Pathophysiological Links Between Myocardial Infarction and Anxiety Disorder, Major Depressive Disorder, Bipolar Disorder and Schizophrenia
Simple Summary
Abstract
1. Introduction
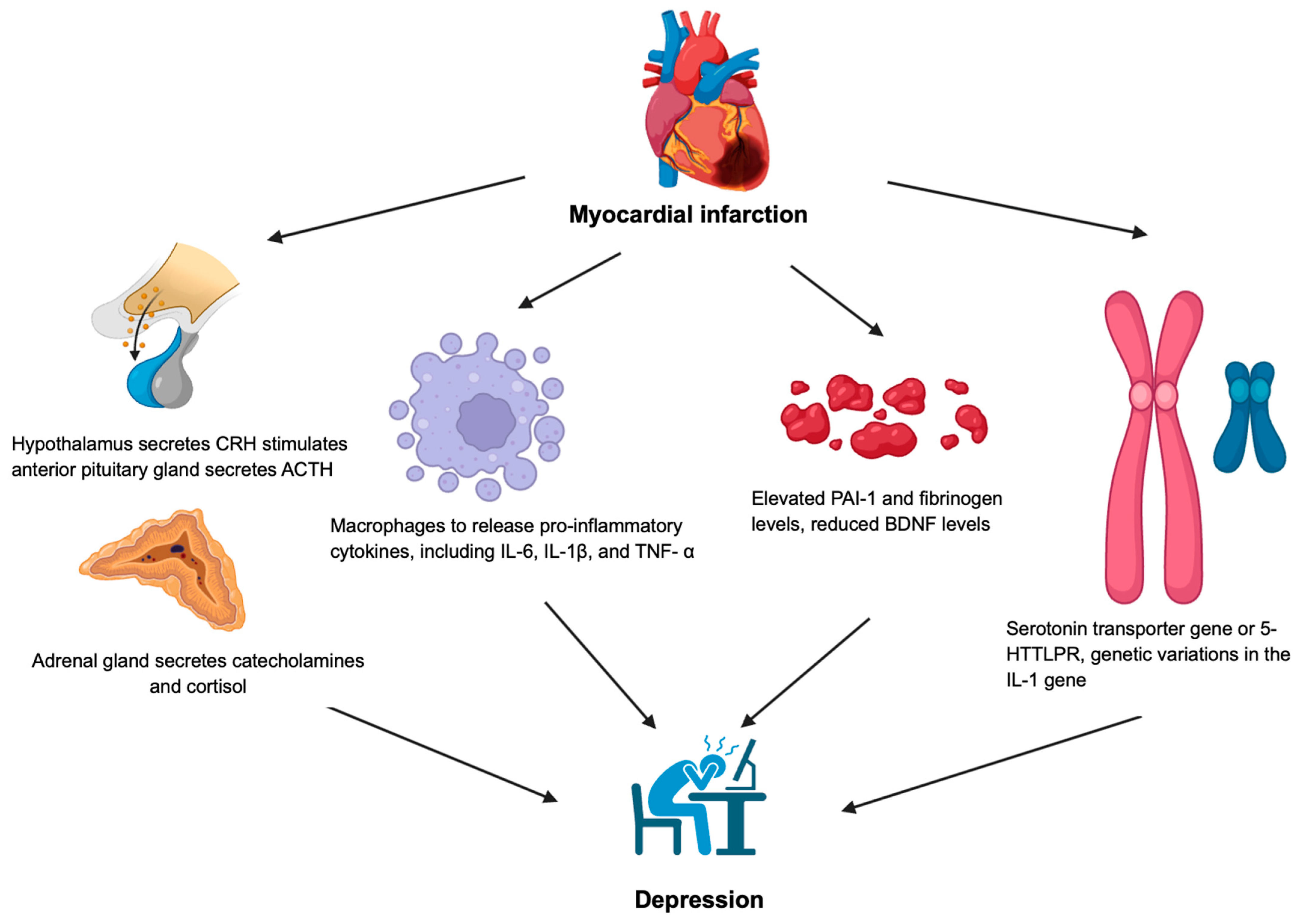
2. Anxiety Disorder and Major Depressive Disorder
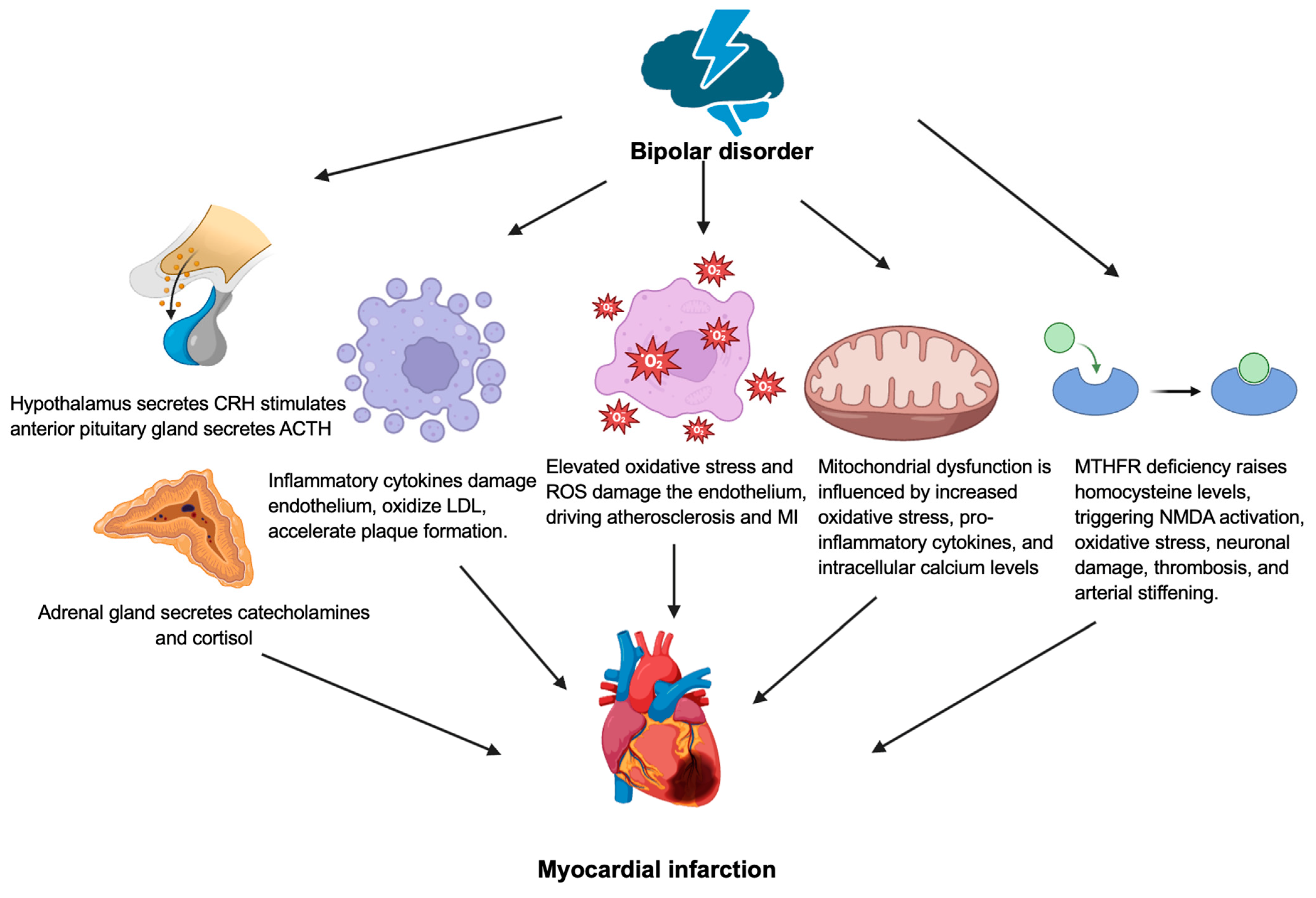
3. Bipolar Disorder
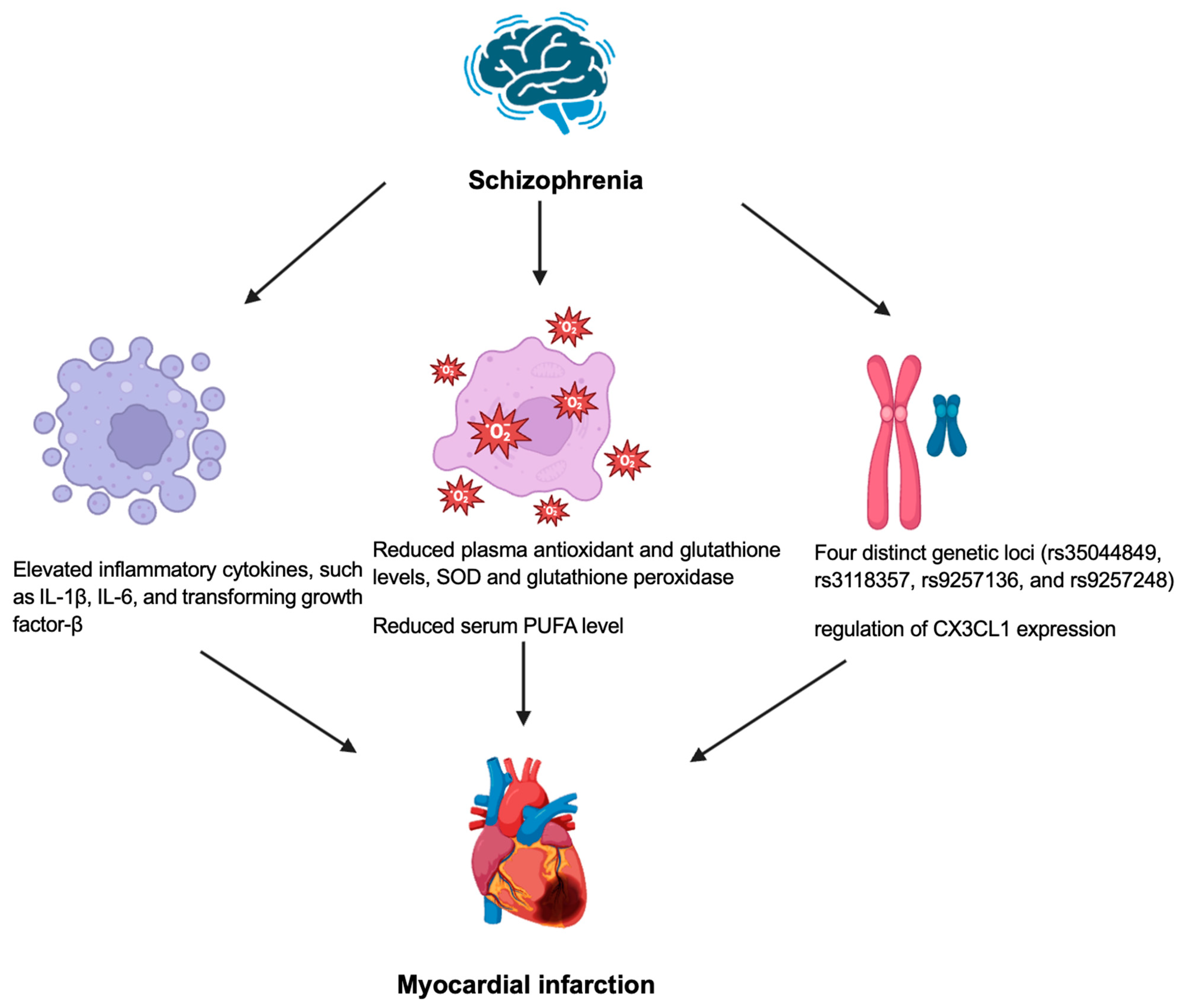
4. Schizophrenia
5. Future Research Directions
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTH | Adrenocorticotropic hormone |
| AGE | Advanced glycation end products |
| AKT | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| ANS | Autonomic nervous system |
| ATP | Adenosine triphosphate |
| BNDF | Brain-derived neurotrophic factor |
| COMT | catechol-O-methyltransferase |
| CRH | Corticotropin-releasing hormone |
| DAMP | Damage-associated molecular patterns |
| EPA | Eicosapentanoate |
| GR-α | Glucocorticoid receptor-alpha |
| HMGB1 | High-mobility group box 1 |
| HPA | Hypothalamic-pituitary-adrenal |
| HSP | Heat shock proteins |
| IL | Interleukin |
| LDL | Low-density lipoprotein |
| MI | Myocardial infarction |
| MTHFR | Methylenetetrahydrofolate reductase |
| MPTP | Mitochondrial permeability transition pore |
| MCU | Mitochondrial calcium uniporter |
| NCX | Na+/Ca2+ exchanger |
| NMDA | N-methyl-D-aspartate |
| NSTEMI | Non-ST elevation MI |
| PAI-1 | Plasminogen activator inhibitor 1 |
| PUFA | Polyunsaturated fatty acid |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| STEMI | ST elevation myocardial infarction |
| TNF | Tumor necrosis factor |
| tPA | tissue plasminogen activator |
References
- Kumar, M.; Nayak, P.K. Psychological sequelae of myocardial infarction. Biomed. Pharmacother. 2017, 95, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Grippo, A.J.; Johnson, A.K. Stress, depression and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress 2009, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ringen, P.A.; Engh, J.A.; Birkenaes, A.B.; Dieset, I.; Andreassen, O.A. Increased mortality in schizophrenia due to cardiovascular disease—A non-systematic review of epidemiology, possible causes, and interventions. Front. Psychiatry 2014, 5, 137. [Google Scholar] [CrossRef]
- Laursen, T.M.; Wahlbeck, K.; Hällgren, J.; Westman, J.; Ösby, U.; Alinaghizadeh, H.; Gissler, M.; Nordentoft, M. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS ONE 2013, 8, e67133. [Google Scholar] [CrossRef]
- Lambert, A.M.; Parretti, H.M.; Pearce, E.; Price, M.J.; Riley, M.; Ryan, R.; Tyldesley-Marshall, N.; Avşar, T.S.; Matthewman, G.; Lee, A.; et al. Temporal trends in associations between severe mental illness and risk of cardiovascular disease: A systematic review and meta-analysis. PLoS Med. 2022, 19, e1003960. [Google Scholar] [CrossRef]
- Goldfarb, M.; De Hert, M.; Detraux, J.; Di Palo, K.; Munir, H.; Music, S.; Piña, I.; Ringen Petter, A. Severe Mental Illness and Cardiovascular Disease. J. Am. Coll. Cardiol. 2022, 80, 918–933. [Google Scholar] [CrossRef]
- Kugathasan, P.; Johansen, M.B.; Jensen, M.B.; Aagaard, J.; Nielsen, R.E.; Jensen, S.E. Coronary Artery Calcification and Mortality Risk in Patients With Severe Mental Illness. Circ. Cardiovasc. Imaging 2019, 12, e008236. [Google Scholar] [CrossRef]
- De Hert, M.; Detraux, J.; Vancampfort, D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin. Neurosci. 2018, 20, 31–40. [Google Scholar] [CrossRef]
- Feng, H.P.; Chien, W.C.; Cheng, W.T.; Chung, C.H.; Cheng, S.M.; Tzeng, W.C. Risk of anxiety and depressive disorders in patients with myocardial infarction: A nationwide population-based cohort study. Medicine 2016, 95, e4464. [Google Scholar] [CrossRef]
- Roest, A.M.; Heideveld, A.; Martens, E.J.; de Jonge, P.; Denollet, J. Symptom dimensions of anxiety following myocardial infarction: Associations with depressive symptoms and prognosis. Health Psychol. 2014, 33, 1468–1476. [Google Scholar] [CrossRef][Green Version]
- Ricci, M.; Pozzi, G.; Caraglia, N.; Chieffo, D.P.R.; Polese, D.; Galiuto, L. Psychological Distress Affects Performance during Exercise-Based Cardiac Rehabilitation. Life 2024, 14, 236. [Google Scholar] [CrossRef] [PubMed]
- Garrels, E.; Kainth, T.; Silva, B.; Yadav, G.; Gill, G.; Salehi, M.; Gunturu, S. Pathophysiological mechanisms of post-myocardial infarction depression: A narrative review. Front. Psychiatry 2023, 14, 1225794. [Google Scholar] [CrossRef] [PubMed]
- Wilkowska, A.; Rynkiewicz, A.; Wdowczyk, J.; Landowski, J. Morning and afternoon serum cortisol level in patients with post-myocardial infarction depression. Cardiol. J. 2019, 26, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, E.; Leanderson, P.; Kristenson, M. Diurnal saliva cortisol levels and relations to psychosocial factors in a population sample of middle-aged Swedish men and women. Int. J. Behav. Med. 2006, 13, 193–200. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Konsman, J.P.; Luheshi, G.N.; Bluthé, R.M.; Dantzer, R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur. J. Neurosci. 2000, 12, 4434–4446. [Google Scholar] [CrossRef]
- Penninx, B.W.; Kritchevsky, S.B.; Yaffe, K.; Newman, A.B.; Simonsick, E.M.; Rubin, S.; Ferrucci, L.; Harris, T.; Pahor, M. Inflammatory markers and depressed mood in older persons: Results from the Health, Aging and Body Composition study. Biol. Psychiatry 2003, 54, 566–572. [Google Scholar] [CrossRef]
- Maes, M.; Bosmans, E.; De Jongh, R.; Kenis, G.; Vandoolaeghe, E.; Neels, H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 1997, 9, 853–858. [Google Scholar] [CrossRef]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J. Role of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in psychological stress and depression. Oncotarget 2017, 8, 113258–113268. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Hong, C.J.; Liou, Y.J.; Yu, Y.W.; Chen, T.J. Plasminogen activator inhibitor-1 gene is associated with major depression and antidepressant treatment response. Pharmacogenet Genom. 2008, 18, 869–875. [Google Scholar] [CrossRef]
- Geiser, F.; Conrad, R.; Imbierowicz, K.; Meier, C.; Liedtke, R.; Klingmüller, D.; Oldenburg, J.; Harbrecht, U. Coagulation activation and fibrinolysis impairment are reduced in patients with anxiety and depression when medicated with serotonergic antidepressants. Psychiatry Clin. Neurosci. 2011, 65, 518–525. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, S.; Li, C.; Lu, N.; Yue, Y.; Yin, Y.; Zhang, Y.; Zhi, X.; Zhang, D.; Yuan, Y. The serum protein levels of the tPA-BDNF pathway are implicated in depression and antidepressant treatment. Transl. Psychiatry 2017, 7, e1079. [Google Scholar] [CrossRef]
- Karege, F.; Perret, G.; Bondolfi, G.; Schwald, M.; Bertschy, G.; Aubry, J.M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002, 109, 143–148. [Google Scholar] [CrossRef]
- Hashimoto, K. Brain-derived neurotrophic factor as a biomarker for mood disorders: An historical overview and future directions. Psychiatry Clin. Neurosci. 2010, 64, 341–357. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Hofer, M.; Pagliusi, S.R.; Hohn, A.; Leibrock, J.; Barde, Y.A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. Embo J. 1990, 9, 2459–2464. [Google Scholar] [CrossRef]
- Otte, C.; McCaffery, J.; Ali, S.; Whooley, M.A. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: The Heart and Soul Study. Am. J. Psychiatry 2007, 164, 1379–1384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schins, A.; Honig, A.; Crijns, H.; Baur, L.; Hamulyák, K. Increased coronary events in depressed cardiovascular patients: 5-HT2A receptor as missing link? Psychosom. Med. 2003, 65, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, D.; Sato, H.; Sakata, Y.; Shiotani, I.; Kinjo, K.; Mizuno, H.; Shimizu, M.; Ito, H.; Koretsune, Y.; Hirayama, A.; et al. Influence of serotonin transporter gene polymorphism on depressive symptoms and new cardiac events after acute myocardial infarction. Am. Heart J. 2005, 150, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Bujak, M.; Frangogiannis, N.G. The role of IL-1 in the pathogenesis of heart disease. Arch. Immunol. Ther. Exp (Warsz) 2009, 57, 165–176. [Google Scholar] [CrossRef]
- Shah, D.; Singh, B.; Varnika, F.; Fredrick, F.C.; Meda, A.K.R.; Aggarwal, K.; Jain, R. Linking hearts and minds: Understanding the cardiovascular impact of bipolar disorder. Future Cardiol. 2024, 20, 709–718. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Porta, A.; Schwartz, P.J. Autonomic Control of the Heart and Its Clinical Impact. A Personal Perspective. Front. Physiol. 2020, 11, 582. [Google Scholar] [CrossRef]
- van Weperen, V.Y.H.; Ripplinger, C.M.; Vaseghi, M. Autonomic control of ventricular function in health and disease: Current state of the art. Clin. Auton. Res. 2023, 33, 491–517. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Carnethon, M.R.; Matthews, K.A.; McIntyre, R.S.; Miller, G.E.; Raghuveer, G.; Stoney, C.M.; Wasiak, H.; McCrindle, B.W. Major Depressive Disorder and Bipolar Disorder Predispose Youth to Accelerated Atherosclerosis and Early Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2015, 132, 965–986. [Google Scholar] [CrossRef]
- Henry, B.L.; Minassian, A.; Paulus, M.P.; Geyer, M.A.; Perry, W. Heart rate variability in bipolar mania and schizophrenia. J. Psychiatr. Res. 2010, 44, 168–176. [Google Scholar] [CrossRef]
- Hillebrand, S.; Gast, K.B.; de Mutsert, R.; Swenne, C.A.; Jukema, J.W.; Middeldorp, S.; Rosendaal, F.R.; Dekkers, O.M. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: Meta-analysis and dose–response meta-regression. EP Eur. 2013, 15, 742–749. [Google Scholar] [CrossRef]
- Fatkhullina, A.R.; Peshkova, I.O.; Koltsova, E.K. The Role of Cytokines in the Development of Atherosclerosis. Biochem. (Mosc) 2016, 81, 1358–1370. [Google Scholar] [CrossRef]
- Steckert, A.V.; Valvassori, S.S.; Moretti, M.; Dal-Pizzol, F.; Quevedo, J. Role of oxidative stress in the pathophysiology of bipolar disorder. Neurochem. Res. 2010, 35, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.I. Bipolar Disorder and the Vascular System: Mechanisms and New Prevention Opportunities. Can. J. Cardiol. 2017, 33, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Cheng, H.; Wang, L. Ferroptosis and Lipid Metabolism in Acute Myocardial Infarction. Rev. Cardiovasc. Med. 2024, 25, 149. [Google Scholar] [CrossRef]
- Morris, G.; Walder, K.; McGee, S.L.; Dean, O.M.; Tye, S.J.; Maes, M.; Berk, M. A model of the mitochondrial basis of bipolar disorder. Neurosci. Biobehav. Rev. 2017, 74, 1–20. [Google Scholar] [CrossRef]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef]
- Hernandez-Resendiz, S.; Prunier, F.; Girao, H.; Dorn, G.; Hausenloy, D.J. Targeting mitochondrial fusion and fission proteins for cardioprotection. J. Cell Mol. Med. 2020, 24, 6571–6585. [Google Scholar] [CrossRef]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabò, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef]
- Salagre, E.; Vizuete, A.F.; Leite, M.; Brownstein, D.J.; McGuinness, A.; Jacka, F.; Dodd, S.; Stubbs, B.; Köhler, C.A.; Vieta, E.; et al. Homocysteine as a peripheral biomarker in bipolar disorder: A meta-analysis. Eur. Psychiatry 2017, 43, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Yuan, S.; Mason, A.M.; Carter, P.; Burgess, S.; Larsson, S.C. Homocysteine, B vitamins, and cardiovascular disease: A Mendelian randomization study. BMC Med. 2021, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Namazi, M.R.; Feily, A. Homocysteine may accelerate skin aging: A new chapter in the biology of skin senescence? J. Am. Acad. Dermatol. 2011, 64, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.C.; Vincenzi, B.; Andrea, N.V.; Ulloa, M.; Copeland, P.M. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry 2015, 2, 452–464. [Google Scholar] [CrossRef]
- Cheslack-Postava, K.; Brown, A.S. Prenatal infection and schizophrenia: A decade of further progress. Schizophr. Res. 2022, 247, 7–15. [Google Scholar] [CrossRef]
- Fond, G.; Lançon, C.; Korchia, T.; Auquier, P.; Boyer, L. The Role of Inflammation in the Treatment of Schizophrenia. Front. Psychiatry 2020, 11, 160. [Google Scholar] [CrossRef]
- van Kesteren, C.F.; Gremmels, H.; de Witte, L.D.; Hol, E.M.; Van Gool, A.R.; Falkai, P.G.; Kahn, R.S.; Sommer, I.E. Immune involvement in the pathogenesis of schizophrenia: A meta-analysis on postmortem brain studies. Transl. Psychiatry 2017, 7, e1075. [Google Scholar] [CrossRef]
- Monji, A.; Kato, T.; Kanba, S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 2009, 63, 257–265. [Google Scholar] [CrossRef]
- Miller, B.J.; Buckley, P.; Seabolt, W.; Mellor, A.; Kirkpatrick, B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol. Psychiatry 2011, 70, 663–671. [Google Scholar] [CrossRef]
- Haybar, H.; Bandar, B.; Torfi, E.; Mohebbi, A.; Saki, N. Cytokines and their role in cardiovascular diseases. Cytokine 2023, 169, 156261. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Rogers, J.C.; Katshu, M.; Liddle, P.F.; Upthegrove, R. Oxidative Stress and the Pathophysiology and Symptom Profile of Schizophrenia Spectrum Disorders. Front. Psychiatry 2021, 12, 703452. [Google Scholar] [CrossRef] [PubMed]
- Aladağ, N.; Asoğlu, R.; Ozdemir, M.; Asoğlu, E.; Derin, A.R.; Demir, C.; Demir, H. Oxidants and antioxidants in myocardial infarction (MI): Investigation of ischemia modified albumin, malondialdehyde, superoxide dismutase and catalase in individuals diagnosed with ST elevated myocardial infarction (STEMI) and non-STEMI (NSTEMI). J. Med. Biochem. 2021, 40, 286–294. [Google Scholar] [CrossRef]
- Solberg, D.K.; Refsum, H.; Andreassen, O.A.; Bentsen, H. A five-year follow-up study of antioxidants, oxidative stress and polyunsaturated fatty acids in schizophrenia. Acta Neuropsychiatr. 2019, 31, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Bentsen, H.; Landrø, N.I. Neurocognitive effects of an omega-3 fatty acid and vitamins E+C in schizophrenia: A randomised controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 57–66. [Google Scholar] [CrossRef]
- Yagi, S.; Fukuda, D.; Aihara, K.I.; Akaike, M.; Shimabukuro, M.; Sata, M. n-3 Polyunsaturated Fatty Acids: Promising Nutrients for Preventing Cardiovascular Disease. J. Atheroscler. Thromb. 2017, 24, 999–1010. [Google Scholar] [CrossRef]
- Lam, M.; Chen, C.Y.; Li, Z.; Martin, A.R.; Bryois, J.; Ma, X.; Gaspar, H.; Ikeda, M.; Benyamin, B.; Brown, B.C.; et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat. Genet. 2019, 51, 1670–1678. [Google Scholar] [CrossRef]
- Shen, J.; Jiang, C. Unraveling the heart-brain axis: Shared genetic mechanisms in cardiovascular diseases and Schizophrenia. Schizophrenia 2024, 10, 113. [Google Scholar] [CrossRef]
- Apostolakis, S.; Spandidos, D. Chemokines and atherosclerosis: Focus on the CX3CL1/CX3CR1 pathway. Acta Pharmacol. Sin. 2013, 34, 1251–1256. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, L.; Bian, C.; Liang, Y.; Xing, R.; Yishakea, M.; Dong, J. Role of CX3CL1 in Diseases. Arch. Immunol. Ther. Exp (Warsz) 2016, 64, 371–383. [Google Scholar] [CrossRef]
- Khokhar, J.Y.; Dwiel, L.L.; Henricks, A.M.; Doucette, W.T.; Green, A.I. The link between schizophrenia and substance use disorder: A unifying hypothesis. Schizophr. Res. 2018, 194, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Jalali, Z.; Khademalhosseini, M.; Soltani, N.; Esmaeili Nadimi, A. Smoking, alcohol and opioids effect on coronary microcirculation: An update overview. BMC Cardiovasc. Disord. 2021, 21, 185. [Google Scholar] [CrossRef]
- Anderson, G. Melatonin, BAG-1 and cortisol circadian interactions in tumor pathogenesis and patterned immune responses. Explor. Target. Antitumor Ther. 2023, 4, 962–993. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Serrano, A.B.; Marquez-Arrico, J.E.; Navarro, J.F.; Martinez-Nicolas, A.; Adan, A. Circadian Characteristics in Patients under Treatment for Substance Use Disorders and Severe Mental Illness (Schizophrenia, Major Depression and Bipolar Disorder). J. Clin. Med. 2021, 10, 4388. [Google Scholar] [CrossRef]
- Sethi, Y.; Padda, I.; Sebastian, S.A.; Malhi, A.; Malhi, G.; Fulton, M.; Khehra, N.; Mahtani, A.; Parmar, M.; Johal, G. Glucocorticoid Receptor Antagonism and Cardiomyocyte Regeneration Following Myocardial Infarction: A Systematic Review. Curr. Probl. Cardiol. 2023, 48, 101986. [Google Scholar] [CrossRef]
- Hadrich, I.; Turki, M.; Chaari, I.; Abdelmoula, B.; Gargouri, R.; Khemakhem, N.; Elatoui, D.; Abid, F.; Kammoun, S.; Rekik, M.; et al. Gut mycobiome and neuropsychiatric disorders: Insights and therapeutic potential. Front. Cell. Neurosci. 2025, 18, 1495224. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, T.; Cao, M.; Yuan, C.; Reiter, R.J.; Zhao, Z.; Zhao, Y.; Chen, L.; Fan, W.; Wang, X.; et al. Gut Microbiota Dysbiosis Induced by Decreasing Endogenous Melatonin Mediates the Pathogenesis of Alzheimer’s Disease and Obesity. Front. Immunol. 2022, 13, 900132. [Google Scholar] [CrossRef]
- Bijla, M.; Saini, S.K.; Pathak, A.K.; Bharadwaj, K.P.; Sukhavasi, K.; Patil, A.; Saini, D.; Yadav, R.; Singh, S.; Leeuwenburgh, C.; et al. Microbiome interactions with different risk factors in development of myocardial infarction. Exp. Gerontol. 2024, 189, 112409. [Google Scholar] [CrossRef]
- Penninx, B.; Lange, S.M.M. Metabolic syndrome in psychiatric patients: Overview, mechanisms, and implications. Dialogues Clin. Neurosci. 2018, 20, 63–73. [Google Scholar] [CrossRef]
- Vangrieken, P.; Scheijen, J.; Schiffers, P.M.H.; van de Waarenburg, M.P.H.; Foulquier, S.; Schalkwijk, C.C.G. Modelling the effects of elevated methylglyoxal levels on vascular and metabolic complications. Sci. Rep. 2025, 15, 6025. [Google Scholar] [CrossRef]
- Md, S.; Hong, S.M.; Lee, J.H.; Park, H.; Chang, K.A.; Kim, H.B.; Park, M.G.; Eo, H.; Oh, M.S.; Kim, S.Y. Depression like-behavior and memory loss induced by methylglyoxal is associated with tryptophan depletion and oxidative stress: A new in vivo model of neurodegeneration. Biol. Res. 2024, 57, 87. [Google Scholar] [CrossRef]
- Manjarrez-Gutiérrez, G.; Valero-Elizondo, G.; Serrano-Hernández, Y.; Mondragón-Herrera, J.A.; Mansilla-Olivares, A. Hypertrophic cardiomyopathy induces changes in the tryptophan-5-hydroxylase, serotonin transporter and serotonergic receptors expressions. Gac. Med. Mex. 2022, 158, 386–392. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, L.T.; Sia, C.-H. Pathophysiological Links Between Myocardial Infarction and Anxiety Disorder, Major Depressive Disorder, Bipolar Disorder and Schizophrenia. Biology 2025, 14, 336. https://doi.org/10.3390/biology14040336
Ong LT, Sia C-H. Pathophysiological Links Between Myocardial Infarction and Anxiety Disorder, Major Depressive Disorder, Bipolar Disorder and Schizophrenia. Biology. 2025; 14(4):336. https://doi.org/10.3390/biology14040336
Chicago/Turabian StyleOng, Leong Tung, and Ching-Hui Sia. 2025. "Pathophysiological Links Between Myocardial Infarction and Anxiety Disorder, Major Depressive Disorder, Bipolar Disorder and Schizophrenia" Biology 14, no. 4: 336. https://doi.org/10.3390/biology14040336
APA StyleOng, L. T., & Sia, C.-H. (2025). Pathophysiological Links Between Myocardial Infarction and Anxiety Disorder, Major Depressive Disorder, Bipolar Disorder and Schizophrenia. Biology, 14(4), 336. https://doi.org/10.3390/biology14040336





