Probiotics as Renal Guardians: Modulating Gut Microbiota to Combat Diabetes-Induced Kidney Damage
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Framework and Study Design
2.2. Sample of Blood
2.3. Biochemical Examinations
2.4. Insulin Sensitivity Test (IST) and Oral Glucose Tolerance Test (OGTT)
2.5. Histological Inspection
2.6. Comet Assay: DNA Fragmentation
2.7. Microbiome Analysis
2.7.1. Collection and Treatment of Samples
2.7.2. DNA Extraction from Microorganisms
2.7.3. 16S rRNA Gene Sequencing
2.7.4. Processing and Filtering of Sequencing Data
2.7.5. Alpha Diversity Analysis
2.7.6. Beta Diversity and Principal Component Analysis (PCA)
2.7.7. Taxonomic Composition and Heatmap Visualization
2.7.8. Firmicutes/Bacteroidetes Ratio
2.8. Analysis of Statistics
3. Results
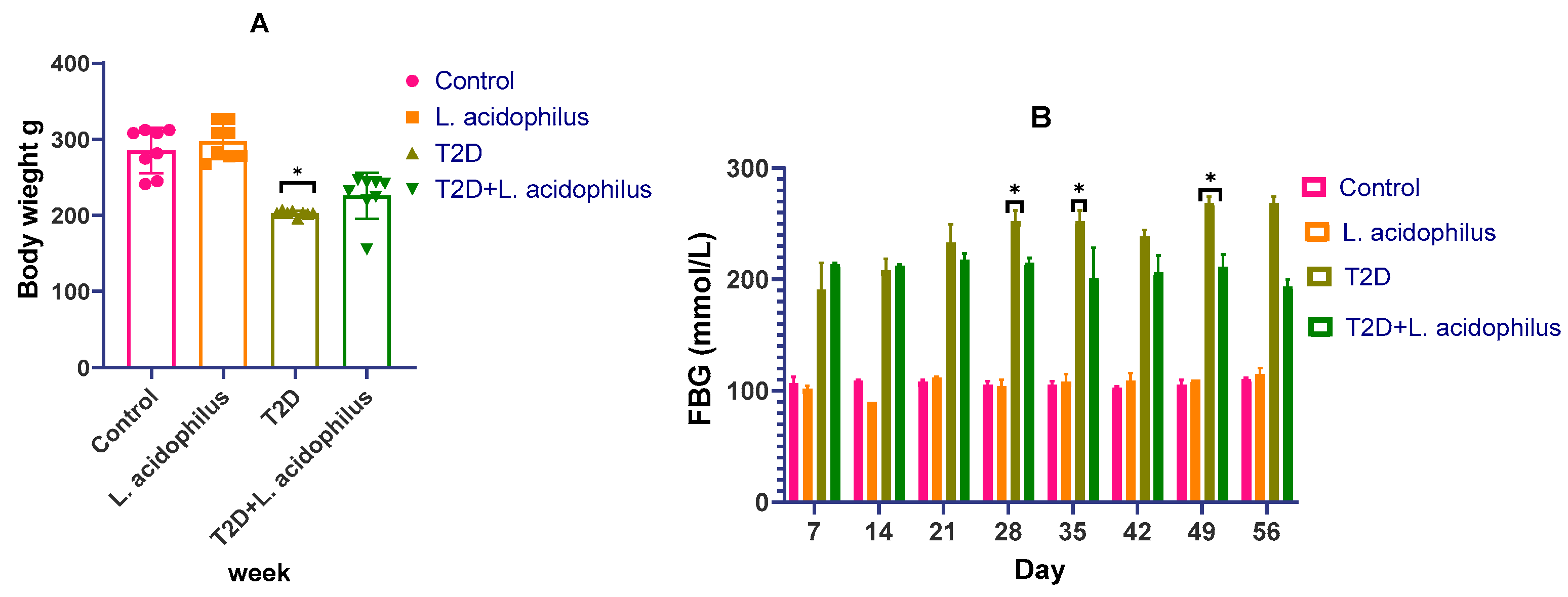
3.1. Effect of Lactobacillus acidophilus on Body Weight and Insulin Levels in Diabetic Rats
3.2. Impact of Lactobacillus acidophilus on T2D Rat Glucose Tolerance and Insulin Sensitivity
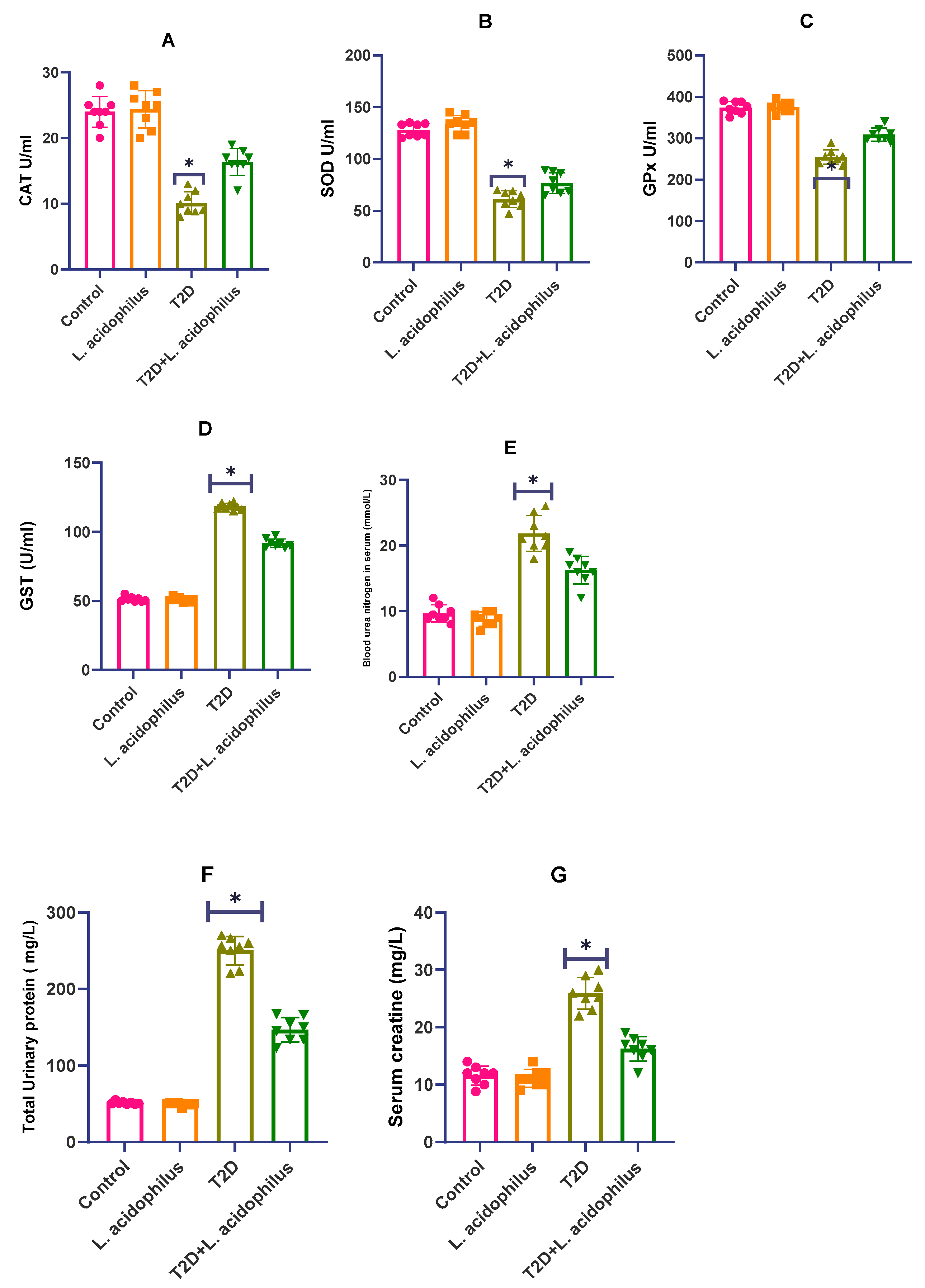
3.3. Impact of Lactobacillus acidophilus on T2D Rat Antioxidant Enzyme Levels
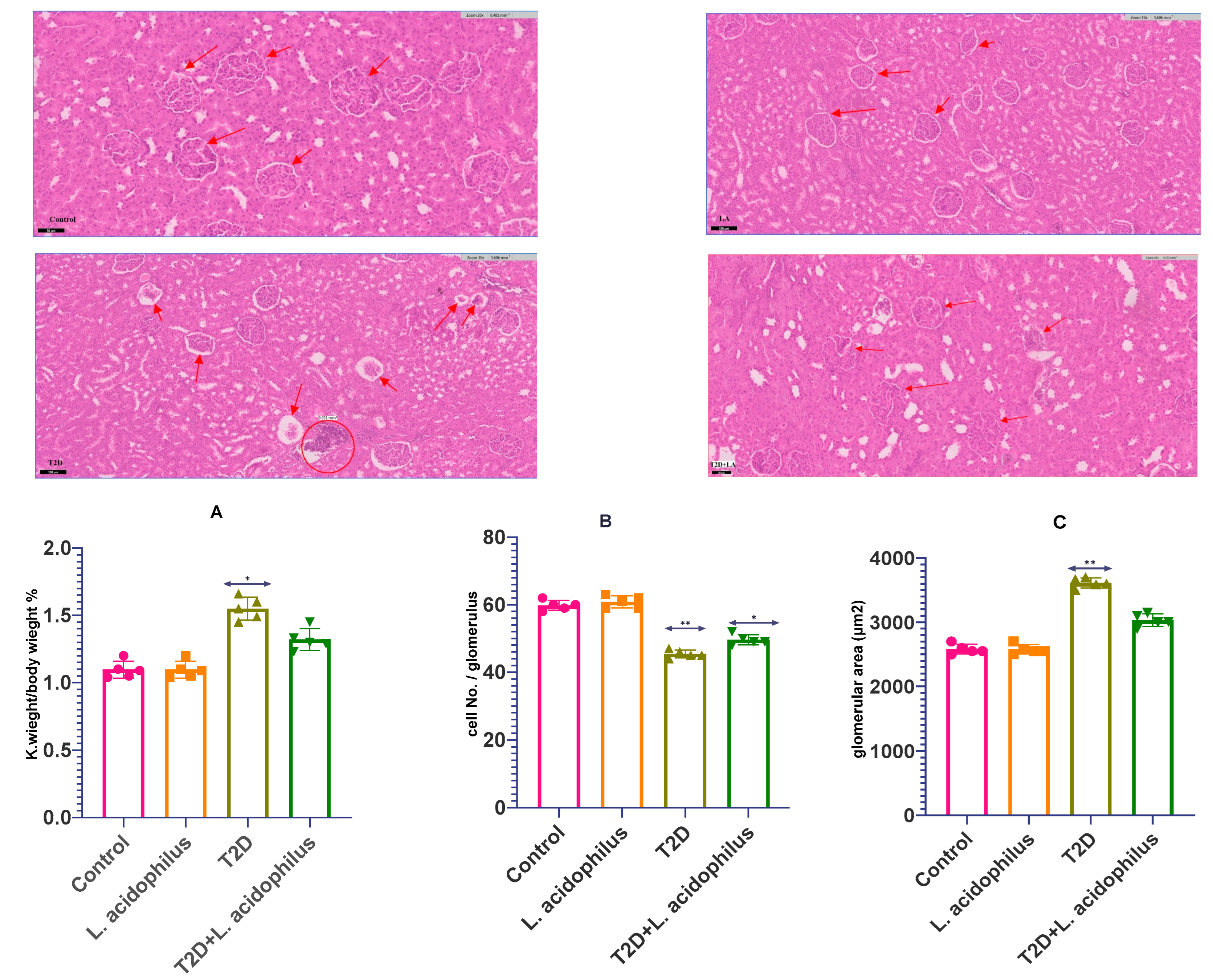
3.4. Assessment of Renal Health Parameters
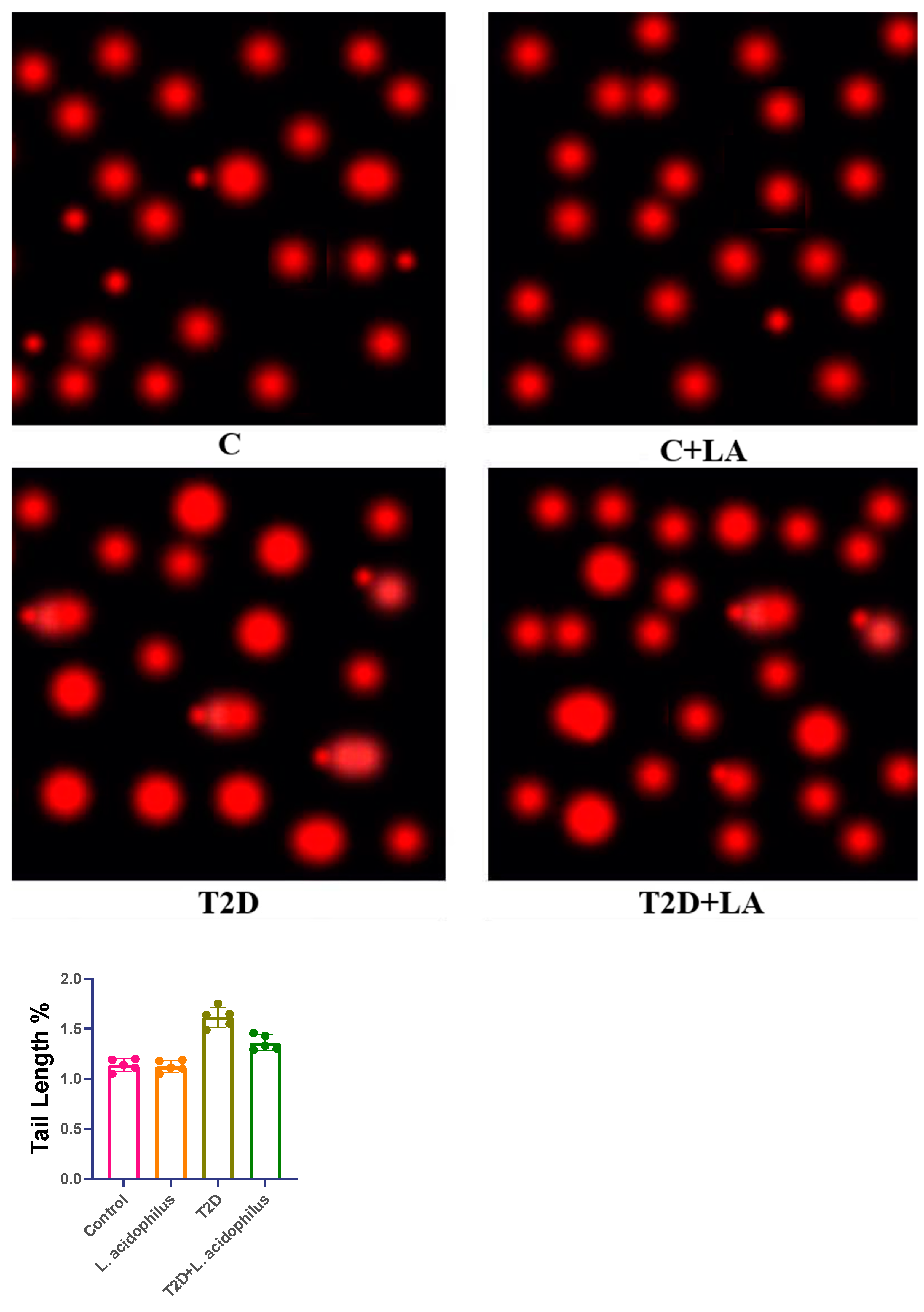
3.5. Comet Assay of Kidney Tissue in T2D Rats
3.6. The Constituents of the Microbiome
3.6.1. Bacterial Phyla Proportions
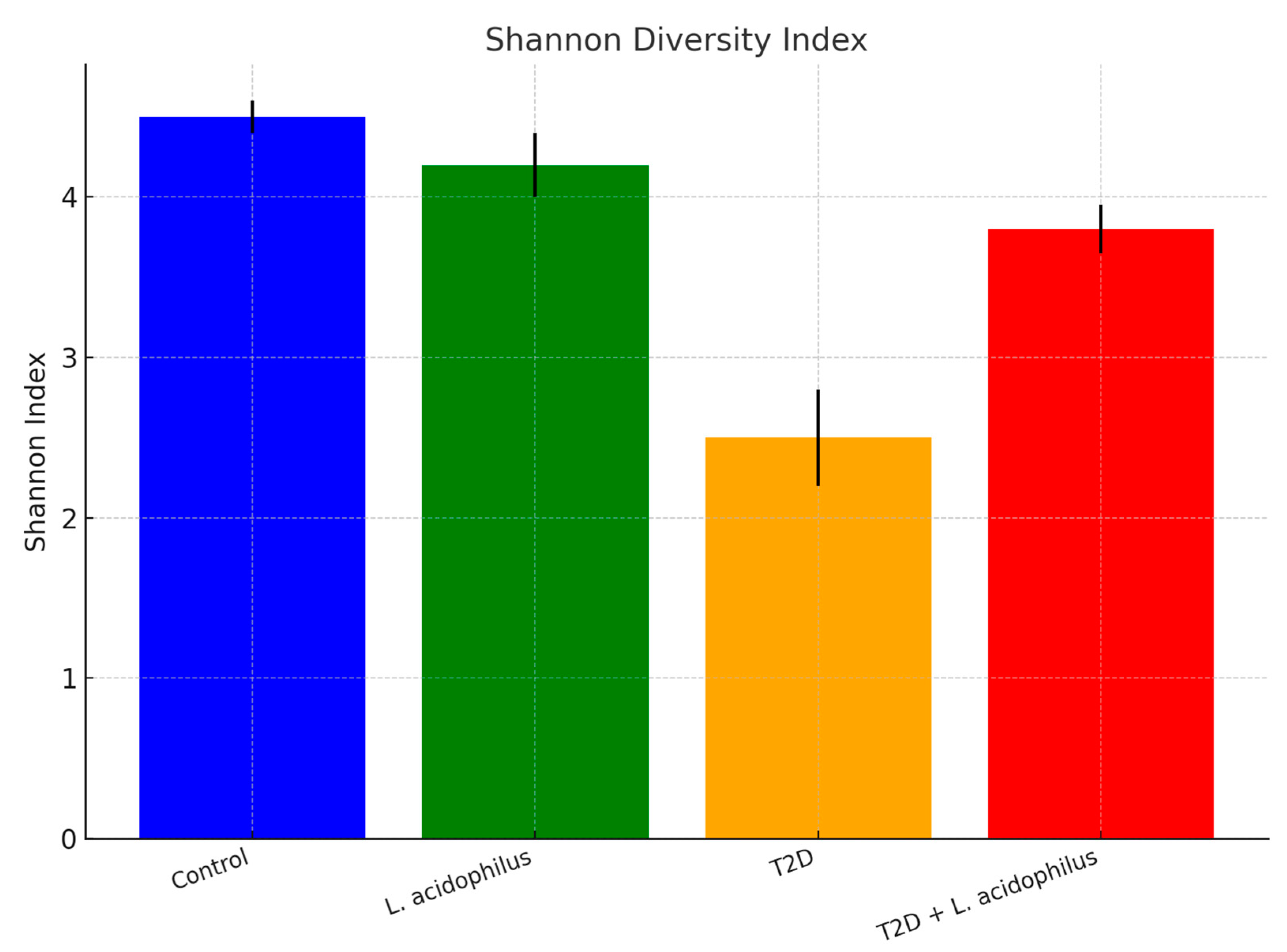
3.6.2. Shannon Diversity Index
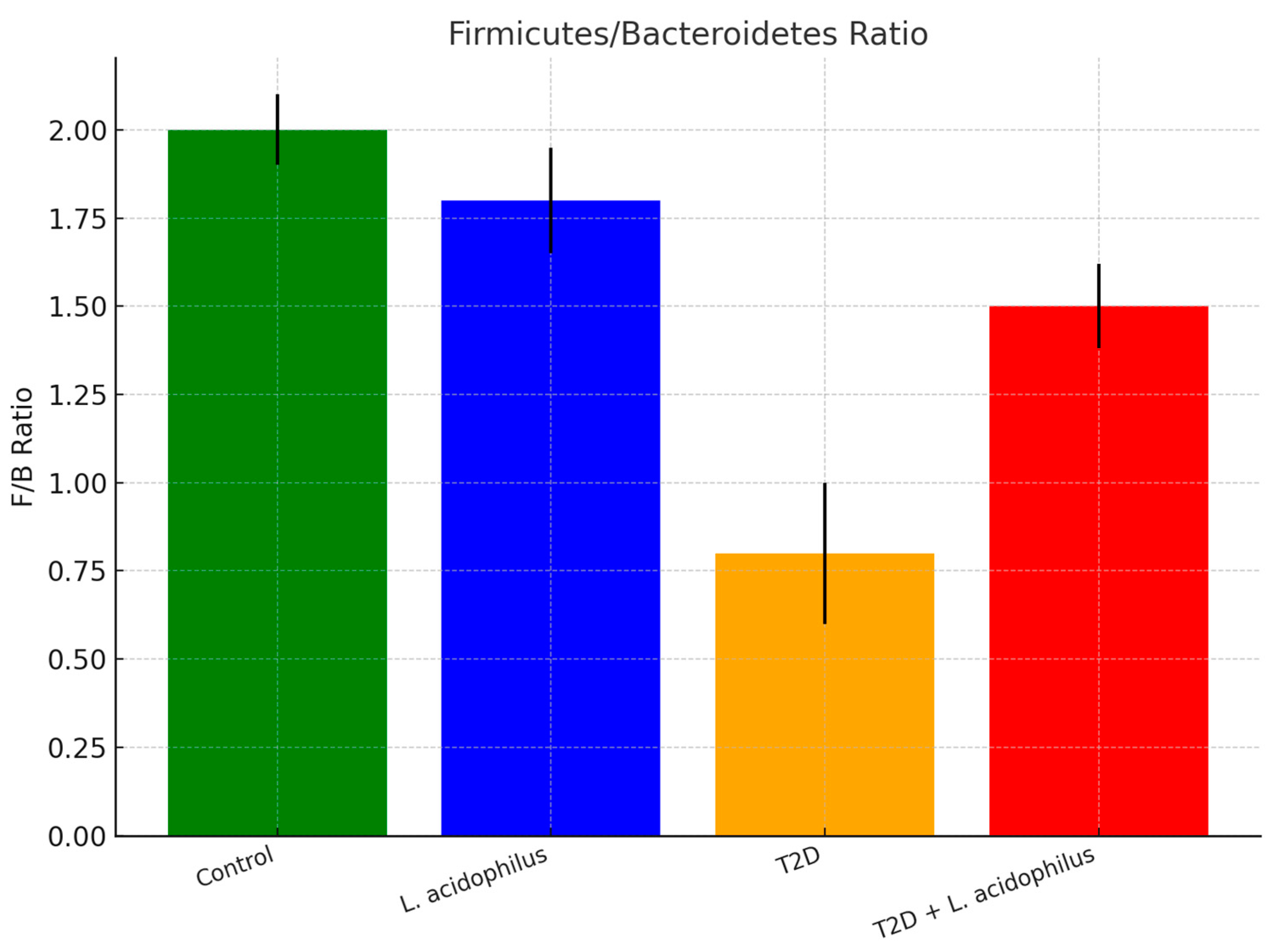
3.6.3. Firmicutes/Bacteroidetes (F/B) Ratio
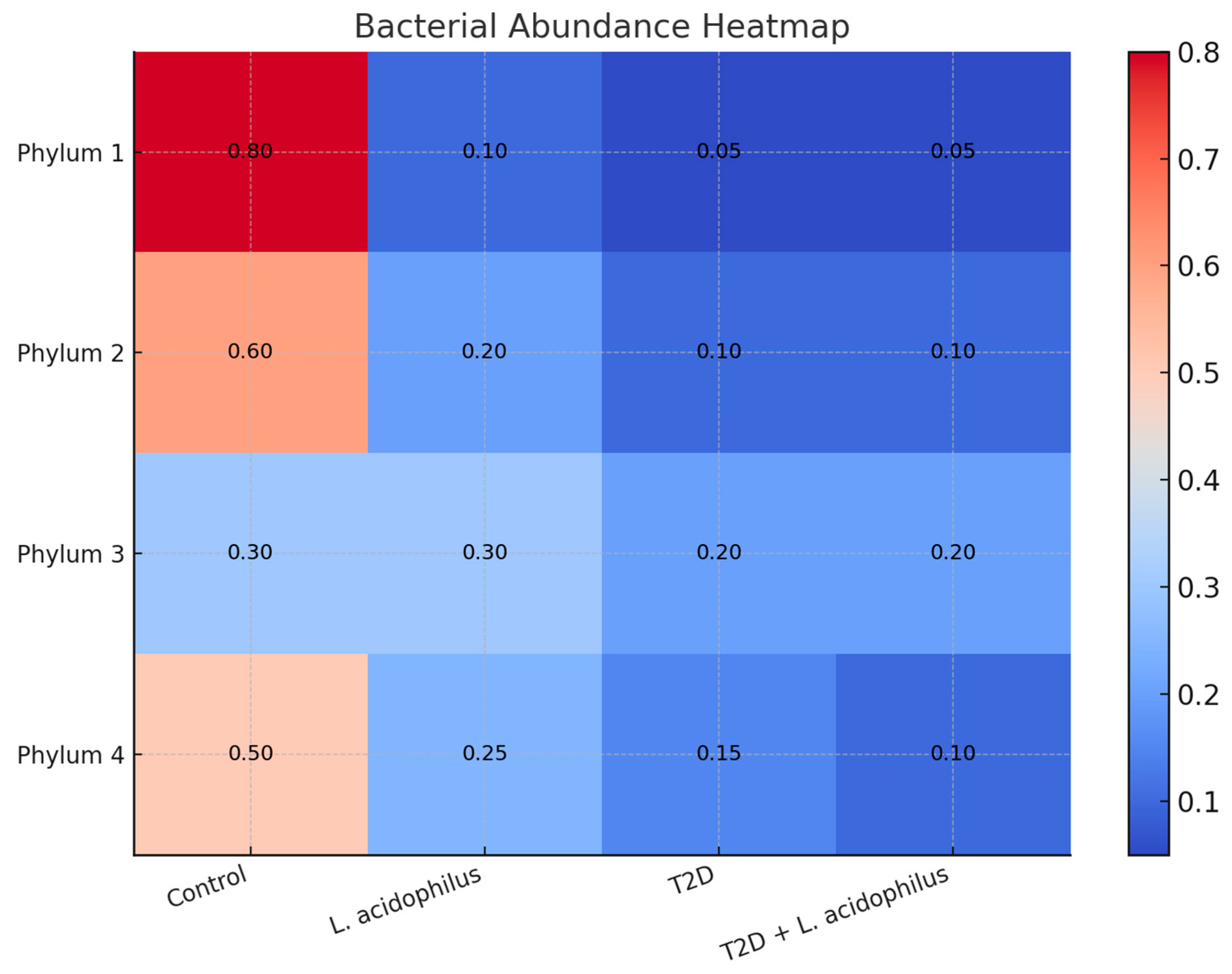
3.6.4. Heatmap Analysis of Bacterial Abundance
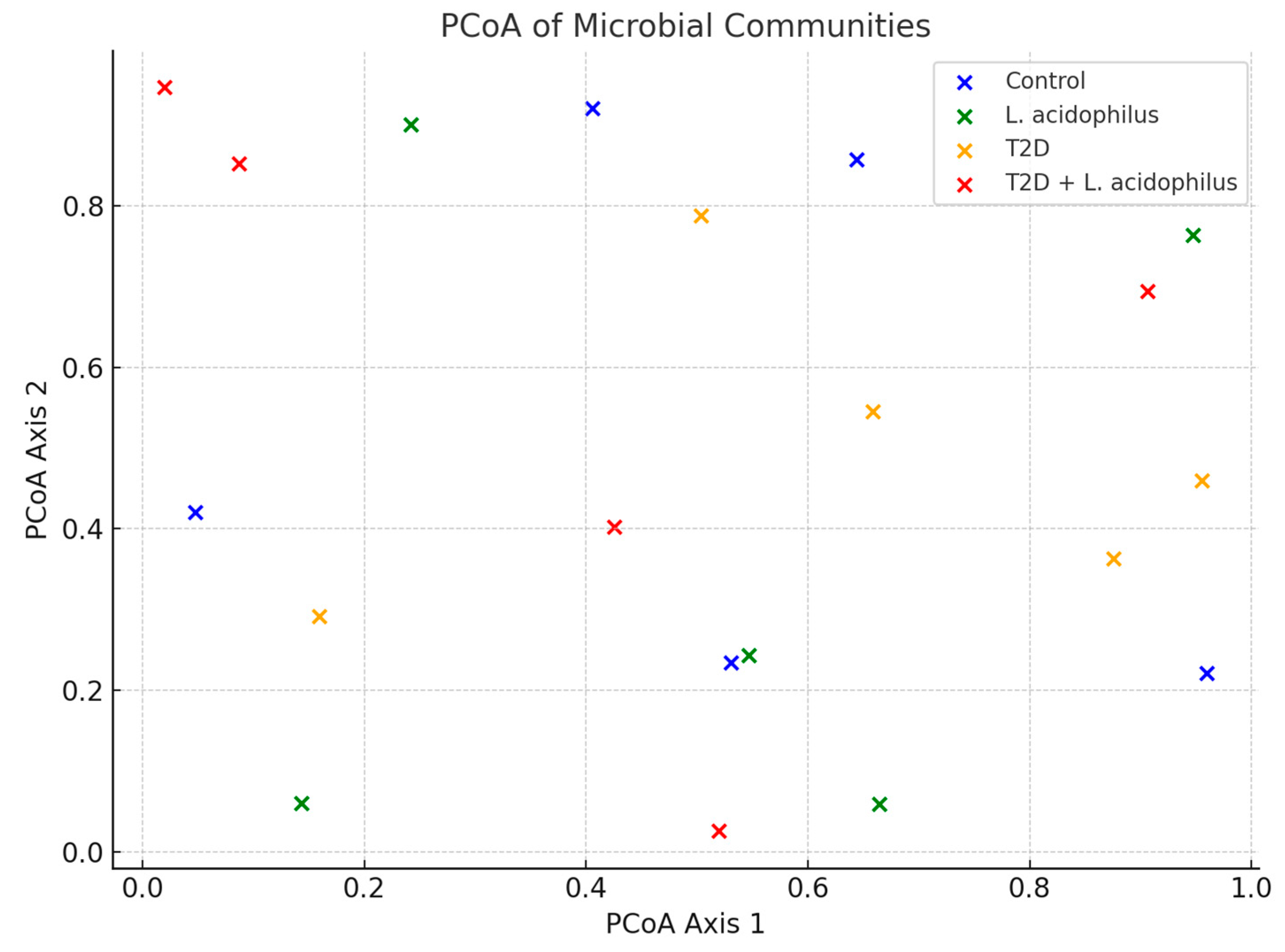
3.6.5. Principal Coordinate Analysis (PCoA)
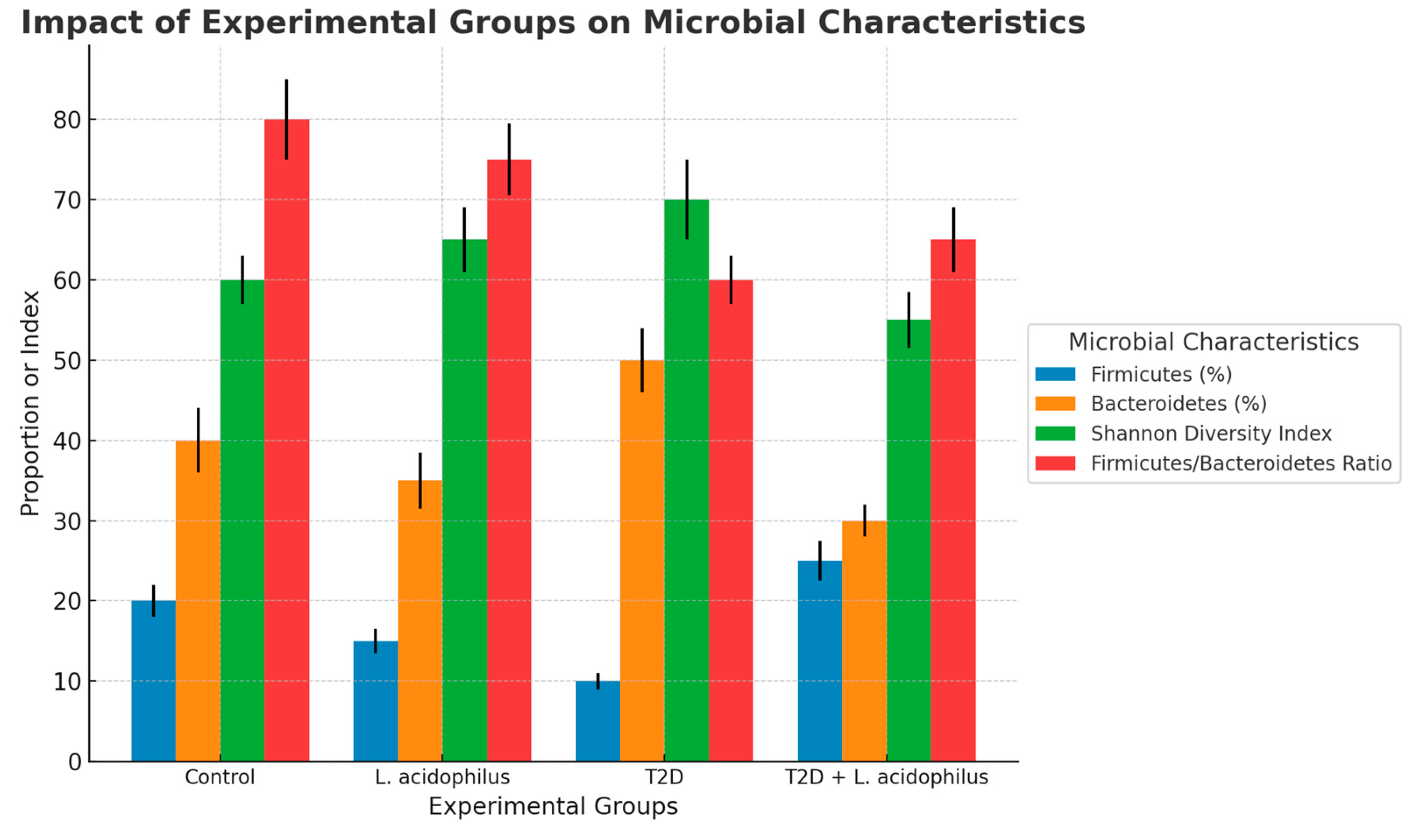
3.6.6. Microbial Characteristics
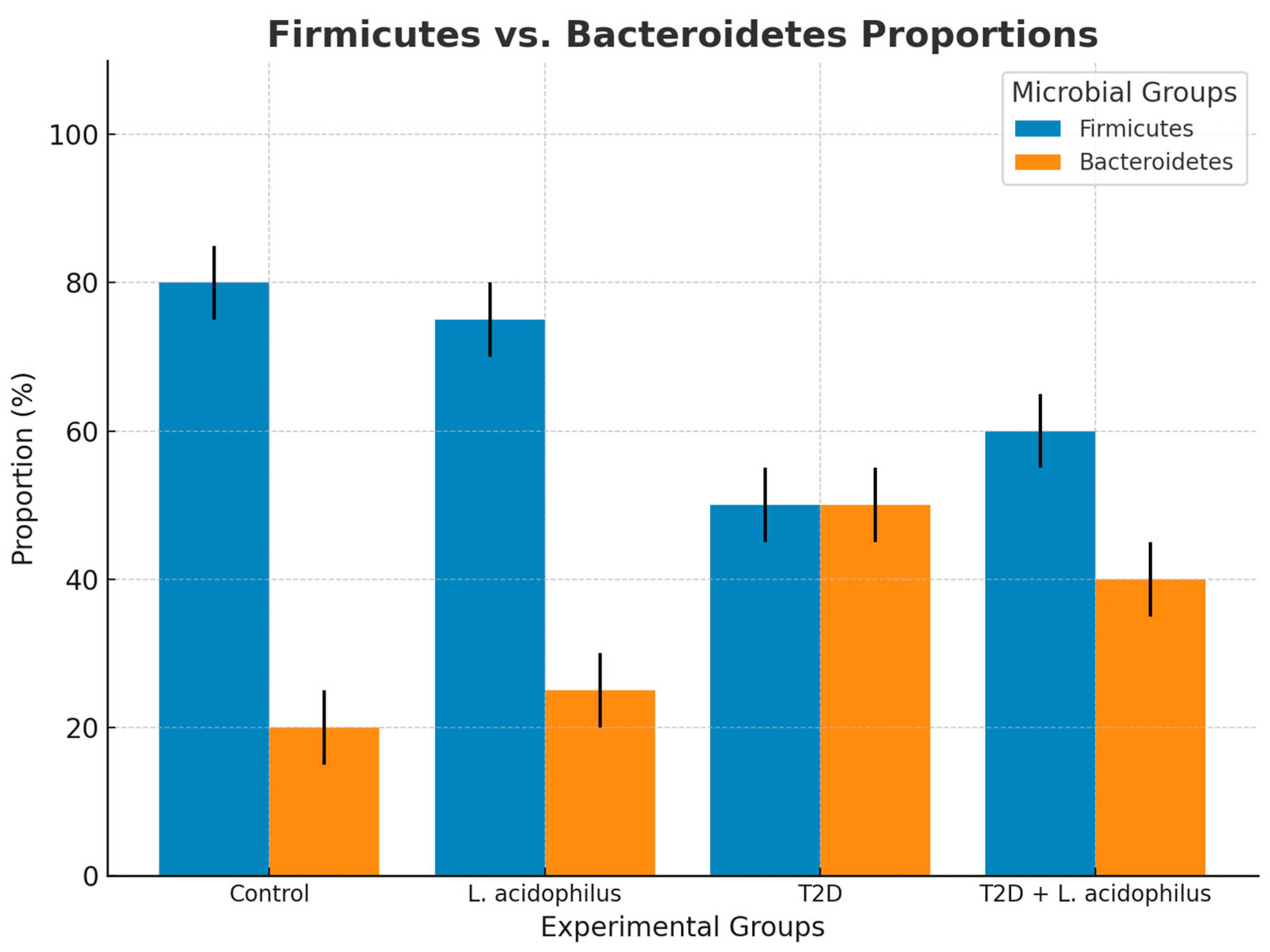
3.6.7. Firmicutes and Bacteroidetes Proportions
4. Discussion
4.1. Impact on Insulin Sensitivity and Metabolic Measures
4.2. Protective Effects on Renal Function
4.3. DNA Protection
4.3.1. Comparing Gut Microbiota Modulation Therapies
4.3.2. The Role of Lactobacillus acidophilus in Modulating Gut Microbiota Dysbiosis in Type 2 Diabetes
4.3.3. Bacterial Phyla Proportions
4.3.4. Microbial Diversity
4.3.5. Firmicutes/Bacteroidetes (F/B) Ratio
4.3.6. Heatmap and Microbial Characteristics
4.3.7. Restoration of Firmicutes and Bacteroidetes Proportions
4.3.8. Principal Coordinate Analysis (PCoA)
4.3.9. Conclusion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gheith, O.; Farouk, N.; Nampoory, N.; Halim, M.; Al-Otaibi, T. Diabetic kidney disease: Worldwide difference of prevalence and risk factors. J. Nephropharmacol. 2015, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Hu, W.; Hu, Z.; Guan, Y.; Zhang, R. A review of nutritional implications of bioactive compounds of Ginger (Zingiber officinale Roscoe), their biological activities and nano-formulations. Ital. J. Food Sci. 2022, 34, 1–12. [Google Scholar] [CrossRef]
- Fernández-Pampín, N.; Plaza, J.J.G.; García-Gómez, A.; Peña, E.; Rumbo, C.; Barros, R.; Martel-Martín, S.; Aparicio, S.; Tamayo-Ramos, J.A. Toxicology assessment of manganese oxide nanomaterials with enhanced electrochemical properties using human in vitro models representing different exposure routes. Sci. Rep. 2022, 12, 20991. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Z.; Xiong, Z.; Li, S.; Liu, W.; Tian, T.; Yang, X. Comparative transcriptomic and lipidomic analyses indicate that cold stress enhanced the production of the long C18–C22 polyunsaturated fatty acids in Aurantiochytrium sp. Front. Microbiol. 2022, 13, 915773. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Jin, Z.; Zhang, D. Microbial Cell Factories for Green Production of Vitamins. Front. Bioeng. Biotechnol. 2021, 9, 661562. [Google Scholar] [CrossRef]
- Tan, H.; Xu, W.; Guo, F.; Liu, Y.; Wang, Y.; Guo, Z.; Zhou, Y.; Wu, Q.; Luo, Q.; Sun, H. Glabridin, a bioactive com-ponent of licorice, ameliorates diabetic nephropathy by regulating ferroptosis and the VEGF/Akt/ERK pathways. Mol. Med. 2022, 28, 58. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Song, B.-L.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef]
- Arcidiacono, M.V.; Carrillo-López, N.; Panizo, S.; Castro-Grattoni, A.L.; Valcheva, P.; Ulloa, C.; Rodríguez-Carrio, J.; Cardús, A.; Quirós-Caso, C.; Martínez-Arias, L.; et al. Barley-ß-glucans reduce systemic inflammation, renal injury and aortic calcification through ADAM17 and neutral-sphingomyelinase2 inhibition. Sci. Rep. 2019, 9, 17810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sui, Z.; Wang, M.; Liu, P. Natural products in attenuating renal inflammation via inhibiting the NLRP3 inflammasome in diabetic kidney disease. Front. Immunol. 2023, 14, 1196016. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, W.; Cai, J.; Liu, W.; Akihisa, T.; Li, W.; Kikuchi, T.; Xu, J.; Feng, F.; Zhang, J. The role of metabolites of steviol glycosides and their glucosylated derivatives against diabetes-related metabolic disorders. Food Funct. 2021, 12, 8248–8259. [Google Scholar] [CrossRef]
- Kawano, K.; Mori, S.; Hirashima, T.; Man, Z.-W.; Natori, T. Examination of the pathogenesis of diabetic nephropathy in OLETF rats. J. Veter-Med. Sci. 1999, 61, 1219–1228. [Google Scholar] [CrossRef]
- Peng, Y.-H.; Sweet, D.H.; Lin, S.-P.; Yu, C.-P.; Chao, P.-D.L.; Hou, Y.-C. Green tea inhibited the elimination of nephro-cardiovascular toxins and deteriorated the renal function in rats with renal failure. Sci. Rep. 2015, 5, 16226. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Addo, T.; Cho, H.J.; Barker, S.L.; Ravikumar, P.; Gillings, N.; Bian, A.; Sidhu, S.S.; et al. Renal production, uptake, and handling of circulating αKlotho. J. Am. Soc. Nephrol. 2016, 27, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Becerra, L.; Cornejo-Granados, F.; García-López, R.; Valdez-Lara, A.; Bikel, S.; Canizales-Quinteros, S.; López-Contreras, B.E.; Mendoza-Vargas, A.; Nielsen, H.; Ochoa-Leyva, A. Metatranscriptomic analysis to define the Secrebiome, and 16S rRNA profiling of the gut microbiome in obesity and metabolic syndrome of Mexican children. Microb. Cell Factories 2020, 19, 61. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing kidney function—Measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Vega Joubert, M.B.; Ingaramo, P.; Oliva, M.E.; D’Alessandro, M.E. Salvia hispanica L. (chia) seed ameliorates liver injury and oxidative stress by modulating NrF2 and NFκB expression in sucrose-rich diet-fed rats. Food Funct. 2022, 13, 7333–7345. [Google Scholar] [CrossRef]
- Abbassi, R.; Pontes, M.C.; Dhibi, S.; Filho, L.A.M.S.D.; Othmani, S.; Bouzenna, H.; Almeida, J.R.G.S.; Hfaiedh, N. Antioxidant properties of date seeds extract (Phoenix dactylifera L.) in alloxan induced damage in rats. Braz. J. Biol. 2023, 83, e274405. [Google Scholar] [CrossRef] [PubMed]
- Sabra, M.S.; Hemida, F.K.; Allam, E.A.H. Adenine model of chronic renal failure in rats to determine whether MCC950, an NLRP3 inflammasome inhibitor, is a renopreventive. BMC Nephrol. 2023, 24, 377. [Google Scholar] [CrossRef] [PubMed]
- Al Za’abi, M.; Ali, H.; Ali, B.H. Effect of flaxseed on systemic inflammation and oxidative stress in diabetic rats with or without chronic kidney disease. PLoS ONE 2021, 16, e0258800. [Google Scholar] [CrossRef]
- Al-Attar, A.M.; Abu Zeid, I.M.; Felemban, L.F.; Omar, A.M.S.; Alkenani, N.A. Protective and antioxidant effects of chia oil and canola oil on testicular injury induced by lead in rats. J. King Saud Univ. Sci. 2023, 35, 102894. [Google Scholar] [CrossRef]
- Hardianto, D. Telaah komprehensif diabetes melitus: Klasifikasi, gejala, diagnosis, pencegahan, dan pengobatan. J. Bioteknol. Biosains Indones. 2020, 7, 304–317. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Wang, P.; Hu, X.; Chen, F. Gut microbiota promotes production of aromatic metabolites through degradation of barley leaf fiber. J. Nutr. Biochem. 2018, 58, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, X.; Deng, H.; Huang, H.; Liu, Y.; Zhong, Z.; Shen, L.; Cao, S.; Ma, X.; Zhou, Z.; et al. Integrated transcriptome and metabolomics to reveal the mechanism of adipose mesenchymal stem cells in treating liver fibrosis. Int. J. Mol. Sci. 2023, 24, 16086. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, G.; Muraleedharan, A.; Sathidevi, P.; Chand, P.; Rajkumar, R. Quantification of DNA damage by the analysis of silver stained comet assay images. IRBM 2015, 36, 306–314. [Google Scholar] [CrossRef]
- Anderson, N.J.; Smith, C.K.; Byrd, J.L. Work-related injury factors and safety climate perception in truck drivers. Am. J. Ind. Med. 2017, 60, 711–723. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Illumina. High-throughput sequencing technology for microbial communities. Nat. Methods 2013, 10, 999–1000. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Ferguson, G.J.; Kulkarni, S.; Damoulakis, G.; Anderson, K.; Bohlooly-Y, M.; Stephens, L.; Hawkins, P.T.; Curi, R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS ONE 2011, 6, e21205. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Marungruang, N.; Fåk, F.; Nyman, M. Effects of two whole-grain barley varieties on caecal SCFA, gut microbiota and plasma inflammatory markers in rats consuming low- and high-fat diets. Br. J. Nutr. 2015, 113, 1558–1570. [Google Scholar] [CrossRef]
- Dong, N.; Xu, B.; Shi, H. Long noncoding RNA MALAT1 acts as a competing endogenous RNA to regulate Amadori-glycated albumin-induced MCP-1 expression in retinal microglia by a microRNA-124-dependent mechanism. Inflamm. Res. 2018, 67, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-K.; Chen, T.-T.; Chen, X.; Liu, Y.; Liu, C.; Li, L. Assessing the product quality and biological activities of barley (Hordeum vulgare L.) grasses at different harvest times. Food Biosci. 2022, 46, 101549. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, J.; Liu, J.; Wang, Z.; Chen, M.; Zhou, S. Metagenome of gut microbiota of children with nonalcoholic fatty liver disease. Front. Pediatr. 2019, 7, 518. [Google Scholar] [CrossRef] [PubMed]
- Shokolenko, I.; Venediktova, N.; Bochkareva, A.; Wilson, G.L.; Alexeyev, M.F. Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res. 2009, 37, 2539–2548. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Redox active metal-induced oxidative stress in biological systems. Transit. Met. Chem. 2012, 37, 127–134. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Yang, C. Evaluating In Vitro DNA damage using Comet assay. J. Vis. Exp. 2017, 128, e56450. [Google Scholar] [CrossRef]
- Saadatzadeh, A.; Fazeli, M.R.; Jamalifar, H.; Dinarvand, R. Probiotic properties of lyophilized cell-free extract of Lactobacillus casei. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 131–137. [Google Scholar] [CrossRef]
- Million, M.; Maraninchi, M.; Henry, M.; Armougom, F.; Richet, H.; Carrieri, P.; Valero, R.; Raccah, D.; Vialettes, B.; Raoult, D. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. 2012, 36, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Panasenko, O.O.; Bezrukov, F.; Komarynets, O.; Renzoni, A. YjbH Solubility Controls Spx in Staphylococcus aureus: Implication for MazEF Toxin-Antitoxin System Regulation. Front. Microbiol. 2020, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project: Exploring the microbial part of ourselves in a changing world. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ghamdi, S.B. Probiotics as Renal Guardians: Modulating Gut Microbiota to Combat Diabetes-Induced Kidney Damage. Biology 2025, 14, 122. https://doi.org/10.3390/biology14020122
Al-Ghamdi SB. Probiotics as Renal Guardians: Modulating Gut Microbiota to Combat Diabetes-Induced Kidney Damage. Biology. 2025; 14(2):122. https://doi.org/10.3390/biology14020122
Chicago/Turabian StyleAl-Ghamdi, Saleh Bakheet. 2025. "Probiotics as Renal Guardians: Modulating Gut Microbiota to Combat Diabetes-Induced Kidney Damage" Biology 14, no. 2: 122. https://doi.org/10.3390/biology14020122
APA StyleAl-Ghamdi, S. B. (2025). Probiotics as Renal Guardians: Modulating Gut Microbiota to Combat Diabetes-Induced Kidney Damage. Biology, 14(2), 122. https://doi.org/10.3390/biology14020122




