Decoding Potential Cuproptosis-Related Genes in Sarcopenia: A Multi-Omics Network Analysis
Simple Summary
Abstract
1. Introduction
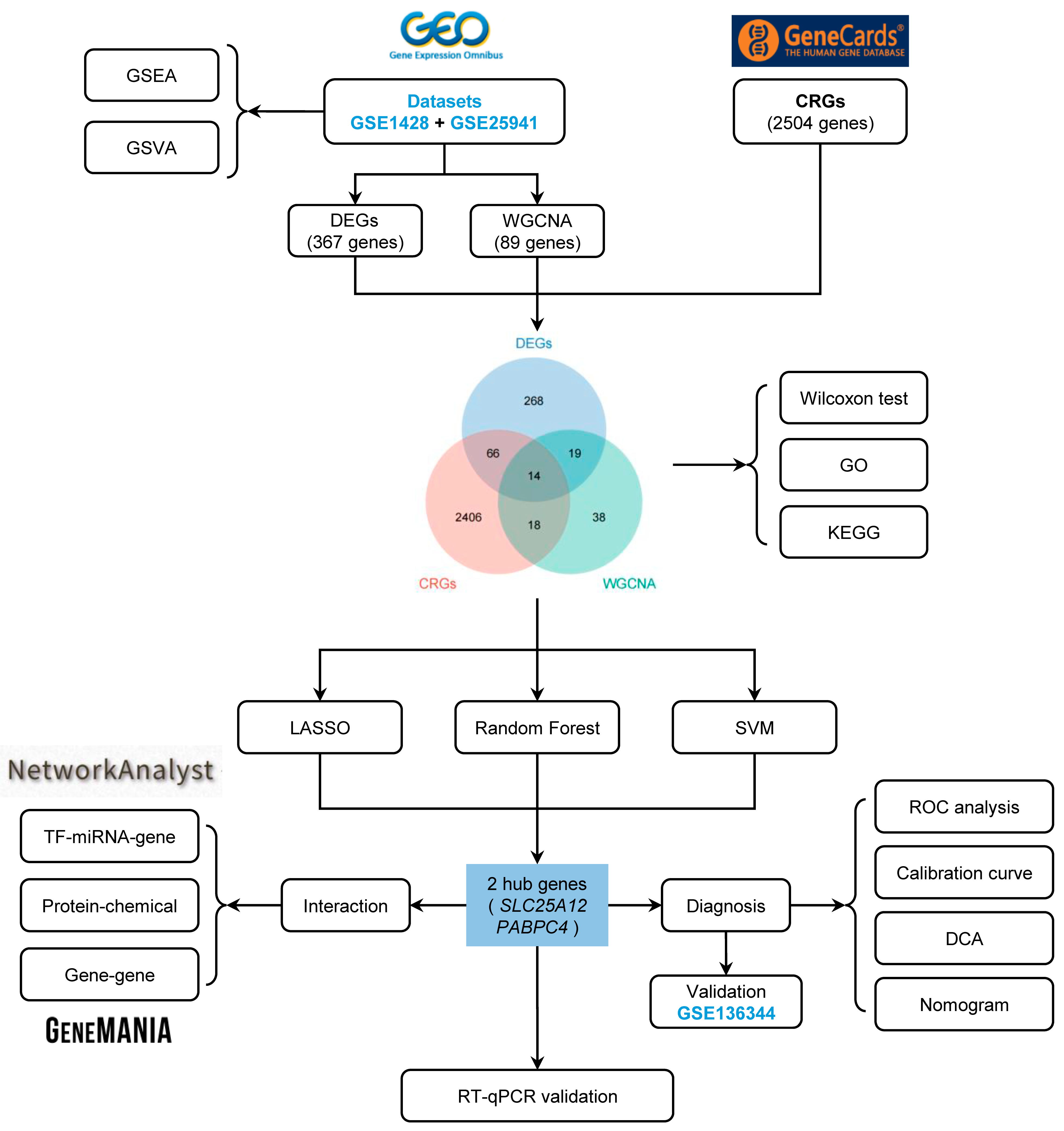
2. Materials and Methods
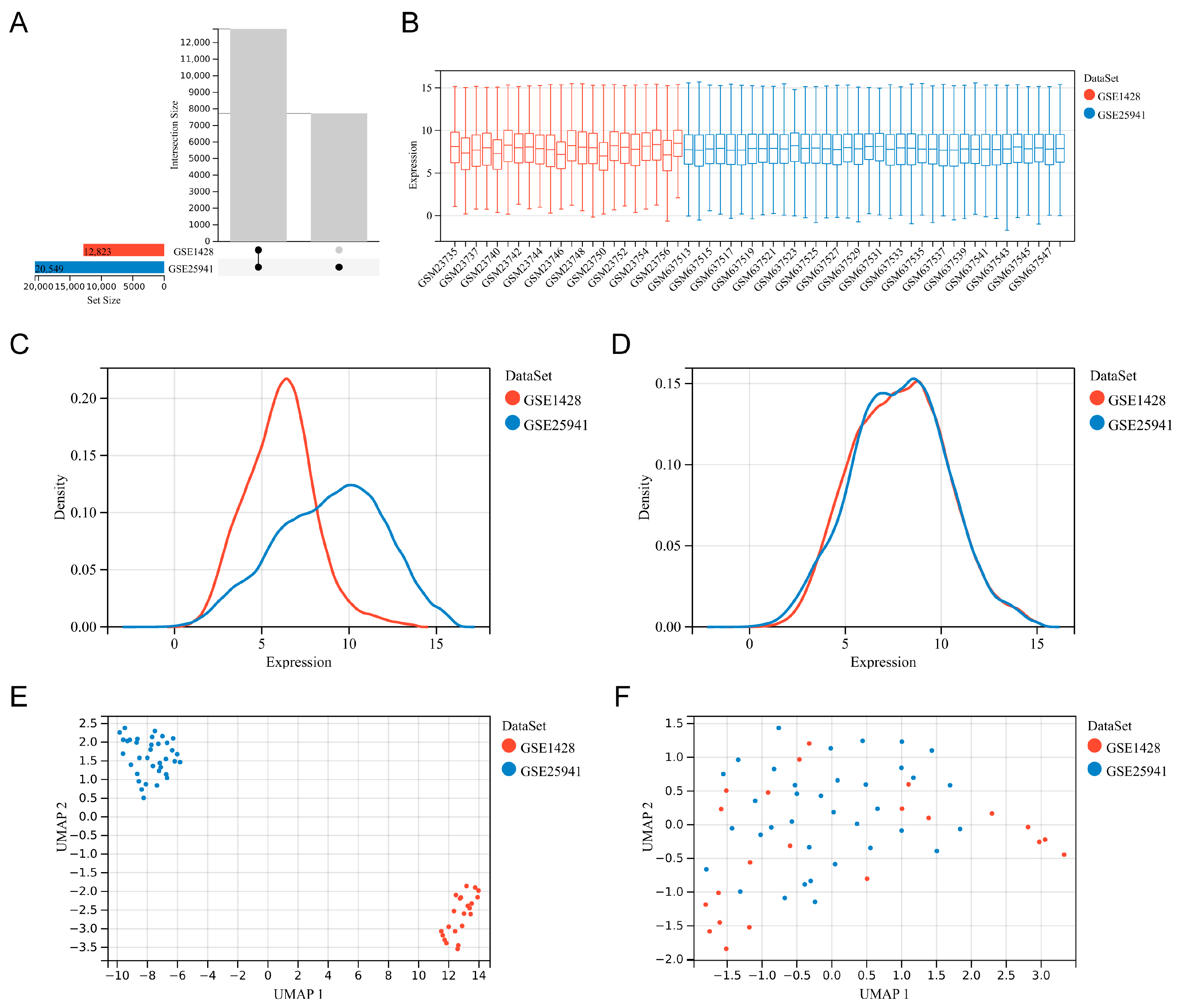
2.1. Data Collection and Preprocessing
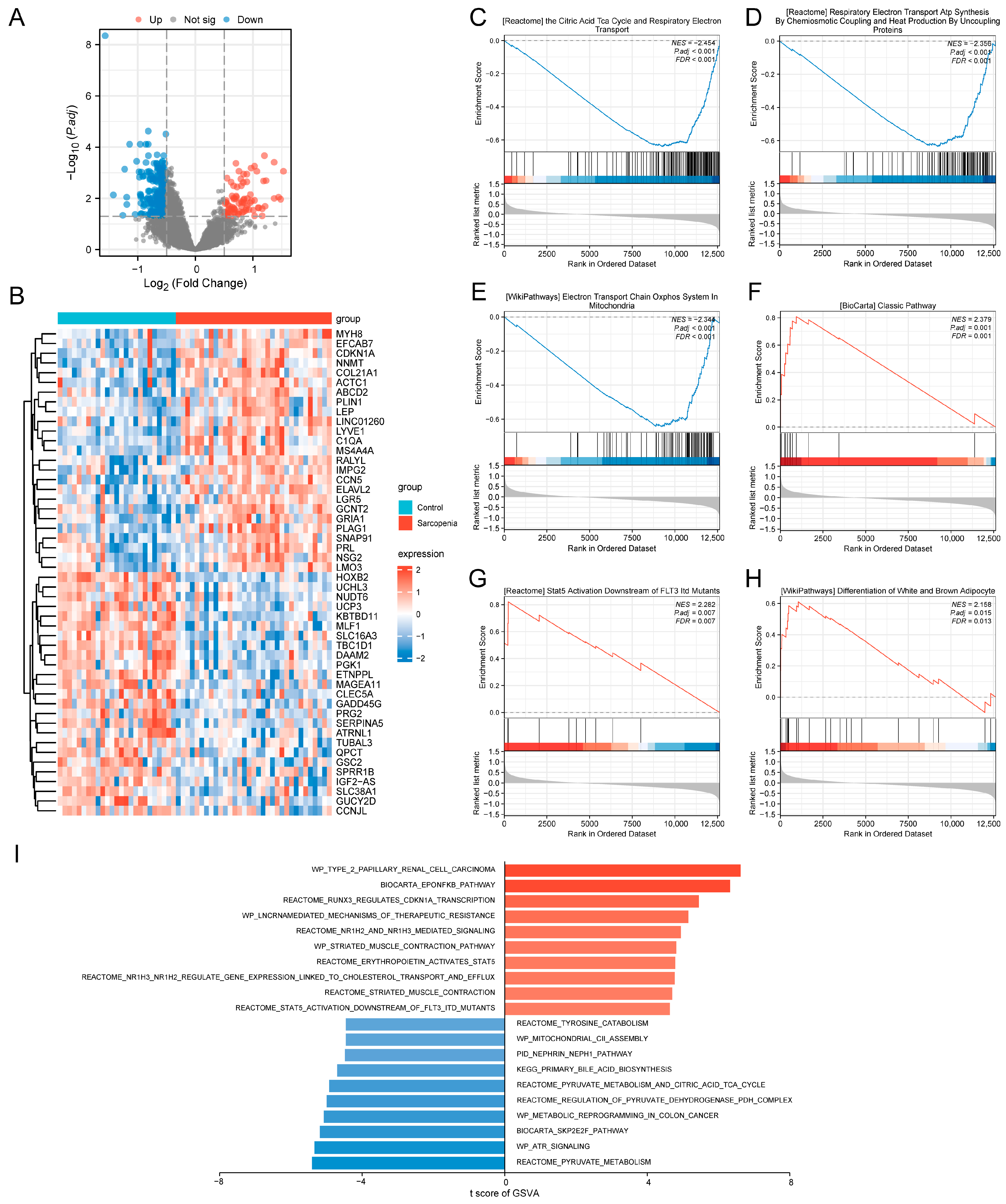
2.2. Differential Expression Analysis
2.3. Gene Set Enrichment Analysis (GSEA)
2.4. Gene Set Variation Analysis (GSVA)
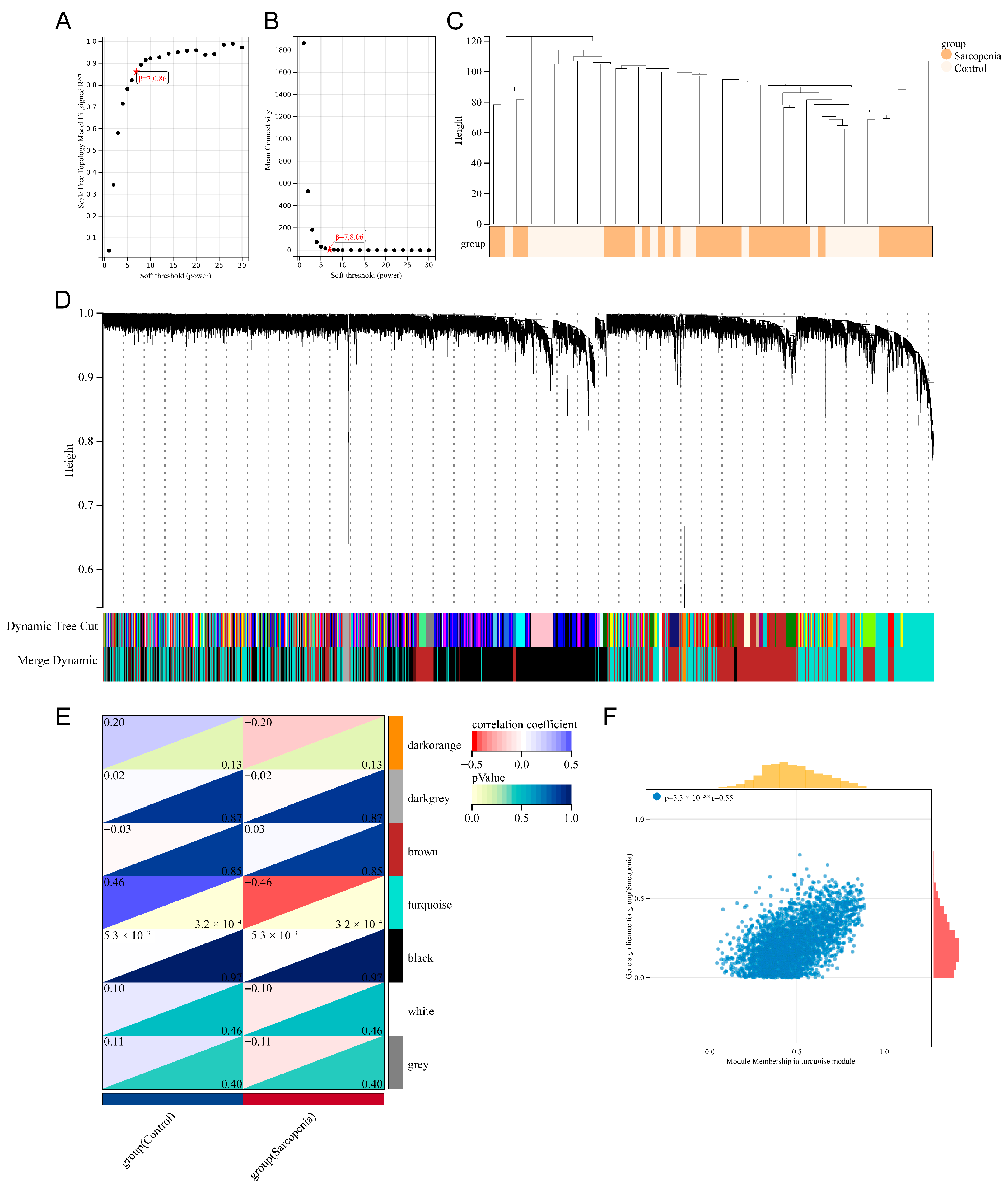
2.5. Weighted Gene Co-Expression Network Analysis (WGCNA)
2.6. Identification and Functional Analysis of SAR-CUP DEGs
2.7. Machine Learning for the Selection of the Hub Genes
2.8. Construction of a Regulatory Network
2.9. Construction of a Gene-Gene Interaction Network of the SAR-CUP DEGs
2.10. Construction and Validation of a Diagnostic Model
2.11. Cell Culture
2.12. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.13. Statistical Analysis
3. Results
3.1. Data Collection and Preprocessing
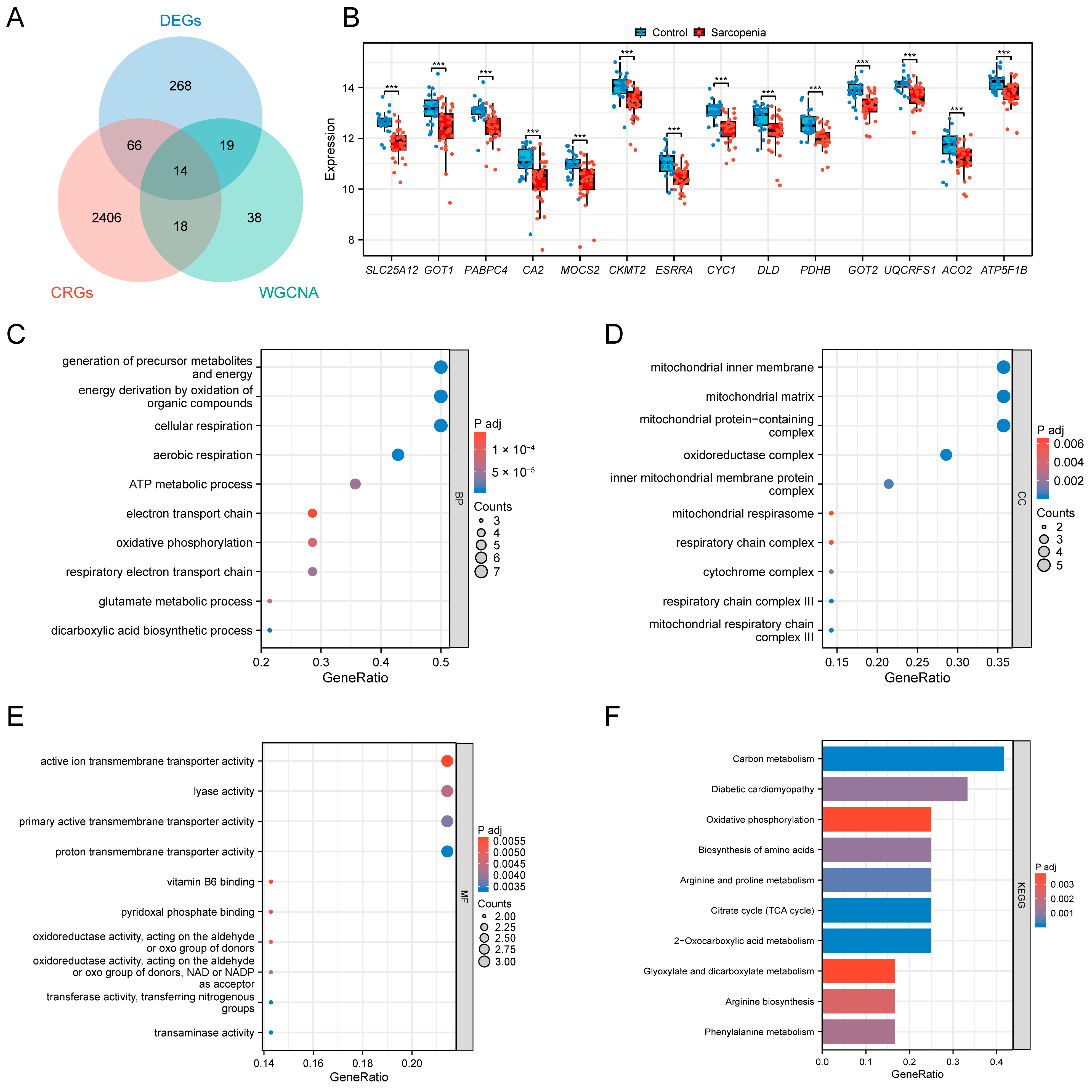
3.2. Identification of the DEGs and Functional Analysis of the Training Set
3.3. Identification of the Key Module Genes by WGCNA
3.4. Selection and Functional Analysis of the SAR-CUP DEGs
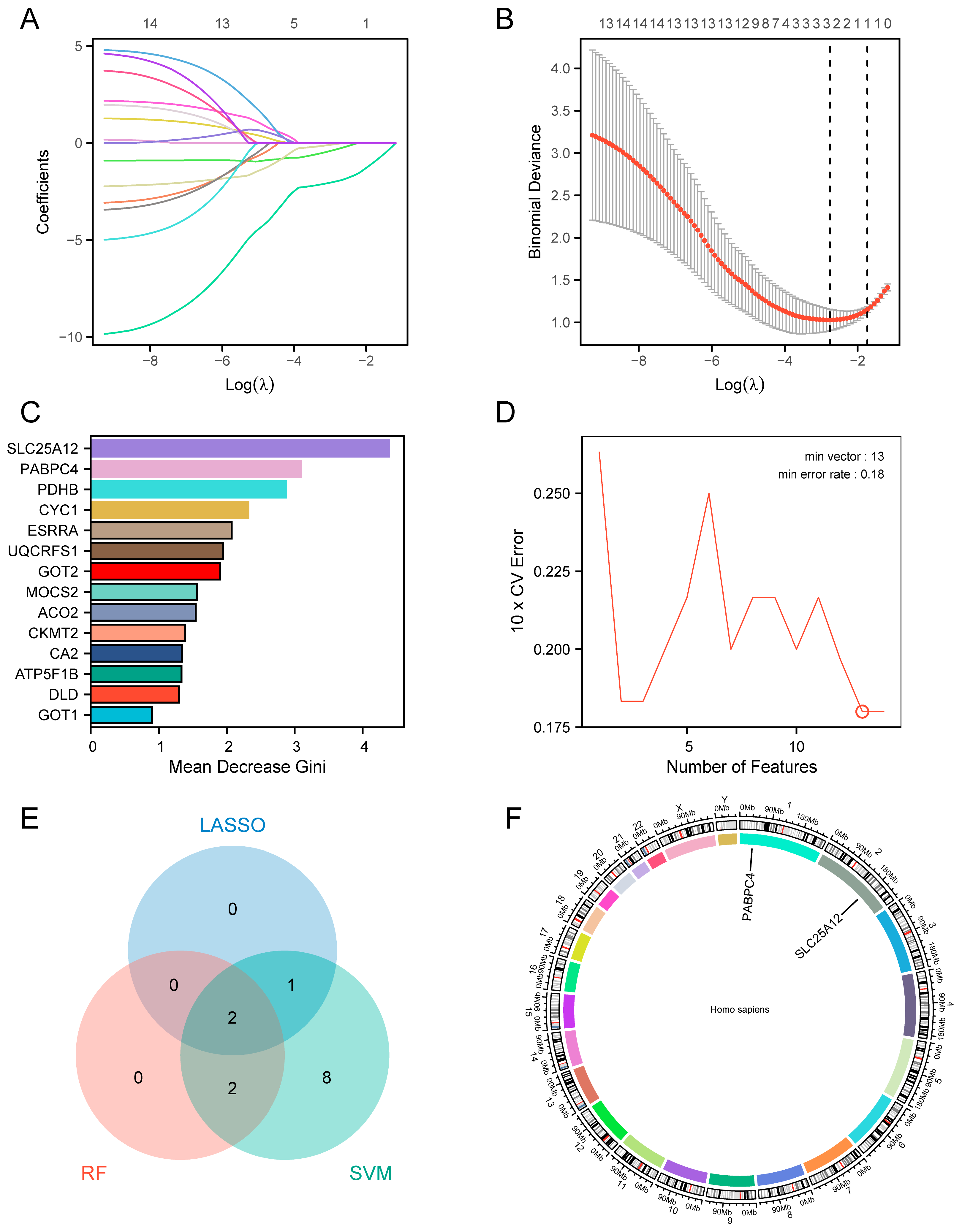
3.5. Identification of the Hub Genes by Machine Learning
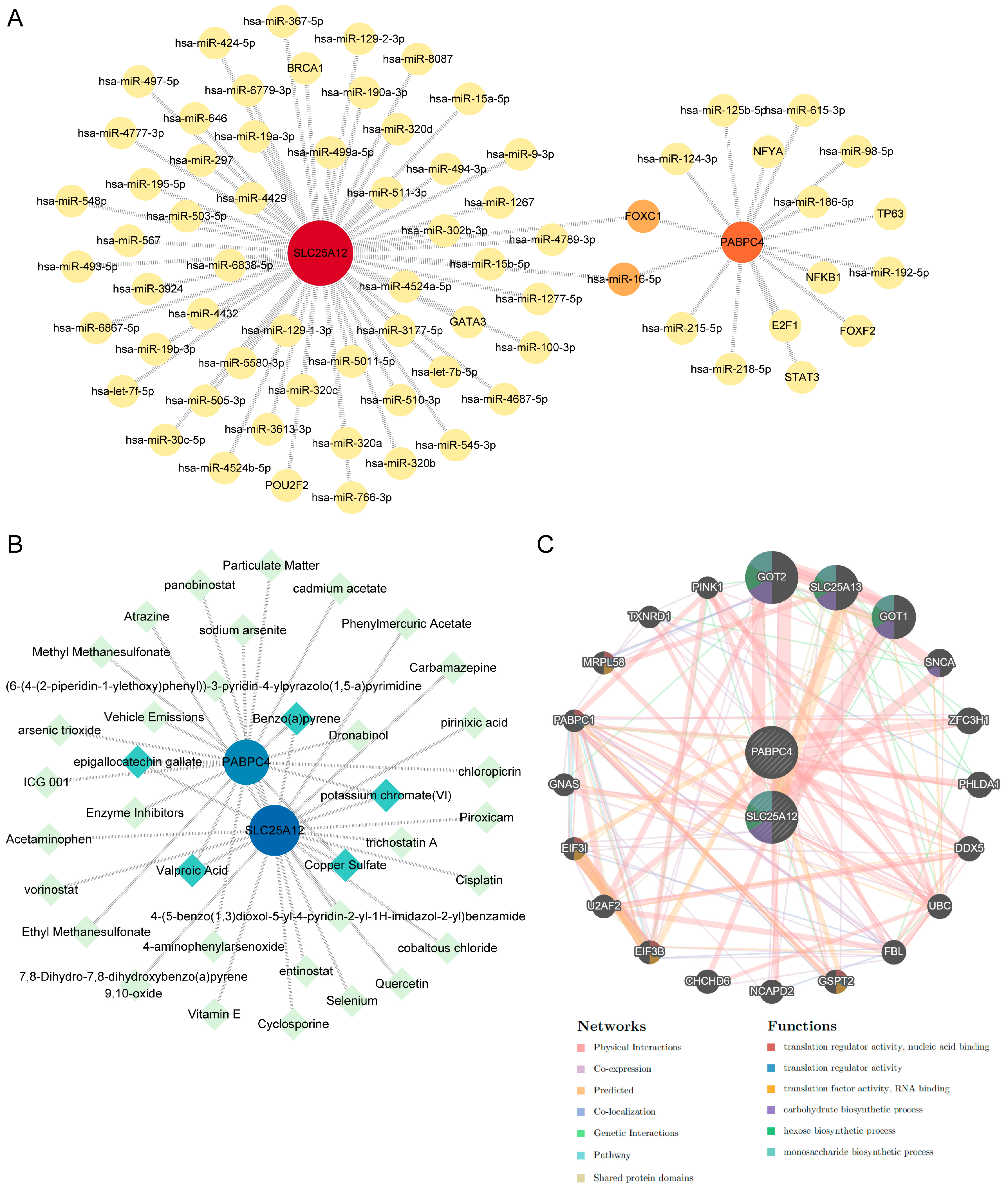
3.6. Construction of the TF-miRNA-Gene, Protein-Chemical, and Gene-Gene Interaction Network
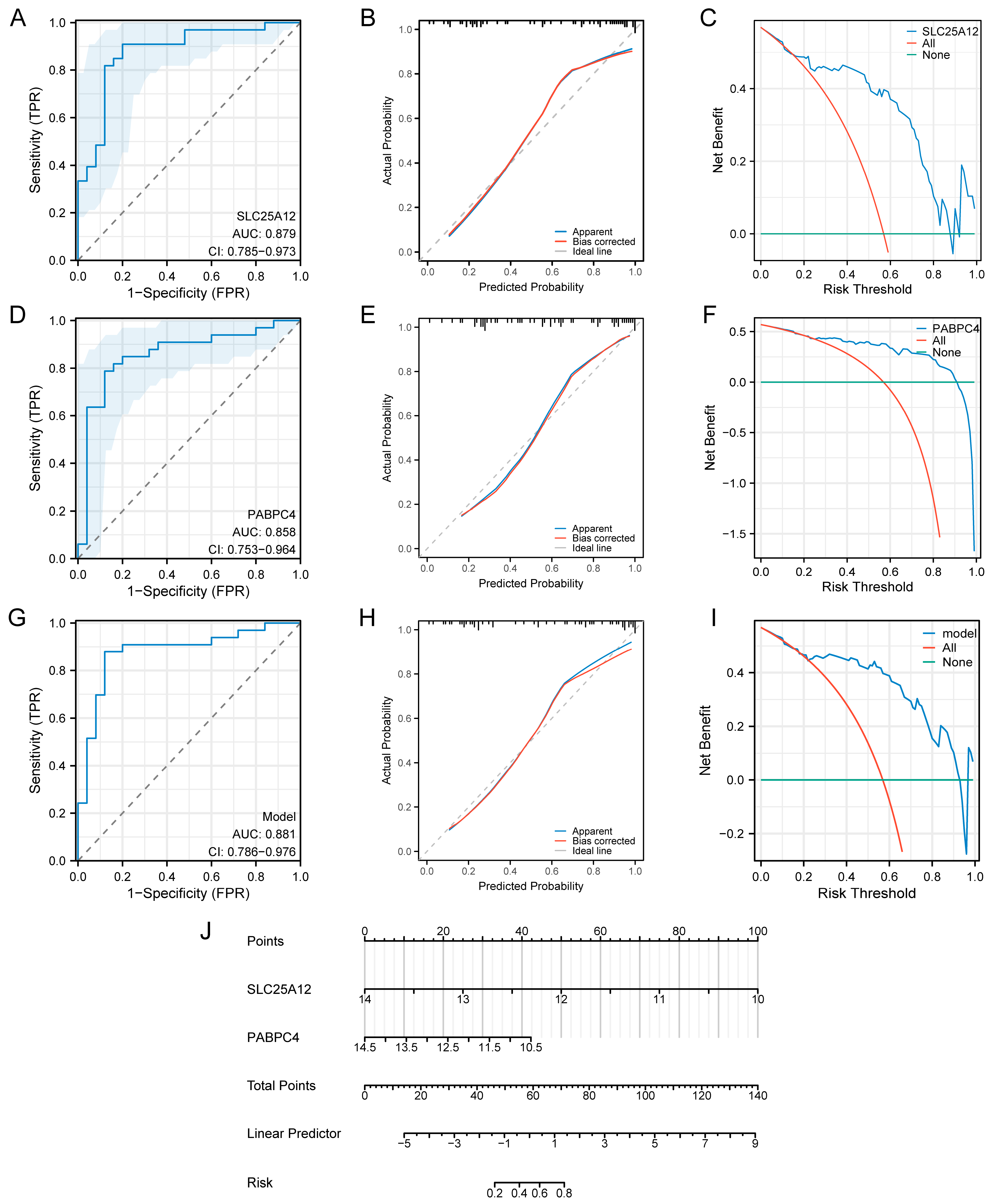
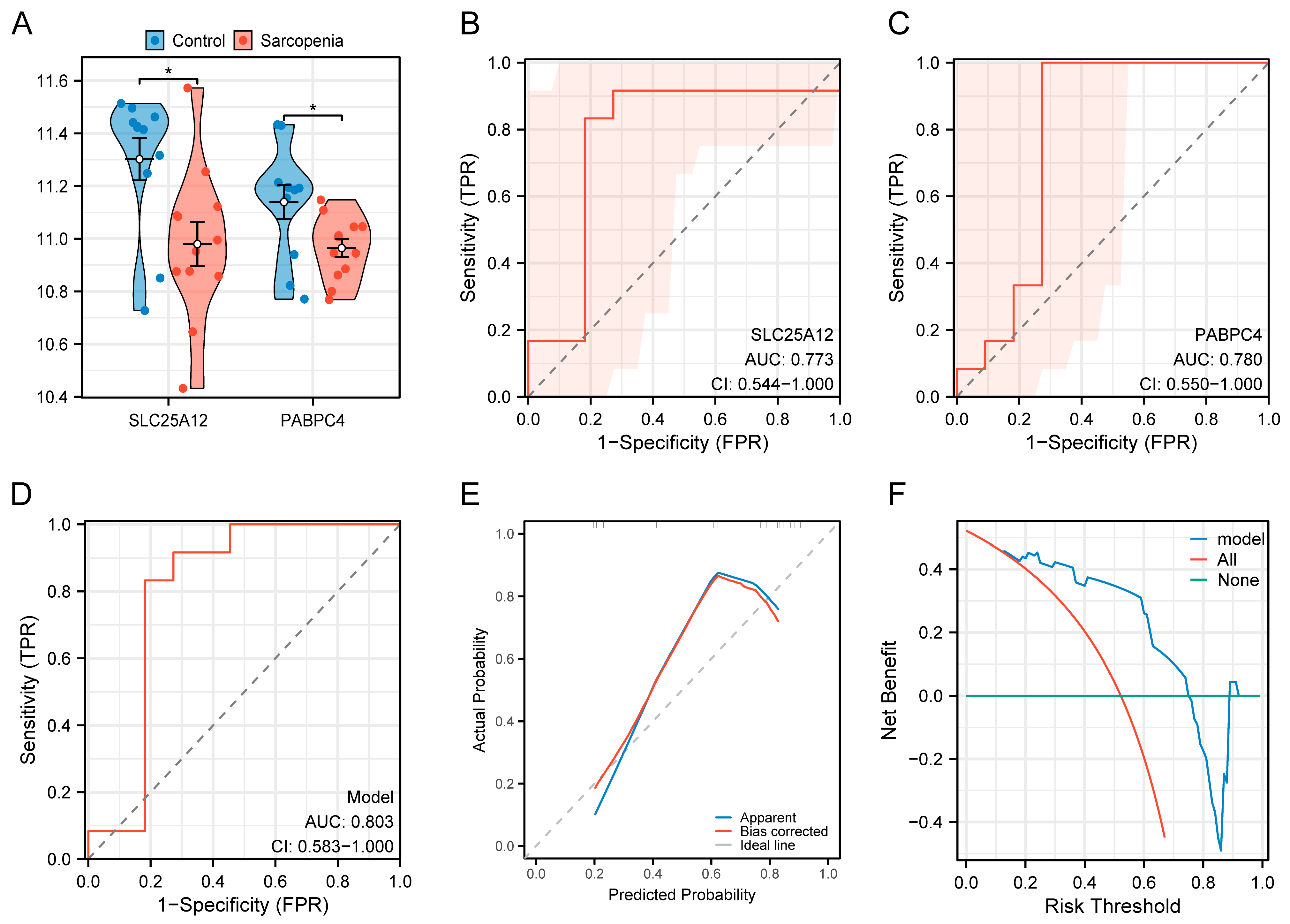
3.7. Construction of the Diagnostic Model
3.8. Validation of the Diagnostic Model
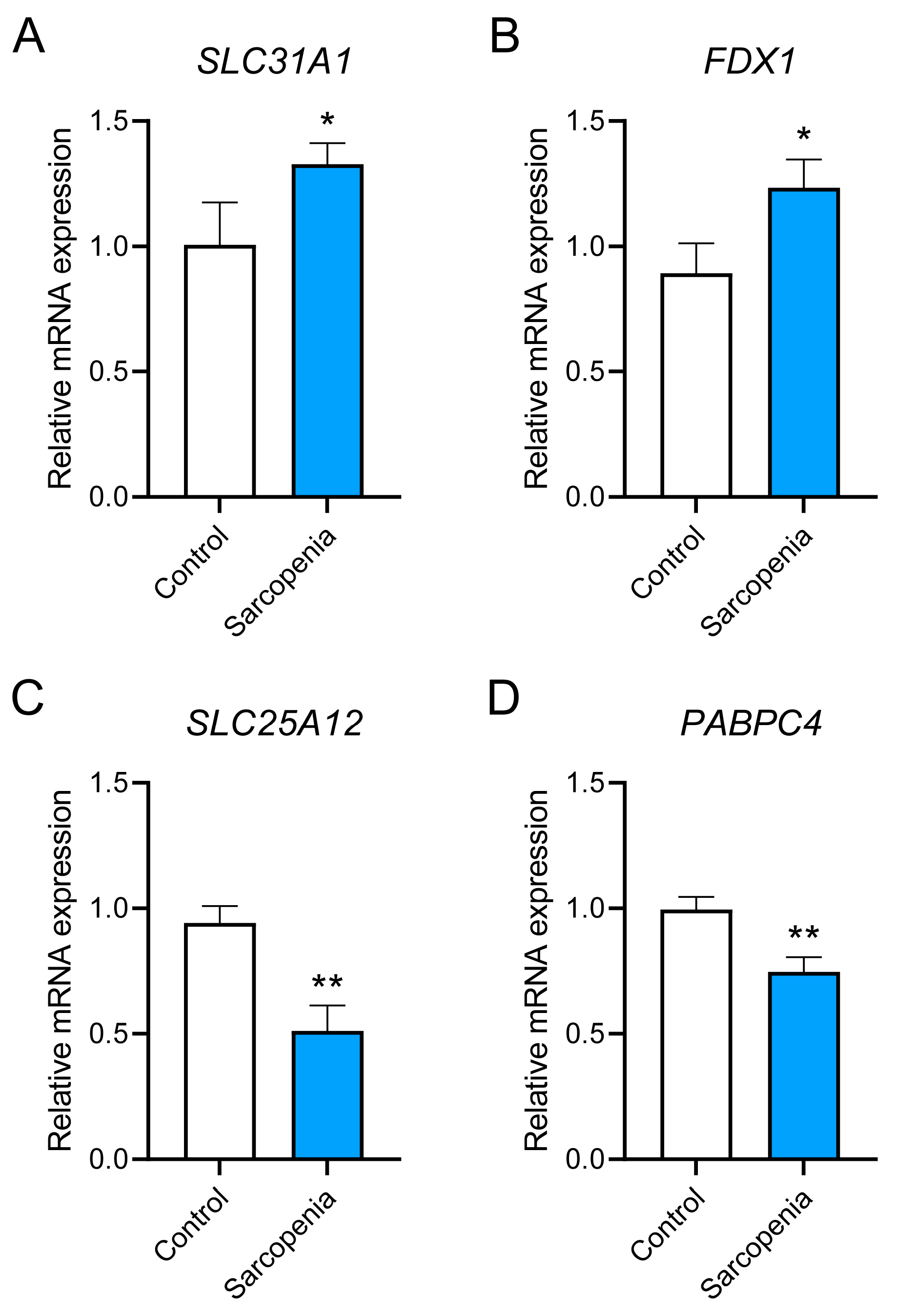
3.9. Preliminary in Vitro Validation of the Hub Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cooper, R.; Arai, H.; Cawthon, P.M.; Ntsama Essomba, M.-J.; Fielding, R.A.; Grounds, M.D.; Witham, M.D.; Cruz-Jentoft, A.J. Sarcopenia. Nat. Rev. Dis. Primers 2024, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Feng, L.; Gao, Q.; Hu, K.; Wu, M.; Wang, Z.; Chen, F.; Mei, F.; Zhao, L.; Ma, B. Prevalence and Risk Factors of Sarcopenia in Patients with Diabetes: A Meta-analysis. J. Clin. Endocrinol. Metab. 2022, 107, 1470–1483. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Hu, K.; Yan, C.; Zhao, B.; Mei, F.; Chen, F.; Zhao, L.; Shang, Y.; Ma, Y.; Ma, B. Associated Factors of Sarcopenia in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4291. [Google Scholar] [CrossRef]
- Amini, N.; Ibn Hach, M.; Lapauw, L.; Dupont, J.; Vercauteren, L.; Verschueren, S.; Tournoy, J.; Gielen, E. Meta-analysis on the interrelationship between sarcopenia and mild cognitive impairment, Alzheimer’s disease and other forms of dementia. J. Cachexia Sarcopenia Muscle 2024, 15, 1240–1253. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Rolland, Y.; Dray, C.; Vellas, B.; Barreto, P.D.S. Current and investigational medications for the treatment of sarcopenia. Metabolism 2023, 149, 155597. [Google Scholar] [CrossRef] [PubMed]
- Coletta, G.; Phillips, S.M. An elusive consensus definition of sarcopenia impedes research and clinical treatment: A narrative review. Ageing Res. Rev. 2023, 86, 101883. [Google Scholar] [CrossRef]
- McCann, C.; Quinteros, M.; Adelugba, I.; Morgada, M.N.; Castelblanco, A.R.; Davis, E.J.; Lanzirotti, A.; Hainer, S.J.; Vila, A.J.; Navea, J.G.; et al. The mitochondrial Cu+ transporter PiC2 (SLC25A3) is a target of MTF1 and contributes to the development of skeletal muscle in vitro. Front. Mol. Biosci. 2022, 9, 1037941. [Google Scholar] [CrossRef]
- Vest, K.E.; Paskavitz, A.L.; Lee, J.B.; Padilla-Benavides, T. Dynamic changes in copper homeostasis and post-transcriptional regulation of Atp7a during myogenic differentiation. Metallomics 2018, 10, 309–322. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, G.; Wang, K.; Yang, J.; Shen, Y.; Yang, X.; Chen, X.; Yao, X.; Gu, X.; Qi, L.; et al. Oxidative stress: Roles in skeletal muscle atrophy. Biochem. Pharmacol. 2023, 214, 115664. [Google Scholar] [CrossRef]
- Pan, L.; Xie, W.; Fu, X.; Lu, W.; Jin, H.; Lai, J.; Zhang, A.; Yu, Y.; Li, Y.; Xiao, W. Inflammation and sarcopenia: A focus on circulating inflammatory cytokines. Exp. Gerontol. 2021, 154, 111544. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Masaldan, S.; Clatworthy, S.A.S.; Gamell, C.; Smith, Z.M.; Francis, P.S.; Denoyer, D.; Meggyesy, P.M.; Fontaine, S.L.; Cater, M.A. Copper accumulation in senescent cells: Interplay between copper transporters and impaired autophagy. Redox Biol. 2018, 16, 322–331. [Google Scholar] [CrossRef]
- Yang, L.; Xie, L.; Li, M.; Miao, Y.; Yang, J.; Chen, S.; Ma, X.; Xie, P. Potential relationship between cuproptosis and sepsis-acquired weakness: An intermediate role for mitochondria. Front. Physiol. 2025, 16, 1520669. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, J.; Liu, N.; Meng, X. Association of Serum Concentrations of Copper, Selenium, and Zinc with Grip Strength Based on NHANES 2013-2014. Biol. Trace Elem. Res. 2024, 202, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, D.; Liu, S.; Yao, N.; Gui, Z.; Huang, Z.; Zeng, Y.; Liu, L.; Wan, H.; Shen, J. Serum essential elements and muscle health in Chinese adults: A community-based study. Nutr. Metab. 2025, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Giresi, P.G.; Stevenson, E.J.; Theilhaber, J.; Koncarevic, A.; Parkington, J.; Fielding, R.A.; Kandarian, S.C. Identification of a molecular signature of sarcopenia. Physiol. Genom. 2005, 21, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Raue, U.; Trappe, T.A.; Estrem, S.T.; Qian, H.-R.; Helvering, L.M.; Smith, R.C.; Trappe, S. Transcriptome signature of resistance exercise adaptations: Mixed muscle and fiber type specific profiles in young and old adults. J. Appl. Physiol. 2012, 112, 1625–1636. [Google Scholar] [CrossRef]
- Gueugneau, M.; Coudy-Gandilhon, C.; Chambon, C.; Verney, J.; Taillandier, D.; Combaret, L.; Polge, C.; Walrand, S.; Roche, F.; Barthélémy, J.-C.; et al. Muscle Proteomic and Transcriptomic Profiling of Healthy Aging and Metabolic Syndrome in Men. Int. J. Mol. Sci. 2021, 22, 4205. [Google Scholar] [CrossRef]
- Taminau, J.; Meganck, S.; Lazar, C.; Steenhoff, D.; Coletta, A.; Molter, C.; Duque, R.; de Schaetzen, V.; Weiss Solís, D.Y.; Bersini, H.; et al. Unlocking the potential of publicly available microarray data using inSilicoDb and inSilicoMerging R/Bioconductor packages. BMC Bioinform. 2012, 13, 335. [Google Scholar] [CrossRef]
- Johnson, W.E.; Li, C.; Rabinovic, A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007, 8, 118–127. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, E.K.; Zhang, D.; Reynolds, R.H.; Garcia-Ruiz, S.; Ryten, M. ggtranscript: An R package for the visualization and interpretation of transcript isoforms using ggplot2. Bioinformatics 2022, 38, 3844–3846. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z. Complex heatmap visualization. Imeta 2022, 1, e43. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef]
- Li, Y.; Ge, X.; Peng, F.; Li, W.; Li, J.J. Exaggerated false positives by popular differential expression methods when analyzing human population samples. Genome Biol. 2022, 23, 79. [Google Scholar] [CrossRef]
- Engebretsen, S.; Bohlin, J. Statistical predictions with glmnet. Clin. Epigenetics 2019, 11, 123. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, H.; Jiang, X.; Zou, R.; Wang, H.; Fan, Z. Unveiling the key genes, environmental toxins, and drug exposures in modulating the severity of ulcerative colitis: A comprehensive analysis. Front. Immunol. 2023, 14, 1162458. [Google Scholar] [CrossRef]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. circlize Implements and enhances circular visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef] [PubMed]
- MFranz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, 46, W60–W64. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Qi, Y.; Yao, Q.; Li, X.; Li, X.; Zhang, W.; Qu, P. Cuproptosis-related gene SLC31A1: Prognosis values and potential biological functions in cancer. Sci. Rep. 2023, 13, 17790. [Google Scholar] [CrossRef]
- Lu, J.; Ling, X.; Sun, Y.; Liu, L.; Liu, L.; Wang, X.; Lu, C.; Ren, C.; Han, X.; Yu, Z. FDX1 enhances endometriosis cell cuproptosis via G6PD-mediated redox homeostasis. Apoptosis 2023, 28, 1128–1140. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Dou, Q.; Zhang, W.; Yang, Y.; Xie, X. Sarcopenia as a predictor of all-cause mortality among older nursing home residents: A systematic review and meta-analysis. BMJ Open 2018, 8, e021252. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, A. Copper-instigated modulatory cell mortality mechanisms and progress in oncological treatment investigations. Front. Immunol. 2023, 14, 1236063. [Google Scholar] [CrossRef]
- Lutsenko, S. Dynamic and cell-specific transport networks for intracellular copper ions. J. Cell Sci. 2021, 134, jcs240523. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Wang, L.; Ji, G.; Dang, Y. Copper homeostasis and cuproptosis in health and disease. MedComm 2024, 5, e724. [Google Scholar] [CrossRef]
- Xiang, Z.; Mei, H.; Wang, H.; Yao, X.; Rao, J.; Zhang, W.; Xu, A.; Lu, L. Cuproptosis and its potential role in musculoskeletal disease. Front. Cell Dev. Biol. 2025, 13, 1570131. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J. Risks of copper and iron toxicity during aging in humans. Chem. Res. Toxicol. 2010, 23, 319–326. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, J.; Wang, Q.; He, Y.; Chen, Y. Copper nanoclusters trigger muscle cell apoptosis and atrophy in vitro and in vivo. J. Appl. Toxicol. 2016, 36, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Napolioni, V.; Persico, A.M.; Porcelli, V.; Palmieri, L. The mitochondrial aspartate/glutamate carrier AGC1 and calcium homeostasis: Physiological links and abnormalities in autism. Mol. Neurobiol. 2011, 44, 83–92. [Google Scholar] [CrossRef]
- Dahlin, M.; Stödberg, T.; Ekman, E.; Töhönen, V.; Wedell, A. Genetic aetiologies in relation to response to the ketogenic diet in 226 children with epilepsy. Brain Commun. 2025, 7, fcaf134. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Ramoz, N.; Barreto, M.; Gazdoiu, M.; Takahashi, N.; Gertner, M.; Dorr, N.; Gama Sosa, M.A.; De Gasperi, R.; Perez, G.; et al. Slc25a12 disruption alters myelination and neurofilaments: A model for a hypomyelination syndrome and childhood neurodevelopmental disorders. Biol. Psychiatry 2010, 67, 887–894. [Google Scholar] [CrossRef]
- Durdiaková, J.; Warrier, V.; Baron-Cohen, S.; Chakrabarti, B. Single nucleotide polymorphism rs6716901 in SLC25A12 gene is associated with Asperger syndrome. Mol. Autism 2014, 5, 25. [Google Scholar] [CrossRef]
- Ramoz, N.; Reichert, J.G.; Smith, C.J.; Silverman, J.M.; Bespalova, I.N.; Davis, K.L.; Buxbaum, J.D. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am. J. Psychiatry 2004, 161, 662–669. [Google Scholar] [CrossRef]
- Qiu, S.; Qiu, Y.; Li, Y.; Cong, X. Genetics of autism spectrum disorder: An umbrella review of systematic reviews and meta-analyses. Transl. Psychiatry 2022, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Jalil, M.A.; Begum, L.; Contreras, L.; Pardo, B.; Iijima, M.; Li, M.X.; Ramos, M.; Marmol, P.; Horiuchi, M.; Shimotsu, K.; et al. Reduced N-acetylaspartate levels in mice lacking aralar, a brain- and muscle-type mitochondrial aspartate-glutamate carrier. J. Biol. Chem. 2005, 280, 31333–31339. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Wei, Z.; Liu, C.; Li, G.; Feng, Y.; Deng, Y. Solute carrier transporter disease and developmental and epileptic encephalopathy. Front. Neurol. 2022, 13, 1013903. [Google Scholar] [CrossRef] [PubMed]
- Shelton, G.D.; Minor, K.M.; Li, K.; Naviaux, J.C.; Monk, J.; Wang, L.; Guzik, E.; Guo, L.T.; Porcelli, V.; Gorgoglione, R.; et al. A Mutation in the Mitochondrial Aspartate/Glutamate Carrier Leads to a More Oxidizing Intramitochondrial Environment and an Inflammatory Myopathy in Dutch Shepherd Dogs. J. Neuromuscul. Dis. 2019, 6, 485–501. [Google Scholar] [CrossRef]
- Liu, D.; Sartor, M.A.; Nader, G.A.; Pistilli, E.E.; Tanton, L.; Lilly, C.; Gutmann, L.; IglayReger, H.B.; Visich, P.S.; Hoffman, E.P.; et al. Microarray Analysis Reveals Novel Features of the Muscle Aging Process in Men and Women. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1035–1044. [Google Scholar] [CrossRef]
- Jiao, Y.; Kong, N.; Wang, H.; Sun, D.; Dong, S.; Chen, X.; Zheng, H.; Tong, W.; Yu, H.; Yu, L.; et al. PABPC4 Broadly Inhibits Coronavirus Replication by Degrading Nucleocapsid Protein through Selective Autophagy. Microbiol. Spectr. 2021, 9, e0090821. [Google Scholar] [CrossRef]
- Kumar, G.R.; Shum, L.; Glaunsinger, B.A. Importin α-Mediated Nuclear Import of Cytoplasmic Poly(A) Binding Protein Occurs as a Direct Consequence of Cytoplasmic mRNA Depletion. Mol. Cell. Biol. 2011, 31, 3113–3125. [Google Scholar] [CrossRef]
- Nikpay, M. Genome-wide search identified DNA methylation sites that regulate the metabolome. Front. Genet. 2023, 14, 1093882. [Google Scholar] [CrossRef]
- Kini, H.K.; Kong, J.; Liebhaber, S.A. Cytoplasmic Poly(A) Binding Protein C4 Serves a Critical Role in Erythroid Differentiation. Mol. Cell. Biol. 2014, 34, 1300–1309. [Google Scholar] [CrossRef]
- Guo, W.; Li, J.; Huang, H.; Fu, F.; Lin, Y.; Wang, C. LncRNA PCIR Is an Oncogenic Driver via Strengthen the Binding of TAB3 and PABPC4 in Triple Negative Breast Cancer. Front. Oncol. 2021, 11, 630300. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, G.; Liu, Y.; Mei, C.; Yao, Y.; Wu, X.; Chen, X.; Ma, W.; Li, K.; Zhang, Z.; et al. A novel long non-coding RNA RP11-286H15.1 represses hepatocellular carcinoma progression by promoting ubiquitination of PABPC4. Cancer Lett. 2021, 499, 109–121. [Google Scholar] [CrossRef]
- Meng, K.; Lu, S.; Li, Y.-Y.; Hu, L.-L.; Zhang, J.; Cao, Y.; Wang, Y.; Zhang, C.Z.; He, Q.-Y. LINC00493-encoded microprotein SMIM26 exerts anti-metastatic activity in renal cell carcinoma. EMBO Rep. 2023, 24, e56282. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Oliveira, L.D.; Cruz, M.V.; Guimarães, D.; Lima, T.I.; Santos-Fávero, B.C.; Luchessi, A.D.; Pauletti, B.A.; Leme, A.P.; Bajgelman, M.C.; et al. Interaction between poly(A)–binding protein PABPC4 and nuclear receptor corepressor NCoR1 modulates a metabolic stress response. J. Biol. Chem. 2023, 299, 104702. [Google Scholar] [CrossRef]
- Gilding, L.N.; Somervaille, T.C.P. The Diverse Consequences of FOXC1 Deregulation in Cancer. Cancers 2019, 11, 184. [Google Scholar] [CrossRef]
- Prabakaran, A.D.; Montecino-Morales, F.; McFarland, K.; Govindarajan, T.; Durumutla, H.B.; Latimer, H.; Akinborewa, O.; Villa, C.; Millay, D.P.; Quattrocelli, M. The human genetic variant rs6190 unveils Foxc1 and Arid5a as novel prometabolic targets of the glucocorticoid receptor in muscle. Sci. Adv. 2025, 11, eadw2593. [Google Scholar] [CrossRef]
- Caddy, J.C.; Luoma, L.M.; Berry, F.B. FOXC1 negatively regulates BMP-SMAD activity and Id1 expression during osteoblast differentiation. J. Cell. Biochem. 2020, 121, 3266–3277. [Google Scholar] [CrossRef]
- Reza, M.S.; Qiu, C.; Lin, X.; Su, K.-J.; Liu, A.; Zhang, X.; Gong, Y.; Luo, Z.; Tian, Q.; Nwadiugwu, M.; et al. An Attention-Aware Multi-Task Learning Framework Identifies Candidate Targets for Drug Repurposing in Sarcopenia. J. Cachexia Sarcopenia Muscle 2025, 16, e13661. [Google Scholar] [CrossRef]
- Cai, B.; Ma, M.; Chen, B.; Li, Z.; Abdalla, B.A.; Nie, Q.; Zhang, X. MiR-16-5p targets SESN1 to regulate the p53 signaling pathway, affecting myoblast proliferation and apoptosis, and is involved in myoblast differentiation. Cell Death Dis. 2018, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, T.; Takehara, N.; Aonuma, T.; Kano, K.; Horiuchi, K.; Nakagawa, N.; Tanaka, H.; Kawabe, J.-I.; Hasebe, N. Sarcopenia-derived exosomal micro-RNA 16-5p disturbs cardio-repair via a pro-apoptotic mechanism in myocardial infarction in mice. Sci. Rep. 2021, 11, 19163. [Google Scholar] [CrossRef] [PubMed]
- Mellis, A.-T.; Misko, A.L.; Arjune, S.; Liang, Y.; Erdélyi, K.; Ditrói, T.; Kaczmarek, A.T.; Nagy, P.; Schwarz, G. The role of glutamate oxaloacetate transaminases in sulfite biosynthesis and H2S metabolism. Redox Biol. 2021, 38, 101800. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Sicińska, P. Influence of Benzo(a)pyrene on Different Epigenetic Processes. Int. J. Mol. Sci. 2021, 22, 13453. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, S.; Hu, W.; Lu, X.; Lou, N.; Yang, Z.; Chen, S.; Zhang, X.; Yang, H. Valproic acid attenuates skeletal muscle wasting by inhibiting C/EBPβ-regulated atrogin1 expression in cancer cachexia. Am. J. Physiol. Cell Physiol. 2016, 311, C101–C115. [Google Scholar] [CrossRef]
- Park, D.-J.; Kang, J.-B.; Koh, P.-O. Epigallocatechin gallate improves neuronal damage in animal model of ischemic stroke and glutamate-exposed neurons via modulation of hippocalcin expression. PLoS ONE 2024, 19, e0299042. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Li, Q.; Lang, W.; Li, W.; Jiang, X.; Wan, Z.; Chen, J.; Wang, H. Epigallocatechin-3-gallate: A phytochemical as a promising drug candidate for the treatment of Parkinson’s disease. Front. Pharmacol. 2022, 13, 977521. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hou, L.; Han, K.; Zhao, C.; Hu, H.; Yin, S. Epigallocatechin Gallate Promotes Cuproptosis via the MTF1/ATP7B Axis in Hepatocellular Carcinoma. Cells 2025, 14, 391. [Google Scholar] [CrossRef] [PubMed]
| Dataset | Control | Sarcopenia | Platform | Species | Tissue | Type |
|---|---|---|---|---|---|---|
| GSE1428 | 10 | 12 | GPL96 | Homo sapiens | vastus lateralis muscle | Expression profiling by array |
| GSE25941 | 15 | 21 | GPL570 | Homo sapiens | vastus lateralis muscle | Expression profiling by array |
| GSE136344 | 11 | 12 | GPL5175 | Homo sapiens | vastus lateralis muscle | Expression profiling by array |
| Gene | Sequence (5′-3′) | Size (bp) |
|---|---|---|
| GAPDH | F: TGTTTCCTCGTCCCGTAGA R: GATGGCAACAATCTCCACTTTG | 116 |
| SLC31A1 | F: AACCACACGGACGACAACAT R: CAGACCCTCTCGGGCTATCT | 246 |
| FDX1 | F: CGCTAACGACCAAGGGGAAA R: GTAGAGCAAGCCAACGTTCC | 109 |
| SLC25A12 | F: GCGGAAATCCTTGCTGGAGGTT R: TGACTCTCGGTCCTGTGGTGAT | 118 |
| PABPC4 | F: GACCAAAGCTGTCACCGAGA R: GGCACAAAATAGCCACCAGC | 195 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Shi, L.; Li, Y.; Zhang, Z. Decoding Potential Cuproptosis-Related Genes in Sarcopenia: A Multi-Omics Network Analysis. Biology 2025, 14, 1642. https://doi.org/10.3390/biology14121642
Yan H, Shi L, Li Y, Zhang Z. Decoding Potential Cuproptosis-Related Genes in Sarcopenia: A Multi-Omics Network Analysis. Biology. 2025; 14(12):1642. https://doi.org/10.3390/biology14121642
Chicago/Turabian StyleYan, Hongyu, Long Shi, Yang Li, and Zhiwen Zhang. 2025. "Decoding Potential Cuproptosis-Related Genes in Sarcopenia: A Multi-Omics Network Analysis" Biology 14, no. 12: 1642. https://doi.org/10.3390/biology14121642
APA StyleYan, H., Shi, L., Li, Y., & Zhang, Z. (2025). Decoding Potential Cuproptosis-Related Genes in Sarcopenia: A Multi-Omics Network Analysis. Biology, 14(12), 1642. https://doi.org/10.3390/biology14121642





