Targeting Atherosclerosis via NEDD4L Signaling—A Review of the Current Literature
Simple Summary
Abstract
1. Introduction
2. An Overview of Atherosclerosis Physiopathology with an Emphasis on NEDD4L Signaling
2.1. Atherosclerosis: A Chronic Inflammatory Condition Predisposing to Cardiovascular Diseases and Outcomes
2.2. General Aspects of the NEDD4 Signaling Pathway
2.3. Exploring Atherosclerosis Physiopathology with an Emphasis on NEDD4L Signaling
3. Implications of Targeting NEDD4L Signaling Against Atherosclerosis
3.1. Literature Search Methodology
3.2. Literature Search Report
3.3. Implications of Targeting NEDD4L Signaling Against Atherosclerosis: Results of the Included Studies and Future Research Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef]
- Li, Z.; Lin, L.; Wu, H.; Yan, L.; Wang, H.; Yang, H.; Li, H. Global, Regional, and National Death, and Disability-Adjusted Life-Years (DALYs) for Cardiovascular Disease in 2017 and Trends and Risk Analysis from 1990 to 2017 Using the Global Burden of Disease Study and Implications for Prevention. Front. Public Health 2021, 9, 559751. [Google Scholar] [CrossRef] [PubMed]
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef]
- Pahwa, R.; Jialal, I. Atherosclerosis. In StatPearls; StatPearls Publishing Copyright © 2025; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wong, N.D.; Budoff, M.J.; Ferdinand, K.; Graham, I.M.; Michos, E.D.; Reddy, T.; Shapiro, M.D.; Toth, P.P. Atherosclerotic cardiovascular disease risk assessment: An American Society for Preventive Cardiology clinical practice statement. Am. J. Prev. Cardiol. 2022, 10, 100335. [Google Scholar] [CrossRef] [PubMed]
- Kumric, M.; Urlic, H.; Bozic, J.; Vilovic, M.; Ticinovic Kurir, T.; Glavas, D.; Miric, D.; Zanchi, J.; Bradaric-Slujo, A.; Lozo, M.; et al. Emerging Therapies for the Treatment of Atherosclerotic Cardiovascular Disease: From Bench to Bedside. Int. J. Mol. Sci. 2023, 24, 8062. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, L.; Huang, Y.; Li, Y.; Liu, C.; Wang, B.; Zuo, Z.; Yao, F. Identification of Key Genes in Atherosclerosis by Combined DNA Methylation and miRNA Expression Analyses. Anatol. J. Cardiol. 2022, 26, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Choi, H.P.; Wang, F.; Su, H.; Fei, Z.; Yang, J.H.; Azadzoi, K.M. Quantitative Proteomic Analysis of Differentially Expressed Proteins and Downstream Signaling Pathways in Chronic Bladder Ischemia. J. Urol. 2016, 195, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Rotin, D.; Prag, G. Physiological Functions of the Ubiquitin Ligases Nedd4-1 and Nedd4-2. Physiology 2024, 39, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.X.; Ren, Z.; Yan, B.J.; Qu, S.L.; Tang, Z.H.; Wei, D.H.; Liu, L.S.; Fu, M.G.; Jiang, Z.S. The Role of Ubiquitin E3 Ligase in Atherosclerosis. Curr. Med. Chem. 2021, 28, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lerman, L.O.; Lerman, A. On to the road to degradation: Atherosclerosis and the proteasome. Cardiovasc. Res. 2010, 85, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Wilck, N.; Ludwig, A. Targeting the Ubiquitin-Proteasome System in Atherosclerosis: Status Quo, Challenges, and Perspectives. Antioxid. Redox Signal. 2014, 21, 2344–2363. [Google Scholar] [CrossRef] [PubMed]
- Poels, K.; Vos, W.G.; Lutgens, E.; Seijkens, T.T.P. E3 Ubiquitin Ligases as Immunotherapeutic Target in Atherosclerotic Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 106. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, X.; Li, J.; Ma, Y.; Zhang, Y. Identification of candidate targets for the diagnosis and treatment of atherosclerosis by bioinformatics analysis. Am. J. Transl. Res. 2021, 13, 4137–4151. [Google Scholar] [PubMed]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef]
- Zhang, R.Y.; He, X.; Chen, J.J.; Wu, C.M.; Lin, Y.L.; Wang, Y.B.; Wang, Q.; Zheng, L.; Hu, X.M. UGP2, a novel target gene of TP53, inhibits endothelial cells apoptosis and atherosclerosis. Life Sci. 2025, 123393. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.Y.; Chen, Y.S.; Yong, S.B.; Yii, C.Y.; Su, Y.T. Blocking ANGPTL3 and CD47 impact on atherosclerosis-correspondence. Pharmacol. Res. 2025, 363, 107601. [Google Scholar] [CrossRef] [PubMed]
- Pavasini, R. Is Myocardial Work a Surrogate Marker for Functional Coronary Artery Stenosis or a Marker of Coronary Atherosclerosis? Echocardiography 2025, 42, e70073. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Seo, J.; Kim, I.S.; Cho, S.; Rim, S.J.; Kown, H.M.; Choi, E.Y. Differential determinants and prognostic value of aortic valve sclerosis over carotid atherosclerosis. Int. J. Cardiol. 2025, 422, 132980. [Google Scholar] [CrossRef] [PubMed]
- Erol, Ç. Atherosclerosis Reviewed. Anatol. J. Cardiol. 2024, 28, 374. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Xu, G.; Huang, H. Correlation between metabolic dysfunction-associated steatotic liver disease and subclinical coronary atherosclerosis in eastern China. Diabetol. Metab. Syndr. 2025, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- de Oliveira Dos Santos, A.R.; de Oliveira Zanuso, B.; Miola, V.F.B.; Barbalho, S.M.; Santos Bueno, P.C.; Flato, U.A.P.; Detregiachi, C.R.P.; Buchaim, D.V.; Buchaim, R.L.; Tofano, R.J.; et al. Adipokines, Myokines, and Hepatokines: Crosstalk and Metabolic Repercussions. Int. J. Mol. Sci. 2021, 22, 2639. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Rodrigues, V.D.; Laurindo, L.F.; Cherain, L.M.A.; de Lima, E.P.; Boaro, B.L.; da Silva Camarinha Oliveira, J.; Chagas, E.F.B.; Catharin, V.C.S.; Dos Santos Haber, J.F.; et al. Targeting AMPK with Irisin: Implications for metabolic disorders, cardiovascular health, and inflammatory conditions—A systematic review. Life Sci. 2025, 360, 123230. [Google Scholar] [CrossRef]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- Milla, A.M.G.; Chagas, E.B.F.; Miola, V.F.B.; Zanuso, B.O.; Guiguer, E.L.; Araújo, A.C.; Tofano, R.J.; Quesada, K.R.; Laurindo, L.F.; Barbalho, S.M. Accuracy of visceral adiposity indices and lipid accumulation products in the identification of adults at high cardiovascular risk. Clínica Investig. Arterioscler. 2023, 35, 236–242. [Google Scholar] [CrossRef]
- d’Aiello, A.; Filomia, S.; Brecciaroli, M.; Sanna, T.; Pedicino, D.; Liuzzo, G. Targeting Inflammatory Pathways in Atherosclerosis: Exploring New Opportunities for Treatment. Curr. Atheroscler. Rep. 2024, 26, 707–719. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Kou, Y.; Shen, L.; Wang, H.; Wang, Y.; Ma, R.; Wu, T.; Yang, X.; Gu, Y.; et al. Mechanisms and treatment of atherosclerosis: Focus on macrophages. Front. Immunol. 2024, 15, 1490387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Liu, D.; Song, H.; Zhang, X.; Yan, C.; Han, Y. Identification of critical endoplasmic reticulum stress-related genes in advanced atherosclerotic plaque. Sci. Rep. 2025, 15, 2107. [Google Scholar] [CrossRef]
- Zhang, Y.J.; He, H.; Sawuer, G.; Ma, X.K.; Ainiwaer, Z.; Wu, D.D.; Zhang, X.X.; An, D.Q. Tianxiangdan suppresses foam cell formation by enhancing lipophagy and reduces the progression of atherosclerosis. Vitr. Cell. Dev. Biol. Anim. 2025. [Google Scholar] [CrossRef] [PubMed]
- Orekhov, A.N.; Orekhov, N.A.; Sukhorukov, V.N.; Khotina, V.A.; Kovianova, T.I.; Sobenin, I.A. Mitochondrial DNA Mutations as a Factor in the Heritability of Atherosclerosis and Other Diseases. Curr. Med. Chem. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, W.; Zhang, K.; Ke, X.; Wang, Z. New insights into the relationship of mitochondrial metabolism and atherosclerosis. Cell. Signal. 2024, 127, 111580. [Google Scholar] [CrossRef]
- Song, F.; Li, J.-Z.; Wu, Y.; Wu, W.-Y.; Wang, Y.; Li, G. Ubiquitinated ligation protein NEDD4L participates in MiR-30a-5p attenuated atherosclerosis by regulating macrophage polarization and lipid metabolism. Mol. Ther.-Nucleic Acids 2021, 26, 1303–1317. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, X.; He, X.; Wei, H.; Li, X.; Tan, Y.; Min, J.; Chen, M.; Zhang, Y.; Dong, M.; et al. METTL4-Mediated Mitochondrial DNA N6-Methyldeoxyadenosine Promoting Macrophage Inflammation and Atherosclerosis. Circulation 2024. [Google Scholar] [CrossRef] [PubMed]
- Wilburn, D.; Fletcher, E.; Papoutsi, E.; Bohannon, W.T.; Haynatzki, G.; Zechmann, B.; Tian, Y.; Pipinos, I.I.; Miserlis, D.; Koutakis, P. Ultrastructural alterations and mitochondrial dysfunction in skeletal muscle of peripheral artery disease patients: Implications for early therapeutic interventions. EXCLI J. 2024, 23, 1208–1225. [Google Scholar] [CrossRef]
- Pulipaka, S.; Singuru, G.; Sahoo, S.; Shaikh, A.; Thennati, R.; Kotamraju, S. Therapeutic efficacies of mitochondria-targeted esculetin and metformin in the improvement of age-associated atherosclerosis via regulating AMPK activation. GeroScience 2024, 46, 2391–2408. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Lv, Y.; Zhang, Z.; He, T.; Hao, X.; Wang, S.; Wang, C.; Meng, J.; Zhong, K.; et al. M2 Macrophage-Derived Exosomes Inhibit Atherosclerosis Progression by Regulating the Proliferation, Migration, and Phenotypic Transformation of Smooth Muscle Cells. Front. Biosci. (Landmark Ed.) 2024, 29, 288. [Google Scholar] [CrossRef]
- Chen, G.; Pei, Y.; Jiang, P.; Ye, Q.; Xie, Z.; Gyawali, L. Exosomal NEDD4L derived from HG+oxLDL-induced vascular endothelial cells accelerates macrophage M1 polarization and oxLDL uptake by ubiquitinating IκBα and PPARγ. Cell Biol. Toxicol. 2025, 41, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, G.; Wang, P.; Wang, W.; Cao, K.; Song, C.; Sun, Y.; Zhang, Y.; Zhang, N. Research progress of Nedd4L in cardiovascular diseases. Cell Death Discov. 2022, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Korn, A.; Simsek, S.; Fiet, M.D.; Waas, I.S.E.; Niessen, H.W.M.; Krijnen, P.A.J. Application of adipose tissue-derived stem cell therapy with a clinically relevant dose does not significantly affect atherosclerotic plaque characteristics in a streptozotocin-induced hyperglycaemia mouse model. J. Mol. Cell. Cardiol. Plus 2024, 9, 100083. [Google Scholar] [CrossRef] [PubMed]
- Blessing, E.; Teichmann, E.; Hinz, B. Anandamide Inhibits Vascular Smooth Muscle Migration, Endothelial Adhesion Protein Expression and Monocyte Adhesion of Human Coronary Artery Cells. Cells 2024, 13, 2108. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhuang, T.T.; Li, C.C.; Li, F.; Shan, S.K.; Zheng, M.H.; Xu, Q.S.; Wang, Y.; Lei, L.M.; Tang, K.X.; et al. MiRNA-132/212 encapsulated by adipose tissue-derived exosomes worsen atherosclerosis progression. Cardiovasc. Diabetol. 2024, 23, 331. [Google Scholar] [CrossRef]
- Nádasy, G.L.; Balla, A.; Dörnyei, G.; Hunyady, L.; Szekeres, M. Direct Vascular Effects of Angiotensin II (A Systematic Short Review). Int. J. Mol. Sci. 2024, 26, 113. [Google Scholar] [CrossRef] [PubMed]
- Lorentzen, L.G.; Yeung, K.; Zitkeviciute, A.; Yang-Jensen, K.C.; Eldrup, N.; Eiberg, J.P.; Davies, M.J. N-Terminal Proteomics Reveals Distinct Protein Degradation Patterns in Different Types of Human Atherosclerotic Plaques. J. Proteome Res. 2025, 24, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Scheffner, M.; Kumar, S. Mammalian HECT ubiquitin-protein ligases: Biological and pathophysiological aspects. Biochim. Biophys. Acta 2014, 1843, 61–74. [Google Scholar] [CrossRef]
- Katz, M.; Shtiegman, K.; Tal-Or, P.; Yakir, L.; Mosesson, Y.; Harari, D.; Machluf, Y.; Asao, H.; Jovin, T.; Sugamura, K.; et al. Ligand-independent degradation of epidermal growth factor receptor involves receptor ubiquitylation and Hgs, an adaptor whose ubiquitin-interacting motif targets ubiquitylation by Nedd4. Traffic 2002, 3, 740–751. [Google Scholar] [CrossRef]
- Yang, B.; Kumar, S. Nedd4 and Nedd4-2: Closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ. 2010, 17, 68–77. [Google Scholar] [CrossRef]
- Kumar, S.; Tomooka, Y.; Noda, M. Identification of a set of genes with developmentally down-regulated expression in the mouse brain. Biochem. Biophys. Res. Commun. 1992, 185, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Harvey, K.F.; Kinoshita, M.; Copeland, N.G.; Noda, M.; Jenkins, N.A. cDNA cloning, expression analysis, and mapping of the MouseNedd4Gene. Genomics 1997, 40, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Ryu, D.; Parmar, M.; Giasson, B.I.; McFarland, N.R. The ER retention protein RER1 promotes alpha-synuclein degradation via the proteasome. PLoS ONE 2017, 12, e0184262. [Google Scholar] [CrossRef]
- Asanuma, M.; Miyazaki, I.; Cadet, J.L. Differentially Expressed Nedd4-binding Protein Ndfip1 Protects Neurons Against Methamphetamine-induced Neurotoxicity. Neurotox. Res. 2025, 43, 4. [Google Scholar] [CrossRef]
- Xu, W.; Hu, P.; Wang, J.; Jiang, H.; Wang, T.; Liu, J.; Li, H. Neural Precursor Cell-Expressed Developmentally Downregulated Protein 4 (NEDD4)-Mediated Ubiquitination of Glutathione Peroxidase 4 (GPX4): A Key Pathway in High-Glucose-Induced Ferroptosis in Corpus Cavernosum Smooth Muscle Cells. Biomolecules 2024, 14, 1552. [Google Scholar] [CrossRef]
- Liu, B.; Song, F.; Zhou, X.; Wu, C.; Huang, H.; Wu, W.; Li, G.; Wang, Y. NEDD4L is a promoter for angiogenesis and cell proliferation in human umbilical vein endothelial cells. J. Cell Mol. Med. 2024, 28, 1–11. [Google Scholar] [CrossRef]
- Liang, J.; Wang, N.; Yao, Y.; Wang, Y.; An, X.; Wang, H.; Liu, H.; Jiang, Y.; Li, H.; Cheng, X.; et al. NEDD4L mediates intestinal epithelial cell ferroptosis to restrict inflammatory bowel diseases and colorectal tumorigenesis. J. Clin. Investig. 2024. [Google Scholar] [CrossRef]
- Lao, J.; Hu, P.; Wan, Y.; Shu, M.; Chen, J.; Yang, H. NEDD4 Knockdown Suppresses Human Endometrial Stromal Cell Growth and Invasion by Regulating PTGS2-Mediated Ferroptosis in Endometriosis. Curr. Mol. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Sun, Y.; Zhu, J.; Wang, X.; Ji, C.; Zhang, J.; Chen, S.; Yu, Y.; Xu, W.; Qian, H. Mesenchymal stem cells-derived small extracellular vesicles alleviate diabetic retinopathy by delivering NEDD4. Stem Cell Res. Ther. 2022, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Guo, L.; Yu, C.; Liu, X.; Lin, Y.; Li, C.; Zhang, W.; Zong, Y.; Yang, W.; Ma, Y.; et al. DCBLD1 Modulates Angiogenesis by Regulation of the VEGFR-2 Endocytosis in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2024, 45, 198–217. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Chong, N.; Chen, D.; Shu, J.; Sun, J.; Sun, Z.; Wang, R.; Wang, Q.; Xu, Y. GDF-15 alleviates diabetic nephropathy via inhibiting NEDD4L-mediated IKK/NF-κB signalling pathways. Int. Immunopharmacol. 2024, 128, 111427. [Google Scholar] [CrossRef]
- Boase, N.A.; Kumar, S. NEDD4: The founding member of a family of ubiquitin-protein ligases. Gene 2015, 557, 113–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, H.; Wu, B.; You, S.; Wu, S.; Lu, S.; Wang, P.; Cao, L.; Zhang, N.; Sun, Y. E3 Ubiquitin ligase NEDD4 family-regulatory network in cardiovascular disease. Int. J. Biol. Sci. 2020, 16, 2727–2740. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Pei, Y.; Ye, Q.; Xie, Z.; Gyawali, L.; Liang, X. NEDD4L-mediated RASGRP2 suppresses high-glucose and oxLDL-induced vascular endothelial cell dysfunctions by activating Rap1 and R-Ras. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119844. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Li, Q.; Bai, Y.; Cavender, S.M.; Miao, Y.; Nguele Meke, F.; Lasse-Opsahl, E.L.; Zhu, P.; Doody, G.M.; Tao, W.A. The PRL2 Phosphatase Upregulates miR-21 through Activation of the JAK2/STAT3 Pathway to Downregulate the PTEN Tumor Suppressor. Biochem. J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Xu, J.; Zhang, Y.; Kong, S.; Cheng, X.; Wu, N.; Han, S.; Yin, J.; Liu, W.; He, X.; et al. Elevated Astrocytic NFAT5 of the hippocampus increases epilepsy susceptibility in hypoxic-ischemic mice. Epilepsia 2024. [Google Scholar] [CrossRef]
- Yuan, W.; Qiu, Z.M.; Li, H.; Huang, M.; Yuan, J.J.; Niu, S.L.; Chen, Q.; Yang, Q.W.; Ouyang, Q. Investigation of the Binding Interaction of Mfsd2a with NEDD4-2 via Molecular Dynamics Simulations. ACS Chem. Neurosci. 2024, 15, 382–393. [Google Scholar] [CrossRef]
- Kawabe, H.; Neeb, A.; Dimova, K.; Young, S.M.; Takeda, M.; Katsurabayashi, S.; Mitkovski, M.; Malakhova, O.A.; Zhang, D.-E.; Umikawa, M. Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development. Neuron 2010, 65, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Oppenheim, R.W.; Sugiura, Y.; Lin, W. Abnormal development of the neuromuscular junction in Nedd4-deficient mice. Dev. Biol. 2009, 330, 153–166. [Google Scholar] [CrossRef]
- Wang, R.; Wang, R.; Zhou, S.; Liu, T.; Dang, J.; Chen, Q.; Chen, J.; Wang, Z. Ubiquitination of angiotensin-converting enzyme 2 contributes to the development of pulmonary arterial hypertension mediated by neural precursor cell-expressed developmentally down-regulated gene 4-Like. Respir. Res. 2024, 25, 326. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chen, S.; Zhang, Y.; Mou, X.; Li, M.; Zhu, S.; Chen, X.; Strandin, T.M.; Jiang, Y.; Xiang, Z.; et al. Hantaan virus glycoprotein Gc induces NEDD4-dependent PTEN ubiquitination and degradation to escape the restriction of autophagosomes and facilitate viral propagation. FASEB J. 2025, 39, e70295. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.M.; Nyenhuis, D.A.; Khan, M.; Ehrlich, L.S.; Ischenko, I.; Powell, M.D.; Tjandra, N.; Carter, C.A. Tsg101 UEV Interaction with Nedd4 HECT Relieves E3 Ligase Auto-Inhibition, Promoting HIV-1 Assembly and CA-SP1 Maturation Cleavage. Viruses 2024, 16, 1566. [Google Scholar] [CrossRef]
- Cui, L.; Ma, J. NEDD4L Promotes IκBα Ubiquitination and Degradation in the Pathogenesis of Diabetic Retinopathy. Curr. Eye Res. 2024, 49, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tan, L.; Qu, Q.; Zhang, W. NEDD4 attenuates oxidized low-density lipoprotein-induced inflammation and dysfunction in vascular endothelial cells via regulating APEX1 expression. Exp. Ther. Med. 2023, 25, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gui, N.; Ma, X.; Zeng, Y.; Mo, T.; Zhang, M. Proliferation, migration and phenotypic transformation of VSMC induced via Hcy related to up-expression of WWP2 and p-STAT3. PLoS ONE 2024, 19, e0296359. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhu, Y.; Zhao, X.; Li, R.; Shao, G.; Gong, D.; Hu, C.; Liu, H.; Xu, K.; Liu, C.; et al. Hepatocytic lipocalin-2 controls HDL metabolism and atherosclerosis via Nedd4-1-SR-BI axis in mice. Dev. Cell 2023, 58, 2326–2337.e2325. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Wu, F.Y.; Liu, Y.J.; Li, L.; Lin, Y.J.; Kang, Y.T.; Peng, Y.M.; Liu, Y.F.; Wang, C.; Ma, Z.S.; et al. Increase of PCSK9 expression in diabetes promotes VEGFR2 ubiquitination to inhibit endothelial function and skin wound healing. Sci. China Life Sci. 2024, 67, 2635–2649. [Google Scholar] [CrossRef]
- Kawashima, S.; Yokoyama, M. Dysfunction of endothelial nitric oxide synthase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.J.; Melista, E.; Cui, J.; DeStefano, A.L.; Bakris, G.L.; Manolis, A.J.; Gavras, H.; Baldwin, C.T. Association of NEDD4L ubiquitin ligase with essential hypertension. Hypertension 2005, 46, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Wang, Y.; Wang, X.; Sun, K.; Zhou, X.; Hui, R. A functional variant of NEDD4L is associated with hypertension, antihypertensive response, and orthostatic hypotension. Hypertension 2009, 54, 796–801. [Google Scholar] [CrossRef]
- Gao, H.; Duan, Y.; Fu, X.; Xie, H.; Liu, Y.; Yuan, H.; Zhou, M.; Xie, C. Comparison of efficacy of SHENQI compound and rosiglitazone in the treatment of diabetic vasculopathy analyzing multi-factor mediated disease-causing modules. PLoS ONE 2018, 13, e0207683. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Luh, F.; Ho, Y.S.; Yen, Y. Exosomes: A review of biologic function, diagnostic and targeted therapy applications, and clinical trials. J. Biomed. Sci. 2024, 31, 67. [Google Scholar] [CrossRef] [PubMed]
- Dharani, S.; Kamaraj, R. A Review of the Regulatory Challenges of Personalized Medicine. Cureus 2024, 16, e67891. [Google Scholar] [CrossRef]
- Zemaitis, M.R.; Boll, J.M.; Dreyer, M.A. Peripheral Arterial Disease. In StatPearls; StatPearls Publishing, Copyright © 2025; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Nunes, Y.C.; Santos, G.O.; Machado, N.M.; Otoboni, A.; Laurindo, L.F.; Bishayee, A.; Fimognari, C.; Bishayee, A.; Barbalho, S.M. Peanut (Arachis hypogaea L.) seeds and by-products in metabolic syndrome and cardiovascular disorders: A systematic review of clinical studies. Phytomedicine 2024, 123, 155170. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Green Healthcare Institutions: Health, Environment, and Economics: Workshop Summary; The National Academies Press: Washington, DC, USA, 2007; p. 128. [Google Scholar]
- Mihaylova, B.; Wu, R.; Zhou, J.; Williams, C.; Schlackow, I.; Emberson, J.; Reith, C.; Keech, A.; Robson, J.; Parnell, R.; et al. Lifetime effects and cost-effectiveness of standard and higher-intensity statin therapy across population categories in the UK: A microsimulation modelling study. Lancet Reg. Health—Eur. 2024, 40, 100887. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Grechko, A.V.; Myasoedova, V.A.; Orekhov, A.N. Potential of anti-inflammatory agents for treatment of atherosclerosis. Exp. Mol. Pathol. 2018, 104, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Ghodeshwar, G.K.; Dube, A.; Khobragade, D. Impact of Lifestyle Modifications on Cardiovascular Health: A Narrative Review. Cureus 2023, 15, e42616. [Google Scholar] [CrossRef]
- Gallucci, G.; Tartarone, A.; Lerose, R.; Lalinga, A.V.; Capobianco, A.M. Cardiovascular risk of smoking and benefits of smoking cessation. J. Thorac. Dis. 2020, 12, 3866–3876. [Google Scholar] [CrossRef]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Trimm, E.; Red-Horse, K. Vascular endothelial cell development and diversity. Nat. Rev. Cardiol. 2023, 20, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Deveza, L.; Choi, J.; Yang, F. Therapeutic angiogenesis for treating cardiovascular diseases. Theranostics 2012, 2, 801–814. [Google Scholar] [CrossRef]
- Everts, P.A.; Lana, J.F.; Onishi, K.; Buford, D.; Peng, J.; Mahmood, A.; Fonseca, L.F.; van Zundert, A.; Podesta, L. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. Biomedicines 2023, 11, 1922. [Google Scholar] [CrossRef]
- Camaré, C.; Pucelle, M.; Nègre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef]
- Ahmadi, A.; Argulian, E.; Leipsic, J.; Newby, D.E.; Narula, J. From Subclinical Atherosclerosis to Plaque Progression and Acute Coronary Events: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Ojha, U.; Lee, Y.M. Pathological angiogenesis and inflammation in tissues. Arch. Pharmacal Res. 2021, 44, 1–15. [Google Scholar] [CrossRef]
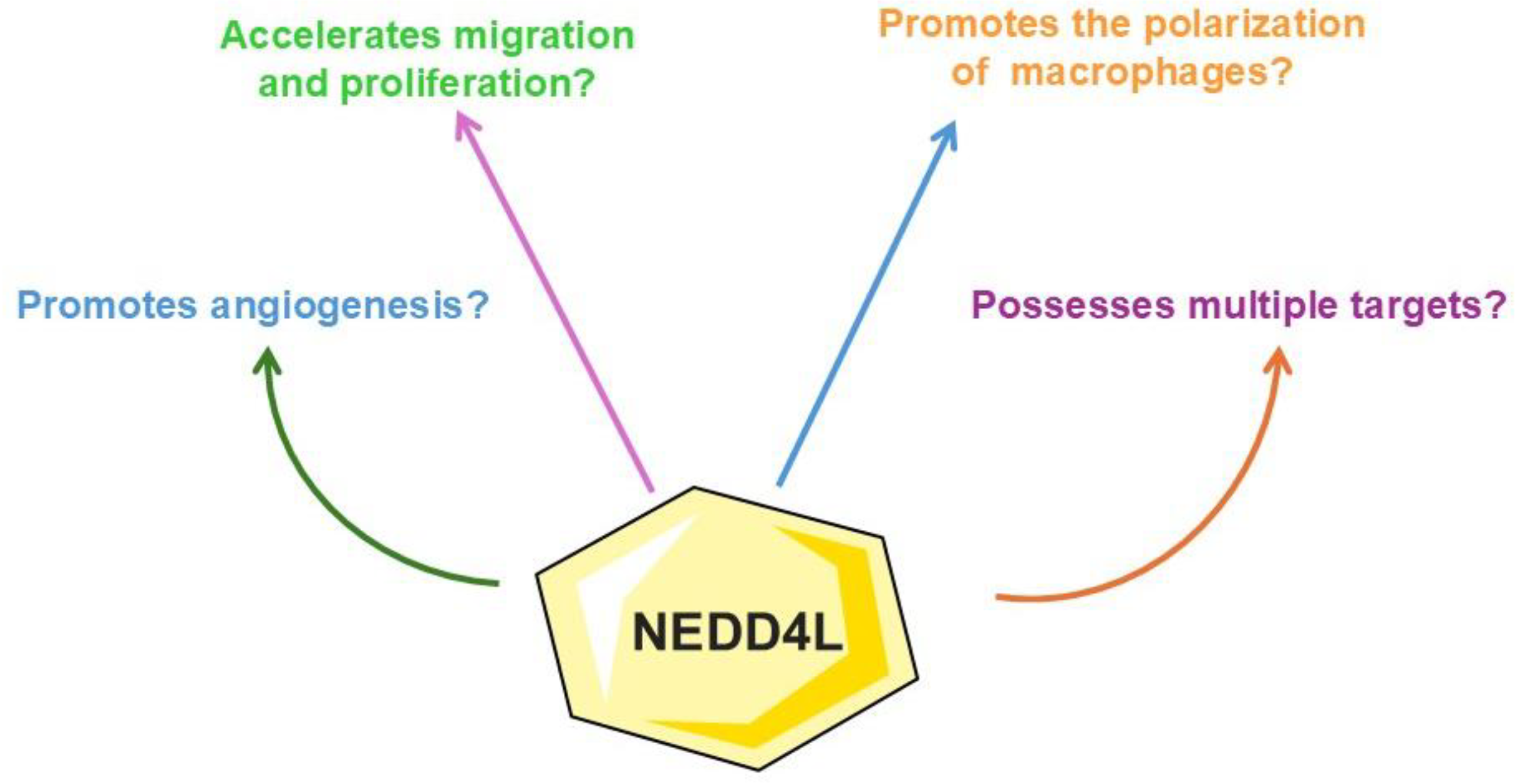
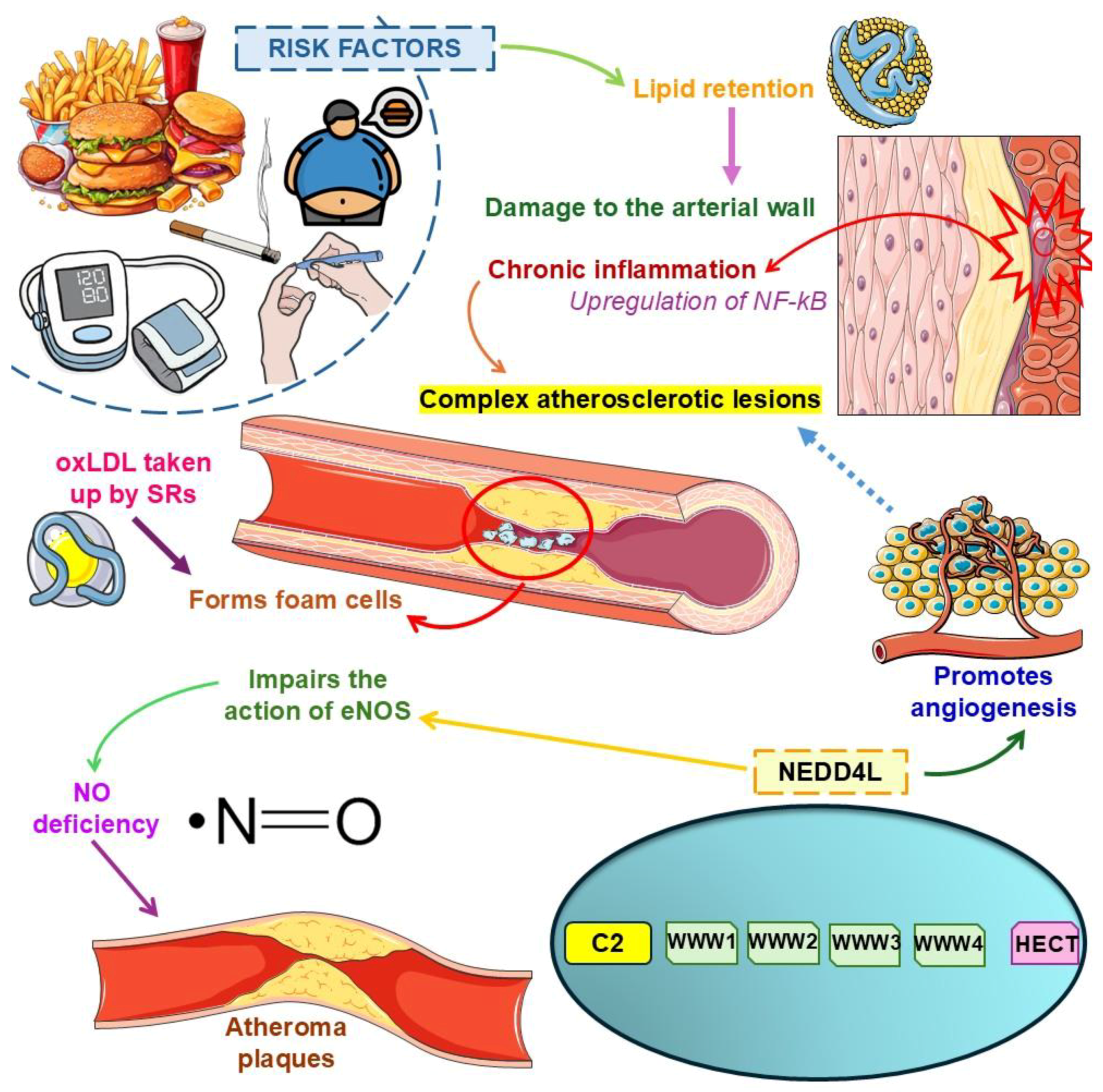



| Ref | Model | Study Aims | Molecular Mechanisms and Outcomes | Possible Clinical Applications | Future Research Endeavors |
|---|---|---|---|---|---|
| [38] | HCMECs exposed to HG and oxLDL. | Investigate whether exosomes containing NEDD4L derived from these cells could negatively interfere with disease progression. | ⬆ IκBα and PPARγ ubiquitination, ⬆ SMAD phosphorylation. In HG + oxLDL-induced HCMECs, NEDD4L is overexpressed, and it promotes ⬆ macrophage M1 polarization, ⬆ oxLDL uptake, and ⬆ foam cell formation. | Targeted therapies to decrease NEDD4L expression in endothelial cells could be developed to mitigate damage caused by cardiovascular diseases. | In vivo experiments are necessary for the translational use of NEDD4L in screening and treating cardiovascular disorders. |
| [61] | HG + oxLDL-induced HCMECs co-cultured with siRNA NEDD4L and ADMSCs exosomes transfected with RASGRP2 overexpression vector. | Evaluate the role of the NEDD4L/RASGRP2 axis in DM-related atherosclerosis progression. | ⬆ RASGRP2 ubiquitination and degradation, ⬇ RASGRP2’s protective effects against atherosclerosis, ⬇ cell viability, ⬇ cell migration, ⬇ cell angiogenesis, ⬆cell permeability, and ⬆ ROS production. | Drugs targeting the knockdown of NEDD4L could be a valuable alternative to combat cardiovascular diseases. | Strategies incorporating NEDD4L signaling must apply to targeted DM-associated atherosclerosis therapy as a first step in animal investigations. |
| [33] | HFD-induced Apo E −/− mice treated with ago-miR-30a-5p and macrophages transfected with miR-30a-5p or NEDD4L siRNA. | Demonstrate whether miR-30a-5p interacts with NEDD4L to attenuate atherosclerosis. | ⬆ M1/M2 ratio, ⬆ oxLDL uptake, ⬆ PPARγ ubiquitination, ⬆ SMAD phosphorylation. miR-30a-5p inhibits NEDD4L, leading to an ⬆ in anti-inflammatory cytokines and a ⬇ in pro-inflammatory factors. | Counteracting the NEDD4L pathway could be valuable in reducing the formation of atherosclerotic lesions and inflammatory responses. | Additional animal experiments are paramount to assess miR-30a-5p and NEDD4L as targets in atherosclerosis management. |
| [53] | HUVECs transfected with NEDD4L-siRNA and infected with NEDD4L-adenovirus. | Evaluate NEDD4L as an endothelial cell function regulator. | ⬆ Akt, ⬆ ERK 1/2 and ⬆ eNOS phosphorylation, ⬆ VEGFR2, ⬆ cell cycle-related proteins cyclin D1 and D3, ⬆ angiogenesis, ⬆ cell proliferation, ⬆ cell migration, ⬆ endothelial function, ⬇ hypertension and atherosclerosis pathology. | NEDD4L emerges as a therapeutic alternative for treating ischemic diseases directly associated with atherosclerosis. | NEDD4L-mediated angiogenesis should be analyzed under several pathological conditions to explore the molecular pathways. |
| [78] | The SHENQI compound was administered to a diabetic model. | Identify NEDD4L transcription factors that may cause diabetic vascular damage. | The SHENQI compound interacts with the NEDD4L signaling. | NEDD4L may offer new and comprehensive therapeutic strategies against DM and atherosclerosis-predominant angiopathy. | More preclinical studies should validate the SHENQI compound’s safety and effectiveness while targeting NEDD4L. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurindo, L.F.; Dogani Rodrigues, V.; de Lima, E.P.; Leme Boaro, B.; Mendes Peloi, J.M.; Ferraroni Sanches, R.C.; Penteado Detregiachi, C.R.; José Tofano, R.; Angelica Miglino, M.; Sloan, K.P.; et al. Targeting Atherosclerosis via NEDD4L Signaling—A Review of the Current Literature. Biology 2025, 14, 220. https://doi.org/10.3390/biology14030220
Laurindo LF, Dogani Rodrigues V, de Lima EP, Leme Boaro B, Mendes Peloi JM, Ferraroni Sanches RC, Penteado Detregiachi CR, José Tofano R, Angelica Miglino M, Sloan KP, et al. Targeting Atherosclerosis via NEDD4L Signaling—A Review of the Current Literature. Biology. 2025; 14(3):220. https://doi.org/10.3390/biology14030220
Chicago/Turabian StyleLaurindo, Lucas Fornari, Victória Dogani Rodrigues, Enzo Pereira de Lima, Beatriz Leme Boaro, Julia Maria Mendes Peloi, Raquel Cristina Ferraroni Sanches, Cláudia Rucco Penteado Detregiachi, Ricardo José Tofano, Maria Angelica Miglino, Katia Portero Sloan, and et al. 2025. "Targeting Atherosclerosis via NEDD4L Signaling—A Review of the Current Literature" Biology 14, no. 3: 220. https://doi.org/10.3390/biology14030220
APA StyleLaurindo, L. F., Dogani Rodrigues, V., de Lima, E. P., Leme Boaro, B., Mendes Peloi, J. M., Ferraroni Sanches, R. C., Penteado Detregiachi, C. R., José Tofano, R., Angelica Miglino, M., Sloan, K. P., Sloan, L. A., & Barbalho, S. M. (2025). Targeting Atherosclerosis via NEDD4L Signaling—A Review of the Current Literature. Biology, 14(3), 220. https://doi.org/10.3390/biology14030220







