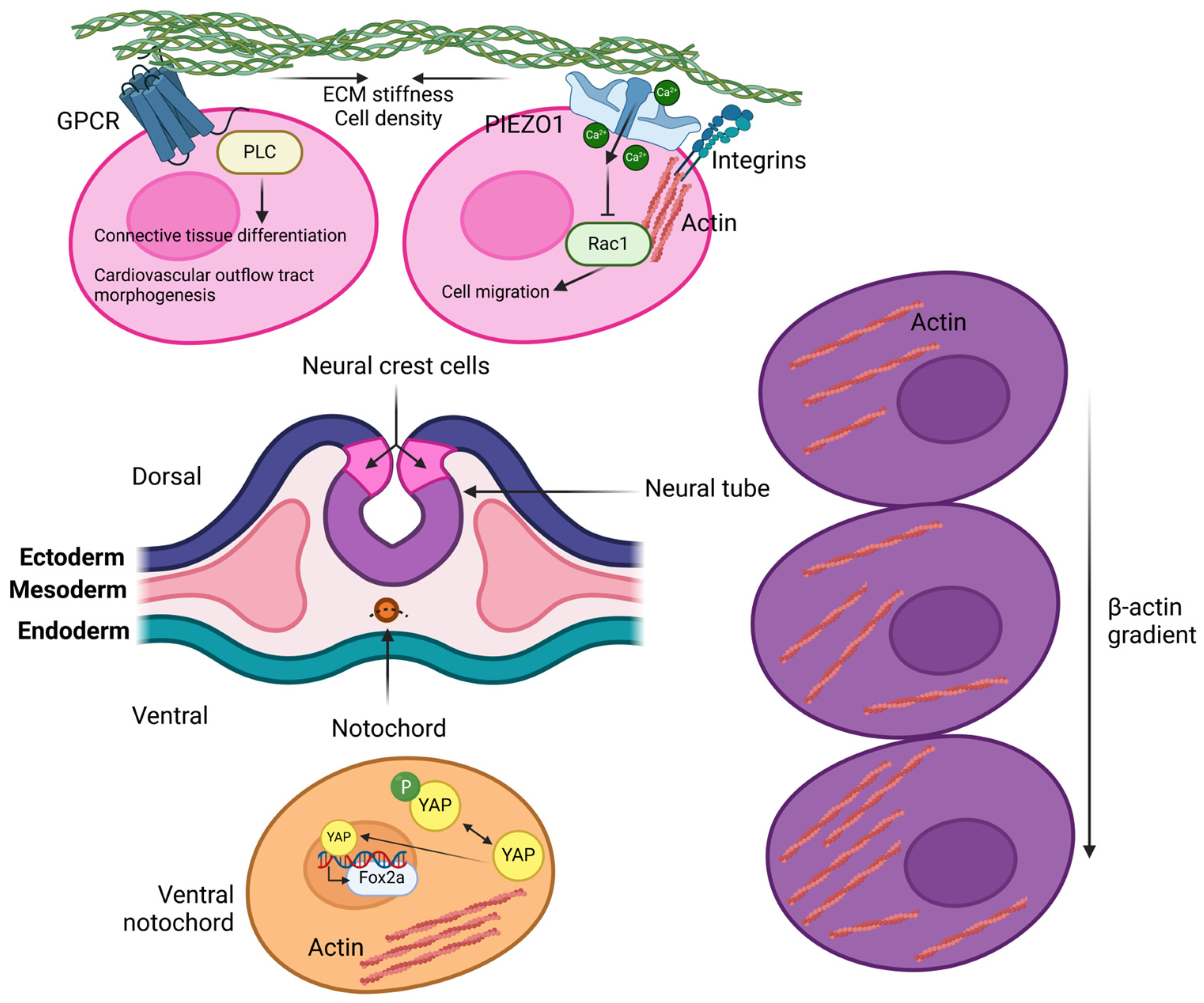
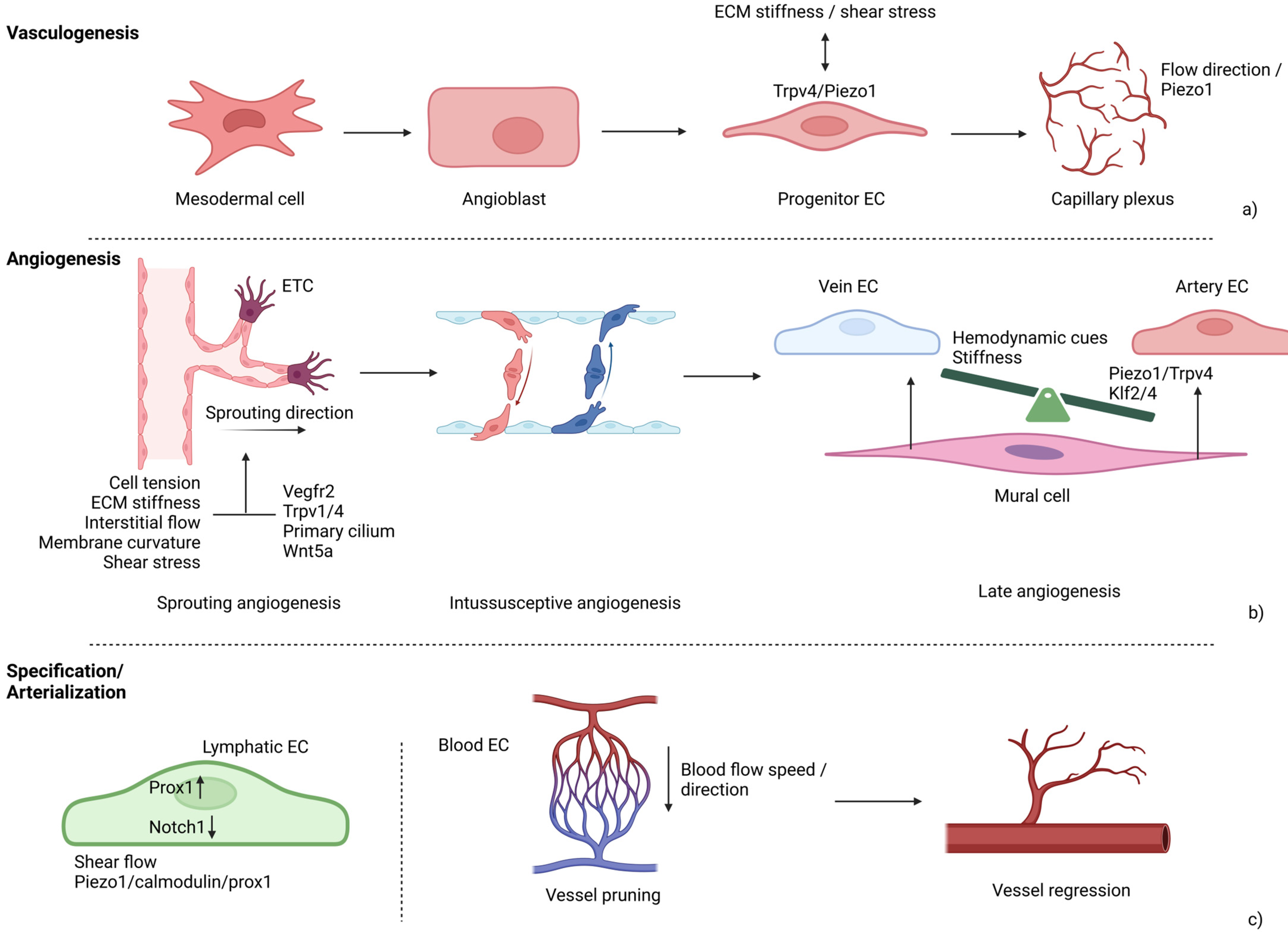
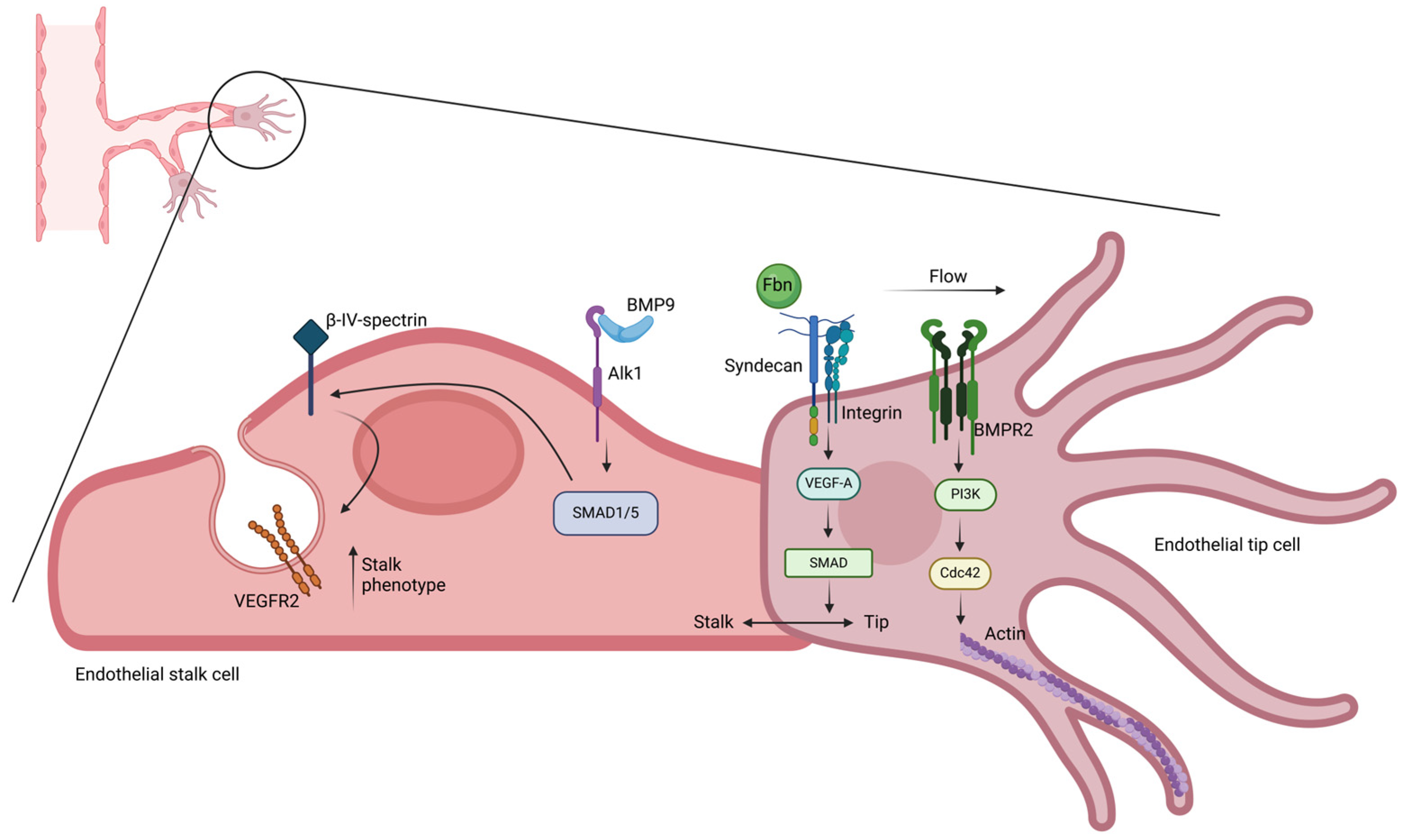
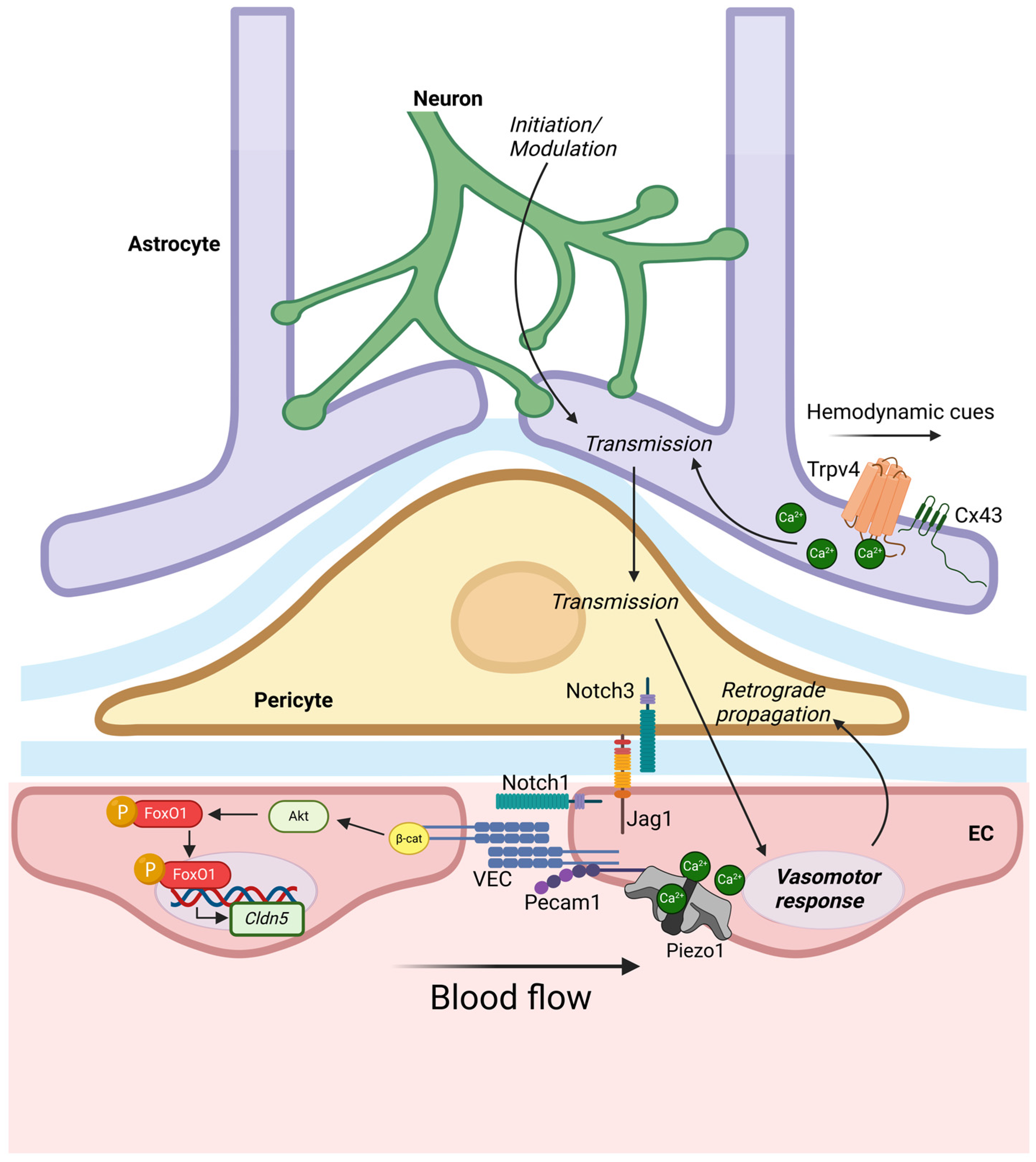
| Mechanosensor | Stimulus | Downstream Effector | Cell Response | Reference |
|---|---|---|---|---|
| ECM-integrin-cytoskeleton proteins | Stiffness, tension, | KLF2/4, YAP/TAZ | Differentiation, apoptosis, proliferation | [52] |
| ENaC | Pressure, shear forces | NO | Neurosensation, blood pressure regulation | [53,54] |
| TREK | Membrane tension | AKAP150, β-COP, Mtap2, sortilin | K+ flow regulation | [55,56] |
| BK | Membrane electric potential | K+ | Cell hyperpolarization, smooth muscle tone | [57] |
| GPCRs | Shear stress, mechanical stretch | Heterogeneous | Cell adhesion | [58] |
| TRPC | Oxidative damage | Calcineurin | Memory, LTP, pain | [59,60] |
| TRPM | Oxidative stress, inflammation, temperature | Heterogeneous | Hormone release, apoptosis | [61,62] |
| TRPV | Mechanical and osmolar stimuli | Phosphatidyl-inositol cascade | ECM remodelling | [63] |
| TRPA | Mechanical stress | Ca2+ | Nociception, temperature sensation | [64] |
| TRPML | Membrane tubulation, pH | CaM, calcineurin | Autophagy | [65] |
| TRPP | Stiffness, cell tension, shear stress | Ca2+ | Tubule morphogenesis | [66] |
| PIEZO1 | Shear stress, stiffness | KLF2/4, YAP/TAZ | Vessel morphogenesis, bone repair | [67] |
| PIEZO2 | Touch, shear forces, stretch | S1P, ERK | Sensory neuron depolarization | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alibrandi, S.; Rinaldi, C.; Vinci, S.L.; Conti, A.; Donato, L.; Scimone, C.; Sidoti, A.; D’Angelo, R. Mechanotransduction in Development: A Focus on Angiogenesis. Biology 2025, 14, 346. https://doi.org/10.3390/biology14040346
Alibrandi S, Rinaldi C, Vinci SL, Conti A, Donato L, Scimone C, Sidoti A, D’Angelo R. Mechanotransduction in Development: A Focus on Angiogenesis. Biology. 2025; 14(4):346. https://doi.org/10.3390/biology14040346
Chicago/Turabian StyleAlibrandi, Simona, Carmela Rinaldi, Sergio Lucio Vinci, Alfredo Conti, Luigi Donato, Concetta Scimone, Antonina Sidoti, and Rosalia D’Angelo. 2025. "Mechanotransduction in Development: A Focus on Angiogenesis" Biology 14, no. 4: 346. https://doi.org/10.3390/biology14040346
APA StyleAlibrandi, S., Rinaldi, C., Vinci, S. L., Conti, A., Donato, L., Scimone, C., Sidoti, A., & D’Angelo, R. (2025). Mechanotransduction in Development: A Focus on Angiogenesis. Biology, 14(4), 346. https://doi.org/10.3390/biology14040346











