Simple Summary
To reduce the influence of chemical fertilizers and pesticides on the cultivation of Fritillaria taipaiensis P. Y. Li, this study adopted the application of microbial fertilizer to mitigate soil damage and enhance the plant’s stress resistance. In this experiment, the growth index, enzyme activity, and gene expression of F. taipaiensis leaves were measured by applying nitrogen-fixing bacteria. The results showed that nitrogen-fixing bacteria could promote the growth and development of F. taipaiensis. This study not only provides a theoretical foundation for the subsequent cultivation technology of F. taipaiensis but also provides a new idea in terms of the realization of green planting of Chinese medicinal materials.
Abstract
The widespread application of chemical fertilizers and pesticides has resulted in environmental pollution. With the growing emphasis on ecological agriculture in traditional Chinese medicine, microbial fertilizers are increasingly recognized for their potential. The aim of this study is to investigate the effect of inoculating nitrogen-fixing bacteria on the soil (yellow loam, river sand, and organic fertilizer in a 2:1:1 ratio) of Fritillaria taipaiensis, with a focus on the leaf changes in terms of physiological parameters, antioxidant enzyme activity, and corresponding gene expression levels. The experiment involved three nitrogen-fixing bacteria, namely Rahnella aquatilis, Pseudomonas chlororaphis, and Paenibacillus stellifer, with a total of eight treatment groups. The objective was to assess how these bacterial treatments influenced physiological parameters, photosynthetic characteristics, pigment content, and both antioxidant enzyme activities and gene expression in the leaves of F. taipaiensis. The experimental results demonstrated statistically significant reductions (p < 0.05) in malondialdehyde (MDA) content and stomatal limitation value (LS) in F. taipaiensis leaves under treatment conditions relative to the control group (CK). The most substantial decreases were observed dual-inoculation with R. aquatilis and P. stellifer (N5), showing reductions of 38.24% and 20.94% in MDA and LS compared to CK values. Additionally, leaf area, leaf thickness, stem thickness, plant height, photosynthetic parameters, pigment content, soluble sugars, soluble proteins, proline levels, and the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) exhibited varying degrees of increase. Compared to the CK group, the SOD, POD, and CAT activities of the N5 group increased by 141.06%, 160.59%, and 106.23%, respectively. The relative gene expression patterns of SOD, POD, and CAT corresponded with the trends observed in their respective antioxidant enzyme activities. Pearson correlation analysis further demonstrated that leaf area and net photosynthetic rate (Pn) were significantly correlated with respect to SOD, POD, and CAT activities, as well as their corresponding gene expression levels. In conclusion, inoculation with nitrogen-fixing bacteria improved the growth and stress tolerance of F. taipaiensis, with the combined application of Rahnella aquatilis and Pseudomonas stellifer yielding the most effective results. This study establishes that different rhizosphere nitrogen-fixing bacteria, either individually or in combination, influence the photosynthetic characteristics, physiological and biochemical parameters, and protective enzyme systems of F. taipaiensis. These findings provide a theoretical foundation for the selection of nitrogen-fixing bacteria as biofertilizers in the artificial cultivation of F. taipaiensis and highlight their potential application in the cultivation of traditional Chinese medicinal materials.
1. Introduction
Fritillaria taipaiensis P. Y. Li is a perennial herbaceous plant of medicinal value belonging to the genus Fritillaria within the family Liliaceae. Traditionally, it has been utilized in the treatment of lung meridian ailments [1]. The wild populations of this species have encountered significant damage, approaching a state of depletion, primarily owing to extensive human excavation and subsequent destruction of the surrounding ecological habitat [2]. In response to the impending scarcity of this resource, the Chinese Pharmacopoeia has incorporated the dried bulb of F. taipaiensis as an acceptable cultivated alternative to F. cirrhosa D. Don [3]. The historical record of cultivating F. taipaiensis can be traced to the “Daning County Annals” [4], wherein it is proclaimed that “F. taipaiensis, produced in Yinchangping, is the best”; the term “Daning County” presently refers to Wuxi County in Chongqing City. Subsequently, domestication of wild F. taipaiensis was undertaken in Shaanxi, Sichuan, and Chongqing provinces [5,6,7].
With the excessive use of fertilizers, the Ministry of Agriculture and Rural Affairs has issued the “Action Plan for Chemical Fertilizer Reduction by 2025” [8], emphasizing the urgency to expedite the substitution of chemical fertilizers with organic fertilizers and promote the use of microbial fertilizers [9]. Continuous cropping obstacles and soil compaction, resulting from the extensive use of chemical fertilizers and pesticides in agricultural production, pose a significant threat to the quality of medicinal plants and soil health. However, the emergence of ecological agriculture principles within traditional Chinese medicine (TCM) cultivation has positioned microbial fertilizers as a strategic response to chemical fertilizer reduction mandates. These biofertilizers concurrently enhance medicinal plant yield and quality, preserve soil ecological integrity, and propel sustainable industrialization of TCM agroecosystems [10].
The screening of highly efficient plant growth-promoting bacteria constitutes a fundamental prerequisite for microbial fertilizer development [11]. Xiucheng Wang [12] identified Herbaspirillum seropedicae DX35, an endophytic nitrogen-fixing bacterium residing in rice root systems, which demonstrates multifunctional agricultural potential through its phosphate-solubilizing capacity and siderophore secretion capabilities. This strain biosynthesizes indole-3-acetic acid (IAA), a phytohormone that enhances root system development and facilitates nutrient absorption efficiency in rice plants. Associative nitrogen-fixing bacteria establish mutualistic symbiosis with sugarcane crops. These diazotrophic microorganisms exhibit a tripartite growth-promoting mechanism encompassing biological nitrogen fixation, inorganic phosphate solubilization, and phytohormone production, collectively contributing to enhanced plant growth and development [13].Additional investigations have revealed that the external inoculation of growth-promoting bacteria, in symbiosis with the root system of F. taipaiensis, can foster an advantageous arbuscular mycorrhizal structure, thus enhancing nutrient accumulation in medicinal materials [14].
Plant growth is fundamentally linked to the administration of nitrogen-containing fertilizers, the predominant source of which is synthetic industrial ammonia. This synthetic process is highly energy-intensive and contributes to greenhouse gas emissions [15], raising environmental concerns. Conversely, biological nitrogen fixation is a cost-effective alternative that is less prone to pollution through volatilization, denitrification, or leaching loss [16]. Biological nitrogen fixation enables the fixation of 100 million tons of nitrogen fertilizer annually [17], signifying its promising potential in cultivation practices. Biological nitrogen fixation relies on nitrogen-fixing bacteria, which convert atmospheric nitrogen into a nitrogen fertilizer that plants can directly utilize and supply for their growth and development [18]. Furthermore, plant growth is intricately connected to photosynthesis, a process whereby light energy is harnessed via chloroplasts to transform carbon dioxide and water into energy-storing organic matter while releasing oxygen. Within this process, chlorophyll functions as the principal agent of light energy absorption, conversion, and transmission; its content notably influences the photosynthetic rate [19]. Research has demonstrated that the application of microbial fertilizers can enhance chlorophyll accumulation [20], augment photosynthetic enzyme activity [21], and expedite the rate of photochemical reactions within plants [22].
When plants are damaged by the external environment, compounds such as malondialdehyde (MDA) and proline (Pro) act to mitigate or minimize physiological damage, while soluble sugar and soluble protein function in osmotic regulation, providing energy to organisms and enhancing their stress resistance [23]. MDA, a byproduct of lipid peroxidation in plants, is an indicator of cell membrane damage, and inversely its levels relate to stress resistance in plants [24].
Elevated SOD activity is frequently advantageous for plants inhabiting low-nitrogen environments [25]. In a drought stress-induced environment, Jiali Xie et al. [26] utilized “Longshu 7” as a research subject and potted plants as test material. By controlling water supply and applying varying amounts of potassium fertilizer, they demonstrated that leaf enzyme activity (superoxide dismutase (SOD), peroxidase (POD), catalase (CAT)) and Solanum tuberosum L. tubers yield could be improved. As indexes of stress resistance, enzymes such as SOD, POD, and CAT play pivotal roles in neutralizing reactive oxygen species and free radicals [27].
At present, the body of research concerning the impact of nitrogen-fixing bacteria inoculation on the physiological indexes and antioxidant capacity of F. taipaiensis leaves remains limited, so this study sought to address this by applying nitrogen-fixing bacteria isolated from the rhizosphere soil of F. taipaiensis to its cultivation process. In this study, the assessment was carried out through the measurement of various parameters, including the leaf area, photosynthetic characteristics, content of photosynthetic pigments, activities of antioxidant enzymes, and alterations in the leaf physiological index pertaining to F. taipaiensis leaves. The objective of this inquiry was to discern the influence of nitrogen-fixing bacteria inoculation on F. taipaiensis and to ascertain the most efficacious inoculation procedure. This empirical approach is aimed at augmenting existing cultivation techniques and furnishing a foundational basis for subsequent experiments.
2. Materials and Methods
2.1. Strain Isolation and Identification
Based on a literature review [28,29], the main distribution areas of F. taipaiensis were in Shaanxi, Hubei, Gansu, Chongqing, Sichuan, and other provinces. The rhizosphere soil of F. taipaiensis was collected from Chongqing, Sichuan, Shaanxi, Hubei, and Yunnan regions. The nitrogen-fixing bacteria were isolated from the rhizosphere soil by the dilution-coating plate method [30]. The isolated bacteria were cultured in a nitrogen-free medium (10 g mannitol, 0.2 g KH2PO4, 0.2 g MgSO4·7H2O, 0.2 g NaCl, 0.1 g CaSO4·2H2O, 5 g CaCO3, 20 g agar, and 1000 mL distilled water) in an incubator at 28 °C for 3 days. The purified strains were stored at −80 °C.
Gene phylogenetic analysis: The full-length 16S rDNA gene was amplified via polymerase chain reaction (PCR) using universal bacterial primers 27F (5′-AGAGTTTGATCCTGGCTGAG-3′) and 1492R (5′-TACGGCTACCTTGTTACGACTT-3′). Size-fractionation of the PCR products on a 1.3% agarose gel revealed a distinct amplified product of approximately 1500 bp. Amplified products were sequenced by Shenggong Biotechnology (Chengdu) Co., Ltd. (Chengdu, China). The obtained sequences were subjected to homology analysis using the BLAST (https://blast.ncbi.nlm.nih.gov/) algorithm against the GenBank nucleotide database. Phylogenetic reconstruction was performed with MEGA 7.0 software. A phylogenetic tree was constructed using the p-distance model, with 1000 bootstrap replicates.
The strains of nitrogen-fixing bacteria were preliminary screened using a nitrogen-fixing ring [31]. Nitrogen-fixation efficiency and indole-3-acetic acid (IAA) production capacity of the strain were determined by liquid fermentation [32] and Salkowski reaction [33]. Three dominant nitrogen-fixing bacteria were comprehensively screened out by using the entropy weight method [34]. We have previously accomplished these tasks, and detailed information can be obtained from a previous paper [33].
2.2. Test Site and Experimental Design
The experimental site was situated in Hongchi Dam, Wuxi County (31°38′8″ N, 108°56′30″ E), China. It features a subtropical warm and humid monsoon climate, sits at an elevation of approximately 1899 m, and has an average annual temperature of 13 °C, with an average annual precipitation that ranges from 1030 to 1950 mm. Moreover, the annual average sunshine duration is 1580 h. The test soil comprising yellow loam, river sand, and organic fertilizer (2:1:1) was obtained from Chongqing Three Gorges University. The fundamental physicochemical properties were as follows: soil pH, 7.52; organic matter content, 43.36 g/kg; total nitrogen content, 0.33 g/kg; alkaline nitrogen content, 37.88 mg/kg; available phosphorus content, 22.29 mg/kg; and available potassium content, 288.34 mg/kg.
F. taipaiensis bulbs were cultivated in 1.5-gallon plastic pots measuring 19.8 cm in outer diameter, 18 cm in base diameter, and 20 cm in depth. Prior to use, the pots were disinfected with 75% ethanol and allowed to dry. Eight discrete treatments were established for this experiment (see Table 1 for detailed information). In October 2022, F. taipaiensis bulbs were transplanted into pots for experimental purposes, with five bulbs per pot (total fresh weight approximately 1.5 g). In March 2023, bacterial strains were inoculated into a sterile beef extract–peptone liquid medium and cultured at 180 rpm for 2 days, individually. Then they were diluted with sterile water and the bacterial content was adjusted to approximately 1 × 108 CFU/mL. Subsequently, bacterial fertilizer was applied via a drenching method directly to the root zone of F. taipaiensis plants under controlled conditions at 18 °C. After a 40-day period, leaf samples from F. taipaiensis were harvested for analysis.

Table 1.
Experimental design.
2.3. Index Measurement
Assessment of growth indices with respect to F. taipaiensis leaves: In this experiment, ten potted plants under sunny weather conditions were selected from each parallel group, and the parameters of one plant in each pot was measured. Parameters such as leaf area, height, stem diameter, and leaf thickness were measured using either a standard ruler or a vernier caliper. The leaf area was ascertained utilizing the length–width ratio method [35].
Analysis of photosynthetic and pigment content in F. taipaiensis leaves: At an external CO2 concentration of 400 μmol/mol, the net photosynthetic rate (Pn), stomatal conductance (Cond), intercellular CO2 concentration (Ci), and transpiration rate (Tr) of F. taipaiensis leaves were evaluated using a photosynthesis analyzer (LI-6400, LI-COR, Inc., Lincoln, NE, USA) from 10:00 a.m. to 12:00 p.m. on 1 May. The stomatal limitation values (LS) were calculated according to the method described by Farquhar and Sharkey [36], and the content of photosynthetic pigments was determined using the methodology of Zhiliang Zhang [37].
Quantification of MDA, soluble sugar, soluble protein, and proline in F. taipaiensis leaves: In this experiment, six potted plants under sunny weather conditions were selected from each parallel group, and the indexes of one plant in each pot were measured. The concentrations of malondialdehyde (MDA) and soluble sugar were quantified by employing the thiobarbituric acid technique [38]; the soluble protein content was ascertained by the coomassie blue staining methodology [39]; and the proline content was measured by the ninhydrin method [40].
Evaluation of antioxidant enzyme activity in F. taipaiensis leaves by referencing the methodology of Kun Ge et al.: Six potted plants under sunny weather conditions were selected from each parallel group, and the indexes of one plant in each pot were measured. SOD activity was gauged through the nitrogen blue tetrazolium method [41]; POD activity was determined by the guaiacol chromogenic method [41]; and CAT activity was evaluated with ultraviolet spectrophotometry [41].
Analysis of gene expression associated with the antioxidant enzyme system in F. taipaiensis leaves: Total RNA was extracted from F. taipaiensis leaves using the TRIzol® Plus RNA Purification Kit. (Thermo Fisher Scientific, Waltham, MA, USA). Its content, purity, and quality were assessed by ultraviolet spectrophotometry and gel electrophoresis, with an optical density value (A260/A280) requirement of 1.8–2.0. RNA was reverse transcribed into cDNA using the SuperScript™ III First-Strand Synthesis Super Mix for qRT-PCR Kit (Thermo Fisher Scientific, Waltham, MA, USA), following a 25 °C incubation for 10 min, a 50 °C incubation for 30 min, and an 85 °C incubation for 5 min. The cDNA was stored at −20 °C for later use. Primer Premier 6.0 and Beacon Designer 7.8 software were utilized to create RT-qPCR primers (Table 2), synthesized by Nanjing Aoqing Biotechnology Co., Ltd. (Nanjing, China), employing the rp116 gene (Accession Numbers: MG525382.1) as an internal reference [42]. The fluorescence quantitative reaction system comprised 10 µL of 2 × Taq Pro Universal SYBR qPCR Master Mix, 1 μL of cDNA template, 0.4 μL each of forward and reverse primers, and an appropriate amount of water, with a total volume of 20 μL. The reaction conditions were as follows: 95 °C for 2 min, 95 °C for 20 s, and 58 °C for 20 s, for 39 cycles. A melting curve was also plotted, each sample was analyzed in triplicate, and the relative expression levels were calculated using the 2−∆∆Ct method [43].

Table 2.
Primer information for RT-qPCR analysis.
2.4. Statistical Analysis of Data
Data processing and organization were conducted using Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA), followed by statistical analyses performed in SPSS 24.0 (IBM Corp., Armonk, NY, USA). Graphical visualizations were generated with Origin Pro 2021 (OriginLab Corp., Northampton, MA, USA). Pearson correlation coefficients were calculated to assess linear relationships between variables. Single-factor analysis of variance (ANOVA) was applied with a 95% confidence interval (α = 0.05) to determine statistical significance.
3. Results
3.1. Strain Isolation and Screen
Fifteen strains of nitrogen-fixing bacteria were identified in the rhizosphere soil of F. taipaiensis (Figure 1). Their 16S ribosomal RNA gene sequences are detailed in Appendix A, Table A1. Phylogenetic analysis revealed that the 15 strains were taxonomically classified into 10 distinct genera spanning nine bacterial families. Notably, strains DY-7 and WXY-1 clustered within the Agrobacterium fabacearum clade, while strains LYX-2, WX-5, CK-1, and DY-2 showed close phylogenetic affinity to Rahnella aquatilis.
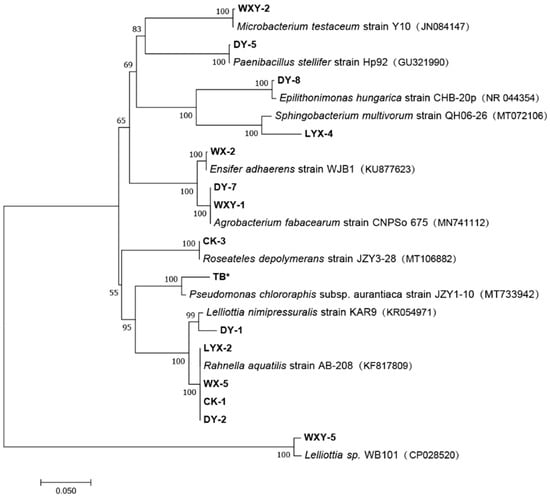
Figure 1.
Phylogenetic tree based on the 16S rDNA gene sequence. Note: numbers denote NCBI Accession Numbers; the scale bar denotes phylogenetic distance. * denotes a type of marker symbol, no functional implications.
The relevant growth-promoting indicators are presented in Table 3, while the detailed process for calculating nitrogen-fixation efficiency values is comprehensively described in Appendix A, Table A2. The nitrogen-fixing efficiency of the 15 strains ranged from 0.38 to 8.49 g/kg. Compared to the non-inoculated seed culture, WX-5 (Rahnella aquatilis) had the best ability at 8.49 g/kg, Pseudomonas chlororaphis was capable of 2.23 g/kg, and Paenibacillus stellifer was capable of 2.57 g/kg. Compared to the non-inoculated medium, Rahnella aquatilis, Pseudomonas chlororaphis, and Paenibacillus stellifer increased indole-3-acetic acid (IAA) levels by 7.42, 10.12, and 2.29 µg/mL, respectively.

Table 3.
Strain growth indices and comprehensive scores.
The comprehensive scores for Rahnella aquatilis, Pseudomonas chlororaphis, and Paenibacillus stellifer were 0.604, 0.494, and 0.470, respectively. The three strains secured first, third, and fifth positions in the composite ranking based on multiple growth-promoting indicators. Therefore, these three nitrogen-fixing bacteria were used in the preparation of bacterial fertilizer applied to F. taipaiensis.
3.2. Influences of Different Treatments on the Growth Index of F. taipaiensis Leaves
As delineated in Table 4, compared to the control (CK) group, distinct treatments were observed to augment the leaf area, leaf thickness, stem thickness, and plant height of F. taipaiensis, which was statistically significant (p < 0.05). Compared to the CK group, in the single-inoculation treatment groups (N1, N2, N3), no statistically significant differences of their effects on leaf area, stem diameter and plant height were observed. In the dual-inoculation treatments (N4, N5, N6), the N5 group exhibited a 61.11% increase in leaf area and a 73.45% increase in plant height compared to CK. Similarly, the N6 group showed a 96.25% enhancement in leaf thickness and a 28.44% increase in stem diameter. In the triple-inoculation treatment group (N7), the stem diameter was 1.29 times that of the CK group, an increase of 29.47%. Notably, dual-inoculation treatments outperformed both single-strain and triple-strain inoculation treatments, with the dual-inoculation of R. Aquatilis and P. stellifer (N5) demonstrating optimal growth promotion in F. taipaiensis.

Table 4.
Growth indexes of Fritillaria taipaiensis P. Y. Li under different treatments.
3.3. Influences of Different Treatments on Photosynthetic Parameters and Photosynthetic Pigment Content of F. taipaiensis Leaves
Table 5 shows the influences of diverse treatments on the photosynthetic parameters of F. taipaiensis leaves. Distinct treatments were observed to significantly increase net photosynthetic rate (Pn), stomatal conductance (Cond), intercellular CO2 concentration (Ci), and transpiration rate (Tr) values (p < 0.05). In contrast with the CK group, in the single-inoculation treatment groups, the Pn increase in the N2 treatment group was the most significant, at 20.21%, while Cond, Ci, and Tr increases in the N3 group were 1.16-fold, 1.22-fold and 1.16-fold those of the CK group, increasing by 16.18%, 21.72%, and 15.85%, respectively. Among the dual-inoculation treatment groups, the N5 group proved to be the most effective, augmenting the Pn, Cond, Ci, and Tr by 29.83%, 61.76%, 46.87%, and 41.93%, respectively, compared to the CK group. Notably, dual-inoculation treatments outperformed both single-strain and triple-strain inoculation treatments, with the dual-inoculation of R. Aquatilis and P. stellifer (N5) demonstrating optimal growth promotion in F. taipaiensis.

Table 5.
Photosynthetic parameters of F. taipaiensis leaves under different treatments.
Table 6 shows the levels of photosynthetic pigments in the F. taipaiensis leaves. In juxtaposition with the control group, chlorophyll a, chlorophyll b, and total chlorophyll content were significantly increased across treatments (p < 0.05). The chlorophyll a content of the treatment groups ranged from 0.82 to 1.02 mg/g, with a 7.91% increase observed in the single-inoculation treatment group (N1) and the largest increase observed in the dual-inoculation treatment group (N5), with a 10.62% increase relative to CK. The chlorophyll b content ranged from 0.24 to 0.35 mg/g, with the highest increase of 44.26% observed in the single-inoculation treatment group (N2) and 49.36% observed in the N5 group relative to CK. Expect for the N5 group, the carotenoid content of the other treatments was observed to be lower than that of the CK group. The total chlorophyll content ranged from 1.15 to 1.37 mg/g, with a 15.63% increase in the N1 group and the largest increase of 18.48% in the N5 group relative to CK. Notably, with the exception of N5, the carotenoid content of the treatments was lower than that of CK. Diverse treatments were capable of elevating the content of photosynthetic pigments in F. taipaiensis leaves, with the N5 group yielding the most pronounced effect.

Table 6.
Photosynthetic pigment levels in F. taipaiensis leaves under different treatments.
3.4. Influences of Different Treatments on MDA, Soluble Sugar, Soluble Protein, and Proline Levels of F. taipaiensis Leaves
As shown in Table 7, the malondialdehyde (MDA) content of F.taipaiensis leaves was significantly lower in the treatment groups than in the control (CK) group, while the concentrations of soluble protein and proline (Pro) were found to be significantly higher compared to the CK group (p < 0.05). All treatment groups exhibited lower MDA content compared to the CK group, with the dual-inoculation treatment group (N5) showing the most significant reduction (38.24%). In single-inoculation treatment groups, the N3 treatment enhanced soluble sugar and soluble protein content by 36.98% and 61.24%, respectively. Notably, the N5 group exhibited a 113.45% increase in soluble protein content relative to the CK group. Proline content in the triple-inoculation group (N7) reached a 1.72-fold increase with respect to CK levels, representing the highest elevation (71.64% increase) among all treatments.

Table 7.
MDA, soluble sugar, soluble protein, and proline content of F. taipaiensis leaves under different treatments.
3.5. Influences of Different Treatments on Antioxidant Enzyme Activities in the Leaves of F. taipaiensis
As shown in Figure 2, specific numerical values were documented in Appendix A, Table A3 and Table A4. The protective enzyme activity values were higher in all treatment groups compared with the CK group. Upon analyzing all the data, we noted that the SOD activity enhancement effect in the dual-inoculation treatment group (N5) was the best, followed by the N6 group. This effect was approximately 141.06% and 82.55% higher in the N5 and N6 groups than in the CK group, respectively. The POD enhancement effect in the N5 group was the best, which was approximately 160.59% higher than that of the CK group, followed by the N4 and N3 groups. The CAT activity enhancement effect in the N5 group was the most obvious, which was 106.23% higher than that of the CK group. The dual-inoculation treatment groups more significantly improved the protective enzyme activity. The SOD, POD, and CAT activities of dual-inoculation treatment group N7 were comparable to those observed in single-inoculation groups, with no significant difference detected (p > 0.05).
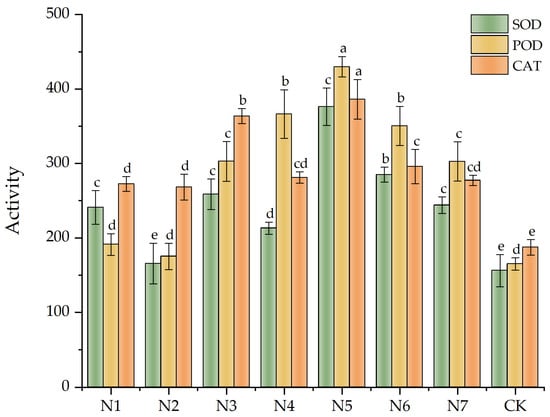
Figure 2.
Effects of antioxidant enzyme activities on Fritillaria taipaiensis P. Y. Li leaves under various treatments. Note: Within the same color group, differing lowercase letters denote statistically significant differences at the p < 0.05 level as determined by Fisher’s Least Significant Difference (LSD) post hoc test. Letter assignments follow a descending order of significance, where ‘a’ indicates the most pronounced difference, followed sequentially by ‘b’, with subsequent letters (c, d, etc.) representing progressively smaller magnitudes of divergence.
3.6. Influences of Different Treatments on Genes Related to Antioxidant Enzyme Systems in the Leaves of F. taipaiensis
RT-qPCR analysis revealed significant variations in the relative mRNA levels of SOD, POD, and CAT genes within the leaves of F. taipaiensis across distinct treatments (Figure 3), with specific numerical values documented in Appendix A, Table A5. The gene expression levels of SOD, POD, and CAT increased in all treatment groups compared with the CK group. The dual-inoculation treatment group N5 exhibited the most pronounced elevations, with SOD, POD, and CAT mRNA levels reaching 5.20-fold, 9.47-fold, and 8.52-fold increases with respect to CK values, respectively. In contrast, the dual-inoculation group N7 showed expression levels comparable to single-inoculation groups, with no statistically significant differences detected (p > 0.05). These findings indicate that dual-inoculation treatments exert differential modulatory effects on antioxidant gene expression, with the N5 group demonstrating the highest transcriptional activation levels among all experimental groups.
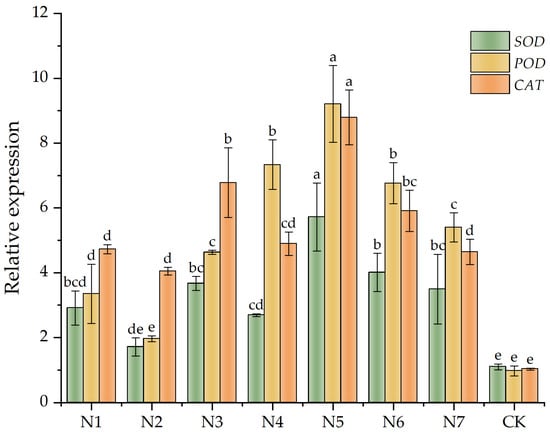
Figure 3.
SOD, POD, and CAT genes related to the protective enzyme systems in leaves of Fritillaria taipaiensis P. Y. Li. Note: Within the same color group, differing lowercase letters denote statistically significant differences at the p < 0.05 level as determined by Fisher’s Least Significant Difference (LSD) post hoc test. Letter assignments follow a descending order of significance, where ‘a’ indicates the most pronounced difference, followed sequentially by ‘b’, with subsequent letters (c, d, etc.) representing progressively smaller magnitudes of divergence.
3.7. The Pearson Correlation Analysis
In this study, the Pearson correlation analysis was performed on the leaf area; total chlorophyll, Pn, MDA, soluble sugar, soluble protein, and free proline content; the activity index of the three protective enzymes; and the relative gene expression levels of the three protective enzymes (Figure 4). Notably, leaf area correlated positively with Pn (r = 0.913, p < 0.01), while exhibiting a negative correlation with MDA (p < 0.01). A discernible negative association between MDA and assorted indicators was detected, with the most pronounced difference occurring with CAT activity (r = −0.863). There was a positive and significant interrelation between soluble protein and SOD, POD, and CAT activities along with their coding gene relative expression levels (p < 0.01). Leaf area and Pn were significantly associated with the activities of three enzyme three enzymes (SOD, POD, CAT) and their corresponding gene expression levels.
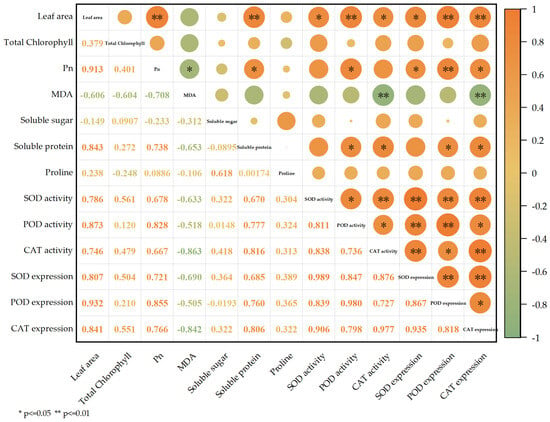
Figure 4.
The Pearson correlation analysis between different indicators. Note: Orange in the figure indicates a positive correlation, green indicates a negative correlation, and numbers indicate correlation coefficients. * denotes p ≤ 0.05, ** denotes p ≤ 0.01.
4. Discussion
The emerging field of rhizosphere growth-promoting bacteria highlights the immense potential these microorganisms hold for plant health and development, as demonstrated by numerous studies on medicinal plants. Compared to other plant growth-promoting rhizobacteria, such as phosphate-solubilizing bacteria, which break down organic phosphorus compounds [44], and potassium-solubilizing bacteria, which decompose insoluble minerals in the soil [45], nitrogen-fixing bacteria offer a distinct advantage due to their high nitrogen utilization efficiency. In this study, 15 strains of nitrogen-fixing bacteria were identified in the soil of F. taipaiensis. The three dominant nitrogen-fixing bacteria screened were R. Aquatilis, P. chlororaphis, and P. stellifer. The nitrogen-fixation efficiency of R. aquatilis was the highest (8.49 g/kg). P. stellifer was 2.57 g/kg; P. chlororaphis was 2.23 g/kg. Theoretically, there was a positive interaction between the nitrogen-fixation efficiency of the strain and the nitrogen-fixation capacity of the plant. For instance, a previous study has shown that inoculation of soil hosting maize with Pseudomonas stutzeri A1501, a diazotrophic strain capable of both biological nitrogen fixation and phytohormone production, significantly enhanced plant biomass and nitrogen content by 25.4% and 7.8%, respectively [46]. Similarly, under low-nitrogen conditions, the soil of wheat inoculated with endophytic Paenibacillus beijingensis BJ-18 showed an 86.1% increase in shoot dry weight, accompanied by a 1.5-fold to 91.9-fold upregulation of nitrogen uptake and metabolism-related genes, thereby improving nutrient absorption efficiency [47]. These findings collectively support the hypothesis that microbial nitrogen-fixing efficacy directly correlates with plant growth promotion and nitrogen utilization efficiency. Chao Ai et al. [48] highlighted that nitrogen-fixing bacteria not only interact with their host plants but may also establish connections with other functional bacteria, fungi, and viruses. In practical applications, nitrogen-fixing bacteria have been incorporated into microbial fertilizers to help reduce environmental pollution caused by excessive fertilizer use while simultaneously improving crop yield and quality. This dual benefit underscores the practical significance of this study.
In this study, the leaf photosynthetic characteristics of F. taipaiensis were analyzed. Plant growth and vitality are closely linked to nitrogen availability, as sufficient nitrogen promotes protein synthesis, which, in turn, stimulates cell division and growth [49]. Under the experimental conditions outlined in this study, growth parameters, including leaf area, leaf thickness, plant height, stem thickness, and total leaf number, showed varying degrees of improvement following inoculation with different nitrogen-fixing bacteria. The largest leaf area was observed in the N5 group, indicating that the dual-inoculation of R. aquatilis and P. stellifer significantly influenced the growth of F. taipaiensis. This finding is generally consistent with previously reported studies [50,51,52]. For instance, the application of microbial fertilizers has been shown to increase plant height in F. pallidiflora Schrenk [53], while compound microbial fertilizers containing nitrogen-fixing, phosphate-solubilizing, and potassium-solubilizing bacteria have been found to enhance the leaf area index and deepen leaf coloration [54]. In this study, combined bacterial treatments proved more effective than individual treatments, likely due to the diverse plant growth-regulating substances secreted by different bacterial strains, such as indole-3-acetic acid (IAA) and cytokinin. Additionally, plant growth is intrinsically tied to photosynthesis [55]. Chlorophyll, the primary pigment involved in photosynthesis, plays a vital role in plant development and yield [56,57]. The net photosynthetic rate (Pn) serves as a key indicator of photosynthetic activity in individual plants. The findings from this study demonstrate that inoculation with nitrogen-fixing bacteria positively influenced chlorophyll, carotenoid, Pn, Cond, Ci, and Tr in F. taipaiensis leaves. This effect may be attributed to sufficient nitrogen availability, which provides essential raw materials for chlorophyll and carotenoid synthesis. The resulting increase in pigment content enhances light absorption and conversion efficiency, ultimately raising Pn. These results align with the findings of Kaichao Wu et al. [58], who reported that inoculating sugarcane seedlings of different genotypes with the endogenous nitrogen-fixing bacterium Pantoea agglomerans led to increased chlorophyll a content and Pn during the elongation phase, surpassing the control group. Similarly, Shunyi Zheng et al. [59] found that inoculation with arbuscular mycorrhizal fungi improved Pn, Cond, and Tr in pepper leaves.
Nitrogen-fixing bacteria can enhance the stress resistance of F. taipaiensis. Under adverse conditions, malondialdehyde (MDA), a terminal product of membrane lipid peroxidation, serves as an indicator of cell membrane damage [60]. Intracellular compounds such as soluble sugars, soluble proteins, and proline, which are essential to cellular metabolism, contribute to plant resilience and survival under stress. This study demonstrated that various treatments significantly reduced MDA concentrations while increasing soluble protein and proline levels, findings that align with previous research [61]. In the dual-inoculation N5 treatment group, MDA levels exhibited a maximum reduction of 38.24% compared to the CK group, while soluble protein content increased by 113.45%. Conversely, in the tri-inoculation N7 treatment group, MDA levels, soluble sugar content, and soluble protein content remained comparable to those observed in the single-inoculation treatment group. Thus, we speculate that R. Aquatilis, P. Chlororaphis, and P. stellifer had a certain degree of antagonism or are related to the number of highly efficient nitrogen-fixing bacteria, which also explains why the enhancement effect on MDA, soluble protein, and proline was not as expected in the N7 group. In summary, combined bacterial inoculation demonstrates superior efficacy compared to single-inoculation treatment groups. This enhanced efficacy may arise from the synergistic effects of dual-strain co-inoculation, which likely induces systemic resistance in plants through distinct mechanisms, thereby improving their stress tolerance.
Antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) play a vital role in preventing the accumulation of reactive oxygen species (O2·− and H2O2), thereby reducing the risk of cell membrane peroxidation [62]. In this study, all treatments significantly enhanced the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), as well as the expression levels of their corresponding genes, compared to the CK group. Furthermore, plants inoculated with dual-strain treatments demonstrated superior antioxidant enzyme activity compared to those receiving single-strain treatments, indicating that combinatorial inoculation enhances physiological robustness. These findings align with previous studies reporting synergistic interactions between microbial inoculants and host antioxidant systems [63]. Notably, the dual-inoculation N5 group exhibited the highest antioxidant enzyme activities and relative gene expression levels among all treatments, while the tri-inoculation N7 group showed enzyme activities and gene expression levels comparable to those of the single-inoculation groups. This divergence may be attributed to strain-specific interactions, such as the secretion of antibiotics or competitive metabolites that interfere with the plant growth-promoting functions of co-inoculated strains, or to a threshold effect where dual-inoculation optimally stimulates antioxidant responses, whereas tri-inoculation triggers negative feedback regulation due to overstimulation. Collectively, our results demonstrate that all bacterial treatments effectively elevate SOD, POD, and CAT activities in F. taipaiensis leaves, underscoring the potential of nitrogen-fixing bacterial fertilizers to enhance plant antioxidant capacity and mitigate cellular damage.
The multifunctional synthetic microbial community based on biological nitrogen fixation has demonstrated significant potential for application in plant nutrition and growth promotion. However, the extent to which nitrogen-fixing bacteria can contribute to the development of a quality evaluation system for Chinese medicinal materials remains uncertain. In this study, we confirmed that different nitrogen-fixing bacteria and their combinations influenced the photosynthetic characteristics, fundamental physiological and biochemical parameters, and protective enzyme systems of F. taipaiensis. Future research should prioritize elucidating the assembly dynamics of rhizosphere microbiota and their co-evolutionary interplay with host plants, particularly through integrating systems-level approaches such as metagenomics, metatranscriptomics, and plant phenomics to decipher genotype × microbiome × environment interactions. By regulating the rhizosphere microbial environment, it may be possible to establish a scientific basis for the ecological cultivation of Chinese medicinal materials, ultimately ensuring the production of high-quality medicinal products.
5. Conclusions
The implementation of distinct nitrogen-fixing bacteria was found to have a pronounced effect on growth indices, photosynthetic parameters, photosynthetic pigment content, soluble sugar, soluble protein, proline, antioxidant enzyme activities, and coding gene relative mRNA levels in F. taipaiensis leaves. Furthermore, a reduction in MDA and LS content was observed. These findings underscore that the inoculation of combined strains demonstrated superior efficacy in fostering the growth of F. taipaiensis compared with the inoculation of single strains. Among the combinations, the dual-inoculation of R. aquatilis and P. stellifer yielded the most favorable outcomes. Therefore, in our study, the dual-inoculation of R. aquatilis and P. stellifer, which has been developed and promoted, serves as an effective technical measure. It can not only enhance the quality of F. taipaiensis and promote plant growth but also improve soil fertility and boost nitrogen utilization efficiency. In addition, it can also reduce fertilizer pollution, improve soil conditions, and create favorable conditions for the further cultivation of F. taipaiensis, forming a virtuous cycle in artificial cultivation.
Author Contributions
Conceptualization, M.Y.; methodology, M.Y.; software, M.Y.; formal analysis, M.Y. and J.L.; investigation, X.K., Z.S. and F.D.; resources, N.Z.; data curation, M.Y.; writing—original draft preparation, M.Y.; writing—review and editing, G.Q., D.G. and N.Z.; visualization, H.Z.; supervision, D.G. and N.Z.; project administration, N.Z.; funding acquisition, N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
A project of the Chongqing Natural Science Foundation, CSTB2023NSCQ-LMX0010, studied the mechanism of the rhizosphere microbiome on the formation quality of Fritillaria taipaiensis P. Y. Li based on the SynCom method; a scientific and technological research project of the Chongqing Municipal Education Commission, KJZD-M202301204, studied the influencing mechanisms of rhizosphere soil factors on the quality of endangered Fritillaria taipaiensis P. Y. Li; a project of the Wanzhou District Science and Technology Bureau of Chongqing, WZSTC-20230202, studied the integration and application of fengtang plum fruit rate improvement, quality, and efficiency improvement technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data from this study can be obtained from the authors upon request.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Appendix A

Table A1.
16S rDNA gene sequences of strains.
Table A1.
16S rDNA gene sequences of strains.
| Strain | 16S rDNA Gene Sequences |
|---|---|
| WX-2 | AACKGGCGGCMGGCTTAACaCATGCgAGTCGAgCGCCCCGCAAGGGGAGYGGCMGACGGGTGAGTAACGCGTGGGAATCTACCSTKYYCTRCGGAATAACKCMKGGAAACKKGWRYTAATACCGYATRMGCCCTWCGGGGGAAAGATTTATCGGGRAAKGATGAGCCCGCGTTGGATTAGCTAGTTGGWGGGGTAAAGGCCTACCAAGGCGACGATCCATAGCTGGTCTGASAGGATGATCARCCACATTGGGACTGARACACGGSCCAAACTCCTACGGGAGGCAGCAGTGGGGAATATTGGACAATGGGCGCAAGCCTGATCCAGCCATGCCGCGTGAGTGATGAAGGCCYTAGGGTTGTAAAGCTCTTTCACCGGTGAAGATAATGACGGTAACCGGAGAAGAAGCCCCGGCTAACTTCGTGCCARCAGCCGCGGTAATACGAAGGGGGCTAGCGTTGTTCGGAATTACTGGGCGTAAAGCGCACGTAGGCGGACATTTAAGTCAGGGGTGAAATCCCGGGGCTCAACCCCGGAACTGCCTTTGATACTGGGTGTCTAGAGTATGGAAGAGGTGAGTGGAATTCCGAGTGTAGAGGTGAAATTCGTAGATATTCGGAGGAACACCAGTGGCGAAGGCGGCTCACTGGTCCATTACTGACGCTGAGGTGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGAATGTTAGCCGTCGGGCAGTWTACTGTTCGGTGGCGCAGCTAACGCATTAAACATTCCGCCTGGGGAGTACGGTCGCAAGATTAAAACTCAAAGGAATTGACGGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTYGAAGCAACGCGCAGAACCTTACCAGCCCTTGACATCCCGATTKGGGcATTacGGAGACGTTTTTCCTTCAGTTTGGCTGGATCGGAgACAGGTGSTGCATGGCTGTTGTCAGCTYGTGTYGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTCSCCCTTAGTTGCCAGCATTTAGTTGGGCMCTYTAAGGGGACTGCCGGTGATAAGCCGAGAGGAAGGTGGGGATGACGTCAAGTCYTCATGGCCCTTACGGGYTGGGCTACACACGTGCTACAATGGTGGTGACAGTGGGCAGCGAGACMGCGAKGTCGAGCTAATCTCCAAAASCCATCTCAGTTCGGATTGCACTCTGCAACTCGAGTGCATGAAGTTGGAATCGCTAGTAATCGCAGATCAGCATGYTGCGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACACCATGGGAGTTGGTTHTMCCCGAAGGTAGTGCGCTAACCGCAAGGAGGCAGCTAACCACaGKAGGTMAMGGGGGG |
| WX-5 | CGCAGTGCGGCAGCTACACATGCAGTCGAGCGGCAGCGGAAAGTAGCTTGCTACTTTGCCGGCGAGCGGCGGACGGGTGAGTAATGTCTGGGAAACTGCCTGATGGAGGGGGATAACTACTGGAAACGGTAGCTAATACCGCATGACCTCGAAAGAGCAAAGTGGGGGATCTTCGGACCTCACGCCATCGGATGTGCCCAGATGGGATTAGCTAGTAGGTGAGGTAATGGCTCACCTAGGCGACGATCCCTAGCTGGTCTGAGAGGATGACCAGCCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAGTGGGGAATATTGCACAATGGGCGCAAGCCTGATGCAGCCATGCCGCGTGTGTGAAGAAGGCCTTAGGGTTGTAAAGCACTTTCAGCGAGGAGGAAGGCATCACACTTAATACGTGTGGTGATTGACGTTACTCGCAGAAGAAGCACCGGCTAACTCCGTGCCAGCAGCCGCGGTAATACGGAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGCACGCAGGCGGTTTGTTAAGTCAGATGTGAAATCCCCGCGCTTAACGTGGGAACTGCATTTGAAACTGGCAAGCTAGAGTCTTGTAGAGGGGGGTAGAATTCCAGGTGTAGCGGTGAAATGCGTAGAGATCTGGAGGAATACCGGTGGCGAAGGCGGCCCCCTGGACAAAGACTGACGCTCAGGTGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCTGTAAACGATGTCGACTTGGAGGTTGTGCCCTTGAGGCGTGGCTTCCGGAGCTAACGCGTTAAGTCGACCGCCTGGGGAGTACGGCCGCAAGGTTAAAACTCAAATGAATTGACGGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGATGCAACGCGAAGAACCTTACCTACTCTTGACATCCACGGAATTCGCCAGAGATGGCTTAGTGCCTTCGGGAACCGTGAGACAGGTGCTGCATGGCTGTCGTCAGCTCGTGTTGTGAAATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTTATCCTTTGTTGCCAGCACGTAATGGTGGGAACTCAAAGGAGACTGCCGGTGATAAACCGGAGGAAGGTGGGGATGACGTCAAGTCATCATGGCCCTTACGAGTAGGGCTACACACGTGCTACAATGGCATATACAAAGAGAAGCGAACTCGCGAGAGCAAGCGGACCTCATAAAGTATGTCGTAGTCCGGATTGGAGTCTGCAACTCGACTCCATGAAGTCGGAATCGCTAGTAATCGTAGATCAGAATGCTACGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACACCATGGGAGTGGGTTGCAAAAGAAGTAGGTAGCTTAACCTTCGGGAGGGCGCTTACCACTTGGAATCCGGGGG |
| DY-1 | TTGCGGGCGGGCTACACATGCAGTCGAGCGGTAGCACAGAGAGCTTGCTCTCGGGTGACGAGCGGCGGACGGGTGAGTAATGTCTGGGAAACTGCCTGATGGAGGGGGATAACTACTGGAAACGGTAGCTAATACCGCATAACGTCTCAAGACCAAAGAGGGGGACCTTCGGGCCTCTTGCCATCTCATGTGCCCAGATGGGATTAGCTAGTAGGTGGGGTAATGGGTCACCTATGCGACAATCCCTATCTGGTCTGAGAGGATGACCACCCACACTGGAACTGAGACACGGTCCACACTCCTACGGGAGGCAGCAGTGGGGAATATTGCGCAATGGGCGCAAGCCTGATGCACCCATGCCGCGTGTATGAAAAAAGCCTTCTGGTTGTAAAGTACTTTCTCCGAGGAGGAAAGCATTGTGGTTAATAACCCCAGTGATTGACGTTACTCTCACAAAAAACACCGGCTAACTCCCTGCCACCAGCCGCGGTAATACAGAGGGTGCAAGCGTTAATCTGAATTACTGGGCGTAAAGCGCACGCASGCGGTCTGTCAAGTCGGATGTGAAATCCCCGGGCTCAACCTGGGAACTGCRTTCGAAACTGGCAGGCTAKAGTCTTGTAGAGGGGGGTAGAATTCCAGGTGTAGCGGTGAAATGCGTAGAGATCTGGAGGAATACCGGTGGCGAARGCGGCCCCCTGGACAAAGACTGACRCTCASGTGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGTCKACTTGGAGGTTGTTCCCTTGAGGAGTGGCTTCCGGAGCTAACGCGTTAAGTCGACCGCCTGGGGAGTACGGCCGCAAGGTTAAAACTCAAATGAATTGACGGGGGCCCGCACAAgCGGTGGAGCATGTGGTTTAATTCGATGCaACGCGAAGAACCTtACCTACTCTTGACATCCACGGAATTTAGCAGAGATGCTTTAGTGCCTTCGGGAACCGTGAGACAGGTGCTGCATGGCTGTCGTCAGCTCGTGTTGTGAAATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTTATCCTTTGTTGCCAGCGGTTCGGCCGGGAACTCAAAGGAGACTGCCAGTGATAAACTGGAGGAAGGTGGGGATGACGTCAAGTCATCATGGCCCTTACGAGTAGGGCTACACACGTGCTACAATGGCGCATACAAAGAGAAGCGACCTCGCGAGAGCAAGCGGACCTCATAAAGTGCGTCGTAGTCCGGATTGGAGTCTGCAACTCGACTCCATGAAGTCGGAATCGCTAGTAATCGTAGATCAGAATGCTACGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACACCATGGGAGTGGGTTGCAAAAGAAGTAGGTAGCTTAACCTTCGGGAGGGCGCTTACCACTTTTGATAA |
| DY-2 | CGCAAGGGGGGCAGCTACACATGCAGTCGAGCGGCAGCGGAAAGTAGCTTGCTACTTTGCCGGCGAGCGGCGGACGGGTGAGTAATGTCTGGGAAACTGCCTGATGGAGGGGGATAACTACTGGAAACGGTAGCTAATACCGCATGACCTCGAAAGAGCAAAGTGGGGGATCTTCGGACCTCACGCCATCGGATGTGCCCAGATGGGATTAGCTAGTAGGTGAGGTAATGGCTCACCTAGGCGACGATCCCTAGCTGGTCTGAGAGGATGACCAGCCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAGTGGGGAATATTGCACAATGGGCGCAAGCCTGATGCAGCCATGCCGCGTGTGTGAAGAAGGCCTTAGGGTTGTAAAGCACTTTCAGCGAGGAGGAAGGCATCATACTTAATACGTGTGGTGATTGACGTTACTCGCAGAAGAAGCACCGGCTAACTCCGTGCCAGCAGCCGCGGTAATACGGAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGCACGCAGGCGGTTTGTTAAGTCAGATGTGAAATCCCCGCGCTTAACGTGGGAACTGCATTTGAAACTGGCAAGCTAGAGTCTTGTAGAGGGGGGTAGAATTCCAGGTGTAGCGGTGAAATGCGTAGAGATCTGGAGGAATACCGGTGGCGAAGGCGGCCCCCTGGACAAAGACTGACGCTCAGGTGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCTGTAAACGATGTCGACTTGGAGGTTGTGCCCTTGAGGCGTGGCTTCCGGAGCTAACGCGTTAAGTCGACCGCCTGGGGAGTACGGCCGCAAGGTTAAAACTCAAATGAATTGACGGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGATGCAACGCGAAGAACCTTACCTACTCTTGACATCCACGGAATTCGCCAGAGATGGCTTAGTGCCTTCGGGAACCGTGAGACAGGTGCTGCATGGCTGTCGTCAGCTCGTGTTGTGAAATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTTATCCTTTGTTGCCAGCACGTAATGGTGGGAACTCAAAGGAGACTGCCGGTGATAAACCGGAGGAAGGTGGGGATGACGTCAAGTCATCATGGCCCTTACGAGTAGGGCTACACACGTGCTACAATGGCATATACAAAGAGAAGCGAACTCGCGAGAGCAAGCGGACCTCATAAAGTATGTCGTAGTCCGGATTGGAGTCTGCAACTCGACTCCATGAAGTCGGAATCGCTAGTAATCGTAGATCAGAATGCTACGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACACCATGGGAGTGGGTTGCAAAAGAAGTAGGTAGCTTAACCTTCGGGAGGGCGCTACCACTTTGAATTCCAGG |
| DY-5 | GAACGGGGGCGGCGTGCTATACATGCAAGTCGAGCGGAGTTATGAAGGAGCTTGCTCCGGATTAACTTAGCGGCGGACGGGTGAGTAACACGTAGGCAACCTGCCCTTCAGACTGGGATAACTACCGGAAACGGTAGCTAATACCGGATAATTCCTTTCTTCTCCTGAGGAAAGGATGAAAGACGGAGCAATCTGTTACTGAGGGATGGGCCTGCGGCGCATTAGCTAGTTGGTGGGGTAACGGCTCACCAAGGCGACGATGCGTAGCCGACCTGAGAGGGTGAACGGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTAGGGAATCTTCCGCAATGGGCGAAAGCCTGACGGAGCAACGCCGCGTGAGTGATGAAGGTTTTCGGATCGTAAAGCTCTGTTGCCAGGGAAGAACGTCTCTTAGAGTAACTGCTAAAAGAGTGACGGTACCTGAGAAGAAAGCCCCGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGGGGCAAGCGTTGTCCGGAATTATTGGGCGTAAAGCGCGCGCAGGCGGCGATTTAAGTCTGGTGTTTAAACCATGGGCTCAACCTGTGGTCGCATCGGAAACTGGATGGCTTGAGTGCAGAAGAGGAAAGTGGAATTCCACGTGTAGCGGTGAAATGCGTAGAGATGTGGAGGAACACCAGTGGCGAAGGCGACTTTCTGGGCTGTAACTGACGCTGAGGCGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGAGTGCTAGGTGTTAGGGGTTTCGATACCCTTGGTGCCGAAGTTAACACAGTAAGCACTCCGCCTGGGGAGTACGGTCGCAAGACTGAAACTCAAAGGAATTGACGGGGACCCGCACAAGCAGTGGAGTATGTGGTTTAATTCGAAGCAACGCGAAGAACCTTACCAGGTCTTGACATCCCCCTGAATACGTTAGAGATAGCGTAGGCCTTCGGGACAGGGGAGACAGGTGGTGCATGGTTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTTGACTTTAGTTGCCAGCAGGTAAGGCTGGGCACTCTAGAGTGACTGCCGGTGACAAACCGGAGGAAGGTGGGGATGACGTCAAATCATCATGCCCCTTATGACCTGGGCTACACACGTACTACAATGGCCGGTACAACGGGAAGCGAAACCGCGAGGTGGAGCCAATCTTATAAAGCCGGTCTCAGTTCGGATTGCAGGCTGCAACTCGCCTGCATGAAGTCGGAATTGCTAGTAATCGCGGATCAGCATGCCGCGGTGAATACGTTCCCGGGTCTTGTACACACCGCCCGTCACACCACGAGAGTTTACAACACCCGAAGTCGGTGGGGTAACCCGTAAGGGAGCCAGCCGCCGAAGGTGGGTAAGATAAGT |
| DY-7 | GAAACCTGGGCCGGGCAGGGCTTAACACATGCAAGTCGAACGCCCCGCAAGGGGAGTGGCAGACGGGTGAGTAACGCGTGGGAATCTACCGTGCCCTGCGGAATAGCTCCGGGAAACTGGAATTAATACCGCATACGCCCTACGGGGGAAAGATTTATCGGGGTATGATGAGCCCGCGTTGGATTAGCTAGTTGGTGGGGTAAAGGCCTACCAAGGCGACGATCCATAGCTGGTCTGAGAGGATGATCAGCCACATTGGGACTGAGACACGGCCCAAACTCCTACGGGAGGCAGCAGTGGGGAATATTGGACAATGGGCGCAAGCCTGATCCAGCCATGCCGCGTGAGTGATGAAGGCCTTAGGGTTGTAAAGCTCTTTCACCGGAGAAGATAATGACGGTATCCGGAGAAGAAGCCCCGGCTAACTTCGTGCCAGCAGCCGCGGTAATACGAAGGGGGCTAGCGTTGTTCGGAATTACTGGGCGTAAAGCGCACGTAGGCGGATATTTAAGTCAGGGGTGAAATCCCAGAGCTCAACTCTGGAACTGCCTTTGATACTGGGTATCTTGAGTATGGAAGAGGTAAGTGGAATTCCGAGTGTAGAGGTGAAATTCGTAGATATTCGGAGGAACACCAGTGGCGAAGGCGGCTTACTGGTCCATTACTGACGCTGAGGTGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGAATGTTAGCCGTCGGGCAGTATACTGTTCGGTGGCGCAGCTAACGCATTAAACATTCCGCCTGGGGAGTACGGTCGCAAGATTAAAACTCAAAGGAATTGACGGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGAAGCAACGCGCAGAACCTTACCAGCTCTTGACATTCGGGGTTTGGGCAGTGGAGACATTGTCCTTCAGTTAGGCTGGCCCCAGAACAGGTGCTGCATGGCTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTCGCCCTTAGTTGCCAGCATTTAGTTGGGCACTCTAAGGGGACTGCCGGTGATAAGCCGAGAGGAAGGTGGGGATGACGTCAAGTCCTCATGGCCCTTACGGGCTGGGCTACACACGTGCTACAATGGTGGTGACAGTGGGCAGCGAGACAGCGATGTCGAGCTAATCTCCAAAAGCCATCTCAGTTCGGATTGCACTCTGCAACTCGAGTGCATGAAGTTGGAATCGCTAGTAATCGCAGATCAGCATGCTGCGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACACCATGGGAGTTGGTTTTACCCGAAGGTAGTGCGCTAACCGCAAGGAGGCAGCTAACCACGTAGTCAGGCGGTGTT |
| DY-8 | GATGCCCCTTGCCGGGGAGGCTACACATGCAAGCCGAGCGGTAGAGGTCCTTCGGGACCTTGAGAGCGGCGCACCCGGTGCGGAACACGTGTGCAACCTGCCTTTATCTGGGGGATAGCCTTTCGAAAGGAAGATTAATACCCCATAATATATTGAATGGCATCATTTGATATTGAAAACTCCGGTGGATAGAGATGGGCACGCGCAAGATTAGATAGTTGGTAGGGTAACGGCCTACCAAGTCGATGATCTTTAGGGGGCCTGAGAGGGTGATCCCCCACACTGGTACTGAGACACGGACCAGACTCCTACGGGAGGCAGCAGTGAGGAATATTGGACAATGGGTGAGAGCCTGATCCAGCCATCCCGCGTGAAGGACGACGGCCCTATGGGTTGTAAACTTCTTTTGTACAGGGATAAACCTTTCCACGTGTGGAAAGCTGAAGGTACTGTACGAATAAGCACCGGCTAACTCCGTGCCAGCAGCCGCGGTAATACGGAGGGTGCAAGCGTTATCCGGATTTATTGGGTTTAAAGGGTCCGTAGGCGGGCCTGTAAGTCAGTGGTGAAATCTCATAGCTCAACTATGAAACTGCCATTGATACTGCAGGCCTTGAGTAGATTTGAAGTGGCTGGAATAAGTAGTGTAGCGGTGAAATGCATAGATATTACTTAGAACACCAATTGCGAAGGCAGGTCACTAAGATCTAACTGACGCTGATGGACGAAAGCGTGGGGAGCGAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGCTAACTCGTTTTTGGGCTTTCGGGCTCAGAGACTAAGCGAAAGTGATAAGTTAGCCACCTGGGGAGTACGTTCGCAAGAATGAAACTCAAAGGAATTGACGGGGGCCCGCACAAGCGGTGGATTATGTGGTTTAATTCGATGATACGCGAGGAACCTTACCAAGACTTAAATGGGAAATGACAGATTTAGAAATAGATCCTTCTTCGGACATTTTCCAAGGTGCTGCATGGTTGTCGTCAGCTCGTGCCGTGAGGTGTTAGGTTAAGTCCTGCAACGAGCGCAACCCCTGTCACTAGTTGCTACCATTAAGTTGAGGACTCTAGTGAGACTGCCTACGCAAGTAGAGAGGAAGGTGGGGATGACGTCAAATCATCACGGCCCTTACGTCTTGGGCCACACACGTAATACAATGGCCGGTACAGAGGGCAGCTACACAGCGATGTGATGCAAATCTCGAAAGCCGGTCTCAGTTCGGATTGGAGTCTGCAACTCGACTCTATGAAGCTGGAATCGCTAGTAATCGCGCATCAGCCATGGCGCGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCAAGCCATGGAAGTCTGGGGTACCTGAAGTCGGTGACCGTAACAGGAGCTGCCTAGGTTAAACCAAGGAATCGT |
| CK-1 | CGCAGTGGGGCAGCTACACATGCAGTCGAGCGGCAGCGGAAAGTAGCTTGCTACTTTGCCGGCGAGCGGCGGACGGGTGAGTAATGTCTGGGAAACTGCCTGATGGAGGGGGATAACTACTGGAAACGGTAGCTAATACCGCATGACCTCGAAAGAGCAAAGTGGGGGATCTTCGGACCTCACGCCATCGGATGTGCCCAGATGGGATTAGCTAGTAGGTGAGGTAATGGCTCACCTAGGCGACGATCCCTAGCTGGTCTGAGAGGATGACCAGCCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAGTGGGGAATATTGCACAATGGGCGCAAGCCTGATGCAGCCATGCCGCGTGTGTGAAGAAGGCCTTAGGGTTGTAAAGCACTTTCAGCGAGGAGGAAGGCATCACACTTAATACGTGTGGTGATTGACGTTACTCGCAGAAGAAGCACCGGCTAACTCCGTGCCAGCAGCCGCGGTAATACGGAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGCACGCAGGCGGTTTGTTAAGTCAGATGTGAAATCCCCGCGCTTAACGTGGGAACTGCATTTGAAACTGGCAAGCTAGAGTCTTGTAGAGGGGGGTAGAATTCCAGGTGTAGCGGTGAAATGCGTAGAGATCTGGAGGAATACCGGTGGCGAAGGCGGCCCCCTGGACAAAGACTGACGCTCAGGTGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCTGTAAACGATGTCGACTTGGAGGTTGTGCCCTTGAGGCGTGGCTTCCGGAGCTAACGCGTTAAGTCGACCGCCTGGGGAGTACGGCCGCAAGGTTAAAACTCAAATGAATTGACGGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGATGCAACGCGAAGAACCTTACCTACTCTTGACATCCACGGAATTCGCCAGAGATGGCTTAGTGCCTTCGGGAACCGTGAGACAGGTGCTGCATGGCTGTCGTCAGCTCGTGTTGTGAAATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTTATCCTTTGTTGCCAGCACGTAATGGTGGGAACTCAAAGGAGACTGCCGGTGATAAACCGGAGGAAGGTGGGGATGACGTCAAGTCATCATGGCCCTTACGAGTAGGGCTACACACGTGCTACAATGGCATATACAAAGAGAAGCGAACTCGCGAGAGCAAGCGGACCTCATAAAGTATGTCGTAGTCCGGATTGGAGTCTGCAACTCGACTCCATGAAGTCGGAATCGCTAGTAATCGTAGATCAGAATGCTACGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACACCATGGGAGTGGGTTGCAAAAGAAGTAGGTAGCTTAACCTTCGGGAGGGCGCTACCACCTTTGATTACCGG |
| CK-3 | GTAGTTGCGGCATGCTTAACATGCAGTCGAACGGTAACGCGGGGCAACCTGGCGACGAGTGGCGAACGGGTGAGTAATATATCGGAACGTGCCCAGTTGTGGGGGATAACTGCTCGAAAGAGCAGCTAATACCGCATACGACCTGAGGGTGAAAGCGGGGGATCGCAAGACCTCGCGCAATTGGAGCGGCCGATATCAGATTAGGTAGTTGGTGGGGTAAAGGCCTACCAAGCCGACGATCTGTAGCTGGTCTGAGAGGACGACCAGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTGGGGAATTTTGGACAATGGGGGCAACCCTGATCCAGCCATGCCGCGTGCGGGAAGAAGGCCTTCGGGTTGTAAACCGCTTTTGTCAGGGAAGAAAAGACTCCTACTAATACTGGGGGTTCATGACGGTACCTGAAGAATAAGCACCGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGTGCGCAGGCGGTTATGCAAGACAGATGTGAAATCCCCGGGCTCAACCTGGGAACTGCATTTGTGACTGCATGGCTAGAGTACGGTAGAGGGGGATGGAATTCCGCGTGTAGCAGTGAAATGCGTAGATATGCGGAGGAACACCGATGGCGAAGGCAATCCCCTGGACCTGTACTGACGCTCATGCACGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCCCTAAACGATGTCAACTGGTTGTTGGGAGGGTTTCTTCTCAGTAACGTAGCTAACGCGTGAAGTTGACCGCCTGGGGAGTACGGCCGCAAGGTTGAAACTCAAAGGAATTGACGGGGACCCGCACAAGCGGTGGATGATGTGGTTTAATTCGATGCAACGCGAAAAACCTTACCTACCCTTGACATGCCAGGAATCCTGCAGAGATGTGGGAGTGCTCGAAAGAGAACCTGGACACAGGTGCTGCATGGCCGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTTGTCATTAGTTGCTACGAAAGGGCACTCTAATGAGACTGCCGGTGACAAACCGGAGGAAGGTGGGGATGACGTCAGGTCATCATGGCCCTTATGGGTAGGGCTACACACGTCATACAATGGCCGGGACAGAGGGCTGCCAACCCGCGAGGGGGAGCTAATCCCAGAAACCCGGTCGTAGTCCGGATCGCAGTCTGCAACTCGACTGCGTGAAGTCGGAATCGCTAGTAATCGCGGATCAGCTTGCCGCGGTGAATACGTTCCCGGGTCTTGTACACACCGCCCGTCACACCATGGGAGCGGGTTCTGCCAGAAGTAGTTAGCCTAACCGCAAGGGGGGCGATACCACCGGCAGGTTCCGTTCCC |
| TB * | GACCTTCGGGCCTTGCGCTATCAGATGAGCCTAGGTCGGATTAGCTAATTGGTGAGGTAATGGCTCACCAAAGGAACGATCCGTAACTGGTCTGAAAGGATGATCAGTCCCTCTGGAACTGAGACACGGGCCATACTCCTACGGGAGGCCGCAGTGGGGAATATTGGACAATGGGCGAAAGCCTGATCCAGCCATGCCGCGTGTGTGAAGAAGGTCTTCGGATTGTAAAGCACTTTAAATTGGGAGGAAGGGTTGTTCATGAATACTCTGCAATTTTGACGTTACCGACTGAATAAGCTCCGAATAACTCTGTGCCAACAGCCGCGGTAATACTAAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGCKCGTAGGTGGTTCGTTAATCTGGATGTGAAATCCCCGGGCTCAACCTGGRAACTGCATCCRAACTGGCGAGCTWKAGTATGGWACAGGGTgGTGGAATTTCCTGTGTAGCGGTGAAATGCRTAKATATAtGRAGGAACACCAGTGGCGAASGCGACCACCTGSACTGATACTGACAcTGASGTGCGAAAaGCGTGRGGAGCAAACASGATTASaTACCCTGGDVgTCCACKCCGTAWaCGATGTCAwCTAGCCKTTGGGAGCCTTGAGCTCTTAGTGKCGCAGCTAACGCATTAAGTTGACCGCCTGGGGAGTACGKCCGCAAGGWTAAAACTCAAATGAATTGACGGKGSCCCGCACAAGCGGTGGAGCWTGTGGTTtAATTMGAAGCAACgCGRAGAACCTTACCAGGCCTTCACATCCAATGAACTTTCCAGAGATGGATTGGTGCATTCGGGAACATTGAGACAGGTGCTGCATGGCTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGTAACGAGAGCACCCCTTTTCCCTAGTTACCACCACGTAATGGTGGGCACTCTAAGGAGACTGCCGGTGACAAACCGGAGGAAGGTGGGGAAGACGTCAAGTCCTCATGGCCCTTACGGCCTGGGCTACCCACGTGCTACCATGGTCGGTACAAAGGGTCACCAAGCCACGAGGTGGAGCTAATTCCATAAAACCGATCGTAGTCCGGATCGCAGTCTGCAACTCGAGTGCGTGAAGTCGGAATCGCTAGTAATCGCGAATCAGAATGTCGCGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACACCATGGGAGTGGGTTGCACCAGAAGTAGTTAGTTTAACCTTAAGGAGGAACGTTTACCCCCGGTGGTTCCAGGGGGT |
| LYX-2 | CATACTTTCCGGCGGGCCTAACACATGCAAGTCGAGCGGCAGCGGAAAGTAGCTTGCTACTTTGCCGGCGAGCGGCGGACGGGTGAGTAATGTCTGGGAAACTGCCTGATGGAGGGGGATAACTACTGGAAACGGTAGCTAATACCGCATGACCTCGAAAGAGCAAAGTGGGGGATCTTCGGACCTCACGCCATCGGATGTGCCCAGATGGGATTAGCTAGTAGGTGAGGTAATGGCTCACCTAGGCGACGATCCCTAGCTGGTCTGAGAGGATGACCAGCCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAGTGGGGAATATTGCACAATGGGCGCAAGCCTGATGCAGCCATGCCGCGTGTGTGAAGAAGGCCTTAGGGTTGTAAAGCACTTTCAGCGAGGAGGAAGGCATCACACTTAATACGTGTGGTGATTGACGTTACTCGCAGAAGAAGCACCGGCTAACTCCGTGCCAGCAGCCGCGGTAATACGGAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGCACGCAGGCGGTTTGTTAAGTCAGATGTGAAATCCCCGCGCTTAACGTGGGAACTGCATTTGAAACTGGCAAGCTAGAGTCTTGTAGAGGGGGGTAGAATTCCAGGTGTAGCGGTGAAATGCGTAGAGATCTGGAGGAATACCGGTGGCGAAGGCGGCCCCCTGGACAAAGACTGACGCTCAGGTGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCTGTAAACGATGTCGACTTGGAGGTTGTGCCCTTGAGGCGTGGCTTCCGGAGCTAACGCGTTAAGTCGACCGCCTGGGGAGTACGGCCGCAAGGTTAAAACTCAAATGAATTGACGGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGATGCAACGCGAAGAACCTTACCTACTCTTGACATCCACGGAATTCGCCAGAGATGGCTTAGTGCCTTCGGGAACCGTGAGACAGGTGCTGCATGGCTGTCGTCAGCTCGTGTTGTGAAATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTTATCCTTTGTTGCCAGCACGTAATGGTGGGAACTCAAAGGAGACTGCCGGTGATAAACCGGAGGAAGGTGGGGATGACGTCAAGTCATCATGGCCCTTACGAGTAGGGCTACACACGTGCTACAATGGCATATACAAAGAGAAGCGAACTCGCGAGAGCAAGCGGACCTCATAAAGTATGTCGTAGTCCGGATTGGAGTCTGCAACTCGACTCCATGAAGTCGGAATCGCTAGTAATCGTAGATCAGAATGCTACGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACACCATGGGAGTGGGTTGCAAAAGAAGTAGGTAGCTTAACCTTCGGGAGGGCGCTTACCCACTTTGGATTCACGGGGT |
| LYX-4 | GAATGCGGAGCTATACATGCAGTCGGACGGGATCCGTCGGAGAGCTTGCTCGAAGACGGTGAGAGTGGCGCACGGGTGCGTAACGCGTGAGCAACCTACCTCTATCAGGGGGATAGCCTCTCGAAAGAGAGATTAACACCGCATAACATATGTGACCGGCATCGGTTGGATATTAAATATTTATAGGATAGAGATGGGCTCGCGTGACATTAGCTAGTTGGTAGGGTAACGGCTTACCAAGGCGACGATGTCTAGGGGCTCTGAGAGGAGAATCCCCCACACTGGTACTGAGACACGGACCAGACTCCTACGGGAGGCAGCAGTAAGGAATATTGGTCAATGGGCGGAAGCCTGAACCAGCCATGCCGCGTGCAGGATGACTGCCCTATGGGTTGTAAACTGCTTTTGTCCAGGAATAAACCTTTCTACGTGTAGGAAGCTGAATGTACTGGAAGAATAAGGATCGGCTAACTCCGTGCCAGCAGCCGCGGTAATACGGAGGATCCGAGCGTTATCCGGATTTATTGGGTTTAAAGGGTGCGTAGGCGGCCTATTAAGTCAGGGGTGAAATACGGTGGCTCAACCATCGCAGTGCCTTTGATACTGATGGGCTTGAATCCATTTGAAGTGGGCGGAATAAGACAAGTAGCGGTGAAATGCATAGATATGTCTTAGAACTCSGATTGCGAAGSCAGYTCAYTAAGCTGGTATTGACGCTGATGCACGAAAGCGTGGGGATCGACCAGGATTAGATACCCTGGTAGTCCACGCCCTAAACGATGATAACTCGATGTTGGGGATAGACACCCAGCGTCCAAGCGAAAGCGTTAAGTTATCCACGTGGGGAGTACGCCCGCAAGGGTGAAATTAAAGGGAATTGCCGGGGCCCCGCACAAGGGGAGGACCATGGGTTTAAATTGAAGAaTCCGGGAGGACCTTTACCCGGGCTGAAAAGTTAGGAAAGGGTGCAGAGCCCCCTTGTTCCTTGGGACcAGGAAATTAGGGCTGTCATGACGTTGTtCAGTTGGTCCCGGGGGGTGTTGGGTTAAGTCCCGCAACGAGCCCACCCCTTTTGTTTATTGCCCAGCTGTTAAGGTGGGGACTCTAAACAGACTGCCTGTGCAAACAGAGAGGAAGGTGGGGACGACGTCAAGTCATCATGGCCCTTACGTCCGGGGCTACACACGTGCTACAATGGATGGTACAGCGGGCAGCTACATAGCAATATGATGCTAATCTCTAAAAGCCATTCACAGTTCGGATTGGGGTCTGCAACTCGACCCCATGAAGTTGGATTCGCTAGTAATCGCGTATCAGCAATGACGCGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCAAGCCATGAAAGTTGGGGGTACCTAAAGCATGTTACCGCAAGGAGCGTGTAGGTAAGCCCAG |
| WXY-1 | GACCATTTGGCCGGGAGGGCCTTAACACATGCAAGTCGAACGCCCCGCAAGGGGAGTGGCAGACGGGTGAGTAACGCGTGGGAATCTACCGTGCCCTGCGGAATAGCTCCGGGAAACTGGAATTAATACCGCATACGCCCTACGGGGGAAAGATTTATCGGGGTATGATGAGCCCGCGTTGGATTAGCTAGTTGGTGGGGTAAAGGCCTACCAAGGCGACGATCCATAGCTGGTCTGAGAGGATGATCAGCCACATTGGGACTGAGACACGGCCCAAACTCCTACGGGAGGCAGCAGTGGGGAATATTGGACAATGGGCGCAAGCCTGATCCAGCCATGCCGCGTGAGTGATGAAGGCCTTAGGGTTGTAAAGCTCTTTCACCGGAGAAGATAATGACGGTATCCGGAGAAGAAGCCCCGGCTAACTTCGTGCCAGCAGCCGCGGTAATACGAAGGGGGCTAGCGTTGTTCGGAATTACTGGGCGTAAAGCGCACGTAGGCGGATATTTAAGTCAGGGGTGAAATCCCAGAGCTCAACTCTGGAACTGCCTTTGATACTGGGTATCTTGAGTATGGAAGAGGTAAGTGGAATTCCGAGTGTAGAGGTGAAATTCGTAGATATTCGGAGGAACACCAGTGGCGAAGGCGGCTTACTGGTCCATTACTGACGCTGAGGTGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGAATGTTAGCCGTCGGGCAGTATACTGTTCGGTGGCGCAGCTAACGCATTAAACATTCCGCCTGGGGAGTACGGTCGCAAGATTAAAACTCAAAGGAATTGACGGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGAAGCAACGCGCAGAACCTTACCAGCTCTTGACATTCGGGGTTTGGGCAGTGGAGACATTGTCCTTCAGTTAGGCTGGCCCCAGAACAGGTGCTGCATGGCTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTCGCCCTTAGTTGCCAGCATTTAGTTGGGCACTCTAAGGGGACTGCCGGTGATAAGCCGAGAGGAAGGTGGGGATGACGTCAAGTCCTCATGGCCCTTACGGGCTGGGCTACACACGTGCTACAATGGTGGTGACAGTGGGCAGCGAGACAGCGATGTCGAGCTAATCTCCAAAAGCCATCTCAGTTCGGATTGCACTCTGCAACTCGAGTGCATGAAGTTGGAATCGCTAGTAATCGCAGATCAGCATGCTGCGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACACCATGGGAGTTGGTTTTACCCGAAGGTAGTGCGCTAACCGCAAGGAGGCAGCTAACCACGGTAGGTACCGGGGGGT |
| WXY-2 | AGGGACCTTGCGGGTTGCTTTAACCATGCAAGTCGAACGGTGAAGCCAAGCTTGCTTGGTGGATCAGTGCCGAACGGGTGAGTAACACGTGAGCAACCTGCCCTGGACTCTGGGATAAGCGCTGGAAACGGCGTCTAATACTGGATATGAGACGTGATCGCATGGTCGTGTTTGGAAAGATTTTTCGGTCTGGGATGGGCTCGCGGCCTATCAGCTTGTTGGTGAGGTAATGGCTCACCAAGGCGTCGACGGGTAGCCGGCCTGAGAGGGTGACCGGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTGGGGAATATTGCACAATGGGCGAAAGCCTGATGCAGCAACGCCGCGTGAGGGATGACGGCCTTCGGGTTGTAAACCTCTTTTAGCATGGAAGAAGCGAAAGTGACGGTACCTGCAGAAAAAGCGCCGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGGSGCAAGCGTTATCSGGAATTAtTGGGCGTAAAGAGCTCGTAGGCGgTTTGTCGCGTCTGcTGTGaAATCCCGAGGCTCAACCTCGGGCCTGCAGTGGgTACKGGCAGACTAGAGTGCGGTAGGGGAGATTGGAATTCCTGGTGTAKCGGTGGAATGCGCAGATATCAGGAgGGAACACCGATGGCGAAGGCAGATCTCTGGGCCGTAACTGACGCTGAGGAGCGAAAGGGTGGGGAGCAAACAGGCTTAGATACCcTGGTAGTCCACCCCGTAAACGTTGGGGAACTAGTTGTGGGGGACCATTCCACGGTTTCCGTGACGCAGCTAACGCATTAAGTTCCCCGCCTGGGGAGTACGGCCGCAAGGCTAAAACTCAAAGGAATTGACGGGGACCCGCACAAGCGGCGGAGCATGCGGATTAATTCGATGCAACGCGAAGAACCTTACCAAGGCTTGACATATACGAGAACGGGCCAGAAATGGTCAACTCTTTGGACACTCGTAAACAGGTGGTGCATGGTTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTCGTTCTATGTTGCCAGCACGTAATGGTGGGAACTCATGGGATACTGCCGGGGTCAACTCGGAGGAAGGTGGGGATGACGTCAAATCATCATGCCCCTTATGTCTTGGGCTTCACGCATGCTACAATGGCCGGTACAAAGGGCTGCAATACCGTGAGGTGGAGCGAATCCCAAAAAGCCGGTCCCAGTTCGGATTGAGGTCTGCAACTCGACCTCATGAAGTCGGAGTCGCTAGTAATCGCAGATCAGCAACGCTGCGGTGAATACGTTCCCGGGTCTTGTACACACCGCCCGTCAAGTCATGAAAGTCGGTAACACCTGAAGCCGGTGGCCCAACCCTTGTGGGAAGGGAAGCCCCGTTTGCT |
| WXY-5 | CGATACAAAGTGGTAGCGCCCTCCCGAAGGTTAAGCTACCTACTTCTTTTGCAACCCACTCCCATGGTGTGACGGGCGGTGTGTACAAGGCCCGGGAACGTATTCACCGTAGCATTCTGATCTACGATTACTAGCGATTCCGACTTCATGGAGTCGAGTTGCAGACTCCAATCCGGACTACGACGCACTTTATGAGGTCCGCTTGCTCTCGCGAGGTCGCTTCTCTTTGTATGCGCCATTGTAGCACGTGTGTAGCCCTACTCGTAAGGGCCATGATGACTTGACGTCATCCCCACCTTCCTCCAGTTTATCACTGGCAGTCTCCTTTGAGTTCCCGGCCGAACCGCTGGCAACAAAGGATAAGGGTTGCGCTCGTTGCGGGACTTAACCCAACATTTCACaACACGAGCTGACGACAGCCATGCAGCACcTGTCTCACGGTTCCCGAAGGCACTAAAGCATCTCTGCTAAATTCCGTGGATGTCAAGAGTAGGTAAGGTTCTTCGCGTTGCATCGAATTAAACCACATGCTCCACCGCTTGTGCGGGCCCCCGTCAATTCATTTGAGTTTTAACCTTGCGGCCGTACTCCCCAGGCGGTCGACTTAACGCGTTAGCTCCGGAAGCCACTCCTCAAGGGAACAACCTCCAAGTCGACATCGTTTACGGCGTGGACTACCAGGGTATCTAATCCTGTTTGCTCCCCACGCTTTCGCACCTGAGCGTCAGTCTTTGTCCAGGGGGCCGCCTTCGCCACCGGTATTCCTCCAGATCTCTACGCATTTCACCGCTACACCTGGAATTCTACCCCCCTCTACAAGACTCTAGCCTGCCAGTTTCGAATGCAGTTCCCAGGTTGAGCCCGGGGATTTCACATCCGACTTGACAGACCGCCTGCGTGCGCTTTACGCCCAGTAATTCCGATTAACGCTTGCACCCTCCGTATTACCGCGGCTGCTGGCACGGAGTTAGCCGGTGCTTTTTCTGCGAGTAACGTCAATCACTGTGGtTATTAACCACAATGCCTTCCTCCTCGCTGAAAGTACTTTACAaCCCGAAGgCcTtCtTCATACACGCGGCATGGCTGCATCAGgCTTGCGCCCATTGTGCAATATTCCCCACTGCTGCCTCCCGTAGGAGTCTGGACCGTGTCTCAGTTCCAGTGTGGCTGGTCATCCTCTCAGACCAGCTAGGGATCGTCGCCTAGGTGAGCCATTACCCCACCTACTAGCTAATCCCATCTGGGCACATCTGATGGCAAGAGGCCCGAAGGTCCCCCTCTTTGGTCTTGCGACGTTATGCGGTATTAGCTACCGTTTCCAGTAGTTATCCCCCTCCATCAGGCAGTTTCCCAGACATTACTCACCCGTCCGCCGCTCGTCACCCGAGAGCAAGCTCTCTGTGCTACCGCTCGACTGCATGGTAGTCTGCGC |
* denotes a type of marker symbol, no functional implications.

Table A2.
Nitrogen-fixation efficiency values of 15 nitrogen-fixing bacteria.
Table A2.
Nitrogen-fixation efficiency values of 15 nitrogen-fixing bacteria.
| Strains Code | Nitrogen Concentration mg/mL | Glucose Concentration g/mL | Nitrogen Fixation Efficiency Value mg/g |
|---|---|---|---|
| WXY-2 | 0.032 | 0.064 | 0.49 ± 0.21 gh |
| DY-5 | 0.126 | 0.049 | 2.57 ± 0.30 cd |
| DY-8 | 0.088 | 0.065 | 1.34 ± 0.18 f |
| LYX-4 | 0.047 | 0.06 | 0.78 ± 0.01 gh |
| WX-2 | 0.066 | 0.022 | 2.97 ± 0.33 c |
| DY-7 | 0.129 | 0.054 | 2.41 ± 0.21 d |
| WXY-1 | 0.030 | 0.064 | 0.47 ± 0.09 gh |
| CK-3 | 0.049 | 0.059 | 0.83 ± 0.06 g |
| TB * | 0.147 | 0.066 | 2.23 ± 0.11 d |
| DY-1 | 0.047 | 0.060 | 0.79 ± 0.07 gh |
| LYX-2 | 0.107 | 0.062 | 1.81 ± 0.14 e |
| WX-5 | 0.317 | 0.037 | 8.49 ± 0.70 a |
| CK-1 | 0.208 | 0.060 | 3.46 ± 0.09 b |
| DY-2 | 0.023 | 0.061 | 0.38 ± 0.07 h |
| WXY-5 | 0.232 | 0.067 | 3.45 ± 0.12 b |
Note: The data for nitrogen concentration and glucose concentration are expressed as means. The data for nitrogen-fixation efficiency values are expressed as means ± standard deviation. Different letters in the same column indicate significant differences at p < 0.05 levels, as detected using the LSD test. * denotes a type of marker symbol, no functional implications.

Table A3.
UV absorption value with respect to CAT activity of F. taipaiensis leaves under different treatments.
Table A3.
UV absorption value with respect to CAT activity of F. taipaiensis leaves under different treatments.
| Treatments | Ack | AS | t (min) | CAT Activity |
|---|---|---|---|---|
| N1 | 0.573 | 0.998 | 1 | 272.448 |
| N2 | 0.576 | 1.226 | 0.5 | 268.145 |
| N3 | 0.575 | 0.947 | 1 | 363.615 |
| N4 | 0.570 | 0.886 | 1 | 281.073 |
| N5 | 0.579 | 0.890 | 1 | 386.264 |
| N6 | 0.570 | 0.967 | 1 | 295.869 |
| N7 | 0.579 | 0.979 | 0.5 | 277.243 |
| CK | 0.576 | 1.221 | 0.5 | 187.301 |
Note: Ack is the light absorption value of a blank control and AS is the light absorption value of the sample. The data are expressed as means.

Table A4.
Content of SOD, POD, and CAT activity of F. taipaiensis leaves under different treatments.
Table A4.
Content of SOD, POD, and CAT activity of F. taipaiensis leaves under different treatments.
| Treatments | SOD Activity | POD Activity | CAT Activity |
|---|---|---|---|
| N1 | 240.893 ± 22.626 c | 191.156 ± 14.512 d | 272.448 ± 9.830 d |
| N2 | 165.450 ± 27.354 e | 175.136 ± 17.594 d | 268.145 ± 17.285 d |
| N3 | 258.581 ± 20.496 c | 302.837 ± 26.645 c | 363.615 ± 10.146 b |
| N4 | 213.118 ± 8.238 d | 366.403 ± 32.585 b | 281.073 ± 7.611 cd |
| N5 | 376.226 ± 25.008 c | 429.907 ± 13.612 a | 386.264 ± 26.560 a |
| N6 | 284.913 ± 10.039 b | 350.404 ± 26.323 b | 295.869 ± 22.913 c |
| N7 | 243.979 ± 11.109 c | 302.666 ± 26.383 c | 277.243 ± 6.843 cd |
| CK | 156.072 ± 21.728 e | 164.975 ± 8.209 d | 187.301 ± 10.351 e |
Note: The data are expressed as means ± standard deviation; n = 6. Different letters in the same column indicate significant differences at p < 0.05 levels, as detected using the LSD test.

Table A5.
Expression levels of SOD, POD, and CAT genes of F. taipaiensis leaves under different treatments.
Table A5.
Expression levels of SOD, POD, and CAT genes of F. taipaiensis leaves under different treatments.
| Treatments | SOD Gene | POD Gene | CAT Gene |
|---|---|---|---|
| N1 | 2.911 ± 0.530 bcd | 3.349 ± 0.914 d | 4.724 ± 0.141 d |
| N2 | 1.712 ± 0.277 de | 1.963 ± 0.092 e | 4.048 ± 0.122 d |
| N3 | 3.668 ± 0.217 bc | 4.630 ± 0.065 cd | 6.781 ± 1.075 b |
| N4 | 2.690 ± 0.044 cd | 7.335 ± 0.766 b | 4.899 ± 0.360 cd |
| N5 | 5.722 ± 1.050 a | 9.212 ± 1.184 a | 8.794 ± 0.844 a |
| N6 | 4.009 ± 0.589 b | 6.765 ± 0.635 b | 5.911 ± 0.637 bc |
| N7 | 3.491 ± 1.076 bc | 5.400 ± 0.451 c | 4.643 ± 0.390 d |
| CK | 1.100 ± 0.088 e | 0.973 ± 0.156 e | 1.032 ± 0.030 e |
Note: The data are expressed as means ± standard deviation; n = 6. Different letters in the same column indicate significant differences at p < 0.05 levels, as detected using the LSD test.
References
- Zhou, Q.; Lei, Q.; Zhao, J.; Fang, Q.; Fang, S. Identification and Quality Research on Fritillariae Cirrhosae Bulbus (Cultivation) of Sichuan Dao-di Herbs. World Chin. Med. 2020, 15, 225–230. [Google Scholar] [CrossRef]
- Luo, M.; Deng, C.; Li, P.; Tan, Q.; Luo, S.; Xu, G.; Zhang, W. Research progress in Medicinal Plant Fritillaria taipaiensis P. Y. Li. Chin. Wild Pl. Resour. 2021, 40, 42–45+56. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020; pp. 38–39.
- Zou, M.; Jiang, R.; Li, J.; Duan, Q.; Liao, H.; Zhou, J. Prediction of potential distribution of Fritillaria taibaiensis based on MaxEnt model. Chin. J. Inf. Tradit. Chin. Med. 2021, 28, 1–5. [Google Scholar]
- Liu, X. Comparative introduction experiment of Fritillaria cirrhosa and Fritillaria taibaiensis. China J. Chin. Mater. Med. 1994, 81–82. [Google Scholar]
- Tian, Q.; Qiang, Y.; Chen, K.; Wang, J. Advance of Valuable Endangered Medicinal Plant Fritillaria taibaiensis. Shaanxi J. Agri. Sci. 2018, 64, 96–98. [Google Scholar]
- Duan, B.; Chen, X.; Huang, L.; Lu, Q.; Li, X.; Chen, S. Overview of Fritillaria taibaiensis resource study. Mod. Chin. Med. 2010, 12, 12–14. [Google Scholar]
- Ministry of Agriculture and Rural Affairs. Action Plan for Chemical Fertilizer Reduction by 2025; Ministry of Agriculture and Rural Affairs: Beijing, China, 16 November 2022.
- Zheng, J.; Li, M.; Shi, F.; Dong, F. Effects of Reducing Chemical Fertilizer and Applying Organic and Microbial Fertilizers on Tomato Photosynthetic Characteristics and Fertilizer Use Efficiency. China Cucurbits Veg. 2024, 37, 74–79. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, M.; Man, B.; Chai, S.; He, Y. Status quo of microbial fertilizer in China and application in Chinese medicinal herb cultivation. Chin. J. Soil Fertil. Sci. 2024, 11, 222–230. [Google Scholar]
- Meng, C.; Zhao, Y.; Chen, J.; Zhang, Y.; Wang, Y.; Feng, L.; Sun, Y.; Guo, C. Screening and identification of two strains of nitrogen-fixing bacteria from the silage maize rhizosphere and their roles in plant growth promotion. Acta Prataculturae Sin. 2024, 33, 174–185. [Google Scholar]
- Wang, X.; Cao, Y.; Tang, X.; Ma, X.; Gao, J.; Zhang, X. Rice endogenous nitrogen fixing and growth promoting bacterium Herbaspirillum seropedicae DX35. Acta Microbiol. Sin. 2014, 54, 292–298. [Google Scholar] [CrossRef]
- Hu, C.; Lin, L.; Shi, G.; Wang, Q.; Wang, Q.; Li, Y. Screening and identification of associative nitrogen fixation bacteria in rhizosphere of sugarcane in Guangxi. Acta Ecol. Sin. 2012, 32, 4745–4752. [Google Scholar]
- Wei, Z.; Pan, X.; Huang, X.; Li, H.; Guo, D.; Zhou, N. Effects of inoculation of Fritillaria Taipaiensis P.Y. Li with growth-promoting bacteria on inorganic elements in rhizosphere soil. Environ. Chem. 2021, 40, 1254–1262. [Google Scholar] [CrossRef]
- Quan, Z.; Zhang, X.; Davidson, E.A.; Zhu, F.; Li, S.; Zhao, X.; Chen, X.; Zhang, L.; He, J.; Wei, W.; et al. Fates and Use Efficiency of Nitrogen Fertilizer in Maize Cropping Systems and Their Responses to Technologies and Management Practices: A Global Analysis on Field 15N Tracer Studies. Earth’s Future 2021, 9, e2020EF001514. [Google Scholar] [CrossRef]
- Herridge, D.; Peoples, M.; Boddey, R. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Q.; Peng, X. Isolation and identification of 2 strains of nitrogen-fixing bacteria. Jiangsu Agric. Sci. 2020, 48, 298–302. [Google Scholar]
- Li, S.; Sun, Y.; Zhao, J.; Liu, X.; Zhao, J.; Ma, C.; Zhang, Q. Effects of Nitrogen Addition on Microbial Quantity, Enzyme Activity in Rhizosphere Soil and Alfalfa Hay Yield. Chin. J. Grassl. 2018, 24, 38–44. [Google Scholar]
- Tang, X.; Jiang, J.; Jin, H.; Zhou, C.; Liu, G.; Yang, H. Effects of shading on chlorophyll content and photosynthetic characteristics in leaves of Phoebe bournei. Chin. J. Appl. Ecol. 2019, 30, 2941–2948. [Google Scholar]
- Negi, S.; Barry, A.N.; Friedland, N.; Sudasinghe, N.; Subramanian, S.; Pieris, S.; Holguin, F.O.; Dungan, B.; Schaub, T.; Sayre, R. Impact of nitrogen limitation on biomass, photosynthesis, and lipid accumulation in Chlorella sorokiniana. J. Appl. Phycol. 2016, 28, 803–812. [Google Scholar] [CrossRef]
- Xu, T.; Lv, T.; Zhang, Y.; Liu, H.; Liu, Y.; Cai, W.; Zhang, R.; Song, W.; Xing, J.; Zhao, J.; et al. Effects of high-temperature stress on photosynthetic characteristics, protective enzyme activity, and yield of maize hybrids along with their parental inbred lines. Chin. J. Eco-Agric. 2024, 32, 1470–1480. [Google Scholar]
- Kumari, K.; Samantaray, S.; Sahoo, D.; Tripathy, B.C. Nitrogen, phosphorus and high CO2 modulate photosynthesis, biomass and lipid production in the green alga Chlorella vulgaris. Photosynth. Res. 2021, 148, 17–32. [Google Scholar] [CrossRef]
- Domonkos, I.; Kis, M.; Gombos, Z.; Ughy, B. Carotenoids, versatile components of oxygenic photosynthesis. Prog. Lipid Res. 2013, 52, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yang, Y.; Li, X.; Lu, J.; Wang, L.; Liu, D. Effect of Various Abiotic Stresses on Growth and Physiological Characteristics of Alfalfa (Medicago sativa L.) WL712 Seedlings. Chin. J. Grassland. 2024, 46, 78–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Jiang, S.; Sun, H.; Yang, P.; Du, J.; Sun, H.; Zhou, Y. Effect of Different Nitrogen Concentrations on Growth and Antioxidant Enzymes Activity in Notopterygium incisum Seedling in Cultivation Matrix. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 38–44. [Google Scholar]
- Xie, J.; Li, M.; Shi, M.; Kang, C.; Zhang, R.; Liu, Y.; Qing, S.; Zhang, W. Effects of Potassium Fertilizer on Physiological Characteristics, Yield and Quality of Potato under Drought Stress. Acta Agric. Boreali-Occident. Sin. 2024, 33, 2059–2069. [Google Scholar]
- Zhan, Z.; Gou, X.; Wang, Z.; Zhang, Y.; Wang, X.; Guo, J. Effects of Seed Soaking with Beauveria bassiana and Metarhizium rileyi on Defense Enzyme Activities and Insect Resistance in Maize Leaves. Chin. J. Biol. Control 2025, 41, 54–62. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, Y.; Hu, P.; Xia, Y. Investigation on the Resources of Fritillaria taipaiensis. J. Anhui Agric. Sci. 2015, 17, 84–85. [Google Scholar] [CrossRef]
- Fu, S.; Chen, H.; Yuan, D.; Jia, H.; Pu, C.; Lu, Q. Investigation of Chongqing Fritillaria taipaiensis Resources. Chin. J. Inf. Tradit. Chin. Med. 2016, 9, 1–4. [Google Scholar]
- Jiang, Y.; Wu, Y.; Wang, G.; Xu, W.; Zhang, Z.; Xu, L.; Hu, F.; Li, H. Plant Growth-promoting Bacterium Variovorax sp. JX14 from Calcareous Alluvial Soil: Characterization and Growth Promotion on Peanuts. Soil 2015, 47, 698–703. [Google Scholar] [CrossRef]
- Wang, B. Screening of Functional Bacteria from Rhizosphere Soil of Allium Plants in Inner Mongolia Grassland and Their Role in the Groeth of Oats. Master’s Thesis, Inner Mongolia University, Hohhot, China, 2021. [Google Scholar] [CrossRef]
- Cai, M. Isolation and Characterization of Associative Nitrogen-Fixing Bacteria in the Rhizosphere of Camellia oleifera. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2011. [Google Scholar]
- Ye, M. Isolation and Screening of Nitrogen-Fixing Bacteria from Fritillaria taibaiensis and Its Nitrogen-Fixing Effect. Master’s Thesis, Chongqing Three Gorges University, Chongqing, China, 2024. [Google Scholar] [CrossRef]
- Ma, N.; Liu, Y.; Li, X.; Chen, X.; Yang, X. Comprehensive Evaluation of Growth Promotion Effect of PGPR Strains on Tobacco Based on Entropy Weight Method. J. Agric. Univ. 2020, 43, 887–895. [Google Scholar]
- Kandiannan, K.; Parthasarathy, U.; Krishnamurthy, K.S.; Thankamani, C.K.; Srinivasan, V. Modeling individual leaf area of ginger (Zingiber officinale Roscoe) using leaf length and width. Sci. Hortic. 2009, 120, 532–537. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal Conductance and Photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, W.; Li, X. Experimental Guide to Plant Physiology; Higher Education Press: Beijing, China, 2009; pp. 78–88. [Google Scholar]
- Bai, B.; Tang, X. Testing Techniques in Plant Physiology; China Science and Technology Press: Beijing, China, 1993. [Google Scholar]
- Zou, Q. Guidance on Plant Physiology; China Agriculture Press: Beijing, China, 2003. [Google Scholar]
- Zhang, Z. Experimental Guidance on Plant Physiology Techniques, 2nd ed.; Higher Education Press: Beijing, China, 1990. [Google Scholar]
- Ge, K.; Wang, P.J.; Shao, H.L.; Chen, D.L.; Du, B. Physiological characteristics of heavy metal accumulation and resistance in leaves of typical urban greening tree species. J. Shanxi Agric. Univ. Nat. Sci. Ed. 2022, 42, 96–104. [Google Scholar]
- Wang, Y.H.; Yuan, L.; Wang, Y.H.; Lang, J.Q.; Ye, M.Y.; Liu, Q.Q.; Ma, Q.; Zhou, N. Phosphorus-solubilizing fungi promote the growth of Fritillaria taipaiensis P. Y. Li by regulating physiological and biochemical reactions and protecting enzyme system–related gene expression. Front. Genet. 2025, 15, 1459191. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, J.Q.; Zhao, G.Y.; Wei, Y.H.; Dong, Y.H.; Hou, L.Y.; Jiao, R.Z. Isolation and screening of multifunctional phosphate solubilizing bacteria and its growth-promoting effect on Chinese fir seedlings. Sci. Rep. 2021, 11, 9081. [Google Scholar] [CrossRef]
- Parmar, P.; Sindhu, S.S. The novel and efficient method for isolating potassium solubilizing bacteria from rhizosphere soil. Geomicrobiol. J. 2019, 36, 130–136. [Google Scholar] [CrossRef]
- Ke, X.; Feng, S.; Wang, J.; Lu, W.; Zhang, W.; Chen, M.; Lin, M. Effect of inoculation with nitrogen-fixing bacterium Pseudomonas stutzeri A1501 on maize plant growth and the microbiome indigenous to the rhizosphere. Syst. Appl. Microbiol. 2019, 42, 248–260. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, H.; Wang, M.; Chen, S. Diazotrophic Paenibacillus beijingensis BJ-18 Provides Nitrogen for Plant and Promotes Plant Growth, Nitrogen Uptake and Metabolism. Front. Microbiol. 2019, 10, 1119. [Google Scholar] [CrossRef]
- Ai, C.; Zhao, Y.; Zhang, L.; Zhang, M.; Huang, S.; Wang, S.; Zhou, W. Research progress on associative nitrogen fixation of gramineous crops. J. Plant Nutr. Fert. 2024, 34, 1307–1321. [Google Scholar]
- Luo, J.; Mu, M.; Wang, Q.; Wen, M.; Zhu, F.; Wu, X.; Zhou, N. Effect of Different Nitrogen Forms and Concentrations on Yield and Quality of Fritillaria thunbergii. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 168–174. [Google Scholar]
- Li, Z.; Luo, L.; Lang, J.; Ye, M.; Yin, F.; Zhao, J.; Zhou, N. Effect of Organophosphate-Solubilizing Bacteria on Photosynthesis, Physiology, and Biochemistry of Paris polyphylla var. yunnanensis. Chin. J. Exp. Tradit. Med. Formulae 2022, 28, 165–171. [Google Scholar]
- Zhang, S.; Ding, D.; He, J.; Wang, X.; Ma, D.; Deng, Y.; Zhang, X. Effects of Two Biofertilizer Mixtures on Growth and Photosynthetic Characteristics of replanted Zanthoxylum bungeanum. Acta Agric. Boreali-Occident. Sin. 2021, 30, 1355–1364. [Google Scholar]
- Zhao, X.; Zhang, X.; Liu, Z.; Lv, Y.; Song, T.; Cui, J.; Chen, T.; Li, J.; Zeng, F.; Zhan, Y. Comparing the Effects of N and P Deficiency on Physiology and Growth for Fast- and Slow-Growing Provenances of Fraxinus mandshurica. Forests 2021, 12, 1760. [Google Scholar] [CrossRef]
- Cheng, B.; Ma, H.; Yang, T.; Cheng, Z.; Niu, X.; Ma, X. Effect of Different Microbial Fertilizer Dosage on the Elements of Fritillaria pallidiflora Schvek. Xinjiang Agric. Sci. 2017, 54, 871–877. [Google Scholar]
- Zhao, J.; Liang, S.; Yang, T.; Wu, X.; Qi, P.; Wang, S.; Wang, Z. Isolation of Growth-Promoting Bacteria and Effect of Compound Bacteria on Yield of Fritillaria przewalskii. Chin. J. Exp. Tradit. 2021, 27, 163–170. [Google Scholar]
- Zhang, J.; Wang, X.; Feng, B.; Zhou, X.; Chen, H.; Pan, S.; Wu, C. Effects of Staggered Planting and Increasing Bacterial Fertilizer on Photosynthetic Characteristics, Yield and Quality of Fritillaria taipaiensis P. Y. Li. Acta Agric. Boreali-Occident. Sin. 2022, 31, 628–639. [Google Scholar]
- Mu, H.; Lin, L.; Yu, J.; Li, S.; Jiang, X.; Xia, F. Photosynthesis of Autotetraploid Betula platyphylla Seedling Responding to NaHCO3 Stress. J. Beihua Univ. Nat. Sci. Ed. 2017, 18, 25–29. [Google Scholar]
- Pei, Z.; Zhou, J.; Xu, X.; Lan, H.; Wang, J.; Lang, S.; Zhang, W. Effects of Drought Treatment on Photosynthesis Rate, Antioxidant Properties of Leaves and Yield of Different Maize Varieties. Crops 2021, 37, 95–100. [Google Scholar]
- Wu, K.; Liang, J.; Wei, L.; Luo, T.; Xing, Y.; Li, Y.; Yang, L. Effects of nitrogen fixing bacteria on photosynthesis and chlorophyll fluorescence in sugarcane at elongating stage. Guihaia 2011, 31, 668–673. [Google Scholar]
- Zheng, S.; Guo, S.; Zhang, Y.; Song, X.; Fang, C.; Zhang, J.; Sun, J. Effects of Arbuscular Mycorrhizal Fungi on Characteristics of Photosynthesis, Microbial Diversity and Enzymes Activity in Rhizosphere of Pepper Plants Cultivated in Organic Substrate. Acta Bot. Boreali-Occident. Sin. 2014, 34, 800–809. [Google Scholar] [CrossRef]
- Tian, F.; Chen, X.; Wang, P.; Zhong, L.; Ou, E. Effect of microbial agents on resistant enzyme system and biochemical indexes of drought resistance in Sophora david. Plant Physiol. J. 2022, 58, 435–446. [Google Scholar]
- Zhang, H.; Zhao, G.; Li, M.; Xia, C.; Wang, C. Physiological responses of Pennisetum longissimum var. intermediumseedlings to PEG, low temperature and salt stress treatments. Acta Prataculturae Sin. 2014, 23, 180–188. [Google Scholar] [CrossRef]
- Yan, M.; Yao, Y.; Mu, K.; Dan, Y.; Li, W.; Liao, W. Involvement of abscisic acid in hydrogen gas-enhanced drought resistance by improving antioxidant enzyme activity and gene expression in tomato seedlings. Acta Agric. Zhejiangensis 2022, 34, 1901–1910. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, Q.; Wang, X.; Li, Z.; Tang, X.; Jiang, L.; Liu, P.; Xing, C. Effects of arbuscular mycorrhizal fungi on antioxidant enzymes activities and photosynthetic characteristics of Solanum lycopersicum L.under salt stress. Acta Agric. Zhejiangensis 2021, 33, 2075–2084. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).