Simple Summary
To investigate the impact of bacteria on the embryonic and larval development of the common toad (Bufo bufo), eggs and tadpoles were exposed under laboratory conditions to bacteria (Bacillus sp., Mesobacillus sp.) isolated from the habitat water. Pure bacterial cultures and their combinations decreased acetylcholinesterase activity but had positive effects on tadpole size and energy budget. These findings suggest that bacteria can influence the larval development of common toads by modifying physiological traits. Future research should elucidate which microbes have beneficial or detrimental effects on amphibian development.
Abstract
Amphibians, as the most threatened group of vertebrates, are the focus of investigation of various agents that could affect their fitness and survival. In this context, we examined the effects of naturally occurring bacteria and their combinations on the embryonic and larval development of common toad tadpoles (Bufo bufo). One egg string that was collected from the wild was disinfected in the lab and divided into short strings, each containing 20 eggs. These strings were exposed to three different control treatments, i.e., a sterile FETAX solution, water collected from the tadpoles’ native habitat, and sterilized habitat water, as well as to three different bacterial cultures isolated from habitat water (Bacillus sp., Mesobacillus sp.). We analyzed several morphometric variables (snout–vent length, total length, and weight), the energy budget by measuring body composition (proteins, carbohydrates, and lipids), and biomarker activity (acetylcholinesterase and lactate dehydrogenase). Our results indicate that the native microbial community had a negative effect on egg and tadpole development, as sterilized habitat water supported their highest development. Additionally, when grown in FETAX, pure bacterial cultures and their combinations decreased acetylcholinesterase activity but positively affected both tadpole size and energy budget. These findings suggest that bacteria can influence the larval development of common toads by modifying physiological traits. Future research should identify which microbes have beneficial or detrimental effects on amphibian development.
1. Introduction
Bacteria are ubiquitously present in nature, persisting across both space and time. They pervade the biosphere, inhabiting environments ranging across oceans and ocean sediments, the lithosphere, and terrestrial and freshwater ecosystems. In all of these habitats, bacteria interact closely with other organisms, some of which form very strong connections with bacteria. For instance, plant-growth-promoting bacteria enhance plant growth and health under various environmental conditions [1]. In other cases, bacteria form obligate endosymbiotic relations with many arthropods and nematodes [2]. Nevertheless, research on bacterial interactions with vertebrates, other than humans or livestock, is still gathering pace [3], although the importance of this relationship has been especially recognized in recent decades [4]. Still, microorganism–amphibian interactions are largely underrepresented and understudied in the scientific literature [5].
Animal–bacterial interactions are gaining increasing attention due to the recognition of their profound impact, ranging from the influence of the human microbiome on human health [6] to the stimulation of settlement and metamorphosis in marine invertebrate larvae [7]. Tadpoles serve as a good model system for studying vertebrate responses to environmental factors. They are often used to assess the effects of various agents on amphibians, including pesticides [8], metals in bulk and nano form [9], and microplastic pollution [10]. In addition to the effects of chemical pollutants, different aspects of tadpole–bacteria interactions were investigated. Jones et al. [11] recognized the importance of colonization order of native bacteria isolates on the microbiome structure of hourglass treefrog tadpoles, Dendropsophus ebraccatus. Furthermore, the developmental stage has been shown to have a much stronger effect on microbiome structure than temperature variations [12]. Similarly, Santos et al. [13] demonstrated that the skin and gut microbiomes of Pelophylax perezi and Bufo spinosus vary depending on both the host species and the water environment. Moreover, the gut microbiome was negatively impacted by glyphosate-based herbicides and antibiotics in Rana berlandieri tadpoles [14]. The impact of pollutants on tadpole microbial communities could result in indirect negative effects on tadpoles, as the interaction between tadpoles and their microbiome is known to be beneficial for their development [15].
Bacteria, as the most widespread microorganisms, exhibit tremendous species richness, providing vast opportunities for interactions. Young animals acquire microbes from their immediate environment. In the case of tadpoles, they interact with microbes from the aquatic environment but also with those that have washed in from the surrounding soil. One common bacterial group in these habitats is the genus Bacillus. These Gram-positive, sporulating microbes contain diverse metabolic, physiological, and ecological traits, such as the production of lipopeptide, antibiotic, hydrogen cyanide, siderophore, extracellular hydrolytic enzymes, and toxins, as well as nitrogen fixation, phosphate solubilization, and phytohormone synthesis [16]. These properties enable them to induce systemic resistance in plants, enhance their health and growth inhabiting the rhizosphere or as endophytes [1,17], and suppress pathogenic fungi [18]. They also colonize the gut of black tiger shrimp, where they help protect from pathogens [19], and some members of the genus are even considered probiotics [20]. Given these characteristics and their effects on plants and invertebrates, it is reasonable to ask whether similar interactions could influence other animals, such as amphibians.
Amphibians are terrestrial vertebrates characterized by highly permeable skin that interacts with microorganisms from the environment, creating a unique microbiome. Its role is very versatile, from maintaining surface integrity to protecting the organisms from numerous pathogens such as the fungus Batrachochytrium dendrobatidis or ranaviruses [21,22,23]. Amphibians are the most threatened group of vertebrates, with over 40% of all species being threatened with extinction; moreover, diseases account for one-third of the total threats facing them [24]. However, despite the critical importance of microbial–skin interactions for amphibian health, most studies refer to various pathogens, and very few studies are available on other roles of microbial–skin interactions.
The common toad, Bufo bufo (Linnaeus, 1758) is a widespread European species, but in most habitats it is not very abundant. It tends to breed in it natal breeding site, and is therefore more susceptible to any changes occurring within the site [25].
Acetylcholinesterase (AChE, EC 3.1.1.7) is an enzyme that catalyzes the hydrolysis of the ester bond of acetylcholine, the most important neurotransmitter. It is one of the fastest enzymes with a unique molecular structure [26], and various stressors can induce or inhibit its activity. Despite its physiological significance, AChE activity has only occasionally been measured in tadpoles. For example, Johnson et al. [27] examined the impact of different water temperatures on AChE activity, while Attademo et al. [28] reported statistically significant inhibition of AChE by microplastics and plastic additives. However, no effect was observed after exposure to pesticide metaldehyde [29].
Lactate dehydrogenase (LDH, EC 1.1.1.27) is a notable enzyme of the anaerobic metabolic pathway. It catalyzes a reversible reaction in which lactate is converted to pyruvate accompanied by the reduction of NAD+ to NADH, and vice versa [30]. Variations in lactate dehydrogenase activity usually indicate some metabolic changes. In amphibians, changes in LDH activities normally occur during the ontogenetic development of embryos, and stabilize later in the tadpole stage [31]. In adult amphibians, changes in LDH activity are observed in response to intensive physical activities, and exposure to stress, e.g., pH and temperature [32,33].
In the present study, we examined the effects of three pure bacterial cultures isolated from the tadpoles’ habitat, as well as combinations of them, on the growth and physiological status of common toad tadpoles. To the best of our knowledge, this is the first time that the impact of bacteria on toad embryonic and larval development has been reported. We hypothesized that bacterial exposure would significantly affect tadpole development. To test this hypothesis, the energetic budget and physiological status of tadpoles were measured.
2. Materials and Methods
2.1. Isolation and Characterization of Bacteria
Tadpoles and water samples were collected from Lake Borovik in eastern Croatia (GPS coordinates 45°23′29.6″ N 18°10′36.2″ E). Water sample was collected on 9 March 2019 using sterilized jar (1 L) and analyzed within 24 h after collection. It was spread plated after serial dilution on nutrient agar (Biolife, Milano, Italy) and incubated at 22 ± 1 °C. Three randomly chosen separated colonies were isolated and preserved for future use on agar slants and in glycerol at −80 °C (Binder, Tuttlingen, Germany).
DNA was extracted from pure colonies of three isolates grown on the nutrient agar plates using the Quick-DNA Miniprep Plus Kit (Zymo Research, Freiburg, Germany). The 16S rRNA marker gene was amplified via PCR (machine PCRmax Alpha Cycler 1, Cole-Parmer, Vernon Hills, IL, USA) using universal primers 27F and 1492R [34] with the following program: 95 °C for 5 min, 30 cycles of 95 °C for 45 s, 55 °C for 1 min, 72 °C for 1.5 min, and a final extension at 72 °C for 10 min. Obtained PCR products were purified and Sanger-sequenced by Macrogen (Amsterdam, The Netherlands). The obtained raw sequences were edited with Chromas Lite v2.6.6 (Technelysium, South Brisbane, Australia) and analyzed using BLASTn 2.16.1+ against the NCBI database.
2.2. Experimental Design
The microcosmic experiment was conducted in heat-sterilized 720 mL round glass jars. Each jar was filled with 500 mL sterile FETAX solution (625 mg NaCl, 96 mg NaHCO3, 75 mg MgSO4, 75 mg CaSO4·2H20, 30 mg KCl, 15 mg CaCl2 per liter distilled water). One string of Bufo bufo eggs was collected from the field on 18 March 2019 and kept in the fridge (4 °C) in a jar filled with lake water until experimental setup the following day. Prior to use, the string was disinfected by submerging the eggs into 70% ethanol for 20 s and rinsing three times in sterile distilled water. In each jar, a string of 20 sterilized eggs (Gosner stage (GS) 11 [35]) was added. After that, each treatment received 1 mL of overnight bacterial culture adjusted to OD600 = 1, or an equal mixture of two cultures, or all three cultures of a total volume of 1 mL. The solution of sterile FETAX without added bacteria was the control (K1). Besides K1, there were two additional controls: one consisting of non-sterile lake water (K2), and the second consisting of autoclaved lake water (K3). These additional controls were taken to assess the suitability of FETAX for tadpole development and to investigate the effects of native microbial communities present in K2 but absent in K3. All jars were on a shelf without direct exposure to sunlight and closed with screw caps. The microcosms were not aerated, but each cap had a mounted 0.2 μm pore size syringe filter.
All treatments were carried out in triplicate. Microcosms were kept at room temperature (22 ± 1 °C) and in a natural light cycle for two weeks. The order of the jars was random, and their position was changed daily. Upon hatching, which occurred after two to three days, all tadpoles were fed daily ad libitum with sterilized commercial fish food (Tubifex, Vitakraft, Bremen, Germany). The medium was not changed during the experiments. After two weeks, from each flask, three tadpoles were randomly chosen (i.e., nine tadpoles per treatment), weighted (precision 0.001 g), had their snout–vent length (from the tip of the snout to the most posterior opening of the cloacal slit; SVL) and tail length (from the posterior edge of the body to the tip of the tail fin; TL) measured using a hand caliper (precision 0.01 mm), and, together with other tadpoles, were frozen at −80 °C until further analysis (some of which are not included in this paper). The experiments were conducted in accordance with the EU legislation for animal experimentation. Eggs that were not used in the experiment were returned to the site where they had been collected.
2.3. Molecular Biomarkers and Energy Budget Analyses
Whole tadpoles were homogenized in phosphate buffer (0.1 M, pH 7.4; ratio 1:5 w/v). Protein content was determined according to the methodology detailed by Bradford [36], homogenate: buffer 1:3, at 595 nm. Total lipid content was determined by applying the phospho-vanillin protocol [37]. Total carbohydrate content was calculated using the spectrophotometric anthrone protocol [38]. Subsequently, the homogenates were centrifuged at 9000× g for 30 min at 4 °C, and supernatants were used for further analysis. The activity of LDH was determined using the protocol set out in [39], and then measured for 60 s at 340 nm. The activity of AChE was measured for 30 s at 412 nm and calculated according to protocol detailed by Ellman et al. [40].
2.4. Statistical Analysis
Before analysis, data were checked for normality of distribution. The Kolmogorov–Smirnov test showed that TL, weight, and AChE did not follow normal distribution and were log x + 1-transformed. Variables were analyzed between treatments via Bayesian one-way ANOVA, after which the post hoc test was used (prior vs. posterior odds) to detect which bacterial treatments differed from the control (K1). The posterior odds were corrected for multiple testing; hence, it is a conservative measure. Bayesian ANOVA does not use one of the factors as reference, but instead calculates the total mean and then compares all other means to the total mean. In this way, the analysis does not depend on the p-value, which is an arbitrary chosen value, and thus subjective, but instead depends on the intrinsic nature of the data set itself. Also, the Bayes factor provides hypothesis testing in terms of probability, which offer continuous conclusions as probability could be anywhere between low and high, while the p-value-based significance test is only dichotomous in the sense that we can only conclude whether the difference exists (on the arbitrary threshold) or not, but without any gradation. The Bayes factor (BF10) was calculated for the null hypothesis of no difference between the treatments and for the alternative hypothesis, both of which were considered equally likely. Also, the robustness of analysis was calculated as a error percentage. This value should be below 20%, with lower values indicating a greater numerical stability of the results [41]. Bayesian tests were conducted in Open Source software JASP 0.19.3.
3. Results
Bacterial isolates were identified as Mesobacillus sp. (culture A) or Bacillus pumillus (culture B), while the third culture (culture C) was left unidentified due to difficulties in sample preparation.
For all tested variables except TL and proteins, the alternative hypothesis was more likely (Table 1), suggesting the existence of differences between the treatments.

Table 1.
Bayesian ANOVA output with prior and posterior odds, Bayes factors, and stability of the modes.
Post hoc tests resulted in prior odds of 0.149 for all comparisons. Hence, all posterior odds larger than 0.149 represent a higher probability of effect.
The pure culture of Mesobacillus sp. significantly decreased AChE activity (posterior odd 0.465, Figure 1). AChE activity was also significantly decreased when exposed to the pure culture of Bacillus pumilus (posterior odd 0.301, Figure 1) and with a combination of Mesobacillus sp. and unidentified bacteria (posterior odd 0.266, Figure 1). Lactate dehydrogenase was increased as a result of the combinations of Mesobacillus sp. and B. pumilus, B. pumilus and unidentified bacteria, and through the combination of all three cultures (posterior odds 0.246, 0.332 and 0.216, respectively, Figure 1).
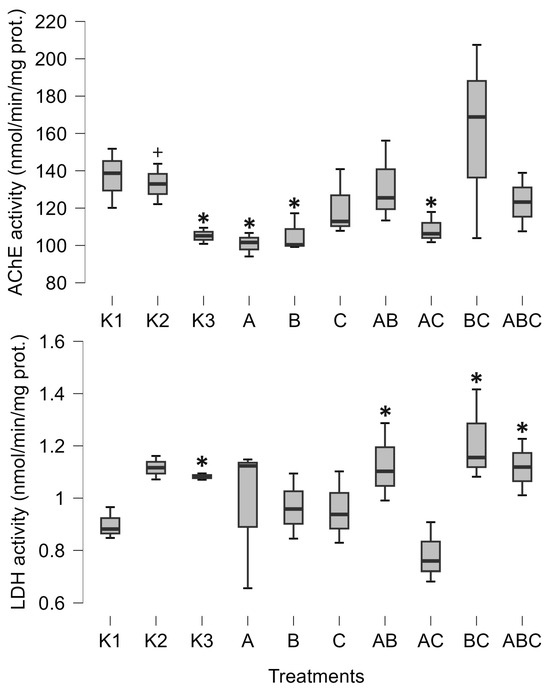
Figure 1.
Minimum, maximum (whiskers), and median (bar) values of tadpoles’ acetylcholinesterase activity (AChE) and lactate dehydrogenase activity (LDH). The asterisk marks significant differences compared to K1, while the plus marks a significant difference compared to K3. K1—sterile FETAX solution, K2—lake water, K3—sterile lake water, A—Mesobacillus sp., B—Bacillus pumilus, C—unidentified bacteria.
Furthermore, the combination of Mesobacillus sp. and Bacillus pumilus significantly increased the amount of lipids in tadpoles (posterior odd 0.425, Figure 2). SVL was positively influenced by the combinations of Mesobacillus sp. and B. pumilus with unidentified bacteria, as well as by the combination of all three cultures (posterior odds 0.846, 0.996, and 0.34, respectively, Figure 3). Although weight and carbohydrates appear to increase as a result of the combination of Mesobacillus sp. and the unidentified bacteria, this was not detected by post hoc analysis as posterior odds were smaller compared to prior.
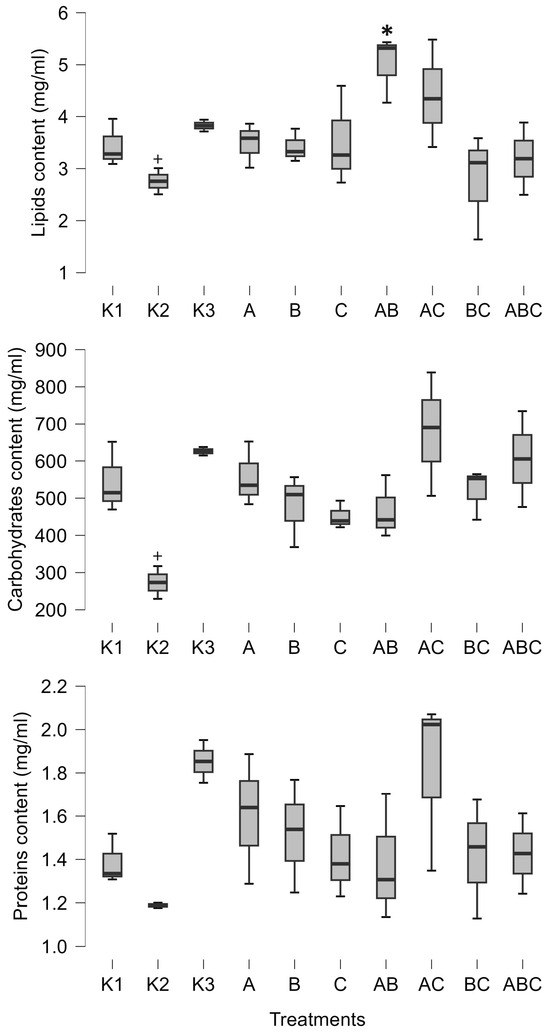
Figure 2.
Minimum, maximum (whiskers), and median (bar) values of tadpoles’ lipids, carbohydrates (C-H), and protein content. The asterisk marks significant differences compared to K1, while the plus marks a significant difference compared to K3. K1—sterile FETAX solution, K2—lake water, K3—sterile lake water, A—Mesobacillus sp., B—Bacillus pumilus, C—unidentified bacteria.
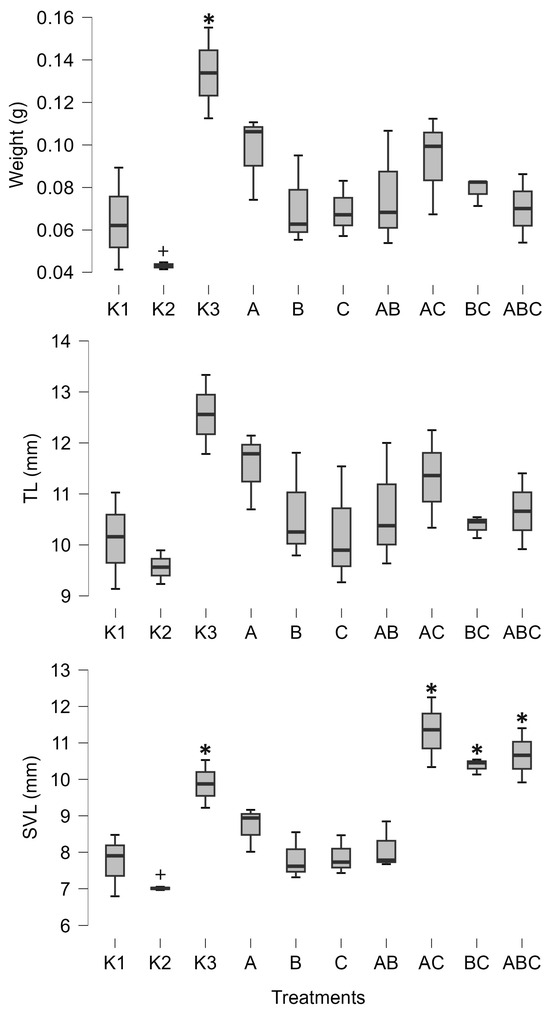
Figure 3.
Minimum, maximum (whiskers), and median (bar) values of tadpoles’ weight, tail length (TL), and snout–vent length (SVL). The asterisk marks significant differences compared to K1, while the plus marks a significant difference compared to K3. K1—sterile FETAX solution, K2—lake water, K3—sterile lake water, A—Mesobacillus sp., B—Bacillus pumilus, C—unidentified bacteria.
The control treatments for the effect of natural microbial communities suggest a negative impact of the aquatic microbial community on tadpole development. Specifically, K2 (non-sterile lake water) significantly decreased weight (posterior odd 0.317, Figure 3), SVL (posterior odd 0.315, Figure 3), carbohydrates (posterior odd 0.523, Figure 2), and lipids (posterior odd 0.286, Figure 2), while AChE activity was increased (posterior odd 0.212, Figure 1) compared to K3 (sterile lake water). LDH activity remained unchanged.
Tadpoles were larger in sterile lake water (K3) compared to FETAX (K1). LDH, weight, and SVL had higher values in K3 (posterior odds 0.409, 0.27, and 0.246, respectively, Figure 1 and Figure 3). AChE activity was decreased in lake water (K3) (posterior odd 0.234, Figure 1). Lipid and carbohydrate levels did not differ between sterile lake water (K3) and FETAX (K1), although they had slightly higher values in K3 (Figure 2).
The microcosms were not probed for bacteria at the end of the experiment. This means that there is a possibility that other bacteria, which survived disinfection, developed during the two-week incubation period alongside the added treatments. Nevertheless, these bacteria were certainly present in substantially lower abundance compared to the introduced bacteria. Therefore, their impact, if any, was probably minimal.
Finally, mortality rates observed during the experiment (Figure 4) did not differ significantly between the groups with FETAX (Bayesian ANOVA), suggesting that it was not related to experimental treatments, i.e., added bacteria. In lake water controls, mortality was highest, especially in the sterile lake water (K3) (Figure 4).
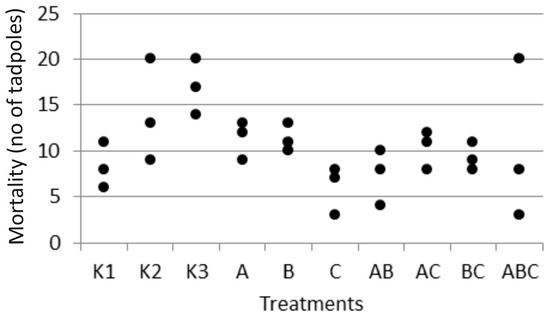
Figure 4.
Mortality occurred during the experiment as number of dead tadpoles in each triplicate of the treatment. There was no difference between groups grown in FETAX (Bayesian ANOVA).
4. Discussion
In this paper, we describe the interactions between Bufo bufo tadpoles and Bacillus pumilus and Mesobacillus sp. One important finding of our study was a decrease in AChE activity induced by Mesobacillus sp. and B. pumilus pure cultures, as well as in combination with Mesobacillus sp. with unidentified bacteria. The activity of AChE is an indicator of environmental stress, such as temperature or exposure to xenobiotics [27,28]. In our study, a significant decrease in AChE activity was observed solely due to the presence of different bacteria. On the other hand, the native microbial community (K2) increased AChE activity compared to sterilized lake water (K3). This is an important finding as it suggests that different members of the aquatic microbial community can either increase or decrease AChE activity in tadpoles. So far, we have confirmed that Mesobacillus sp. and B. pumilus decrease its activity. This enzyme is responsible for the normal functioning of neuromuscular junctions, and its inhibition can lead to muscular paralysis. In our study, AChE activity was not completely inhibited, but was decreased. In such circumstances, it is possible that tadpoles were less active and hence able to accumulate biomass. Our results support this idea, as decreased AChE activity was accompanied by an increase in tadpoles’ size and energetic status (Figure 2 and Figure 3). Mesobacillus sp. is one of the new genera derived from Bacillus. Members of this genus are aerobic or facultatively anaerobic, Gram-positive, rod-shaped microbes inhabiting different environments including soil [42] and freshwater lake sediment [43]; however, further information is scarce [44]. On the other hand, B. pumilus is a well-studied bacterial species, particularly in the context of plant growth promotion [1]. In plant interactions, these bacteria provide protection against fungal phytopathogens [18], produce phytohormones [45], and synthesize ACC deaminase [46], supporting plant development through the rhizosphere [18] or as endophytes [47]. Murugappan et al. [47] found increased root and shoot length, as well as an increased number of leaves, in plants inoculated with B. pumilus. Furthermore, B. pumilus induces systemic resistance in plants, indicating its ability to modulate the plant immune system [48]. There is a wide surface through which B. pumilus interacts with plants, and it is highly probable that such a wide span of interactions also exists with animals [4]. Dökenel and Özer [49] detected B. pumilus on marsh frogs (Pelophylax ridibundus) from a frog farm, but not in water or feed samples. This result suggests that B. pumilus exhibits some degree of association with at least one frog species. Similarly, Scalvenzi et al. [5] reported the constant presence or dominance of Firmicutes (including the Bacillus genus) in different developmental stages of Xenopus tropicalis.
Our results suggest that B. pumilus and Mesobacillus sp. may influence tadpole physiology. Furthermore, the interaction between B. pumilus and unidentified bacteria appears to mitigate the negative effect of B. pumilus on AChE activity. Moreover, the overall microbial community significantly increased AChE activity, highlighting the importance of microbial interactions in their effects on tadpoles [50]. AChE activity is seldomly measured in tadpoles and serves as an indicator of chemical environmental contamination. Attademo et al. [29] assessed the effects of metaldehyde on AChE activity in common toad (Rhinella arenarum) tadpoles but found no significant effects, similar to the lack of effects observed with the use of glufosinate-ammonium-based herbicide on tadpoles of the same species [51]. Conversely, the same authors recorded an increase in AChE activity due to glyphosate-based herbicides [51]. However, Attademo et al. [28] noted a significant decrease in AChE activity caused by polyethylene microplastics (40–48 µm particle size, 60 mg L−1) and by plastic additive tetrabromobisphenol A in tadpoles of the same species. In their investigation, AChE activity decreased by approximately 25% compared to the control, which is comparable to our findings. Depending on the treatments (pure culture or mixture), our work resulted in a 25% to 35% decrease in AChE activity. This suggests that both chemical pollution and bacteria present in the environment are equally important in shaping the physiological response of tadpoles. Nevertheless, we would like to emphasize that we used only one string of eggs in our experiment; hence, the inclusion of more egg strings would more precisely describe the variability in bacteria–tadpole interactions. Finally, LDH activity was stimulated by a combination of B. pumilus and unidentified bacteria, which is the same mixture that mitigated the negative effect of B. pumilus on AChE activity. Therefore, unidentified bacteria, which had no impact on AChE or LDH activity in pure culture, in combination with B. pumilus mitigated its negative effect on AChE activity and induced an increase in LDH activity. This emergent situation greatly complicates the investigation of host–microbe interactions, as their effects are altered by the development of microbe–microbe interactions. Due to the tremendous species richness of bacteria, a myriad of underlying interactions could exist. The nature of these microbe–microbe interactions, which differentially impact amphibians, remains to be discovered.
5. Conclusions
Our results demonstrate that bacteria present in the tadpole environment play a significant role, under laboratory conditions, in embryonic and larval development by modulating their physiology. Exposure of common toad (Bufo bufo) tadpoles to the whole microbial community resulted in increased AChE activity and decreased weight, SVL, lipids, and carbohydrates compared to sterile lake water. Conversely, the exposure of eggs and tadpoles to Mesobacillus sp. and B. pumilus, or unidentified bacteria, resulted in decreased AChE activity, while LDH, SVL, and lipids increased. However, these findings should be taken with caution, as tadpoles in their natural environment are not exposed to pure bacterial cultures or simple combinations thereof, but rather to complex microbial communities. Future research should perhaps focus on investigating the effects of different community structures.
Author Contributions
The design of the experiment was conceived by O.J.G. and G.P., sampling O.J.G., laboratory analyses O.J.G., I.S.P. and G.P., statistical analysis G.P., writing of the manuscript O.J.G., I.S.P. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
The investigation was supported by the institutional project VFZ310520 of the University of Osijek—Department of Biology.
Institutional Review Board Statement
Ethical review and approval were waived for this study because at the time no authorized committee existed to grant approval. Nevertheless, the lead author (OJG) holds a FELASA Category C license.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to Luca Zelić for assistance in biomarker analyses.
Conflicts of Interest
The authors declare no competing interest.
References
- Dobrzyński, J.; Jakubowska, Z.; Dybek, B. Potential of Bacillus pumilus to Directly Promote Plant Growth. Front. Microbiol. 2022, 13, 1069053. [Google Scholar] [CrossRef] [PubMed]
- Manoj, R.R.S.; Latrofa, M.S.; Epis, S.; Otranto, D. Wolbachia: Endosymbiont of Onchocercid Nematodes and Their Vectors. Parasit. Vectors 2021, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Hoye, B.J.; Fenton, A. Animal Host–Microbe Interactions. J. Anim. Ecol. 2018, 87, 315–319. [Google Scholar] [CrossRef]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a Bacterial World, a New Imperative for the Life Sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef]
- Scalvenzi, T.; Clavereau, I.; Bourge, M.; Pollet, N. Gut microbial ecology of Xenopus tadpoles across life stages. Peer Community J. 2021, 1, e41. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Sig Transduct. Target. Ther. 2022, 7, 1–28. [Google Scholar] [CrossRef]
- Hadfield, M.G. Biofilms and Marine Invertebrate Larvae: What Bacteria Produce That Larvae Use to Choose Settlement Sites. Annu. Rev. Mar. Sci. 2011, 3, 453–470. [Google Scholar] [CrossRef]
- Brühl, C.A.; Schmidt, T.; Pieper, S.; Alscher, A. Terrestrial Pesticide Exposure of Amphibians: An Underestimated Cause of Global Decline? Sci. Rep. 2013, 3, 1135. [Google Scholar] [CrossRef]
- Jovanović Glavaš, O.; Stjepanović, N.; Hackenberger, B.K. Influence of Nano and Bulk Copper on Agile Frog Development. Ecotoxicology 2022, 31, 357–365. [Google Scholar] [CrossRef]
- Karaoğlu, K.; Gül, S. Characterization of Microplastic Pollution in Tadpoles Living in Small Water-Bodies from Rize, the Northeast of Turkey. Chemosphere 2020, 255, 126915. [Google Scholar] [CrossRef]
- Jones, K.R.; Hughey, M.C.; Belden, L.K. Colonization Order of Bacterial Isolates on Treefrog Embryos Impacts Microbiome Structure in Tadpoles. Proc. Biol. Sci. 2023, 290, 20230308. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-K.; Park, W.-B.; Do, Y. Tadpole Growth Rates and Gut Bacterial Community: Dominance of Developmental Stages over Temperature Variations. PLoS ONE 2023, 18, e0292521. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.; Martins, F.M.S.; Sabino-Pinto, J.; Licata, F.; Crottini, A. Skin and Gut Microbiomes of Tadpoles Vary Differently with Host and Water Environment: A Short-Term Experiment Using 16S Metabarcoding. Sci. Rep. 2023, 13, 16321. [Google Scholar] [CrossRef]
- Villatoro-Castañeda, M.; Forsburg, Z.R.; Ortiz, W.; Fritts, S.R.; Gabor, C.R.; Carlos-Shanley, C. Exposure to Roundup and Antibiotics Alters Gut Microbial Communities, Growth, and Behavior in Rana Berlandieri Tadpoles. Biology 2023, 12, 1171. [Google Scholar] [CrossRef]
- Bishop, T.F.; Beck, C.W. Bacterial Lipopolysaccharides Can Initiate Regeneration of the Xenopus Tadpole Tail. iScience 2021, 24, 103281. [Google Scholar] [CrossRef]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus Species in Soil as a Natural Resource for Plant Health and Nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef]
- Thomas, P. Isolation of Bacillus pumilus from in Vitro Grapes as a Long-Term Alcohol-Surviving and Rhizogenesis Inducing Covert Endophyte. J. Appl. Microbiol. 2004, 97, 114–123. [Google Scholar] [CrossRef]
- Sari, E.; Etebarian, H.R.; Aminian, H. The Effects of Bacillus pumilus, Isolated from Wheat Rhizosphere, on Resistance in Wheat Seedling Roots against the Take-All Fungus, Gaeumannomyces graminis var. tritici. J. Phytopathol. 2007, 155, 720–727. [Google Scholar] [CrossRef]
- Hill, J.E.; Baiano, J.C.F.; Barnes, A.C. Isolation of a Novel Strain of Bacillus pumilus from Penaeid Shrimp That Is Inhibitory against Marine Pathogens. J. Fish. Dis. 2009, 32, 1007–1016. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as Potential Probiotics: Status, Concerns, and Future Perspectives. Front. Microbiol. 2017, 8, 01490. [Google Scholar] [CrossRef]
- Bates, K.A.; Friesen, J.; Loyau, A.; Butler, H.; Vredenburg, V.T.; Laufer, J.; Chatzinotas, A.; Schmeller, D.S. Environmental and Anthropogenic Factors Shape the Skin Bacterial Communities of a Semi-Arid Amphibian Species. Microb. Ecol. 2023, 86, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.N.; James, T.Y.; Lauer, A.; Simon, M.A.; Patel, A. Amphibian Pathogen Batrachochytrium dendrobatidis Is Inhibited by the Cutaneous Bacteria of Amphibian Species. EcoHealth 2006, 3, 53–56. [Google Scholar] [CrossRef]
- McGrath-Blaser, S.; Steffen, M.; Grafe, T.U.; Torres-Sánchez, M.; McLeod, D.S.; Muletz-Wolz, C.R. Early Life Skin Microbial Trajectory as a Function of Vertical and Environmental Transmission in Bornean Foam-Nesting Frogs. Anim. Microbiome 2021, 3, 83. [Google Scholar] [CrossRef]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing Declines for the World’s Amphibians in the Face of Emerging Threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef]
- AmphibiaWeb Bufo bufo: Common Toad. 2024. Available online: https://amphibiaweb.org/species/127 (accessed on 7 January 2025).
- Massoulié, J.; Sussman, J.; Bon, S.; Silman, I. Chapter 15: Structure and Functions of Acetylcholinesterase and Butyrylcholinesterase. In Progress in Brain Research; Cuello, A.C., Ed.; Cholinergic Function and Dysfunction; Elsevier: Amsterdam, The Netherlands, 1993; Volume 98, pp. 139–146. [Google Scholar]
- Johnson, C.S.; Schwarzbach, S.E.; Henderson, J.D.; Wilson, B.W.; Tjeerdema, R.S. Influence of Water Temperature on Acetylcholinesterase Activity in the Pacific Tree Frog (Hyla regilla). Environ. Toxicol. Chem. 2005, 24, 2074–2077. [Google Scholar] [CrossRef]
- Attademo, A.M.; Curi, L.M.; Boccioni, A.P.C.; Barrios, C.E.; Peltzer, P.M.; Simoniello, M.F.; Lajmanovich, R.C.; Michlig, M.P.; Repetti, M.R.; Ríos, J.M. Microplastics and Plastic Additives as Contaminants of Emerging Concern: A Multi-Biomarker Approach Using Rhinella arenarum Tadpoles. Environ. Adv. 2023, 14, 100444. [Google Scholar] [CrossRef]
- Attademo, A.M.; Lajmanovich, R.C.; Peltzer, P.M.; Junges, C.M. Acute Toxicity of Metaldehyde in the Invasive Rice Snail Pomacea Canaliculata and Sublethal Effects on Tadpoles of a Non-Target Species (Rhinella arenarum). Water Air Soil. Pollut. 2016, 227, 400. [Google Scholar] [CrossRef]
- Vanderlinde, R.E. Measurement of Total Lactate Dehydrogenase Activity. Ann. Clin. Lab. Sci. 1985, 15, 13–31. [Google Scholar]
- Adams, E.; Finnegan, C.V. An Investigation of Lactate Dehydrogenase Activity in Early Amphibian Development. J. Exp. Zool. 1965, 158, 241–251. [Google Scholar] [CrossRef]
- Bennett, A.F. Enzymatic Correlates of Activity Metabolism in Anuran Amphibians. Am. J. Physiol.-Leg. Content 1974, 226, 1149. [Google Scholar] [CrossRef]
- Mendiola, P.; De Costa, J. The Effects of pH and Temperature on the Kinetic Properties of Skeletal Muscle Lactate Dehydrogenase from Anuran Amphibians. J. Comp. Physiol. B 1990, 160, 105–111. [Google Scholar] [CrossRef]
- Senko, H.; Kajić, S.; Huđ, A.; Palijan, G.; Petek, M.; Rajnović, I.; Šamec, D.; Udiković-Kolić, N.; Mešić, A.; Brkljačić, L.; et al. Will the Beneficial Properties of Plant-Growth Promoting Bacteria Be Affected by Waterlogging Predicted in the Wake of Climate Change: A Model Study. Appl. Soil. Ecol. 2024, 198, 105379. [Google Scholar] [CrossRef]
- Gosner, K.L. A Simplified Table for Staging Anuran Embryos and Larvae with Notes on Identification. Herpetologica 1960, 16, 183–190. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Frings, C.S.; Fendley, T.W.; Dunn, R.T.; Queen, C.A. Improved Determination of Total Serum Lipids by the Sulfo-Phospho-Vanillin Reaction. Clin. Chem. 1972, 18, 673–674. [Google Scholar] [CrossRef]
- Jermyn, M.A. Increasing the Sensitivity of the Anthrone Method for Carbohydrate. Anal. Biochem. 1975, 68, 332–335. [Google Scholar] [CrossRef]
- Bergmeyer, H.-U.; Bernt, E.; Hess, B. Lactic Dehydrogenase. In Methods of Enzymatic Analysis; Bergmeyer, H.-U., Ed.; Academic Press: Cambridge, MA, USA, 1965; pp. 736–743. ISBN 978-0-12-395630-9. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- van Doorn, J.; van den Bergh, D.; Böhm, U.; Dablander, F.; Derks, K.; Draws, T.; Etz, A.; Evans, N.J.; Gronau, Q.F.; Haaf, J.M.; et al. The JASP Guidelines for Conducting and Reporting a Bayesian Analysis. Psychon. Bull. Rev. 2021, 28, 813–826. [Google Scholar] [CrossRef]
- Ahmed, I.; Yokota, A.; Fujiwara, T. A Novel Highly Boron Tolerant Bacterium, Bacillus boroniphilus Sp. Nov., Isolated from Soil, That Requires Boron for Its Growth. Extremophiles 2007, 11, 217–224. [Google Scholar] [CrossRef]
- Müller, N.; Scherag, F.D.; Pester, M.; Schink, B. Bacillus stamsii Sp. Nov., a Facultatively Anaerobic Sugar Degrader That Is Numerically Dominant in Freshwater Lake Sediment. Syst. Appl. Microbiol. 2015, 38, 379–389. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, R.; Chen, T.; Zhang, B.; Yang, H.; Wu, X.; Gao, H.; Zhang, W.; Liu, G. Mesobacillus harenae Sp. Nov., Isolated from the Sandy Soil of a Cold Desert. Int. J. Syst. Evol. Microbiol. 2021, 71, 004594. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Mañero, F.J.; Ramos-Solano, B.; Probanza, A.; Mehouachi, J.; Tadeo, F.R.; Talon, M. The Plant-Growth-Promoting Rhizobacteria Bacillus pumilus and Bacillus licheniformis Produce High Amounts of Physiologically Active Gibberellins. Physiol. Plant. 2001, 111, 206–211. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-Tolerant Plant Growth-Promoting Bacillus pumilus Strain JPVS11 to Enhance Plant Growth Attributes of Rice and Improve Soil Health under Salinity Stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef]
- Murugappan, R.M.; Begum, S.B.; Roobia, R.R. Symbiotic Influence of Endophytic Bacillus pumilus on Growth Promotion and Probiotic Potential of the Medicinal Plant Ocimum Sanctum. Symbiosis 2013, 60, 91–99. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Dökenel, G.; Özer, S. Bacterial Agents Isolated from Cultured Marsh Frog (Pelophylax ridibundus, Pallas 1771). EgeJFAS 2019, 36, 115–124. [Google Scholar] [CrossRef]
- Burmølle, M.; Ren, D.; Bjarnsholt, T.; Sørensen, S.J. Interactions in Multispecies Biofilms: Do They Actually Matter? Trends Microbiol. 2014, 22, 84–91. [Google Scholar] [CrossRef]
- Cuzziol Boccioni, A.P.; Lener, G.; Peluso, J.; Peltzer, P.M.; Attademo, A.M.; Aronzon, C.; Simoniello, M.F.; Demonte, L.D.; Repetti, M.R.; Lajmanovich, R.C. Comparative Assessment of Individual and Mixture Chronic Toxicity of Glyphosate and Glufosinate Ammonium on Amphibian Tadpoles: A Multibiomarker Approach. Chemosphere 2022, 309, 136554. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).