1. Introduction
In 2018, it was estimated that approximately one million insect species had been described and identified, accounting for more than 50% of all eukaryotes. The predicted number of new species described each year is around 20.000. The estimated total number of insect species, including those not yet identified, is believed to be 5.5 million, representing about 80% of all eukaryotes. Insects are classified into 24 orders. In terms of the number of species, Coleoptera (beetles: 386.500), Lepidoptera (butterflies and moths: 157.338), Diptera (flies and mosquitoes: 155.477), Hymenoptera (ants, bees, and wasps: 116.861), Hemiptera (true bugs: 103.590), and Orthoptera (locusts, grasshoppers, and crickets 23.855) are the dominant orders, while Mantophasmatodea (15) is the least abundant [
1].
It was reported that some insect populations are in decline in their abundance, biomass, and species richness in many studied areas, while other insect populations are not. Generally, each insect species is affected by changes in the environment in different ways compared to other species (depending on the success of adaptation to survive). The declines of insect abundance have been attributed to reasons such as habitat loss (urbanization), pesticide (insecticides and herbicides) use on crops, and invasive species, which compete with the indigenous species [
2]. During 2017, about 66 insect species were recorded as extinction species [
3] all around the world, and many other insect species were documented by IUCN (the International Union for Conservation of Nature). This includes 97 species of Odanata, 91 species of Orthoptera, 72 species of Coleoptera, 51 species of Lepidoptera, 18 species of Hymenoptera, and 7 species of Blattodea [
4]. Nowadays, more than 166.000 species of both fauna and flora populations have been documented within IUCN red list [
5].
A nature reserve is a protected ecological area, mainly for fauna and flora [
6]. It is also known as wildlife refuge, wildlife sanctuary, or nature conservation area. These areas usually fall into IUCN in different categories in addition to the local laws [
7]. The establishment of nature reserves is an ecological way to protect biodiversity. It is also considered an effective method to protect the ecological environment from human activities that disturb habitat quality [
8]. The assessment of biotic and abiotic components will help for planning different conservation scenarios [
9] in the context of systematic conservation to form a resilient ecological system [
10].
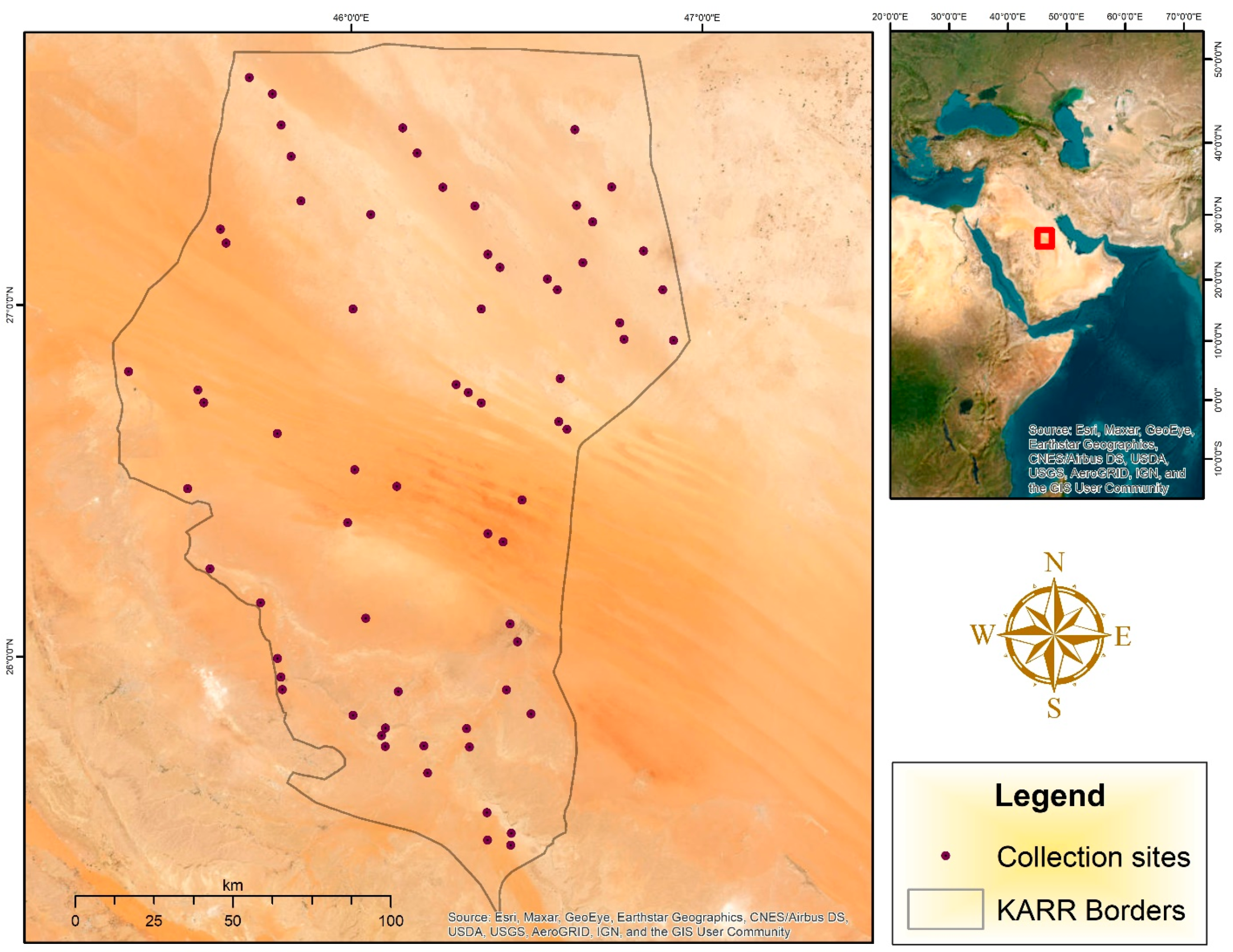
The Kingdom of Saudi Arabia (KSA) occupies an area of about 2.2 million km
2. This area is characterized by different ecological systems, but it is classified as arid and semiarid. There are various ecosystems including valley, hills, deserts (rocky and sandy), and coastal areas that provide the optimal conditions for different fauna and flora to flourish. The ecological system of KSA hosts about 78 species of mammals, 550 species of birds, 130 species of reptiles, 7 species of amphibians, and an undefined number of fish, arthropods, and other invertebrates [
11]. In 2018, the King Abdulaziz Royal Nature Reserve (KARR) was established. It occupies an area of 28.345 km
2 (about 13% of KSA total area). It represents all the ecosystem of the KSA, except the coastal one. The KARR is working within its ability, under laws and legislations set by IUCN organization, to conserve biomass and to assess biodiversity [
12].
The evolution of soil arthropod diversity in desert areas has been studied using both active and passive trapping techniques [
13]. Arid conditions are driven by plant size, plant morphological characters, soil fertilization, plant nutritional content, and prey–predator interactions [
14].
In arid areas, climate change (e.g., the elevation of temperature) increases the frequency of droughts and fluctuates rainfall pattern and rates, which in turn directly affects living organisms such as insect and plant populations. The unstable and unpredicted weather conditions may alter the normal physiological process of all living taxa. Temperature shifts may alter the biochemical process (enzyme activities) within insects, and consequently deteriorate fertility, feeding patterns, survival rates, population dynamics, and dispersal patterns, and accordingly, change and modify the abundance, distribution, densities, and lifecycle of insects. The effect of climate change on insect ecology may disrupt pollination and hence, food security [
15] and crop yield [
16], because crop production depends mainly on climate variables and insect pests.
A similar study conducted at Rawdhat Khorium National Park (25°23’ N, and 47°17’ E), Northern Riyadh, Central Saudi Arabia, was conducted in order to determine the population of beetles in that area. The collection methods involved pitfall, UV-light, Malaise traps, net sweeping, beating vegetation, vacuuming, and hand-picking. The results of this study show that Tenebrionidae and Scarabeidae were the most abundant families [
17].
Because of a lack of records in the KARR about the exact components of flora and fauna species, in addition to the needs for constructing base-line data, this study examined the insect occurrence and biodiversity in association with seasonal variation (spatio-temporal) during January–November 2023. The study was also interested in reflecting the presence and distribution of beneficial (pollinators, decomposers, and predators), and pest insects in order to assist in designing management plans within the study area. The study also constructs baseline data involving other categories such as non-insect arthropods, various classes of vertebrates, and plants.
4. Discussion
The aim of this study was to evaluate the biodiversity of insects (in terms of density and abundance) within the designated study area in addition to reflecting the abundance of some key species. Similar studies has been conducted in the Kingdom of Saudi Arabia in the years 2017 [
17], 2018 [
34], 2020 [
35], 2021 [
36], 2022 [
37], and 2023 [
38]. All these studies agreed that Coleoptera and Hymenoptera are the most abundant insects in the KSA.
Similar studies were conducted in some Arabic countries, e.g., the UAE, during 2014 [
39] and 2023 [
40], and Algeria [
41] and they share similar results with the KSA regarding the flourishing of darkling beetles and some ant species. This finding may be attributed to the similarity of arid habitat in the above-mentioned countries.
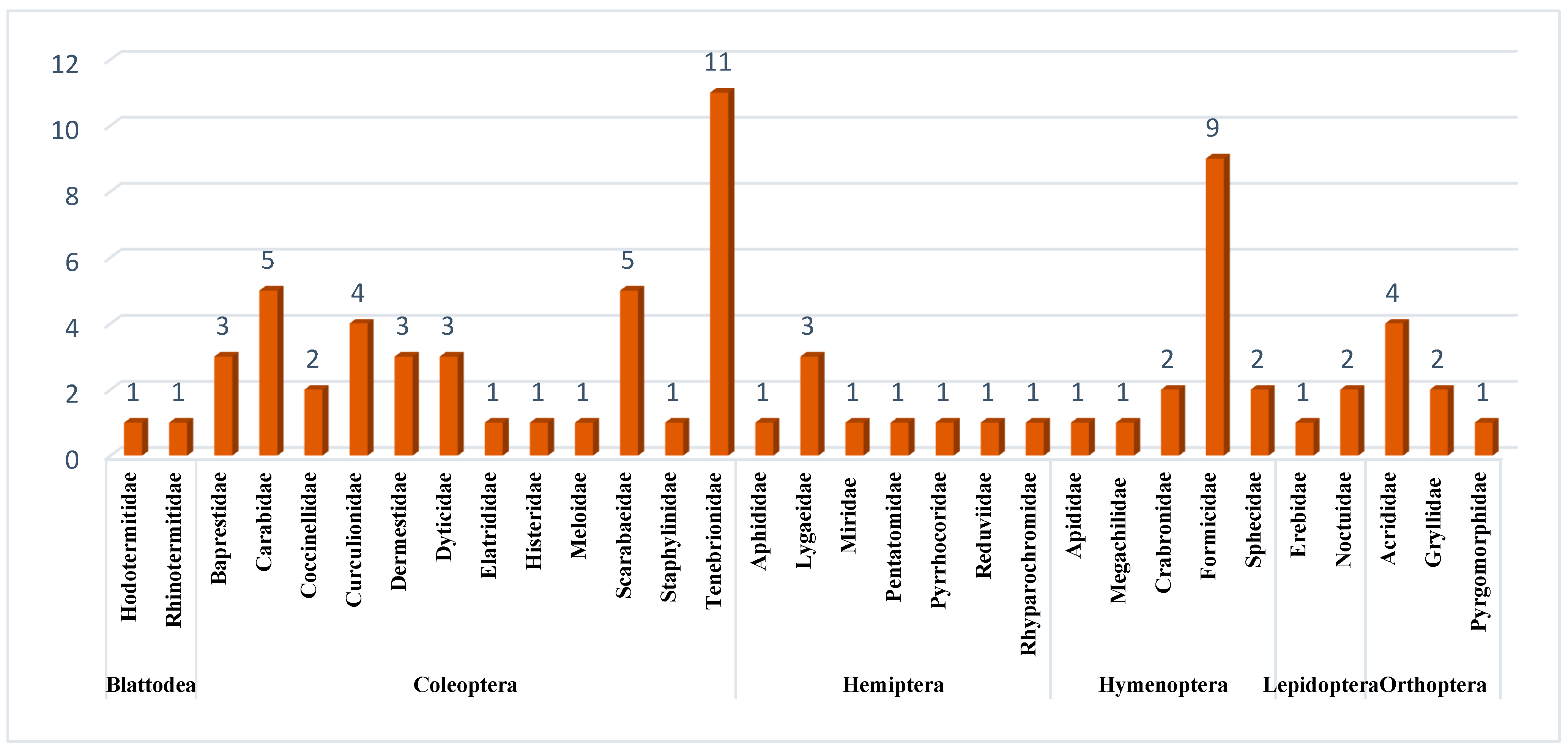
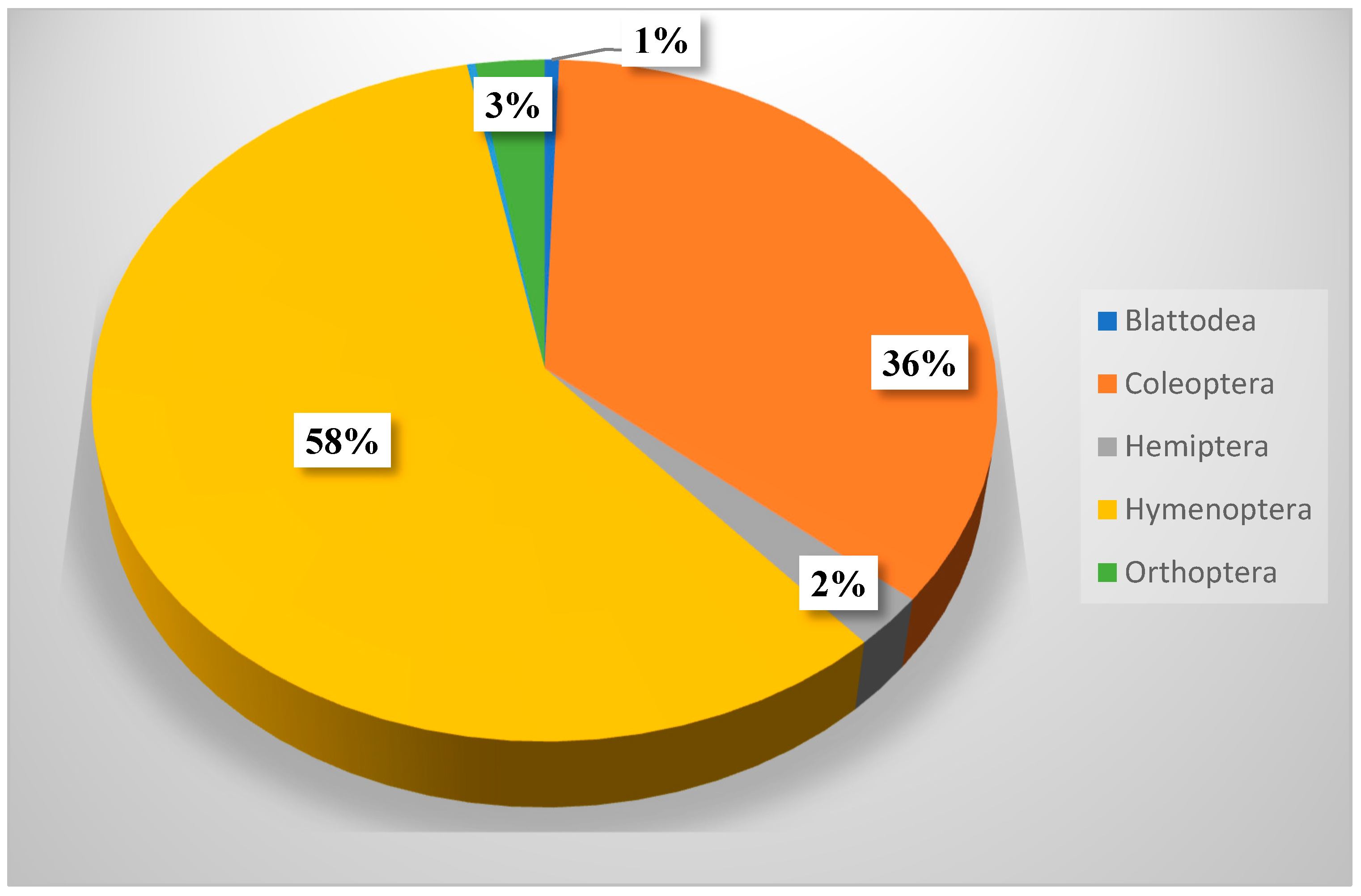
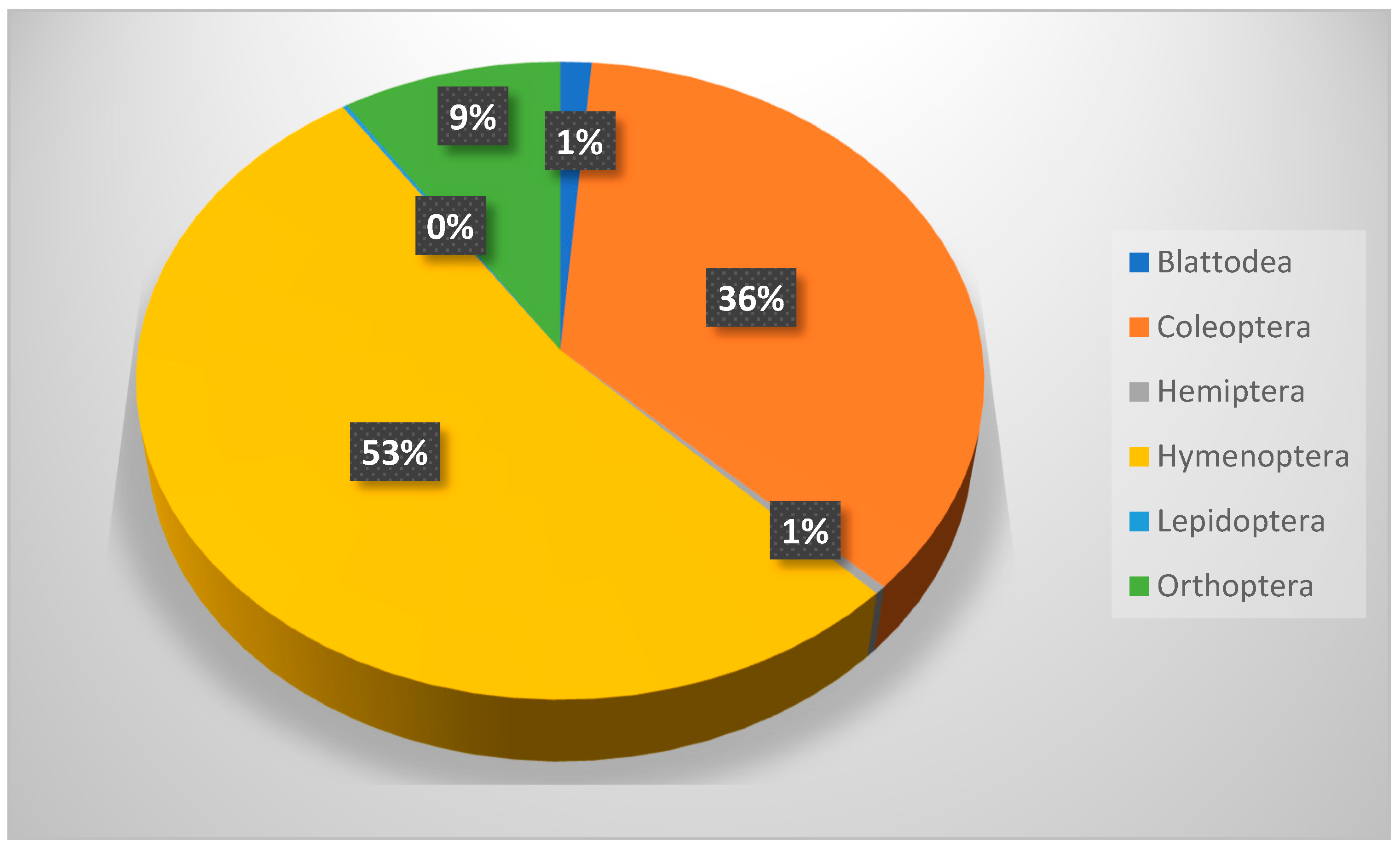
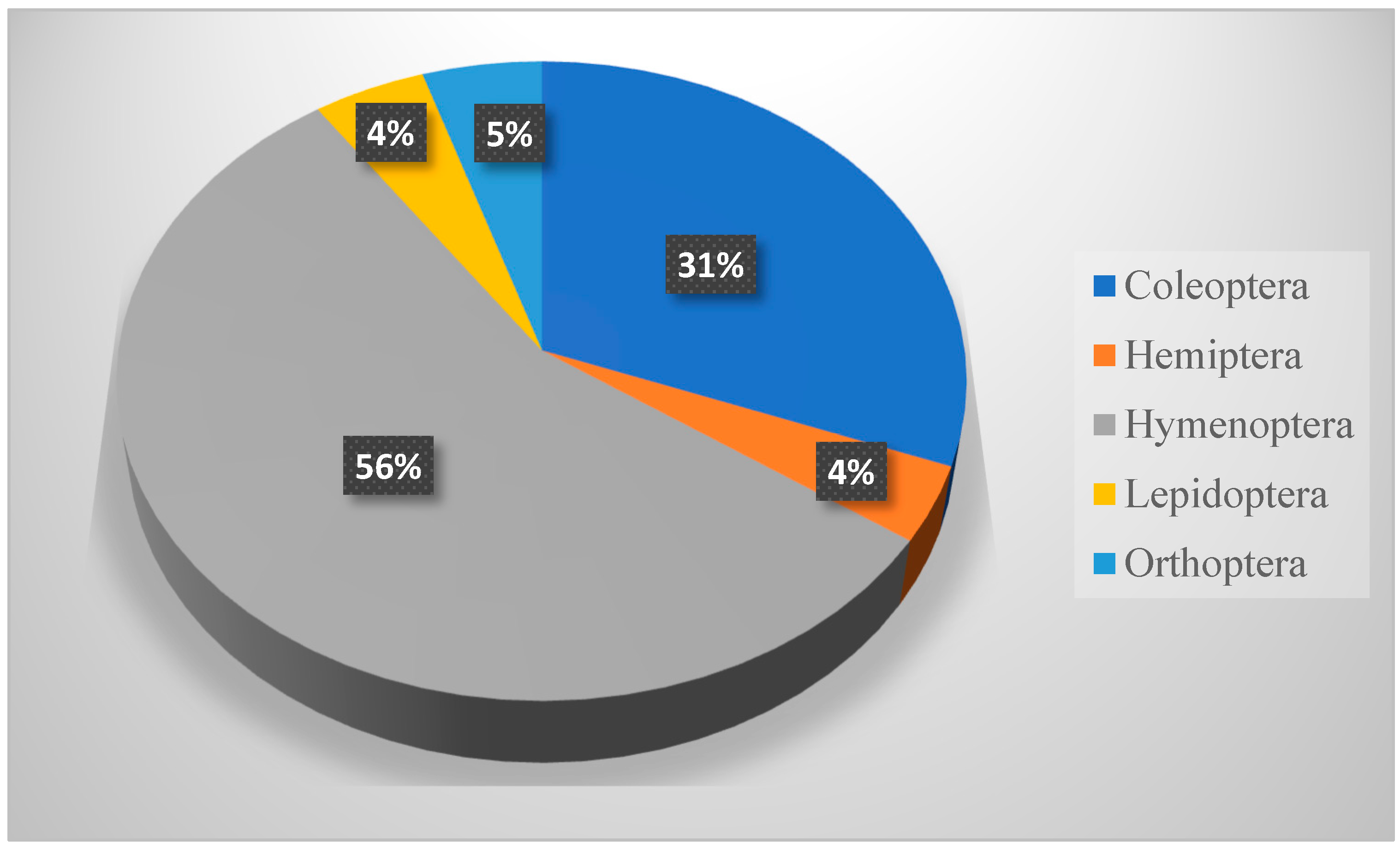
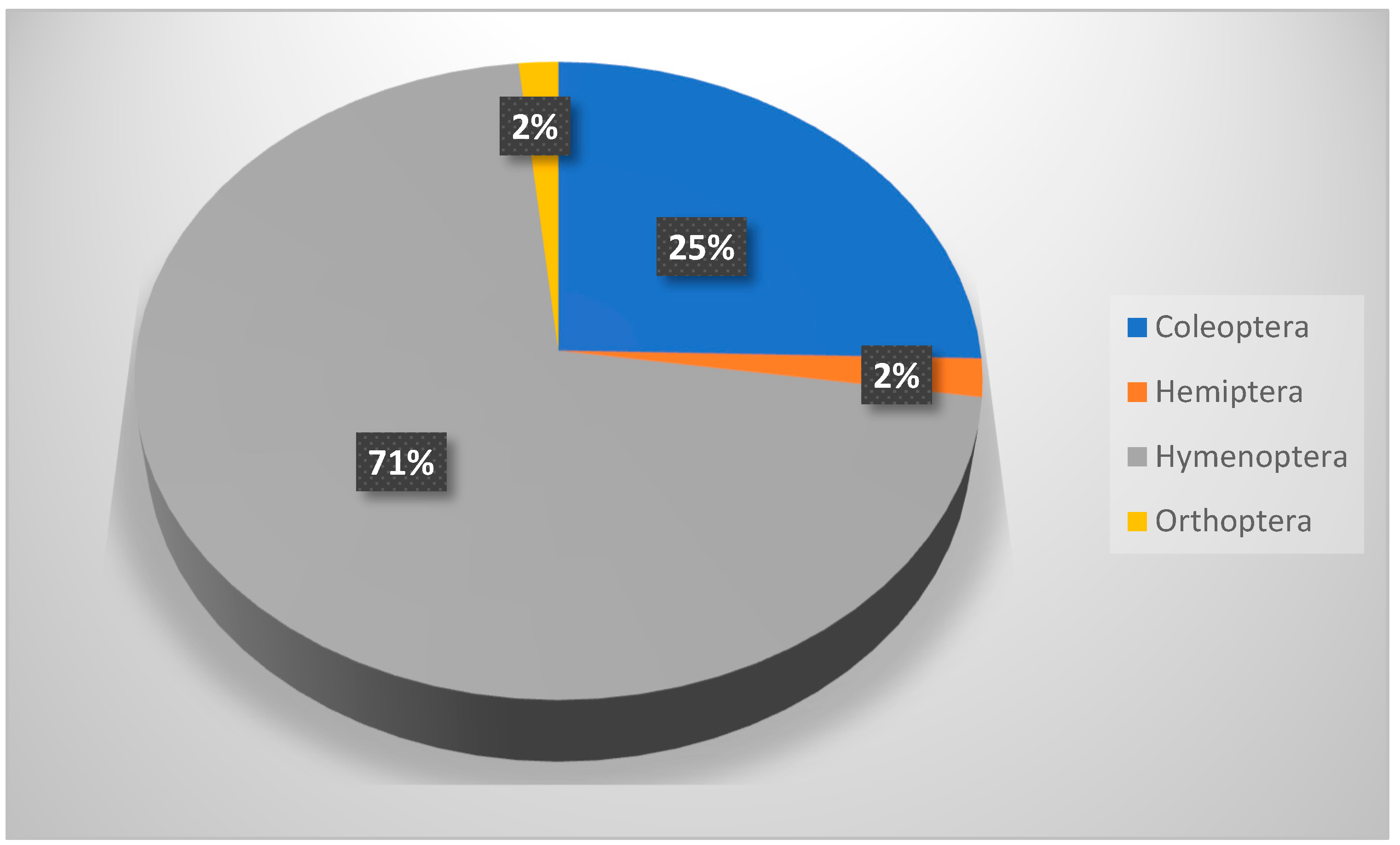
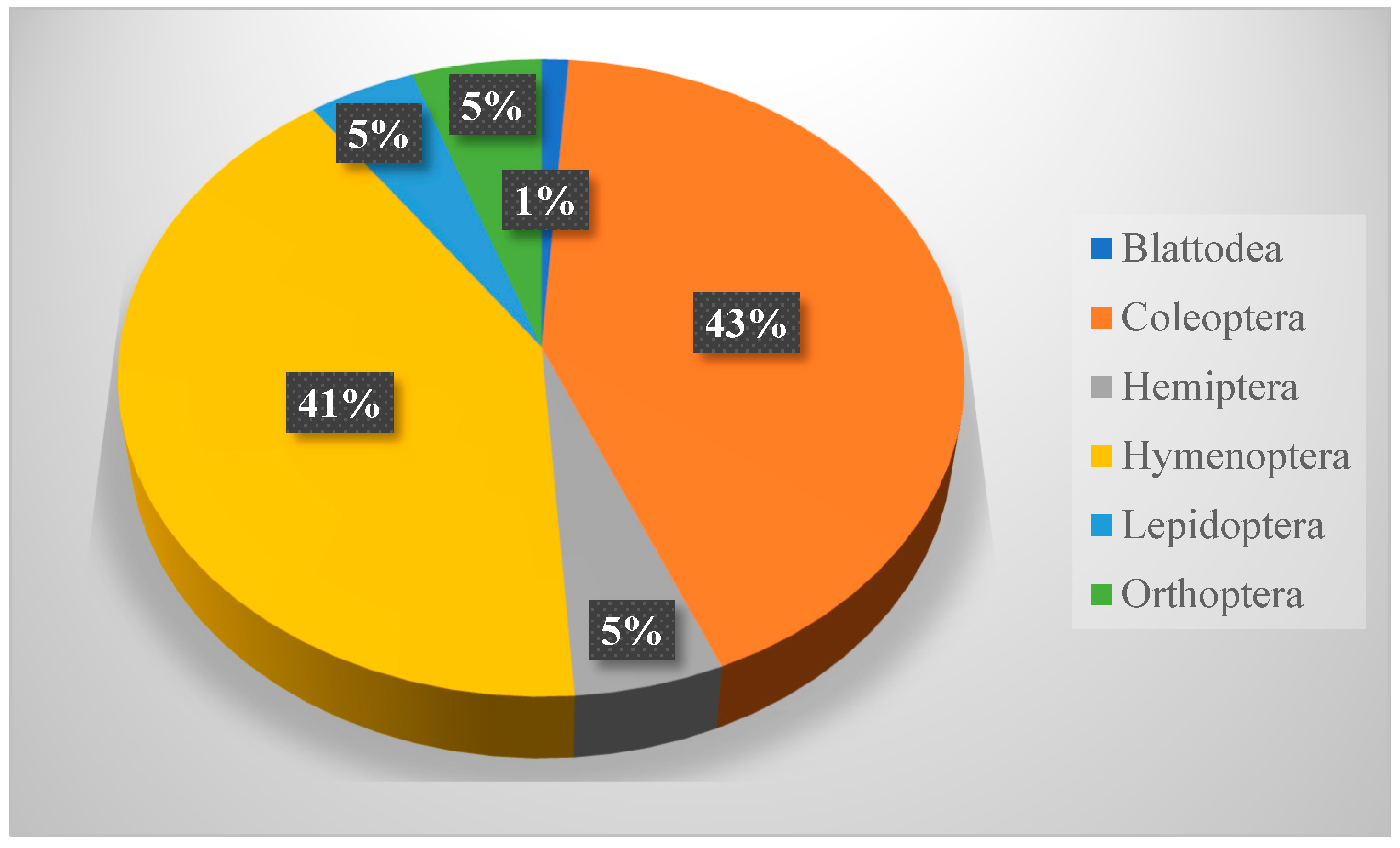
Table 8 shows that members of Hymenoptera (about 58% of all collection) were the most dominant insect throughout this study. It also shows a significant difference between insect orders in their occurrence, but not in their distribution across seasons. Hymenopteran insects (ants, bees, and wasps) played important roles as pollinators, parasites, honey makers, and carnivores [
36].
It was noticed that the immature stages of all orders, except Hymenoptera and Blattodea, were recorded during autumn, winter, and spring, but not during the summer season and this may be attributed to the shortage of food source and plant cover, while the temperature was higher and humidity was lower, as shown in
Table 1. Biodiversity in KSA is mainly influenced by the arid climate, vast desert, extensive mountain ranges, and the long coastlines along the Red Sea and the Arabian Gulf [
35]. Global climate change has affected abundance by altering various abiotic and biotic factors which in turn impacts species’ physiology, morphology, and life pattern. As such, systematic monitoring, assessment, and conservation are necessary to protect biodiversity in Saudi Arabia’s aquatic and terrestrial ecosystems, in line with Vision 2030 [
12].
In respect to microhabitat, few species of insects are restricted to certain plants. One of these insects is
Steraspis speciosa (the xylophagus beetle) which was recorded in this study. It is a potential cause of significant damage and death to
Acacia trees in Saudi Arabia in the driest environments. This beetle is considered a pest of
Acacia tree [
22], while
Hypolixus pica beetles (recorded in this study) are considered as beneficial insects and a potential biological control agent against
Amaranthus weeds [
42].
Few species of beetles with specialized adaptations appeared in certain seasons. One of these species is the carrion beetle
Dermestes maculatus, which was recorded at two sites (four individuals; 0.18% of all Coleoptera) during the spring season only. It is an example of a spring-flourishing insect. This beetle feeds on dead vertebrate bodies and decomposes the flesh [
43], so it has been used for the preparation of vertebrate skeletons [
44]. The abundance of this insect is very important to decompose dead vertebrates within the KARR.
Another example highlighting the same concern is the
Hydroglyphus signatellus beetle (seven individuals; 0.31% of all Coleoptera), which appeared only during the autumn season, swimming in stagnant rainwater. This species is known as a predaceous diving beetle [
45].
Five species belonging to the Scarabaeidea family were recorded during this study. These beetles feed exclusively on herbivore and omnivore feces, and hence, are named dung beetles. These beetles inhabit and are adapted to live in desert, savanna, and forest ecosystems, but do not like very cold or hot habitats. These beetles play important ecological roles, first by burying and consuming dung, which helps to improve soil structure and plant growth; second by helping to distribute seeds present in dung; and third by helping in environmental control by removing the dung of cattle and assisting animal husbandry against vectors and flies, saving millions of dollars spent in cattle industries [
46].
Table 4,
Table 5,
Table 6 and
Table 7 show that
Blaps polychresta dominated across the study periods (466 individuals; 20.58% of all Coleoptera).
Blaps is a genus of darkling beetle, represented in this study by two genera. It was trapped within 54 sites out of 68. Generally, there are more than 30 known species of
Blaps, mainly distributed in Eurasia and Australia [
21].
Table 2 shows that the family Tenebrionidae (the darkling beetles) has a high species richness across this study (represented by 12 species, 1668 individuals, and 73.67% of all Coleoptera), and this may be as a result of its special adaptations to live in arid and semiarid habitats. They prefer vegetated (shrub or grass) habitats. This finding can be attributed to the fact that vegetation tends to reduce the risk of desiccation and predation and offers better oviposition location; additionally, these beetles are detritivores [
47]. The predatory darkling beetle can move easily between the vegetation to pursue prey. It is well known that the microorganisms which live in the digestive system of darkling beetles are used to decompose plant litter and recycle the nutrients into the soil to improve its nutrient composition, and hence, help in increasing plant production. Some darkling beetles (e.g.,
Calosoma) are more important predators than many other desert predators. The abundance of these beetles year-round in desert areas makes them an available source of food for reptiles, birds, and small mammals. It is very important to mention that none of the
Blap species were recorded as pests [
48].
Another member of Tenebrionidae beetle family that was widely distributed within the KARR is the beetle
Mesostena angustata (recorded within 52 sites out of 68, with a total of 347 individuals (20.8% of all darkling beetles) trapped across the study period). In some reports, this beetle is well known as a predator and was noticed to feed on
Messor intermedius ant [
49]. In the current study, this beetle was noticed within some termite colonies, feeding on them. Beetles (specially Tenebrionidae) are widely distributed and dominate across several Provinces in the KSA [
33,
34,
35,
37], UAE [
40], Algeria [
41], Iran [
49], Qatar [
50], Iraq [
51], Kuwait [
52], and Israel [
53], but not in India [
54] where Lepidoptera is dominates over Coleoptera and Hymenoptera.
The
Pimelia beetle was also recorded in this study with a remarkable abundance (227 individuals; 13.61% of all darkling beetles). It is an example of a good desert-adapted beetle. It survives in arid climates and desert environments (high temperature, low humidity, excessive radiant energy, low and irregular rainfall, long periods of drought, strong winds, and unstable sand substrates) in virtue of some adaptations, including it losing its ability to fly. Its morphological adaptations include the waxy layers of the epicuticle, the fused sclerites (that minimize water loss), the subelytral cavity (that allowed relative humidity to spiracles and reduce water loss), and the structure of the body surface (which reflects and scatters radiant energy). Diurnal activity early morning and late evening in addition to burrowing behavior helps a lot in heat regulation during hot days [
53]. It was suggested that the abundance of darkling beetles can be used as an environmental bioindicator for pesticides [
55].
Concerning the ecological adaptations, the darkling beetle,
Prionotheca coronata (61 individuals distributed within 24 sites), has unique morphological and behavioral adaptations to the desert area. It possesses sharp spines along its abdominal margin and along its inner hind legs (for this reason it is named urchin beetle), used against vertebrate or invertebrate enemies [
56].
Two individuals of
triatoma (Hemiptera) were recorded in only one site within the KARR.
Triatoma is a blood-sucking bug and can transmit Chagas disease that infects human and other mammals. Its saliva may cause allergic reactions to some people; hence, it was ranked with the medical and veterinary insects [
57]. Although only two
triatoma bugs were recorded, attention must be focused on their medical value to the local people and their domestic animals.
Aphis nerii was also reported in this study (73 individuals; 46.79% of all Hemiptera insects). It is considered to be a plant pest that causes serious damage to various crops due to its feeding habit, resulting in economic losses. Some Hemiptera insects can transmit various plant pathogens (e.g., viruses, bacteria, and phytoplasmas) [
58]. Their successive feeding process in addition to their potential to transmit plant pathogens may hurt crops and cause a negative impact on agricultural production. The interactions between plants and Hemiptera are multi-faceted; part of this interaction is related to the plant biosynthesis of some molecules and the adaptation of some defense mechanisms. Insects struggle to obtain nutrients from plants and to assure shelter and oviposition place within plants. The plant then responds to insect infestation by developing physical barriers to prevent insect gaining access to plants and by producing anti-nutrition (deterrent) compounds. In turn, insects adapt to avoid plants’ defense strategies. The development of competing strategies that benefit both plants and insects will never stop, as it is a concept of survival [
59,
60].
Two species of termites were recorded in this study, but there are 30 termite species distributed in the Arabian Peninsula, belonging to 4 families and 9 genera, of which 27 species are known in Saudi Arabia (three of them are previously recorded in Riyadh Province:
Anacathotermes ochraceous, Psammotermes hypostoma and
Coptotermes heimi) [
29].
Anacathotermes and
Psammotermes termites are distributed in the desert and semi-desert areas of North Africa, Middle East, and Southwest Asia [
61].
Although termites are considered as wood pests in forests, they are also considered as main decomposers in arid and semi-arid habitats. Termites also act as soil engineers, since they improve the physical (texture) and chemical properties of the soil through acting as bioturbators and as weathering agents of clay minerals (e.g., iron and calcium) [
62,
63].
Table 2 shows the relatively high abundance of ant species:
Camponotus (2233 individuals, 60.81% of all Hymenoptera and 35.22% of all collected insects),
Messor (817 individuals, 22.25% of all Hymenoptera and 12.88% of all collected insects), and
Cataglyphis (599 individuals, 16.38% of all Hymenoptera and 9.45% of all collected insects). These species are reported within some Provinces of the KSA [
36]. Ants played an important ecological and agricultural role in seedling establishment and plant composition, specifically in the arid habitat [
64]. Ants are also considered to be cheap, clean, and accurate bio-indicator tools for diagnosing soil fertility. The physical properties and chemical contents (e.g., nitrogen and phosphorus) of soil are significantly improved within three weeks of ant presence [
65]. Concerning the distribution of ants, they are widely found in agricultural land, dry land, vegetative land, and human houses, without any significant differences between these habitats [
66]. The distribution of ants across a trees is correlated with tree trunk size, topography, and tree species [
67].
Schistocerca. gregaria (the desert locust), which was recorded at five sites in the KARR, is a highly voracious and polyphagous insect, and is considered to be one of the most dangerous and destructive migratory pests in the world against various crops. It can travel hundreds of kilometers a day; thus, its population size cannot be easily estimated. Integrated control methods, in addition to monitoring techniques, should be adopted against this serious pest [
68,
69] and in the KARR.
Few members of bees, wasps, and Lepidoptera’s were recorded in this study. Bees (honey bees and megachilids), Lepidoptera (butterfly and moth), and aculeate wasps, are the main pollinators. Flowering plants in turn encourage these insects by providing nectar and high-protein foods in the form of pollen grains. Their adhesive and hairy legs or bodies, in addition to the presence of some basket-like structures in some insect species, assists in pollination. It was noticed that insecticide application against some plant pests occasionally hurt these pollinators (as non-target organisms) and the consequences will be reduction in pollinator activity and a collapse in plant production [
70,
71].
The variation in species composition in any ecosystem is dependent on its assemblage, dynamics, inter and intra-specific interactions, etc., which changes among different microhabitats [
72], which is very obvious in the biodiversity of insect species reported in this study in respect to the variation in habitats across different sites and seasons within the KARR. No specific patterns or features are noticed during this study to indicate climate change since the same species with the same morphological features are distributed within the KSA and neighboring countries. The occurrence and distribution of various pollinators, seed distributors, decomposers, predators, plant pests, and medical and veterinary insects, in addition to considerable numbers of immature stages and other mentioned insects across the seasons within the KARR, in addition to other neighboring provinces and countries, reflects the status of the ecological system and its capacity to host this diversity. It is also worth noting that none of the mutation signs or features were noticed during the identification process.
The integration of the obtained data with that of other parallel survey studies (non-insect arthropods, reptiles, birds, and mammals), will help the KARR in planning suitable conservation plans. The data of the further surveys will help to determine whether the occurrence line of insects is stable, increases, or declines.