Burn-Related Glycocalyx Derangement and the Emerging Role of MMP8 in Syndecan Shedding
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment and Sample Collection
2.2. Quantification of Serum Analytes via Enzyme-Linked Immunosorbent Assay
2.3. Statistical Analysis
2.4. Single-Cell RNA Sequencing (scRNA-Seq) Analysis
2.5. DNA Microarray and Gene Set Enrichment Analysis
2.6. Bioinformatics Data Visualization
2.7. Cell Culture and MMP8 In Vitro Assay
2.8. Immunofluorescence Staining and Microscopy
3. Results
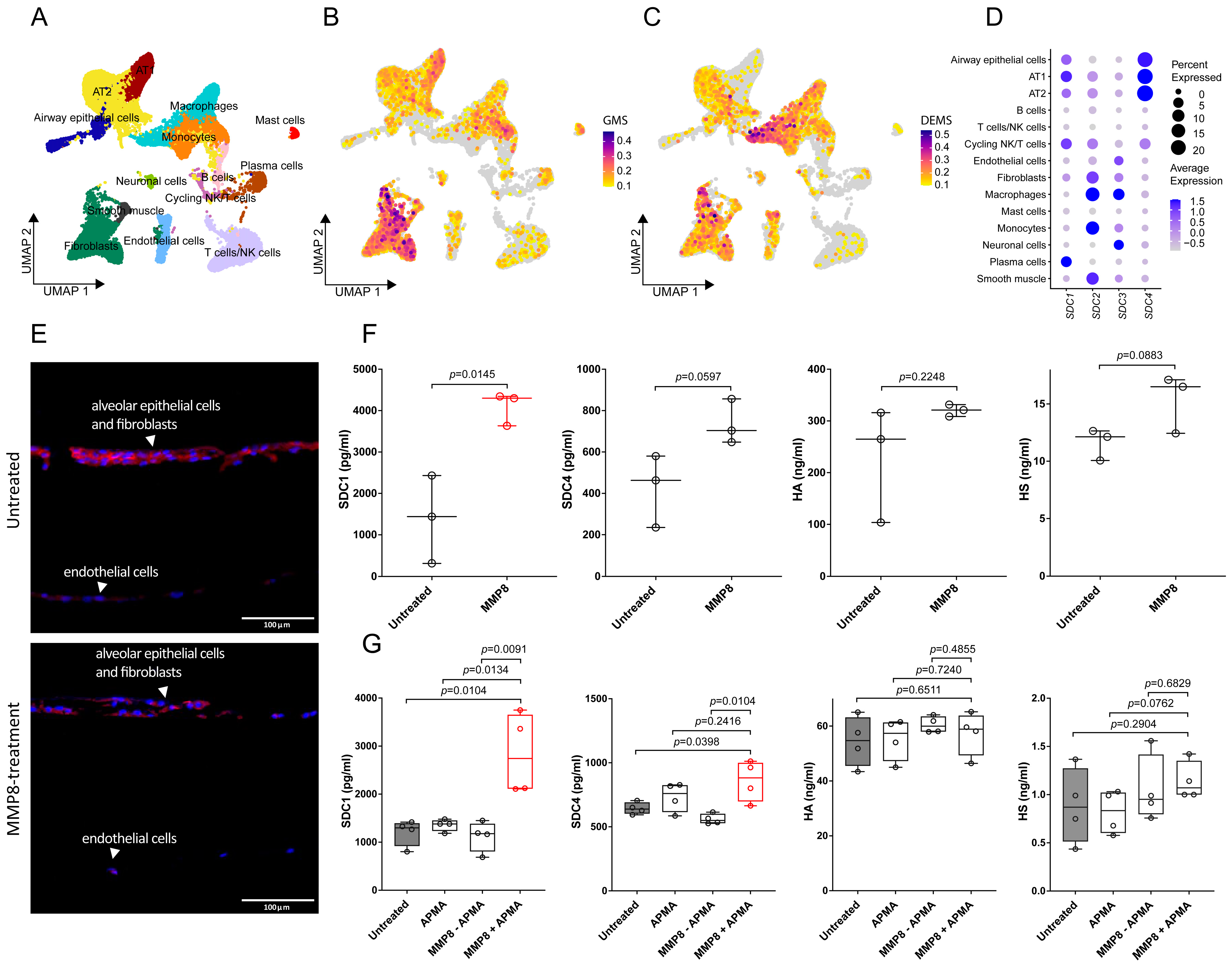
3.1. Transcriptomics Analysis Reveals Glycocalyx Derangement in Response to Burn Injury
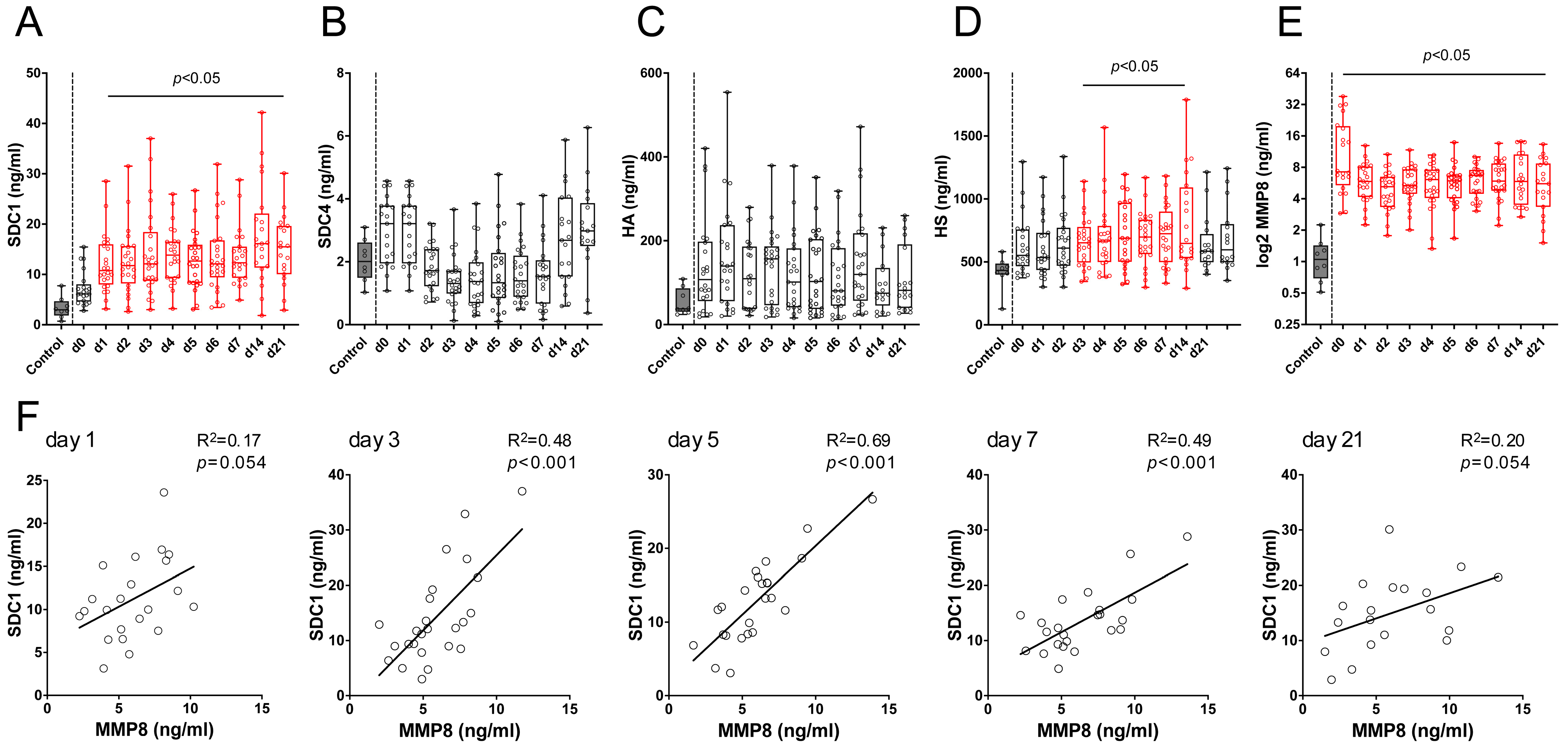
3.2. SDC1 and MMP8 Serum Levels Are Increased in Burn Patients
3.3. MMP8 Induces SDC1 Shedding in Alveolar Epithelial Cells but Not in Lung-Derived Endothelial Cells In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABSI | Abbreviated Burn Severity Index |
| ADAM | A Disintegrin and Metalloproteinase |
| ADAMTS | A Disintegrin and Metalloproteinase with Thrombospondin Motifs |
| APACHE II | Acute Physiology and Chronic Health Evaluation II |
| ARDS | Acute respiratory distress syndrome |
| BAL | Bronchoalveolar lavage |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| DEG | Differentially expressed gene |
| ELISA | Enzyme-linked immunosorbent assay |
| GAG | Glycosaminoglycan |
| HA | Hyaluronic Acid |
| HMVEC | Human lung microvascular endothelial cells |
| HPAEC | Human pulmonary artery endothelial cells |
| HS | Heparan sulfate |
| LIX | Lipopolysaccharide-Induced CXC Chemokine |
| LOH | Length of Hospitalization |
| MMP | Matrix metalloproteinase |
| MS | Module score |
| NETosis | Neutrophil extracellular trap formation |
| PCA | Principal Component Analysis |
| RPCA | Robust Principal Component Analysis |
| ROS | Reactive oxygen species |
| SAECs | Small airway epithelial cells |
| SAPS | Simplified Acute Physiology Score |
| SDC | Syndecan |
| scRNA-seq | Single-cell RNA sequencing |
| sST2 | Soluble Suppression of Tumorigenicity 2 |
| TBSA | Total body surface area |
| TGFβ1 | Transforming Growth Factor Beta 1 |
| UMAP | Uniform Manifold Approximation and Projection |
| UMIs | Unique molecular identifiers |
References
- Association, A.B. National Burn Repository 2019 Update: Report of Data from 2009 to 2018; American Burn Association: Chicago, IL, USA, 2019. [Google Scholar]
- Brusselaers, N.; Monstrey, S.; Vogelaers, D.; Hoste, E.; Blot, S. Severe Burn Injury in Europe: A Systematic Review of the Incidence, Etiology, Morbidity, and Mortality. Crit. Care 2010, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn Injury. Nat. Rev. Dis. Primer 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.; Loh, E.-W.; Hsu, C.-C.; Lin, H.-J.; Huang, C.-C.; Chou, Y.-Y.; Lien, C.-C.; Tam, K.-W. Fluid Resuscitation in Patients with Severe Burns: A Meta-Analysis of Randomized Controlled Trials. Acad. Emerg. Med. Off. J. Soc. Acad. Emerg. Med. 2018, 25, 320–329. [Google Scholar] [CrossRef]
- Staud, C.J.; Resch, A.; Christ, A.; Borger, A.; Zaussinger, M.; Teufelsbauer, M.; Worel, N.; Radtke, C. Skin Bank Establishment in Treatment of Severe Burn Injuries: Overview and Experience with Skin Allografts at the Vienna Burn Center. J. Clin. Med. 2023, 12, 4717. [Google Scholar] [CrossRef]
- Young, A.W.; Dewey, W.S.; King, B.T. Rehabilitation of Burn Injuries: An Update. Phys. Med. Rehabil. Clin. 2019, 30, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Sierawska, O.; Małkowska, P.; Taskin, C.; Hrynkiewicz, R.; Mertowska, P.; Grywalska, E.; Korzeniowski, T.; Torres, K.; Surowiecka, A.; Niedźwiedzka-Rystwej, P.; et al. Innate Immune System Response to Burn Damage—Focus on Cytokine Alteration. Int. J. Mol. Sci. 2022, 23, 716. [Google Scholar] [CrossRef]
- Ravat, F.; Payre, J.; Peslages, P.; Fontaine, M.; Sens, N. La Brûlure: Une Pathologie Inflammatoire. Pathol. Biol. 2011, 59, e63–e72. [Google Scholar] [CrossRef]
- Hacker, S.; Dieplinger, B.; Werba, G.; Nickl, S.; Roth, G.A.; Krenn, C.G.; Mueller, T.; Ankersmit, H.J.; Haider, T. Increased Serum Concentrations of Soluble ST2 Predict Mortality after Burn Injury. Clin. Chem. Lab. Med. 2018, 56, 2079–2087. [Google Scholar] [CrossRef]
- Laggner, M.; Lingitz, M.-T.; Copic, D.; Direder, M.; Klas, K.; Bormann, D.; Gugerell, A.; Moser, B.; Radtke, C.; Hacker, S.; et al. Severity of Thermal Burn Injury Is Associated with Systemic Neutrophil Activation. Sci. Rep. 2022, 12, 1654. [Google Scholar] [CrossRef]
- Steinberg, K.P.; Milberg, J.A.; Martin, T.R.; Maunder, R.J.; Cockrill, B.A.; Hudson, L.D. Evolution of Bronchoalveolar Cell Populations in the Adult Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 1994, 150, 113–122. [Google Scholar] [CrossRef]
- Scozzi, D.; Liao, F.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The Role of Neutrophil Extracellular Traps in Acute Lung Injury. Front. Immunol. 2022, 13, 953195. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Kinoshita, M.; Ono, S.; Seki, S.; Saitoh, D. Burn-Evoked Reactive Oxygen Species Immediately After Injury Are Crucial to Restore the Neutrophil Function Against Postburn Infection in Mice. Shock 2015, 44, 252–257. [Google Scholar] [CrossRef]
- Kajita, Y.; Terashima, T.; Mori, H.; Islam, M.M.; Irahara, T.; Tsuda, M.; Kano, H.; Takeyama, N. A Longitudinal Change of Syndecan-1 Predicts Risk of Acute Respiratory Distress Syndrome and Cumulative Fluid Balance in Patients with Septic Shock: A Preliminary Study. J. Intensive Care 2021, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Ochs, M.; Hegermann, J.; Lopez-Rodriguez, E.; Timm, S.; Nouailles, G.; Matuszak, J.; Simmons, S.; Witzenrath, M.; Kuebler, W.M. On Top of the Alveolar Epithelium: Surfactant and the Glycocalyx. Int. J. Mol. Sci. 2020, 21, 3075. [Google Scholar] [CrossRef] [PubMed]
- Lingitz, M.-T.; Wollner, G.; Bauer, J.; Kuehtreiber, H.; Mildner, M.; Copic, D.; Bormann, D.; Direder, M.; Krenn, C.G.; Haider, T.; et al. Elevation of Neutrophil-Derived Factors in Patients after Multiple Trauma. J. Cell. Mol. Med. 2023, 27, 1859–1866. [Google Scholar] [CrossRef]
- Hasty, K.A.; Pourmotabbed, T.F.; Goldberg, G.I.; Thompson, J.P.; Spinella, D.G.; Stevens, R.M.; Mainardi, C.L. Human Neutrophil Collagenase. A Distinct Gene Product with Homology to Other Matrix Metalloproteinases. J. Biol. Chem. 1990, 265, 11421–11424. [Google Scholar] [CrossRef]
- Wang, B.; Chenru, W.; Jiang, Y.; Hu, L.; Fang, H.; Zhu, F.; Yu, Q.; Zhu, B.; Wu, G.; Sun, Y.; et al. Incidence and Mortality of Acute Respiratory Distress Syndrome in Patients With Burns: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 709642. [Google Scholar] [CrossRef]
- Cartotto, R.; Li, Z.; Hanna, S.; Spano, S.; Wood, D.; Chung, K.; Camacho, F. The Acute Respiratory Distress Syndrome (ARDS) in Mechanically Ventilated Burn Patients: An Analysis of Risk Factors, Clinical Features, and Outcomes Using the Berlin ARDS Definition. Burns 2016, 42, 1423–1432. [Google Scholar] [CrossRef]
- Jones, S.W.; Williams, F.N.; Cairns, B.A.; Cartotto, R. INHALATION INJURY: Pathophysiology, Diagnosis, and Treatment. Clin. Plast. Surg. 2017, 44, 505–511. [Google Scholar] [CrossRef]
- Bittner, E.; Sheridan, R. Acute Respiratory Distress Syndrome, Mechanical Ventilation, and Inhalation Injury in Burn Patients. Surg. Clin. N. Am. 2023, 103, 439–451. [Google Scholar] [CrossRef]
- Rizzo, A.N.; Schmidt, E.P. The Role of the Alveolar Epithelial Glycocalyx in Acute Respiratory Distress Syndrome. Am. J. Physiol. Cell Physiol. 2023, 324, C799–C806. [Google Scholar] [CrossRef]
- Tarbell, J.M.; Cancel, L.M. The Glycocalyx and Its Significance in Human Medicine. J. Intern. Med. 2016, 280, 97–113. [Google Scholar] [CrossRef]
- Aldecoa, C.; Llau, J.V.; Nuvials, X.; Artigas, A. Role of Albumin in the Preservation of Endothelial Glycocalyx Integrity and the Microcirculation: A Review. Ann. Intensive Care 2020, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Manon-Jensen, T.; Multhaupt, H.A.B.; Couchman, J.R. Mapping of Matrix Metalloproteinase Cleavage Sites on Syndecan-1 and Syndecan-4 Ectodomains. FEBS J. 2013, 280, 2320–2331. [Google Scholar] [CrossRef]
- Qu, J.; Cheng, Y.; Wu, W.; Yuan, L.; Liu, X. Glycocalyx Impairment in Vascular Disease: Focus on Inflammation. Front. Cell Dev. Biol. 2021, 9, 730621. [Google Scholar] [CrossRef] [PubMed]
- LaRivière, W.B.; Schmidt, E.P. The Pulmonary Endothelial Glycocalyx in ARDS: A Critical Role for Heparan Sulfate. Curr. Top. Membr. 2018, 82, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.S.; Wickersham, N.; McNeil, J.B.; Shaver, C.M.; May, A.K.; Bastarache, J.A.; Ware, L.B. Endothelial Glycocalyx Degradation Is More Severe in Patients with Non-Pulmonary Sepsis Compared to Pulmonary Sepsis and Associates with Risk of ARDS and Other Organ Dysfunction. Ann. Intensive Care 2017, 7, 102. [Google Scholar] [CrossRef]
- Rizzo, A.N.; Haeger, S.M.; Oshima, K.; Yang, Y.; Wallbank, A.M.; Jin, Y.; Lettau, M.; McCaig, L.A.; Wickersham, N.E.; McNeil, J.B.; et al. Alveolar Epithelial Glycocalyx Degradation Mediates Surfactant Dysfunction and Contributes to Acute Respiratory Distress Syndrome. JCI Insight 2022, 7, e154573. [Google Scholar] [CrossRef]
- Sorkin, M.; Huber, A.K.; Hwang, C.; Carson, W.F.; Menon, R.; Li, J.; Vasquez, K.; Pagani, C.; Patel, N.; Li, S.; et al. Regulation of Heterotopic Ossification by Monocytes in a Mouse Model of Aberrant Wound Healing. Nat. Commun. 2020, 11, 722. [Google Scholar] [CrossRef]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary Learning for Integrative, Multimodal and Scalable Single-Cell Analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef]
- Finak, G.; McDavid, A.; Yajima, M.; Deng, J.; Gersuk, V.; Shalek, A.K.; Slichter, C.K.; Miller, H.W.; McElrath, M.J.; Prlic, M.; et al. MAST: A Flexible Statistical Framework for Assessing Transcriptional Changes and Characterizing Heterogeneity in Single-Cell RNA Sequencing Data. Genome Biol. 2015, 16, 278. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Meltzer, P.S. GEOquery: A Bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinforma. Oxf. Engl. 2007, 23, 1846–1847. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2. In Use R! Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Blighe, K.; Rana, S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. 2023. Available online: https://github.com/kevinblighe/EnhancedVolcano (accessed on 3 March 2025).
- Marsh, S.; Tang, M.; Kozareva, V.; Graybuck, L. scCustomize: Custom Visualizations & Functions for Streamlined Analyses of Single Cell Sequencing. 2023. Available online: https://samuel-marsh.github.io/scCustomize/articles/FAQ.html (accessed on 3 March 2025).
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic Responses in Mouse Models Poorly Mimic Human Inflammatory Diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Welling, H.; Henriksen, H.H.; Gonzalez-Rodriguez, E.R.; Stensballe, J.; Huzar, T.F.; Johansson, P.I.; Wade, C.E. Endothelial Glycocalyx Shedding in Patients with Burns. Burns 2020, 46, 386–393. [Google Scholar] [CrossRef]
- Butler, K.L.; Ambravaneswaran, V.; Agrawal, N.; Bilodeau, M.; Toner, M.; Tompkins, R.G.; Fagan, S.; Irimia, D. Burn Injury Reduces Neutrophil Directional Migration Speed in Microfluidic Devices. PLoS ONE 2010, 5, e11921. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Guo, Z.; Li, L.; Shao, Y.; Song, M.; Sun, B. Investigation and Assessment of Neutrophil Dysfunction Early after Severe Burn Injury. Burns 2021, 47, 1851–1862. [Google Scholar] [CrossRef]
- Stengle, J.; Meyers, R.; Pyle, J.; Dries, D.J. Neutrophil Recruitment after Remote Scald Injury. J. Burn Care Rehabil. 1996, 17, 14–18. [Google Scholar] [CrossRef]
- Arbak, S.; Ercan, F.; Hürdağ, C.G.; Karabulut, O.; Gürbüz, V.; Corak, A.; Alican, I. Acute Lung Injury Following Thermal Insult to the Skin: A Light and Transmission Electron Microscopial Study. Acta Histochem. 1999, 101, 255–262. [Google Scholar] [CrossRef]
- Zemans, R.L.; Matthay, M.A. What Drives Neutrophils to the Alveoli in ARDS? Thorax 2017, 72, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Okada, H.; Takemura, G.; Takada, C.; Kuroda, A.; Yano, H.; Zaikokuji, R.; Morishita, K.; Tomita, H.; Oda, K.; et al. Neutrophil Elastase Damages the Pulmonary Endothelial Glycocalyx in Lipopolysaccharide-Induced Experimental Endotoxemia. Am. J. Pathol. 2019, 189, 1526–1535. [Google Scholar] [CrossRef]
- Yang, S.-C.; Tsai, Y.-F.; Pan, Y.-L.; Hwang, T.-L. Understanding the Role of Neutrophils in Acute Respiratory Distress Syndrome. Biomed. J. 2021, 44, 439–446. [Google Scholar] [CrossRef] [PubMed]
- DeLeon-Pennell, K.Y.; Meschiari, C.A.; Jung, M.; Lindsey, M.L. Chapter Two—Matrix Metalloproteinases in Myocardial Infarction and Heart Failure. In Progress in Molecular Biology and Translational Science; Khalil, R.A., Ed.; Matrix Metalloproteinases and Tissue Remodeling in Health and Disease: Cardiovascular Remodeling; Academic Press: Cambridge, MA, USA, 2017; Volume 147, pp. 75–100. [Google Scholar]
- Van Lint, P.; Libert, C. Chemokine and Cytokine Processing by Matrix Metalloproteinases and Its Effect on Leukocyte Migration and Inflammation. J. Leukoc. Biol. 2007, 82, 1375–1381. [Google Scholar] [CrossRef]
- Tester, A.M.; Cox, J.H.; Connor, A.R.; Starr, A.E.; Dean, R.A.; Puente, X.S.; López-Otín, C.; Overall, C.M. LPS Responsiveness and Neutrophil Chemotaxis in Vivo Require PMN MMP-8 Activity. PLoS ONE 2007, 2, e312. [Google Scholar] [CrossRef] [PubMed]
- Pruessmeyer, J.; Martin, C.; Hess, F.M.; Schwarz, N.; Schmidt, S.; Kogel, T.; Hoettecke, N.; Schmidt, B.; Sechi, A.; Uhlig, S.; et al. A Disintegrin and Metalloproteinase 17 (ADAM17) Mediates Inflammation-Induced Shedding of Syndecan-1 and -4 by Lung Epithelial Cells. J. Biol. Chem. 2010, 285, 555–564. [Google Scholar] [CrossRef]
- Echtermeyer, F.; Bertrand, J.; Dreier, R.; Meinecke, I.; Neugebauer, K.; Fuerst, M.; Lee, Y.J.; Song, Y.W.; Herzog, C.; Theilmeier, G.; et al. Syndecan-4 Regulates ADAMTS-5 Activation and Cartilage Breakdown in Osteoarthritis. Nat. Med. 2009, 15, 1072–1076. [Google Scholar] [CrossRef]
- Haeger, S.M.; Liu, X.; Han, X.; McNeil, J.B.; Oshima, K.; McMurtry, S.A.; Yang, Y.; Ouyang, Y.; Zhang, F.; Nozik-Grayck, E.; et al. Epithelial Heparan Sulfate Contributes to Alveolar Barrier Function and Is Shed during Lung Injury. Am. J. Respir. Cell Mol. Biol. 2018, 59, 363–374. [Google Scholar] [CrossRef]
- LaRivière, W.B.; Liao, S.; McMurtry, S.A.; Oshima, K.; Han, X.; Zhang, F.; Yan, S.; Haeger, S.M.; Ransom, M.; Bastarache, J.A.; et al. Alveolar Heparan Sulfate Shedding Impedes Recovery from Bleomycin-Induced Lung Injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1198–L1210. [Google Scholar] [CrossRef]
- Kliment, C.R.; Englert, J.M.; Gochuico, B.R.; Yu, G.; Kaminski, N.; Rosas, I.; Oury, T.D. Oxidative Stress Alters Syndecan-1 Distribution in Lungs with Pulmonary Fibrosis. J. Biol. Chem. 2009, 284, 3537–3545. [Google Scholar] [CrossRef]
- Parimon, T.; Yao, C.; Habiel, D.M.; Ge, L.; Bora, S.A.; Brauer, R.; Evans, C.M.; Xie, T.; Alonso-Valenteen, F.; Medina-Kauwe, L.K.; et al. Syndecan-1 Promotes Lung Fibrosis by Regulating Epithelial Reprogramming through Extracellular Vesicles. JCI Insight 2019, 4, e129359. [Google Scholar] [CrossRef]
- Gao, W.; Fang, F.; Xia, T.J.; Zhang, Y.; Sun, J.; Wu, Q.; Wang, W. Doxycycline Can Reduce Glycocalyx Shedding by Inhibiting Matrix Metalloproteinases in Patients Undergoing Cardiopulmonary Bypass: A Randomized Controlled Trial. Microvasc. Res. 2022, 142, 104381. [Google Scholar] [CrossRef] [PubMed]
- Beer, L.; Warszawska, J.M.; Schenk, P.; Debreceni, T.; Dworschak, M.; Roth, G.A.; Szerafin, T.; Ankersmit, H.J. Intraoperative Ventilation Strategy during Cardiopulmonary Bypass Attenuates the Release of Matrix Metalloproteinases and Improves Oxygenation. J. Surg. Res. 2015, 195, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Kormi, I.; Alfakry, H.; Tervahartiala, T.; Pussinen, P.J.; Sinisalo, J.; Sorsa, T. The Effect of Prolonged Systemic Doxycycline Therapy on Serum Tissue Degrading Proteinases in Coronary Bypass Patients: A Randomized, Double-Masked, Placebo-Controlled Clinical Trial. Inflamm. Res. 2014, 63, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Garcia, L.; Oliveira, B.; Tanita, M.; Festti, J.; Cardoso, L.; Lavado, L.; Grion, C. Acute Respiratory Distress Syndrome in Burn Patients: Incidence and Risk Factor Analysis. Ann. Burns Fire Disasters 2016, 29, 178. [Google Scholar]
- Kreuger, J.; Jemth, P.; Sanders-Lindberg, E.; Eliahu, L.; Ron, D.; Basilico, C.; Salmivirta, M.; Lindahl, U. Fibroblast Growth Factors Share Binding Sites in Heparan Sulphate. Biochem. J. 2005, 389, 145–150. [Google Scholar] [CrossRef]
- Forsten-Williams, K.; Chu, C.L.; Fannon, M.; Buczek-Thomas, J.A.; Nugent, M.A. Control of Growth Factor Networks by Heparan Sulfate Proteoglycans. Ann. Biomed. Eng. 2008, 36, 2134–2148. [Google Scholar] [CrossRef]
- Altemeier, W.A.; Schlesinger, S.Y.; Buell, C.A.; Brauer, R.; Rapraeger, A.C.; Parks, W.C.; Chen, P. Transmembrane and Extracellular Domains of Syndecan-1 Have Distinct Functions in Regulating Lung Epithelial Migration and Adhesion. J. Biol. Chem. 2012, 287, 34927–34935. [Google Scholar] [CrossRef]
- Grotberg, J.C.; Reynolds, D.; Kraft, B.D. Management of Severe Acute Respiratory Distress Syndrome: A Primer. Crit. Care 2023, 27, 289. [Google Scholar] [CrossRef]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Carney, D.E.; McCann, U.G.; Schiller, H.J.; Gatto, L.A.; Steinberg, J.; Picone, A.L.; Nieman, G.F. Metalloproteinase Inhibition Prevents Acute Respiratory Distress Syndrome. J. Surg. Res. 2001, 99, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.; Goldklang, M.; Stearns, K.; Ma, X.; Xiao, R.; Zelonina, T.; D’Armiento, J. Attenuation of Pulmonary Injury by an Inhaled MMP Inhibitor in the Endotoxin Lung Injury Model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L1036. [Google Scholar] [CrossRef] [PubMed]
- Sela-Passwell, N.; Kikkeri, R.; Dym, O.; Rozenberg, H.; Margalit, R.; Arad-Yellin, R.; Eisenstein, M.; Brenner, O.; Shoham, T.; Danon, T.; et al. Antibodies Targeting the Catalytic Zinc Complex of Activated Matrix Metalloproteinases Show Therapeutic Potential. Nat. Med. 2011, 18, 143–147. [Google Scholar] [CrossRef]
- Davey, A.; McAuley, D.F.; O’Kane, C.M. Matrix Metalloproteinases in Acute Lung Injury: Mediators of Injury and Drivers of Repair. Eur. Respir. J. 2011, 38, 959–970. [Google Scholar] [CrossRef]

| Variable | Burn Patients | Controls |
|---|---|---|
| n | 28 | 8 |
| Age (years) | 49.6 (42.5) ± 21.8 [32.25–72.75] | 40.5 (36) ± 19.9 [23–55.75] |
| F:M ratio (%) | 7:21 (25:75) | 3:5 (37.5:62.5) |
| ABSI | 7.7 (8) ± 2.8 [5–9] | |
| APACHE 2 | 18.4 (18) ± 8.3 [11.5–26.5] | |
| LOH (days) | 41.1 (33) ± 34.0 [15–67.75] | |
| SAPS II | 38.3 (35) ± 16.9 [24.75–48.5] | |
| SAPS III | 31.7 (31.5) ± 10.8 [23–37.25] | |
| TBSA (%) | 32.5 (30) ± 20.2 [16.25–39.50] | |
| Deceased (%) * | 3 (10.7) | |
| Inhalation injury * | 6 (21.4) | |
| 3rd-degree burn (%) * | 19 (67.9) |
| Days After Burn | ABSI | Inhalation Injury | TBSA | 3rd-Degree Burn | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson’s r | 95% CI | p-Value | OR | 95% CI | p-Value | Pearson’s r | 95% CI | p-Value | OR | 95% CI | p-Value | |
| 0 | 0.37 | −0.07–0.69 | 0.097 | 1.91 | 1.25–4.16 | 0.001 | 0.17 | −0.28–0.56 | 0.462 | 1.23 | 0.90–1.95 | 0.212 |
| 1 | 0.27 | −0.15–0.61 | 0.202 | 1.47 | 1.12–2.68 | 0.002 | 0.04 | −0.37–0.43 | 0.862 | 1.19 | 0.98–1.56 | 0.087 |
| 2 | 0.62 | 0.29–0.82 | 0.001 | 1.58 | 1.15–2.88 | <0.001 | 0.54 | 0.18–0.78 | 0.006 | 1.15 | 0.98–1.43 | 0.106 |
| 3 | 0.50 | 0.13–0.75 | 0.011 | 1.39 | 1.13–2.00 | <0.001 | 0.29 | −0.12–0.61 | 0.161 | 1.12 | 0.99–1.35 | 0.091 |
| 4 | 0.46 | 0.07–0.73 | 0.023 | 1.42 | 1.11–2.17 | 0.002 | 0.30 | −0.12–0.63 | 0.158 | 1.20 | 1.00–1.55 | 0.056 |
| 5 | 0.29 | −0.12–0.62 | 0.164 | 1.31 | 1.06–1.84 | 0.009 | 0.28 | −0.13–0.60 | 0.182 | 1.14 | 0.96–1.41 | 0.138 |
| 6 | −0.04 | −0.42–0.36 | 0.860 | 1.08 | 0.94–1.24 | 0.266 | 0.00 | −0.39–0.39 | 0.991 | 1.03 | 0.91–1.20 | 0.636 |
| 7 | −0.05 | −0.45–0.37 | 0.832 | 1.11 | 0.93–1.35 | 0.250 | 0.05 | −0.37–0.45 | 0.817 | 1.04 | 0.88–1.29 | 0.662 |
| 14 | 0.04 | −0.40–0.46 | 0.874 | 1.14 | 1.02–1.33 | 0.022 | −0.09 | −0.50–0.35 | 0.695 | 0.99 | 0.89–1.12 | 0.844 |
| 21 | 0.14 | −0.33–0.56 | 0.564 | 1.16 | 0.98–1.48 | 0.090 | 0,06 | −0.40–0.50 | 0.792 | 0.95 | 0.79–1.13 | 0.588 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kühtreiber, H.; Bormann, D.; Salek, M.; Auer, L.; Haider, T.; Mildner, C.S.; Lingitz, M.-T.; Aigner, C.; Radtke, C.; Zimpfer, D.; et al. Burn-Related Glycocalyx Derangement and the Emerging Role of MMP8 in Syndecan Shedding. Biology 2025, 14, 269. https://doi.org/10.3390/biology14030269
Kühtreiber H, Bormann D, Salek M, Auer L, Haider T, Mildner CS, Lingitz M-T, Aigner C, Radtke C, Zimpfer D, et al. Burn-Related Glycocalyx Derangement and the Emerging Role of MMP8 in Syndecan Shedding. Biology. 2025; 14(3):269. https://doi.org/10.3390/biology14030269
Chicago/Turabian StyleKühtreiber, Hannes, Daniel Bormann, Melanie Salek, Lisa Auer, Thomas Haider, Caterina Selina Mildner, Marie-Therese Lingitz, Clemens Aigner, Christine Radtke, Daniel Zimpfer, and et al. 2025. "Burn-Related Glycocalyx Derangement and the Emerging Role of MMP8 in Syndecan Shedding" Biology 14, no. 3: 269. https://doi.org/10.3390/biology14030269
APA StyleKühtreiber, H., Bormann, D., Salek, M., Auer, L., Haider, T., Mildner, C. S., Lingitz, M.-T., Aigner, C., Radtke, C., Zimpfer, D., Ankersmit, H. J., & Mildner, M. (2025). Burn-Related Glycocalyx Derangement and the Emerging Role of MMP8 in Syndecan Shedding. Biology, 14(3), 269. https://doi.org/10.3390/biology14030269









