Short-Term Heat Stress Differentially Affects the Photosynthetic Thermotolerance of Cotyledons and Early Orthotropic Leaves in Coffea arabica L. Seedlings
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth
2.2. Heat Stress Treatments
2.3. Gas Exchange and Chlorophyll Fluorescence Measurement
2.4. Photosystem II Heat Tolerance (PHT) Thresholds
2.5. Data Analysis
3. Results
3.1. Analysis of Gas Exchange and PSII Responses to Heat Stress and Recovery Reveal Differential Acclimation Between Leaf Types
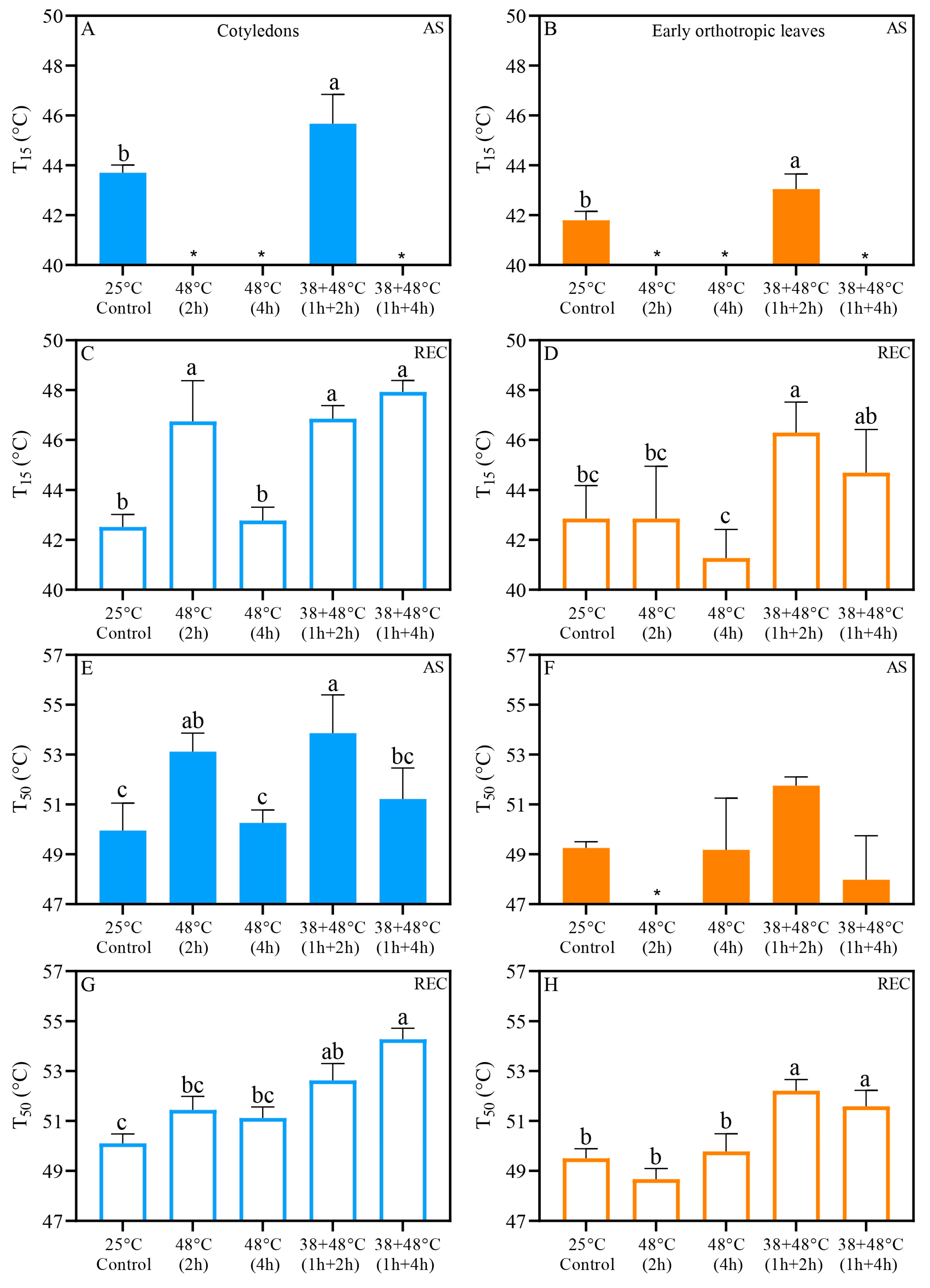
3.2. Photosynthetic Heat Tolerance Thresholds (T15 and T50) Vary Between Acclimated and Non-Acclimated Leaves and Between Leaf Types
3.3. Evaluation of the Photosynthetic Response of Cotyledons and Early-Emitted Orthotropic Leaves Reveals Higher PSII Heat Tolerance in Cotyledons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | After heat stress |
| REC | 5 days of recovery after heat stress |
| CL | Cotyledons |
| EOL | Early orthotropic leaves |
| PSII | Photosystem II |
| PHT | Photosynthetic heat tolerance |
| Pn | Net photosynthetic rate µmol CO2 m−2 s−1 |
| E | Transpiration rate mmol H2O m−2 s−1 |
| gs | Stomatal conductance mol H2O m−2 s−1 |
| Ci | Intercellular CO2 concentration µmol mol−1 |
| PPFD | Photosynthetic photon flux density µmol photons m−2 s−1 |
| CO2 | Atmospheric CO2 concentration µmol mol−1 |
| WUE | Water use efficiency µmol CO2 mmol−1 H2O |
| Fv/Fm | Maximum quantum yield of PSII |
| T15 | Temperature at which there is a 15% reduction in Fv/Fm compared to initial values |
| T50 | Temperature at which there is a 50% reduction in Fv/Fm compared to initial values |
References
- Vegro, C.L.R.; De Almedia, L.F. Global coffee market: Socio-economic and cultural dynamics. In Coffee Consumption and Industry Strategies in Brazil; Woodhead Publishing: Cambridge, UK, 2020; pp. 3–19. [Google Scholar] [CrossRef]
- Vartan, J. Coffee Cultivation and Industry in Brazil: A Comprehensive Review. Int. J. Sci. Soc. 2023, 5, 323–332. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Avila, R.T.; Cardoso, A.A.; Martins, S.C.V.; Ramalho, J.C. Physiological and Agronomic Performance of the Coffee Crop in the Context of Climate Change and Global Warming: A Review. J. Agric. Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef]
- ICO. Sustainability & Resilience of the Coffee Global Value Chain: Towards a Global Investment Vehicle; International Coffee Organization: London, UK, 2024. [Google Scholar]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Ecophysiology of coffee growth and production. Braz. J. Plant Physiol. 2007, 19, 485–510. [Google Scholar] [CrossRef]
- Bilen, C.; El Chami, D.; Mereu, V.; Trabucco, A.; Marras, S.; Spano, D. A Systematic Review on the Impacts of Climate Change on Coffee Agrosystems. Plants 2022, 12, 102. [Google Scholar] [CrossRef]
- Jayakumar, M.; Rajavel, M.; Surendran, U.; Gopinath, G.; Ramamoorthy, K. Impact of climate variability on coffee yield in India—With a micro-level case study using long-term coffee yield data of humid tropical Kerala. Clim. Change 2017, 145, 335–349. [Google Scholar] [CrossRef]
- Pham, Y.; Reardon-Smith, K.; Mushtaq, S.; Cockfield, G. The impact of climate change and variability on coffee production: A systematic review. Clim. Change 2019, 156, 609–630. [Google Scholar] [CrossRef]
- Gomes, L.C.; Bianchi, F.J.J.A.; Cardoso, I.M.; Fernandes, R.B.A.; Filho, E.I.F.; Schulte, R.P.O. Agroforestry systems can mitigate the impacts of climate change on coffee production: A spatially explicit assessment in Brazil. Agric. Ecosyst. Environ. 2020, 294, 106858. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef]
- WMO. State of the Global Climate 2024; World Meteorological Organization: Geneva, Switzerland, 2025. [Google Scholar]
- Assad, E.D.; Pinto, H.S.; Zullo Junior, J.; Ávila, A.M.H. Climatic changes impact in agroclimatic zoning of coffee in Brazil. Pesqui. Agropecu. Bras. 2004, 39, 1057–1064. [Google Scholar] [CrossRef]
- Camargo, M.B.P. The impact of climatic variability and climate change on arabic coffee crop in Brazil. Bragantia 2010, 69, 239–247. [Google Scholar] [CrossRef]
- Zullo, J., Jr.; Pinto, H.S.; Assad, E.D.; de Ávila, A.M.H. Potential for growing Arabica coffee in the extreme south of Brazil in a warmer world. Clim. Change 2011, 109, 535–548. [Google Scholar] [CrossRef]
- Bunn, C.; Läderach, P.; Ovalle Rivera, O.; Kirschke, D. A bitter cup: Climate change profile of global production of Arabica and Robusta coffee. Clim. Change 2015, 129, 89–101. [Google Scholar] [CrossRef]
- Martins, M.Q.; Rodrigues, W.P.; Fortunato, A.S.; Leitao, A.E.; Rodrigues, A.P.; Pais, I.P.; Martins, L.D.; Silva, M.J.; Reboredo, F.H.; Partelli, F.L.; et al. Protective Response Mechanisms to Heat Stress in Interaction with High [CO2] Conditions in Coffea spp. Front. Plant Sci. 2016, 7, 947. [Google Scholar] [CrossRef]
- Menezes-Silva, P.E.; Sanglard, L.; Avila, R.T.; Morais, L.E.; Martins, S.C.V.; Nobres, P.; Patreze, C.M.; Ferreira, M.A.; Araujo, W.L.; Fernie, A.R.; et al. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot. 2017, 68, 4309–4322. [Google Scholar] [CrossRef]
- Venancio, L.P.; Filgueiras, R.; Mantovani, E.C.; do Amaral, C.H.; da Cunha, F.F.; dos Santos Silva, F.C.; Althoff, D.; dos Santos, R.A.; Cavatte, P.C. Impact of drought associated with high temperatures on Coffea canephora plantations: A case study in Espírito Santo State, Brazil. Sci. Rep. 2020, 10, 1979. [Google Scholar] [CrossRef]
- Rodrigues, A.P.; Pais, I.P.; Leitão, A.E.; Dubberstein, D.; Lidon, F.C.; Marques, I.; Semedo, J.N.; Rakocevic, M.; Scotti-Campos, P.; Campostrini, E.; et al. Uncovering the wide protective responses in Coffea spp. leaves to single and superimposed exposure of warming and severe water deficit. Front. Plant Sci. 2024, 14, 1320552. [Google Scholar] [CrossRef]
- World Coffee Research (WCR). Forging the Future of Coffee; World Coffee Research (WCR): Portland, OR, USA, 2023. [Google Scholar]
- Camargo, Â.P.d.; Camargo, M.B.P. Definição e esquematização das fases fenológicas do cafeeiro arábica nas condições tropicais do Brasil. Bragantia 2001, 60, 65–68. [Google Scholar] [CrossRef]
- Bertrand, B.; Boulanger, R.; Dussert, S.; Ribeyre, F.; Berthiot, L.; Descroix, F.; Joet, T. Climatic factors directly impact the volatile organic compound fingerprint in green Arabica coffee bean as well as coffee beverage quality. Food Chem. 2012, 135, 2575–2583. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Guihur, A.; Rebeaud, M.E.; Goloubinoff, P. How do plants feel the heat and survive. Trends Biochem. Sci. 2022, 47, 824–838. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher Plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Vegetation Stress: An Introduction to the Stress Concept in Plants. J. Plant Physiol. 2012, 148, 4–14. [Google Scholar] [CrossRef]
- Yeh, C.H.; Kaplinsky, N.J.; Hu, C.; Charng, Y.Y. Some like it hot, some like it warm: Phenotyping to explore thermotolerance diversity. Plant Sci. 2012, 195, 10–23. [Google Scholar] [CrossRef]
- Bokszczanin, K.L.; Fragkostefanakis, S. Perspectives on deciphering mechanisms underlying plant heat stress response and thermotolerance. Front. Plant Sci. 2013, 4, 315. [Google Scholar] [CrossRef]
- Li, Z.; Howell, S.H. Heat Stress Responses and Thermotolerance in Maize. Int. J. Mol. Sci. 2021, 22, 948. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Way, D.A.; Sharkey, T.D. Plant heat stress: Concepts directing future research. Plant Cell Environ. 2021, 44, 1992–2005. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.R.; Gao, J.; Lin, H.X.; Lin, Y. The molecular basis of heat stress responses in plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Doring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Larkindale, J.; Vierling, E. Core genome responses involved in acclimation to high temperature. Plant Physiol. 2008, 146, 748–761. [Google Scholar] [CrossRef]
- Song, L.; Jiang, Y.; Zhao, H.; Hou, M. Acquired thermotolerance in plants. Plant Cell Tissue Organ Cult. 2012, 111, 265–276. [Google Scholar] [CrossRef]
- Gallas, G.; Waters, E.R. Boechera species exhibit species-specific responses to combined heat and high light stress. PLoS ONE 2015, 10, e0129041. [Google Scholar] [CrossRef]
- Halter, G.; Simonetti, N.; Suguitan, C.; Helm, K.; Soroksky, J.; Waters, E.R. Patterns of thermotolerance, chlorophyll fluorescence, and heat shock gene expression vary among four Boechera species and Arabidopsis thaliana. Botany 2017, 95, 9–27. [Google Scholar] [CrossRef]
- Rodrigues, W.P.; Martins, M.Q.; Fortunato, A.S.; Rodrigues, A.P.; Semedo, J.N.; Simões-Costa, M.C.; Pais, I.P.; Leitão, A.E.; Colwell, F.; Goulao, L.; et al. Long-term elevated air [CO2] strengthens photosynthetic functioning and mitigates the impact of supra-optimal temperatures in tropical Coffea arabica and C. canephora species. Glob. Change Biol. 2016, 22, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Marias, D.E.; Meinzer, F.C.; Still, C. Impacts of leaf age and heat stress duration on photosynthetic gas exchange and foliar nonstructural carbohydrates in Coffea arabica. Ecol. Evol. 2017, 7, 1297–1310. [Google Scholar] [CrossRef]
- Yamane, K.; Nishikawa, M.; Hirooka, Y.; Narita, Y.; Kobayashi, T.; Kakiuchi, M.; Iwai, K.; Iijima, M. Temperature tolerance threshold and mechanism of oxidative damage in the leaf of Coffea arabica ‘Typica’ under heat stress. Plant Prod. Sci. 2022, 25, 337–349. [Google Scholar] [CrossRef]
- Perez, T.M.; Feeley, K.J. Photosynthetic heat tolerances and extreme leaf temperatures. Funct. Ecol. 2020, 34, 2236–2245. [Google Scholar] [CrossRef]
- Marias, D.E.; Meinzer, F.C.; Still, C. Leaf age and methodology impact assessments of thermotolerance of Coffea arabica. Trees—Struct. Funct. 2017, 31, 1091–1099. [Google Scholar] [CrossRef]
- Vilas-Boas, T.; Duarte, A.A.; della Torre, F.; Lovato, M.B.; Lemos-Filho, J.P. Does acclimation in distinct light conditions determine differences in the photosynthetic heat tolerance of coffee plants? Plant Biol. 2023, 25, 1101–1108. [Google Scholar] [CrossRef]
- Vilas-Boas, T.; Almeida, H.A.; della Torre, F.; Modolo, L.V.; Lovato, M.B.; Lemos-Filho, J.P. Intraspecific variation in the thermal safety margin in Coffea arabica L. in response to leaf age, temperature, and water status. Sci. Hortic. 2024, 337, 113455. [Google Scholar] [CrossRef]
- Burke, J.J. Integration of Acquired Thermotolerance within the Developmental Program of Seed Reserve Mobilization. In Biochemical and Cellular Mechanisms of Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 1994; pp. 191–200. [Google Scholar]
- Zhao, K.; Villar-Salvador, P.; Li, G. Legacy effects of early cotyledon removal on the growth, carbon and nitrogen storage, and drought response of Quercus variabilis seedlings. For. Ecol. Manag. 2024, 561, 121923. [Google Scholar] [CrossRef]
- Eira, M.T.S.; Amaral Da Silva, E.A.; de Castro, R.D.; Dussert, S.; Walters, C.; Bewley, J.D.; Hilhorst, H.W.M. Coffee seed physiology. Braz. J. Plant Physiol 2006, 18, 149–163. [Google Scholar] [CrossRef]
- Rosa, S.D.V.F.; McDonald, M.B.; Veiga, A.D.; Vilela, F.d.L.; Ferreira, I.A. Staging coffee seedling growth: A rationale for shortening the coffee seed germination test. Seed Sci. Technol. 2010, 38, 421–431. [Google Scholar] [CrossRef]
- Matiello, J.B.; Satinato, R.; Almeida, S.R.; Garcia, A.W.R. Cultura de Café no Brasil: Manual de recomendações; Editora, F., Ed.; Futurama Editora: São Paulo, Brazil, 2015. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Drake, J.E.; Tjoelker, M.G.; Varhammar, A.; Medlyn, B.E.; Reich, P.B.; Leigh, A.; Pfautsch, S.; Blackman, C.J.; Lopez, R.; Aspinwall, M.J.; et al. Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Glob. Change Biol. 2018, 24, 2390–2402. [Google Scholar] [CrossRef]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Change Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Hikosaka, K.; Ishikawa, K.; Borjigidai, A.; Muller, O.; Onoda, Y. Temperature acclimation of photosynthesis mechanisms involved in the changes in temperature dependence of photosynthetic rate. J. Exp. Bot. 2006, 57, 291–302. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Posch, B.C.; Evans, J.R.; Farquhar, G.D.; Atkin, O.K. RuBisCO deactivation and chloroplast electron transport rates co-limit photosynthesis above optimal leaf temperature in terrestrial plants. Nat. Commun. 2023, 14, 2820. [Google Scholar] [CrossRef]
- Scafaro, A.P.; Fan, Y.; Posch, B.C.; Garcia, A.; Coast, O.; Atkin, O.K. Responses of leaf respiration to heatwaves. Plant Cell Environ. 2021, 44, 2090–2101. [Google Scholar] [CrossRef]
- Wijewardene, I.; Shen, G.; Zhang, H. Enhancing crop yield by using RuBisCO activase to improve photosynthesis under elevated temperatures. Stress Biol. 2021, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Wang, Y.; Jie, W.; Peng, X.; Hua, X.; Yan, X.; Zhou, Z.; Lin, J. Physiological adaptive strategies of oil seed crop Ricinus communis early seedlings (Cotyledon vs. True leaf) under salt and alkali stresses: From the growth, photosynthesis and chlorophyll fluorescence. Front. Plant Sci. 2019, 9, 1939. [Google Scholar] [CrossRef]
- Sameena, P.P.; Puthur, J.T. Cotyledonary leaves effectively shield the true leaves in Ricinus communis L. from copper toxicity. Int. J. Phytoremediat. 2020, 23, 492–504. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Chen, L.; He, M.; Lan, H. The Developmental Delay of Seedlings with Cotyledons Only Confers Stress Tolerance to Suaeda aralocaspica (Chenopodiaceae) by Unique Performance on Morphology, Physiology, and Gene Expression. Front. Plant Sci. 2022, 13, 844430. [Google Scholar] [CrossRef] [PubMed]
- Milberg, P.; Lamont, B.B. Seed/cotyledon size and nutrient content play a major role in early performance of species on nutrient-poor soils. New Phytol. 1997, 137, 665–672. [Google Scholar] [CrossRef]
- Santos, H.P.; Buckeridge, M.S. The role of the storage carbon of cotyledons in the establishment of seedlings of Hymenaea courbaril under different light conditions. Ann. Bot. 2024, 94, 819–830. [Google Scholar] [CrossRef]
- Sameena, P.P.; Puthur, J.T. Differential modulation of photosynthesis and defense strategies towards copper toxicity in primary and cotyledonary leaves of Ricinus communis L. J. Photochem. Photobiol. 2021, 8, 100059. [Google Scholar] [CrossRef]
- Olas, J.J.; Apelt, F.; Annunziata, M.G.; John, S.; Richard, S.I.; Gupta, S.; Kragler, F.; Balazadeh, S.; Mueller-Roeber, B. Primary carbohydrate metabolism genes participate in heat-stress memory at the shoot apical meristem of Arabidopsis thaliana. Mol. Plant 2021, 14, 1508–1524. [Google Scholar] [CrossRef] [PubMed]

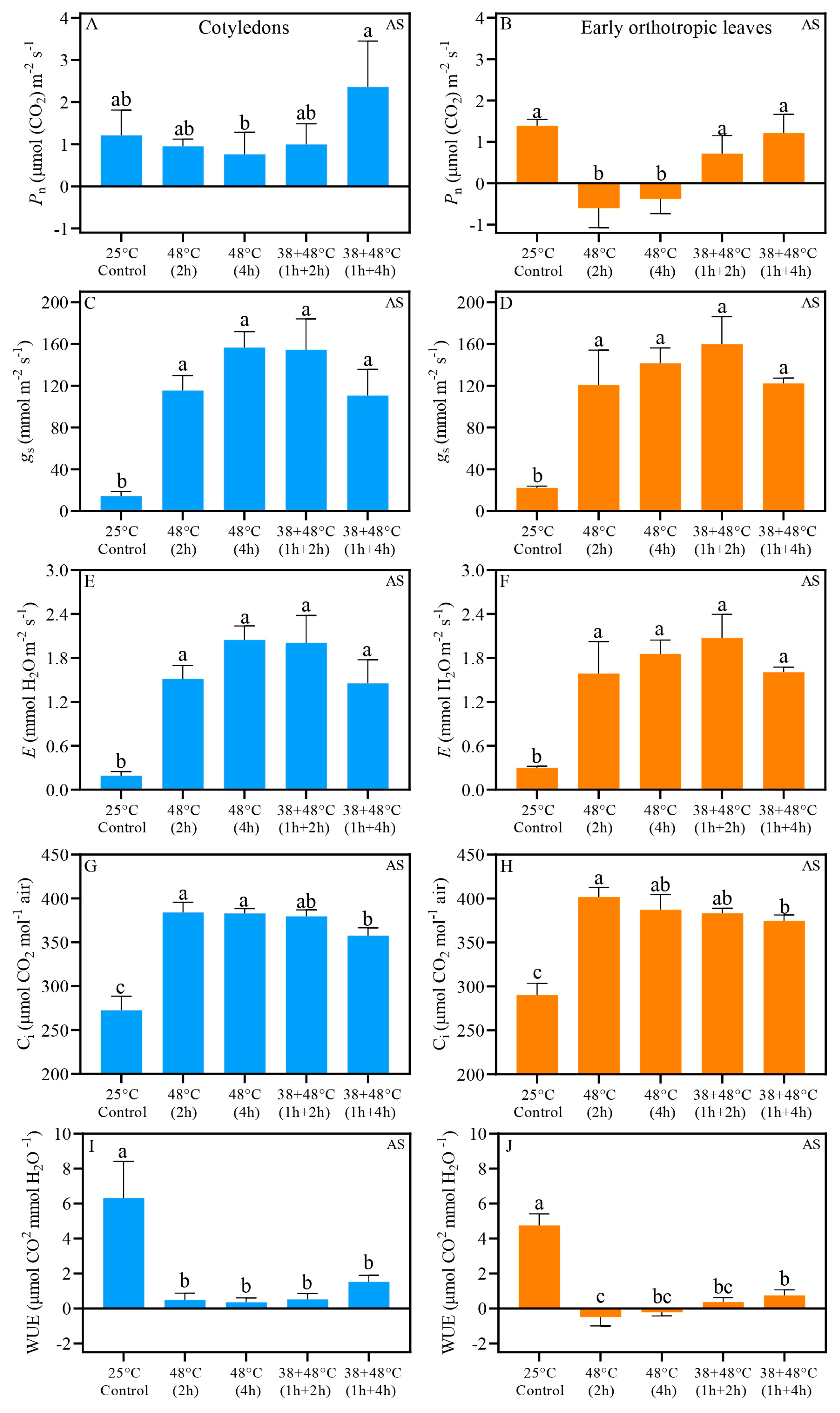
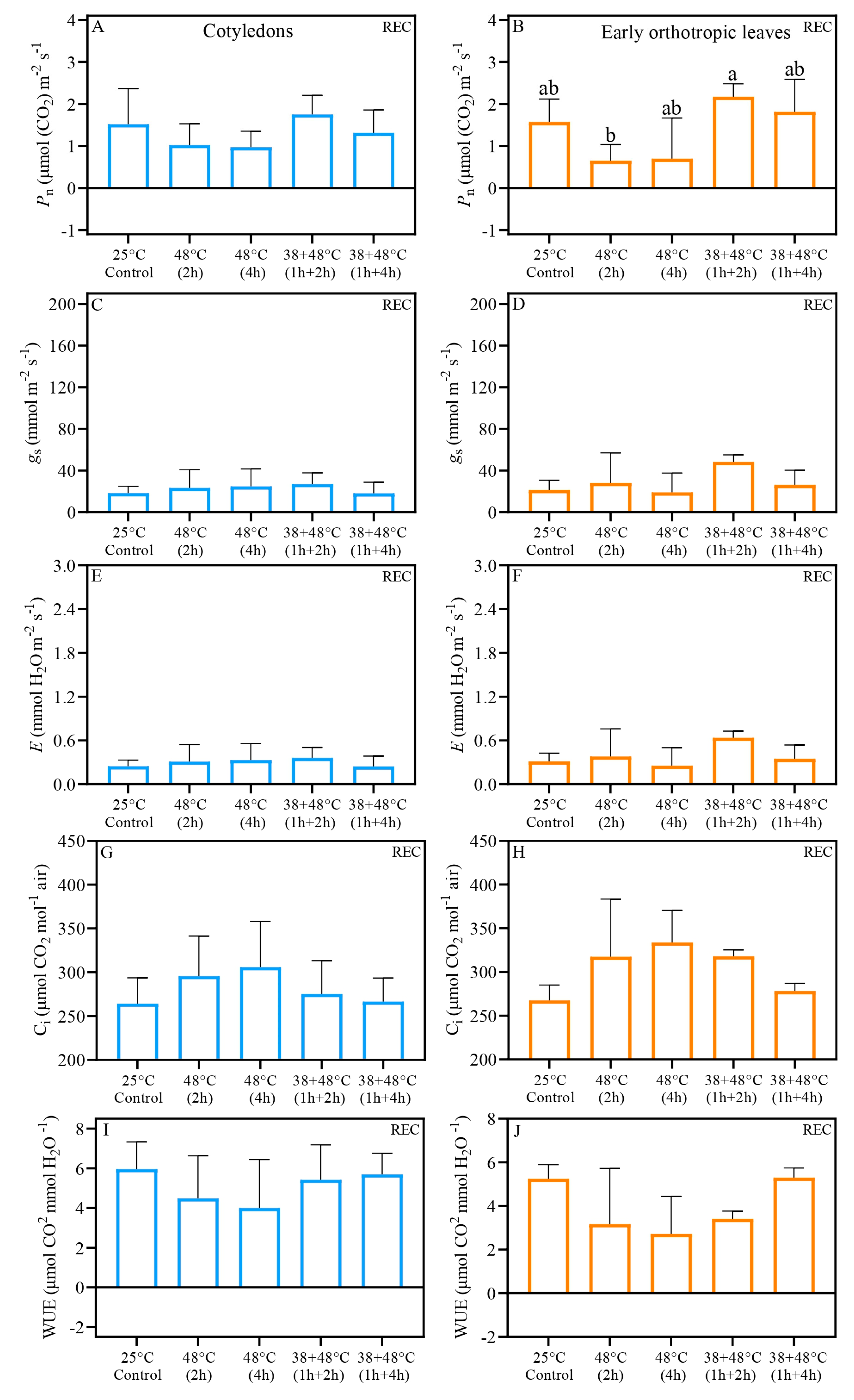
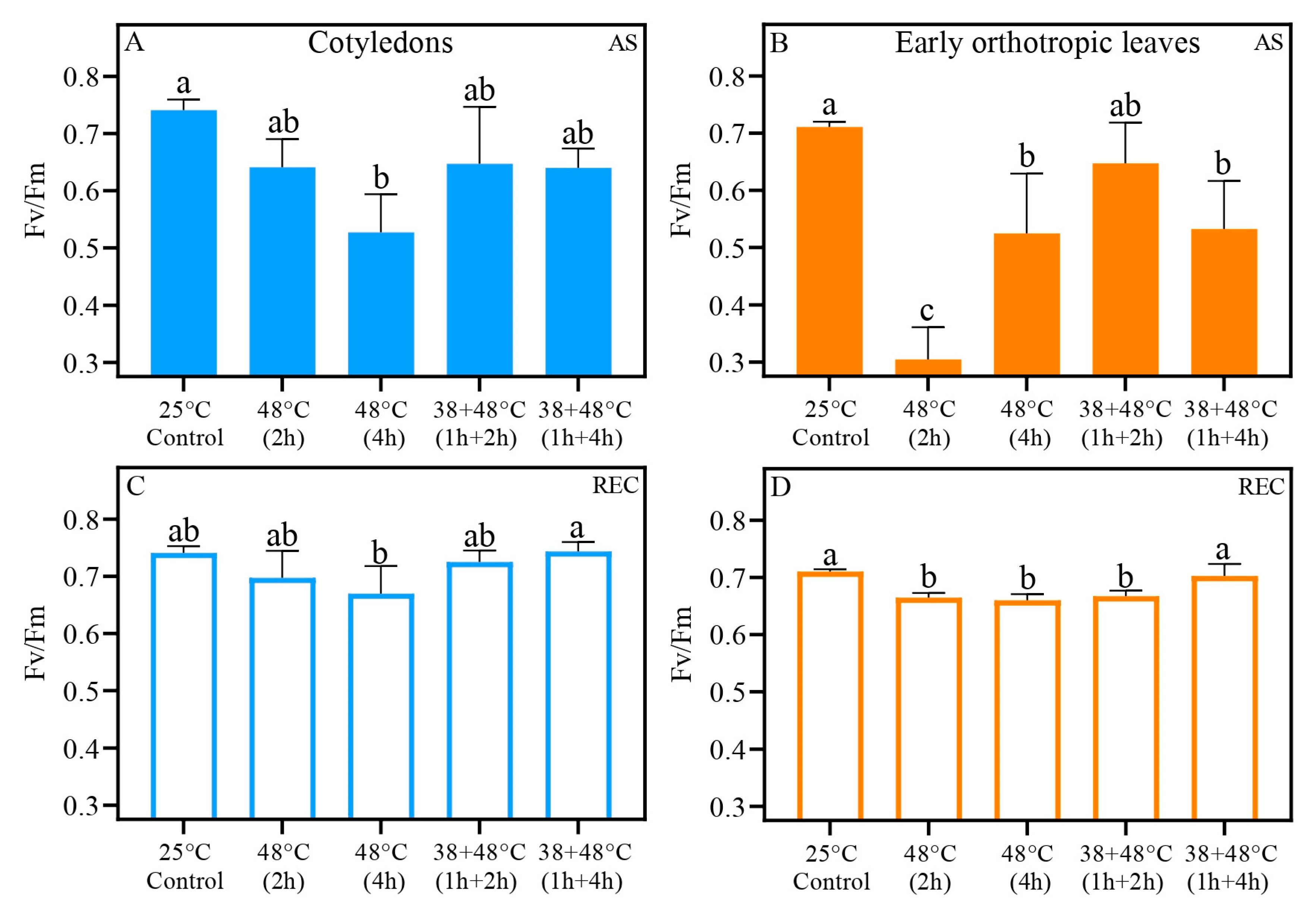
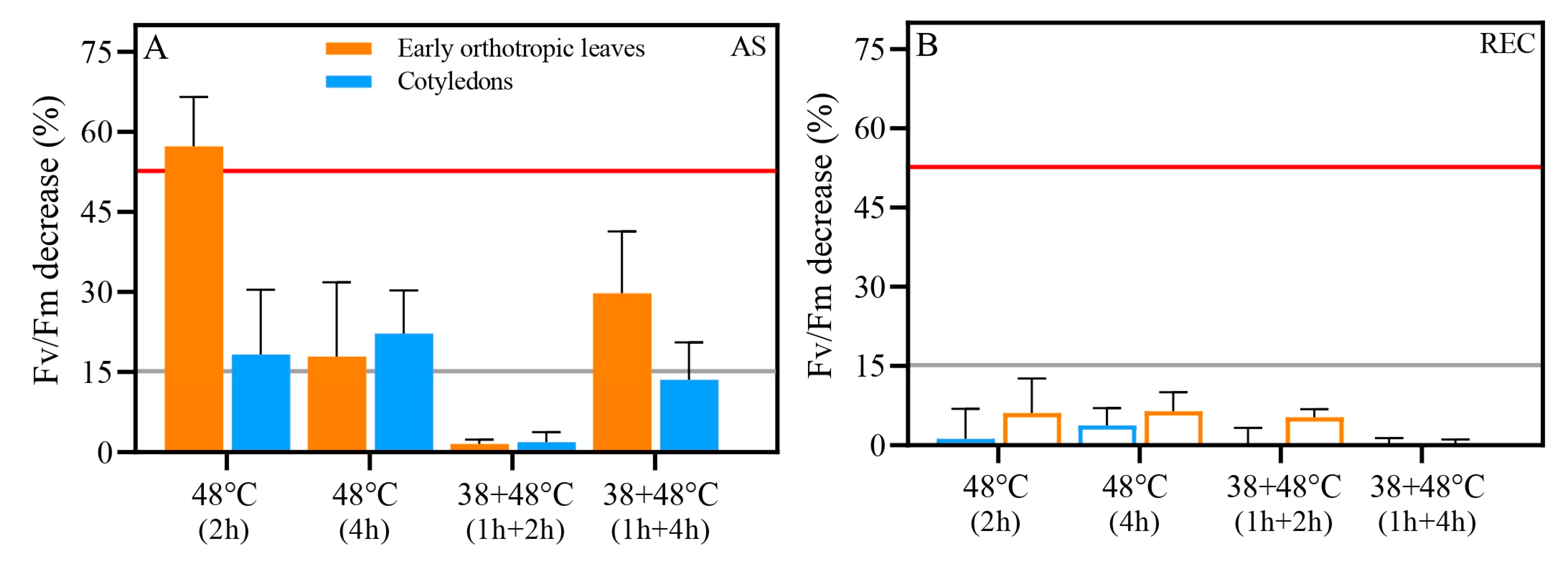
| Trait | Treatment | After Stress (AS) | Five Days After Stress (REC) | ||||
|---|---|---|---|---|---|---|---|
| Cotyledons | Orthotropic Leaves | p | Cotyledons | Orthotropic Leaves | p | ||
| Pn 1 | Control (25 °C) | 1.21 ± 0.29 | 1.39 ± 0.08 | 0.6261 | 1.52 ± 0.42 | 1.57 ± 0.27 | 0.8379 |
| 48 °C (2 h) | 0.95 ± 0.08 a | −0.60 ± 0.02 b | 0.0023 | 1.03 ± 0.25 | 0.65 ± 0.19 | 0.0758 | |
| 48 °C (4 h) | 0.76 ± 0.26 a | −0.38 ± 0.17 b | 0.0140 | 0.98 ± 0.19 | 0.70 ± 0.48 | 0.6545 | |
| 38 + 48 °C (1 h + 2 h) | 0.99 ± 0.24 | 0.71 ± 0.21 | 0.3985 | 1.76 ± 0.22 | 2.17 ± 0.15 | 0.1258 | |
| 38 + 48 °C (1 h + 4 h) | 2.36 ± 0.54 a | 1.22 ± 0.22 b | 0.0401 | 1.32 ± 0.27 | 1.81 ± 0.38 | 0.1424 | |
| gs 2 | Control (25 °C) | 14.4 ± 2.16 b | 22.2 ± 0.83 a | 0.0264 | 18.3 ± 3.26 | 21.3 ± 4.71 | 0.1884 |
| 48 °C (2 h) | 115 ± 7.18 | 120 ± 16.7 | 0.7973 | 23.4 ± 8.67 | 28.1 ± 14.4 | 0.5409 | |
| 48 °C (4 h) | 156 ± 7.65 | 141 ± 7.40 | 0.3502 | 24.8 ± 8.36 | 19.0 ± 9.27 | 0.7398 | |
| 38 + 48 °C (1 h + 2 h) | 154 ± 14.8 | 159 ± 13.2 | 0.7028 | 27.0 ± 5.34 b | 48.6 ± 3.42 a | 0.0149 | |
| 38 + 48 °C (1 h + 4 h) | 110 ± 12.6 | 122 ± 2.55 | 0.4574 | 18.2 ± 5.35 | 26.2 ± 7.08 | 0.2187 | |
| E 3 | Control (25 °C) | 0.19 ± 0.03 b | 0.30 ± 0.01 a | 0.0254 | 0.25 ± 0.04 | 0.31 ± 0.05 | 0.1078 |
| 48 °C (2 h) | 1.52 ± 0.09 | 1.59 ± 0.22 | 0.7816 | 0.31 ± 0.11 | 0.38 ± 0.19 | 0.4876 | |
| 48 °C (4 h) | 2.05 ± 0.09 | 1.85 ± 0.09 | 0.3385 | 0.33 ± 0.11 | 0.25 ± 0.12 | 0.7378 | |
| 38 + 48 °C (1 h + 2 h) | 2.01 ± 0.18 | 2.07 ± 0.16 | 0.7095 | 0.36 ± 0.07 b | 0.64 ± 0.04 a | 0.0159 | |
| 38 + 48 °C (1 h + 4 h) | 1.45 ± 0.16 | 1.60 ± 0.03 | 0.4472 | 0.24 ± 0.07 | 0.35 ± 0.09 | 0.2246 | |
| Ci 4 | Control (25 °C) | 272 ± 8.03 | 290 ± 6.87 | 0.2935 | 264 ± 14.6 | 267 ± 8.83 | 0.8890 |
| 48 °C (2 h) | 384 ± 5.81 b | 401 ± 5.56 a | 0.0002 | 295 ± 22.8 | 317 ± 33.0 | 0.5124 | |
| 48 °C (4 h) | 383 ± 2.68 | 387 ± 8.67 | 0.6432 | 306 ± 25.9 | 333 ± 18.4 | 0.2206 | |
| 38 + 48 °C (1 h + 2 h) | 379 ± 3.57 | 383 ± 2.92 | 0.4406 | 275 ± 19.0 | 318 ± 3.67 | 0.1465 | |
| 38 + 48 °C (1 h + 4 h) | 357 ± 4.52 b | 374 ± 3.30 a | 0.0011 | 266 ± 13.5 | 278 ± 4.39 | 0.4205 | |
| WUE 5 | Control (25 °C) | 6.32 ± 1.05 | 4.76 ± 0.32 | 0.3052 | 5.97 ± 0.68 | 5.26 ± 0.32 | 0.4835 |
| 48 °C (2 h) | 0.50 ± 0.19 a | −0.48 ± 0.26 b | 0.0013 | 4.49 ± 1.07 | 3.17 ± 1.28 | 0.3252 | |
| 48 °C (4 h) | 0.36 ± 0.12 a | −0.22 ± 0.10 b | 0.0148 | 4.01 ± 1.21 | 2.72 ± 0.86 | 0.2375 | |
| 38 + 48 °C (1 h + 2 h) | 0.53 ± 0.16 | 0.37 ± 0.13 | 0.4415 | 5.42 ± 0.88 | 3.41 ± 0.17 | 0.1443 | |
| 38 + 48 °C (1 h + 4 h) | 1.52 ± 0.19 a | 0.76 ± 0.15 b | 0.0006 | 5.70 ± 0.53 | 5.30 ± 0.22 | 0.5070 | |
| Trait | Treatment | After Stress (AS) | Five Days After Stress (REC) | ||||
|---|---|---|---|---|---|---|---|
| Cotyledons | Orthotropic Leaves | p Value | Cotyledons | Orthotropic Leaves | p Value | ||
| Fv/Fm | Control (25 °C) | 0.74 ± 0.01 a | 0.71 ± 0.00 b | 0.0434 | 0.74 ± 0.01 a | 0.71 ± 0.01 b | 0.0164 |
| 48 °C (2 h) | 0.64 ± 0.05 a | 0.30 ± 0.05 b | 0.0030 | 0.69 ± 0.01 | 0.67 ± 0.04 | 0.2662 | |
| 48 °C (4 h) | 0.53 ± 0.06 | 0.52 ± 0.10 | 0.9460 | 0.66 ± 0.01 | 0.67 ± 0.04 | 0.6788 | |
| 38 + 48 °C (1 h + 2 h) | 0.64 ± 0.10 | 0.64 ± 0.07 | 0.5973 | 0.73 ± 0.02 a | 0.67 ± 0.01 b | 0.0061 | |
| 38 + 48 °C (1 h + 4 h) | 0.64 ± 0.03 a | 0.53 ± 0.08 b | 0.0361 | 0.74 ± 0.01 a | 0.70 ± 0.02 b | 0.0222 | |
| T15 (°C) | Control (25 °C) | 43.7 ± 0.30 a | 41.8 ± 0.30 b | 0.0101 | 42.5 ± 1.11 | 42.9 ± 1.32 | 0.7176 |
| 48 °C (2 h) | * | * | 0.6823 | 46.7 ± 3.26 | 42.8 ± 2.10 | 0.3065 | |
| 48 °C (4 h) | * | * | 0.6252 | 42.8 ± 1.05 | 41.3 ± 1.15 | 0.2805 | |
| 38 + 48 °C (1 h + 2 h) | 45.7 ± 1.11 a | 43.0 ± 0.60 b | 0.0303 | 46.8 ± 1.10 | 46.3 ± 1.22 | 0.4483 | |
| 38 + 48 °C (1 h + 4 h) | * | * | 0.3484 | 47.9 ± 0.90 | 44.6 ± 1.74 | 0.1343 | |
| T50 (°C) | Control (25 °C) | 49.9 ± 1.10 | 49.3 ± 0.50 | 0.2926 | 50.1 ± 0.38 | 49.5 ± 0.77 | 0.3104 |
| 48 °C (2 h) | 53.1 ± 0.74 | * | 0.4472 | 51.4 ± 0.94 a | 48.7 ± 0.83 b | 0.0071 | |
| 48 °C (4 h) | 50.2 ± 0.51 | 49.2 ± 4.15 | 0.6232 | 51.1 ± 0.46 | 49.8 ± 1.40 | 0.1612 | |
| 38 + 48 °C (1 h + 2 h) | 54.3 ± 1.31 a | 51.7 ± 0.71 b | 0.0135 | 52.6 ± 0.46 | 52.1 ± 0.90 | 0.6200 | |
| 38 + 48 °C (1 h + 4 h) | 51.2 ± 1.24 | 49.9 ± 3.54 | 0.1338 | 54.3 ± 0.52 a | 51.5 ± 1.29 | 0.0136 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilas-Boas, T.; Della Torre, F.; Dalton, Z.; Lovato, M.B.; de Lemos-Filho, J.P.; Waters, E.R. Short-Term Heat Stress Differentially Affects the Photosynthetic Thermotolerance of Cotyledons and Early Orthotropic Leaves in Coffea arabica L. Seedlings. Biology 2025, 14, 1659. https://doi.org/10.3390/biology14121659
Vilas-Boas T, Della Torre F, Dalton Z, Lovato MB, de Lemos-Filho JP, Waters ER. Short-Term Heat Stress Differentially Affects the Photosynthetic Thermotolerance of Cotyledons and Early Orthotropic Leaves in Coffea arabica L. Seedlings. Biology. 2025; 14(12):1659. https://doi.org/10.3390/biology14121659
Chicago/Turabian StyleVilas-Boas, Tiago, Felipe Della Torre, Zachary Dalton, Maria Bernadete Lovato, José Pires de Lemos-Filho, and Elizabeth R. Waters. 2025. "Short-Term Heat Stress Differentially Affects the Photosynthetic Thermotolerance of Cotyledons and Early Orthotropic Leaves in Coffea arabica L. Seedlings" Biology 14, no. 12: 1659. https://doi.org/10.3390/biology14121659
APA StyleVilas-Boas, T., Della Torre, F., Dalton, Z., Lovato, M. B., de Lemos-Filho, J. P., & Waters, E. R. (2025). Short-Term Heat Stress Differentially Affects the Photosynthetic Thermotolerance of Cotyledons and Early Orthotropic Leaves in Coffea arabica L. Seedlings. Biology, 14(12), 1659. https://doi.org/10.3390/biology14121659





